Abstract
Background: Percutaneous coronary intervention (PCI) is widely used in treatment of acute coronary syndrome (ACS) clinically. It is believed that Danhong injection (DHI) extracted from salviae miltiorrhizae and flos carthami combined with PCI could increase the therapeutic efficacy on ACS. We provide an updated meta-analysis with detailed information on combination of DHI and PCI therapy.
Materials and Methods: Electronic databases were searched for appropriate articles without language limitations on key words before October 22, 2017. All trails were screened according to certain criteria. Quality of eligible studies was also assessed. We made a detailed record of outcome measurements. RevMan 5.3 software was used to perform the meta-analysis.
Results: 14 articles involving 1533 patients with ACS were selected. Compared to PCI treatment alone, total efficacy rate (TER) was enhanced and major adverse cardiovascular events (MACE) were reduced significantly for the combination of DHI and PCI (P < 0.00001). Vascular endothelial function was improved by significantly decreasing the contents of ET-1, vWF and increasing the levels of NO and FMD (P < 0.00001). The serum levels of IL-1, IL-6, IL-18, TNF-α, LpPLA2, MMP-9, and pentraxin-3 were significantly decreased (P < 0.00001), whereas IL-10 in serum was increased (P < 0.00001), indicating a stronger anti-inflammatory effect of the combination. The combination therapy decreased the serum levels of CD62P, PAGT, PADT, FIB-C significantly (P < 0.05), which was beneficial for preventing coagulation of platelets. Blood lipid was also affected by regulating TC, TG, LDL, and HDL, but the results were not statistically significant (P > 0.05). Cardiac function was improved by increasing LEVF (P = 0.006) but not LVED (P = 0.08). The combination treatment was associated with an improvement in antioxidant effect by decreasing MDA and increasing SOD significantly (P < 0.00001).
Conclusion: Combination of DHI and PCI in treatment of ACS could improve TER and reduce incidence of MACE after PCI therapy. These effects may be mediated by combined actions of several mechanisms. However, these results of this study should be handled cautiously due to the limitations of this research. Several rigorous RCTs are in need to confirm these findings.
Keywords: Danhong injection, percutaneous coronary intervention, acute coronary syndrome, systematic review, meta-analysis
Introduction
Acute coronary syndrome (ACS) is the most severe form of Cardiovascular disease (CVD) and CVD accounts for approximately one-third of all global deaths (Chu et al., 2017). Percutaneous coronary intervention (PCI) has become the most effective treatment for ACS (Sun et al., 2017). Despite reestablishing the epicardial coronary vessel patently, PCI may associate with some pathological mechanisms including vascular endothelial dysfunction, aggregation of platelets, diffuse myocardial edema, and neutrophilic plugging which would lead to some major adverse cardiac events (MACE) and/or poor prognosis (Movahed and Butman, 2008; Kirtane et al., 2014; Bouleti et al., 2015).
Danhong injection (DHI) is a Chinese patent medicine extracted from salviae miltiorrhizae and flos carthami. DHI was approved by China Food and Drug Administration (CFDA) in 2002. Its major function is promoting blood circulation to remove blood stasis and dredging meridians. In the theory of TCM, the mechanism of ACS belongs to stagnant blood block and DHI serves to invigorate blood circulation and eliminate stasis which was proven to be efficacious in treating ACS (Wu et al., 2015). Modern pharmacological researches also have shown that DHI can improve the coronary circulation, reduce blood viscosity, and scavenge free radicals to control the occurrence of angina pectoris (Chen and Wang, 2011; Wu et al., 2015). DHI is believed to have obvious therapeutic effects for patients with CVD including coronary heart disease (CHD), angina, myocardial infarction (MI), and cerebral infarction (CI) (Wang et al., 2014). Several recent studies have found that DHI is beneficial to patients with ACS after PCI (Dong et al., 2014; Wu et al., 2015; Zheng et al., 2015; Xie et al., 2016; Zhang Z.H. et al., 2016). A newest meta-analysis reported DHI combined with PCI was superior to PCI therapy alone in treatment for periprocedural myocardial injury (Zhang et al., 2017). But the outcome measures in this paper were not comprehensive enough. Therefore, we provide an updated and extended meta-analysis with detailed information for the combination of DHI and PCI on patients with ACS (Figure 1).
FIGURE 1.
Work flow of present study.
Methods and Program
Literature Retrieval Strategy
Keywords “Danhong injection (DHI) or Danhong” [Title/Abstract] AND “percutaneous coronary intervention (PCI)” [Title/Abstract] AND “acute coronary syndrome (ACS)” [Title/Abstract] were used as search items in electronic databases including Pubmed, Wanfang, the China National Knowledge Infrastructure (CNKI), the VIP medicine information system (VMIS), Embase, the Cochrane Library and the Chinese Biomedical Database (CBM). Articles published before October 22, 2017 was examined without language limitations in order to obtain a comprehensive retrieval. All relevant articles were downloaded into Endnote software (version X7, Thomson Reuters, Inc., New York, NY, United States) for further exploring. Duplicate records were removed. Full-text review was performed while the title/abstract thought to be thematic. The job above was executed by two investigators independently. Conflicts were resolved by the consensus and discussion.
Inclusion and Exclusion Criteria
According to the suggestions of a cardiologist, we designed the inclusion criteria as follows: (1) Patients in RCTs were diagnosed with ACS by meeting the criteria of Diagnostic Criteria of European Society of Cardiology Congress (DCESCC) version 2000, or Diagnosis and treatment of unstable angina pectoris(DTUAP) version 2000, or Diagnostic Criteria of World Health Organization (DTWHO) version 1981, or Clinical guideline of new drugs for traditional Chinese medicine(CGNDTCM), or Diagnostic Criteria of American College of Cardiology (DCACC) version 2007, or guidelines for diagnosis and treatment of acute myocardial infarction (GDTAMI) version 2001, or guidelines for diagnosis and treatment of coronary heart disease (GDTCHD) version 2007, or carrying out coronary arteriography or echocardiogram. (2) All trails mentioned were described as RCTs. (3) Patients in experimental group received PCI-based therapy with DHI, whereas patients in control group received PCI therapy only. (4) Outcome measurements of each study must have included a minimum of two of the following indices: P-selectin CD62(CD62P), fibrinogen C(FIB-C), high-sensitivity C-reactive protein(hsCRP), total cholesterol (TC), triglyceride(TG), low density lipoprotein (LDL), high density lipoprotein (HDL), left ventricular end-diastolic volume (LVED/LVEDV), left ventricular end-systolic volume (LVESV), left ventricular fraction (LVEF), Platelet aggregation (PAGT), Platelet Adhesion rate (PADT), major adverse cardiovascular events (MACE, mainly included MI, angina, sudden cardiac deaths and heart failure), ST segment resolution (STR), total Efficacy Rate (TER), endothelin-1 (ET-1), fibrinogen (Fg), von Willebrand factor (vWF), nitric oxide (NO), flow-mediated dilation (FMD), tumor necrosis factor-α (TNF-α), interleukin (IL), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), brain natriuretic peptide (BNP), lipoprotein-associated phospholipase A2 (LpPLA2), matrix metalloproteinase (MMP).
An exclusion criterion was designed as follows: (1) Articles such as reviews, animal experiments, case report and comments et al. were thought to be unrelated with the topic. (2) Trails were not RCTs or Diagnostic criteria in statement were ambiguous. (3) The intervention of ACS patients was not based on PCI treatment.
Data Extraction and Quality Assessment
Information of eligible studies including authors, year of publication, sample size, interventions and outcome measurements et al. were extracted and arranged to tables. Quality of included studies was assessed by two investigators independently according to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks et al., 2011). Disagreement was resolved by the consensus. Quality assessment was evaluated as follows: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Each term was judged with three levels. “Low risk” of bias means the description of methods or procedures was adequate, “High risk” indicates the description of methods or procedures was not adequate or incorrect while “Unclear risk” of bias means there was no description of methods and/or procedures.
