Abstract
As “Immunotherapy age” is coming, immune checkpoint inhibitors (CPI) therapies have shown favorable clinical benefits and low toxicity profiles in patients with advanced non-small cell lung cancer (NSCLC). While it is a pity that there is a little clear clinical trials evidence about immunotherapy among Asian population. Moreover, since there is an ethnic difference for targeted therapy, what about immunotherapy? Which factors may associate with ethnic differences from Caucasian population to Asiatic population? In this review, we supposed that the characteristics of the much higher proportion of EGFR mutation, hepatitis B virus infection and unexpected immune-related toxicity among Asian patients should be considered.
Keywords: Immunotherapy, PD-1/PD-L1 inhibitors, non-small cell lung cancer (NSCLC), ethnic difference, Asiatic population
Introduction
Non-small cell lung cancer (NSCLC) is the primary cause of cancer death worldwide. It is also the leading cause of cancer-related death, taking a staggering 1.6 million lives each year (1). “Chemotherapy age” with a platinum-doublet provides a median overall survival (OS) of 10 months for advanced-stage NSCLC (2). With “IPASS” published in 2004, “TKI (tyrosine kinase inhibitor) age” has significantly prolonged progression-free survival (PFS) and OS in selected patients carrying actionable genes such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), but resistance to TKIs inevitably occurs (3-6), and 5-year survival rates still remain low (7), which underscoring the need for novel and more effective treatment strategies for NSCLC. After the first remarkable reports of immune checkpoint inhibitors (CPI) shrinking advanced melanoma, since 2015, “Immunotherapy age” is coming.
In 2017, ASCO has named Immunotherapy 2.0 as the advance of the year with two characteristics. The first is expanded indications. Up to now, the FDA has approved more ten uses for immune CPI: melanoma (8), lung cancer (9), Hodgkin’s Lymphoma (10), urothelial carcinoma (11), head and neck cancer (12), renal cell carcinoma (13,14), Merck cell carcinoma (15), colorectal cancer (16), gastric cancer (17), liver cancer (18). More indications will be confirmed.
The second is longer overall survival. CPI demonstrated a long duration of response in responders, usually more than 1 or 2 years (19,20). According to data from National Cancer Institute’s SEER, 5-year overall survival rate in advanced NSCLC is only 4.8% and 1.6% (non-squamous carcinoma and squamous carcinoma, respectively). In 2017AACR, with new data emerging from the NSCLC cohort of CA209-003, the estimated of 5-year overall survival rate in nivolumab-treated patients up to about 16% (20). In 2017 ASCO, group with pembrolizumab exhibited that 3-year overall survival rate is 26.4% in treatment-naïve patients and 19.0% in previously-treated patients and 5-year overall survival rate was even up to 21.5% (21). Compared with docetaxel, group with nivolumab had almost 3 times (CheckMate017) or 2 times (CheckMate057) 3-year OS, about 16–18% (Table 1) (22) .
Table 1. Long-term survival in NSCLC with PD-1/PD-L1 inhibitors.
| Drugs | Clinical trials | 2-year OS (%) | 3-year OS (%) | 5-year OS (%) |
|---|---|---|---|---|
| Pembrolizumab | Keynote 001 (treated native pts vs. previously treated pts) | 44.5 vs. 31.3 | 26.4 vs. 19.0 | Keynote 010 21.5 |
| Nivolumab | CM017 (SQ) (Nivo vs. docetaxel) | 23.0 vs. 8.0 | 16.0 vs. 6.0 | CA209-003 SQ vs. non-SQ: 16.0 vs. 15.0 |
| CM057 (non-SQ) (Nivo vs. docetaxel) | 29.0 vs. 16.0 | 18.0 vs. 9.0 | ||
| Atezolizumab | OAK (Atezo vs. docetaxel) | 31.0 vs. 21.0 | – | – |
NSCLC, non-small cell lung cancer; Pts, patients; CM, checkmate; SQ, squamous lung cancer; non-SQ, non-squamous lung cancer; Nivo, nivolumab; Atezo, atezolizumab; OS, overall survival.
Until recently, four CPI have received US Food and Drug Administration approval for use in advanced NSCLC: nivolumab (March 4, 2015), pembrolizumab (October 2,2015) (23,24). Atezolizumab (October 18,2016) and durvalumab (July, 2017) (25). In addition, nivolumab will be the first CPI coming into the market in China.
