Abstract
Liver fibrosis is an important pathological precondition for hepatocellular carcinoma. The degree of hepatic fibrosis is positively correlated with liver cancer. Liver fibrosis is a series of pathological and physiological process related to liver cell necrosis and degeneration after chronic liver injury, which finally leads to extracellular matrix and collagen deposition. The early detection and precise staging of fibrosis and cirrhosis are very important for early diagnosis and timely initiation of appropriate therapeutic regimens. The risk of severe liver fibrosis finally progressing to liver carcinoma is >50%. It is known that biopsy is the gold standard for the diagnosis and staging of liver fibrosis. However, this method has some limitations, such as the potential for pain, sampling variability, and low patient acceptance. Furthermore, the necessity of obtaining a tissue diagnosis of liver fibrosis still remains controversial. An increasing number of reliable non-invasive approaches are now available that are widely applied in clinical practice, mostly in cases of viral hepatitis, resulting in a significantly decreased need for liver biopsy. In fact, the non-invasive detection and evaluation of liver cirrhosis now has good accuracy due to current serum markers, ultrasound imaging, and magnetic resonance imaging quantification techniques. A prominent advantage of the non-invasive detection and assessment of liver fibrosis is that liver fibrosis can be monitored repeatedly and easily in the same patient. Serum biomarkers have the advantages of high applicability (>95%) and good reproducibility. However, their results can be influenced by different patient conditions because none of these markers are liver-specific. The most promising techniques appear to be transient elastography and magnetic resonance elastography because they provide reliable results for the detection of fibrosis in the advanced stages, and future developments promise to increase the reliability and accuracy of the staging of hepatic fibrosis. This article aims to describe the recent progress in the development of non-invasive assessment methods for the staging of liver fibrosis, with a special emphasize on computer-aided quantitative and deep learning methods.
Keywords: Liver fibrosis, non-invasive detection, computer-aided quantitative, deep learning
Introduction
Approximately 55%–60% of hepatocellular carcinoma (HCC) cases worldwide1 are caused by chronic hepatitis B virus (HBV) infection. The infection progresses to fibrosis, cirrhosis, and hepatocellular carcinoma in these patients. In HBV-related cirrhosis, the 5-year cumulative HCC risk is 15% in high endemic areas and 10% in the West2. Liver fibrosis is the early stage of cirrhosis and its degree is important for predicting the occurrence and recurrence of liver cancer3. In a retrospective study of 1,079 patients with chronic HBV infection, multivariate analyses showed that fibrosis level but not antiviral regimen was independently associated with the risk of HCC (P < 0.05) 4. Fibrosis is a dynamic process and many studies have suggested that liver fibrosis is actually reversible when the underlying condition is treated5,6. In the early stages of fibrosis, it may be possible to achieve a total curative effect. Therefore, the early diagnosis and prevention of liver fibrosis is of great importance in the clinical setting.
From the surgeons' perspective, the patient's liver function is directly related to the feasibility of the surgical plan. Thus, it is of great clinical value to determine whether there will be a serious risk of liver failure after surgery. Accurate assessment of liver fibrosis can predict the patient's liver function, which is useful for planning liver surgery. However, there is controversy regarding the use of tissue detection for the assessment of liver fibrosis in clinical practice. Liver biopsy is still the gold standard for staging fibrosis using the Metavir score7 or New Inuyama classification8 to assign a score ranging from F0 (no fibrosis) to F4 (cirrhosis). The Metavir scoring system is commonly used in Europe. The fibrosis score is based on a five-point scale (F0, no fibrosis; F1, portal fibrosis without septa; F2, few septa; F3, numerous septa without cirrhosis; F4, cirrhosis) (Table 1).
1.
The Metavir score system and fibrosis stage
| Metavir score system | Fibrosis stage |
| F0 | No fibrosis can be detected |
| F1 | Fibrosis exists with expansion of portal zones |
| F2 | Fibrosis exists with expansion of most portal zones, and occasional bridging |
| F3 | Fibrosis exists with expansion of most portal zones, marked bridging, and occasional modules |
| F4 | Presence of cirrhosis |
Liver biopsy is currently the gold standard for the assessment of liver fibrosis in clinical practice. Histological evaluation is important for identifying the underlying liver disease, assessing the necro-inflammatory grade, and staging the patient's fibrosis. However, this method has many disadvantages because it is invasive, with relatively high costs and the potential for sampling errors9. Histological examination is subject to intra- and inter-observer variation and does not always predict disease progression. Furthermore, liver biopsy cannot be repeated easily10. Therefore, many non-invasive methods, including serum markers, ultrasound (US)-based transient elastography (TE), and magnetic resonance (MR)-based approaches, have been evaluated and shown to have promise in the diagnosis and staging of liver fibrosis11,12.
Imaging methods for the diagnosis of liver fibrosis include color US, computed tomography (CT), and MR imaging (MRI). US is a widespread, low-cost, user-friendly, and accurate technique. However, it lacks specificity due to limitations related to the patient or operator and is mostly applied according to the patient's preference and follow-up requirements13. MRI represents the latest technology in this field as it allows the diagnosis and characterization of fibrosis and the overall assessment of chronic liver disease. In addition, MRI is more research-oriented due to its multiparametric potential, which allows not only various fibro-steatosic alterations to be distinguished but also metabolic assessment. Thus, MRI promotes research into etiology and medicine. However, there are some limitations of MRI. For example, high-quality imaging equipment resources and professional expertise are relatively rare14.
All non-invasive radiologic modalities are capable of distinguishing cirrhosis from less serious types of fibrosis. However, it is still difficult to precisely stage fibrosis. The most promising techniques at present are TE and MR elastography because they provide reliable results for the detection of severe fibrosis, and future developments promise to increase the reliability and accuracy of liver fibrosis staging11.
Considering these factors, this review aimed to discuss the recent progress in non-invasive detection of liver fibrosis.
Serum markers
Quantification of fibrosis biomarkers in serum represents a “biological” approach, which is in contrast to the “physical” approach based on the measurement of liver stiffness using elastography-based technologies. Detection and quantification of serum biomarkers has advantages of high applicability (>95%) and good reproducibility. Serum markers also have some disadvantages because none of them is liver specific. The results can be influenced by the patient's condition. For example, there is a risk of false positive results with the FibroTest in patients with Gilbert's syndrome or with aspartate aminotransferase to platelet ratio index (APRI) in patients with acute hepatitis. TE can be performed at the bedside or in an outpatient clinic with high performance for detecting cirrhosis. This method is very user friendly. However, its applicability (80%) is not as high as that of serum biomarkers, especially in the case of ascites, obesity, and an inexperienced operator. Serum biomarkers also have the risk of false positive results in case of alanine aminotransferase (ALT) flares15.
The serum parameters for the diagnosis of liver fibrosis include hyaluronic acid (HA)16, procollagen II N-terminal propeptide (PIINP)17, type-IV collagen18,19, laminin, and so on.20,21. Table 2 summarized several serum markers for the diagnosis of liver fibrosis. Leroy et al.22 found that combining two serum markers reflecting both fibrogenesis (PIIINP) and fibrolysis (matrix metalloproteinase-1) is a potentially useful tool for assessing liver fibrosis. Recently, newly-detected serum markers related to liver fibrosis are promising to improve the diagnosis of liver fibrosis. Jazwinski et al.23 found that CK-18 levels were much higher in chronic hepatitis C (CHC) patients than in controls. The fibrosis stage was associated with increased CK-18 levels. Parfieniuk-Kowerda et al.24 found that elevated serum M30-CK18 level was an indicator of severe apoptosis of hepatocytes and corralated with active hepatic inflammation and fibrosis. Thus, it may serve as a non-invasive marker of disease activity.