Data Analysis
Data analysis was performed using Review Manager 5.3 (Cochrane Collaboration). Outcome measures such as TER and MACE were regarded as dichotomous variables and presented as the odds ratio (OR) with 95% confidence intervals (95% CI), Contents of inflammatory cytokines (IL-1, IL-6, IL-10, IL-18, TNF-α, LpPLA2, MMP-9, and pentraxin-3), indices of platelet (CD62P, sP-sel, PAGT, PADT, and FIB-C) and factors of blood lipid (TC, TG, LDL, and HDL) et al. were continuous variables that presented as the mean difference (MD) with 95% CI. Q statistic and I2 tests were applied to assess the heterogeneity among studies. A fixed-effects model was used to analyze data with low heterogeneity (P ≥ 0.1 and I2 ≤ 50%) and data with high heterogeneity (P < 0.1 or I2 > 50%) was estimated using random-effects model. Potential publication bias was revealed by funnel plots.
Results
Characteristics of the Eligible Studies
One hundred and twenty articles were identified through database searching, in which 58 articles were removed for duplicates. 21 articles in 62 remaining were excluded for thematic disqualification. Then, 41 articles remained for further full-text review. 21 studies were excluded in this procedure for the following reasons: Diagnosis in 9 articles was vague, 6 studies mentioned unfit interventions and 12 studies were single-arm designs. 14 studies (Feng et al., 2007; Chen et al., 2009, 2015; Xiao and Cao, 2010; Wang et al., 2011; Zhao and Jiang, 2011; Zhang and Zhang, 2012; Dong and Du, 2015; Guo J.F. et al., 2015; Guo L.L. et al., 2015; Liu et al., 2016, 2017; Zhang W.W. et al., 2016; Ye et al., 2017) were included in quantitative synthesis finally (Figure 2).
FIGURE 2.
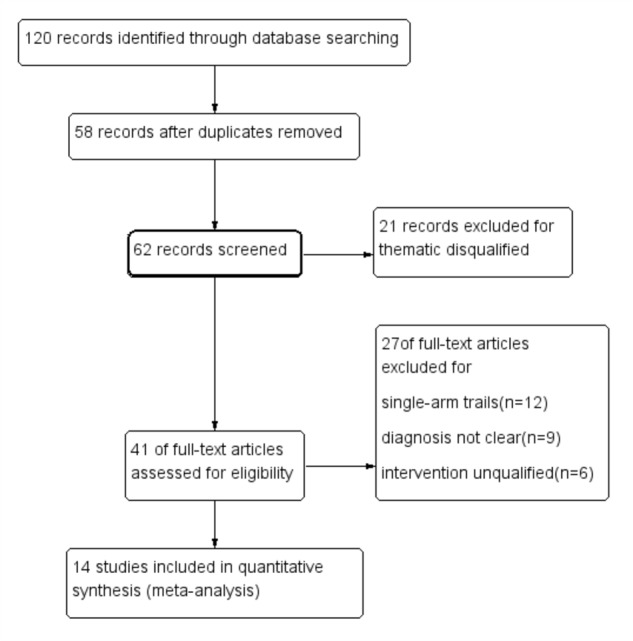
Process of study extracted for the meta-analysis.
One thousand, five hundred and thirty-three patients diagnosed with ACS (769 cases in the experimental group and 764 cases in the control group) were taken in this meta-analysis. The age of the patients ranged from 45 to 87 years, and there was no obvious difference in terms of age and sex between the two groups (Table 1). Trails were conducted between 2007 and 2017; all were RCTs with a comparison between a combination of DHI and PCI therapy and PCI therapy only. The PCI therapy in eligible trails varied slightly and the usual regimen was the combination of clopidogrel, aspirin, low molecular heparin (LMH), β-receptor blocker or ACEI in PCI treatment. The dose of DHI ranged from 20 to 40 mL/day via intravenous drip. Thirteen studies reported the duration of treatment lasted for 2 weeks. Four trails reported a follow up ranged from 2 to 6 months (Table 2).
Table 1.
Characteristics of included studies.
| Author, year | Cases T/C | Diagnostic standard | Age (years) Range, mean | Sex Male/female | ||
|---|---|---|---|---|---|---|
| Chen et al., 2009 | 50/50 | DCESCC(2000)&CA | T:50–72, 63.1 | C: 51–74,67.5 | T: 32/18 | C: 30/20 |
| Chen et al., 2015 | 60/60 | EC | T:61.4 | C: 61.5 | T: 36/24 | C: 39/21 |
| Dong and Du, 2015 | 90/90 | DTUAP(2000) | T: 45–76, 62.4 | C: 46–78, 62.5 | T: 48/42 | C: 54/36 |
| Feng et al., 2007 | 45/46 | CA | T: 67.2 | C: 65.6 | T: 34/12 | C: 32/13 |
| Guo et al., 2015 | 63/62 | DCWHO(1981)& CGNDTCM | T: 55–79, 62.1 | C: 53–76, 61.5 | T: 33/30 | C: 36/26 |
| Guo et al., 2015 | 40/38 | NR | T:61.6 | C: 60.1 | T: 23/17 | C: 22/16 |
| Liu et al., 2016 | 52/52 | EC | T: 47–73, 58.5 | C: 48–72, 59.2 | T: 28/24 | C: 27/25 |
| Liu et al., 2017 | 90/90 | GDTAMI(2001) | NR | NR | NR | NR |
| Wang et al., 2011 | 60/60 | DCESCC(2000)&CA | T: 56–79, 67.8 | C: 54–76, 65.8 | T: 31/29 | C: 28/32 |
| Xiao and Cao, 2010 | 50/50 | DCESCC(2000)&CA | T: 56–79, 67.8 | C: 54–76, 65.8 | T: 26/24 | C: 27/23 |
| Ye et al., 2017 | 49/48 | DCWHO(1981) | T: 52–87, 64.1 | C: 51–86, 64.3 | T: 28/21 | C: 26/22 |
| Zhang and Zhang, 2012 | 34/34 | DCACC(2007)&CA | T: 55.7 | C: 54.5 | T: 18/16 | C: 19/15 |
| Zhang et al., 2016 | 50/50 | GDTCHD(2007) | T: 61–80, 71.3 | C: 61–79, 68.3 | T: 33/17 | C: 34/16 |
| Zhao and Jiang, 2011 | 36/34 | DCACC(2007) | T: 54.0 | C: 54.0 | T: 19/17 | C: 18/16 |
T, trial group; C, control group; NR, no report. DCESCC, Diagnostic Criteria of European Society of Cardiology Congress; CA, coronary arteriography; EC, echocardiogram; DTUAP, diagnosis and treatment of unstable angina pectoris; DCWHO, Diagnostic Criteria of World Health Organization; CGNDTCM, clinical guideline of new drugs for traditional Chinese medicine; DCACC, Diagnostic Criteria of American College of Cardiology; GDTCHD, guidelines for diagnosis and treatment of coronary heart disease; GDTAMI, guideline for diagnosis and treatment of acute myocardial infarction.
Table 2.
Intervention characteristics of included studies.