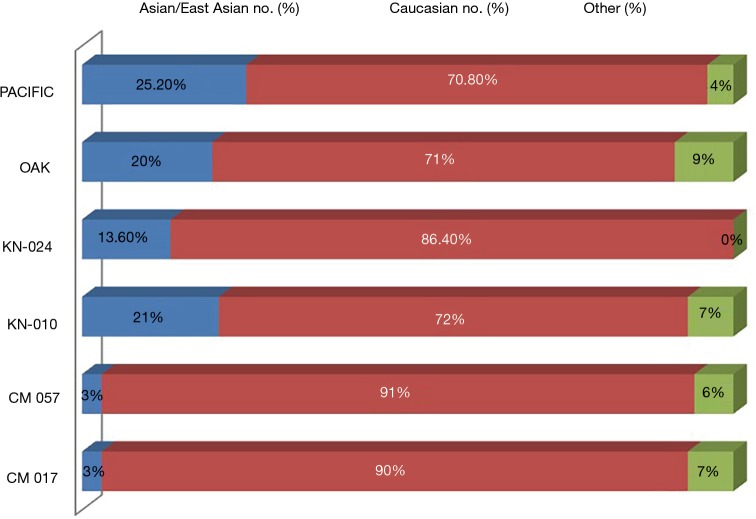
Despite these promising outcomes, the clinical trials results for Asiatic population is still rare now. Either Checkmate or Keynote or OAK, Caucasian population is the majority of almost all clinical trials (Figure 1). It is reported that there are 270 ongoing international clinical trials of PD-1/PD-L1(programmed cell death 1)/(programmed death-ligand 1) inhibitors in last three years, among that, clinical trials performed in East Asia was only taken up in 22.6% and China was even 5.2% (26). Up to December, 2017, fifty-one phase 3 clinical trials of PD-1/PD-L1 blockades in NSCLC were signed up on ClinicalTrials.gov, covering eight drugs: pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, IBI308, SHR-1210 and BGB-A317. IBI308, SHR-1210 and BGB-A317 were developed by China, which accounts for a small percentage in these phase 3 clinical trials, about 6%. Moreover, only KEYNOTE-024 has subgroup analysis of the response between East Asian population and non-East Asian population. Checkmate078 is the first trial demonstrates that nivolumab shows superior overall survival to predominantly Chinese population with NSCLC (27), and the specific data was opened in 2018 AACR.
Figure 1.
The proportion of Asiatic population in previous PD-1/PD-L1 clinical trials. KN, keynote; CM, checkmate.
Since there is an ethnic difference for targeted therapy (28), what about immunotherapy? Which factors may associate with it? Herein, we aim to provide a review about the current understanding of the role of immunotherapy in NSCLC from Asiatic population to Caucasian population.
Pharmacokinetics
Previous clinical trials demonstrate that between Japanese patients and non-Japanese patients, pharmacokinetic characteristics of ipilimumab and pembrolizumab were similar (29-32), which was consistent with results with nivolumab: demographic factor also has little impact on pharmacokinetics in patients with solid tumors (33). In short, pharmacokinetics profile of PD-1/PD-L1 inhibitors has no significant difference from Asiatic population to Caucasian population.
Clinical response and toxicity
For first line setting, KEYNOTE-024 shows that East Asia population is more sensitive to Pembrolizumab than to non-East Asia. For second line setting, two phase II studies evaluated the safety and efficacy of nivolumab in Japanese patients with advanced non-squamous lung cancer (JapicCTI-132073) and squamous lung cancer (JapicCTI-132073) respectively (34,35). Above results (Table 2) of clinical trials are consistent that the safety and the efficacy of PD-1/PD-L1 inhibitors is superior to docetaxel. It was reported that the objective response rate (ORR) of Chinese population (CheckMate078) was 17%, which was also in accordance with Caucasian population (CheckMate017/057). Besides, the clinical benefits from nivolumab were independent of pathological types and expression of PD-L1. Interestingly, responses in Asiatic population especially in Japanese patients seems a little better than Caucasian population. In all, more large sample clinical trials for immunotherapy in Asiatic patients are urgently needed.
Table 2. Comparisons different PD-1/PD-L1 clinical trials results between Asiatic and Caucasian population.
| Trial results | Squamous NSCLC | Non-squamous NSCLC | |||||
|---|---|---|---|---|---|---|---|
| Checkmate017 | Japic CTI-132072 | Checkmate078 | Checkmate057 | Japic CTI-132072 | Checkmate078 | ||
| N | 135 | 35 | 133 | 292 | 76 | 205 | |
| ORR, % | 20.0 | 25.7 | 16.6 | 19.0 | 22.4 | 16.6 | |
| Median PFS (months) | 3.5 | 4.2 | 2.8 | 2.3 | 2.8 | 2.8 | |
| OS (months) | 9.2 | 16.3 | 12.3 | 12.2 | 17.1 | 11.9 | |
| 3–4 grade Tr-AEs (%) | 7.0 | 5.7 | 10.4 | 10.0 | 6.5 | 10.4 | |
| All grades Tr-AEs (%) | 58.0 | 68.6 | 64.1 | 69.0 | 84.2 | 64.1 | |
| Hepato-toxicity | Unknown | 5.7 | 18.0 | Unknown | 6.6 | 18.0 | |
| Pulmonary toxicity | 5.0 | 5.7 | 7.0 | 3.0 | 7.9 | 7.0 | |
NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; Tr-AEs, treatment-related adverse events; ORR, objective response rate.