2.
The serum markers for diagnosis of liver fibrosis
| Serum parameter | Correlation to liver fibrosis | Significance |
| HA16 | Positive correlation | Accurately reflect the fibrosis and damage to the liver cells. Serum HA is slightly elevated with acute hepatitis, and significantly elevated with chronic active hepatitis, and extremely elevated with liver cirrhosis. |
| PIINP17 | Positive correlation, but not specific | Is suitable for early diagnosis of liver fibrosis. |
| Type IV collagen18,19 | Positive correlation | Usually diagnose early liver fibrosis and chronic liver disease |
| Laminin20,21 | Positive correlation with fibrosis activity
and portal pressure |
Reflect degree of liver fibrosis. Elevated laminin (LN) content is also related to tumor invasion and metastasis. |
However, single parameters are not sufficient to accurately reflect the degree of liver fibrosis. Thus, combining multiple serum markers is becoming a focus of interest (Table 3). Many non-invasive models (AST/ALT ratio, APRI, fibrosis-cirrhosis index, fibrosis index, fibrosis-4 score, fibrosis quotient, King, and von Willebrand factor antigen/thrombocyte ratio) for predicting fibrosis were compared with liver biopsy. Many new scores for predicting fibrosis stages with better accuracy have been developed25 (Table 3).
3.
Non-invasive diagnostic model for liver fibrosis
| Model | Formula |
| ALB: albumin; AST: aspartate aminotransferase; ALT: alanine transaminase; INR, international normalized ratio; GGT: gamma-glutamyl transferase; PLT: platelet; ULN: upper limit of normal; FibroQ: fibrosis quotient. | |
| AAR26,27 | AST(IU/L) / AST(IU/L) |
| APRI28 | AST(IU/L) / PLT(109/l) |
| GPRI | GGT(IU/L) / PLT(109/l) |
| S index | 1,000 × GGT (IU/L) / [PLT(109/l) × ALB2(g/L)] |
| APRI29 | AST(IU/L) / ULN(IU/L) / PLT(109/l) |
| FIB-4 score | [Age (years) × AST(IU/L)] / PLT (109/l) × ALT1/2 (IU/L) |
| Fibro-Q | [10× AST(IU/L) × age (years) × INR] /
PLT (109/l) × ALT (IU/L) |
| API
(Age plus PLT) |
Age (years): >70=5, 61–70=4, 51–60=3, 41–50=2, 31–40=1, ≤30=0 PLT (10 9/l): <125=5, 125–149=4, 150–174=3, 175–199=2, 200–224=1, ≥225=0 |
| VITRO | von Willebrand factor antigen (vWF-Ag)/thrombocyte ratio |
| APRG30 | ALP/PLT/RDW-SD/globulin |
Most of the non-invasive methods were initially developed and validated in patients with CHC, and most of the published studies were performed in the context CHC. However, non-invasive models have been established for the assessment of liver fibrosis in patients with chronic hepatitis B (CHB)15.
Radiological techniques
US, CT, and MRI have traditionally been used to explore the liver31. They are able to detect biological changes in the liver parenchyma when there is significant fibrosis (bridging fibrosis and cirrhosis) and signs of portal hypertension. Common imaging methods are US, spiral CT, and MRI [including diffusion-weighted imaging (DWI), diffusion tensor imaging, etc.]. These methods can detect advanced portal hypertension with advanced liver fibrosis with high sensitivity and specificity. Another commonly used diagnostic method is TE.
US technology
Color Doppler US
Color Doppler US utilizes the changes in hepatic blood flow during fibrosis to check for fibrosis. The main indicators for detection are hepatic artery flow rate, portal vein flow rate, and the ratio of the two. Some studies have pointed out32 that color Doppler US has high accuracy for the diagnosis of cirrhosis > stage F2. However, Doppler US examination still has poor stability and is strongly influenced by equipment performance, operator skill, and the patient's physical condition.
Contrast-enhanced US
Contrast-enhanced US is commonly used for the diagnosis of liver tumors. Because the hepatic blood flow and the appearance of microbubble acoustical contrast agent change with liver fibrosis, information related to hepatic perfusion can be obtained through the variations in microbubbles, including the portal vein arrival time, hepatic vein arrival time, and hepatic artery arrival time. The interval between hepatic artery arrival time and hepatic vein arrival time indirectly reflects the degree of liver fibrosis. It has been suggested that portal vein arrival time and the interval between hepatic artery arrival time and hepatic vein arrival time are significantly reduced in patients with significant fibrosis and are negatively correlated with the degree of fibrosis33,34. However, due to the need to inject contrast medium, which may be associated with safety hazards such as allergic reactions, the diagnostic value of contrast-enhanced US requires further evaluation.
US elastography
US elastography was first proposed by Ophir et al.35. The main imaging principles include the following: 1, application of arterial or static stimulation to the tissue; 2, the tissue produces displacement, strain, and other changes; 3, US imaging indirectly or directly reflects the hardness of the tissue.
US elastography has been recommended for the non-invasive staging of liver fibrosis by the clinical practice guidelines of the European Association for the Study of the Liver and the Asian-Pacific Association for the Study of the Liver36. US elastography encompasses mainly TE, point quantification shear wave elastography (pSWE), and two-dimensional SWE37.
TE is a US-based technique that has been applied in the clinical diagnosis of liver fibrosis and has been shown to have advantages in many original studies. However, it has the disadvantage of involving no direct image guidance. A 3.5-MHz “M” probe, a 2.5-MHz “XL” probe (for obese patients), or a 5.0-MHz probe (for children) is placed in the region dullest to percussion, typically in the 9th–11th intercostals space, and a portion of liver about 6 cm deep is assessed. This has numerous advantages, such as a well-defined technique, shallow learning curve, and repeatability, although the technique is not presently recommended for spleen measurements. Conversely, the method requires a dedicated machine, the probe must be recalibrated every 6–12 months depending on the probe, assessment may not be possible in the case of ascites and obesity (obesity can be resolved using an extra-large probe), no grayscale image of the liver is obtained (A-mode images are available), and performance is lower than acoustic radiation force impulse (ARFI) techniques36-39. Tsochatzis et al.40 reported in their meta-analysis that the hierarchical summary receiver operating characteristic (HSROC) model has been performed in CHC and CHB patients. The results showed that the sensitivity of TE for diagnosing liver fibrosis stage F ≥ 2, F ≥ 3, and F = 4 was 0.79, 0.82, and 0.83 and the summary specificity was 0.78, 0.86, and 0.89, respectively. Elastography is defined by good sensitivity and specificity for cirrhosis and lower sensitive and specificity for lesser degrees of fibrosis. However, the technique should be carefully applied because it is difficult to validate the cut-off values for the different stages of fibrosis. Steadman et al.41 also assessed the diagnostic accuracy of elastography using an HSROC model in patients with various characteristics. The results of subgroup analyses in patients with CHB showed that the sensitivity of elastography was 0.77, 0.83, and 0.67 and the specificity was 0.72, 0.81, and 0.87 for predicting fibrosis stages F ≥ 2, F ≥ 3, and F = 4, respectively. The authors suggested that TE is more accurate in patients with moderate fibrosis or cirrhosis and that TE is less effective and less expensive than liver biopsy.