| Study ID (name, year) | Essential treatment and drugs for ACS | DHI dosage and method | Duration/follow up | Outcome measures |
|---|---|---|---|---|
| Chen et al., 2009 | PCI + Clopidogrel + Aspirin et al. | 40 mL/day, intravenous drip | 2 weeks/NR | CD62P, GP IIb/IIIa, FIB-C, hs-CRP |
| Chen et al., 2015 | PCI + Clopidogrel + Aspirin et al. | 40 mL/day, intravenous drip | 2 weeks/6 months | TC, TG, LDL, HDL, LVED, LVEF, PAGT, PADT, CD62P, MACE |
| Dong and Du, 2015 | PCI + Clopidogrel + Aspirin + LMH et al. | 20 mL/day, intravenous drip | 2 weeks/NR | TER, vWF, ET-1, NO, FMD, SOD, MDA |
| Feng et al., 2007 | PCI + Clopidogrel + Aspirin et al. | 40 mL/day, intravenous drip | 4 weeks/NR | TC, TG, LDL, HDL, hs-CRP, ET, Fg, MACE |
| Guo et al., 2015 | PCI + Clopidogrel + Aspirin et al. | 40 mL/day, intravenous drip | 2 weeks/2 months | vWF, ET-1, NO, FMD, TNF-α, IL-1, CRP, MACE |
| Guo et al., 2015 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 14 days/NR | hs-CRP, ICAM-1, VCAM-1 |
| Liu et al., 2016 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 2 weeks/NR | vWF, ET-1, NO, FMD, NTG, IL-18, IL-10, LpPLA2, LVESV, LVEDV, LVEF, BNP, pentraxin-3 |
| Liu et al., 2017 | PCI + Clopidogrel + Aspirin + LMH et al. | 20 mL/day, intravenous drip | 14 days/NR | TER, hsCRP, ET, LVEF, LVEDV |
| Wang et al., 2011 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 2 weeks/NR | vWF, ET-1, NO |
| Xiao and Cao, 2010 | PCI + Clopidogrel + Aspirin et al. | 20 mL/day, intravenous drip | 14 days/NR | vWF, ET-1, NO, FMD |
| Ye et al., 2017 | PCI + Clopidogrel + Aspirin et al. | 40 mL/day, intravenous drip | 3 months/6 months | TER, ET-1, NO, MACE |
| Zhang and Zhang, 2012 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 14 days/NR | ET-1, CD62P, hs-CRP, STR |
| Zhang et al., 2016 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 2 weeks/2 months | vWF, ET-1, NO, FMD, IL-6, MMP-9, hs-CRP |
| Zhao and Jiang, 2011 | PCI + Clopidogrel + Aspirin + LMH et al. | 40 mL/day, intravenous drip | 2 weeks/NR | ET-1, sP-sel, hs-CRP, MACE |
ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; DHI, Danhong injection; CD62P, P-selectin CD62P; GP, glucose protein; FIB-C, fibrinogen C; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; TG, triglyceride; LDL, low density lipoprotein; HDL, high density lipoprotein; LVED/LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular fraction; PAGT, platelet aggregation; PADT, platelet adhesion rate; MI: myocardial infarction; MACE, major adverse cardiac events; LMH, low molecular heparin; MACE, major adverse cardiovascular events; STR, ST segment resolution; TER, total efficacy rate; ET-1, endothelin-1; Fg, fibrinogen; vWF, von Willebrand factor; NO, nitric oxide; FMD, flow-mediated dilation; TNF-α, tumor necrosis factor-α; IL, interleukin; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; NTG, endothelial non-dependent vascular dilation reactions; BNP, brain natriuretic peptide; LpPLA2, lipoprotein-associated phospholipase A2; MMP, matrix metalloproteinase; sP-sel, soluble P-selectin; NR, no report.
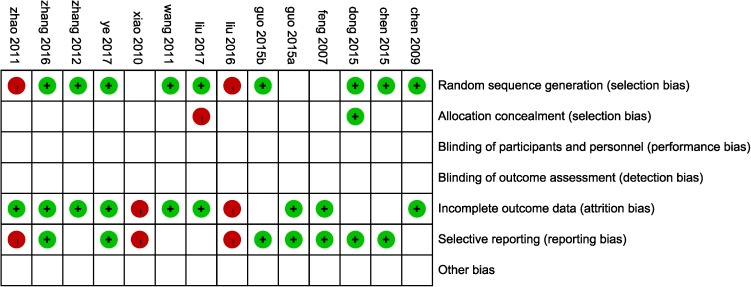
Quality of Included Trials Assessment
According to the Cochrane risk of bias estimation, all trails mentioned a randomized allocation of participants while nine trails (Chen et al., 2009, 2015; Wang et al., 2011; Zhang and Zhang, 2012; Dong and Du, 2015; Guo L.L. et al., 2015; Zhang W.W. et al., 2016; Liu et al., 2017; Ye et al., 2017) described the appropriate generation of the random allocation sequence. Detailed information on allocation concealment of majority studies was missing. Blinding of participants and outcome assessment of all studies was not mentioned. Nine studies (Feng et al., 2007; Chen et al., 2009; Wang et al., 2011; Zhao and Jiang, 2011; Zhang and Zhang, 2012; Guo J.F. et al., 2015; Zhang W.W. et al., 2016; Liu et al., 2017; Ye et al., 2017) were at low risk of attrition bias for having been given a complete outcome data. Seven trails (Feng et al., 2007; Chen et al., 2015; Dong and Du, 2015; Guo J.F. et al., 2015; Guo L.L. et al., 2015; Zhang W.W. et al., 2016; Ye et al., 2017) reported the result of detailed indices indicated a low risk of reporting bias (Figure 3).
FIGURE 3.
Risk of bias assessment in eligible studies. The quality assessment was conducted by Review Manager 5.3 according to Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Red circle, high risk of bias; green circle, low risk of bias; blank, unclear risk of bias.
Outcome Measures With Subgroup Analysis
TER and MACE of DHI Combined With PCI vs. PCI Therapy Alone
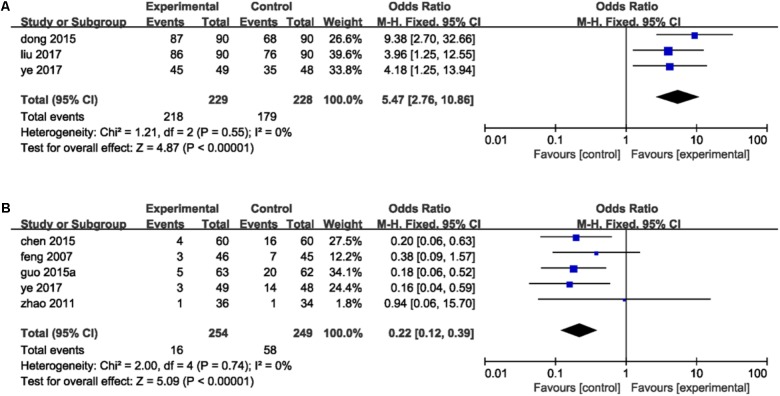
Criterion of TER was set as follows: ecovery was defined as the absence of symptoms while anginal attacks decreased significantly and duration of attacks decreased more than 80%. Effectiveness was identified that the symptoms were ameliorated and lower frequency of anginal attacks and duration decreased between 50 and 80%. Symptoms and anginal attacks remaining unchanged or worsening were defined as invalidation. TER refers to the proportion of patients who were evaluated to recovery and effectiveness in total groups. Three studies (Dong and Du, 2015; Liu et al., 2017; Ye et al., 2017) reported the total efficacy rate. A meta-analysis of these trails using a fixed-effect model demonstrated that DHI combined with PCI treatment significantly improved TER in the treatment of ACS (OR = 5.47, 95%CI: 2.76, 10.86; P < 0.00001). There was no statistically significant heterogeneity among individual trails (P = 0.55, I2 = 0%; Figure 4A). Five trails (Feng et al., 2007; Zhao and Jiang, 2011; Chen et al., 2015; Guo J.F. et al., 2015; Ye et al., 2017) provided descriptions on MACE after PCI therapy such as MI, angina, sudden cardiac deaths and heart failure. A fixed-effect model analysis certified that the combination of DHI and PCI treatment reduced the incidence of MACE significantly (OR = 0.22, 95%CI: 0.12, 0.39; P < 0.00001). No statistically significant heterogeneity was found among individual studies (P = 0.74, I2 = 0%; Figure 4B).
FIGURE 4.
Forest plot of TER and MACE in patients treated with DHI + PCI and PCI alone. (A) The plot of TER, (B) the plot of MACE. I2 and P are the criterion for the heterogeneity test. ♦ Pooled odds ratio, — — odds ratio and 95%CI.
— odds ratio and 95%CI.
