Abstract
Myelination of the central nervous system is important for normal motor and sensory neuronal function and recent studies also link it to efficient learning and memory. Cyclin-dependent kinase 5 (Cdk5) is required for normal oligodendrocyte development, myelination and myelin repair. Here we show that conditional deletion of Cdk5 by targeting with CNP (CNP;Cdk5 CKO) results in hypomyelination and disruption of the structural integrity of Nodes of Ranvier. In addition, CNP;Cdk5 CKO mice exhibited a severe impairment of learning and memory compared to controls that may reflect perturbed neuron-glial interactions. Co-culture of cortical neurons with CNP;Cdk5 CKO oligodendrocyte lineage cells resulted in a significant reduction in the density of neuronal dendritic spines. In short term fear-conditioning studies, CNP;Cdk5 CKO mice had decreased hippocampal levels of immediate early genes such as Arc and Fos, and lower levels of p-CREB and p-cofilin suggested these pathways are affected by the levels of myelination. The novel roles of Cdk5 in oligodendrocyte lineage cells may provide insights for helping understand the cognitive changes sometimes seen in demyelinating diseases such as multiple sclerosis.
Keywords: oligodendrocyte, Cdk5, myelin, Node of Ranvier, memory
Introduction
Normal learning and memory are dependent on the orchestrated actions of a neuron-glia network in the central nerve system (CNS). Oligodendrocytes (OLs) generate myelin that wraps axons and enhances axonal conduction velocity in the CNS. Oligodendrocyte lineage cells also provide trophic and metabolic support for neurons and other CNS cells (Lee et al., 2012; Nave, 2010). Myelinated axons are found in white and gray matter and recent studies suggest that demyelination and subtle changes in the integrity of both white and gray matter are related to the impairment of cognitive and neuronal function by regulating the velocity and synchrony of impulse conduction in multiple sclerosis (MS) and other neurodegenerative diseases including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ASL) and psychiatric diseases (Desai et al., 2010; Fields, 2008; Kang et al., 2013; Tognatta and Miller, 2016; Yarnykh et al., 2014). Furthermore, recent studies suggested that new oligodendrocyte formation and potentially myelination was required for motor skill learning in adult animals (McKenzie et al., 2014; Xiao et al., 2016).
The function of myelinated axons is dependent on the structure of nodes of Ranvier and the localization of ion channels (Na+ and K+) at nodes. For example, the density and organization of nodes of Ranvier along with the distribution and concentration of Na+ and K+ channels markedly influences axonal conduction velocity and axonal excitability (Fields, 2008). During development, an optimal level of myelination is achieved and hyper - or - hypomyelination results in functional deficits that may reflect perturbation of neuronal connectivity and network activity resulting from changes in myelination such as internodal lengths and/or nodal organization (Edgar and Sibille, 2012; Ritter et al., 2013).
Within the CNS, cyclin-dependent kinase 5 (Cdk5) regulates the development of neurons and OLs. For example, Cdk5 modulates neuronal differentiation, migration, synaptic plasticity and dendritic spine formation during brain development (Su and Tsai, 2011) and is also important for learning and memory formation and hippocampal plasticity through changes in cAMP signaling (Guan et al., 2011), and NR2B trafficking and metabolism (Hawasli et al., 2007; Plattner et al., 2014). Dysregulation of Cdk5 has also been implicated in the pathology of drug addiction, psychiatric disorders and neurodegenerative diseases (Cheung and Ip, 2012). Oligodendrocyte development and myelination are also regulated by Cdk5 and its activators (Luo et al., 2016). For example, targeted deletion of Cdk5 to Olig1+ cells disrupted the transition of OPCs to mature OLs, compromised process formation in mature OLs and delayed myelination (He et al., 2011; Yang et al., 2013). Targeting deletion of Cdk5 in CNP+ oligodendrocyte lineage cells had less effect on myelin development but inhibited myelin repair following focal LPC induced demyelination through modulation of the Akt and Gsk-3beta signaling pathways (Luo et al., 2014) and inhibited myelin repair in the chronic cuprizone toxicity demyelinating model (Bankston et al., 2013). In current study, we examine the consequences of Cdk5 deletion in CNP+ oligodendrocyte lineage cells (CNP;Cdk5 CKO) in the absence of any additional insult. We show a significant reduction in the level of myelination and perturbations in the number and organization of nodes of Ranvier in multiple CNS regions of older animals. These changes are associated with severely impaired learning and memory and decreased levels of immediate early genes (Arc and Fos), p-CREB and p-cofilin in the hippocampus. Co-culture studies demonstrate changes in neuronal dendritic spine and synapse maturation in the absence of Cdk5 in oligodendrocyte lineage cells. Such changes likely contribute to abnormal cognitive function seen in CNP;Cdk5 CKO animals, and may offer new insights for enhancing myelin repair and neuronal function in demyelinating diseases and other neurodegenerative diseases.
Materials and Methods
Animals
All animal experiments were done in compliance with approved animal policies of the Institutional Animal Care and Use Committee (IACUC) at CWRU University. Floxed Cdk5 and CNP Cre/+ mouse breeding pairs were obtained from Dr. LH Tsai (Massachusetts Institute of Technology) and Dr. K.A. Nave (Max Planck Institute, Germany) respectively. To produce conditional knockout of CNP Cre/+;Cdk5 fl/fl CKO mice (CNP;Cdk5 CKO), floxed Cdk5fl/fl mice were crossed with double-transgenic CNPCre/+;Cdk5fl/+mice as previously described (Luo et al., 2014) and genotyped using tailed genomic DNA PCR (Lappe-Siefke et al., 2003; Luo et al., 2014). For comparative experiments, CNPCre/+;Cdk5fl/fl animals are used as CNP;Cdk5 CKO; and sex- and age-matched animals of heterozygotes CNPCre/+;Cdk5+/+ served as controls as they had no detectable phenotype. The animals were generated and maintained on a C57BL/6 background and housed in a vivarium with a 12 -h light/dark cycle. No more than 4 adult mice were housed in the same cage. Mice of both sexes were used with littermate controls unless otherwise indicated.
Behavior tests
At least a 16–18 group of sex- and age-matched (8 weeks) controls and CNP;Cdk5 CKO animals were used for cognitive functional tests including: learning and memory (T-maze, passive avoidance test and contextual fear conditioning), motor function (open field and rotarod test) and anxiety test (elevated plus maze). All tests were conducted in a blinded fashion at the Case Rodent Animal Behavior Core and performed during the light phase. In the first week, the mice underwent rotarod test on day 1, open field assayed on day 3 and elevated plus maze on day 5. In the second week, the mice were subjected to the T-maze on day 1 and passive avoidance test on day 3. In the third week, the mice underwent contextual fear conditioning test. To minimize stresses, animals were transported and acclimated to a testing room 2 hours before the tests. All test apparatus was cleaned with 5% ethanol to avoid instinctive odorant between animals.
T-maze test
A clear-plexiglass T-maze apparatus (with 60 cm length of arms) was used and recorded using Ethovision video tracking system. A tested animal was placed in the apparatus with one blocked arm and allowed to explore the maze freely for 10 min. To avoid any arm preference bias, the blocked arm was switched randomly between tested animals. After 10 minutes the test mouse was returned to the home -cage for 3h before being placed back in T-maze with two opened arms for 5min. The time spent and the frequency in the previously blocked arm, and the total number of arm entry were recorded indicating a challenge for short-term spatial memory of hippocampal and forebrain function.
Passive avoidance test
A two-compartment step-through passive avoidance apparatus was used to evaluate memory based contextual fear conditioning and instrumental learning memory (Ogren et al., 1985). A decrease in retention latency indicates impaired memory. On training day 1, mice were placed into the light compartment and allowed to freely explore light and dark chambers. On the following day 2, the animal was placed in the light compartment and when it stepped into the dark compartment, the inter-compartment door closed and a foot shock (0.5 mA/2 sec) delivered. The mouse was returned to the home cage after 1 min in the dark. The time taken to enter the dark compartment (training latency) was recorded. On the day 3 (24 hours after the acquisition trial), in the retention trial, animals were placed in the illuminated compartment without foot shock and the step-through latency recorded (maximum a cutoff time of 300s) and used as an index of retention of learned experiences.
Contextual and Cued Fear Conditioning Test
The contextual fear-conditioning test was performed as previous described (Yu et al., 2014). In the training phase on day 1, an individual mouse was placed in a conditioning chamber (Med Associates, Burlington, VT) equipped with stainless grids and allowed to explore freely for 2 min (preconditioned phase). A tone (80dB, 2800 Hz) was presented as a conditioned stimulus for 30 sec, and a foot shock (0.5mA, 2 sec) was delivered as an unconditional stimulus through a shock generator 2.5 sec after the tone stimulus. A total of 4 tone-shock pairs were repeated and the mouse-freezing response recorded. After 24 hours, retention tests were performed with mice placed into the conditioning chamber and the freezing response recorded for 5 min in the absence of the tone. Two hours after the contextual freezing was measured, mice were introduced in the contextually altered chamber with flatted plastic floor for 6 minutes. During the first 3 minutes, freezing response was measured in the absence of the tone; and in the remaining 3 minutes, the tone stimulus was delivered and freezing behavior measured to assess cued fear conditioning.