US elastography has shown promise as a non-invasive, inexpensive, and portable technique. Particularly, SWE-derived estimates of shear wave speed and hepatic Young’s modulus measured in kilopascal (kPa) have been shown to be associated with liver fibrosis stage42. Recently, a meta-analysis of SWE studies summarized 12 reports and generated 12 different cut-off values for sensitivity and specificity43. Earlier SWE studies have proposed various cut-off values to distinguish the staging of fibrosis, such as early fibrosis, no fibrosis, advanced fibrosis, and cirrhosis44. Dhyani et al.45 validated the previously established US SWE cut-off values (≥ F2 fibrosis) in a cohort of patients with chronic liver disease. In this previous cross-sectional study, 338 patients undergoing liver biopsy underwent SWE using an Aixplorer US machine (SuperSonic Imagine, Aix-en-Provence, France). Median SWE values were calculated from sets of 10 elastograms. A single-blinded pathologist referred to METAVIR fibrosis staging as the gold standard. On pathological examination, 212 of 277 enrolled patients (76.5%) had F0–F1 fibrosis and 65 (23.5%) had ≥ F2 fibrosis. Applying the SuperSonic Aixplorer system (SuperSonic Imagine), the authors validated the test performance of a cut-off value of 7.29 kPa on liver SWE and distinguished higher stages of liver fibrosis (METAVIR stages F2–F4) from lower stages of fibrosis (METAVIR stages F0–F1) in patients with chronic liver disease.
In pSWE, shear waves in the liver in a small region of interest (ROI) (approximately 1 cm3) are generated by applying an ARFI pulse. Monitoring the displacement of liver tissue caused by the shear waves is achieved by B-mode imaging. pSWE can be an independent procedure or an add-on during liver US or direct visualization of liver insonation, with the possibility of quantitatively lower variability than 2D SWE. pSWE can also be used to assess the spleen. However, this method has been reported less in the literature than TE because it has been used for a shorter period of time37.
In 2D SWE, multiple measurements with ARFI technology are performed in a large field of view. This can be performed either as a single image or in real time. Within this large field of view, an ROI can be placed to obtain measurements from the chosen location37. The procedure can be an add-on during liver US, direct visualization of the liver region being insolated, or color display of a larger field of view. However, it also has less published material than TE as it has not been used for a long time37.
Elastography techniques can be used to distinguish patients with no or minimal fibrosis (METAVIR stages F0/F1) and those with severe fibrosis or cirrhosis (METAVIR stages F3/F4)37.
MRI
DW-MRI
MRI has been applied for the detection and quantification of hepatic fibrosis using DW sequences. Several encouraging studies have shown this method to be promising for the detection of liver fiborosis46. At present, DWI is a routine procedure in the liver MRI protocol. In the context of chronic liver disease, important studies have evaluated the capability of DWI to quantify the extent of hepatic fibrosis48,49.
Based on the sensitivity of MRI to motion, diffusion imaging consists of a spin-echo sequence, in which the main 180° focalization pulse is preceded and followed by two additional gradient pulses. A proton response to these gradient pulses is strongly related to their movements and follows a Brownian motion. The first gradient field is applied before 180° refocusing of the RF pulse, leading to the phase shift of protons. For static molecules, the second gradient pulse compensates for the phase shift produced by the first. As such, no additional shift is generated from movement. The refocusing introduced by the second gradient is visible on MRI DWIs as high signal intensity.
As the main parameter for quantifying proton diffusion motion in tissues, apparent diffusion coefficient (ADC) is estimated using images acquired with two different b-values. ADC is calculated by the following formula: ADC = Ln (S0/S1) [b1–b2], where S0 is the signal intensity with b = 0, S1 is the signal intensity after the application of a given b gradient, and b is the strength of the applied gradient50. On DWIs, tissues containing molecules with a high degree of movement and diffusion are represented as dark areas of low signal intensity, whereas tissues in which protons are unable to move freely have high signal intensity. Fibrosis can also decrease the width of the interstitial spaces. Fibrotic tissues generally develop as a consequence of chronic inflammation, with narrowing of the interstitial spaces and consequent reductions in proton motion51.
DWI of the liver is influenced by motions induced by breathing and cardiac pulsations and lower T2 signal52. Accordingly, a different technique has been developed to improve the quality of DWIs and the precision of ADC measurement. Taouli et al.46 evaluated their preliminary experience of using DW-MRI to quantify the degree of liver fibrosis. They performed DW-MRI with the single-shot echo-planar technique with b values of 50, 300, 500, 700, and 1,000 s/mm2 in 23 patients with chronic hepatitis and seven healthy volunteers. ADC was measured in four locations in the liver. Liver biopsy results (n = 19) were retrospectively reviewed by two independent hepatopathologists who came to a consensus regarding the criteria used to determine the different stages of liver fibrosis and the grade of liver inflammation. They found that hepatica was a significant predictor of ≥ stage 2 and ≥ stage 3 fibrosis, with areas under the curve of 0.896 and 0.896, sensitivity of 83.3% and 88.9%, and specificity of 83.3% and 80.0%, respectively. The study demonstrated that DW-MRI is good for predicting moderate and advanced liver fibrosis. Bonekamp et al.53 evaluated the diagnostic accuracy of DWI for hepatic fibrosis by retrospectively comparing DWIs from clinically-acquired MRI scans with histological methods. Liver biopsy specimens were staged as F0–F4 in accordance with the Metavir score. Liver ADC values were inversely correlated with fibrosis stage: P = –0.54 (P < 0.0001). The authors found that the differences in ADC values according to METAVIR stages F0 vs. F1–4, F0–1 vs. F > 1, F0–2 vs. F3–4, and F0–3 vs. F4 were all significant. Liver ADC can be used to predict liver fibrosis with acceptable diagnostic accuracy.
Intravoxel incoherent motion (IVIM) DWI
IVIM reflects the random microscopic motion that occurs in voxels on MR images of water molecules (either extracellular or intracellular) and the microcirculation of blood54. Notably, as a DWI-based imaging technique, IVIM analyzes the signal decay of multiple b values to simultaneously evaluate the perfusion-related diffusivity (demonstrated by parameters D*, f) and pure molecular diffusivity (demonstrated by the parameter D)55. Le Bihan et al.56 proposed the IVIM model in 1986. They believed that there are two kinds of microscopic movement within living animals: the diffusion of water molecules and blood perfusion. IVIM calculates the corresponding parameters using a nonlinear regression function
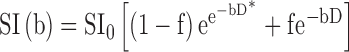 , that includes a pure diffusion coefficient, pseudo-diffusion coefficient, and perfusion fraction54,57.
, that includes a pure diffusion coefficient, pseudo-diffusion coefficient, and perfusion fraction54,57.
According to the theory of IVIM, diffusion and perfusion are influenced by several tissue characteristics, including the presence of restrictive barriers within the tissue, the viscosity of the fluid in which the spins are diffusing, and the velocity and fractional volume of perfusing spins58. IVIM imaging was limited to neuroradiologic applications at first because the abdominal organs can be influenced by respiratory and other motion artifacts.
IVIM MRI has been used for the evaluation of the abdominal organs, including the liver. These analyses make use of respiratory gating and parallel imaging to improve signal intensity59-61. Yamada et al.59 first assessed the use of IVIM MRI for the abdominal organs. However, they used only a limited number of b values (30, 300, 900, and 1,100 s/mm2) and did not calculate pseudo-diffusion values. Grech-Sollars et al.62 demonstrated that the IVIM parameter PF had a high intra-scanner coefficient of variation (CoV) of 8.4% and inter-scanner CoV of 24.8%.
