Abstract
The introduction of immune-checkpoint blockade in the cancer therapy led to a paradigm change of the management of late stage cancers. There are already multiple FDA approved checkpoint inhibitors and many other agents are undergoing phase 2 and early phase 3 clinical trials. The therapeutic indication of immune checkpoint inhibitors expanded in the last years, but still remains unclear who can benefit. MicroRNAs are small RNAs with no coding potential. By complementary pairing to the 3' untranslated region of messenger RNA, microRNAs exert posttranscriptional control of protein expression. A network of microRNAs directly and indirectly controls the expression of checkpoint receptors and several microRNAs can target multiple checkpoint molecules, mimicking the therapeutic effect of a combined immune checkpoint blockade. In this review, we will describe the microRNAs that control the expression of immune checkpoints and we will present four specific issues of the immune checkpoint therapy in cancer: (1) imprecise therapeutic indication, (2) difficult response evaluation, (3) numerous immunologic adverse-events, and (4) the absence of response to immune therapy. Finally, we propose microRNAs as possible solutions for these pitfalls. We consider that in the near future microRNAs could become important therapeutic partners of the immune checkpoint therapy.
Keywords: MicroRNA, PD-1, PD-L1, CTLA-4, checkpoint inhibitors
Introduction
The introduction of immune checkpoint blockade (ICB) in cancer therapy led to a paradigm change of the management of late stage cancers. This new therapy inhibits the cancer mediated suppression of the immune system. The first checkpoint inhibitor approved for cancer treatment was ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) antibody which was initially used only for the treatment of metastatic melanoma and more recently also as an adjuvant therapy for stage III melanoma patients1,2. The FDA approved other two agents, pembrolizumab and nivolumab, both of which are anti-programmed cell death protein 1 (PD-1) monoclonal antibodies. These two new agents were initially indicated for the treatment of stage IV melanoma3,4 and for non-small cell lung cancer (NSCLC)5,6. Additionally, in late 2016 and early 2017, FDA approved atezolizumab [anti-programmed death-ligand 1 (PD-L1) monoclonal antibody] for the management of advanced and metastatic urothelial carcinoma (UC)7 and for stage IV NSCLC8; avelumab (anti-PD-L1 monoclonal antibody) for the management of stage IV Merkel cell carcinoma9 and durvalumab (also an anti-PD-L1 monoclonal antibody) for the treatment of late stage UC10.
The therapeutic indication of immune checkpoint antibodies expanded in the last few years. Based on recent clinical trials, pembrolizumab received FDA approval for any type of late stage solid tumor with microsatellite instability-high or DNA mismatch repair deficiencies11. Furthermore, nivolumab was also accepted for treating renal cell carcinoma, urothelial bladder cancer, metastatic epidermoid carcinoma of the head and neck and classical Hodgkin lymphoma12.
MicroRNAs (miRNAs) are small RNAs with no coding potential, produced from long transcripts named primary miRNAs13. By complementary pairing to the 3’ untranslated region (UTR) of messenger RNA (mRNA), miRNAs exert a posttranscriptional control of protein expression, usually leading to a protein repression14. MiRNAs differ in their origin from other small non-coding RNAs [small interfering RNA (siRNA) and Piwi-interacting RNA (piRNA)]: miRNAs derive from transcripts forming stem-loops, siRNAs derive from double strand RNA precursors and piRNAs are the product of single strand fragments15. MiRNAs involvement in human diseases started being intensely studied after Calin et al. demonstrated their importance in chronic lymphatic leukemia development16,17. Afterwards, altered miRNA expression was linked with a variety of human diseases, including infectious, autoimmune, degenerative and any type of neoplastic pathology18. Intriguingly, most miRNAs can target multiple mRNAs and most mRNAs are targeted by several miRNAs19. Hence, in order to understand the underling biological phenomenon and to be able to therapeutically manipulate, it is important to study more than one miRNA that controls the expression of one protein. Using molecular networks, it is possible to characterize not only the relationship between the inhibitor and its target, but also the interaction between the different inhibitors20,21. A network of miRNAs directly and indirectly controls the expression of immune checkpoint receptors. The level of each of these negative regulators of the immune system is fine-tuned by several miRNAs (direct targeting) and by other proteins, which themselves are regulated by miRNAs (indirect targeting).
The roles of miRNAs as regulators of immune checkpoints were already discussed in other reviews22,23. In this review, we will describe the miRNA network that controls the expression of the immune checkpoints and we will present four specific issues of the ICB: (1) imprecise therapeutic indication, (2) difficult response evaluation, (3) numerous immunologic adverse-events, and (4) the absence of response to immune checkpoint therapy. Finally, we propose miRNAs as possible solutions for these pitfalls. We consider that in the near future miRNAs could become important therapeutic partners of the ICB.
MiRNAs control immune checkpoints expression
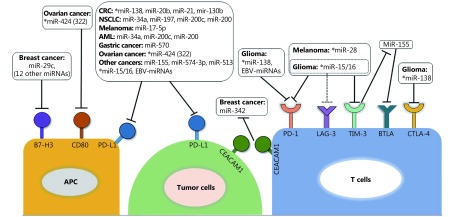
MiRNAs fine tune the expression of immune checkpoint receptors and their ligands. One miRNA can target several checkpoint molecules, mimicking the therapeutic effect of a combined ICB22. It is crucial to understand which are the hubs of the miRNA regulatory network (Figure 1) in order to design therapies that target these super-connected nodes24. On the other hand, the immune checkpoint molecules can control the expression of miRNAs, making the network robust and complex. A list of the miRNAs that target the immune checkpoints and of the immune checkpoints that control the expression of miRNAs can be found in Table 1.
1.
A network of miRNAs directly controls the expression level of the immune checkpoint molecules. Some of these miRNAs (*) target multiple immune checkpoints and are suitable therapeutic targets. Increasing the level of these hubs can lead to a multiple checkpoint blockade. Similarly, immune checkpoint can also change the expression of microRNAs.
1.