However, a meta-analysis demonstrated that PD-1 inhibitors were associated with increasing risk of immune-associated pneumonitis in cancer patients regardless of dose (36). Moreover, NSCLC have the highest risk of pneumonitis, about 5% (37). In terms of pulmonary toxicity, previously studies showed that Asiatic patients with NSCLC may be more susceptible to the onset of pneumonitis (Tables 2,3), consistent results were found in subgroup data of Japanese patient in PACIFIC (38), a phase III study of durvalumab in stage III, unresectable NSCLC patients. Compared with all patients, pneumonitis or radiation pneumonitis is much higher in Japanese patients, (33.9% vs. 73.6% in all grades Tr-AEs, respectively) (Table 3). Incidence of interstitial lung disease (ILD) in combination osimertinib with durvalumab could reach to 38% and the majority of patients with ILD is Asian (39). In terms of hepatotoxicity, though the incidence of hepatotoxicity in Japanese patients was about 6%, it up to 18% in Chinese patients, which suggested that hepatotoxicity is also a question should be seriously considered.
Table 3. Safety summary in PACIFIC.
| Pneumonitis or radiation pneumonitis, n (%) | All patients durvalumab (n=475) | Japanese patients durvalumab (n=72) |
|---|---|---|
| Any grade | 161 (33.9) | 53 (73.6) |
| Grade 3–4 | 16 (3.4) | 4 (5.6) |
In brief, relatively high incidence of adverse events especially pulmonary toxicity and hepatotoxicity among Asiatic population deserves great attention. So how to balance clinical response and toxicity? What is the optimal dose for immunotherapy? Although the 2 mg/kg Q3W regimen for pembrolizumab was authorized by the FDA to treat advanced NSCLC following first-line therapy, whether the weight-based or fixed-dose strategies were preferable remains controversial. With regard to the economic burden and pervasive Asian body weight, the lowest dose is usually recommended. More clinical trials are expected to answer these questions.
HBV
A study showed that Asia accounted for nearly two-thirds (62%) of global HBV burden attributable to injecting drug use, particularly east Asia, which took up of 44% of total HBV burden (40) China has a major impact on the global burden of liver diseases, with the number of patients with hepatitis B is reaching 93 million, far more than those in Euro-America with less than 10 million (41). It is raised a question that what about response to NSCLC with HBV infection? Dose HBV infection really matters to immunotherapy?
Checkmate040 demonstrated that advanced hepatocellular carcinoma has durable objective responses to nivolumab. A subgroup analysis in Checkmate025 of Japanese patients even found that higher Median PFS and ORR than Checkmate040 (4.6 vs. 4.0 months; 26% vs. 20%, respectively) (13). Moreover, it is gratifying that in patients with advanced hepatocellular carcinoma, there is no significant different between HBV infected subgroup and uninfected subgroup (18). A recent study published on Nature found that in patients with liver chronic inflammation and fibrosis, inflammation-induced IgA+ cells, which express PD-L1 and interleukin-10, suppressing liver cytotoxic CD8+ T lymphocytes and resulting in tumor-promoting. This study may explain why PD-L1 inhibitors could induce HCC regression (42).
In a word, HBV infection has litter impact on the treatment of patients with advanced hepatocellular carcinoma, so we suppose that patients with NSCLC also have response to PD-L1 inhibitors. While up to now, HBV infected patients are excluded by the majority of clinical trials in NSCLC. Further study should be investigational.
Gene mutation
It is well known that EGFR mutations were more frequent in Asian populations and KRAS mutations were more frequent in Western populations. Up to 47.9% of Asian population harbor EGFR mutation while this rate was only about 15% in Caucasian population. When it comes to KRAS mutation, the mutation rate is higher in Caucasian population (30% vs. 7%) (28).
In terms of KRAS, subgroup analysis from Checkmate057 and OAK suggest that KRAS positive patients have a better clinical response to PD-1 inhibitors. It was reported that either median PFS or 24-week PFS rate, patients with KRAS-mutant was numerically prior to patients with KRAS-wild type (11.8 vs. 2.3 months; 88% vs. 29%, respectively) (9). A study from Guangdong Lung Cancer In statute also discovered that TP53 or KRAS mutation patients, especially those with co-occurring TP53/KRAS mutations, showed remarkable clinical benefit to PD-1 inhibitors (43). Similar results were found in the latest evidence and clinical consequences by Pilar Garrido and his colleagues, however, patients with LKB1-deficient in KRAS-mutated exhibited low response (44).