Open Field Test
Spontaneous motor activity was assessed in a bright and illuminated wooden, square, arena. Mice were placed in the center of the clear arena in standard room-lighting conditions for evaluation of locomotor activity. The total distance travelled was tracked and quantitated by a video EthoVision system for 15-min test period. The number of entries, the time spent in the center zone and the total distance traveled were monitored.
Rotarod test
Motor learning and motor coordination were evaluated with the accelerating-rotarod test. Controls and CNP;Cdk5 CKO mice were kept in their home cages and acclimate in the testing room for at least 15 min. In training phase, mice were placed on the rotarod with a constantly speed at 4rpm walking forward for 60 sec. All animals were given a session consisting of three training trails with a 10 min inter -trial interval and at least a 30 min break between training and testing phases. In the test phase, the speed of the rotarod gradually accelerated from 4 to 40 rpm within 5 min. The latency and speed of each mouse falling off from the rotarod was recorded. Three test trials were performed and recorded with at least 30 min inter-trial intervals.
Elevated Plus-Maze Test
The elevated plus-maze apparatus consisted of total four arms of equal dimensions (50cm × 10cm). Two arms were enclosed by 40-cm-high blocks and were arranged perpendicularly to two opposite open arms. Following acclimatization an individual mouse was placed in the center of the maze facing an open arm. Entries and time spent in the open arm were recorded for 5 min using a video system and used as measures of anxiety-like behavior.
Immunohistochemistry
Mice were anaesthetized with Avertin and perfused intracardially with 4% paraformaldehyde in PBS. Dissected optic nerves and brain were post-fixed in 4% paraformaldehyde overnight at 4°C and 20μm frozen sections of optic nerve (longitudinal) and brain sections (coronal) collected. For immunohistochemistry, antigen retrieval was applied with reveal decloaker solution (Biocare Medical). Sections were incubated with primary antibodies overnight at 4°C. Primary antibodies include: MBP (SMI-99, 1:500, Covance); Olig2 (AB9610, Millipore, 1:250) and GFAP (MAB360, Millipore, 1:250); CC1 (OP80, Millipore, 1:250); Iba1 (019-19741, WAKO, 1:250); Caspr (MABN69, Millipore, 1:100); Nav1.6 (ASC-009, Alomone Labs, 1:100) and Kv1.1 (APC-009, Alomone Labs, 1:100); Neurofilament 200 (N4142, Sigma, 1:250); Cre (#69050-3, Novagen, 1:250); NeuN (ab104224, Abcam, 1:250); Cdk5(sc-173, Santa Cruz, 1:200); NG2 (AB5320, Millipore, 1:250); Ki67 (#556003, BD pharmingen, 1:100). Primary antibodies were visualized with fluorescence-conjugated secondary goat anti-mouse IgG or goat anti-rabbit IgG FluoAlexa-594 or 488 antibodies (A-11032, A-11001, A-11008, A-11012, Invitrogen, 1:500). Nuclei were stained with DAPI (1:1000, sigma) and images collected using a Leica DFC500 fluorescence microscope.
Electron microscopy analysis and Toluidine blue staining
For ultrastructural analyses, animals were perfused with 2% glutaraldehyde/4% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH7.4 (Electron Microscopy Sciences). Brain and optic nerves were post-fixed in 1% OsO4, dehydrated through a series of graded ethanols, stained using saturated uranyl acetate and embedded in a Poly/Bed812 resin (polysciences Inc.). For Toluidine blue staining, 2um-thick transverse sections were cut using a Leica Ultracut UCT ultramicrotome (Leica Microsystems, Germany) and stained with 1% Toluidine blue and washed with PBS. To analysis myelination and the structure of node of Ranvier, thin section (1um) and ultrathin section (0.1um) from matching areas of CNP;Cdk5 CKO and control tissue blocks were cut and visualized using an electron microscope (JEOL100CX) at 80 kV. The g -ratio, defined as the ratio of the inner axonal diameter to the total outer diameter, was calculated from at least 150–200 randomly selected myelinated axons from 3–5 controls and CNP;Cdk5 CKO animals.
Black-Gold II myelin staining
20μm frozen sections were incubated in pre-warmed 0.3% Black-Gold II solution (Histochem, Jefferson AR) at 60°C for 30–40 min according to the manufacture’s instruction. Sections were washed in distilled water, fixed in 2% sodium thiosulfate for 3 min. Staining was visualized using a Leica DFC500 fluorescence microscope.
In situ hybridization
A 969 bp probe for proteolipid protein (PLP) was generated using PCR primers from Allen Brain Atlas flanked with T7/T3 (T3 forward, 5′-AATTAACCCTCACTAAAGGGGGGGATGCCTGAGAAGGT3′; and T7 reverse, 5′-GTAATACGACTCACTATAGGGCGTGTGATGCTTTCTGCCCA-3′). PCR products were generated using isolated RNA and the SuperScript One-Step RT-PCR kit (Invitrogen), re-amplified, and confirmed by sequencing. Probes were then transcribed using the Ambion Maxiscript transcription kit (Invitrogen) and digoxigenin-11-UTP (Roche), using either T3 for the sense control probe or T7 for the antisense probe. In situ hybridization was performed according to the published protocol (www.mskcc.org/research/lab/alexandra-joyner/laboratory-protocols). Probes were diluted 1:50 in hybridization solution and hybridized overnight at 55°C–60°C.
Nissl staining
Coronal sections (20um) were mounted on slides and dried overnight at room temperature. Sections were stained subsequently with 0.1% cresyl violet (sigma) for 20 min. Slides were then dehydrated in 100%, 95% and 70% ethanol, and cleared in histo-clear II (National Diagnostics), coversliped with cytoseal™ 60 (Thermo Scientific).
Biochemical analysis
Tissues (optic nerve, hippocampus, cortex and corpus callosum) were micro-dissected and homogenized with RIPA lysis buffer. Equal amounts of proteins were loaded and separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 0.1% Tween20 PBS buffer containing 5% BSA overnight at 4°C and incubated with primary antibodies followed by anti-mouse or anti-rabbit IgG conjugated to HRP. Enhanced chemiluminescence was performed with a West Pico Kit and detected by FluorChem E system (ProteinSimple, USA). The following primary antibodies were used: Cdk5 (sc-173, Santa Cruz, 1:1000); PLP (ab28486, Abcam, 1:1000); Caspr (MABN69, Millipore, 1:1000); MBP (SMI-99P, Covance, 1:1000); CREB (#9197, Cell signaling, 1:1000) and p-CREB (#9198, Cell signaling, 1:1000); Cofilin (#5175, Cell signaling, 1:1000) and p-cofilin (#3311, Cell signaling, 1:1000); Fos (#4384, Cell signaling, 1:1000) and Arc (sc-17839, Santa Cruz, 1:1000) and β-actin (sc-47778, Santa Cruz, 1:1000). The density of immunoblots were quantified against normalized internal loading control using ImageJ software.
OPC cultures
Mouse OPC cultures were prepared using immunopanning as previously described (Yang et al., 2011). Postnatal day 1–2 mouse brains were dissected and cerebral cortices were minced and digested in 0.1% Trypsin-EDTA at 37 °C for 30 min. After titration and centrifugation, dissociated cells were incubated on pre -coated culture dishes with secondary antibody IgM (10 μg/ml) (Millipore, Cat # 55460) and primary antibody A2B5 for 30 min at 37 °C. Following wash-off non-adherent cells, OPC were released by 0.05% Trypsin in DMEM and collected at a purity of approximately 96%. OPCs were expanded in DMEM/F12 based-medium supplemented with N2, 5 ng/ml NT-3, 10 ng/ml CNTF, 20 ng/ml bFGF and 20 ng/ml PDGF-AA.
Oligodendrocyte conditional media
Enriched OPCs were plated at a high density of 6×105/well in 6-well plate in serum-free DMEM/F12 based medium supplemented with N2, Glutamax, 5 ng/ml NT-3 and 10 ng/ml CNTF. After 2 days, cells were gently washed with DMEM/F12 medium and conditioning medium (DMEM/F12 containing N2 and Glutamax) added in the culture. After 24 hours, the conditioned medium was harvested and passed through centrifugal filter (Amicon ultra-4 3K, Cat#: UFC800324) at 1000rpm for 5 min. The supernatant was stored at −80°C and used at a dilution of 1:25 for neuronal cell culture.