A panel of miRNAs controls the expression of the immune checkpoints
| Item | Tissue/cell line | Relationship to immune checkpoints | Function | Ref. |
| MiRNAs | ||||
| MiR-424 (322) | Ovarian cancer tissue and ovarian cancer cell lines | Anticorrelates with CD80
and PD-L1 |
Low levels of miR-424(322) are associated with chemoresistance | 26 |
| MiR-15/16 family | Glioma mouse model | Correlates with PD-1, TIM-3, LAG-3 | Low levels of miR-15a/16 prolongs mice survival | 32 |
| MPM tissue and
MPM cell lines |
Anticorrelates with PD-L1 | High PD-L1 is associated with low miR-15/16 levels and short overall survival | 48 | |
| MiR-138 | Glioma mouse model | Anticorrelates with PD-1,
CTLA-4 |
High level of miR-138 inhibit tumor progression | 33 |
| CRC patient samples and
CRC cell lines |
Anticorrelates with PD-L1 | Low levels of miR-138 are associated with shorter overall survival | 34 | |
| MiR-28 | Exhausted T-cells from
mice melanoma |
Anticorrelates with PD-1,
TIM3 and BTLA |
Low levels of miR-28 induces T-cell exhaustion | 35 |
| MiR-155 | Mouse T-cells | Anticorrelates with BTLA | Low levels of miR-155 decrease
CD4+ T cell activation |
37 |
| Dermal lymphatic
endothelial cells |
Anticorrelates with PD-L1 | MiR-155 is part of a regulatory loop which controls the expression of PD-L1 | 57 | |
| MiR-29c and
other 12 miRNAs |
Breast cancer cell lines and tissue from breast cancer patients | Anticorrelates with B7-H3 | High levels of miR-29c associate with a decreased risk of dying from breast cancer | 39 |
| MiR-570 | Gastric cancer tissue | Anticorrelates with PD-L1 | The inability of miR-570 to bind the PD-L1 mRNA leads to an aggressive gastric cancer phenotype | 42 |
| MiR-34a
(and miR-34 family) |
TCGA lung adenocarcinoma, p53 (R172HΔ)g/+K-ras (LA1/+) mouse model and various
cell lines |
Anticorrelates with PD-L1 | P53 regulates the anti-tumor immunity by overexpressing miR-34, an inhibitor of PD-L1 | 43 |
| AML patient samples and leukemia cell lines | Anticorrelates with PD-L1 | High levels of miR-34 decrease T-cell apoptosis | 44 | |
| MiR-34a and
MiR-200c |
AML cell lines
and AML mouse model |
Anticorrelates with PD-L1 | High levels of miR-34a and miR-200c leads to increased immune mediated killing of the tumor | 45 |
| MiR-197 | NSCLC patient samples and human lung cancer cell lines | Anticorrelates with PD-L1 | Low level of miR-197 predict low survival in NSCLC | 46 |
| Oral squamous cell carcinoma | Anticorrelates with PD-L1 | High levels of miR-197 predict poor overall survival | 52 | |
| MiR-200 | Lung adenocarcinoma databases, different mouse models and cell models | Anticorrelates with PD-L1 | MiR-200 simultaneously inhibits neoplastic invasion and immunosuppression | 49 |
| MiR-20b, miR-21
and miR-130b |
CRC tissue | Correlate with PD-L1 | MiR-20b, miR-21 and miR-130b inhibit PTEN, which is an inhibitor of PD-L1 | 50 |
| MiR-574-3p | Spinal chordoma tissue | Anticorrelates with PD-L1 | Low levels of miR-574-3p are associated with worse local recurrence-free survival | 51 |
| MiR-25-93-106b
cluster |
Primary pancreatic cancer cells from murine models | Anticorrelates with PD-L1 | The miRNA cluster controls the bone marrow metastasis | 53 |
| Continued | ||||
CTLA-4
CTLA-4 is expressed solely on T-cells and inhibits their function by binding to its ligand CD80. CTLA-4 is the first therapeutically targeted immune checkpoint molecule25. The function of CTLA-4-CD80 pair is controlled by miR-424 that directly binds the 3’UTR of two mRNAs involved in the immune suppressive system, CD80 and PD-L1. MiR-424 down-regulates CD80 in dendritic cell, thus increases the efficacy of chemotherapy by improving T cells immune toxicity. Further analysis revealed that higher miR-424 was correlated to the lower expression of CTLA-4 (R=–0.1, P=0.0273, n=489), and CD80 (R=–0.1148, P=0.00111, n=489)26.
TIM-3, CEACAM1 and galactine-9
T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), another immune regulator, expressed on activated T effector cells, negatively controls the responses of T effector cells by inducing T cell tolerance and exhaustion. Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is expressed on activated T-cells endowing TIM-3 immunosuppressive function27. Galectin-9 is the most common ligand of TIM-3 that facilitates its negative immune regulatory function28. These checkpoints can also exert their function by changing the expression of multiple miRNAs. Over 100 miRNAs were identified dysregulated in TIM-3 knock-down macrophages suggesting that miRNAs are pivotal for TIM-3’s biological function. TIM-3 negatively regulates miR-155 both in vitro and colon cancer mouse models. Signal transducer and activator of transcription-1 (STAT1) was confirmed as the signaling adaptor, connecting TIM-3 with miR-155 to induce M2 macrophage polarization29. CEACAM1 and galactine-9 can also control the expression of miRNAs. MiR-342 is a target of CEACAM1; this miRNA is down-regulated in MCF7 breast cancer cells when CEACAM1 is overexpressed. The interaction between CEACAM1 and miR-342 partially explains the mechanism by which this immune checkpoint maintains the luminal orientation in epithelial breast cells30. Similarly, galectin-9 can regulate 42 miRNAs in human liver metastatic cancer cell lines31. These data further support that the function of immune checkpoints is interconnected to the miRNA regulatory network through a dual relationship: while miRNAs controls the expression of the checkpoints, these can also change the level of miRNAs and influence their functions.
MiRNA hubs
Some miRNAs target immune checkpoints from different cells of the tumor microenvironment and have a profound regulatory effect. In glioma, knock-out of miR-15a/16 alleviates glioma progression and prolongs mice survival by decreasing the PD-1, TIM-3 and lymphocyte-activation gene 3 (LAG-3) expression, and promotes the secretion of several cytokines from tumor-infiltrating CD8+ T cells32.
