Abstract
Revision hip arthroplasty in the setting of periacetabular bone loss presents a significant challenge, as options for restoring bone loss are limited. Recombinant human bone morphogenetic protein-2 may offer a solution by promoting bone growth to restore bone stock before implant reimplantation. Here we present a case of a patient with a periprosthetic acetabulum fracture, resulting in pelvic discontinuity as the result of significant periacetabular bone loss. Using a staged approach, periacetabular bone stock was nearly entirely reconstituted using recombinant BMPs and allograft, which resulted in stable fixation, but with abundant heterotopic bone formation. Recombinant BMP-2 offers a useful tool for restoring bone stock in complex hip arthroplasty revision cases with periacetabular bone loss; however, caution must be used as overabundant bone growth as heterotopic ossification may result.
Keywords: Revision arthroplasty, Bone defects, rhBMP-2
Introduction
Periacetabular bone loss in revision hip arthroplasty presents a significant challenge, with limited options for reconstruction. Here we present a case where recombinant human bone morphogenetic protein (rhBMP)-2 was utilized to promote bone reconstitution, however was associated with abundant heterotopic ossification.
Case history
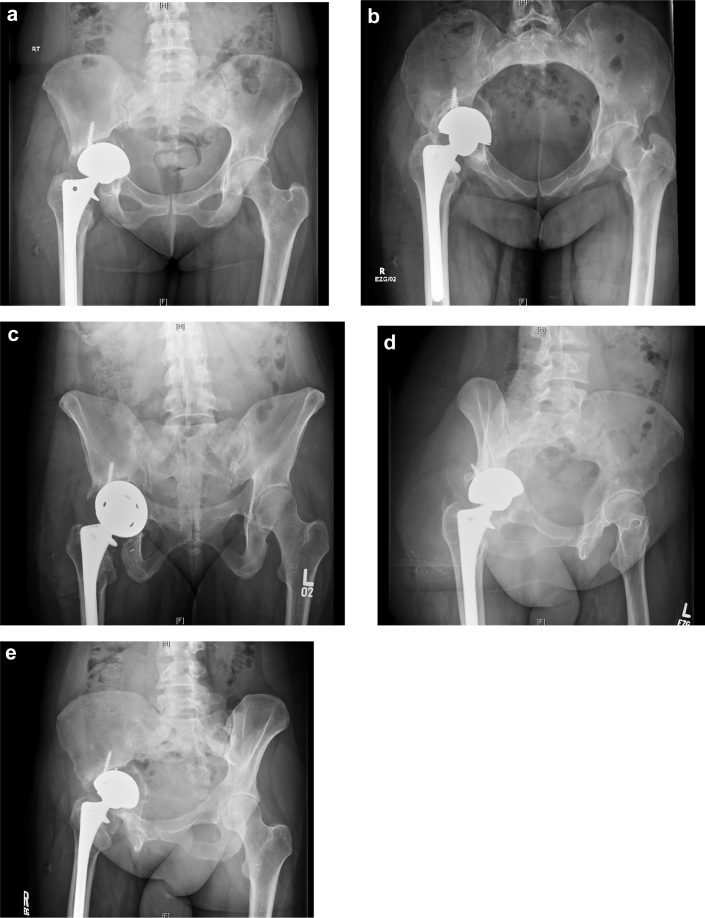
A 42-year-old woman with rheumatoid arthritis on prednisone presented to the emergency department with severe right hip pain and inability to bear weight after sustaining a twisting injury while stepping off a curb. She previously had a right total hip arthroplasty performed 20 years ago and revised 1 year prior for loosening. She reported never having a pain-free interval after her revision. Physical examination revealed significant pain with any right hip range of motion and inability to perform active straight leg raise. Radiographs revealed a right transverse periprosthetic acetabulum fracture with an acetabular component that was grossly loose and protruded. Extensive osteolysis and bone loss were noted in both the ischium and ilium (Fig. 1). Laboratory workup at that time yielded erythrocyte sedimentation rate and C-reactive protein levels that were within normal limits. Hip aspiration was also performed, which was negative for infection.
Figure 1.
Preoperative pelvic radiographs. Anteroposterior (AP) (a), inlet (b), outlet (c), obturator oblique (d), and iliac oblique (e) images.
