Abstract
Alzheimer’s disease (AD) is a chronic progressive neurodegenerative disease in adults characterized by the deposition of extracellular plaques of β-amyloid protein (Aβ), intracellular neurofibrillary tangles (NFTs), synaptic loss and neuronal apoptosis. AD has a strong and complex genetic component that involving into multiple genes. With recent advances in whole-exome sequencing (WES) and whole-genome sequencing (WGS) technology, UNC5C was identified to have association with AD. Emerging studies on cell and animal models identified that aberrant UNC5C may contribute to AD by activating death-associated protein kinase 1 (DAPK1) which is a new component involved in AD pathogenesis with an extensive involvement in aberrant tau, Aβ and neuronal apoptosis/autophagy. In this review, we briefly summarize the biochemical properties, genetics, epigenetics, and the speculative role of UNC5C in AD. We hope our review would bring comprehensive understandings of AD pathogenesis and provide new therapeutic targets for AD.
Keywords: Alzheimer’s disease (AD), apoptosis/autophagy, activating death-associated protein kinase 1 (activating DAPK1), tau, UNC5C
Introduction
Alzheimer’s disease (AD) is the most common chronic neurodegenerative disorder which is characterized by progressive memory loss and ultimately dementia (1,2). The pathogenesis of AD is multifactorial which involves in the interaction of complex genetic, epigenetic, and environmental factors (3). AD is commonly categorized into two types, early-onset AD (EOAD) and late-onset AD (LOAD) based on the onset time (4). The EOAD cases (<60 years old; 5–10%) are Mendelian forms of the disease caused by rare and dominantly inherited mutations in the amyloid-β protein precursor (APP), presenilin 1 (PSEN1) and PSEN2 (5). The LOAD (onset ≥65 years), is the major type of AD accounting for >95% of all cases (6). The pathology of LOAD is multi-factorial with biological, genetic and environmental factors interacting with each other to aggravate the process of AD pathology (7). Up to now, the ɛ4 isoform of apolipoprotein E (ApoE4) has been widely accepted as the only genetic risk factor for LOAD (8). With recent advances in whole-exome sequencing (WES) and whole-genome sequencing (WGS) technology, to identify rare variants with large effect sizes associated with the disease has been proven to be feasible (9,10). Compared with this putative variant, recently the UNC5C gene was also revealed to have significant association with AD pathogenesis via combining WES, WGS and linkage analyses in large LOAD pedigrees in European populations (11). UNC5C plays an important physiological role during neural development by directing axon extension and cell migration (12,13). Thus, discussion on in which way the aberrant expression of UNC5C contributes to the mechanisms of LOAD is essential.
Accumulation of evidence from cell models showed that a UNC5C variant could lead to AD pathogenesis by activating death-associated protein kinase 1 (DAPK1) which was involved in modulating tau protein accumulation, β-amyloid protein (Aβ) toxicity and neuronal apoptosis/autophagy (11,14). However, in view of the complexity of AD pathogenesis, there are still no complete cellular or animal models demonstrating all pathological traits of AD. In addition, the definition of pathogenesis and effective therapies remain unsolved (15). Therefore, it is more essential to define the specific role of any molecule in AD pathogenesis. In this review, we focus on the role of UNC5C in AD pathogenesis. We briefly summarize the current genetics findings, the potential epigenetics and speculative roles of UNC5C in AD. Besides, we believe that further studies on UNC5C may be of great value to understand the mechanism of AD and UNC5C-targeted therapeutics will present the recent challenges and advances.
Structure and biochemical properties of UNC5C
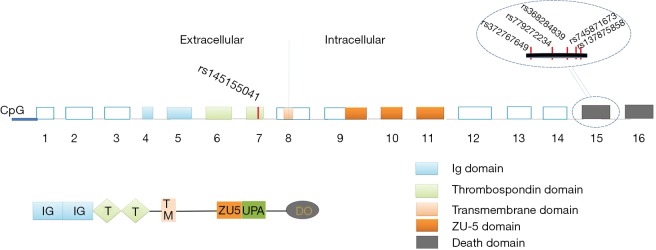
UNC5C was the first to be identified to have association with AD in 2014 by combining WGS, WES and linkage analyses in large LOAD pedigrees in European populations (11). UNC5C gene localizes on chromosome 4q22.3 and encodes 16 exons, which can translate into 950 amino acid polypeptides (16). The UNC5C protein is a typical transmembrane protein that contains two Ig domains (Ig), two thrombospondin domains (TS), a transmembrane domain (TM), a zona occludens-5 domain (ZU-5), a UPA domain, and a death domain (DD) (16) (Figure 1). UNC5C is a member of UNC-5 family including UNC5A, UNC5B, UNC5C and UNC5D, which are widely expressed in the nervous system as well as the heart and seems to be enriched in the hippocampus of AD brain (17). So far, among these members, only UNC5C is reported to be associated with AD (18). UNC5C is universally known as the receptor of neurin-1, playing a crucial role in mediating axon repulsion of neuronal growth cones and cell migration in the developing nervous system (19,20). UNC5C is also known as a dependence receptor which is responsible for the regulation of neuronal apoptosis and whether UNC5C acts the role of pro- or anti-apoptotic molecule depends on its binding to netrin-1 (21,22). Dysfunctional UNC5C will cause relative cells to be misrouted and to fail to receive survival signals, ultimately triggering cell death (23). Previous studies revealed that mice homozygous for mutations in UNC5C are ataxic and have cerebellar hypoplasia and laminar structure defects (16,17), which indicated that UNC5C plays an indispensable role in the development of nervous system. Additionally, the altered UNC5C expression is associated with many types of cancers including colorectal, breast, stomach, lung, ovary, uterus, or kidney cancers (22). Recently, a UNC5C variant (T835M) was found to markedly shorten cell survival time up to 50% compared with controls in vitro experiment, suggesting a significant role of UNC5C in the regulation of cell survival (11). More recently, a genome-wide association study (GWAS) revealed a single nucleotide polymorphism (SNP) in the 3’UTR of UNC5C has close association with embryonic development in mammals, which may affect the binding site of microRNAs and further change the expression levels of mRNA and UNC5C protein (24). Given those mentioned above, aberrant expression of UNC5C is likely to cause diverse disease development. In view of the important function of UNC5C in nervous system, a more comprehensive study on it may offer a deeper understanding of the AD pathogenesis as well as helpful insights into tumor context.