MiR-138 was reported to inhibit glioma progression and increases the survival of tumor-bearing mice by evoking an anti-tumor immune response, by binding to the 3’UTR of PD-1 and CTLA-4. Further analysis revealed that miR-138 decreases PD-1, CTLA-4, and forkhead box protein 3 (FOXP3) in transfected CD4+ T cells. In addition, no anti-glioma effect of miR-138 treatment was found in immune-incompetent mice or in an in vivo T-cell depletion model, which revealed that its anti-cancer efficacy is immune system dependent33. In a different study, miR-138 was also reported as a direct inhibitor of PD-L1 in colorectal cancer (CRC), being able to inhibit cell growth and tumorigenesis in vitro and in vivo34. Similarly, miR-28 can inhibit the expression of TIM-3, B- and T-lymphocyte attenuator (BTLA), PD-1, and the secretion of cytokines IL-2 and TNF-α to modulate exhaustive differentiation of T cells35.
BTLA
BTLA is one of the immune checkpoints that is induced during the activation of T cells. Its activation obstructs the anti-neoplastic function of CD8+ cancer-specific T cell36. One study showed that miR-155 targets the BTLA 3’UTR and decreases the surface BTLA expression by about 60%. As expected, knockdown of miR-155 resulted in up-regulation of surface BTLA37. Further studies are required to determine the function of the miR-155-BTLA interaction in neoplastic pathology.
B7-H3
B7-H3 is a B7 family member which is expressed on the surface of many cell types. The function of B7-H3 remains controversial, being unclear if its overexpression has an anti-tumor effect or an immunosuppressive function38. Numerous miRNAs downregulate the protein levels of B7-H3 in breast cancer cell lines. Thirteen of these miRNAs (miR-214, miR-363, miR-326, miR-940, miR-29c, miR-665, miR-34b, miR-708, miR-601, miR-124a, miR-380-5p, miR-885-3p, and miR-593) bind to the 3’UTR of B7-H3 and inhibit its translation. From these miRNAs, high expression of miR-29c was identified to show the best correlation with a substantial diminished risk of mortality from breast cancer in both discovery and validation groups39.
PD-L1 and PD-1
The level of PD-L1 is intimately controlled by the miRNA network. PD-L1 is expressed on different types of cells, mainly on immune cells [T-cells, B-cells, monocytes, antigen-presenting cells (APCs)], but also epithelial cells. PD-L1 is overexpressed when inflammatory cytokines (e.g. IFN-γ and IL-4) stimulate the transcription factors STAT1 and IFN regulatory factor-140. As an immunosuppressive mechanism, the level of PD-L1 is high in various types of neoplasia and is often linked to poor prognosis and predicts favorable responses to anti-PD-1/PD-L1 antibodies41.
The 3’UTR of the PD-L1 mRNA harbors multiple cis-acting segments implicated in mRNA decay, as well as an adenylate-uridylate (AU)-rich element and some possible miRNA-binding sites. A single nucleotide mutation at the 3′-UTR of PD-L1 leads to the overexpression of PD-L1 by disrupting the complementarity between miR-570 and its 3’UTR binding site. This mutation is associated with high PD-L1 levels in gastric cancer and also with the aggressive phenotype42. The interaction between miRNAs and PD-L1 3’-UTR is dependent on the structural variations of PD-L1 3’-UTR and this type of mutation is one of the mechanism by which tumor cells can escape immune surveillance.
P53 directly controls the expression level of miR-34a, miR-34b, and miR-34c in different cell lines and tissues. The interaction between p53 and PD-L1 is mediated by miR-34, which binds to the PD-L1 3'-UTR in NSCLC models43. Additionally, using leukemia cell lines, Wang et al.44 also demonstrate that miR-34a can directly bind the 3’UTR of PD-L1, downregulating its expression. Furthermore, the PD-L1 induced T cell apoptosis was decreased after transfection with miR-34a mimic. The authors also found a positive feedback mechanism among PD-L1 level and AKT activation. Another molecule that interferes with miR-34a-PD-L1 regulatory axis is mucin1 (MUC1). The inhibition of MUC1 in acute myeloid leukemia cell lines leads to a decrease of PD-L1 by overexpressing miR-34a and miR-200c, both negative regulators of PD-L1. MUC1 controls the expression of these two miRNAs by altering the level of DICER, the RNase-Ⅲ enzyme that processes precursor miRNAs into mature miRNA45.
PD-L1 is also regulated by miR-197 through a complex regulatory mechanism involving the cyclin-dependent kinases regulatory subunit 1/signal transducer and activator of transcription 3 (CKS1B/STAT3) pathway. The expression of the immune checkpoint PD-L1 is controlled by STAT3, which is activated by CKS1B. CKS1B is a direct target of miR-197, therefore not surprisingly, low level of miR-197 correlates with high expression of PD-L1 and predicts shorter survival in NSCLC46. STAT3 is also a well-known inhibitor of p5347, therefore STAT3 is a strategic regulatory component of the network that controls the expression of PD-L1 directly and indirectly through p53-miR-34 regulatory axis.
Kao et al.48 confirmed the role of the miR-15/16 family as an important element of the network. In malignant pleural mesothelioma (MPM) cell lines, the authors demonstrated that miR-15a, miR-15b and miR-16 directly bind to the 3’UTR of PD-L1 reducing the expression of this immune checkpoint molecule. Additionally, miR-193a-3p can directly inhibit the expression of PD-L1 in MPM.
A robust correlation between the level of epithelial-to-mesenchymal transition (EMT) involved in cancer metastasis, miR-200 and PD-L1 expression in lung adenocarcinomas have been demonstrated, where PD-L1 is directly controlled by miR-200. Additionally, miR-200 is anticorrelated with most of the EMT markers, and inhibit the phenotypical transition and reduce tumor invasion. MiR-200 forms a negative feedback-loop with the zinc finger E-box-binding homeobox 1 (ZEB1), a positive regulator of EMT49. Hence, we can perceive miR-200 as a node of the network that links two important hallmarks of cancer, immunosuppression and invasion.
MiR-20b, miR-21, and miR-130b are positive regulators of PD-L1 in advanced CRC. By inhibiting phosphatase and tensin homolog (PTEN), these miRNAs cause an indirect upregulation of PD-L1. These three miRNAs are one of the few that positively correlate with the expression of an immune checkpoint and would be suitable for anti-miRNA therapy50.
For some of the miRNAs that regulate the expression of PD-L1 mechanistic insight is lacking and only statistical correlations are available. For example, in spinal chordoma, miR-574-3p was recognized to inversely correlate with PD-L1 expression: patients with high PD-L1 and low miR-574-3p chordoma were significantly associated with worse local recurrence-free survival51. For another miRNA only statistical inverse correlations to PD-L1 levels are available: high miR-197 anticorrelates to PD-L1 in oral squamous cell carcinoma and predicts poor overall survival52.