In terms of EGFR, for one thing, though preclinical findings indicated EGFR mutation increased the PD-L1 expression (45-48). In real world clinical trials, immune checkpoint inhibitors were of significant benefits to EGFR wide type patients but no improvement in OS to EGFR mutant advanced NSCLC compared with docetaxel (9,38,39,49,50). A retrospective analysis performed by Dr. Gainor and colleagues also showed that NSCLCs harboring EGFR mutations is in relation to poor response rates to PD-1 blockade in NSCLCs (51). In addition, EGFR alterations result in potentially higher risk of hyper-progressors after immunotherapy (52). What possible reasons may underlie these clinical observations? For one hand, most of the patients harboring EGFR gene mutation are of low non-synonymous mutation burden owing to light or never smoking (53,54). For the other hand, patients with EGFR mutation exhibited an uninflamed tumor microenvironment (TME) (55) with fewer of CD8-positive tumor infiltrating Lymphocytes and lower tumor mutation burden (TMB) (56). As it was found that CD73 (57) and IFNG signature (58) play a role in PD-1/PD-L1 pathway. In 2017 ASCO, it was reported that overexpression of CD73 and low expression of IFNG signature in EGFR mutant tumors result in “cold tumor”, which may provide an explanation for poor response to PD-1/PD-L1 inhibitors (59). Finally, based on East Asian patients, EGFR mutation was correlated with decreased PD-L1 expression (56), which is opposite to some previously studies (60). Ethnic differences may underline the diversities of PD-L1 expression from Caucasian population to Asiatic population.
Since EGFR-mutant NSCLCs manifested poorer response to PD-1/PD-L1 inhibitors, what about patients acquiring resistance after EGFR-TKIs?
It was reported that patients after EGFR inhibitor therapy is associated with increased PD-L1 expression (61), which indicate the potential of application for PD-1/PD-L1 inhibitors, while results from CheckMate057 and OAK showed that response to PD-1/PD-L1 inhibitors is not better or even worse than chemotherapy in patients who took PD-1/PD-L1 inhibitors after acquiring resistance to EGFR-TKI (9,49). Interestingly, contradictory results were found in small sample clinical trials: object response rate could up to 20% (62) or was only 3.6% (51) in those patients.
In regard to combination EGFR-TKIs with PD-1/PD-L1 inhibitors, TATTON trial suggested an encouraging clinical activity of combination of osimertinib with durvalumab, the incidence of ILD was 26% in patients of EGFR-TKI pretreated with dose escalation while this rate up to 64% in patients of EGFR-TKI treatment naive with dose expansion (39). Erlotinib plus atezolizumab was not onset of interstitial lung disease, but treatment-related G3–4 AEs and treatment-related serious AEs occurred in 39% and 50% of patients respectively (63). Gefitinib combined with durvalumab demonstrated markedly increased ALT/AST (64). Thus, the safety profile of combination EGFR-TKIs with PD-1/PD-L1 inhibitors warrants additional consideration.
In short, to EGFR mutation NSCLCs, for the first line, EGFR-TKI is still the best option. For the second line, PD-1/L1 inhibitors have no significant advantage over chemotherapy. Combination PD-1/PD-L1 inhibitors with EGFR TKIs may be an effective way to extend the clinical response and improve durability, but the relatively high incidence of adverse events limiting the further development.
Current issues
It is obvious that comprehensive factors contributing to ethnic differences from Asiatic population to Caucasian population in immunotherapy, not a single factor. Furthermore, there are still some problem remains unknown. Some questions remain controversial.
Firstly: who is the superior population for immunotherapy? The response rate to PD-1/PD-L1 inhibitors was around 19% to 25% in unselected patients while up to 92% in some selected patients (9,65-69). Many other patients either do not benefit from immunotherapy at all or just experience a short period of response. The low ORR highlighting the need to refine patient selection by predictive markers.