Indices of Vascular Endothelial Function of DHI Combined With PCI Therapy vs. PCI Therapy Alone
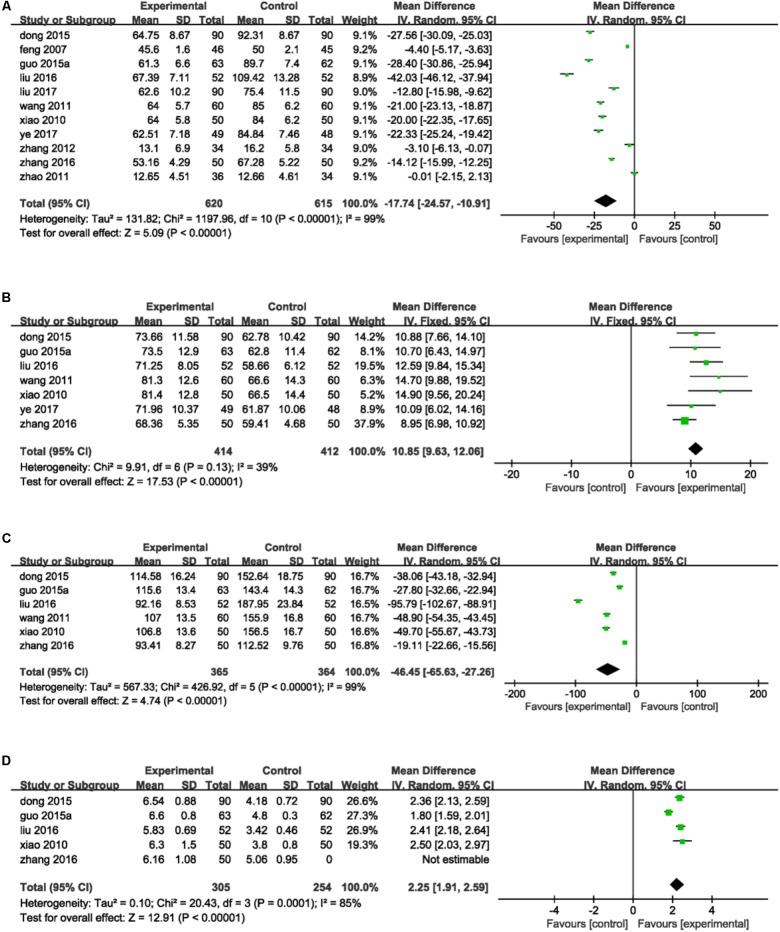
ET-1, NO, vWF, and FMD were the main indices that mentioned in included studies reflected vascular endothelial function. Eleven studies (Feng et al., 2007; Xiao and Cao, 2010; Wang et al., 2011; Zhao and Jiang, 2011; Zhang and Zhang, 2012; Dong and Du, 2015; Guo J.F. et al., 2015; Liu et al., 2016, 2017; Zhang W.W. et al., 2016; Ye et al., 2017) reported the detection of ET-1. There was statistically significant heterogeneity among individual studies (P < 0.00001, I2 = 99%), so a random-effect model was applied to take a meta-analysis which demonstrated that the combination of DHI and PCI treatment significantly decreased the level of ET-1 in serum (MD = -17.74, 95%CI: -24.57, -10.91; P < 0.00001; Figure 5A). Seven trails (Xiao and Cao, 2010; Wang et al., 2011; Dong and Du, 2015; Guo J.F. et al., 2015; Liu et al., 2016; Zhang W.W. et al., 2016; Ye et al., 2017) provided the contents of NO. A fixed-effect analysis certified that DHI combined with PCI significantly increased the serum level of NO compared to PCI alone (MD = 10.85, 95%CI: 9.63, 12.06; P < 0.00001). No statistical significant was observed among individual studies (P = 0.13, I2 = 39%; Figure 5B). Detection of vWF was reported in six trails (Xiao and Cao, 2010; Wang et al., 2011; Dong and Du, 2015; Guo J.F. et al., 2015; Liu et al., 2016; Zhang W.W. et al., 2016). Heterogeneity was found among individual studies (P < 0.00001, I2 = 99%) and then a random-effect analysis was applied to demonstrate that DHI combined with PCI significantly decreased the serum content of vWF (MD = -46.45, 95%CI: -65.63, -27.26; P < 0.00001; Figure 5C). Five studies (Xiao and Cao, 2010; Dong and Du, 2015; Guo J.F. et al., 2015; Liu et al., 2016; Zhang W.W. et al., 2016) provided data of FMD. There was heterogeneity among individual trails (P = 0.0001, I2 = 85%) and a meta-analysis using a random-effect proved that combination of DHI and PCI could significantly increase the level of FMD of vascular endothelium (MD = 2.25, 95%CI: 1.91, 2.59; P < 0.00001; Figure 5D).
FIGURE 5.
Forest plot of indices of vascular endothelial function in patients treated with DHI + PCI and PCI alone. (A) The plot of ET-1, (B) the plot of NO, (C) the plot of vWF, and (D) the plot of FMD. I2 and P are the criterion for the heterogeneity test. ♦ Pooled odds ratio, — —mean difference and 95%CI.
—mean difference and 95%CI.
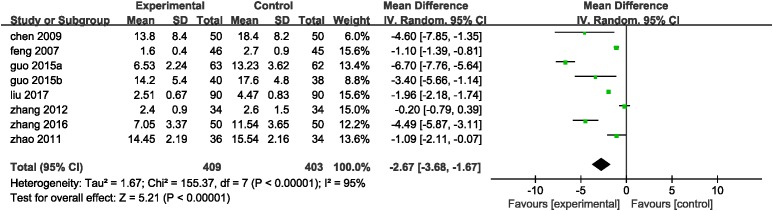
Inflammatory Indices of DHI Combined With PCI Therapy vs. PCI Therapy Alone
Inflammation hypothesis of ACS proposed by Ross (1999) indicates that inflammation plays an important role in plaque buildup within the coronary arteries. Inflammatory indices reported in eligible studies including hsCRP, IL-1, IL-6, IL-10, IL-18, TNF-α, LpPLA2, MMP-9, and pentraxin-3. Eight trails (Feng et al., 2007; Chen et al., 2009; Zhao and Jiang, 2011; Zhang and Zhang, 2012; Guo J.F. et al., 2015; Guo L.L. et al., 2015; Zhang W.W. et al., 2016; Liu et al., 2017) mentioned the investigation on hsCRP. A random-effect model was used because of heterogeneity existence (P < 0.00001, I2 = 95%). A meta-analysis demonstrated that DHI combined with PCI significantly decreased the serum level of hsCRP (MD = -2.67, 95%CI: -3.68, -1.67; P < 0.00001; Figure 6). One trail (Guo J.F. et al., 2015) reported the serum IL-1 and TNF-α level. The serum levels of IL-10, IL-18, LpPLA2, and pentraxin-3 were measured in one study (Liu et al., 2016), and another one trail (Zhang W.W. et al., 2016) provided the IL-6 and MMP-9 level. The MD with 95%CI for IL-1, IL-6, IL-18, TNF-α, LpPLA2, MMP-9, and pentraxin-3 were (MD = -33.87, 95%CI: -37.45, -30.29), (MD = -14.41, 95%CI: -16.79, -12.03), (MD = -46.88, 95%CI: -60.46, -33.30), (MD = -27.79, 95%CI: -31.88, -23.70), (MD = -55.16, 95%CI: -60.00, -50.32), (MD = -107.93, 95%CI: -137.84, -78.02), and (MD = -2.41, 95%CI: -2.68, -2.14), respectively, indicating a significant decrease in the inflammatory indices in the experimental group compared with control group (P < 0.00001). The MD with 95%CI for IL-10 was (MD = 7.63, 95%CI: 5.82, 9.44) certifying a significant increase in the DHI + PCI group compared with PCI therapy alone (P < 0.00001; Table 3).
FIGURE 6.
Forest plot of hsCRP in patients treated with DHI + PCI and PCI alone. I2 and P are the criterion for the heterogeneity test. ♦ Pooled odds ratio, — —mean difference and 95%CI.
—mean difference and 95%CI.
Table 3.
Danhong injection (DHI) combined with PCI vs. essential treatment on inflammatory factors.
| Inflammatory factors | Number of studies | Study ID | Cases of experimental group | Cases of control group | MD [95%CI] | Z-value | P-value |
|---|---|---|---|---|---|---|---|
| IL-1 | 1 | Guo et al., 2015 | 63 | 62 | -33.87 [-37.45, -30.29] | 18.54 | <0.00001 |
| IL-6 | 1 | Zhang et al., 2016 | 50 | 50 | -14.41 [-16.79, -12.03] | 11.89 | <0.00001 |
| IL-10 | 1 | Liu et al., 2016 | 52 | 52 | 7.63 [5.82, 9.44] | 8.26 | <0.00001 |
| IL-18 | 1 | Liu et al., 2016 | 52 | 52 | -46.88 [-60.46, -33.30] | 6.77 | <0.00001 |
| TNF-α | 1 | Guo et al., 2015 | 63 | 62 | -27.79 [-31.88, -23.70] | 13.31 | <0.00001 |
| LpPLA2 | 1 | Liu et al., 2016 | 52 | 52 | -55.16 [-60.00, -50.32] | 22.34 | <0.00001 |
| MMP-9 | 1 | Zhang et al., 2016 | 50 | 50 | -107.93 [-137.84, -78.02] | 7.07 | <0.00001 |
| Pentraxin-3 | 1 | Liu et al., 2016 | 52 | 52 | -2.41 [-2.68, -2.14] | 17.68 | <0.00001 |
Platelet Activation Indices of DHI Combined With PCI Therapy vs. PCI Therapy Alone
CD62P, sP-sel, PAGT, PADT, and FIB-C were the indices of platelet activation recorded in eligible studies. Three trail (Chen et al., 2009, 2015; Zhang and Zhang, 2012) mentioned the determination of serum CD62P. One study (Zhao and Jiang, 2011) reported sP-sel, one trail (Chen et al., 2015) provided PAGT and PADT, and two trails (Feng et al., 2007; Chen et al., 2009) recorded FIB-C. The MD with 95% CI for CD62P, PAGT, PADT, and FIB-C were (MD = -1.82, 95%CI: -2.59, -1.06), (MD = -2.49, 95%CI: -3.73, -1.25), (MD = -1.07, 95%CI: -1.95, -0.19), and (MD = -1.50, 95%CI: -2.18, -0.83), respectively, indicating a significant decrease in experimental group in coagulation of platelet (P < 0.05). Compared to control group, sP-sel level of experimental group decreased slightly (MD = -0.80, 95%CI: -6.98, 5.38) but without statistical significance (P = 0.80; Table 4).