The level of PD-L1 is controlled also by the miR-25-93-106b cluster. MiR-25-93-106b knockout mice have 50% higher level of PD-L1 +/CD11b + bone marrow cells compared to WT mice. Moreover, treatment with miR-93-5p and miR-106b-5p mimics or OTX015 (inhibitor of the bromodomain and extraterminal family of proteins) which is an upstream positive regulator of the same miRNA cluster, decreases the expression of PD-L1 in the peripheral blood cells of mice or primary cancer cells53.
Very intriguing, a cluster of EBV-miRNAs is correlated with the upregulation of PD-1 and PD-L1 in solid malignancies. A large population based study from The Cancer Genome Atlas (TCGA) project showed that patients with higher level of EBV-miRNA have also high expression of PD-1, PD-L1, TGFβ1, IL-10, IFN-γ and TGFβ2, and may be candidates for immune checkpoint therapy. Additional studies are necessary to explain the mechanism behind this surprising correlation54.
Based on the interaction between miRNAs and IFN-γ/STAT1 pathway, Baer et al.55 found that DICER deficiency in tumor-associated macrophages (TAMs) induces an anti-tumorigenic phenotype, with tumors populated largely by M1-like TAMs. Moreover, tumors populated by DICER deficient TAMs can be completely eradicated by treatment with anti-PD-1 antibodies or CD40 agonistic antibodies. The rescue of let-7 activity in DICER-/- macrophages leads to an increased M2-like macrophage population and reduces the number of tumor-infiltrating cytotoxic T lymphocytes. These observations sustain that DICER/let-7 activity antagonizes the IFN-γ induced anti-neoplastic effect.
Recently, it was also shown that the oncogenic miR-155 is necessary to limit tumor growth and activate IFN-γ synthesis by T cells within the neoplastic microenvironment. ICB against PD-1, PD-L1 and CTLA-4 restored the antitumor immunity in conditionally deleted miR-155 in T-cells. This data suggests that the ICB and miR-155 control overlapping signaling pathways. Additionally, miR-155 deficiency leads to a decrease expression of IFN-γ genes in TAMs56.
The interaction between IFN-γ - miR-155 and PD-L1 was proven in dermal lymphatic endothelial cells and dermal fibroblast. Treating the cells with IFN-γ or TNF-α induced an upregulation of PD-L1 and miR-155, while miR-155 inhibits the expression of PD-L1. Hence, it seems that miR-155 is controlling the expression of PD-L1 activation, building a regulatory loop57. On the contrary, IFN-γ stimulation of biliary epithelial cells leads to the decrease of miR-513 (in fact miR-513a-5p) and upregulation of PD-L1. MiR-513 targets PD-L1 and miR-513 transfection downregulates the IFN-γ induced PD-L1 protein expression58. It is not clear if these regulatory mechanisms are tissue specific or are present simultaneously when PD-L1 is activated. It has been shown by others that etoposide can increase the expression of PD-1, indicating a potential association between chemotherapy and neoplastic avoidance of immune destruction. MiR-513a-5p expression is downregulated after treatment of retinoblastoma cells with etoposide and the PD-1 expression is reduced gradually with the increasing dose of miR-513a-5p mimics. MiR-513a-5p directly inhibits the expression of PD-1 creating a connection between the response to chemotherapy and inactivation of the immune system59. Additional studies are necessary to describe the interaction between miRNAs and IFN-γ pathway and the role of miRNAs on promoting specific TAM phenotypes.
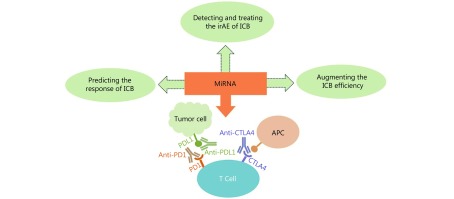
MiRNAs from the network which specifically inhibit only one checkpoint are suitable for assessing if a patient will benefit from the therapy and for evaluating the response to immune checkpoint blockade after the initiation of therapy (i.e. biomarkers). The miRNAs which were reported to be dysregulated in autoimmune disease are probably suited to monitor and predict the immune related adverse events. Finally, the miRNAs which target multiple checkpoints are ideal therapeutic targets, because they mimic the blockade with multiple immune checkpoint inhibitors which proved to be superior to single antibody therapy (Figure 2).
2.
The ICB is a promising therapy, but clinicians encounter several difficulties. MiRNAs can be suitable partners of the ICB and be used to predict the response to therapy, detect and treat the side effects of anti-immune checkpoint antibodies and potentiate the effect of the ICB (ICB – immune checkpoint blockade; irAE – immune related adverse events).
Translational perspectives
The therapeutic indications of checkpoint inhibitors are imprecise and the evaluation of the therapy response is difficult
The therapeutic indication of immune checkpoint antibodies is expanding rapidly. This new therapy is approved for numerous types of cancer, but only a subset of patient can benefit from it60. Adding other genetic and epigenetic markers could further delimitate the indication of the ICB. For the clinician, an important challenge is to decide who can benefit. Significant research is carried out to discover new biomarkers specific for the molecular mechanism of immune checkpoint inhibition or use routinely available markers (e.g. leucocyte count, lactate dehydrogenase, C-reactive protein) that can predict the response to therapy, but none proved efficient enough61.
Therapy with antibodies against the immune checkpoints can lead to an atypical response. In a subgroup of patients, the initial phases of treatment are accompanied by tumor growth/or the appearance of secondary lesions, but shortly after the tumor burden decreases. This unique tumor response pattern is termed “pseudoprogression”62. The atypical response mechanism opens new challenges for the clinician, which encounters difficulties in evaluating the treatment and also taking future therapeutic decisions. Moreover, recent studies have demonstrated that the classical tumor response criteria [WHO criteria and Response Evaluation Criteria In Solid Tumors (RECIST)] are not suitable for assessing the tumor burden in case of the ICB63-65. Hence, there is an unmet need for novel biomarkers which can be used to assess the response to immune checkpoint inhibitor therapy.