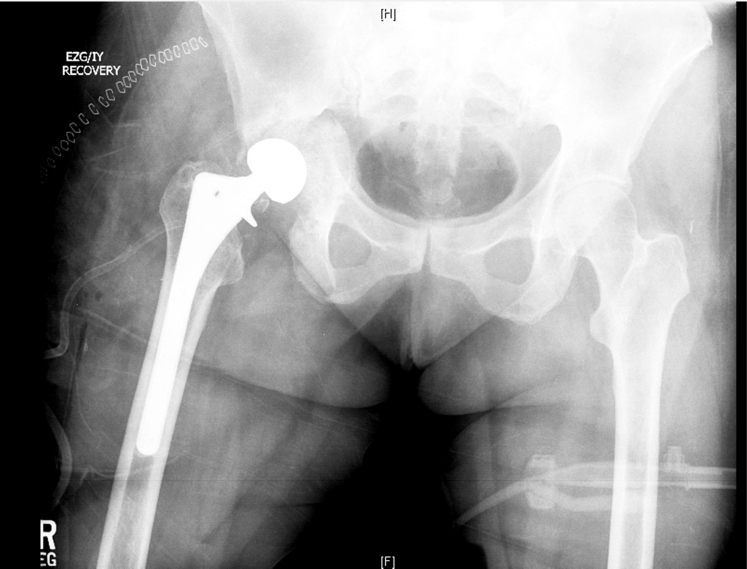
After discussion with the patient, the decision was made to proceed with acetabular component revision for aseptic loosening. Prednisone was held before her surgery. At the time of surgery, she was found to have a grossly loose acetabular component with a complex pelvic discontinuity involving transverse fractures through the anterior and posterior columns as well as a large segmental posterior wall fragment. There was significant osteolysis, which precluded stable fixation of an immediate revision acetabular component. The decision was made to perform the revision in a staged fashion because the extent of bone loss would have limited bony contact (<30%) with the implant as well as insufficient screw fixation. In addition, there was concern for the ability to achieve bony union of the discontinuity, given her history of rheumatoid arthritis. A 2.8-cc dose of rhBMP-2 and 10 mL of demineralized bone matrix were placed overlying the discontinuity site and 150 cc of allograft bone chips within the acetabulum. The femoral component was retained. (Fig. 2) She remained toe-touch weight-bearing postoperatively.
Figure 2.
Postoperative radiograph taken immediately after the first staged surgery.
The patient underwent the second stage of her revision 7 months later.
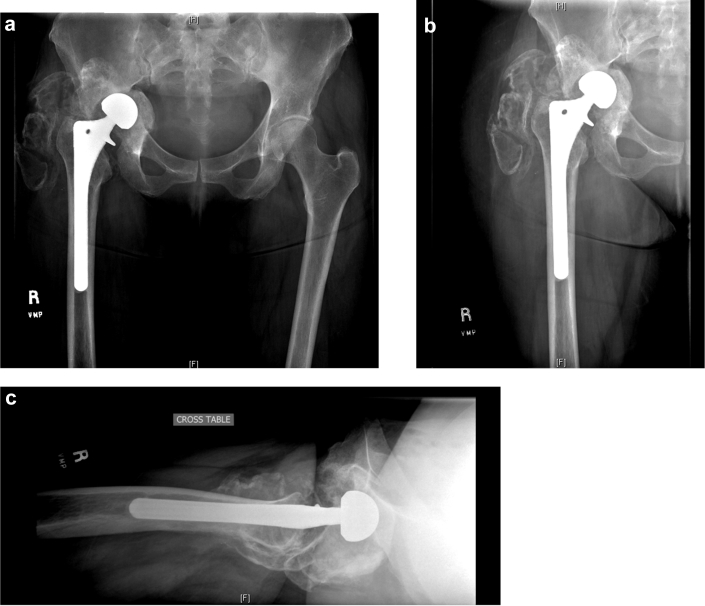
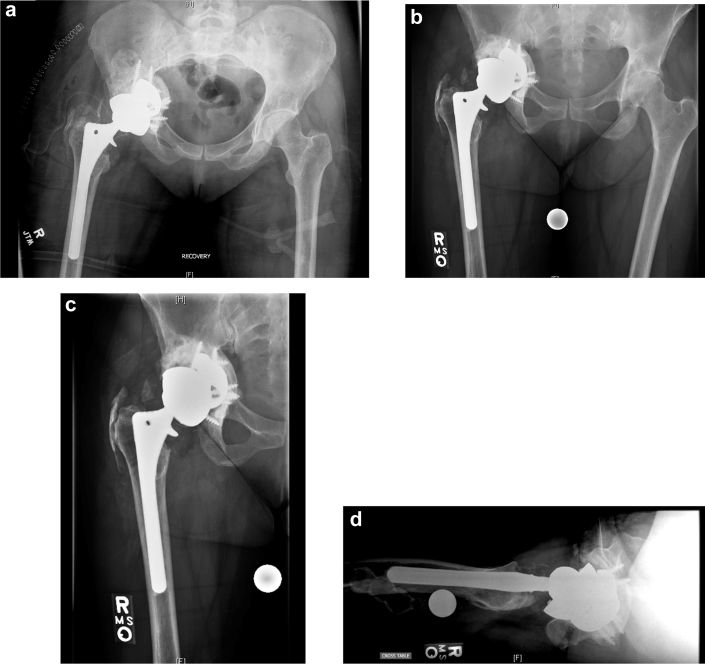
Intraoperatively, she was found to have completely healed the pelvic discontinuity with reconstitution of the entire acetabulum with restoration of the anterior and posterior columns as well as dome and medial wall. Furthermore, an abundance of heterotopic ossification (HO) had also formed around the acetabulum, which was excised. (Fig. 3) An acetabular component with trabecular metal augments and 5 screws for fixation were placed and supplemented with autograft from the excised HO. (Fig. 4)
Figure 3.