Figure 1.
Genomic features of the UNC5C gene with some reported SNPs in corresponding exon domains. The schematic representation of the UNC5C gene is shown in this study including exon-intron structure, location of CpG island, corresponding encoded protein domains and the site of filtered SNPs. The locations of rs137875858, rs145155041, rs372767649, rs368284839, rs779272234, rs745871673 highlighted by the red line means six main risk loci among UNC5C variants in AD. AD, Alzheimer’s disease; DD, death domain.
Genetics of UNC5C in AD
To date, more than 20 loci have been revealed to be associated with AD risk, among which the total APOE with frequent [1–5% minor allele frequency (MAF)]variants are irrefutably recognized as the major susceptibility gene for LOAD (25). What’s more, in 2014, a UNC5C SNP rs137875858 T835M was identified to predispose to LOAD with a similar effect size to that of APOE ε4 allele (26) by linkage analyses, segregating with disease in two independent families (11). Wetzel-Smith et al. also demonstrated that a UNC5C SNP predisposed to increasing risk of sporadic AD (odds ratio =2.15, P=0.0095, 95% CI: 1.21–3.84) in four independent data sets including 8,050 LOAD cases and 98,194 controls (11). Furthermore, Jiao et al. replicated UNC5C in Chinese population with 360 AD cases and 400 controls and revealed four highly conserved UNC5C SNPs associated with LOAD (27). However, UNC5C T835M failed to be replicated in Chinese population, but four novel loci (p.S843G, p.Q860H, p.V836V, p.T837K) showed to confer certain risk of AD (27). The four loci only existed in sporadic AD cases but not in controls, among which p.Q860H variant showed stronger association with AD risk with a P value 0.017 (27). More recently, rs145155041 (D353N) of UNC5C located in exon 7 which encodes an extracellular TS was found to be involved in AD occasionally during the study focusing on TREM2 (28). Apparently, UNC5C variants were found in different datasets and close association between UNC5C and AD was highlighted. The detailed information of UNC5C variants are shown in Table 1.
Table 1. Rare coding variants of UNC5C related to LOAD.
| UNC5C SNPs | Risk allele | AA variation | Exon location | P | Population |
|---|---|---|---|---|---|
| rs137875858 | A | T835M | Exon 15 | 0.0095 | European population |
| rs145155041 | T | D353N | Exon 7 | N/A | European population |
| rs372767649 | G | Q860H | Exon 15 | 0.017 | Han Chinese |
| rs368284839 | A | T837K | Exon 15 | 0.13 | Han Chinese |
| rs779272234 | G | S843G | Exon 15 | 0.36 | Han Chinese |
| rs745871673 | G | V836V | Exon 15 | 0.36 | Han Chinese |
P value was determined using Fisher exact test. LOAD, late-onset Alzheimer’s disease; SNP, single-nucleotide polymorphism; N/A, not applicable.
Recently, a neuroimaging study by Sun et al. demonstrated that UNC5C gene polymorphisms had a notable effect on the brain structure in AD-associated regions (29). Some loci near rs145155041 and rs137875858 showed significant association with brain atrophy; rs34585936 is related to the volume atrophy in right middle temporal; rs72672784, rs13120458, and rs34875919 would promote the atrophy rate in crucial regions especially the left hippocampus; rs72672784, rs74690179 and rs2001246 are associated with right precuneus atrophy (29). What’s more, the study by Sun et al. (29) also showed that effect of UNC5C gene polymorphisms on brain structures was independent of APOE genotype, indicating that UNC5C may be an independent risk factor for AD. Given those mentioned above, UNC5C gene polymorphisms play a significant role in AD and thus deeper studies will be of great value in exploring UNC5C induced pathways in AD.
The epigenetics of UNC5C in AD
Epigenetics is referred to as the processes involving changing gene expression without altering the DNA sequence (30). The UNC5C promoter contains a special methyl group which is called CpG dinucleotides (31), and methylation in these CpG islands or any correlated modifications in histone complexes can disorder the process of gene transcription in an epigenetic manner (30,32) (Figure 1). In addition, research showed that the aberrant methylation of UNC5C was a universal phenomenon in cancers, and the level of mRNA was markedly reduced by the aberrant methylation (13,33). In the context of tumors, the aberrant UNC5C methylation is negatively correlated with its protein expression (34). However, due to the bidirectional effect of aberrant DNA methylation in protein expression, methylation in gene promoter would lead to the increase in protein expression and reversely methylation in gene bodies would lead to the reduction in protein expression (30,35). Following this logic, the aberrant UNC5C methylation in neurons seems warranted.