Luciani et al.55 retrospectively evaluated a respiratory-triggered DW-MRI sequence that was combined with parallel acquisition. The technique allowed the calculation of pure molecular-based (D) and perfusion-related (D*, f) diffusion parameters based on the IVIM theory. The authors found that Dfast was significantly decreased in patients with liver fibrosis compared to that in patients with a healthy liver. However, there was no significant difference between Dslow and PF measurements in the healthy liver (n = 25) and liver fibrosis (n = 12) groups.
Guiu et al.61 compared pure molecular diffusion (D), perfusion-related diffusion (D*), and perfusion fraction (f) determined from DW-MRI based on the IVIM theory and reported that Dslow and Dfast were significantly lower in steatotic compared with non-steatotic livers. However, PF was significantly higher in steatotic compared with non-steatotic livers. Therefore, steatosis can affect diffusion parameters obtained with IVIM.
In another study, Patel et al.63 reported their preliminary experience with the use of IVIM DW-MRI and dynamic contrast-enhanced (DCE)-MRI alone and in combination for the diagnosis of liver cirrhosis. The authors prospectively assessed 30 participants (16 with non-cirrhotic livers, 14 with cirrhosis) with IVIM DW-MRI (n = 27) and DCE-MRI (n = 20). The diagnostic performance for cirrhosis was evaluated for each modality alone and in combination using logistic regression and receiver operating characteristic analyses. IVIM and DCE-MR parameters were compared using a generalized set of estimation equations. Finally, the study revealed that the values of Dslow, PF, and Dfast in cirrhotic livers were lower than those in non-cirrhotic livers. However, no further grading was performed within the liver cirrhosis patients as only three had histopathology data available for analysis.
In a rat model of diethylnitrosamine-induced liver fibrosis, Zhang et al.64 compared ADC and perfusion fraction measured by IVIM MRI among rodents with different degrees of fibrosis. Both diffusion and perfusion contributed to ADC. ADC and f values decreased significantly with increasing fibrosis level (correlation coefficient for ADC: rho = –0.781, P < 0.001; for f: rho = –0.720, P = 0.001). However, D was poorly correlated with fibrosis level (rho = –0.502, P = 0.040). It has been reported that PF values decrease significantly with increasing fibrosis level. However, Dslow was poorly correlated with fibrosis level.
In a carbon tetrachloride-induced rat liver fibrosis model, Chow et al.65 characterized longitudinal changes in molecular water diffusion, blood microcirculation, and their contributions to the apparent diffusion changes using IVIM analysis in an experimental mouse model of liver fibrosis. The authors reported that as liver fibrosis progressed, Dslow and Dfast decreased. Both molecular water diffusion and blood microcirculation contribute to alteration of the apparent diffusion changes in liver fibrosis. Reduction in D (true) and D (pseudo) values resulted from diffusion and perfusion changes, respectively, during the progression of liver fibrosis. IVIM analysis may serve as a valuable and robust tool for the detection and characterization of liver fibrosis in its early stages, allowing monitoring of progression in a non-invasive manner.
To date, the actual values of PF and Dfast are still unclear, and the best clinical settings for IVIM imaging are still under debate. Li et al.54 reviewed the mean values and variability of Dslow, PF, and Dfast of the liver in the published papers and analyzed how the data acquisition set-up may influence these values. This previous paper showed that IVIM technique is still not capable of detecting liver fibrosis in the early stage and diagnosing the extent liver fibrosis. Furthermore, the method is far from capable of differentiating liver tumors.
MR spectroscopy
MR spectroscopy is a non-invasive technique that facillitates the easy study of cellular metabolism. MR spectroscopy is widely used by biochemists for the in vitro investigation of physiological processes. Recently, radiologists have used this method for the in vivo detection of abnormalities66. Phosphorus-31 (31P) MR spectroscopy has been used to study liver metabolism in vivo67-69. In contrast to 31P-MR spectroscopy, hydrogen-1-MR spectroscopy is accurate and suitable for the in vivo quantification of liver fat deposition70,71. Hydrogen-1 MR spectroscopy is not useful for the complete evaluation of hepatic fibrosis but can be an alternative to liver biopsy for the evaluation of steatosis and necroinflammatory activity in liver disease72.
Magnetic resonance elastography (MRE)
MRE is used to measure the mechanical characteristics of tissues (such as stiffness, elasticity, and viscosity) by acquiring images of the propagation of a shear wave created by an external source of motion73. MRE is based on spin-echo, echo-planar imaging, and gradient-recalled echo. By detecting the displacement of particles generated by external forces in human tissues74, it takes advantage of propagating mechanical shear waves (20–200 Hz) to detect the mechanical properties of tissues75. Such waves propagate more quickly in stiffer tissue and more slowly in softer tissue, and the wavelength becomes shorter as the tissue stiffness decreases. Low-frequency mechanical shear waves are generated with a special acoustic driver system and propagated throughout the body. MRE is a technique with three steps: first, mechanical waves are generated in the tissue; second, images of the waves are obtained with a special MRI sequence; finally, the wave information is processed and elastograms are generated. During this process, images are generated and used to quantitatively depict tissue stiffness76.
In prior studies, MRE could not be directly compared to liver biopsy because biopsy was used as a reference standard14,77-80. However, Morisaka et al.81 compared the diagnostic accuracy of liver fibrosis staging between MRE-based methods and liver biopsy using pathological results from resected liver specimens as reference standards, and found that MRE can be an alternative to liver biopsy for fibrosis staging.
MRE has been standardized and yields repeatable results across sites82,83. MRE also has the advantage of being technically feasible in larger patients or those with ascites14. MRE is associated with several biological confounders, such as concomitant liver steatosis, inflammation, cholestasis, hepatic venous congestion, postprandial state, and right heart failure84. MRE may be affected by moderate-to-severe iron deposition in the liver, which leads to a low signal-to-noise ratio and sometimes inconclusive measurements.
Currently, the use of MRE for the staging of liver fibrosis still requires further use in more clinical patients and clinical trials.
Perfusion imaging
Perfusion imaging measures quantitative or semi-quantitative perfusion parameters of the liver using contrast agents. Gadolinium-based contrast agents are the most frequently used. Signal enhancement in the liver tissue and vessels (abdominal aorta or hepatic artery and portal vein) following the injection of one of these contrast agents is measured at different time points85. Perfusion imaging can be performed on any imaging modality (MRI, US, and CT) and shows potential for prognostic significance. Thus, perfusion constants could be used to predict treatment outcomes in fibrosis patients. However, perfusion imaging has some limitations. It is more invasive than other MRI-based liver fibrosis quantification techniques as it requires the injection of a contrast agent. It also requires full patient cooperation and several breath holds to achieve good results, especially for the proper timing of image acquisition to record the arterial and portal venous peaks73.
Application of computer-aided quantitative techniques in the active staging of liver fibrosis
Computer-aided quantitative technology has developed in recent years. These methods make use of the fact that image texture analysis is a very common diagnostic method for liver fibrosis. For this reason, many scholars focus on the analysis of the image texture features of different fibrosis stage31. By using a series of mathematical equations to generate a range of parameters associated with image texture, texture analysis characterizes the spatial variation of gray levels throughout an image86.
CT
CT texture analysis quantifies the heterogeneity of a ROI by analyzing the distribution and/or relationship of pixel or voxel gray levels in the image87.