Cortical or hippocampal neuronal cultures
Cortical and hippocampal neuronal cell cultures were prepared from E18 mouse brain as described previously (Romito-DiGiacomo et al., 2007). Tissues were dissected and trypsinized for 15–20 min at 37 °C. Dissociated neuronal cells were plated on PLL-Laminin-coated, 24-well coverslip, respectively, and grown in Neurobasal media supplemented with 2% B27, 2mM Glutamax and 1% penicillin/streptomycin. For co-culture, 48 hours after initial plating cortical or hippocampal neurons were co-cultured with purified WT or Cdk5−/− OPCs; or treated with conditional media derived from either WT or Cdk5−/− OPCs and the density and maturation of neuronal dendritic spine and synapse formation assayed.
Immunocytochemistry
Cultured cells were fixed in 4% paraformaldehyde, blocked with 5% normal goat serum and 0.1% Triton X-100 in PBS and incubated in primary antibodies (MAP2, M4403, sigma; Synapsin-1, #106103, Synaptic Systems) overnight at 4 °C. After rising in PBS, Alexa fluoresces-conjugated secondary antibodies 488 or 594 (1:500, Invitrogen) were incubated for 2 hours. The coverslips were mounted with Vecta Shield mounting medium (Vector Laboratories) and visualized by fluorescent microscopy.
Statistical analyses
Data were collected from control and CNP-Cdk5 CKO animals (N=3–5 in each group) and presented as mean±SEM. Statistical analysis was performed by two-tailed unpaired Student’s t tests, one-way ANOVA or two-way repeated measures ANOVA with Sidak’s multiple comparisons test or Tukey’s multiple comparison test. Quantifications were performed in a blinded fashion.
Results
CNP;Cdk5 CKO mice exhibit a maturation dependent reduction of myelination
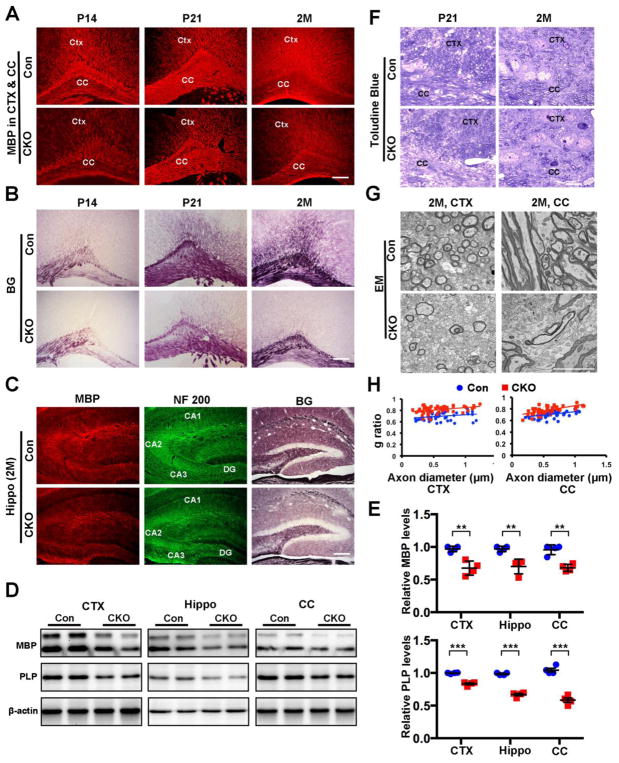
Early oligodendrocyte development and myelination were only slightly affected in CNP;Cdk5 CKO animals and comparison of the levels of myelination in different brain regions of CNP;Cdk5 CKO and control animals points showed no detectable differences at P7 and only slightly reduced MBP expression at P14. The differences in myelin expression in the corpus callosum and cortex of control and CNP;Cdk5 CKO animals became evident at P14 and increasingly pronounced at P21 and 2M suggesting a late onset developmental defect (Fig. 1A and Supplementary Fig. S1A, B). This age-related reduction in myelination of CNP;Cdk5 CKO animals was confirmed with Black Gold II myelin staining, which labels both normal and pathological myelin (Fig. 1B).
Figure 1. Decreased expression of MBP and PLP myelin protein and reduced myelin thickness in brain of CNP;Cdk5 CKO animals.
(A–B) Representative immunostained images of MBP (A) and Black Gold II (B) myelin staining in the cortex (CTX) and corpus callosum (CC) of CNP;Cdk5 CKO mice at P14, P21 and 2M and their age-matched control littermates. Quantitative analysis of MBP intensity level at P7, P14, P21 and 2 months is shown Fig S1B. (C) Immunostaining of 2M specimens for MBP and Black Gold II (BG) shows hypomyelination in CNP;Cdk5 CKO hippocampus (Hippo). Axons stained with neurofilament (NF 200) are unaffected. (D) Western blot analysis of MBP and PLP protein expression in CTX, Hippo and CC of 2-month-old CNP;Cdk5 CKO mice and control littermates. (E) Quantification of relative protein expression level of MBP and PLP in CNP;Cdk5 CKO and control animals. (F -G) Reduction of myelin and fewer myelinated axon in CTX and CC of CNP;Cdk5 CKO at P21 and 2-month old was confirmed by toluidine blue staining (F) and EM analysis (G). (H) G-ratio analysis revealed the reduction of myelin thickness in the CTX and CC of P 21 and 2-month-old CNP;Cdk5 CKO mice compared with controls. Values are mean ± SEM. In E: **P < 0.01. ***P < 0.001. n = 4 animals per genotype. two-tailed unpaired Student’s t-test. Scale bars: a-c = 200 μm; f = 25 μm and g = 10 μm.
Reduced myelination was widespread throughout the CNS of CNP;Cdk5 CKO animals compared to controls at 2 months of age including in the hippocampus as visualized by anti-MBP and Black Gold II myelin staining (Fig. 1C). Likewise, semi-quantitative analysis by western blots confirmed significant reductions of MBP and PLP protein levels in the corpus callosum, cortex, and hippocampus of 2-month-old CNP;Cdk5 CKO mice compared with age-matched littermate controls (Fig. 1D,E). The extent of myelin reduction was evident by ultrastructural analysis. For example, at 2 months of age the proportion of myelinated axons in the corpus callosum and cortex was reduced (Fig. 1F,G) and g-ratio analyses revealed thinner myelin sheaths in CNP;Cdk5 CKO mice compared with controls (Fig. 1H. CTX: Con, g-ratio= 0.696844324 ±0.071197221; CKO, g-ratio=0.813446314±0.063471558; P<0.05. CC: Con, g-ratio= 0.706150444 ±0.052582414; CKO, g-ratio=0.777315548±0.066164728; P<0.05. Two-tailed unpaired Student’s t test). These data suggest that selective loss of Cdk5 in oligodendrocyte lineages results in a persistent reduction of myelination.
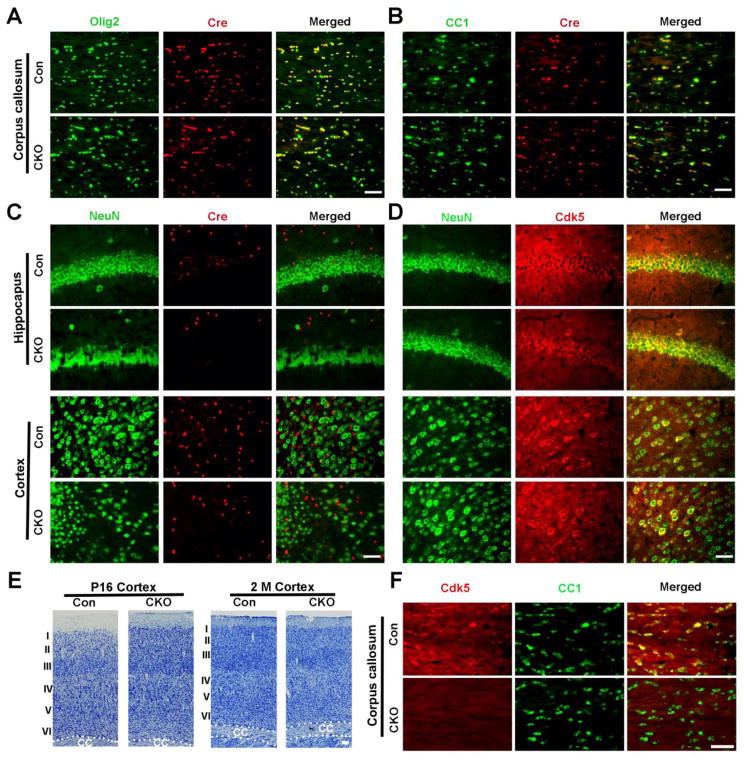
Deletion of Cdk5 through targeting with the CNP promoter results in selective loss of Cdk5 in oligodendrocytes. Previous studies suggest that in some CNP promoter -driven transgenic lines, multipotent precursors and a subset of hippocampal cells may express GFP in addition to oligodendrocytes (Aguirre and Gallo, 2004; Belachew et al., 2003). In the present study however, Cre expression was restricted to and expressed in the majority of Olig2+ cells and CC1+ OLs in both control and CNP;Cdk5 CKO mice (Fig. 2A, B; Supplementary Fig. 1C, D). Cdk5 was expressed in CC1+ cells in the corpus callosum of control animals, but absent in CNP;Cdk5 CKO mice (Fig. 2F; Supplementary Fig. 1E). In addition western blot analysis demonstrated reduced Cdk5 expression in the brain of CNP;Cdk5 CKO mice compared to controls (Supplementary Fig. 2A) that was specific to the oligodendrocyte lineage. Double labeling of CNP;Cdk5 CKO tissue sections with the neuronal marker NeuN and anti-Cre recombinase shown a lack of coincident expression in either hippocampal or cortical (NeuN+) neurons (Fig. 2C) even though Cdk5 was clearly expressed in those cells (Fig. 2D) and cortical lamination appeared relatively normal (Fig. 2E). Cre expression was also absent from GFAP+ astrocytes (Supplementary Fig. 2B) suggesting the morphological changes seen in CNP;Cdk5 CKO animals reflected loss of Cdk5 in oligodendrocyte lineage cells.