Table 4.
Danhong injection combined with PCI vs. essential treatment on platelet activation.
| Platelet activation indices | Number of studies | Study ID | Cases of experimental group | Cases of control group | MD [95%CI] | Z-value | P-value |
|---|---|---|---|---|---|---|---|
| CD62P | 3 | Chen et al., 2009, 2015; Zhang and Zhang, 2012 | 144 | 144 | -1.82 [-2.59, -1.06] | 4.66 | <0.00001 |
| sP-sel | 1 | Zhao and Jiang, 2011 | 36 | 34 | -0.80 [-6.98, 5.38] | 0.25 | 0.80 |
| PAGT | 1 | Chen et al., 2015 | 60 | 60 | -2.49 [-3.73, -1.25] | 3.94 | <0.0001 |
| PADT | 1 | Chen et al., 2015 | 60 | 60 | -1.07 [-1.95, -0.19] | 2.40 | 0.02 |
| FIB-C | 2 | Feng et al., 2007; Chen et al., 2009 | 96 | 95 | -1.50 [-2.18, -0.83] | 4.35 | <0.0001 |
Influence on Blood Lipid of DHI Combined With PCI vs. PCI Alone
Overweight and dyslipidaemia were the 1st and 2nd prevalent factors leading to ACS (Vazquez-Arce and Marques-Sule, 2017). TC, TG, LDL, and HDL were highly related to overweight and dyslipidaemia which were reported in two trails (Feng et al., 2007; Chen et al., 2015). The MD with 95% CI for TC, LDL, and HDL were (MD = -0.11, 95%CI: -0.40, 0.18), (MD = -0.14, 95%CI: -0.38, 0.09) and (MD = -0.07, 95%CI: -0.32, 0.18), respectively, revealing a decrease in blood lipid but without statistical significance (P > 0.05). The MD with 95%CI (MD = 0.06, 95%CI: -0.14, 0.27) indicating a slightly increase in TG without significance (P > 0.05; Table 5).
Table 5.
Danhong injection combined with PCI vs. essential treatment on blood lipid.
| Indices of blood lipid | Number of Studies | Study ID | Cases of experimental group | Cases of control group | MD [95%CI] | Z-value | P-value |
|---|---|---|---|---|---|---|---|
| TC | 2 | Feng et al., 2007; Chen et al., 2015 | 105 | 106 | -0.11 [-0.40, 0.18] | 0.77 | 0.44 |
| TG | 2 | Feng et al., 2007; Chen et al., 2015 | 105 | 106 | 0.06 [-0.14, 0.27] | 0.63 | 0.53 |
| LDL | 2 | Feng et al., 2007; Chen et al., 2015 | 105 | 106 | -0.14 [-0.38, 0.09] | 1.18 | 0.24 |
| HDL | 2 | Feng et al., 2007; Chen et al., 2015 | 105 | 106 | -0.07 [-0.32, 0.18] | 0.57 | 0.57 |
Influence on Cardiac Function and Oxidation State of DHI Combined With PCI vs. PCI Alone
Three trails (Chen et al., 2015; Liu et al., 2016; Liu et al., 2017) reported the LVEF and LVED reflecting cardiac function. One trail (Dong and Du, 2015) provided measures of SOD and MDA in representation of oxidation state. There were heterogeneity in LVEF and LVED (P < 0.00001, I2= 93%; P < 0.0001, I2= 90%, respectively) and a random effect model was thus used for analysis. The MD and 95% CI for LVEF was (MD = 5.68, 95%CI: 1.61, 9.76), indicating a significant increase of LVEF in experimental group (P = 0.006). A meta-analysis demonstrated that DHI combined with PCI decreased LVED (MD = -5.89, 95%CI: -12.51, 0.74) non-significantly (P = 0.08). An analysis certified that the combination of DHI and PCI increased SOD level (MD = 22.17, 95%CI: 15.07, 29.27) and decreased MDA (MD = -1.09, 95%CI: -1.38, -0.80) significantly (P < 0.00001; Table 6).
Table 6.
Danhong injection combined with PCI vs. essential treatment on cardiac function and oxidation state.
| Item | Indices | Number of studies | Study ID | Cases of experimental group | Cases of control group | MD [95%CI] | Z-value | P-value |
|---|---|---|---|---|---|---|---|---|
| Cardiac function | LVEF | 3 | Chen et al., 2015; Liu et al., 2016, 2017 | 202 | 202 | 5.68 [1.61, 9.76] | 2.73 | 0.006 |
| LVED | 3 | Chen et al., 2015; Liu et al., 2016, 2017 | 202 | 202 | -5.89 [-12.51, 0.74] | 1.74 | 0.08 | |
| Oxidation state | SOD | 1 | Dong and Du, 2015 | 90 | 90 | 22.17 [15.07, 29.27] | 6.12 | <0.00001 |
| MDA | 1 | Dong and Du, 2015 | 90 | 90 | -1.09 [-1.38, -0.80] | 7.37 | <0.00001 |
Publication Bias
Publication bias was expressed by a funnel plot. In this study, funnel plots of combination of DHI and PCI vs. PCI therapy alone on ET-1, hs-CRP, NO and vWF were applied. The plots were generally symmetric, suggesting that there was no obvious publication bias (Figures 7A–D).
FIGURE 7.
Funnel plot for the publication bias. (A) The plot of ET-1, (B) the plot of hsCRP, (C) the plot of NO, and (D) the plot of vWF. The funnel plots of these factors were symmetric, indicating that the publication was small.
Discussion
Cardiovascular Disease (CVD) is the main cause of death which will be the first “killer” for human beings by 2020 (Lopez and Murray, 1998). CVD is the most common disease in China and ACS is the most severe form accompanied by highly disability rate, fatality rate and multi-complications which places gravely threat on human health (Zheng et al., 2014). Despite PCI is more effective in restoring coronary blood flow compared with other interventions, itself would be a dangerous factor that results in poor prognosis. MI associated with PCI now is a new type in the new classification for acute myocardial infarction (Makki et al., 2015). There are some management of PCI-related complications in patients who have undergone PCI. However, they usually bring some new risks such as bleeding (Hansen et al., 2010), intracranial hemorrhage (Hess et al., 2015), in-stent restenosis (Chen et al., 2015) and some other considerable harms (Cannon et al., 2017) which make PCI a difficult challenge in treatment of ACS for patients.
Though there are some limitations for TCM due to the lack of enough basic research, increasingly effective evidence-based practice makes it an eye-catching therapy system for many diseases. Danhong injection extracted from salviae miltiorrhizae and flos carthami, is a star representative in TCM for treating cardiovascular and cerebrovascular diseases. Salviae miltiorrhizae serves as monarch drug in the prescription, taking on effectiveness including antithrombus, improving microcirculation and antioxidant (Qi et al., 2017). Flos carthami plays a role of ministerial drug taking effects of improving hemorheology, inhibiting the aggregation of platelets, decreasing myocardial reperfusion and enhancing vascular endothelial function (Pei, 2005).