In view of PD-L1 expression, though many studies demonstrate higher PD-L1 expression is associated with better response to PD-1/PD-L1 inhibitors in the context of advanced melanoma and NSCLC (11,70-74), this finding is not consistent among all studies. For first-line treatment, group with PD-L1 ≥50% with better repose to pembrolizumab has been confirmed. For second-line treatment, TC2/3 or IC2/3 (PD-L1 expression ≥5% on tumor cells or tumor-infiltrating immune cells) may serve as a predictive biomarker to atezolizumab (75). Moreover, constitutive expression of PD-L1 and the presence of TILs is also of potential predictive value (76). Interestingly, compared 3-year OS rate between Checkmate057 and Checkmate017, we can have observed that PD-L1 expression levels have little impact on response to immunotherapy in squamous lung cancer excepting when PD-L1 expression ≥50% while have great effects on no squamous lung cancer, which indicates PD-L1 expression may be of predictive value in different histologic type. Regarding the high PD-L1 expression in relation to better response, patients with squamous cell carcinomas in East Asian while patients in no squamous lung cancer in western countries may be the prior population for immunotherapy (77). In view of gene mutation, TP53 or KRAS mutation patients, especially those with co-occurring TP53/KRAS mutations, showed remarkable clinical benefit to PD-1 inhibitors (43,78). While in a recent meta-analysis, KRAS was failure to be recommended as a predictive biomarker owing to the absence of significant statistical differences (79). Furthermore, it also suggested that immune checkpoint inhibitors have no overall survival benefit to NSCLCs with EGFR mutation in second-line therapy (79).
In view of TMB, Checkmate026 is the first pivotal randomized phase 3 trial to incorporate an analysis evaluating TMB and clinical benefit with PD-1 inhibitor therapy, suggesting that nivolumab improves ORR and PFS compared with chemotherapy in patients with high TMB (80) which is consistent with findings in CheckMate032 (81) and Checkmate 227 (82). Investigation of other potential predictive biomarkers such as MSI-H and dMMR are also ongoing.
Secondly, how to improve the low overall response rate? For one thing, it is essential for exploring the best predictive markers to identify patients who are most likely to get curative effect from CPI. For another thing, combination treatment strategies would be a feasible research direction.
Either PFS or OR, patients with pembrolizumab who previously received any radiotherapy was twofold longer than those without previous radiotherapy (4.4 vs. 2.1 months; 10.7 vs. 5.3 months, respectively) (83). In CheckMate012, ORR of nivolumab plus ipilimumab group reached to 92% in patients with PD-L1 expression 50%. To date, results from many previous studies supporting synergistic efficacy to administer immunotherapy concurrently with chemotherapy (84-90), targeted therapies (39,86,87,91,92), radiotherapy (93-97) and CTLA-4 checkpoint inhibitors (98-100), as well as angiogenesis inhibitors (68,73,90,101).
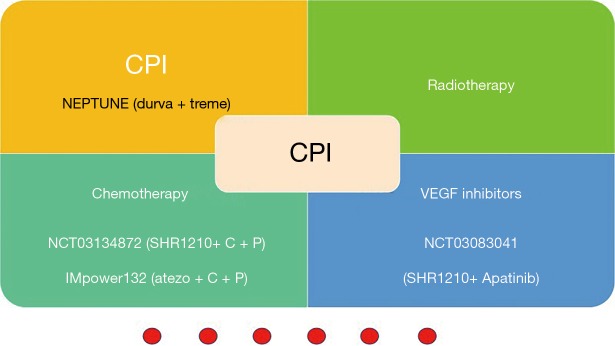
Clinical trials about CPI combination therapies that recruiting Asiatic patients are also underway. It seems that SHR-1210 is taking the leading position in domestic medicines of China (Figure 2). However, the management of immune-related adverse events (Tr-AEs) merits serious consideration.
Figure 2.
Combination CPI with other treatments recruiting Asiatic patients in lung cancer. CPI, immune checkpoint inhibitors. durva, durvalumab; treme, tremelimumab; atezo, atezolizumab; C, carboplatin; P, pemetrexed.
Thirdly, when is the best time for immunotherapy? Since the median PFS of durvalumab in patients after chemoradiotherapy has up to 16.8 months. How is the response to immunotherapy for first-line treatment especially for driver gene-negative patients? What is the optimal sequence for treatment for NSCLC? So far pembrolizumab has established priority position in driver gene-negative NSCLCs so long as PD-L1 ≥1% (102). Pembrolizumab in combination with chemotherapy also has already been approved by the FDA for patients with no squamous NSCLC at frontline treatment based on data from Keynote189 (103) and the cohort G of KEYNOTE-021 (104). In the foreseeable future, Pembrolizumab in combination with chemotherapy will be approved for patients with metastatic NSCLC in virtue of findings from KEYNOTE-407 trial (105). According to IMpower131 and IMpower150 (101), combination therapy of Atezolizumab also showed great superiority in NSCLC at first line treatment. Many related clinical trials such as MYSTIC and CheckMate227, deserve great anticipation.
Finally, as the existence of EGFR mutations among Chinese patients with NSCLC is approximately 50% (106,107), while clinical trials results for immunotherapy in Asiatic population stills rare. It is raised some questions such as how is the clinical response and toxicity for EGFR-mutation patients? CheckMate078 exhibited the results in Asiatic population, but it ruled out EGFR-mutation patients (27).