Two months after explant and bone grafting postoperative radiographs of the pelvis (a), hip AP (b), and lateral (c).
Figure 4.
Radiographs taken after the second stage of the procedure. Immediately postoperative (a), 8 months postoperative AP pelvis (b), AP hip (c), and lateral hip (d).
The patient had an uneventful perioperative recovery and was discharged from the hospital. By 4 months postoperatively, her pain had nearly resolved, incisions were healed, and radiographs demonstrated early osteointegration of the acetabular augment and implant. At 2-year follow-up, she was doing very well without pain and walking without limp.
Discussion
Basic science
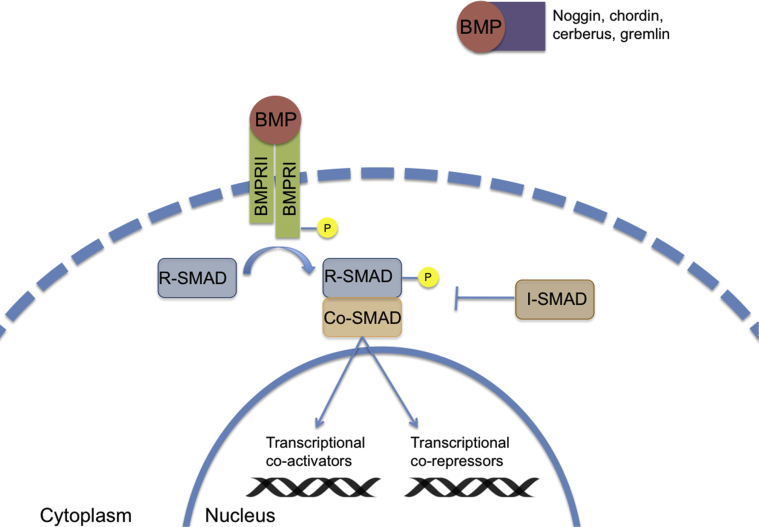
Bone morphogenetic proteins (BMPs) were discovered in 1965 when implantation of demineralized bone matrix led to new bone formation, suggesting unknown local factors resulted in osteogenesis by autoinduction [1]. BMPs are now understood to be members of the TGF-β supergene family and play a crucial role in the differentiation of the osteogenic lineage and fracture repair [2]. Osteogenic BMPs, particularly BMP-2 and BMP-7, function through tyrosine-kinase receptors to activate the Smad protein-signaling pathway (Fig. 5). This, in-turn, activates gene transcription factors to differentiate mesenchymal cells into osteogenic and chondrogenic phenotypes [2], [3], [4], [5]. Induced bone formation occurs because a chemical gradient leads to chemotaxis of chondrogenic and osteogenic cells, followed by vascular invasion and cell differentiation [6], [7]. In addition to its primary function in regulating bone tissues, the BMP cascade also functions to regulate cell growth, movement, and homeostasis [8], [9]. During fracture healing, BMP subtype expression occurs in a specific temporal fashion to regulate cartilage calcification and osteoblast recruitment [10].
Figure 5.
BMP gene transcription pathway.
Two BMP subtypes have been approved by the US Food and Drug administration (FDA) for clinical use in humans: rhBMP-7 (also known as osteogenic protein-1, initially distributed by Stryker Biotech, Hopkinton, MA, but now not available in the United States) and rhBMP-2 (INFUSE, Medtronic, Inc. Fridley, MN) [11]. rhBMP-2 is approved by the US FDA for 3 specific indications: 1. Treatment of lumbar interbody fusion using metallic cage, rhBMP-2, and collagen sponge carrier; 2. Treatment of open tibial shaft fractures treated with intramedullary nail fixation; and 3. As an alternative to autogenous bone graft for sinus augmentation and localized alveolar ridge augmentation for defects associated with extraction sockets.