In clinical trials, a surrogate endpoint (or marker) is a measure of effect of a specific treatment that may correlate with a real clinical endpoint but does not necessarily have a guaranteed relationship. Interestingly, we find that surrogate endpoints were used in most of the analyzed trials (cytokines, endothelin etc…), while clinical endpoints were used only in some trials in this meta-analysis. The popularity of surrogate endpoints in ACS is for many reasons. Firstly, the primary clinical endpoints (such as MACE) may cause life threatening problems to patients, thus making it impractical to conduct a clinical trial to assess the antianginal effects of a drug. Secondly, surrogate endpoints selected in eligible trails could be easily measured prior to the occurrence of MACE and highly correlated with the process of ACS which often leads to dramatic reductions in sample size and much shorter studies than use of the clinical endpoints. What’s more important, the use of surrogate endpoints could speed up the arrival of scientific conclusion and is in favor of making early decisions on clinical protocols in ACS.
The endothelium plays an important role in the pathogenesis of ACS (Cieslik-Guerra et al., 2014), and circulating endothelial cells (CECs) have been put forward as a promising biomarker for diagnosis and prognosis of coronary artery disease and ACS (Schmidt et al., 2015). The increased ET-1 level in active coronary lesions may lead to vasospasm and to the progression of atherosclerosis (Ihling, 1998). NO maintains endothelial balance by controlling cellular processes of vascular smooth muscle cells. The variations in the NO pathway could include atherosclerotic events (Umman et al., 2015). High level of vWF is partly due to endothelial dysfunction and atherosclerosis, and it is associated with an increased risk of coronary artery disease (Sonneveld et al., 2015). FMD of the brachial artery has been recommended as non-invasive methods to assess endothelial structure and function (Kalay et al., 2010). Here we certified that the combination of DHI and PCI could improve the vascular endothelial function by decreasing the contents of ET-1 and vWF, increasing the levels of NO and FMD (P < 0.00001).
Inflammatory response has been considered as an important mechanism for ACS (Santos-Gallego et al., 2014). Thrombolytic therapy in essential treatment of PCI would up-regulate inflammatory mediators including hs-CRP and TNF-α compared with PCI alone, which suggests an increasing risk of detrimental effects on myocardium (Garjani et al., 2016). An increase of hs-CRP is a reflex of unstable plaque and poor prognosis (Ross, 1999). TNF-α is a pro-inflammatory cytokine that could be a potential biomarker in ACS due to its multiple functions (Sandoval-Pinto et al., 2016). Here we found that DHI combined with PCI could not only decrease the serum level of hs-CRP (MD = -2.67, 95%CI: -3.68, -1.67; P < 0.00001) and TNF-α (MD = -27.79, 95%CI: -31.88, -23.70; P < 0.00001), but also decrease other inflammatory factors including IL-1, IL-6, IL-18, LpPLA2, MMP-9, and pentraxin-3 significantly (P < 0.00001). IL-6 is an independent predictor of adverse events in low-moderate risk patients with NSTE-ACS and troponin-negative (Garcia-Salas et al., 2014). IL-18 level is a valuable parameter for risk of MACE in patients with ACS (Zhou et al., 2014). Lp-PLA2 levels are related to plaque stability (Chung et al., 2014) which represents the crossroad between lipid metabolism and inflammatory response (Abbate et al., 2012). Serum MMP-9 could be an early marker that discriminates MI from UA and predicts poor outcome in terms of disease severity and extent of disease complications (Hamed and Fattah, 2015). What’s more, the combination further enhanced anti-inflammatory effects via increasing serum level of IL-10 (MD = 7.63, 95%CI: 5.82, 9.44; P < 0.00001).
Antiplatelet agents form the cornerstone of medical therapy in patients with ACS (Makki et al., 2015). CD62P expressing functional thymic stromal lymphopoietin receptors (TSLPR) which promote platelet activation may be one of the mechanisms involved in thrombosis in ACS (Wang et al., 2013). PAGT and PADT play important parts in the occurrence and development of CHD as manifested by the enhancement of platelet’s adherence and aggregation (Jia, 1989). Increased FIB levels are independently associated with intermediate-high syntax score which is related to more serious disease and worse prognosis in patients with ACS (Kurtul et al., 2016). sP-sel is demonstrated to have prognostic values in predicting the cardiac events in patients with preserved left ventricular systolic function (Chen et al., 2013). We provided that DHI combined with PCI significantly decreased serum levels of CD62P, PAGT, PADT, and FIB-C (P < 0.05). The combination also decreased the level of sP-sel but without statistical significance (P = 0.80).
Hyperlipidemia is a major risk factor for CHD and potential benefit can be obtained by early treatment of hyperlipidemia following ACS (Balci, 2011). A series of changes in TC, TG, LDL, and HDL occur during acute phase response. However, DHI did not influence the serum levels of lipid significantly in this report (P > 0.05). We can partly explain this from the using of statins in the PCI essential treatment, whose effects on blood lipid may overwhelm the influence of DHI. The fluctuation of lipid in determination was also blamed for the result.
Oxidative stress leading to modification of LDL is a central paradigm of atherogenesis and plaque destabilization (Armstrong et al., 2006). DHI alleviated the oxidative stress via increasing SOD level and decreasing MDA content significantly (P < 0.00001). Cardiac function was also improved by DHI through increasing LVEF in patients (P = 0.006).
We have checked the methods used for the measurement of different biomarkers across the selected studies. All the indexes except NO were detected by the same or similar methods to keep the methodological consistency. NO was detected by nitrate reductase method in six trails (Xiao and Cao, 2010; Wang et al., 2011; Dong and Du, 2015; Guo J.F. et al., 2015; Liu et al., 2016; Zhang W.W. et al., 2016) whereas by ELISA in one study (Ye et al., 2017). No statistical significant was observed among individual studies, which mean the difference in methodological inconsistency didn’t add heterogeneity to the analysis of NO.
Acute coronary syndrome is a pathology characterized by complicated changes of inflammatory response, dyslipidemia, endothelium, platelet activation, oxidative stress, and cardiac functions et al. DHI exerts its pharmacological effects through a multi-components and targets way including anti-inflammatory, antioxidant, adjusting functions of vascular endothelial and cardiac, anti-agglutination of platelets to improve symptoms of ACS in patients after PCI. However, there are limitations to this research, such as the low quality of eligible trails, the lack of strict methodologies and the employed of sole race rather than a more varied population sample. It is necessary to examine the results using other rigorous and large-scale RCTs.
Conclusion
These findings indicate that the combination of DHI and PCI significantly improve the TER and reduce incidence of MACE after PCI therapy. These effects are mediated by combined action of several mechanisms. In the present study, the combination could enhance the protection of vascular endothelial function through decreasing the ET-1 and vWF while increasing the level of NO and FMD. DHI combined with PCI plays an anti-inflammatory role by decreasing the level of IL-1, IL-6, IL-18, TNF-α, LpPLA2, MMP-9, pentraxin-3 and increasing level of IL-10 in serum. The combination displays an anticoagulation effect by decreasing level of CD62P, PAGT, PADT, FIB-C but not sP-sel in serum. It is likely that the combination also affects the blood lipid by regulating the contents of TC, TG, LDL, and HDL, but the results were not statistically significant. Cardiac function is improved by increasing LEVF but not LVED. The combination shows a regulation of oxidation state by decreasing content of MDA and increasing level of SOD. However, our findings must be handled with care because of the small sample size and low quality of clinic trials cited. Other rigorous and large-scale RCTs are in need to confirm these results.
Author Contributions
J-BZ, X-FZ, and JW searched articles in electronic databases and wrote the manuscript. FW, J-XC, F-YY, and XS analyzed the data. J-BZ, YW, and Y-LL performed the data extraction. Y-JS designed the study and amended the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by Chinese Medicine Pharmaceutical Key Discipline of Shaanxi Province (303061107). This work was also supported by the Key Research and Development Program of Shaanxi Province of China (No. 2017ZDXM-SF-008).