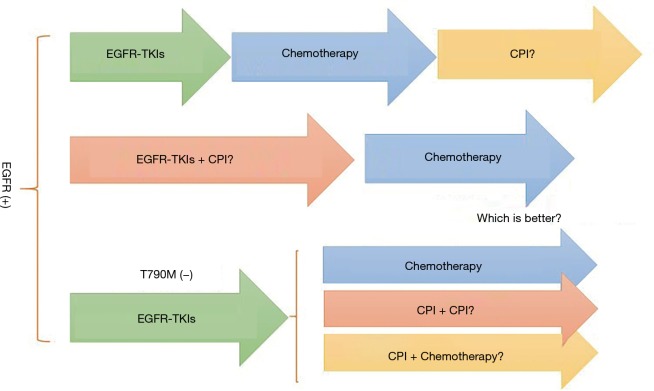
Furthermore, when is the best time point to give EGFR mutant patients with CPI to extend the duration of treatment response (Figure 3)? It is also observed that EGFR-mutation patients acquiring resistance to EGFR-TKIs due to T790M are less likely to receive benefit from PD-1 inhibitors than T790M-negative patients, possibly owing to lower PD-L1 expression level in T790M-positive patients (62). So, for patients acquiring resistance to EGFR-TKIs but T790M were negative, could CPI make a difference (Figure 3)? CheckMate722 adopted CPI monotherapy, CPI plus chemotherapy and CPI plus CPI three regimens respectively, to explore the best immunotherapy regimens for these patients.
Figure 3.
When and how to combine EGFR-TKIs with CPI. CPI, immune checkpoint inhibitors.
In addition, which is the optimal PD-L1 cut-off value for Asian patients? Without question, PD-L1 cut-off value is a key factor to success for the clinical trial. Up to now, 1%, 5%, 25%, and 50% have been taken as cut-off value in previous trials, however, research on this matter is far from conclusive. A phase II single-arm study of SHR-1210 is exploring the definitions of ‘‘positive’’ PD-L1 test result among Asian patients. Above all, results of these studies are of great significance to address current problems and worth great anticipating.
Conclusions
In conclusion, immunotherapy has undoubtedly turned over a new leaf in both understanding as well as therapeutic patterns to cancer. Though PD-1/PD-L1 inhibitors international multicenter clinical trials have increased greatly during the last 3 years, it is a pity that there is a little clear clinical trials evidence about immunotherapy among Asian people. The characteristics of the much higher proportion of EGFR mutation, hepatitis B virus infection among Asian patients should be considered when designing Asiatic immunotherapy trials. This review highlighted an insight into the ethnic difference from Asiatic population to Caucasian population and problems in immunotherapy remains unsolved. Further efforts will be taken aiming to illustrate current issues and extend the benefits for more patients.
Acknowledgements
Funding: This study is supported by the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (grant No. 2012A061400006), the Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (grant No. 201402031), and the Research Fund from Guangzhou Science and Technology Bureau (grant No. 2014Y2-00050).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 3.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. 10.1126/scitranslmed.3003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol 2016;27 Suppl 3:iii42-iii50. 10.1093/annonc/mdw305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. 10.1056/NEJMoa044238 [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 7.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236-71. 10.6004/jnccn.2012.0130 [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558-68. 10.1016/S1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283-94. 10.1016/S1470-2045(16)30167-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita Y, Fukasawa S, Shinohara N, et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin Oncol 2017;47:639-46. 10.1093/jjco/hyx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 2016;374:2542-52. 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA Approves Merck’s KEYTRUDA® (pembrolizumab) for Previously Treated Patients with Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer Whose Tumors Express PD-L1 (CPS Greater Than or Equal to 1). Available online: http://wwwmrknewsroomcom/news-release/prescription-medicine-news/fda-approves-mercks-keytruda-pembrolizumab-previously-treate.
- 18.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramalingam S, Hui R, Gandhi L, et al. P2.39: Long-Term OS for Patients With Advanced NSCLC Enrolled in the KEYNOTE-001 Study of Pembrolizumab: Track: Immunotherapy. J Thorac Oncol 2016;11:S241-S2. 10.1016/j.jtho.2016.08.11027676573 [DOI] [Google Scholar]
- 20.Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018:JCO2017770412. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Helwick C. Pembrolizumab Affords Long-Term Survival to One-Fourth of Selected Patients With NSCLC, Alternative Statistical Model Suggests. THE ASCO POST.
- 22.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. 10.1093/annonc/mdy041 [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration: FDA approves Keytruda for advanced non-small cell lung cancer: First drug approved in lung cancer for patients whose tumors express PD-L1. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm.
- 24.US Food and Drug Administration: FDA expands approved use of Opdivo to treat lung cancer,3/15 update. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436534.htm.