One of the most studied method to predict the response and outcomes of anti-PD-1 agents is by assessing the expression of PD-L1 receptors by immunohistochemistry in tumor samples. The results are controversial, but FDA approved, based on several positive studies, the treatment of metastatic NSCLC with pembrolizumab if the expression of PD-L1 receptors in metastatic tumors is over 50%66. This method has several limitations: (a) requires biopsy; (b) because of tumor heterogeneity some samples do not express PD-L1, although the overall expression is high; (c) most antibodies target the membranous and cytoplasmic PD-L1, leading to imprecise results62.
A different strategy to determine if a patient will respond to checkpoint inhibitors could be the expression of miRNAs that control the level of immune checkpoints. Therefore, finding miRNAs that correlate with the expression of immune checkpoints is a good alternative. A possible biomarker to determine if a patient will respond to anti-PD-L1 therapy is miR-34a. Furthermore, using bone marrow samples from 44 acute myeloid leukemia and 5 healthy controls, Wang et al.42 confirmed that the level of miR-34a is statistically inversely correlated with that of PD-L1. In a different study, using the TCGA database for 181 NSCLC it has been showed that in WT TP53 patients, the PD-L1 level is low and the level of miR-34a is high, suggesting that WT TP53 inhibits PD-L1 via miR-34a43.
MiR-17-5p from sera of metastatic melanoma is inversely correlated with the expression of PD-L1 in tumor tissue and with the appearance of BRAF mutations. The authors propose low miR-17-5p to assess the level of PD-L1 in metastatic melanoma tumors67.
Kao et al.48 showed that in metastatic pleural mesothelioma tumor samples with high levels of PD-L1 correlate with a low level of miR-15b, miR-16, miR-193a-3p and miR-200c predicting a poor prognosis. Additionally, using the TCGA lung adenocarcinoma database (n = 230), Chen et al.,49 discovered that the miR-200 family anticorrelates with the mRNA level of PD-L1 and high PD-L1 associates with a high mesenchymal score. The authors speculate that low miR-200 is a suitable biomarker for lung adenocarcinomas which responds to immune checkpoint blockade.
Two studies confirmed that the level of PD-L1 is anticorrelated with that of miR-197 in two tumor types, NSCLC and oral squamous carcinoma, respectively46,52. In recurrent, platinum-resistant NSCLC, miR-197 is downregulated in tumor samples compared to chemotherapy responsive tumors. Regarding the prognostic value of miR-197, the results are controversial between the studies. In NSCLC high miR-197 was linked to a good overall survival46, while in oral squamous carcinoma high miR-197 was linked to worse overall survival52. These observations suggest a different mechanism for miR-197-PD-L1 regulation in the two tumor types. Additionally, Fujita et al.46 demonstrate that knock down of miR-197 in vitro and in vivo promotes an aggressive pulmonary cancer phenotype. Taken together, the data from the NSCLC study prove the potential therapeutic role of miR-197 mimetics, at least in chemoresistant NSCLC.
We envision that miRNA could solve the problem of imprecise indication of ICB. By assessing the level of miRNAs that anticorrelate with the expression of immune checkpoint receptors, before the start of the therapy, the clinician could predict the potential response of this novel treatment. Additionally, the diagnostic approaches used now to determine the response to ICB are invasive. As shown, most of the studies measure the expression of immune checkpoint regulatory miRNAs in the tumor sample. It would be interesting to evaluate if these miRNAs also circulate in plasma and would be suitable for noninvasive methods to determine the response to ICB. If the expression of miRNAs is appropriate to predict the therapeutic response, than the same miRNAs could be tools to assess the response to treatment after the initiation of the ICB. We imagine that the miRNAs which are downregulated before the initiation of the treatment, will be restored, if the therapy is effective and could be markers of beneficial response. Further studies expanded on larger sets of samples (that are building rapidly with the advance of ICB use), are necessary to show how powerful these miRNAs are as potential noninvasive biomarkers to predict the response to ICB.
The immune checkpoint therapy is characterized by numerous immunologic adverse-events
Following the treatment with checkpoint inhibitors, one should not perceive the immune system to be in a new state of hemostasis. The immune system after being stimulated by checkpoint inhibitors reacts in an aggressive manner not only against the tumor cells, but also against self-tissues. This new state of the immunity resembles with that of an autoimmune disease. This observation is enforced by the numerous side effects related to the treatment with checkpoint inhibitors, side effects which are very different from those associated with conventional chemotherapy68. The checkpoint inhibitors related side effects are named immune-related adverse events (irAEs). The most frequent irAEs consist in skin reactions rush and/or pruritus, reaching an incidence of 40%–60%, depending on the type of targeted receptor69-71. Diarrhea and/or colitis are also side effects of the ICB: around 7% of patients treated with CTLA-4 ICB develop high grade colitis (grade 3–4) and only 1.8% of those treated with PD-1 antibodies3. Also common are the immune related endocrinopathyes: hypophysitis and thyroiditis, which occur in approximately 10% of treated patients72 and adrenalitis, a more rare, but life threatening toxic side-effect73. Furthermore, the incidence of irAEs increases if two immune checkpoint inhibitors are combined. The treatment of irAEs differs based on the rate of adverse reaction grade. Often, the immune stimulation with checkpoint inhibitors is interrupted and immunosuppression with corticosteroids is required68. Hence, the immune system is inhibited and the tumor progresses all over again. From a clinical perspective there are two important questions: (1) how can the oncologist promptly recognize irAEs? And (2) how can the side effects be managed without discontinuing the immune blockade and without turning the immune mechanism in favor of the tumor (by using steroids and other immunosuppressive agents)?
MiRNAs could be one of the elements that maintain the balance between immune tolerance and autoimmunity. By now, several studies demonstrated that miR-155 is a PD-1 suppressor57. Zhang and Braun74 showed in vivo that autoimmune encephalomyelitis does not occur in miR-155 deficient mice, but in double knockout Pdcd1-/miR-155- mice the susceptibility to autoimmune disease is restored, accompanied by an increase pro-inflammatory cytokine production and T-cell infiltration. In a clinical study, Sonkoly et al.75 showed that miR-155 is overexpressed in the dermal lesions of patients with atopic dermatitis and this miRNA suppresses CTLA-4 in T-cells. The role of the miR-155-CTLA-4 interaction as an element of the pathogenic chain of autoimmune disease was also proven in allergic asthma, where high miR-155 downregulates CTLA-4 expression and induces T-cell activation76. On the other hand, Huffaker et al.56 underlined the function of miR-155 in immune tolerance, showing that the antitumor immunity of T cells is defective in miR-155 deficient mice, and ICB can restore the immunity in this mouse model. These data show the importance of miR-155 in regulating the interplay between T-cells and self-tissues, including neoplastic tissue, by controlling the expression of checkpoint molecules. Hence, it would be a promising approach to evaluate the expression level of miR-155 in patients treated with ICB that present irAEs and establish if high miR-155 expression level is a suitable biomarker for the onset of irAEs.