Kayaalti et al.88 selected a 32 × 32-pixel ROI on CT and obtained a comprehensive set of texture features using a gray level co-occurrence matrix (GLCM), Laws' method, discrete wavelet transform (DWT), and Gabor filters. The authors used sequential floating forward selection and exhaustive search methods in different combinations for the selection of the most discriminating features. Finally, the selected texture features were classified using two methods: support vector machines (SVM) and k-nearest neighbors (k-NN). The mean classification accuracy in the pairwise group comparisons was approximately 95% for both classification methods using only five features. However, when this approach was performed for classifying the liver fibrosis stage of participants in the test set into seven possible stages, both SVM and k-NN methods had relatively low classification accuracy. Pairwise group classification results showed that DWT, Gabor, GLCM, and Laws' texture features were more successful than the others. As the features extracted from these methods were used in the feature fusion process, fusing features from these better performing families will further improve the classification performance.
Lubner et al.89 applied quantitative texture analysis of the liver performed using abdominal multidetector (MD) CT scans to evaluate CT texture analysis (CTTA) for the staging of liver fibrosis. The study included 289 people. By using the filtration-histogram statistic-based technique, they correlated CTTA parameters with fibrosis stage (F0–F4), with biopsy performed within 1 year for all cases with intermediate fibrosis (F1–F3) using commercially available software (TexRAD). Mean gray-level intensity increased with fibrosis stage, resulting in a receiver operating characteristics (ROC) area under the curve (AUC) of 0.78 at medium filtration for fibrosis F0 vs. fibrosis F1–4, with sensitivity and specificity of 74% and 74%, respectively. For cirrhosis (equal to F4), kurtosis and skewness showed AUCs of 0.86 and 0.87. Thus, it is believed that CTTA may be helpful for detecting the presence of hepatic fibrosis and discriminating between the stages of fibrosis, especially at advanced levels. Daginawala et al.90 assessed the ability of texture analyses of contrast-enhanced CT images for distinguishing between liver fibrosis of different extents in patients with chronic liver disease using histopathology as a standard. This cohort contained 83 patients who underwent contrast-enhanced 64-MDCT and had undergone a liver biopsy within 6 months of the CT scan. The authors assembled three analysis groups and compared Ishak scales of 0–2 with 3–6, 0–3 with 4–6, and 0–4 with 5–6. Finally, a total of 19 different texture features with seven histogram features, one grey level co-occurrence matrix, six gray level run lengths, one Laws feature, and four gray level gradient matrices demonstrated significant differences for discriminating between fibrosis groupings. The highest AUCs had fair performance for distinguishing between distinct fibrosis groups. These findings suggest that texture-based analysis of contrast-enhanced CT images offers a potential approach to the non-invasive evaluation of liver fibrosis.
US and MR
Some groups have applied texture analysis to US or MRI91. Texture analysis of MR images included the analysis of T2-weighted MR, arterial phase, venous phase, and DW images.
House et al.86 studied the ability of texture analysis based on MRI images to stage hepatic fibrosis by enrolling 49 patients with different extents of liver disease and biopsy-confirmed fibrosis. For texture analysis, all patients were scanned with a T2-weighted, high-resolution, spin echo sequence, and Heraldic texture features were applied. The best mean ROC AUC achieved for separating mild from severe fibrosis was 0.81. The authors found that the combination of MRI measures, including selected texture features from T2-weighted images, was useful for excluding fibrosis in patients with liver disease. However, they also found that texture analysis from MRI only had a modest effect when applied to the classification of patients in the mild and intermediate stages of fibrosis.
Using 11.7 Tesla (T) MRI, Anderson et al.92 evaluated the effects of hepatic fibrosis on ADC and T2 values of imaging for in vitro murine liver specimens. The degrees of fibrosis were assessed by a pathologist and digital image analysis system. Scatter plot graphs were generated by comparing ADC and T2 according to the extent of fibrosis and correlation coefficients were calculated. A strong correlation was found between the degree of hepatic fibrosis and ADC, with a greater severity of fibrosis associated with lower hepatic ADC values. A moderate correlation was seen between hepatic fibrosis and T2 values, with higher degrees of fibrosis associated with lower T2 values. It has been reported that inverse relationships exist between the degree of fibrosis and both ADC and T2 values, suggesting that MRI quantification of liver fibrosis is promising.
Gao et al.13 used a gray-level gradient co-occurrence matrix for the texture analysis of US liver images first before using a grey level co-occurrence matrix. The authors obtained 22 features using these two methods. The seven most prominent features were selected for classification using a back propagation neural network. Fibrosis was divided into five stages (F0–F4), with classification accuracy for the stages F0–F4 of 100%, 90%, 70%, 90%, and 100%, respectively.
Han et al.93 extracted liver textures based on the densely-sampled DAISY descriptor and then used principal component analysis for feature dimensionality reduction. Then, a Fisher Vector encoding method was used to encode the local features of DAISY. The eigenvector of each liver image was then obtained. Staging of liver fibrosis was performed based on a SVM classification model. However, the classification system included "normal, " "liver fibrosis, " and "cirrhosis, " and the normal and abnormal cirrhosis and non-cirrhosis accuracy was 89.1% and 91.64%.
In the above method, pre-processing and selection of ROIs are usually required prior to feature extraction. A large ROI is usually best for the extraction of liver fibrosis features. ROI selection follows these principles: 1) avoid vascular and rib artifacts; 2) choose a more homogeneous liver parenchyma; 3) select the appropriate ROI size. At present, the methods for extracting image texture features of liver fibrosis mainly include statistical methods of gray histograms and gray level co-occurrence matrices, Markov random field models, fractal models, time-frequency domain transform of Fourier transform, Gabor transform, and wavelet transform analysis. Texture features based on a gray level co-occurrence matrix are the most commonly used and most effective features for the quantitative staging of liver fibrosis.
Application of deep learning
Deep learning has gained attention as an artificial intelligence strategy. It allows the production of a model composed of many processing layers for the study of multiple levels of abstraction in data representation94. Current approaches based on traditional machine learning have some limitations: (1) the features used for classification are usually based on human subjective experience and (2) a very limited number of features are extracted. The use of deep convolution networks has led to breakthroughs in the processing of image, video, voice, and audio, while regular networks tend to be used for text and voice94.
Miotto et al.95 proposed a new unsupervised deep feature learning approach that allows a general-purpose patient representation to be produced from electronic health record (EHR) data. This method aims to promote clinical predictive modeling. The authors used a three-layer stack noise auto encoder to obtain stratified rule polymerization of EHR data in approximately 700,000 patients from the Mount Sinai data warehouse. The method was tested in 76,214 patients from different clinical areas and time periods. The test cohort included patients with 78 different diseases. The results were significantly better than those obtained using the representation implementation based on the original EHR data and the alternative feature learning strategy. The predictive performance was superior for serious diabetes, schizophrenia, and various cancers. These findings suggest that deep learning applied to EHR data can provide patient representations that improve clinical predictions. This approach can facilitate the extraction of the general characteristics of patients from EHR data to make clinical predictive modeling more convenient. ConvNets is designed to deal with multiple array data and is composed of a two-dimensional array of pixel intensity containing three color channels of color images94.