- 25.Durvalumab "Game-Changer" for Locally Advanced Lung Cancer. Available online: http://wwwonclivecom/conference-coverage/esmo-2017/durvalumab-gamechanger-for-locally-advanced-lung-cancer.
- 26.Liu SY, Wu YL. Ongoing clinical trials of PD-1 and PD-L1 inhibitors for lung cancer in China. J Hematol Oncol 2017;10:136. 10.1186/s13045-017-0506-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CheckMate -078, a Pivotal, Multinational Phase 3 Opdivo (nivolumab) Lung Cancer Trial with Predominantly Chinese Patients, Stopped Early for Demonstrating Superior Overall Survival. Available online: https://newsbmscom/press-release/corporatefinancial-news/checkmate-078-pivotal-multinational-phase-3-opdivo-nivolumab-l.
- 28.Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. 10.1093/annonc/mdt205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horinouchi H, Yamamoto N, Fujiwara Y, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest New Drugs 2015;33:881-9. 10.1007/s10637-015-0243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950-6. 10.1200/JCO.2008.16.1927 [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, Seto T, Hirai F, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs 2016;34:347-54. 10.1007/s10637-016-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber J, Hamid O, Amin A, et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer Immun 2013;13:7. [PMC free article] [PubMed] [Google Scholar]
- 33.Bajaj G, Wang X, Agrawal S, et al. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacometrics Syst Pharmacol 2017;6:58-66. 10.1002/psp4.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 2017;1:e000108. 10.1136/esmoopen-2016-000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hida T, Nishio M, Nogami N, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci 2017;108:1000-6. 10.1111/cas.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Hong D, Zhang X, et al. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Sci Rep 2017;7:44173. 10.1038/srep44173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 39.Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. 10.1016/S1556-0864(16)30246-527198274 [DOI] [Google Scholar]
- 40.Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016;16:1385-98. 10.1016/S1473-3099(16)30325-5 [DOI] [PubMed] [Google Scholar]
- 41.Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099-108. 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017;551:340-5. 10.1038/nature24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- 44.Garrido P, Olmedo ME, Gomez A, et al. Treating KRAS-mutant NSCLC: latest evidence and clinical consequences. Ther Adv Med Oncol 2017;9:589-97. 10.1177/1758834017719829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. 10.1097/JTO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 46.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gettinger S, Politi K. PD-1 Axis Inhibitors in EGFR- and ALK-Driven Lung Cancer: Lost Cause? Clin Cancer Res 2016;22:4539-41. 10.1158/1078-0432.CCR-16-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin K, Cheng J, Yang T, et al. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kappaB. Biochem Biophys Res Commun 2015;463:95-101. 10.1016/j.bbrc.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 49.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 51.Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol 2016;27:LBA44._PR. 10.1093/annonc/mdw435.43 [DOI] [Google Scholar]
- 54.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 56.Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017;6:e1356145. 10.1080/2162402X.2017.1356145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin PSA, Wu S, Widmaier M, et al. Mutually exclusive expression of CD73 and PDL1 in tumors from patients (pt) with NSCLC, gastroesophageal (GE) and urothelial bladder carcinoma (UBC). J Clin Oncol 2017;35:3079. [Google Scholar]
- 58.Streicher KMC, Sebastian Y, Kuziora M, et al. Gene expression analysis of tumor biopsies from a trial of durvalumab to identify subsets of NSCLC with shared immune pathways. J Clin Oncol 2017;35:3041. [Google Scholar]
- 59.Streicher K, Higgs BW, Wu S, et al. Increased CD73 and reduced IFNG signature expression in relation to response rates to anti-PD-1(L1) therapies in EGFR-mutant NSCLC. J Clin Oncol 2017;35:11505. [Google Scholar]
- 60.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. 10.1093/annonc/mdu242 [DOI] [PubMed] [Google Scholar]
- 61.Han JJ, Kim DW, Koh J, et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:263-70.e2. 10.1016/j.cllc.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 62.Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 2017;28:1532-9. 10.1093/annonc/mdx183 [DOI] [PubMed] [Google Scholar]
- 63.Ma BBY, Rudin CM, Cervantes A, et al. Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Ann Oncol 2016;27:S9 10.1093/annonc/mdw594.005 [DOI] [Google Scholar]
- 64.Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 antiprogrammed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. 10.1016/S1556-0864(16)30171-X27198414 [DOI] [Google Scholar]
- 65.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015;10:985-9. 10.1097/JTO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 68.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control 2014;21:80-9. 10.1177/107327481402100112 [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 2015;10:e0130142. 10.1371/journal.pone.0130142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 73.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. 10.1200/JCO.2016.71.9476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong ZY, Wu SP, Liao RQ, et al. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour Biol 2016;37:4251-61. 10.1007/s13277-016-4812-9 [DOI] [PubMed] [Google Scholar]
- 77.Pan Y, Zheng D, Li Y, et al. Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer. J Thorac Dis 2017;9:2579-86. 10.21037/jtd.2017.08.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma Clin Cancer Res 2017;23:3012-24. 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- 79.Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.AACR 2017. Impact of Tumor Mutation Burden on the Efficacy of First-LineNiv in Stage IV or Recurrent Non-Small Cell Lung Cancer: An Exploratory Analysis of CheckMate 026.