As a matter of fact, the AD pathogenesis is multifactorial. The risk factors are not only genetic variants and environmental factors but also epigenetic abnormalities, such as changes in DNA methylation or modifications of the proteins that package the DNA (36). Previous studies have revealed that epigenetic modifications may affect AD pathogenesis, such as increased microtubule-associated protein tau (MAPT). Methylation could suppress the MAPT expression, which could affect the levels of tau protein (37). However, whether aberrant UNC5C DNA methylation alters in AD requires more in-depth investigation. Interestingly, the epigenetic changes can be reversible under certain circumstances (34), thus further research is needed on the epigenetics of UNC5C. Targeting the epigenome may provide new opportunities in neuroprotection and therapy for AD.
The speculative role of UNC5C in AD
The initial finding of UNC5C in AD was detected by Wetzel-Smith et al. (11) with the variant T835M, suggesting the involvement of UNC5C-induced neuronal death pathways, tau pathology and Aβ-associated pathways. Subsequently, a study by Hashimoto et al. focusing on molecular mechanisms of the association between UNC5C and AD, demonstrated that UNC5C leading to neuron death was mediated by an intracellular death-signaling cascade through the sequentially activating DAPK1/protein kinase D (PKD)/apoptosis signal-regulating kinase 1 (ASK1)/JNK/NADPH oxidase/caspases (14). Among the signal transduction processes, activated DAPK1 is the central component in initiating the pathology of AD. Interestingly, the death-signaling cascade in UNC5C partially overlapped with APP-mediated death-signaling pathway at ASK1 (14). However, as UNC5C is a novel risk gene in AD, the correlative study on it is modest compared with that of APOE, MAPT, and clusterin (CLU) for less available data. In view of the fact that UNC5C variants play a pronounced role in neuronal death, in-depth investigation of potential molecular pathways in AD is required. Following this logic, we reviewed the current available studies on UNC5C, which summarized thoughts in AD with tumors contexts and elaborated the following speculative pathways.
UNC5C-induced signaling pathway and Aβ
Abnormal Aβ aggregation and accumulation forming the amyloid plaques is well known as a major pathological hallmark of AD (38). As Aβ accumulation acts as a putative central factor in initiating the AD pathogenesis, it is essential to explore the role of UNC5C in Aβ pathways. Accordingly, the initial study was to explore the effect of UNC5C on the production of Aβ. Aβ is generated by sequential proteolytic cleavages of amyloid precursor protein (APP) by β-secretase and γ-secretase (39) (Figure 2). Aβ is 38–43 amino acid residue peptides including two major isoforms Aβ1-40 (account for 90%) and Aβ1–42 (account for 5–10%), in which Aβ1–42 is more prone to assemble into neurotoxic oligomers (40,41). However, cell models to alter the expression levels of UNC5C showed no evidence of affecting the generation of Aβ1–40 and Aβ1–42, indicating that UNC5C-induced neuronal death did not affect the process of APP cleavage (11). Additionally, a deeper investigation of the association between UNC5C and Aβ showed that overexpressed UNC5C and UNC5C-T835M cell had higher propensity to death compared with normal controls in Aβ-incubated rat hippocampal neurons, indicating that the aberrant UNC5C increased the susceptibility to Aβ-induced neurotoxicity (11). Meanwhile, the study also revealed that the aberrant UNC5C increases the susceptibility of neurons not only to Aβ but also to other neurotoxic insults in a similar manner (11), indicating that the role of UNC5C in neuronal death did not depend on Aβ. However, deeper exploration is required for the explanation of why UNC5C-induced neuronal death was intensified in the existence of Aβ. Until 2016, Hashimoto et al. revealed the signaling pathway of UNC5C contributing to neuronal death was involved in DAPK1/PKD/ASK1/JNK/NADPH oxidase/caspases pathways (14). During the caspases-dependent cell death pathway, DAPK1 played an initial role in this signaling transduction and acted as a regulator of PKD and was indispensable for the activation of JNK signaling in oxidative stress condition (42). It is well recognized that Aβ showed wide neurotoxicity including increasing oxidative stress in cells (41,43). Based on these concepts, we hypothesize that the Aβ-induced oxidative stress condition would promote the signaling activation of PDK and JNK by DAPK1. That seems to be a plausible explanation of why UNC5C variant increased cell death in the existence of Aβ without affecting Aβ levels. Taken together, aberrant UNC5C-induced neuronal death was independent of Aβ. Meanwhile, Aβ-induced oxidative stress condition would promote DAPK1-induced death signaling (Figure 2). However, the direct molecular mechanism of the association between UNC5C and Aβ pathways remains to be further verified.