Nguyen et al.96 used a deep convolutional neural network (DCNN) to construct Deep record (Deepr), which aims to improve clinical diagnostic accuracy. The authors showed that Deepr, which is a new end-to-end deep learning system, can learn to extract features from medical records and automatically predict future risks. Deepr converts medical records to a series of discrete elements separated from the transfer encoding time intervals and the hospital. Above the sequence, it is a convolutional neural network that attempts to detect predictive local clinical motifs to facilitate risk stratification. Deepr allows transparent inspection and visualization of its internal workings. Compared with traditional technologies, Deepr detects more meaningful clinical patterns and reveals the potential structure of the disease and intervention space.
A new paradigm of computer-aided medical treatment is emerging with the development of deep learning technology. The application of deep learning methods in the staging of liver fibrosis will gather more attention in the near future.97
Meng et al.98 put forward a new classification method of liver fibrosis based on transfer learning, which uses VGGNet and a deep classifier called a fully connected network (FCNet). Based on this framework, the deep function combined with FCNet can provide appropriate information. A more accurate prediction model can be constructed using this method than with alternative approaches. Liver fibrosis is divided into three stages: normal, fibrosis, and early cirrhosis. However, this method cannot distinguish the F1–F3 stages of liver fibrosis. Yasaka et al.97 investigated the performance of the DCNN model for staging liver fibrosis using hepatobiliary phase MRI with gadoxetic acid enhancement. This previous retrospective study assigned patients for whom input data [hepatobiliary phase MRIs, static magnetic field of the imaging unit, and hepatitis B and C virus testing results if available (either positive or negative)] and reference standard data (liver fibrosis stage evaluated from biopsy or surgical specimens obtained within 6 months of the MR examinations) were available to the training (534 patients) and test (100 patients) groups. For the training group, MRIs with three different section levels were augmented 90-fold (rotated, parallel-shifted, brightness-changed, and contrast-changed images were generated; a total of 144,180 images). Supervised training was performed using the DCNN model to minimize the difference between the output data [fibrosis score obtained through deep learning (FDL score)] and the staging of hepatic fibrosis. The use of the DCNN model was evaluated in the test group with ROC analyses. The FDL score was mutually related with fibrosis stage (Spearman's rank correlation coefficient: 0.63;P < 0.001). Fibrosis stages F4, F3, and F2 were diagnosed with ROC AUCs of 0.84, 0.84, and 0.85. The DCNN model exhibited high diagnostic efficiency for the staging of hepatic fibrosis.
Perspective
Traditional imaging analysis is often dependent on human cognition, limited to one or two-dimensional lesion size analysis, or involves the assessment of subjective and qualitative features, such as mild non-uniformity, large areas of necrosis, and so on. With the progress of image acquisition and analysis methods, a large number of objective and quantitative image feature descriptors can be extracted, which are expected to represent effective non-invasive diagnostic biomarkers. Image-omics is an emerging research direction to solve the above problems. This method uses a large number of automatic data feature extraction algorithms to transform the image data into a multi-dimensional, extractable feature space.
US images are created due to different absorption levels of sound propagation, while sonar digital simulation uses computational theory to transform image results into digital results. The advantage of sonar over US is that sonar can facilitate digital simulation models and predict the development of diseases. Data from sonar can be transformed into a mathematical model. These mathematical models can assess the status quo in a similar manner to elastic US and predict the speed and extent of disease development relative to the status quo, which is not possible using elastic US.
The image feature space, including information related to density, morphology, and texture based on nuclear magnetic imaging and sonar with autonomous feature mining based on convolutional neural networks, should be established. Artificial intelligence analysis tools will then be used to identify features that are strongly associated with liver fibrosis and improve the accuracy of the non-invasive assessment of liver fibrosis. Clinical detection features such as serological diagnosis to the image-based feature space should also be added to further improve the accuracy of liver fibrosis assessment.
Conclusions
A patient's liver function is directly related to the feasibility of the patient's surgical plan and whether there will be a serious risk of liver failure after the operation, making this information very clinically useful. Accurate assessment of liver fibrosis can predict the patient's liver function, which is important when planning liver surgery. The assessment of fibrosis is not only important for diagnosis but also increasingly significant for management decisions and follow-up. Non-invasive detection of liver fibrosis allows the dynamic evaluation and assessment of liver fibrosis and function during surgery and internal treatment using medication. In the future, artificial intelligence and deep learning methods capable of integrating serum markers, US, and MRI findings will be the focus of research.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911–5. doi: 10.1016/j.gcb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Yoo S, Wang W, Wang Q, Fiel MI, Lee E, Hiotis SP, et al. A pilot systematic genomic comparison of recurrence risks of hepatitis B virus-associated hepatocellular carcinoma with low- and high-degree liver fibrosis. BMC Med. 2017;15:214. doi: 10.1186/s12916-017-0973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HS, Kim BK, Kim SU, Park JY, Kim DY, Song KJ, et al. Association between level of fibrosis, rather than antiviral regimen, and outcomes of patients with chronic hepatitis b. Clin Gastroenterol Hepatol. 2016;14:1647–56 e6. doi: 10.1016/j.cgh.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 6.Carrion JA, Navasa M, Garcia-Retortillo M, Garcia-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–56. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 8.Yamada G. Histopathological characteristics and clinical significance of New Inuyama Classification in chronic hepatitis B. Nihon Rinsho. 2004;62 Suppl 8:290–2. [PubMed] [Google Scholar]
- 9.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 10.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–81. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 11.Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–35. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 12.Castera L. Noninvasive assessment of liver fibrosis. Dig Dis. 2015;33:498–503. doi: 10.1159/000374097. [DOI] [PubMed] [Google Scholar]
- 13.Gao S, Peng Y, Guo H, Liu W, Gao T, Xu Y, et al. Texture analysis and classification of ultrasound liver images. Biomed Mater Eng. 2014;24:1209–16. doi: 10.3233/BME-130922. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology. 2016;278:114–24. doi: 10.1148/radiol.2015142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castera L. Hepatitis B: are non-invasive markers of liver fibrosis reliable? Liver Int. 2014;34 Suppl 1:91–6. doi: 10.1111/liv.12393. [DOI] [PubMed] [Google Scholar]