Figure 2. CNP-targeted deletion of Cdk5 is restricted to oligodendrocyte lineages in CNP;Cdk5 CKO mice.
(A–B) Double immunostaining showed co-localization of Cre and olig2+ (A) and CC1+ (B) in the corpus callosum of 2-month-old CNP;Cdk5 CKO and controls. (C) Lack of Cre expression in either hippocampal or cortical neurons identified by NeuN in CNP;Cdk5 CKO mice and controls. (D) Cdk5 expression presents in NeuN+ cells in the cortex and hippocampus of CNP;Cdk5 CKO mice. (E) Representative Nissl-stained images show relatively normal cortical lamination in CNP;Cdk5 CKO at P16 and 2-months of age. (F) Lack of Cdk5 expression in CC+ cells in the corpus callosum of 2-month-old CNP;Cdk5 CKO mice. Scale bars = 50 μm.
The proliferation of OPCs was not significantly affected in CNP;Cdk5 CKO animals (Supplementary Fig. 4A, B) neither was the total number of NG2+ cells and mature CC1+ OLs (Supplementary Fig. 3A–F) affected at any age examined. In situ hybridization confirmed that there were similar numbers of differentiated PLP+ cells at P21 and 2M in the corpus callosum of CNP;Cdk5 CKO and controls (Supplementary Fig. 4C, D). The thinner myelin sheaths seen in CNP;Cdk5 CKO mice may reflect reduced cytoskeletal motility since Cdk5-deficient OLs develop fewer cellular processes and branches (Yang et al., 2013). No obvious changes in the morphology or number of GFAP+ astrocytes (Supplementary Fig. 5A), Iba1+ microglia (Supplementary Fig. 5B) or NF200+ axons were detected in CNP;Cdk5 CKO animals compared to controls (Supplementary Fig. 5C).
Disrupted myelination and nodes of Ranvier in the optic nerve of CNP;Cdk5 CKO mice
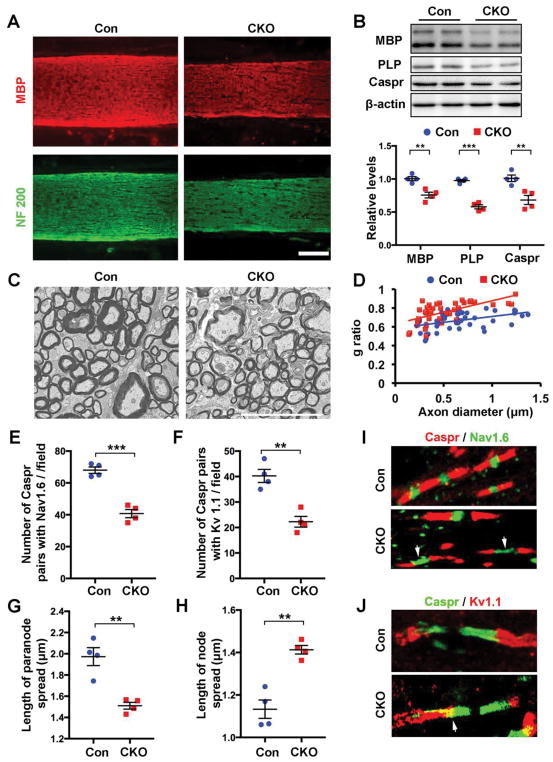
A critical element of myelinated axons are nodes of Ranvier, whose formation is dependent on myelination and myelin maintenance (Poliak and Peles, 2003). The onset of myelination coincides with the clustering of voltage-dependent Na+ channels at developing nodes while paranodal regions contribute to establishing the size and composition of nodes (Rasband and Shrager, 2000; Rasband et al., 1999; Trapp and Kidd, 2000). Given that optic nerve myelination was reduced in CNP;Cdk5 CKO as evidenced by reduced expression of PLP and MBP (Fig. 3A,B), thinner myelin sheaths and significant changes in g-ratio (Fig. 3C,D. Con: g-ratio= 0.662837±0.087069; CKO: g-ratio=0.756208±0.110571; P<0.05. Two-tailed unpaired Student’s t test) while neurofilament (NF200) labeling was normal suggested relatively intact axonal organization (Fig. 3A), the architecture and number of optic nerve nodes were assayed. In CNP;Cdk5 CKO optic nerves the architecture of nodes of Ranvier was disrupted. Analysis of Caspr+ pairs associated with the Na+ channel protein Nav1.6 and juxtaparanodal region associated with the K+ channel protein Kv1.1 revealed disorganization in CNP;Cdk5 CKO optic nerve with significantly reduced expression and numbers of Caspr+ pairs compared to controls (Fig. 3E–J; Supplementary Fig. 6A). The distance between Caspr+ pairs (node spread or nodal gap) was significantly larger (Fig. 3F, I) and the paranodal length was decreased in CNP;Cdk5 CKOs compared to controls (Fig. 3G, I). The distribution of Na+ and K+ channels was also perturbed in CNP;Cdk5 CKO nodes including a decrease in the total number of double -labeled Caspr/Nav1.6 nodes and a dispersal of Nav1.6 channel distribution compared to controls (Fig. 3E, F; Supplementary Fig. 6A). Similarly, Kv1.1 channel protein was diffuse and its distribution overlapped with Caspr+ in paranode domains (Fig. 3J). Ultrastructural analysis confirmed the abnormality of nodal structures in CNP;Cdk5 CKO (Supplementary Fig. 6B) including less compact and thinner myelin and disorganized paranodal junctions, suggesting a reduction of axon-oligodendrocyte interactions in Cdk5 deficient OLs.
Figure 3. Disrupted myelination and nodes of Ranvier in the optic nerve of CNP;Cdk5 CKO mice.
(A–B) Reduction of MBP and PLP expression in optic nerve of 2-month-old CNP;Cdk5 CKO mice shown by immunostaining of MBP (A, red) and Western blot analysis (B) compared with controls. Quantitative analysis is shown below the gels. (C) EM and (D) G-ratio analysis reveals reduced myelin thickness in the optic nerve of 2-month-old CNP;Cdk5 CKO mice compared to controls. (E–H) Disorganized and reduced number of Caspr/Nav1.6 or Caspr/Kv1.1 pairs in the optic nerve of 2-month-old CNP;Cdk5 CKO compare to controls. (I–J) Confocal images showed disrupted organization and abnormal distribution of Nav1.6 and Kv1.1 in CNP;Cdk5 CKO (arrows) compared to control. Values are mean ± SEM. n = 4 animals per genotype. **P < 0.01. ***P < 0.001. In 3B, E–H: two-tailed unpaired Student’s t-test. Scale bars: B = 100 μm; D = 10μm.
Widespread expression of node abnormalities in CNP;Cdk5 CKO mice
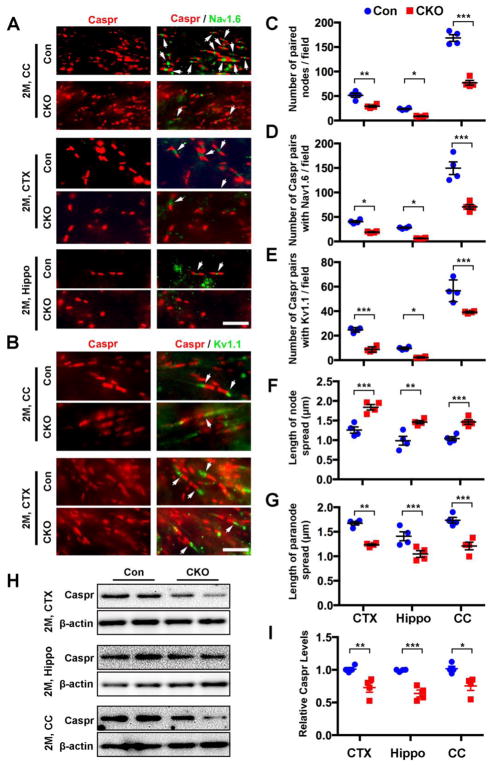
The perturbation of node architecture was not restricted to the optic nerve. Disorganized and significantly decreased numbers of Caspr+ pairs, with elongated nodal gaps and shorted paranodes were found in the corpus callosum, prefrontal cortex and dorsal hippocampus of CNP;Cdk5 CKO mice (Fig. 4A–G). Quantitative analysis revealed significant differences in the length of nodes and the length of paranodes between CNP;Cdk5 CKO and control mice (Fig. 4F,G). Biochemical analysis demonstrated significantly reduced Caspr protein levels in CNP;Cdk5 CKO mice (Fig. 4H,I). Likewise, fewer Caspr+/Nav1.6 pairs and Caspr+/Kv1.1 pairs were detected in corpus callosum, prefrontal cortex and dorsal hippocampus of CNP;Cdk5 CKO compared with controls (Fig. 4A–E) suggesting the loss of Cdk5 in OLs results in aberrant distribution of ion channels at nodes of Ranvier in both gray and white matter.