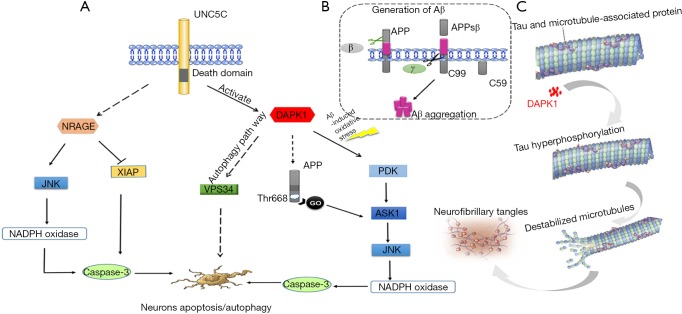
Figure 2.
Summary of the speculative roles of UNC5C in AD pathogenesis. (A) Aberrant UNC5C would activate the expression of DAPK1 then activated DAPK1 would sequential activate its downstream of PKD, ASK1, JNK, NADPH oxidase, caspases and ultimately led to a common caspases-dependent neuronal apoptosis. DAPK1-induced Vps34 pathways also may be a possible pathway to trigger neuronal autophagy. In addition, activated DAPK1 could phosphorylate APP at Thr668, which would lead to neuronal death by Aβ-independent GO protein pathway. On the other hand, the UNC5C also may be possible to be related to NRAGE-associated pathway. The aberrant NRAGE may activate its downstream of JNK, which would ultimately lead to caspases-dependent neuronal death. What’s more, the NRAGE may also degrade the caspase inhibitor XIAP, which would trigger the increase of caspases and result in promoting to neuronal apoptosis; (B) Aβ is generated via cleaving fragment of APP via β-secretase and γ-secretase. Firstly, APP is cleaved by β-secretase at the corresponding site and then releases APPsβ ectodomain. Secondly, the remaining carboxy-terminal fragment is cleaved by γ-secretase and then Aβ is released; (C) aberrant UNC5C over activate DAPK1, overexpression of DAPK1 will lead to tau protein hyper phosphorylation and ultimately promote to the form of NFT. AD, Alzheimer’s disease; DAPK1, death-associated protein kinase 1; PKD, protein kinase D; ASK1, apoptosis signal-regulating kinase 1; APP, amyloid-β protein precursor; Aβ, β-amyloid protein; NRAGE, neurotrophin receptor p75-interacting melanoma-associated antigen homolog; XIAP, X-chromosome-linked inhibitor of apoptosis protein; NFT, neurofibrillary tangle.
Interestingly, previous studies revealed that APP also had an Aβ-independent pathway associated with neuronal apoptosis and lysosomal-autophagy in AD by a heterotrimeric G protein Go pathways (44-48) (Figure 2). What’s more, UNC5C revealed a common death-associated signaling pathway with APP-induced cell death at ASK1 during the G protein Go pathways (14). However, there is still no direct link between APP and UNC5C-induced signaling pathway. Based on the important roles of both in AD, it will be of great value in exploring the possible link between UNC5C and APP. Hence, an in-depth investigation of whether UNC5C interacts with APP is required in further study.
The speculative role of UNC5C in tau
Recently, aberrant UNC5C expression was revealed to notably increase the levels of extracellular tau proteins in cell models (11). Tau is a natively microtubule-associated protein widely distributed in the neurons of central nervous system, playing a role in the assembly and stabilization of microtubules (49). Tau protein phosphorylation is a physiological process to regulate microtubule dynamics and promote neuronal differentiation (50,51). However, hyperphosphorylated tau triggering aberrant tau accumulation in neurons was the center of many neurodegenerative diseases including AD (15,52). Overall, when tau protein is abnormally hyperphosphorylated and modified, it will lose its physiological function in binding to microtubules and then generate pathological insults in neurons. The recent study by Wetzel-Smith et al. revealed that a UNC5C variant (T835M) would notably increase the levels of extracellular tau proteins and decrease the cell survival in vitro cell models (11). It is obvious that the aberrant UNC5C expression had a close association with increasing tau levels. Though the underlying mechanisms of the association between UNC5C and tau remain unclear, prospective correlative pathways were introduced in the following.
Emerging studies revealed that DAPK1 could notably increase tau protein phosphorylation and improve tau stability (53). In cell and animal model studies, the DAPK1 knockout mice showed an evident decrease of tau protein stability and even an abolition of phosphorylated tau (53), which indicated that DAPK1 played a critical role in the regulation of tau protein. The cellular molecule studies revealed that DAPK1 would phosphorylate tau protein at Ser262, Thr231 and Ser396 sites by inhibiting the function of Pin1 (53). Pin1, a phosphorylation-dependent peptidyl-prolyl cis-trans isomerase, played an important role in restoring the conformation of phosphorylated tau (53,54). Obviously, the DAPK1 inhibited the protective function of Pin1 and thus led to enhancing tau stability and promoting tau phosphorylation which was prone to form pathological aggregation and trigger neurodegeneration (53) (Figure 2). Given that aberrant UNC5C could activate DAPK1 expression and increase tau levels, UNC5C-induced DAPK1 activation may be a prospective mechanism associated with tauopathies. Although aberrant UNC5C elevated tau levels, the underlying mechanisms involved in DAPK1 remains to be further verified in cell and animal experiments.