- 16.Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem. 2016;49:302–15. doi: 10.1016/j.clinbiochem.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Nojgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–86. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 18.Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology. 1994;20:780–7. doi: 10.1002/hep.1840200403. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi M, Urashima S, Matsuda Y, Takase S, Takada A. Changes in type IV collagen content in livers of patients with alcoholic liver disease. Hepatology. 1993;17:820–7. [PubMed] [Google Scholar]
- 20.Lebensztejn DM, Kaczmarski M, Sobaniec-Lotowska M, Bauer M, Voelker M, Schuppan D. Serum laminin-2 and hyaluronan predict severe liver fibrosis in children with chronic hepatitis B. Hepatology. 2004;39:868–9. doi: 10.1002/hep.20147. [DOI] [PubMed] [Google Scholar]
- 21.Korner T, Kropf J, Gressner AM. Serum laminin and hyaluronan in liver cirrhosis: markers of progression with high prognostic value. J Hepatol. 1996;25:684–8. doi: 10.1016/s0168-8278(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 22.Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271–9. doi: 10.1111/j.1572-0241.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 23.Jazwinski AB, Thompson AJ, Clark PJ, Naggie S, Tillmann HL, Patel K. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J Viral Hepat. 2012;19:278–82. doi: 10.1111/j.1365-2893.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parfieniuk-Kowerda A, Lapinski TW, Rogalska-Plonska M, Swiderska M, Panasiuk A, Jaroszewicz J, et al. Serum cytochrome c and m30-neoepitope of cytokeratin-18 in chronic hepatitis C. Liver Int. 2014;34:544–50. doi: 10.1111/liv.12297. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed Hassan E, Sharaf El-Din Abd El-Rehim A, Ahmed Sayed ZE, Farah Mohamed Kholef E, Sabry A, Abd El-Rehim Abo Elhagag N. VAP score as a novel non-invasive liver fibrosis model in patients with chronic hepatitis C. Hepatol Res. 2017;47:1408–16. doi: 10.1111/hepr.12884. [DOI] [PubMed] [Google Scholar]
- 26.Ding D, Li H, Liu P, Chen L, Kang J, Zhang Y, et al. FibroScan, aspartate aminotransferase and alanine aminotransferase ratio (AAR), aspartate aminotransferase to platelet ratio index (APRI), fibrosis index based on the 4 factor (FIB-4), and their combinations in the assessment of liver fibrosis in patients with hepatitis B. Int J Clin Exp Med. 2015;8:20876–82. [PMC free article] [PubMed] [Google Scholar]
- 27.Deng H, Qi X, Peng Y, Li J, Li H, Zhang Y, et al. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, and king scores for diagnosis of esophageal varices in liver cirrhosis: a retrospective study. Med Sci Monit. 2015;21:3961–77. doi: 10.12659/MSM.895005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebensztejn DM, Skiba E, Sobaniec-Lotowska M, Kaczmarski M. A simple noninvasive index (APRI) predicts advanced liver fibrosis in children with chronic hepatitis B. Hepatology. 2005;41:1434–5. doi: 10.1002/hep.20736. [DOI] [PubMed] [Google Scholar]
- 29.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, Sanchez-Avila F, Vargas-Vorackova F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350–7. [PubMed] [Google Scholar]
- 30.Wang J, Yan X, Yang Y, Chang H, Jia B, Zhao XA, et al. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget. 2017;8:59257–67. doi: 10.18632/oncotarget.19501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagan M, Asrani SK, Talwalkar J. Non-invasive assessment of liver fibrosis and prognosis. Expert Rev Gastroenterol Hepatol. 2015;9:1251–60. doi: 10.1586/17474124.2015.1075391. [DOI] [PubMed] [Google Scholar]
- 32.Liu CH, Hsu SJ, Lin JW, Hwang JJ, Liu CJ, Yang PM, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol. 2007;5:1199–1206 e1. doi: 10.1016/j.cgh.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Abbattista T, Ridolfi F, Ciabattoni E, Marini F, Bendia E, Brunelli E, et al. Diagnosis of liver cirrhosis by transit-time analysis at contrast-enhanced ultrasonography. Radiol Med. 2008;113:860–74. doi: 10.1007/s11547-008-0292-3. [DOI] [PubMed] [Google Scholar]
- 34.Ridolfi F, Abbattista T, Marini F, Vedovelli A, Quagliarini P, Busilacchi P, et al. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007;39:929–35. doi: 10.1016/j.dld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 36.European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, et al. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology. 2015;276:845–61. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 38.Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125–33. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 39.Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: consensus guidelines from the canadian association for the study of the liver. Can J Gastroenterol. 2012;26:359–75. doi: 10.1155/2012/947676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–9. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Steadman R, Myers RP, Leggett L, Lorenzetti D, Noseworthy T, Rose S, et al. A health technology assessment of transient elastography in adult liver disease. Can J Gastroenterol. 2013;27:149–58. doi: 10.1155/2013/684982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol. 2015;62:317–24. doi: 10.1016/j.jhep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Feng JC, Li J, Wu XW, Peng XY. Diagnostic Accuracy of SuperSonic Shear Imaging for Staging of Liver Fibrosis: A Meta-analysis. J Ultrasound Med. 2016;35:329–39. doi: 10.7863/ultra.15.03032. [DOI] [PubMed] [Google Scholar]
- 44.Beland MD, Brown SF, Machan JT, Taliano RJ, Promrat K, Cronan JJ. A pilot study estimating liver fibrosis with ultrasound shear-wave elastography: does the cause of liver disease or location of measurement affect performance? AJR Am J Roentgenol. 2014;203:W267–73. doi: 10.2214/AJR.13.11718. [DOI] [PubMed] [Google Scholar]
- 45.Dhyani M, Grajo JR, Bhan AK, Corey K, Chung R, Samir AE. Validation of shear wave elastography cutoff values on the supersonic aixplorer for practical clinical use in liver fibrosis staging. Ultrasound Med Biol. 2017;43:1125–1133. doi: 10.1016/j.ultrasmedbio.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 47.Palmucci S, Mauro LA, Messina M, Russo B, Failla G, Milone P, et al. Diffusion-weighted MRI in a liver protocol: its role in focal lesion detection. World J Radiol. 2012;4:302–10. doi: 10.4329/wjr.v4.i7.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89–95. doi: 10.1002/jmri.21227. [DOI] [PubMed] [Google Scholar]
- 49.Pasquinelli F, Belli G, Mazzoni LN, Regini F, Nardi C, Grazioli L, et al. MR-diffusion imaging in assessing chronic liver diseases: does a clinical role exist? Radiol Med. 2012;117:242–53. doi: 10.1007/s11547-011-0730-5. [DOI] [PubMed] [Google Scholar]
- 50.Colagrande S, Carbone SF, Carusi LM, Cova M, Villari N. Magnetic resonance diffusion-weighted imaging: extraneurological applications. Radiol Med. 2006;111:392–419. doi: 10.1007/s11547-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 51.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol. 1992;159:591–9. doi: 10.2214/ajr.159.3.1503032. [DOI] [PubMed] [Google Scholar]
- 52.Murtz P, Flacke S, Traber F, van den Brink JS, Gieseke J, Schild HH. Abdomen: diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology. 2002;224:258–64. doi: 10.1148/radiol.2241011117. [DOI] [PubMed] [Google Scholar]
- 53.Bonekamp S, Torbenson MS, Kamel IR. Diffusion-weighted magnetic resonance imaging for the staging of liver fibrosis. J Clin Gastroenterol. 2011;45:885–92. doi: 10.1097/MCG.0b013e318223bd2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li YT, Cercueil JP, Yuan J, Chen W, Loffroy R, Wang YX. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg. 2017;7:59–78. doi: 10.21037/qims.2017.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008;249:891–9. doi: 10.1148/radiol.2493080080. [DOI] [PubMed] [Google Scholar]