References
- Abbate R., Cioni G., Ricci I., Miranda M., Gori A. M. (2012). Thrombosis and acute coronary syndrome. Thromb. Res. 129 235–240. 10.1016/j.thromres.2011.12.026 [DOI] [PubMed] [Google Scholar]
- Armstrong E. J., Morrow D. A., Sabatine M. S. (2006). Inflammatory biomarkers in acute coronary syndromes: part III: biomarkers of oxidative stress and angiogenic growth factors. Circulation 113 e289–e292. 10.1161/circulationaha.105.595546 [DOI] [PubMed] [Google Scholar]
- Balci B. (2011). The modification of serum lipids after acute coronary syndrome and importance in clinical practice. Curr. Cardiol. Rev. 7 272–276. 10.2174/157340311799960690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouleti C., Mewton N., Germain S. (2015). The no-reflow phenomenon: state of the art. Arch. Cardiovasc. Dis. 108 661–674. 10.1016/j.acvd.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Cannon C. P., Bhatt D. L., Oldgren J., Lip G. Y. H., Ellis S. G., Kimura T., et al. (2017). Dual Antithrombotic therapy with Dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 377 1513–1524. 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- Chen J., Xu H., Zhou D. P., Dong M., Du H. L. (2015). The curative effect of danhong injection in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Liaoning J. Tradit. Chin. Med. 42 341–344. 10.13192/j.issn.1000-1719.2015.02.048 [DOI] [Google Scholar]
- Chen W. S., Chen S. J., Lee C. C., Cherng W. J., Liu M. H., Wang C. H. (2013). Plasma P-selectin predicts long-term cardiovascular events in hospitalized patients with suspected coronary artery disease and preserved left ventricular function: a 10-year follow-up study. Biomed. J. 36 137–143. 10.4103/2319-4170.113231 [DOI] [PubMed] [Google Scholar]
- Chen X. G., Wang H. P. (2011). Efficacy observation of Danhong injection for the treatment of elderly patients with acute coronary syndrome. Chin. J. Clin. Res. 24 156–157. [Google Scholar]
- Chen Z. Q., Hong L., Wang H., Yin Q. L., Lai H. L., Lu L. X. (2009). Effect of Danhong injection on platelet activation and inflammatory factors in patients of acute coronary syndrome after intervention therapy. Chin. J. Integr. Tradit. Western Med. 29 692–694. [PubMed] [Google Scholar]
- Chu C. Y., Lin T. H., Lai W. T. (2017). The management and prognostic factors of acute coronary syndrome: evidence from the taiwan acute coronary syndrome full spectrum registry. Acta Cardiol. Sin. 33 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H., Kwon H. M., Kim J. Y., Yoon Y. W., Rhee J., Choi E. Y., et al. (2014). Lipoprotein-associated phospholipase A(2) is related to plaque stability and is a potential biomarker for acute coronary syndrome. Yonsei Med. J. 55 1507–1515. 10.3349/ymj.2014.55.6.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik-Guerra U. I., Fila M., Kaminski M., Kotas R., Wroblewski J., Trzos E., et al. (2014). Correlation between the activity of the autonomic nervous system and endothelial function in patients with acute coronary syndrome. Pol. Arch. Med. Wewn. 124 509–515. 10.20452/pamw.2456 [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. P. T., Altman D. G., Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://training.cochrane.org/handbook [Google Scholar]
- Dong C., Du L. H. (2015). Protective effect with vascular endothelial of Danhong injection on unstable angina after coronary intervention in patients. Chin. J. Prim. Med. Pharm. 22 3407–3409. 10.3760/cma.j.issn.1008-6706.2015.22.016 [DOI] [Google Scholar]
- Dong J., Chen Y. D., Liu H. X., Chen J. S., Zhou Y., Wang J., et al. (2014). Effects of Danhong injection on endothelial function of perioperative unstable angina patients with blood stasis syndrome undergoing percutaneous coronary intervention. J. Chin. Med. 55 1109–1112. 10.13288/j.11-2166/r.2014.13.009 [DOI] [Google Scholar]
- Feng K., Ji X. B., Qiu W. W., Jiang M. Y., Shen W., Jiang W., et al. (2007). The effect of Danhong injection on cardiovascular event in earlier period and inflammatory reaction of the patients of ACS with PCI. J. Chin. Microcirc. 11 390–392. [Google Scholar]
- Garcia-Salas J. M., Tello-Montoliu A., Manzano-Fernandez S., Casas-Pina T., Lopez-Cuenca A., Perez-Berbel P., et al. (2014). Interleukin-6 as a predictor of cardiovascular events in troponin-negative non-ST elevation acute coronary syndrome patients. Int. J. Clin. Pract. 68 294–303. 10.1111/ijcp.12245 [DOI] [PubMed] [Google Scholar]
- Garjani A., Sohrabi B., Movassaghpour A. A., Andalib S., Shokri M., Taherkhanchi B., et al. (2016). Thrombolytic therapy up-regulates inflammatory mediators compared to percutaneous coronary intervention (PCI). Iran. J. Allergy Asthma Immunol. 15 257–263. [PubMed] [Google Scholar]
- Guo J. F., Wu S. B., Yang J. P., Sun G. D., Wang Y. F. (2015). Treatment of Danhong injection with atorvastatin on endothelial function and inflammation factors in patients with acute coronary syndrome after percutaneous coronary intervention. Chin. J. Exp. Tradit. Med. Formulae 21 154–157. [Google Scholar]
- Guo L. L., An Y., Sun B., Chu X. M., Li T. D. (2015). Influence of Danhong injection combining external counterpulsation on inflammatory factors in patients with acute coronary syndrome after percutaneous coronary intervention. Chin. J. Evid. Based Cardiovasc. Med. 7 631–634. 10.3969/j.issn.1674-4055.2015.05.14 [DOI] [Google Scholar]
- Hamed G. M., Fattah M. F. (2015). Clinical Relevance of matrix metalloproteinase 9 in patients with acute coronary syndrome. Clin. Appl. Thromb. Hemost. 21 705–711. 10.1177/1076029614567309 [DOI] [PubMed] [Google Scholar]
- Hansen M. L., Sorensen R., Clausen M. T., Fog-Petersen M. L., Raunso J., Gadsboll N., et al. (2010). Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch. Intern. Med. 170 1433–1441. 10.1001/archinternmed.2010.271 [DOI] [PubMed] [Google Scholar]
- Hess C. N., Peterson E. D., Peng S. A., de Lemos J. A., Fosbol E. L., Thomas L., et al. (2015). Use and outcomes of triple therapy among older patients with acute myocardial infarction and atrial fibrillation. J. Am. Coll. Cardiol. 66 616–627. 10.1016/j.jacc.2015.05.062 [DOI] [PubMed] [Google Scholar]
- Ihling C. (1998). [Pathomorphology of coronary atherosclerosis]. Herz 23 69–77. 10.1007/BF03044538 [DOI] [PubMed] [Google Scholar]
- Jia B. S. (1989). [Relation of the level of platelet membrane glycoproteins, platelet adhesion, platelet aggregation and traditional Chinese medicine syndrome patterns of coronary heart disease]. Zhong Xi Yi Jie He Za Zhi 9 717–719, 707. [PubMed] [Google Scholar]
- Kalay N., Yarlioglues M., Ardic I., Duran M., Kaya M. G., Inanc T., et al. (2010). The assessment of atherosclerosis on vascular structures in patients with acute coronary syndrome. Clin. Invest. Med. 33 E36–E43. 10.25011/cim.v33i1.11836 [DOI] [PubMed] [Google Scholar]
- Kirtane A. J., Sandhu P., Mehran R., McEntegart M., Cristea E., Brener S. J., et al. (2014). Association between intraprocedural thrombotic events and adverse outcomes after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (a Harmonizing Outcomes With RevasculariZatiON and Stents in Acute Myocardial Infarction [HORIZONS-AMI] Substudy). Am. J. Cardiol. 113 36–43. 10.1016/j.amjcard.2013.08.034 [DOI] [PubMed] [Google Scholar]
- Kurtul A., Yarlioglues M., Murat S. N., Duran M., Oksuz F., Koseoglu C., et al. (2016). The association of plasma fibrinogen with the extent and complexity of coronary lesions in patients with acute coronary syndrome. Kardiol. Pol. 74 338–345. 10.5603/KP.a2015.0196 [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu J. P., Di W. Y., Li J., Xu Z. W., Zhao X. Z., et al. (2016). Effect of danhong injection on endothelial injury, degree of inflammation and cardiac function of patients with acute coronary syndrome after intervention therapy. J. Hainan Med. Univ. 22 1619–1622. 10.13210/j.cnki.jhmu.20160427.002 [DOI] [Google Scholar]