The speculative role of UNC5C in apoptosis/autophagy
Accumulative evidence suggested that inappropriate apoptosis and impaired autophagic processes were extensively involved in neurodegenerative diseases including AD (55-57). A recent study revealed that UNC5C overexpression and UNC5C-T835M in neurons brought higher levels of annexin V, an early apoptotic marker (11,58), which indicated that aberrant UNC5C was involved in LOAD by mediating neuronal apoptosis process. UNC5C emerges as a dependence receptor including a DD which is responsible for cell death (59) and the knockout of the C-terminal DD would markedly abolish cell death (14). Accordingly, it is clear that UNC5C-induced neuronal death depends on the DD. The DD contains a caspase cleavage site in the intracellular region where it is cleaved by caspase-3 (60). This cleavage will lead to the generation of a pro-apoptotic fragment called addiction/dependence domain (ADD) (61,62), which acts as a scaffold to recruit and activate caspases which have been termed “executioner” proteins, ultimately leading to cell apoptosis, or programmed cell death (63). In addition, a mechanistic study in cell models identified that DAPK1 was activated in the aberrant UNC5C cell and the interaction of activated DAPK1 with the DD was the initial component in triggering neuronal death. DAPK1 interacted with the DD and triggered a series of highly regulated steps including sequential activation of PKD, ASK1, JNK, NADPH oxidases and caspases, ultimately leading to caspases-dependent neuronal apoptosis (14,64). As for the role of DAPK1 in UNC5C-induced neuronal apoptosis, DAPK1 was an initial factor which was indispensable for PDK and JNK phosphorylation and DAPK1 inhibitor could almost nullify the UNC5C variant-induced cell death in cell culture model (42). DAPK1 was identified to be involved in AD pathogenesis with an up-regulated expression in AD brain (65). In addition, SNPs (rs4878104 and rs4877365) in DAPK1 were identified to be risk factors for AD, which indicated the negative association of DAPK1 with AD (66-68).
As a death-associated kinase protein, DAPK1 is identified as a new component of the neuronal death signaling complex involved in a wide range of cellular processes, including apoptosis and autophagy (69). Therefore, DAPK1 could not only mediate apoptotic caspase-dependent cell death pathway but also be recognized as a mediator of cell autophagy to mediate a non-apoptotic caspases-independent programmed cell death (69). The molecular mechanisms of DAPK1 in cell autophagy were mainly associated with the Vps34-associated signaling pathways and the process of Vps34 activation included two independent pathways (69). The first pathway involved a kinase cascade, in which DAPK1 bound and phosphorylated PKD and further phosphorylated and activated Vps34. In the other pathway, DAPK1 can directly phosphorylate Beclin1, a necessary component of the Vps34 complex, and further activate Vps34 by releasing Beclin1 from its inhibitors, B-cell lymphoma 2 (Bcl-2) (69). Although DAPK1 can induce cell autophagy, the mechanisms underlying UNC5C-induced cell autophagy in AD context remain to be further elucidated.
In addition, based on the studies on UNC5A-induced death-signaling pathway, other possible signaling molecules may also be involved in the UNC5C-induced neuronal apoptosis. Neurotrophin receptor p75-interacting melanoma-associated antigen homolog (NRAGE), a UNC-5 family interacting protein, was revealed to participate in UNC5A-induced apoptosis via two ways (70). In one way, NRAGE is involved in the degradation of the X-chromosome-linked inhibitor of apoptosis protein (XIAP) which is a caspase inhibitor, and in another way, NRAGE could participate in activating pro-apoptotic JNK signaling pathway (70). However, it is a fact that the binding affinity of NRAGE to UNC5C is weaker than that to UNC5A (70). Thereby whether these signaling molecules participated in UNC5C-induced apoptosis needs further study. In conclusion, the role of UNC5C in neuronal death was involved in several mechanisms including cell apoptosis and autophagy process (Figure 2). The UNC5C-induced DAPK1 pathway in apoptosis has been identified in AD associated cell and animal models. However, the UNC5C-induced DAPK1 pathway in autophagy and the UNC5C-induced NRAGE pathway in AD are still unclear and remain to be elucidated in future research. In view of the importance role of DAPK1 in triggering neuronal death, we sincerely expect that blocking DAPK1 can be a new therapeutic target for AD.
UNC5C as a therapeutic target for AD
Given together, the potential pathways underpinning roles of UNC5C in AD pathogenesis demonstrated above have provided new insights into further investigations and intervention in the disease.
As demonstrated above, aberrant UNC5C-induced LOAD is mainly mediated by DAPK1-dependent cell death signaling pathways. Therefore, blocking the DAPK1 signal transduction seems feasible in UNC5C-associated AD. Calmodulin-like skin protein (CLSP) acts on JNK, the downstream molecular of DAPK1, as an endogenous inhibitor was identified to have marked neuroprotective effects in aberrant UNC5C cell models. For this reason, the deeper study of applying CLSP in UNC5C-associated AD treatment will be of great value. Interestingly, studies focusing on netrin-1 revealed that it prevented cell apoptosis by binding to UNC5C on the cell surface and suppressed UNC5C-induced death (14). Additionally, previous studies also reported netrin-1 could interact with APP and regulate Aβ levels in animal models (71). Combining the above evidence with the fact that netrin-1 has been successfully applied as an anti-apoptotic agent against hypoxic injury of the brain tissue (72,73), we can conclude that netrin-1 is promising to present a new therapeutic approach for UNC5C-induced AD. In view of the strong risk factor of UNC5C gene polymorphism for LOAD, UNC5C gene targeted therapy such as antisense oligonucleotides and RNA interference would offer a new therapeutic opportunity for UNC5C-associated AD. Finally, given the potential roles of UNC5C to AD pathogenesis, we sincerely hope that these new findings of UNC5C may open up avenues for further novel therapeutic approaches.