One of the limitations of the ICB therapy is the appearance of irAEs, which often leads to the interruption of the treatment. Interestingly, the miRNAs that are deregulated in autoimmune diseases show an opposite expression pattern in vivo studies and clinical samples of patients who present immune tolerance. This being a supplementary argument that confirms that the irAEs are a phenomenon similar to autoimmunity. We consider that a good understanding of the function of miRNAs in autoimmunity and irAEs could lead to a new therapy of the side effects of ICB. High miR-155 is frequently associated with autoimmunity, during the ICB we can hypothesize that miR-155 is overexpressed because its targets are downregulated. The role of miR-155 is not strictly depended on the immune checkpoint receptors and its overexpression will lead to an augmentation of the irAEs. Hence we believe that by manipulating the miRNAs that coordinate the side effects, the clinician will not be obligated to interrupt the therapy with immune checkpoint inhibitors in case of irAEs, he will be able to control the undesirable effects. Additionally, one can presume that in the near future by analyzing the expression of miRNAs, clinicians could be able to recognize and promptly tackle irAEs, but further preclinical studies are necessary in order to implement this strategy in the clinical arena.
Absence of response to immune checkpoint therapy
Optimistic is not the percentage of patients who can benefit from ICB, which is relatively modest, but the very low mortality rate of those who respond. Analyzing the survival curve of melanoma patients treated with ipilimumab one can observe a plateau after 3 years of follow up, but only an approximate 20% of treated patients reach this point77. Patients who respond appear to be cured. Therefore, finding new methods to increase the response rate of patients to ICB is highly necessary. We hypothesize that a miRNA therapy combined with ICB could increase the efficiency of the established monotherapeutic approach. The miRNA therapy is classified in miRNA mimetics (overexpression of a specific suppressor miRNA) and miRNA inhibitors (blocking the expression of a specific oncogenic miRNA)78. The great advantage of using a miRNA based therapy is the capacity of these short transcripts to target multiple molecules involved in the same pathway or from different pathways with synergistic global effect (e.g. immune inhibition). Therefore, choosing miRNAs that target multiple immune checkpoint molecules is the best strategy.
Xu et al.26 demonstrate that, in ovarian cancer tumors, the expression of miR-424 is negatively associated with the level of PD-L1 and CD80 (the receptor for CTLA-4) and that a high expression of this miRNA is correlated with progression-free survival. They showed that restoration of miR-424 levels leads to a T cell activation and reverses chemoresistance. Therefore, adding miR-424 mimetics to the ICB has the potential to increase the therapeutic efficiency of immunotherapy and chemotherapy.
Another miRNA capable to target two distinct immune checkpoints is miR-138. MiR-138 binds to the 3’UTR of PD-1 and CTLA-4 and downregulates the expression of these checkpoints in vitro and in vivo. Furthermore, miR-138 treatment activated the T-cells and consequently increased the survival of immune competent glioma mice with 43%. As expected the results were not reproducible in nude immunocompromised mice33. Zhao et al.34 underlined the importance of miR-138-5p in CRC. Using CRC samples and corresponding adjacent normal tissues they observed that the expression of miR-138-5p is inversely associated with that of PD-L1, where low miR-138-5p and high PD-L1 predict shorter overall survival. Another miRNA with high therapeutic potential in the context of ICB, is miR-28. MiR-28 is an important hub of the tumor immune evasion regulatory network, being capable to inhibit multiple immune checkpoints. In vitro studies demonstrated that miR-28 mimics are capable to decrease the expression of PD-1 while miR-28 inhibition leads to an increases expression of PD-1, TIM3 and BTLA35. Future in vivo and clinical studies are necessary to prove the usefulness of miR-28 mimics as an additive therapy for ICB.
Because only a subset of patients respond to ICB and using combinations of immune checkpoint molecules increases also the number and gravity of adverse effects, we consider it is highly necessary to find new approaches to augment the response to ICB. One possible class of molecules which are suitable to increase the efficiency of ICB are miRNAs. The best miRNAs for this job are the ones who can target multiple immune checkpoints simultaneously, mimicking a multi-checkpoint blockade. Because physiologically miRNAs lead to a modest downregulation of their target and because high levels of exogenously administrated miRNAs can trigger immunologic side effects (citation), we consider that a miRNA monotherapy is not a good option. The best solution in the case of the non-responders to ICB would be the addition of high physiological levels of miRNA based therapy to the already approved immune checkpoint treatment. We hypothesize that such an approach could boost the immune response against the treatment and convert non-responders to responders.
Final remarks
The molecular regulatory network we describe is far from complete. There are at least two other layers of complexity which are not explored yet and need to be further researched. In this review we presented miRNAs as regulatory elements of the immune checkpoints expression. Most probably the network contains also non-coding RNAs, [i.e. long non-coding RNAs (lncRNAs), circular RNAs] which add a supplementary level of regulation to the network. To our knowledge there is only a study reporting the role of lncRNAs in tumor immune evasion. Tang et al.79 show that the level of the lncRNA actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1) is positively correlated with that of PD-1 in nasopharyngeal cancer tissues, but the study lacks any mechanistic details regarding the interaction of the two molecules. In order to have a comprehensive understanding of regulatory network of immune checkpoints future research should also be directed towards describing the role of lncRNAs and also other types of ncRNAs in immune tolerance.
It is well known that ncRNAs80, especially miRNAs travel via exosomes in the tumor microenvironment and change the phenotype of neighboring cells81. Therefore, it is crucial to evaluate if the miRNAs that control the expression of immune checkpoints are transcribed in the same cell where they perform their function or are imported from neighboring cells. Finding out that the tumor tissue secrets exosomes containing miRNAs capable to modulate the immune response would bring new insights to the mechanism of neoplastic immune tolerance. These details would help improve the design of future therapies.