- Liu Z. Q., Li Y. B., Zhao L. P., Zhang F. C. (2017). Clinical Observation of Danhong Injection on patients with non-ST elevation ACS after emergency PCI intervention. Shaanxi J. Tradit. Chin. Med. 38 869–870. 10.3969/j.issn.1000-7369.2017.07.028 [DOI] [Google Scholar]
- Lopez A. D., Murray C. C. (1998). The global burden of disease, 1990-2020. Nat. Med. 4 1241–1243. 10.1038/3218 [DOI] [PubMed] [Google Scholar]
- Makki N., Brennan T. M., Girotra S. (2015). Acute coronary syndrome. J. Intensive Care Med. 30 186–200. 10.1177/0885066613503294 [DOI] [PubMed] [Google Scholar]
- Movahed M. R., Butman S. M. (2008). The pathogenesis and treatment of no-reflow occurring during percutaneous coronary intervention. Cardiovasc. Revasc. Med. 9 56–61. 10.1016/j.carrev.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Pei Y. N. (2005). The pharmacological action and clinical application of safflower. Lishizhen Med. Mater. Med. Res. 16 144–146. [Google Scholar]
- Qi T. T., Bao Y. M., Liu A. H. (2017). Research advance in protective effect of water-soluble ingredients in Salviae miltiorrhizae radix et rhizoma on myocardial ischemia and reperfusion injury. Chin. J. Exp. Tradit. Med. Formulae 23 257–263. [Google Scholar]
- Ross R. (1999). Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340 115–126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- Sandoval-Pinto E., Padilla-Gutierrez J. R., Valdes-Alvarado E., Garcia-Gonzalez I. J., Valdez-Haro A., Munoz-Valle J. F., et al. (2016). Association of the -1031T > C polymorphism and soluble TNF-alpha levels with Acute Coronary Syndrome. Cytokine 78 37–43. 10.1016/j.cyto.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Santos-Gallego C. G., Picatoste B., Badimon J. J. (2014). Pathophysiology of acute coronary syndrome. Curr. Atheroscler. Rep. 16:401. 10.1007/s11883-014-0401-9 [DOI] [PubMed] [Google Scholar]
- Schmidt D. E., Manca M., Hoefer I. E. (2015). Circulating endothelial cells in coronary artery disease and acute coronary syndrome. Trends Cardiovasc. Med. 25 578–587. 10.1016/j.tcm.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Sonneveld M. A., Cheng J. M., Oemrawsingh R. M., de Maat M. P., Kardys I., Garcia-Garcia H. M., et al. (2015). Von Willebrand factor in relation to coronary plaque characteristics and cardiovascular outcome. Results of the ATHEROREMO-IVUS study. Thromb. Haemost. 113 577–584. 10.1160/th14-07-0589 [DOI] [PubMed] [Google Scholar]
- Sun B., Liu Z., Yin H., Wang T., Chen T., Yang S., et al. (2017). Intralesional versus intracoronary administration of glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention in patients with acute coronary syndromes: a meta-analysis of randomized controlled trials. Medicine 96:e8223. 10.1097/md.0000000000008223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umman B., Cakmakoglu B., Cincin Z. B., Kocaaga M., Emet S., Tamer S., et al. (2015). Identification of gene variants related to the nitric oxide pathway in patients with acute coronary syndrome. Gene 574 76–81. 10.1016/j.gene.2015.07.081 [DOI] [PubMed] [Google Scholar]
- Vazquez-Arce M. I., Marques-Sule E. (2017). Descriptive and comparative study of cardiovascular risk factors and physical activity in patients with acute coronary syndrome. Aten. Primaria 10.1016/j.aprim.2017.06.008 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Peng Y., Dong J., Lin J., Wu C., Su Y., et al. (2013). Human platelets express functional thymic stromal lymphopoietin receptors: a potential role in platelet activation in acute coronary syndrome. Cell Physiol. Biochem. 32 1741–1750. 10.1159/000356608 [DOI] [PubMed] [Google Scholar]
- Wang S., He S. F., Zhai J. B., Zhang Y., Kang L. Y., Ren M. (2014). Research progress in pharmacological effects and clinical applications of Danhong Injection. Chin. J. Inform. Tradit. Chin. Med. 21 128–131. 10.3969/j.issn.1005-5304.2014.03.046 [DOI] [Google Scholar]
- Wang Z. X., Qiao Z. R., Yang L. H., Han J., Dong Q. J., Wang R. Q. (2011). “Effect of Danhong injection on vWF ′ ET-1 ′ NO level in patients with acute coronary syndrome undergoing percutaneous coronary intervention,” in Paper Presented at the 2nd National Central and Western Medicine Conference of China Central and Western Medicine Association, NanJing. [Google Scholar]
- Wu J. R., Zhang X. M., Zhang B. (2015). Danhong injection in the treatment of acute coronary syndrome: a systematic review and meta-analysis. Am. J. Chin. Med. 43 199–214. 10.1142/s0192415x15500135 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Cao D. R. (2010). Effect of Danhong injection on vascular endothelial function in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Med. J. West China 22 1214–1216. 10.3969/j.issn.1672-3511.2010.07.016 [DOI] [Google Scholar]
- Xie X., Zhang D. W., Liu H. X., Dong J., Chen Y. D., Zhang Z. H., et al. (2016). Protective effects of Danhong injection on perioperative myocardial injury in patients with unstable angina pectoris and blood stasis syndrome during seleetive percutaneous coronary intervention. Chin. J. Integr. Med. Cardio Cerebrovasc. Dis. 14 1185–1188. 10.3960/j.issn.1672-1340.2016.11.001 [DOI] [Google Scholar]
- Ye S. Q., Ma D. M., Yang J. (2017). Impact of Danhong injection combined with clopidogrel on postoperative serum levels of et-1 and no in acute coronary syndrome patients undergoing percutaneous coronary intervention. Pract. J. Card. Cereb. Pneumal Vasc. Dis. 25 148–150. 10.3969/j.issn.1008-5971.2017.06.043 [DOI] [Google Scholar]
- Zhang W. W., Dong H. W., Zhang W. Q. (2016). Influence of Danhong Injection on endothelial function and inflammatory factor in treatment of elderly patients with acute coronary syndrome after PCI intervention. Chin. J. Gerontol. 22 5591–5593. 10.3969/j.issn.1005-9202.2016.22.042 [DOI] [Google Scholar]
- Zhang Y. L., Shang J. J., Xing W. L., Li L., Zhou Q., Lai X. L., et al. (2017). Danhong injection for Periprocedural myocardial injury in elective percutaneous coronary: a systematic review. World Chin. Med. 12 258–265. 10.3696/j.issn.1673-7202.2017.02.006 [DOI] [Google Scholar]
- Zhang Y. X., Zhang Q. (2012). Effects of Danhong injection on protect myocardium of acute coronary syndrome patients after selective percutaneous coronary artery intervention. Chin. J. Exp. Tradit. Med. Formulae 18 308–311. 10.13422/j.cnki.syfjx.2012.23.097 [DOI] [Google Scholar]
- Zhang Z. H., Liu H. X., Zhang D. W., Chen Y. D., Shang J. J., Dai M., et al. (2016). The impact of Danhong injection on unstable angina patients after percutaneous coronary intervention reflected upon velocity vector imaging. World Chin. Med. 11 388–391. [Google Scholar]
- Zhao P. X., Jiang S. (2011). Effect of Danhong injection on ET-1, sP-sel and hsCRP in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin. J. Integr. Tradit. West. Med. 31 11–14. [PubMed] [Google Scholar]
- Zheng H. C., Long F., Yang H., Li P. (2014). The pathogenesis and treatment of acute coronary syndrome. China Pharm. 25 2846–2848. 10.6039/j.issn.1001-0408.2014.30.24 [DOI] [Google Scholar]
- Zheng W. H., Wu X. Z., Zhang A. P. (2015). Influence of Danhong injection on IL-17 expression after PCI in STEMI patients. Chin. J. Cardiovasc. Rehabil. Med. 24 84–87. 10.3969/j.issn.1008-0074.2015.01.25 [DOI] [Google Scholar]
- Zhou J., Deng G., Yang T., Ma Q., Luo X. (2014). [Association between interleukin-18 and Global Registry of Acute Coronary Events score in patients with acute coronary syndrome]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 39 570–576. 10.11817/j.issn.1672-7347.2014.06.005 [DOI] [PubMed] [Google Scholar]