Conclusions
UNC5C is widely expressed in the adult hippocampus and cerebellum neurons. As for its biological function, it acts as a chemotropic molecule in mediating axon growth and neuronal migration in neuronal development and acts as a dependence receptor in the regulation of cell apoptosis. Up to now, six SNPs in UNC5C have been reported to increase the risk of LOAD. Although the association between UNC5C and LOAD risk has been well replicated in Chinese population, all these findings need to be further identified in more ethnic groups. To our knowledge, this review is the first study to summarize the role of UNC5C in AD. Although the potential mechanisms of UNC5C in AD are unclear, emerging data suggests that aberrant UNC5C predisposing to LOAD was mediated by DAPK1-induced cell death signaling pathway. However, further study should be conducted in cell culture and animal models to validate the speculative pathological pathways including DAPK1-induced APP phosphorylation, aberrant tau accumulation, neuronal autophagy in UNC5C-associated AD. Other possible pathways, such as synaptic plasticity, iron homeostasis and lipid metabolism, also remain to be further investigated before being translated into clinical practice. Finally, we sincerely hope that in-depth studies on the association of UNC5C with AD will point to novel pathogenesis and thus provide insights into novel therapeutic targets for AD.
Acknowledgements
This work was supported by grants from the Shandong Taishan Scholar, Qingdao Key Health Discipline Development Fund, and Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci 2014;37:79-100. 10.1146/annurev-neuro-071013-014300 [DOI] [PubMed] [Google Scholar]
- 2.Muller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci 2017;18:281-98. 10.1038/nrn.2017.29 [DOI] [PubMed] [Google Scholar]
- 3.Athanasopoulos D, Karagiannis G, Tsolaki M. Recent Findings in Alzheimer Disease and Nutrition Focusing on Epigenetics. Adv Nutr 2016;7:917-27. 10.3945/an.116.012229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez MF. Early-Onset Alzheimer Disease. Neurol Clin 2017;35:263-81. 10.1016/j.ncl.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanoiselee HM, Nicolas G, Wallon D, et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med 2017;14:e1002270. 10.1371/journal.pmed.1002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu NN, Tan MS, Yu JT, et al. The Role of Reelin Signaling in Alzheimer's Disease. Mol Neurobiol 2016;53:5692-700. 10.1007/s12035-015-9459-9 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura YV, Sekine K, Chihama K, et al. Dissecting the factors involved in the locomotion mode of neuronal migration in the developing cerebral cortex. J Biol Chem 2010;285:5878-87. 10.1074/jbc.M109.033761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane-Donovan C, Herz J., Apo E, Apo E. Receptors, and the Synapse in Alzheimer's Disease. Trends Endocrinol Metab 2017;28:273-84. 10.1016/j.tem.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng SB, Buckingham KJ, Lee C, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 2010;42:30-5. 10.1038/ng.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del-Aguila JL, Koboldt DC, Black K, et al. Alzheimer's disease: rare variants with large effect sizes. Curr Opin Genet Dev 2015;33:49-55. 10.1016/j.gde.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 11.Wetzel-Smith MK, Hunkapiller J, Bhangale TR, et al. A rare mutation in UNC5C predisposes to late-onset Alzheimer's disease and increases neuronal cell death. Nat Med 2014;20:1452-7. 10.1038/nm.3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kury S, Garrec C, Airaud F, et al. Evaluation of the colorectal cancer risk conferred by rare UNC5C alleles. World J Gastroenterol 2014;20:204-13. 10.3748/wjg.v20.i1.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mur P, Sánchez-Cuartielles E, Aussó S, et al. Scarce evidence of the causal role of germline mutations in UNC5C in hereditary colorectal cancer and polyposis. Sci Rep 2016;6:20697. 10.1038/srep20697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Toyama Y, Kusakari S, et al. An Alzheimer Disease-linked Rare Mutation Potentiates Netrin Receptor Uncoordinated-5C-induced Signaling That Merges with Amyloid beta Precursor Protein Signaling. J Biol Chem 2016;291:12282-93. 10.1074/jbc.M115.698092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet 2016;388:505-17. 10.1016/S0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- 16.Ackerman SL, Knowles BB. Cloning and mapping of the UNC5C gene to human chromosome 4q21-q23. Genomics 1998;52:205-8. 10.1006/geno.1998.5425 [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Ackerman SL. The UNC5C netrin receptor regulates dorsal guidance of mouse hindbrain axons. J Neurosci 2011;31:2167-79. 10.1523/JNEUROSCI.5254-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang B, Peng G, Gao J. Expression of unc5 family genes in zebrafish brain during embryonic development. Gene Expr Patterns 2013;13:311-8. 10.1016/j.gep.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 19.Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol 2010;22:46-54. 10.1097/CCO.0b013e328333dcd1 [DOI] [PubMed] [Google Scholar]