- 81.Opdivo Alone or Combined with Yervoy Shows Encouraging Response and Survival Rates in Recurrent Small Cell Lung Cancer Patients with High Tumor Mutation Burden, in Exploratory Analysis from Phase 1/2 Study CheckMate -032. Available online: https://investorsbmscom/iframes/press-releases/press-release-details/2017/Opdivo-Alone-or-Combined-with-Yervoy-Shows-Encouraging-Response-and-Survival-Rates-in-Recurrent-Small-Cell-Lung-Cancer-Patients-with-High-Tumor-Mutation-Burden-in-Exploratory-Analysis-from-Phase-12-Study-CheckMate--032/defaultaspx.
- 82.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018;33:843-52.e4. 10.1016/j.ccell.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maio M, Di Giacomo AM, Robert C, et al. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol 2013;25:166-72. 10.1097/CCO.0b013e32835dae4f [DOI] [PubMed] [Google Scholar]
- 86.Galluzzi L, Buqué A, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690-714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 87.Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74-88. 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 88.Apetoh L, Ladoire S, Coukos G, et al. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol 2015;26:1813-23. 10.1093/annonc/mdv209 [DOI] [PubMed] [Google Scholar]
- 89.Gadgeel SM, Stevenson J, Langer CJ, et al. Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. J Clin Oncol 2016;34:abstr9016.
- 90.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. 10.1200/JCO.2016.66.9861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu L, Mayes PA, Eastman S, et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 2015;21:1639-51. 10.1158/1078-0432.CCR-14-2339 [DOI] [PubMed] [Google Scholar]
- 92.Tabchi S, Kourie HR, Kattan J. Adding checkpoint inhibitors to tyrosine kinase inhibitors targeting EGFR/ALK in non-small cell lung cancer: a new therapeutic strategy. Invest New Drugs 2016;34:794-6. 10.1007/s10637-016-0383-2 [DOI] [PubMed] [Google Scholar]
- 93.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. 10.1172/JCI69219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67:65-85. 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
- 97.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanvetyanon T, Gray JE, Antonia SJ. PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer? Expert Opin Biol Ther 2017;17:305-12. 10.1080/14712598.2017.1280454 [DOI] [PubMed] [Google Scholar]
- 99.Gubens MA, Sequist LV, Stevenson J, et al. Phase I/II study of pembrolizumab (pembro) plus ipilimumab (ipi) as second-line therapy for NSCLC: KEYNOTE-021 cohorts D and H,. J Clin Oncol 2016;34:abstr 9027.
- 100.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. 10.1016/S1470-2045(15)00544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phase III IMpower150 Study Showed Genentech’s TECENTRIQ (Atezolizumab) and Avastin (Bevacizumab) Plus Carboplatin and Paclitaxel Helped People With Advanced Lung Cancer Live Longer Compared to Avastin Plus Carboplatin and Paclitaxel. Available online: https://wwwbusinesswirecom/news/home/20180325005099/en/Phase-III-IMpower150-Study-Showed-Genentech%E2%80%99s-TECENTRIQ.
- 102.Mok T, Wu YL, Sadowski S, et al. 195TiP: Pembrolizumab (MK-3475) versus platinum-based chemotherapy for PD-L1+ NSCLC in a phase 3, randomized, open-label study: KEYNOTE-042. J Thorac Oncol 2016;11:S142. 10.1016/S1556-0864(16)30304-527198333 [DOI] [Google Scholar]
- 103.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018. [Epub ahead of print]. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 104.Papadimitrakopoulou V, Gadgeel SM, Borghaei H, et al. First-line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: Updated results of KEYNOTE-021 cohort G. J Clin Oncol 2017;35:9094 [Google Scholar]
- 105.Broderick JM. FDA Approval Sought for Frontline Pembrolizumab Combo in Squamous NSCLC. Available online: https://wwwonclivecom/web-exclusives/fda-approval-sought-for-frontline-pembrolizumab-combo-in-squamous-nsclc.
- 106.Shi Y, Li J, Zhang S, et al. Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology - Mainland China Subset Analysis of the PIONEER study. PLoS One 2015;10:e0143515. 10.1371/journal.pone.0143515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. 10.1097/JTO.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]