Multiple animal studies have demonstrated that rhBMP-2 leads to local bone and cartilage formation [7] and has a time- and dosage-dependent effect to accelerate cartilage formation and bone mineralization [7], [12]. BMPs improve bony ingrowth into porous metal implants and lead to more rapid initial callus formation, maturation, and greater early torsional strength in animal models [13], [14], [15], [16], [17].
Clinical utilization of BMPs
Advantages of BMPs include the potential to achieve similar union rates as cancellous autograft but with decreased rates of donor site pain [18], [19], [20]. In the spine literature, initial reports found that BMPs provide comparable posterolateral fusion rates as local autograft [21], [22], [23], [24], [25], [26]. A randomized study by Hurlbert et al [27] found that patients receiving rhBMP-2 demonstrated a higher rate of radiographic fusion than those who received autograft, but found no differences in clinical outcomes. Michielsen et al [28] found analogous fusion rates in patients receiving either rhBMP-2 or autologous iliac crest graft. In the trauma literature, Govender et al [29] randomized 450 patients with open tibia fractures to intramedullary nail fixation with or without rhBMP-2. Though patients who received high-dose rhBMP-2 required fewer secondary interventions and demonstrated accelerated fracture healing, intramedullary reaming was also performed more frequently in the BMP group. In patients treated with reamed intramedullary nails for open tibial shaft fractures. Lyon et al [30] randomized patients with closed tibial shaft fractures to reamed, locked intramedullary nails with or without rhBMP-2. No differences in infection rates, time to radiographic union or pain-free weight-bearing was noted; however, patients receiving rhBMP-2 had increased rates of venous thromboembolism and postoperative HO.
As the clinical usage of BMPs has increased, its safety has been called into question [31], [32]. Carragee et al initially reported concerns, pointing out that many initial trials were sponsored by industry and may have under-reported adverse events [19], [32], [33], [34]. In some studies, BMPs have shown no advantages over autograft and may lead to greater risk of complications such as osteolysis and bony resorption, ectopic bone formation, retrograde ejaculation, wound complications, life-threatening cervical soft-tissue swelling, and nerve root radiculitis [34], [35], [36], [37]. Caution should be taken during placement of BMPs because displacement or migration has led to ectopic bone formation, which can potentially affect nearby critical structures [38]. Through December 2011, the FDA received 62 reports of adverse events following rhBMP-2 use in nonspinal applications, including wound complications, HO formation, and local inflammation [39].
Though concerns of BMPs as a pro-oncogenic protein have been raised, a large study using the Medicare database found no increased risk of malignancy [40].
BMPs have been studied in the treatment of acetabular bone defects due to osteolysis, as the alternatives of bone allograft or autograft have not reliably produced bony ingrowth in a porous implant [41], [42]. Bragdon et al implanted porous-coated titanium acetabular components after creating central acetabular defects in a canine model. Those receiving rhBMP-2 had near-complete bony bridging, whereas defects in the control group remained empty with fibrous tissue [43]. Hoshino et al [44] performed total hip arthroplasty after creating proximal femur and acetabular defects in a canine model. Hips receiving rhBMP-2 demonstrated complete radiographic healing of both femoral and acetabular defects, as compared to none in groups receiving carrier only. Histologic examination of hips receiving rhBMP-2 demonstrated bony ingrowth of woven bone and fibrous tissue compared to minimal ingrowth and persistent bony defect in the control group. Barrack et al [45] created central acetabular defects in a canine model and performed porous-coated titanium total hip arthroplasties. Hips treated with rhBMP-7 had more complete radiographic bony healing, greater histologic new bone formation, and increased ingrowth into the porous metal implant. Hips receiving rhBMP-7 had even greater bony ingrowth into the implant than hips treated without an acetabular defect. Jensen et al found the addition of rhBMP-7 increased bone formation and mechanical stability of a hydroxyapatite-coated implant in a canine model [46], [47].
Cook et al reported a clinical case where rhBMP-7 was used with femoral head allograft to successfully reconstruct a large acetabular defect [48], [49]. Similarly, Jager et al reported the successful usage of rhBMP-2 in a staged fashion to bridge a large acetabular defect [50]. Kärrholm et al [51] published a retrospective case–control series investigating the use of rhBMP-7 for bone loss in acetabular revision. Impaction grafting using morselized femoral head allograft in conjunction with 3.5 mg of rhBMP-7 was performed. Control cases were matched for age, gender, type of defect, type of implant, and volume of allograft used. The authors found no differences in Harris hip score or reported pain between groups, but 2 patients in the BMP group required acetabular revision for loosening and proximal component migration.