- 20.Poliak S, Morales D, Croteau LP, et al. Synergistic integration of Netrin and ephrin axon guidance signals by spinal motor neurons. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonardo ED, Hinck L, Masu M, et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 1997;386:833-8. 10.1038/386833a0 [DOI] [PubMed] [Google Scholar]
- 22.Thiebault K, Mazelin L, Pays L, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A 2003;100:4173-8. 10.1073/pnas.0738063100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llambi F, Causeret F, Bloch-Gallego E, et al. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J 2001;20:2715-22. 10.1093/emboj/20.11.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto M, Gotoh Y, Kawahara T, et al. Molecular Effects of Polymorphism in the 3'UTR of Unc-5 homolog C Associated with Conception Rate in Holsteins. PLoS One 2015;10:e0131283. 10.1371/journal.pone.0131283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 2011;16:903-7. 10.1038/mp.2011.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrer LA. Expanding the genomic roadmap of Alzheimer's disease. Lancet Neurol 2015;14:783-5. 10.1016/S1474-4422(15)00146-5 [DOI] [PubMed] [Google Scholar]
- 27.Jiao B, Liu X, Tang B, et al. Investigation of TREM2, PLD3, and UNC5C variants in patients with Alzheimer's disease from mainland China. Neurobiol Aging 2014;35:2422.e9-11. 10.1016/j.neurobiolaging.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 28.Korvatska O, Leverenz JB, Jayadev S, et al. R47H Variant of TREM2 Associated With Alzheimer Disease in a Large Late-Onset Family: Clinical, Genetic, and Neuropathological Study. JAMA Neurol 2015;72:920-7. 10.1001/jamaneurol.2015.0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JH, Wang HF, Zhu XC, et al. The Impact of UNC5C Genetic Variations on Neuroimaging in Alzheimer's Disease. Mol Neurobiol 2016;53:6759-67. 10.1007/s12035-015-9589-0 [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Mut JV, Graff J. Epigenetic Alterations in Alzheimer's Disease. Front Behav Neurosci 2015;9:347. 10.3389/fnbeh.2015.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin SK, Nagasaka T, Jung BH, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology 2007;133:1849-57. 10.1053/j.gastro.2007.08.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanker S, Hu Z, Wilkinson MF. Epigenetic regulation and downstream targets of the Rhox5 homeobox gene. Int J Androl 2008;31:462-70. 10.1111/j.1365-2605.2008.00904.x [DOI] [PubMed] [Google Scholar]
- 33.Hibi K, Sakuraba K, Shirahata A, et al. Methylation of the UNC5C gene is frequently detected in hepatocellular carcinoma. Hepatogastroenterology 2012;59:2573-5. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Wang G, He B, et al. Methylation of the UNC5C gene and its protein expression in colorectal cancer. Tumour Biol 2017;39:1010428317697564. 10.1177/1010428317697564 [DOI] [PubMed] [Google Scholar]
- 35.Wood H. Alzheimer disease: AD-susceptible brain regions exhibit altered DNA methylation. Nat Rev Neurol 2014;10:548. 10.1038/nrneurol.2014.164 [DOI] [PubMed] [Google Scholar]
- 36.Dyukov YV, Bachinskaya NY, Cholin VA, et al. [Genetic And Epigenetic Determinants Of Alzheimer's Disease]. Adv Gerontol 2015;28:299-306. [PubMed] [Google Scholar]
- 37.Zhang CC, Xing A, Tan MS, et al. The Role of MAPT in Neurodegenerative Diseases: Genetics, Mechanisms and Therapy. Mol Neurobiol 2016;53:4893-904. 10.1007/s12035-015-9415-8 [DOI] [PubMed] [Google Scholar]
- 38.Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 2017;134:187-205. 10.1007/s00401-017-1709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 2011;34:185-204. 10.1146/annurev-neuro-061010-113613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of beta-amyloid in Alzheimer's disease. Pathol Int 2017;67:185-93. 10.1111/pin.12520 [DOI] [PubMed] [Google Scholar]
- 41.Grimm MO, Mett J, Grimm HS, et al. APP Function and Lipids: A Bidirectional Link. Front Mol Neurosci 2017;10:63. 10.3389/fnmol.2017.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenberg-Lerner A, Kimchi A. DAP kinase regulates JNK signaling by binding and activating protein kinase D under oxidative stress. Cell Death Differ 2007;14:1908-15. 10.1038/sj.cdd.4402212 [DOI] [PubMed] [Google Scholar]
- 43.Ganguly G, Chakrabarti S, Chatterjee U, et al. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer's disease and Parkinson's disease. Drug Des Devel Ther 2017;11:797-810. 10.2147/DDDT.S130514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamatsuji T, Matsui T, Okamoto T, et al. G protein-mediated neuronal DNA fragmentation induced by familial Alzheimer's disease-associated mutants of APP. Science 1996;272:1349-52. 10.1126/science.272.5266.1349 [DOI] [PubMed] [Google Scholar]
- 45.Tachi N, Hashimoto Y, Matsuoka M. MOCA is an integrator of the neuronal death signals that are activated by familial Alzheimer's disease-related mutants of amyloid beta precursor protein and presenilins. Biochem J 2012;442:413-22. 10.1042/BJ20100993 [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto Y, Chiba T, Yamada M, et al. Transforming growth factor beta2 is a neuronal death-inducing ligand for amyloid-beta precursor protein. Mol Cell Biol 2005;25:9304-17. 10.1128/MCB.25.21.9304-9317.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto Y, Matsuoka M. A mutation protective against Alzheimer's disease renders amyloid beta precursor protein incapable of mediating neurotoxicity. J Neurochem 2014;130:291-300. 10.1111/jnc.12717 [DOI] [PubMed] [Google Scholar]