In conclusion, numerous studies describe miRNAs as key regulatory elements of tumor immune evasion by changing the expression of immune checkpoints. These miRNAs build an intricate network that partially controls the immune response via immune checkpoints against the tumor cells. We propose that the miRNAs can be used to predict and evaluate the response of ICB, control irAEs and potentiate the effect of the immune checkpoint inhibitors.
Acknowledgments
This work is supported by National Institutes of Health (NIH/NCATS) grant UH3TR00943-01 through the NIH Common Fund, Office of Strategic Coordination (OSC), the NIH/NCI grant 1 R01 CA182905-01, a U54 grant—UPR/MDACC Partnership for Excellence in Cancer Research 2016 Pilot Project, a Team DOD (Grant No. CA160445P1) grant, a Ladies Leukemia League grant, a CLL Moonshot Flagship project, a SINF 2017 grant, and the Estate of C. G. Johnson, Jr. The work of Mihnea Dragomir is supported by a POC grant, entitled “Clinical and economical impact of personalized targeted anti-microRNA therapies in reconverting lung cancer chemoresistance”—CANTEMIR, Competitively Operational Program, 2014–2020, Grant No. 35/01.09.2016, MySMIS 103375. The authors want to thank Diana Gulei for the helpful discussions during the preparation of the manuscript.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced Squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. FDA approves new, targeted treatment for bladder cancer. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm.
- 8.U.S. Food and Drug Administration. Atezolizumab (TECENTRIQ). https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525780.htm.
- 9.U.S. Food and Drug Administration. FDA approves first treatment for rare form of skin cancer. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm548278.htm.
- 10.U.S. Food and Drug Administration. Durvalumab (Imfinzi). https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555930.htm.
- 11.U.S. Food and Drug Administration. FDA approves first cancer treatment for any solid tumor with a specific genetic feature. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htm.
- 12.Bristol-Myers-Squibb. Bristol-myers squibb receives FDA approval for opdivo (nivolumab) in previously treated locally advanced or metastatic urothelial carcinoma, a type of bladder cancer. https://news.bms.com/press-release/bladdercancer/bristol-myers-squibb-receives-fda-approval-opdivo-nivolumab-previously-t.
- 13.Bullrich F, Fujii H, Calin G, Mabuchi H, Negrini M, Pekarsky Y, et al. Characterization of the 13q14 tumor suppressor locus in CLL: identification of ALT1, an alternative splice variant of the LEU2 gene . Cancer Res. 2001;61:6640–8. [PubMed] [Google Scholar]
- 14.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia . Proc Natl Acad Sci USA. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci. 2011;121:141–58. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 18.Hesse M, Arenz C. MicroRNA maturation and human disease. In: Arenz C. miRNA Maturation: Methods and Protocols. Totowa, NJ: Humana Press. 2014; 1095: 11-25.
- 19.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasilescu C, Dragomir M, Tanase M, Giza D, Purnichescu-Purtan R, Chen M, et al. Circulating miRNAs in sepsis-A network under attack: An in-silico prediction of the potential existence of miRNA sponges in sepsis . PLoS One. 2017;12:e0183334. doi: 10.1371/journal.pone.0183334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, et al. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010;20:589–99. doi: 10.1101/gr.098046.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smolle MA, Calin HN, Pichler M, Calin GA. Noncoding RNAs and immune checkpoints-clinical implications as cancer therapeutics. FEBS J. 2017;284:1952–66. doi: 10.1111/febs.14030. [DOI] [PubMed] [Google Scholar]
- 23.Grenda A, Krawczyk P. New dancing couple: PD-L1 and MicroRNA. Scand J Immunol. 2017;86:130–4. doi: 10.1111/sji.12577. [DOI] [PubMed] [Google Scholar]
- 24.Giza DE, Vasilescu C, Calin GA. MicroRNAs and ceRNAs: therapeutic implications of RNA networks. Expert Opin Biol Ther. 2014;14:1285–93. doi: 10.1517/14712598.2014.920812. [DOI] [PubMed] [Google Scholar]
- 25.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu SH, Tao Z, Hai B, Liang HG, Shi Y, Wang T, et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun. 2016;7:11406. doi: 10.1038/ncomms11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–90. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, et al. The Tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang XW, Zhou TT, Xiao Y, Yu JH, Dou SJ, Chen GJ, et al. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology. 2016;5:e1211219. doi: 10.1080/2162402X.2016.1211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng CY, Nguyen T, Shively JE. miRNA-342 regulates CEACAM1-induced lumen formation in a three-dimensional model of mammary gland morphogenesis. J Biol Chem. 2016;291:16777–86. doi: 10.1074/jbc.M115.710152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadokoro T, Fujihara S, Chiyo T, Oura K, Samukawa E, Yamana Y, et al. Induction of apoptosis by Galectin-9 in liver metastatic cancer cells: in vitro study . Int J Oncol. 2017;51:607–14. doi: 10.3892/ijo.2017.4053. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Liu RH, Deng YT, Qian JW, Lu Z, Wang YD, et al. MiR-15a/16 deficiency enhances anti-tumor immunity of glioma-infiltrating CD8+ T cells through targeting mTOR . Int J Cancer. 2017;141:2082–92. doi: 10.1002/ijc.30912. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu S, Gabrusiewicz K, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2016;18:639–48. doi: 10.1093/neuonc/nov292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L, Yu HB, Yi SJ, Peng XW, Su P, Xiao ZM, et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370–84. doi: 10.18632/oncotarget.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Johnston N, Zheng XF, Wang HM, Zhang XS, Gao D, et al. miR-28 modulates exhaustive differentiation of T cells through silencing programmed cell death-1 and regulating cytokine secretion. Oncotarget. 2016;7:53735–50. doi: 10.18632/oncotarget.10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torphy RJ, Schulick RD, Zhu YW. Newly emerging immune checkpoints: promises for future cancer therapy. Int J Mol Sci. 2017;18:2642. doi: 10.3390/ijms18122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YA, Nie W, Jin Y, Zhuo AS, Zang YS, Xiu QY. B and T lymphocyte attenuator is a target of miR-155 during naive CD4+ T cell activation . Iran J Immunol. 2016;13:89–99. [PubMed] [Google Scholar]