Heterotopic ossification
We present a case where the therapeutic use of recombinant BMPs was associated with HO [52], [53]. Conditions such as fibrodysplasia ossificans progressiva, where mRNA expression of BMP-4 is upregulated, and progressive osseous heteroplasia have shed light on the pathways for ectopic bone formation [54], [55]. Current animal models of HO are based on the surgical implantation of BMPs, which induce heterotopic bone formation through endochondral ossification. rhBMP has been shown to create ectopic bone when implanted in subcutaneous tissue in a dose-dependent fashion [7], [56], [57]. Though the exact mechanism of HO remains unknown, 3 key phases for its development have been identified: 1. Inductive signaling pathways, 2. Inducible osteoprogenitor cells, and 3. A microenvironment conducive to osteogenesis [52].
Rates of significant HO formation following total hip arthroplasty ranges from 1% to 27% and is associated with male gender, advanced patient age, increased soft-tissue trauma, and hematoma formation [58], [59], [60]. Fracture morphology, early timing of surgery, and associated abdominal and thoracic injuries correlate with the development of heterotopic bone formation following acetabular surgery [61], [62]. Both nonsteroidal anti-inflammatory medications and perioperative radiation therapy have been used for HO prophylaxis following major hip surgery; randomized trials have shown both to be effective [60], [63], [64], [65]. Though some reviews contend radiation as superior [66], a recent meta-analysis of more than 1200 patients found similar efficacy between the 2 treatment modalities in preventing heterotopic bone formation [67]. Patient compliance in completing 6 weeks of nonsteroidal anti-inflammatory medication prophylaxis are thought to contribute to differences seen between the 2 treatment methods [68], [69].
Other reports have been published describing heterotopic bone formation following the therapeutic use of rhBMPs. In a randomized trial, Haid et al reported that the use of rhBMP-2 in lumbar interbody cages was associated with new bone formation into the spinal canal or neuroforamina in 71% patients [70], [71]. Wysocki and Cohen reported pathologic bone formation in the triceps musculature following rhBMP-7 use when treating a distal humerus fracture nonunion [72]. Axelrad et al reported 4 cases of substantial heterotopic bone formation in the upper extremity following the use of rhBMPs for fractures [73]. Boraiah et al reported heterotopic bone formation in 59% of patients when rhBMP-2 was used during surgical treatment of acute tibial plateau fractures [74].
Summary
Management of periacetabular bone loss presents a challenge during total hip arthroplasty; we present a case of staged acetabular revision using recombinant BMPs and allograft to effectively restore bone stock, followed by implantation of an acetabular component with trabecular metal augments, leading to stable fixation and satisfactory clinical results at 1 year postoperatively. However, abundant heterotopic bone formation was noted within the soft tissues around the hip after the first stage and thus we urge caution when using recombinant BMPs in this application.
Footnotes
One or more of the authors of this article have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field, which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2017.12.004.
Appendix A. Supplementary data
References
- 1.Urist M.R. Bone: formation by autoinduction. Science. 1965;150(3698):893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Ebara S., Nakayama K. Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S10. doi: 10.1097/00007632-200208151-00004. [DOI] [PubMed] [Google Scholar]
- 3.ten Dijke P., Fu J., Schaap P., Roelen B.A. Signal transduction of bone morphogenetic proteins in osteoblast differentiation. J Bone Joint Surg Am. 2003;85-A Suppl 3:34. doi: 10.2106/00004623-200300003-00007. [DOI] [PubMed] [Google Scholar]
- 4.Sampath T.K., Reddi A.H. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983;80(21):6591. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wozney J.M., Rosen V., Celeste A.J. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 6.Reddi A.H. Initiation of fracture repair by bone morphogenetic proteins. Clin Orthop Relat Res. 1998;(355 Suppl):S66. doi: 10.1097/00003086-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wang E.A., Rosen V., D'Alessandro J.S. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87(6):2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D., Zhao M., Mundy G.R. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 9.Wozney J.M., Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;(346):26. [PubMed] [Google Scholar]
- 10.Cho T.J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 11.Corporation O. http://www.olympus-global.com/en/news/2010b/nr101207op1e.jsp [accessed 22.11.14].