- 48.Lauritzen I, Pardossi-Piquard R, Bourgeois A, et al. Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology. Acta Neuropathol 2016;132:257-76. 10.1007/s00401-016-1577-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 2016;12:15-27. 10.1038/nrneurol.2015.225 [DOI] [PubMed] [Google Scholar]
- 50.Mandell JW, Banker GA. Microtubule-associated proteins, phosphorylation gradients, and the establishment of neuronal polarity. Perspect Dev Neurobiol 1996;4:125-35. [PubMed] [Google Scholar]
- 51.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 2004;117:5721-9. 10.1242/jcs.01558 [DOI] [PubMed] [Google Scholar]
- 52.Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 2015;349:1255555. 10.1126/science.1255555 [DOI] [PubMed] [Google Scholar]
- 53.Kim BM, You MH, Chen CH, et al. Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function. Cell Death Dis 2014;5:e1237. 10.1038/cddis.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driver JA, Zhou XZ, Lu KP. Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer's disease. Biochim Biophys Acta 2015;1850:2069-76. 10.1016/j.bbagen.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francois A, Rioux Bilan A, Quellard N, et al. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J Neuroinflammation 2014;11:139. 10.1186/s12974-014-0139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiriyama Y, Nochi H. The Function of Autophagy in Neurodegenerative Diseases. Int J Mol Sci 2015;16:26797-812. 10.3390/ijms161125990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su JH, Anderson AJ, Cummings BJ, et al. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport 1994;5:2529-33. 10.1097/00001756-199412000-00031 [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Liu Y, Wang X, et al. The Role of (99m) Tc-Annexin V Apoptosis Scintigraphy in Visualizing Early Stage Glucocorticoid-Induced Femoral Head Osteonecrosis in the Rabbit. Biomed Res Int 2016;2016:7067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao Y, Pei J, Cheng H, et al. An ancient autoproteolytic domain found in GAIN, ZU5 and Nucleoporin98. J Mol Biol 2014;426:3935-45. 10.1016/j.jmb.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang X, Jang SW, Okada M, et al. Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol 2008;10:698-706. 10.1038/ncb1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bordeaux MC, Forcet C, Granger L, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 2000;19:4056-63. 10.1093/emboj/19.15.4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thibert C, Teillet MA, Lapointe F, et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science 2003;301:843-6. 10.1126/science.1085405 [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez D, Espino J, Bejarano I, et al. Caspase-3 and -9 are activated in human myeloid HL-60 cells by calcium signal. Mol Cell Biochem 2010;333:151-7. 10.1007/s11010-009-0215-1 [DOI] [PubMed] [Google Scholar]
- 64.Bell RAV, Megeney LA. Evolution of caspase-mediated cell death and differentiation: twins separated at birth. Cell Death Differ 2017;24:1359-68. 10.1038/cdd.2017.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.You MH, Kim BM, Chen CH, et al. Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ 2017;24:238-50. 10.1038/cdd.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Grupe A, Rowland C, et al. DAPK1 variants are associated with Alzheimer's disease and allele-specific expression. Hum Mol Genet 2006;15:2560-8. 10.1093/hmg/ddl178 [DOI] [PubMed] [Google Scholar]
- 67.Gaj P, Paziewska A, Bik W, et al. Identification of a late onset Alzheimer's disease candidate risk variant at 9q21.33 in Polish patients. J Alzheimers Dis 2012;32:157-68. 10.3233/JAD-2012-120520 [DOI] [PubMed] [Google Scholar]
- 68.Wu ZC, Zhang W, Yu JT, et al. Association of DAPK1 genetic variations with Alzheimer's disease in Han Chinese. Brain Res 2011;1374:129-33. 10.1016/j.brainres.2010.12.036 [DOI] [PubMed] [Google Scholar]
- 69.Singh P, Ravanan P, Talwar P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front Mol Neurosci 2016;9:46. 10.3389/fnmol.2016.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams ME, Strickland P, Watanabe K, et al. UNC5H1 induces apoptosis via its juxtamembrane region through an interaction with NRAGE. J Biol Chem 2003;278:17483-90. 10.1074/jbc.M300415200 [DOI] [PubMed] [Google Scholar]
- 71.Lourenco FC, Galvan V, Fombonne J, et al. Netrin-1 interacts with amyloid precursor protein and regulates amyloid-beta production. Cell Death Differ 2009;16:655-63. 10.1038/cdd.2008.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodrigues S, De Wever O, Bruyneel E, et al. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene 2007;26:5615-25. 10.1038/sj.onc.1210347 [DOI] [PubMed] [Google Scholar]
- 73.Son TW, Yun SP, Yong MS, et al. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin alpha6beta4-dependent Akt, GSK-3beta, and HSF-1 in mesenchymal stem cells. Cell Death Dis 2013;4:e563. 10.1038/cddis.2013.94 [DOI] [PMC free article] [PubMed] [Google Scholar]