Figure 4. Widespread expression of node abnormalities in white and grey matter regions of CNP;Cdk5 CKO mice.
(A–B) Reduction in the number of Caspr/Nav1.6 (A) and Caspr/Kv1.1 (B) pairs in the prefrontal cortex (CTX), dorsal hippocampal (Hippo) and corpus callosum (CC) of 2-month-old CNP;Cdk5 CKO mice. (C–G) Quantification of the number of paired nodes (C), pairs of Caspr/Nav1.6 (D) and Caspr/Kv1.1 (E), node spread (F), and paranode length (G) in CNP;Cdk5 CKO mice. (H–I) Western blot analysis showed reduction of Caspr expression level in CTX, Hippo and CC of adult CNP;Cdk5 CKO mice. Values are mean ± SEM, n = 4 animals/group. *p < 0.05. **P < 0.01. *** P < 0.001. In C–G: Two-way ANOVA with Sidak’s multiple comparisons test. In I: two-tailed unpaired Student’s t-test. Scale bars = 100 μm.
A previous study demonstrated that reduced expression of CNP disturbs axoglia interaction resulting in mis-localized Caspr and decreased clustering of Na+ channels in aged CNP1-null mice (Rasband et al., 2005). To ensure the changes in nodal architecture in CNP;Cdk5 CKO animals reflected loss of Cdk5 and not reduced CNP protein, nodal numbers and architecture were analyzed in CNP+/−;Cdk5+/+ mice and no significant changes were observed in the expression of Caspr or Nav1.6 or in the number of Caspr+/Nav1.6 pairs in CNP+/−;Cdk5+/+ compared to CNP+/+;Cdk5+/+ mice (Supplementary Fig. 7A, B) suggesting the abnormal phenotypes in CNP;Cdk5 CKO mice were unlikely to be caused by reduced levels of CNP.
Impairment of learning and memory in CNP;Cdk5 CKO mice
Changes in myelination levels have recently been linked to learning and memory (Fields, 2008; McKenzie et al., 2014; Yarnykh et al., 2014). To determine whether the changes seen in myelination and node formation in CNP;Cdk5 CKO animals influenced CNS function, animals were subjected to a battery of behavioral tests including T-maze, passive avoidance and fear conditioning tests.
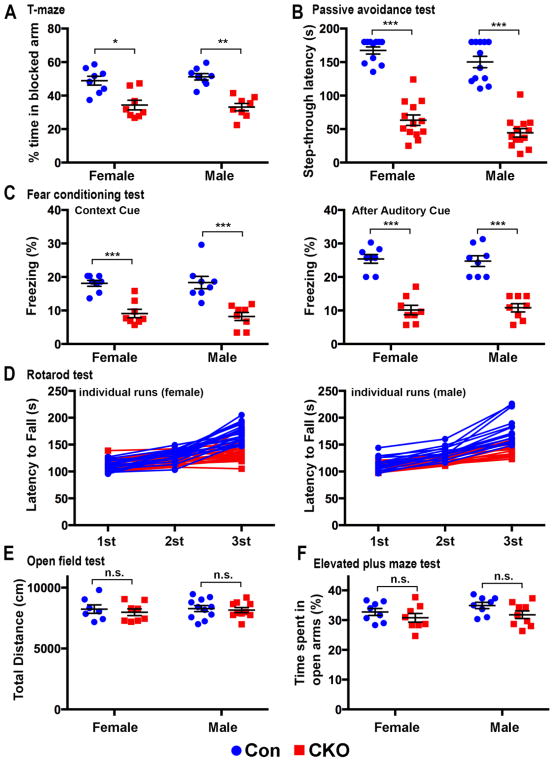
In the T-maze test for short-term spatial memory, CNP;Cdk5 CKO mice (8 weeks of age) spent significantly less time exploring the previously blocked arm than controls (Fig. 5A), consistent with impaired hippocampal and forebrain function. In a passive avoidance test to assess memory consolidation and storage, CNP;Cdk5 CKO mice showed significantly decreased latency to enter the dark box consistent with a decreased ability to store long-term memory (Fig. 5B). In the fear-conditioning test to evaluate the hippocampus- and amygdala-dependent associative learning. CNP;Cdk5 CKO mice had significantly decreased freezing responses compared with controls in both context-dependent and cued-dependent memory (Fig. 5C). Although male animals were more variable, the differences between the genotypes were statistically significant for both sexes. In rotarod tests of motor abilities both CNP;Cdk5 CKO mice and controls performed comparably in the first two trials on the accelerating rotarod (Fig. 5D), however while control animals showed improved performance during the third trial, CNP;Cdk5 CKO animals did not (Fig. 5D. female -con vs female-CKO: 170.9±3.8 vs 135.5±3.3, p<0.0001; male-con vs male-CKO: 177.8±6.8 vs 141.3±3.3, p<0.0001. two-way ANOVA with Sidak’s multiple comparison test). Evaluation of overall locomotor activity in an open field test indicated no significant differences between CNP;Cdk5 CKO and controls (Fig. 5E). No significant differences between CNP;Cdk5 CKO and controls in the time spent exploring open arms (Fig. 5F) were seen in an elevated plus maze test to assess anxiety-like behavior. Together, these data suggest the selective deletion of Cdk5 in oligodendrocyte lineage cells results in selective impairment of learning and memory.
Figure 5. Impairment of learning and memory in CNP;Cdk5 CKO mice.
(A) T maze test showed that CNP;Cdk5 CKO mice (8 weeks of age) spent significantly less time in blocked arm (n = 8 mice/group). (B) The passive avoidance test: CNP;Cdk5 CKO mice spent significantly less time in the dark compartment (n = 11–13 mice/group). (C) Fear conditioning test: CNP;Cdk5 CKO had significant lower levels of freezing in the context-dependent associative memory and cued-dependent memory tests (n = 8 mice/group). (D) Rotarod test: control mice showed a significant improvement in motor coordination while CNP;Cdk5 CKO mice did not (n = 13–23 mice/group). (E) No changes were seen in locomotor activities in an open field test. (F) Elevated plus maze: anxiety-like behavior was equivalent in CNP;Cdk5 CKO mice and controls (n = 7–11 animals/group). Values are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Two-way ANOVA with Sidak’s multiple comparisons test. n.s. = not significant.
Reduction of synaptic and dendritic spine density in CNP;Cdk5 CKO mice
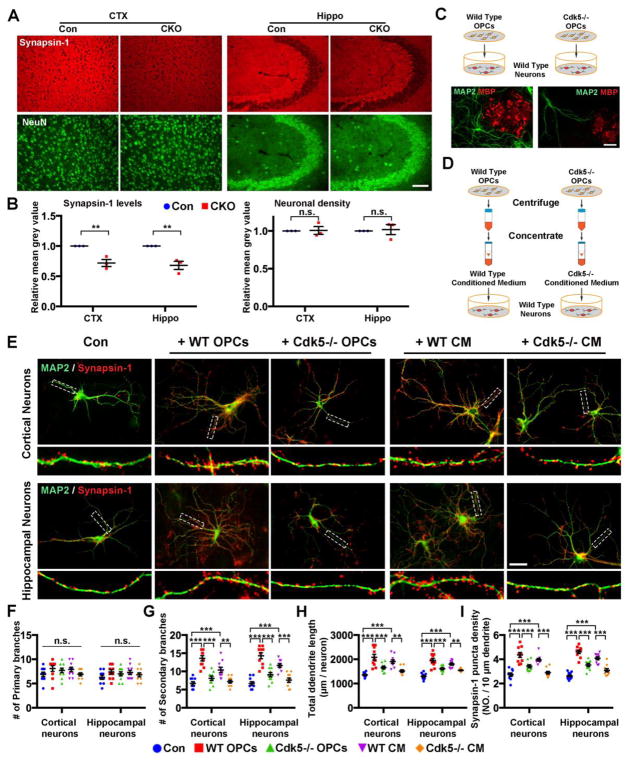
Synaptic density was reduced in CNP Cdk5 CKO animals compared to controls. A significantly lower expression of synapsin-1 in cortical and hippocampal neurons (Fig. 6A, B) was apparent even though the total number of neurons appears normal suggesting that OL Cdk5 deficiency does not affect neurogenesis. Co-culture of WT cortical or hippocampal neurons with WT OPCs (Fig. 6C, D) enhanced their survival, maturation, synaptic density and process outgrowth as shown by increased secondary branching and synaptic density (Fig. 6E–I, red dots). The effect was mediated by diffusible factors and largely reproduced by OPC-derived conditioned medium (Fig. 6E–I, magenta dots). Cultures of CNP;Cdk5 CKO OPCs while not neurotoxic were far less effective in stimulating dendritic growth or synaptic density (Fig. 6E–I, green dots) and their CM was ineffective (Fig. 6E–I, orange dots). These results suggest that expression of Cdk5 in OPCs/OLs modulates their capacity to influence neuronal dendritic spine maturation and dendritic synaptic development.