- 12.Chen D., Harris M.A., Rossini G. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60(3):283. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 13.Saran N., Zhang R., Turcotte R.E. Osteogenic protein-1 delivered by hydroxyapatite-coated implants improves bone ingrowth in extracortical bone bridging. Clin Orthop Relat Res. 2011;469(5):1470. doi: 10.1007/s11999-010-1573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumner D.R., Turner T.M., Urban R.M., Turek T., Seeherman H., Wozney J.M. Locally delivered rhBMP-2 enhances bone ingrowth and gap healing in a canine model. J Orthop Res. 2004;22(1):58. doi: 10.1016/S0736-0266(03)00127-X. [DOI] [PubMed] [Google Scholar]
- 15.Murakami N., Saito N., Horiuchi H., Okada T., Nozaki K., Takaoka K. Repair of segmental defects in rabbit humeri with titanium fiber mesh cylinders containing recombinant human bone morphogenetic protein-2 (rhBMP-2) and a synthetic polymer. J Biomed Mater Res. 2002;62(2):169. doi: 10.1002/jbm.10236. [DOI] [PubMed] [Google Scholar]
- 16.Bouxsein M.L., Turek T.J., Blake C.A. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83-A(8):1219. doi: 10.2106/00004623-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Li R.H., Bouxsein M.L., Blake C.A. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res. 2003;21(6):997. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 18.Friedlaender G.E., Perry C.R., Cole J.D. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S151. [PMC free article] [PubMed] [Google Scholar]
- 19.Jones A.L., Bucholz R.W., Bosse M.J. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(7):1431. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 20.Garrison K.R., Shemilt I., Donell S. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;(6):CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanayama M., Hashimoto T., Shigenobu K., Yamane S., Bauer T.W., Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine (Phila Pa 1976) 2006;31(10):1067. doi: 10.1097/01.brs.0000216444.01888.21. [DOI] [PubMed] [Google Scholar]
- 22.Johnsson R., Strömqvist B., Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2654. doi: 10.1097/00007632-200212010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Vaccaro A.R., Patel T., Fischgrund J. A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine (Phila Pa 1976) 2004;29(17):1885. doi: 10.1097/01.brs.0000137062.79201.98. [DOI] [PubMed] [Google Scholar]
- 24.Vaccaro A.R., Patel T., Fischgrund J. A 2-year follow-up pilot study evaluating the safety and efficacy of op-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2005;14(7):623. doi: 10.1007/s00586-004-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delawi D., Dhert W.J., Rillardon L. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine (Phila Pa 1976) 2010;35(12):1185. doi: 10.1097/BRS.0b013e3181d3cf28. [DOI] [PubMed] [Google Scholar]
- 26.Dawson E., Bae H.W., Burkus J.K., Stambough J.L., Glassman S.D. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1604. doi: 10.2106/JBJS.G.01157. [DOI] [PubMed] [Google Scholar]
- 27.Hurlbert R.J., Alexander D., Bailey S. rhBMP-2 for posterolateral instrumented lumbar fusion: a multicenter prospective randomized controlled trial. Spine (Phila Pa 1976) 2013;38(25):2139. doi: 10.1097/BRS.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 28.Michielsen J., Sys J., Rigaux A., Bertrand C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J Bone Joint Surg Am. 2013;95(10):873. doi: 10.2106/JBJS.L.00137. [DOI] [PubMed] [Google Scholar]
- 29.Govender S., Csimma C., Genant H.K. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A(12):2123. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Lyon T., Scheele W., Bhandari M. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: a double-blind, randomized, controlled phase-II/III trial. J Bone Joint Surg Am. 2013;95(23):2088. doi: 10.2106/JBJS.L.01545. [DOI] [PubMed] [Google Scholar]
- 31.Fu R., Selph S., McDonagh M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158(12):890. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers M.A., Brown J.V., Heirs M.K. Reporting of industry funded study outcome data: comparison of confidential and published data on the safety and effectiveness of rhBMP-2 for spinal fusion. BMJ. 2013;(346):f3981. doi: 10.1136/bmj.f3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., Ba G., Shen T., Fu Q. Recombinant human bone morphogenetic protein-2 versus autogenous iliac crest bone graft for lumbar fusion: a meta-analysis of ten randomized controlled trials. Arch Orthop Trauma Surg. 2012;132(12):1725. doi: 10.1007/s00402-012-1607-3. [DOI] [PubMed] [Google Scholar]