- 38.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125:3335–46. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nygren MK, Tekle C, Ingebrigtsen VA, Mäkelä R, Krohn M, Aure MR, et al. Identifying microRNAs regulating B7-H3 in breast cancer: the clinical impact of microRNA-29c. Br J Cancer. 2014;110:2072–80. doi: 10.1038/bjc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 41.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang WP, Sun J, Li F, Li R, Gu YP, Liu CP, et al. A frequent somatic mutation in CD274 3'-UTR leads to protein over-expression in gastric cancer by disrupting miR-570 binding . Hum Mutat. 2012;33:480–4. doi: 10.1002/humu.22014. [DOI] [PubMed] [Google Scholar]
- 43.Cortez MA, Ivan C, Valdecanas D, Wang XH, Peltier HJ, Ye YP, et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108:djv303. doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Li JG, Dong K, Lin F, Long M, Ouyang YR, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27:443–52. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Pyzer AR, Stroopinsky D, Rosenblatt J, Anastasiadou E, Rajabi H, Washington A, et al. MUC1 inhibition leads to decrease in PD-L1 levels via upregulation of miRNAs. Leukemia. 2017;31:2780–90. doi: 10.1038/leu.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita Y, Yagishita S, Hagiwara K, Yoshioka Y, Kosaka N, Takeshita F, et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther. 2015;23:717–27. doi: 10.1038/mt.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu GL, Wright KL, Ma YH, Wright GM, Huang M, Irby R, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–40. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao SC, Cheng YY, Williams M, Kirschner MB, Madore J, Lum T, et al. Tumor suppressor microRNAs contribute to the regulation of PD-L1 expression in malignant pleural mesothelioma. J Thorac Oncol. 2017;12:1421–33. doi: 10.1016/j.jtho.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Chen LM, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu JJ, Chen LX, Zou LT, Yang PP, Wu RR, Mao Y, et al. MiR-20b, -21, and-130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol. 2014;75:348–53. doi: 10.1016/j.humimm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Zou MX, Guo KM, Lv GH, Huang W, Li J, Wang XB, et al. Clinicopathologic implications of CD8+/Foxp3+ ratio and miR-574-3p/PD-L1 axis in spinal chordoma patients . Cancer Immunol Immunother. 2018;67:209–24. doi: 10.1007/s00262-017-2080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn H, Yang JM, Kim H, Chung JH, Ahn SH, Jeong WJ, et al. Clinicopathologic implications of the miR-197/PD-L1 axis in oral squamous cell carcinoma. Oncotarget. 2017;8:66178–94. doi: 10.18632/oncotarget.19842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cioffi M, Trabulo SM, Vallespinos M, Raj D, Kheir TB, Lin ML, et al. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget. 2017;8:21609–25. doi: 10.18632/oncotarget.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandya D, Mariani M, He SQ, Andreoli M, Spennato M, Dowell-Martino C, et al. Epstein-barr virus MicroRNA expression increases aggressiveness of solid malignancies. PLoS One. 2015;10:e0136058. doi: 10.1371/journal.pone.0136058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, et al. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016;18:790–802. doi: 10.1038/ncb3371. [DOI] [PubMed] [Google Scholar]
- 56.Huffaker TB, Lee SH, Tang WW, Wallace JA, Alexander M, Runtsch MC, et al. Antitumor immunity is defective in T cell-specific microRNA-155-deficient mice and is rescued by immune checkpoint blockade. J Biol Chem. 2017;292:18530–41. doi: 10.1074/jbc.M117.808121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yee D, Shah KM, Coles MC, Sharp TV, Lagos D. MicroRNA-155 induction via TNF-α and IFN-γ suppresses expression of programmed death ligand-1 (PD-L1) in human primary cells. J Biol Chem. 2017;292:20683–93. doi: 10.1074/jbc.M117.809053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong AY, Zhou R, Hu GK, Li XQ, Splinter PL, O'Hara SP, et al. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-γ-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182:1325–33. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu L, Chen Z, Zhang J, Xing YQ. Effect of miR-513a-5p on etoposide-stimulating B7-H1 expression in retinoblastoma cells. J Huazhong Univ Sci Technol. 2012;32:601–6. doi: 10.1007/s11596-012-1004-8. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 61.Hopkins AM, Rowland A, Kichenadasse G, Wiese MD, Gurney H, McKinnon RA, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117:913–20. doi: 10.1038/bjc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–68. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 64.Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol. 2015;84:1259–68. doi: 10.1016/j.ejrad.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.U.S. Food and Drug Administration. Pembrolizumab (KEYTRUDA) checkpoint inhibitor. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm526430.htm.
- 67.Audrito V, Serra S, Stingi A, Orso F, Gaudino F, Bologna C, et al. PD-L1 up-regulation in melanoma increases disease aggressiveness and is mediated through miR-17-5p. Oncotarget. 2017;8:15894–911. doi: 10.18632/oncotarget.15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–53. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 69.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 70.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hodi FS, Postow MA, Chesney JA, Pavlick AC, Robert C, Grossmann KF, et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. J Clin Oncol. 2015;33:9004. [Google Scholar]
- 72.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–75. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 73.Sarnaik AA, Yu B, Yu DH, Morelli D, Hall M, Bogle D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang JY, Braun MY. PD-1 deletion restores susceptibility to experimental autoimmune encephalomyelitis in miR-155-deficient mice. Int Immunol. 2014;26:407–15. doi: 10.1093/intimm/dxu043. [DOI] [PubMed] [Google Scholar]
- 75.Sonkoly E, Janson P, Majuri ML, Savinko T, Fyhrquist N, Eidsmo L, et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 2010;126:581–9. doi: 10.1016/j.jaci.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 76.Zhang YY, Sun ET, Li XQ, Zhang M, Y Tang ZS, He L, et al. miR-155 contributes to Df1-induced asthma by increasing the proliferative response of Th cells via CTLA-4 downregulation. Cell Immunol. 2017;314:1–9. doi: 10.1016/j.cellimm.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–6. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–65. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang YY, He Y, Shi L, Yang LT, Wang JP, Lian Y, et al. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:39001–11. doi: 10.18632/oncotarget.16545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dragomir M, Chen BQ, Calin GA. Exosomal lncRNAs as new players in cell-to-cell communication. Transl Cancer Res. 2017;7:S243–52. doi: 10.21037/tcr.2017.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ. 2015;22:34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]