Figure 6. Decreased synaptic and dendritic spine density in the hippocampus and cortex of CNP;Cdk5 CKO mice.
(A) Decrease in expression of Synapsin-1 in cortex and hippocampus of CNP;Cdk5 CKO mice compared to controls with no obvious change in the number of neurons. (B) Quantification of relative density of Synapsin-1 and NeuN+ neurons. Values are mean ± SEM (n = 3 mice/group, 12 sections/brain/genotype). (C–D) Diagrams illustrate the experimental design of the co-culture experiments (C) or conditioned medium experiments (D). The photomicrograph shows neurons (Map2, green) cultured with OPCs (MBP, red) from either WT or Cdk5−/− animals. (E) Double immunostaining of synapsin-1 (red) and MAP2 (green) revealed reduction of synapsin-1 expression in co-culture of WT cortical/hippocampal neurons and Cdk5−/− OPCs or Cdk5−/− OPC-conditioned media (CM). Magnified images from the boxed regions are shown under each image. (F–I) Quantification of primary (F) and secondary branches (G), total dendritic length (H) and the density of synapsin-1 puncta (I) of WT neuron co-cultured with WT OPCs (red), Cdk5−/− OPCs (green), WT OPC-CM (megenta) or Cdk5−/−-CM (orange). Values are mean ±SEM (n = 10 neurons/group and repeated three times). **p < 0.01, ***p < 0.001. In B: two-tailed unpaired Student’s t-test. In F-I: One-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant. Scale bars = 50 μm.
Induction of Arc, Fos, p-CREB and p-cofilin were suppressed in CNP;Cdk5 CKO mice
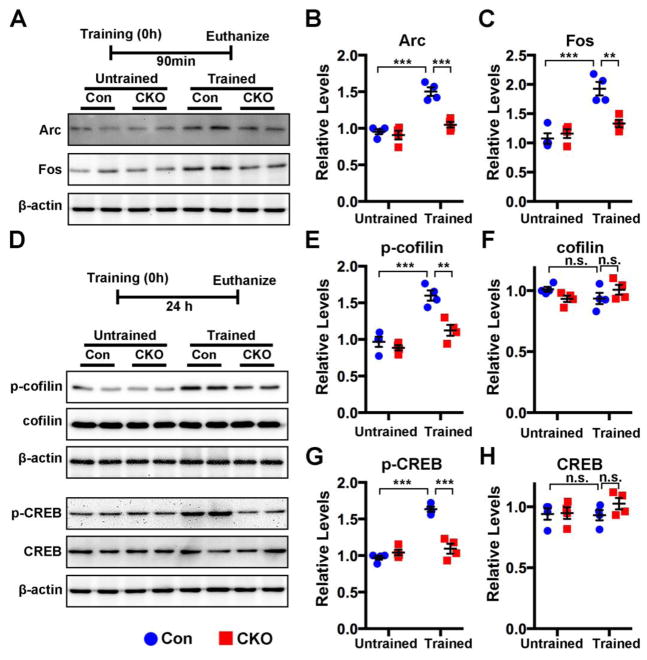
The expression levels of several proteins associated with memory formation and maintenance are altered in CNP;Cdk5 CKO animals. Proteins such as activity-regulated cytoskeletal-associated protein (Arc), Fos, CREB phosphorylation and cofilin are involved in memory (Johansen et al., 2011). Arc and Fos are immediate early gene (IEG) products whose levels are increased following behavioral tasks (Alberini, 2009; Bramham et al., 2008). The phosphorylation of CREB at Serine 133 (Ser133) and cofilin at Ser3 accompany the formation of long-term memory and plasticity and persist for several hours or days (Alberini, 2009; Gu et al., 2010; Suzuki et al., 2011). Consistently, in control animals a significant increase in the expression of Arc and Fos was seen at 90 mins following training in the fear conditioning task (Fig. 7A–C). By contrast, CNP;Cdk5 CKO mice showed no equivalent change in hippocampal Arc and Fos (Fig. 7A–C) following training. Similar results were seen in the expression of CREB and cofilin. While levels were increased in control animals, the levels of phospho -CREB and phospho-cofilin were unchanged after training in CNP;Cdk5 CKO mice, as were levels of total protein (Fig. 7D–H). These data suggest the lack of memory consolidation in CNP;Cdk5 CKO mice reflects a lack of induction of immediate early genes (Arc, Fos) and the phosphorylation of CREB and cofilin.
Figure 7. Training-induced Arc, Fos, p-CREB and p-cofilin are suppressed in CNP;Cdk5 CKO mice.
(A–D) After fear conditioning control mice showed a significant increase in hippocampal Arc, Fos at 90 min (A), and p-CREB and p-cofilin at 24 hours (D) after training. After the same conditioning, CNP;Cdk5 CKO mice showed little or no increase in any of these proteins. Quantification of the western bands is shown in B, C, E–H. All proteins values were normalized to internal control β-actin. Data are expressed as mean ± SEM. n = 4 mice per genotype. * p < 0.05, ** p < 0.01, *** p < 0.001. two-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant.
Discussion
In the CNS, the appropriate level of myelination is important for normal motor and sensory functions although the mechanisms that regulate myelination are not well understood. Here we show that CNP-targeted deletion of Cdk5 in the oligodendrocyte lineage results in thinner myelin sheaths as well as a reduction in the number and disruption of the architecture of nodes of Ranvier. These perturbations become more prevalent as animals mature and are associated with impaired learning and memory seen in multiple cognitive functional tests as well as reductions in the expression levels of Fos, p-CREB, Arc and p-cofilin during the induction of memory formation. These observations suggest that Cdk5-dependent functions in the oligodendrocyte lineage are important for normal neuronal function in the CNS.
The CNP-Cdk5 CKO mice developed severe hypomyelination in the optic nerve, corpus callosum, cortex and hippocampus by 2 months of age. The effect on myelination in the spinal cord was less pronounced than seen in previous studies (Luo et al., 2014) suggesting there may region-specific effects of OL Cdk5 on myelination. The underlying mechanisms are currently unclear although it may reflect the differential expression and kinase activities of Cdk5 and its co-activators of p35 and p39 in brain and spinal cord. For example, mRNA levels of p35 have been proposed to be generally higher in brain compared to spinal cord in the adult rat (Wu et al., 2000). Furthermore, p35 protein levels and Cdk5 kinase activity are significantly higher in the cerebral cortex and hippocampus, but lower in the cerebellum and striatum (Wu et al., 2000) and may influence the success of localized myelination. The hypomyelination seen in the corpus callosum and cortex of CNP;Cdk5 CKO mice are consistent with that reported in Emx1 or Olig1-cre mediated Cdk5 conditional knockout mice (He et al., 2011; Yang et al., 2013). In contrast to the Emx1 or Olig1-cre mediated Cdk5 CKOs, hypomyelination in CNP;Cdk5 CKO mice did not reflect impaired differentiation of OPCs, which may result from differences in the timing or cellular target of Cdk5 deletion.
Nodes of Ranvier were profoundly disrupted in CNP-Cdk5 CKO animals. The total number of nodes was reduced, and the width of the nodes was increased. In addition, the distribution of sodium and potassium channels in the axolemma was significantly disrupted. Such perturbations are likely to have significant effects on the ability of affected axons to efficiently propagate action potentials and as such may contribute to the deficits in behavior of CNP;Cdk5 CKO animals. One mechanism that might account for such morphological changes is Cdk5 kinase functions that target proteins regulating cytoskeletal dynamics. These include FAK, WAVE1, NF proteins and the microtubule-associated protein tau (Dhavan and Tsai, 2001; Su and Tsai, 2011). Cdk5 is also important in ErbB signaling transduction and ErbB deletion results in a higher number of smaller oligodendrocytes each myelinating less axonal surface (Roy et al., 2007). Cdk5 is also proposed to link Fyn kinase to WAVE2 after PDGF stimulation and modulates OPC migration (Miyamoto et al., 2008), raising the possibility that Cdk5 may transduce extracellular stimuli to the cytoskeletal network, thereby affecting oligodendrocyte morphology, myelination and neuron-glial interactions.