- 34.Carragee E.J., Hurwitz E.L., Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Villavicencio A.T., Burneikiene S., Nelson E.L., Bulsara K.R., Favors M., Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436. doi: 10.3171/spi.2005.3.6.0436. [DOI] [PubMed] [Google Scholar]
- 36.Vaidya R., Sethi A., Bartol S., Jacobson M., Coe C., Craig J.G. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557. doi: 10.1097/BSD.0b013e31815ea897. [DOI] [PubMed] [Google Scholar]
- 37.Simmonds M.C., Brown J.V., Heirs M.K. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158(12):877. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 38.Poynton A.R., Lane J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S40. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 39.Woo E.J. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin Orthop Relat Res. 2013;471(5):1707. doi: 10.1007/s11999-012-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly M.P., Savage J.W., Bentzen S.M., Hsu W.K., Ellison S.A., Anderson P.A. Cancer risk from bone morphogenetic protein exposure in spinal arthrodesis. J Bone Joint Surg Am. 2014;96(17):1417. doi: 10.2106/JBJS.M.01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook S.D., Barrack R.L., Patron L.P., Salkeld S.L. Osteoinductive agents in reconstructive hip surgery: a look forward. Clin Orthop Relat Res. 2003;(417):195. doi: 10.1097/01.blo.0000096809.78689.b4. [DOI] [PubMed] [Google Scholar]
- 42.Søballe K., Hansen E.S., Brockstedt-Rasmussen H., Pedersen C.M., Bünger C. Bone graft incorporation around titanium-alloy- and hydroxyapatite-coated implants in dogs. Clin Orthop Relat Res. 1992;(274):282. [PubMed] [Google Scholar]
- 43.Bragdon C.R., Doherty A.M., Rubash H.E. The efficacy of BMP-2 to induce bone ingrowth in a total hip replacement model. Clin Orthop Relat Res. 2003;(417):50. doi: 10.1097/01.blo.0000096811.78689.2b. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino M., Namikawa T., Kato M., Terai H., Taguchi S., Takaoka K. Repair of bone defects in revision hip arthroplasty by implantation of a new bone-inducing material comprised of recombinant human BMP-2, Beta-TCP powder, and a biodegradable polymer: an experimental study in dogs. J Orthop Res. 2007;25(8):1042. doi: 10.1002/jor.20424. [DOI] [PubMed] [Google Scholar]
- 45.Barrack R.L., Cook S.D., Patrón L.P., Salkeld S.L., Szuszczewicz E., Whitecloud T.S. Induction of bone ingrowth from acetabular defects to a porous surface with OP-1. Clin Orthop Relat Res. 2003;(417):41. doi: 10.1097/01.blo.0000096808.78689.fd. [DOI] [PubMed] [Google Scholar]
- 46.Jensen T.B., Overgaard S., Lind M., Rahbek O., Bünger C., Søballe K. Osteogenic protein 1 device increases bone formation and bone graft resorption around cementless implants. Acta Orthop Scand. 2002;73(1):31. doi: 10.1080/000164702317281378. [DOI] [PubMed] [Google Scholar]
- 47.Jensen T.B., Overgaard S., Lind M., Rahbek O., Bünger C., Søballe K. Osteogenic protein-1 increases the fixation of implants grafted with morcellised bone allograft and ProOsteon bone substitute: an experimental study in dogs. J Bone Joint Surg Br. 2007;89(1):121. doi: 10.1302/0301-620X.89B1.17077. [DOI] [PubMed] [Google Scholar]
- 48.Cook S.D., Barrack R.L., Santman M., Patron L.P., Salkeld S.L., Whitecloud T.S. The Otto Aufranc Award. Strut allograft healing to the femur with recombinant human osteogenic protein-1. Clin Orthop Relat Res. 2000;(381):47. doi: 10.1097/00003086-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Cook S.D., Barrack R.L., Shimmin A., Morgan D., Carvajal J.P. The use of osteogenic protein-1 in reconstructive surgery of the hip. J Arthroplasty. 2001;16(8 Suppl 1):88. doi: 10.1054/arth.2001.28363. [DOI] [PubMed] [Google Scholar]
- 50.Jäger M., Emami R., Thorey F., Krauspe R. Saving implants BMP-2 application in revision total hip surgery. Int J Biomed Sci. 2006;2(2):187. [PMC free article] [PubMed] [Google Scholar]
- 51.Kärrholm J., Hourigan P., Timperley J., Razaznejad R. Mixing bone graft with OP-1 does not improve cup or stem fixation in revision surgery of the hip: 5-year follow-up of 10 acetabular and 11 femoral study cases and 40 control cases. Acta Orthop. 2006;77(1):39. doi: 10.1080/17453670610045687. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan F.S., Glaser D.L., Hebela N., Shore E.M. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12(2):116. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 53.O'Connor J.P. Animal models of heterotopic ossification. Clin Orthop Relat Res. 1998;(346):71. [PubMed] [Google Scholar]