- 56.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 57.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 58.Murphy P, Hooker J, Ang B, Wolfson T, Gamst A, Bydder M, et al. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging. 2015;41:1629–38. doi: 10.1002/jmri.24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617–23. doi: 10.1148/radiology.210.3.r99fe17617. [DOI] [PubMed] [Google Scholar]
- 60.Guiu B, Cercueil JP. Liver diffusion-weighted MR imaging: the tower of Babel? Eur Radiol. 2011;21:463–7. doi: 10.1007/s00330-010-2017-y. [DOI] [PubMed] [Google Scholar]
- 61.Guiu B, Petit JM, Capitan V, Aho S, Masson D, Lefevre PH, et al. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012;265:96–103. doi: 10.1148/radiol.12112478. [DOI] [PubMed] [Google Scholar]
- 62.Grech-Sollars M, Hales PW, Miyazaki K, Raschke F, Rodriguez D, Wilson M, et al. Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain. NMR Biomed. 2015;28:468–85. doi: 10.1002/nbm.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010;31:589–600. doi: 10.1002/jmri.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Jin N, Deng J, Guo Y, White SB, Yang GY, et al. Intra-voxel incoherent motion MRI in rodent model of diethylnitrosamine-induced liver fibrosis. Magn Reson Imaging. 2013;31:1017–21. doi: 10.1016/j.mri.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow AM, Gao DS, Fan SJ, Qiao Z, Lee FY, Yang J, et al. Liver fibrosis: an intravoxel incoherent motion (IVIM) study. J Magn Reson Imaging. 2012;36:159–67. doi: 10.1002/jmri.23607. [DOI] [PubMed] [Google Scholar]
- 66.Orlacchio A, Bolacchi F, Cadioli M, Bergamini A, Cozzolino V, Angelico M, et al. Evaluation of the severity of chronic hepatitis C with 3-T1H-MR spectroscopy. AJR Am J Roentgenol. 2008;190:1331–9. doi: 10.2214/AJR.07.2262. [DOI] [PubMed] [Google Scholar]
- 67.Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788–94. doi: 10.1053/jhep.2003.50149. [DOI] [PubMed] [Google Scholar]
- 68.Angus PW, Dixon RM, Rajagopalan B, Ryley NG, Simpson KJ, Peters TJ, et al. A study of patients with alcoholic liver disease by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond) 1990;78:33–8. doi: 10.1042/cs0780033. [DOI] [PubMed] [Google Scholar]
- 69.Dezortova M, Taimr P, Skoch A, Spicak J, Hajek M. Etiology and functional status of liver cirrhosis by 31P MR spectroscopy. World J Gastroenterol. 2005;11:6926–31. doi: 10.3748/wjg.v11.i44.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Longo R, Ricci C, Masutti F, Vidimari R, Croce LS, Bercich L, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297–302. [PubMed] [Google Scholar]
- 71.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–95. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 72.Cho SG, Kim MY, Kim HJ, Kim YS, Choi W, Shin SH, et al. Chronic hepatitis: in vivo proton MR spectroscopic evaluation of the liver and correlation with histopathologic findings. Radiology. 2001;221:740–6. doi: 10.1148/radiol.2213010106. [DOI] [PubMed] [Google Scholar]
- 73.Petitclerc L, Gilbert G, Nguyen BN, Tang A. Liver Fibrosis Quantification by Magnetic Resonance Imaging. Top Magn Reson Imaging. 2017;26:229–241. doi: 10.1097/RMR.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dresner MA, Rose GH, Rossman PJ, Muthupillai R, Manduca A, Ehman RL. Magnetic resonance elastography of skeletal muscle. J Magn Reson Imaging. 2001;13:269–76. doi: 10.1002/1522-2586(200102)13:2<269::aid-jmri1039>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 75.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–7. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 76.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–55. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–8. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichikawa S, Motosugi U, Morisaka H, Sano K, Ichikawa T, Enomoto N, et al. Validity and reliability of magnetic resonance elastography for staging hepatic fibrosis in patients with chronic hepatitis B. Magn Reson Med Sci. 2015;14:211–21. doi: 10.2463/mrms.2014-0150. [DOI] [PubMed] [Google Scholar]
- 79.Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24:70–8. doi: 10.1007/s00330-013-2978-8. [DOI] [PubMed] [Google Scholar]
- 80.Batheja M, Vargas H, Silva AM, Walker F, Chang YH, De Petris G, et al. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging. 2015;40:760–5. doi: 10.1007/s00261-014-0321-8. [DOI] [PubMed] [Google Scholar]
- 81.Morisaka H, Motosugi U, Ichikawa S, Nakazawa T, Kondo T, Funayama S, et al. Magnetic resonance elastography is as accurate as liver biopsy for liver fibrosis staging. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25868. [DOI] [PubMed] [Google Scholar]
- 82.Trout AT, Serai S, Mahley AD, Wang H, Zhang Y, Zhang B, et al. Liver stiffness measurements with mr elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology. 2016;281:793–804. doi: 10.1148/radiol.2016160209. [DOI] [PubMed] [Google Scholar]
- 83.Serai SD, Yin M, Wang H, Ehman RL, Podberesky DJ. Cross-vendor validation of liver magnetic resonance elastography. Abdom Imaging. 2015;40:789–94. doi: 10.1007/s00261-014-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: part 2, diagnostic performance, confounders, and future directions. AJR Am J Roentgenol. 2015;205:33–40. doi: 10.2214/AJR.15.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Materne R, Van Beers BE, Smith AM, Leconte I, Jamart J, Dehoux JP, et al. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 2000;99:517–25. [PubMed] [Google Scholar]
- 86.House MJ, Bangma SJ, Thomas M, Gan EK, Ayonrinde OT, Adams LA, et al. Texture-based classification of liver fibrosis using MRI. J Magn Reson Imaging. 2015;41:322–8. doi: 10.1002/jmri.24536. [DOI] [PubMed] [Google Scholar]
- 87.Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging. 2013;13:140–9. doi: 10.1102/1470-7330.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kayaalti O, Aksebzeci BH, Karahan IO, Deniz K, Ozturk M, Yilmaz B, et al. Liver fibrosis staging using CT image texture analysis and soft computing. Applied Soft Computing. 2014;25:399–413. [Google Scholar]
- 89.Lubner MG, Malecki K, Kloke J, Ganeshan B, Pickhardt PJ. Texture analysis of the liver at MDCT for assessing hepatic fibrosis. Abdom Radiol (NY) 2017;42:2069–78. doi: 10.1007/s00261-017-1096-5. [DOI] [PubMed] [Google Scholar]
- 90.Daginawala N, Li B, Buch K, Yu H, Tischler B, Qureshi MM, et al. Using texture analyses of contrast enhanced CT to assess hepatic fibrosis. Eur J Radiol. 2016;85:511–7. doi: 10.1016/j.ejrad.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45:1276–95. doi: 10.1002/jmri.25550. [DOI] [PubMed] [Google Scholar]
- 92.Anderson SW, Jara H, Ozonoff A, O'Brien M, Hamilton JA, Soto JA. Effect of disease progression on liver apparent diffusion coefficient and T2 values in a murine model of hepatic fibrosis at 11.7 Tesla MRI. J Magn Reson Imaging. 2012;35:140–6. doi: 10.1002/jmri.22807. [DOI] [PubMed] [Google Scholar]
- 93.Han Z, Wan J, Deng L, Liu K. Oil Adulteration Identification by Hyperspectral Imaging Using QHM and ICA. PLoS One. 2016;11:e0146547. doi: 10.1371/journal.pone.0146547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 95.Miotto R, Li L, Kidd BA, Dudley JT. Deep Patient: An Unsupervised Representation to Predict the Future of Patients from the Electronic Health Records. Sci Rep. 2016;6:26094. doi: 10.1038/srep26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen P, Tran T, Wickramasinghe N, Venkatesh S. Deepr: A Convolutional Net for Medical Records. IEEE J Biomed Health Inform. 2017;21:22–30. doi: 10.1109/JBHI.2016.2633963. [DOI] [PubMed] [Google Scholar]
- 97.Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S. Liver Fibrosis: Deep Convolutional Neural Network for Staging by Using Gadoxetic Acid-enhanced Hepatobiliary Phase MR Images. Radiology. 2017:171928. doi: 10.1148/radiol.2017171928. [DOI] [PubMed] [Google Scholar]
- 98.Meng D, Zhang LB, Cao GT, Cao WM, Zhang GX, Hu B. Liver Fibrosis Classification Based on Transfer Learning and FCNet for Ultrasound Images. Ieee Access. 2017;5:5804–10. [Google Scholar]