Several lines of evidence suggest the lack of Cdk5 in OL lineages has wide spread effects on CNS function. The loss of Cdk5 in oligodendrocytes decreases Fos expression in the hippocampus after the training phase of a fear conditioning test, as well as decreasing the level of both learning -dependent p-CREB and Arc and the levels of the structural protein, p-cofilin. These data suggest a critical functional link between oligodendrocytes and the regulation of neuronal gene expression during the formation of long-term memories. The coordinate loss of these four proteins is likely to be very significant: Fos is an immediate early gene that is involved in memory consolidation(Alberini, 2009). Both p-CREB and Arc have been shown to underlie long-term synaptic plasticity and its related synaptic structural changes (Alberini, 2009; Bramham et al., 2008), while p-cofilin levels correlate with spine morphological changes induced during memory acquisition (Gu et al., 2010; Suzuki et al., 2011). In addition, recent studies implicate myelination in cognition, learning and memory (Fields, 2008; Xiao et al., 2016) and the structure of white matter is known to be dynamic and regulated by experience and electrical activity (Barres and Raff, 1993; Demerens et al., 1996; Young et al., 2013). It seems likely that in patients with demyelinating diseases, defects in myelin may lead to impaired cognitive function in addition to the more widely recognized motor deficits (Kujala et al., 1997).
One potential mechanism by which perturbations in myelination may affect neural function is through disruption of action potential synchrony between distant brain regions. Myelin can influence conduction velocity by regulating axon diameter, myelin thickness, the number and spacing of nodes of Ranvier, as well as the molecular composition of ion channels in the node and paranodal region (Fields, 2008). Oligodendrocytes also monitor neural activity through a variety of receptors including glutamatergic (AMAP, NMDA, and kainate) and GABAergic (GABA A) receptors that depolarize the cell through elevation of intracellular levels of Cl− at rest (Edgar and Sibille, 2012). Activation of glutamate receptors on oligodendrocytes leads to depolarization that rapidly modulates axonal conduction velocity (Karadottir et al., 2005; Yamazaki et al., 2007). Thus, it may be that the reduced level of myelination and disorganization of nodes of Ranvier in CNP;Cdk5 CKO mice alters conduction velocity, thereby affecting the processes of learning and memory. In addition to controlling conduction velocity, a number of other oligodendrocyte-related mechanisms may contribute to reduced cognition in CNP;Cdk5 CKO mice. These include regulation or signaling of other ion channels and lactate in neuronal or non-neuronal cells. The interaction of myelin-associated glycoprotein (MAG) with an axonal receptor(s) induces a signal transduction cascade that modulates expression and phosphorylation of neuronal cytoskeletal elements by Cdk5 and ERK1/2 (Dashiell et al., 2002). OPCs and OLs also secret various factors (i.e. BDNF, FGF2, NGF, IGF-1 and NT-3) that may affect synaptogenesis (Birey et al., 2015; Dai et al., 2003; Kim et al., 2014; Wilkins et al., 2001; Zhang et al., 2006). Further, Cdk5 has been implicated in synaptic vesicle exocytosis and endocytosis and it remains to be determined whether the Cdk5 loss in OLs affects oligodendroglial exosome composition or secretion, a novel mode of neuron-glia communication contributing to neuronal integrity (Fruhbeis et al., 2013).
Our findings have potential implications for the cognitive deficits seen in numerous neurological and neuropsychiatric disorders. Myelin aberrations have been implicated in the pathophysiology of many neurological and neurodegenerative diseases, such as AD, MS, depression and schizophrenia (Edgar and Sibille, 2012; Fields, 2008; Nave and Ehrenreich, 2014). Moreover, cognitive decline in aging parallels subtle changes in the integrity of white matter (Gootjes et al., 2004). While the direct cause of these white matter changes is unknown, our results suggest a critical role for oligodendrocyte Cdk5 in cognitive function and its loss may underlie behavioral changes in a number of neurological conditions.
Supplementary Material
(A) Representative image of MBP immunostaining of P7 brain sections of control and CNP;Cdk5 CKO mice. (B) Quantitative analysis of MBP intensity at P7, P14, P21 and 2 months (for Figure 1A and Figure S1A). Values are mean ± SEM. n=4 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. ***p < 0.001. n.s. = not significant. (C) Percentage of Cre+/Olig2+ positive cells relative to Olig2+ cells only in corpus callosum. (D) Percentage of Cre+/CC1+ positive cells relative to CC1+ cells only in corpus callosum. (E) Percentage of Cdk5+/CC1+ positive cells relative to CC1+ cells in corpus callosum. Values are mean ± SEM. n=3 mice per genotype. two-tailed unpaired Student’s t-test. ***p < 0.001. n.s. = not significant. Scale bars, 100 μm.
(A) Western blot analysis and quantitation of relative Cdk5 protein expression levels showed significantly reduced expression of Cdk5 in the brain of 2-month-old CNP;Cdk5 CKO mice compared to matched control littermates. (B) No Cre co -localized in GFAP+ cells in corpus callosum of either 2-month-old CNP;Cdk5 CKO or control mice. Values are mean ± SEM. n=3 mice per genotype. *** p < 0.001. two-tailed unpaired Student’s t-test. Scale bar = 50 μm.
(A–C) Representative immuostained images of NG2 (A), Olig2 (B) and CC1 (C) in the cortex (Ctx), corpus callosum (CC) (A–C), and hippocampus (D–E) demonstrate no significant differences in the number of NG2+, Olig2+ and CC1+ cells between 2-month-old CNP;Cdk5 CKO mice and controls. Data are expressed as mean ± SEM. n = 3 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant. Scale bars = 100 μm.
(A) Representative images of double immunostaining of Ki67 (red) and Olig2 (green) of brain sections of control and CNP;Cdk5 CKO mice at ages of P7 and P14. Quantitative analysis of the number of Ki67+/Olig2+ positive cells in (B). Values are mean ± SEM. n=4 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant. (C) In situ hybridization confirmed no difference in the number of total PLP+ cells in the corpus callosum of CNP;Cdk5 CKO and control. Quantification analysis showed in (D). Values are mean ± SEM; n=4 mice/group for p21; n=3 mice/group for 2M. two-tailed unpaired Student’s t-test. n.s. = not significant. Scale bars =100 μm.
(A–C) Representative images of GFAP+ (A), Iba1+ (B) and neurofilament 200 (C) in cortex and corpus callosum showing no significant changes in morphology and number of GFAP+, Iba1+ cells and axons (NF 200) between 2-month-old CNP;Cdk5 CKO mice and controls. Scale bar = 100 μm.
(A) Representative images of Caspr, Nav1.6 and Kv1.1 in optic nerve showing a significant decrease in the number of paired nodes, Caspr/Nav1.6 pairs, Caspr/Kv1.1 pairs and the length of paranode spread and node spread (node gap) in CNP;Cdk5 CKO mice compared to controls. (B) Representative EM images confirmed abnormal node structure in optic nerve with increased node spread, less compact and missing paranodal junction structures. Scale bars: A = 100 μm; B = 10 μm.
(A–B) Double immunostaining revealed normal distribution of Nav1.6 at nodes, and no change in the number of Caspr/Nav1.6 pairs in the corpus callosum and optic nerve of 2-month-old Cdk5+/+; CNP+/− mice compared to WT Cdk5+/+; CNP+/+ mice. Quantitative data are showed below the images (B). Data are presented as mean ± SEM. n=3 mice per group. two-tailed unpaired Student’s t-test. n.s. = not significant. Scale bar = 20 μm.
Highlights.
Loss of Cdk5 in oligodendrocyte lineages reduces myelin.
Ablation of Cdk5 in oligodendrocyte lineages disrupts the nodes of Ranvier.
Delete Cdk5 in oligodendrocyte lineages results in learning and memory deficits.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers R01 NS077942 and R01 NS030800].