- 54.Shafritz A.B., Shore E.M., Gannon F.H. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335(8):555. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 55.Shore E.M., Glaser D.L., Gannon F.H. Osteogenic induction in hereditary disorders of heterotopic ossification. Clin Orthop Relat Res. 2000;(374):303. doi: 10.1097/00003086-200005000-00028. [DOI] [PubMed] [Google Scholar]
- 56.Love D.A., Lietman S.A. The effect of osteogenic protein-1 dosing regimen on ectopic bone formation. Clin Orthop Relat Res. 2004;(423):264. doi: 10.1097/01.blo.0000129161.54751.8c. [DOI] [PubMed] [Google Scholar]
- 57.Sampath T.K., Maliakal J.C., Hauschka P.V. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267(28):20352. [PubMed] [Google Scholar]
- 58.Ahrengart L. Periarticular heterotopic ossification after total hip arthroplasty. Risk factors and consequences. Clin Orthop Relat Res. 1991;(263):49. [PubMed] [Google Scholar]
- 59.Tannous O., Stall A.C., Griffith C., Donaldson C.T., Castellani R.J., Pellegrini V.D. Heterotopic bone formation about the hip undergoes endochondral ossification: a rabbit model. Clin Orthop Relat Res. 2013;471(5):1584. doi: 10.1007/s11999-013-2801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Board T.N., Karva A., Board R.E., Gambhir A.K., Porter M.L. The prophylaxis and treatment of heterotopic ossification following lower limb arthroplasty. J Bone Joint Surg Br. 2007;89(4):434. doi: 10.1302/0301-620X.89B4.18845. [DOI] [PubMed] [Google Scholar]
- 61.Ghalambor N., Matta J.M., Bernstein L. Heterotopic ossification following operative treatment of acetabular fracture. An analysis of risk factors. Clin Orthop Relat Res. 1994;(305):96. [PubMed] [Google Scholar]
- 62.Chémaly O., Hebert-Davies J., Rouleau D.M., Benoit B., Laflamme G.Y. Heterotopic ossification following total hip replacement for acetabular fractures. Bone Joint J. 2013;95-B(1):95. doi: 10.1302/0301-620X.95B1.29721. [DOI] [PubMed] [Google Scholar]
- 63.Moore K.D., Goss K., Anglen J.O. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998;80(2):259. doi: 10.1302/0301-620x.80b2.8157. [DOI] [PubMed] [Google Scholar]
- 64.Burd T.A., Lowry K.J., Anglen J.O. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am. 2001;83-A(12):1783. doi: 10.2106/00004623-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Schneider D.J., Moulton M.J., Singapuri K. The Frank Stinchfield Award. Inhibition of heterotopic ossification with radiation therapy in an animal model. Clin Orthop Relat Res. 1998;(355):35. doi: 10.1097/00003086-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Blokhuis T.J., Frölke J.P. Is radiation superior to indomethacin to prevent heterotopic ossification in acetabular fractures?: a systematic review. Clin Orthop Relat Res. 2009;467(2):526. doi: 10.1007/s11999-008-0532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vavken P., Castellani L., Sculco T.P. Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res. 2009;467(12):3283. doi: 10.1007/s11999-009-0924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vielpeau C., Joubert J.M., Hulet C. Naproxen in the prevention of heterotopic ossification after total hip replacement. Clin Orthop Relat Res. 1999;(369):279. doi: 10.1097/00003086-199912000-00029. [DOI] [PubMed] [Google Scholar]
- 69.Gebuhr P., Soelberg M., Orsnes T., Wilbek H. Naproxen prevention of heterotopic ossification after hip arthroplasty. A prospective control study of 55 patients. Acta Orthop Scand. 1991;62(3):226. doi: 10.3109/17453679108993597. [DOI] [PubMed] [Google Scholar]
- 70.McKay B., Sandhu H.S. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S66. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- 71.Haid R.W., Branch C.L., Alexander J.T., Burkus J.K. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527. doi: 10.1016/j.spinee.2004.03.025. discussion 538-9. [DOI] [PubMed] [Google Scholar]
- 72.Wysocki R.W., Cohen M.S. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32(5):647. doi: 10.1016/j.jhsa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Axelrad T.W., Steen B., Lowenberg D.W., Creevy W.R., Einhorn T.A. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90(12):1617. doi: 10.1302/0301-620X.90B12.20975. [DOI] [PubMed] [Google Scholar]
- 74.Boraiah S., Paul O., Hawkes D., Wickham M., Lorich D.G. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467(12):3257. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.