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. Transcription Factors in Long-Term Memory and Synaptic Plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankston AN, Li W, Zhang H, Ku L, Liu G, Papa F, Zhao L, Bibb JA, Cambi F, Tiwari-Woodruff SK, Feng Y. p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. The Journal of biological chemistry. 2013;288:18047–18057. doi: 10.1074/jbc.M113.453688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, Maffei A, Aguirre A. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron. 2015;88:941–956. doi: 10.1016/j.neuron.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The Immediate Early Gene Arc/Arg3.1: Regulation, Mechanisms, and Function. Journal of Neuroscience. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22:169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, Yang L, Shen MM, Dreyfus CF. The trophic role of oligodendrocytes in the basal forebrain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiell SM, Tanner SL, Pant HC, Quarles RH. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J Neurochem. 2002;81:1263–1272. doi: 10.1046/j.1471-4159.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. The American journal of pathology. 2010;177:1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nature reviews. Molecular cell biology. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Translational psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS biology. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Moller HJ, Hampel H. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dementia and geriatric cognitive disorders. 2004;18:180–188. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nature neuroscience. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Su SC, Gao J, Joseph N, Xie Z, Zhou Y, Durak O, Zhang L, Zhu JJ, Clauser KR, Carr SA, Tsai LH. Cdk5 is required for memory function and hippocampal plasticity via the cAMP signaling pathway. Plos One. 2011;6:e25735. doi: 10.1371/journal.pone.0025735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nature neuroscience. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Takahashi S, Suzuki H, Hashikawa T, Kulkarni AB, Mikoshiba K, Ohshima T. Hypomyelination phenotype caused by impaired differentiation of oligodendrocytes in Emx1-cre mediated Cdk5 conditional knockout mice. Neurochemical research. 2011;36:1293–1303. doi: 10.1007/s11064-010-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nature neuroscience. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Kim D, Cui J, Jang HH, Kim KS, Lee HJ, Kim SU, Ahn SM. Secretome analysis of human oligodendrocytes derived from neural stem cells. Plos One. 2014;9:e84292. doi: 10.1371/journal.pone.0084292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis. A controlled 3-year follow-up. Brain. 1997;120(Pt 2):289–297. doi: 10.1093/brain/120.2.289. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature genetics. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Zhang J, Burke K, Miller RH, Yang Y. The Activators of Cyclin-Dependent Kinase 5 p35 and p39 Are Essential for Oligodendrocyte Maturation, Process Formation, and Myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:3024–3037. doi: 10.1523/JNEUROSCI.2250-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo FC, Burke K, Kantor C, Miller RH, Yang Y. Cyclin-Dependent Kinase 5 Mediates Adult OPC Maturation and Myelin Repair through Modulation of Akt and GsK-3 beta Signaling. Journal of Neuroscience. 2014;34:10415–10429. doi: 10.1523/JNEUROSCI.0710-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Ehrenreich H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA psychiatry. 2014;71:582–584. doi: 10.1001/jamapsychiatry.2014.189. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Johansson C, Magnusson O. Forebrain serotonergic involvement in avoidance learning. Neuroscience letters. 1985;58:305–309. doi: 10.1016/0304-3940(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Plattner F, Hernandez A, Kistler TM, Pozo K, Zhong P, Yuen EY, Tan C, Hawasli AH, Cooke SF, Nishi A, Guo A, Wiederhold T, Yan Z, Bibb JA. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron. 2014;81:1070–1083. doi: 10.1016/j.neuron.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Shrager P. Ion channel sequestration in central nervous system axons. J Physiol-London. 2000;525:63–73. doi: 10.1111/j.1469-7793.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Tayler R, Kaga Y, Yang Y, Lappe-Siefke C, Nave KA, Bansal R. CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia. 2005;50:86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. Journal of neurocytology. 1999;28:319–331. doi: 10.1023/a:1007057512576. [DOI] [PubMed] [Google Scholar]
- Ritter J, Schmitz T, Chew LJ, Buhrer C, Mobius W, Zonouzi M, Gallo V. Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:8990–9002. doi: 10.1523/JNEUROSCI.5528-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romito-DiGiacomo RR, Menegay H, Cicero SA, Herrup K. Effects of Alzheimer’s disease on different cortical layers: the role of intrinsic differences in Abeta susceptibility. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:8496–8504. doi: 10.1523/JNEUROSCI.1008-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O’Donnell P, Brunner D, Corfas G. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Tsai LH. Cyclin-Dependent Kinases in Brain Development and Disease. Annu Rev Cell Dev Bi. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognatta R, Miller RH. Contribution of the oligodendrocyte lineage to CNS repair and neurodegenerative pathologies. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Kidd GJ. Axo-glial septate junctions: The maestro of nodal formation and myelination? J Cell Biol. 2000;150:F97–F99. doi: 10.1083/jcb.150.3.f97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Chandran S, Compston A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia. 2001;36:48–57. doi: 10.1002/glia.1094. [DOI] [PubMed] [Google Scholar]
- Wu DC, Yu YP, Lee NT, Yu AC, Wang JH, Han YF. The expression of Cdk5, p35, p39, and Cdk5 kinase activity in developing, adult, and aged rat brains. Neurochemical research. 2000;25:923–929. doi: 10.1023/a:1007544106645. [DOI] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nature neuroscience. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Sugihara T, Fujii S, Goto K, Kato H. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 2007;3:325–334. doi: 10.1017/S1740925X08000070. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang H, Zhang J, Luo F, Herrup K, Bibb JA, Lu R, Miller RH. Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation. Developmental biology. 2013;378:94–106. doi: 10.1016/j.ydbio.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnykh VL, Bowen JD, Samsonov A, Repovic P, Mayadev A, Qian P, Gangadharan B, Keogh BP, Maravilla KR, Henson LK. Fast Whole-Brain Three-dimensional Macromolecular Proton Fraction Mapping in Multiple Sclerosis. Radiology. 2014:140528. doi: 10.1148/radiol.14140528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Levi L, Casadesus G, Kunos G, Noy N. Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) in the brain. The Journal of biological chemistry. 2014;289:12748–12758. doi: 10.1074/jbc.M114.559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem cells and development. 2006;15:943–952. doi: 10.1089/scd.2006.15.943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative image of MBP immunostaining of P7 brain sections of control and CNP;Cdk5 CKO mice. (B) Quantitative analysis of MBP intensity at P7, P14, P21 and 2 months (for Figure 1A and Figure S1A). Values are mean ± SEM. n=4 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. ***p < 0.001. n.s. = not significant. (C) Percentage of Cre+/Olig2+ positive cells relative to Olig2+ cells only in corpus callosum. (D) Percentage of Cre+/CC1+ positive cells relative to CC1+ cells only in corpus callosum. (E) Percentage of Cdk5+/CC1+ positive cells relative to CC1+ cells in corpus callosum. Values are mean ± SEM. n=3 mice per genotype. two-tailed unpaired Student’s t-test. ***p < 0.001. n.s. = not significant. Scale bars, 100 μm.
(A) Western blot analysis and quantitation of relative Cdk5 protein expression levels showed significantly reduced expression of Cdk5 in the brain of 2-month-old CNP;Cdk5 CKO mice compared to matched control littermates. (B) No Cre co -localized in GFAP+ cells in corpus callosum of either 2-month-old CNP;Cdk5 CKO or control mice. Values are mean ± SEM. n=3 mice per genotype. *** p < 0.001. two-tailed unpaired Student’s t-test. Scale bar = 50 μm.
(A–C) Representative immuostained images of NG2 (A), Olig2 (B) and CC1 (C) in the cortex (Ctx), corpus callosum (CC) (A–C), and hippocampus (D–E) demonstrate no significant differences in the number of NG2+, Olig2+ and CC1+ cells between 2-month-old CNP;Cdk5 CKO mice and controls. Data are expressed as mean ± SEM. n = 3 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant. Scale bars = 100 μm.
(A) Representative images of double immunostaining of Ki67 (red) and Olig2 (green) of brain sections of control and CNP;Cdk5 CKO mice at ages of P7 and P14. Quantitative analysis of the number of Ki67+/Olig2+ positive cells in (B). Values are mean ± SEM. n=4 mice per genotype. two-way ANOVA with Tukey’s multiple comparisons test. n.s. = not significant. (C) In situ hybridization confirmed no difference in the number of total PLP+ cells in the corpus callosum of CNP;Cdk5 CKO and control. Quantification analysis showed in (D). Values are mean ± SEM; n=4 mice/group for p21; n=3 mice/group for 2M. two-tailed unpaired Student’s t-test. n.s. = not significant. Scale bars =100 μm.
(A–C) Representative images of GFAP+ (A), Iba1+ (B) and neurofilament 200 (C) in cortex and corpus callosum showing no significant changes in morphology and number of GFAP+, Iba1+ cells and axons (NF 200) between 2-month-old CNP;Cdk5 CKO mice and controls. Scale bar = 100 μm.
(A) Representative images of Caspr, Nav1.6 and Kv1.1 in optic nerve showing a significant decrease in the number of paired nodes, Caspr/Nav1.6 pairs, Caspr/Kv1.1 pairs and the length of paranode spread and node spread (node gap) in CNP;Cdk5 CKO mice compared to controls. (B) Representative EM images confirmed abnormal node structure in optic nerve with increased node spread, less compact and missing paranodal junction structures. Scale bars: A = 100 μm; B = 10 μm.
(A–B) Double immunostaining revealed normal distribution of Nav1.6 at nodes, and no change in the number of Caspr/Nav1.6 pairs in the corpus callosum and optic nerve of 2-month-old Cdk5+/+; CNP+/− mice compared to WT Cdk5+/+; CNP+/+ mice. Quantitative data are showed below the images (B). Data are presented as mean ± SEM. n=3 mice per group. two-tailed unpaired Student’s t-test. n.s. = not significant. Scale bar = 20 μm.