Abstract
Background
Crimean Congo haemorrhagic fever (CCHF) is a tick‐borne disease that occurs in parts of Asia, Europe and Africa. Since 2000 the infection has caused epidemics in Turkey, Iran, Russia, Uganda and Pakistan. Good‐quality general supportive medical care helps reduce mortality. There is uncertainty and controversy about treating CCHF with the antiviral drug ribavirin.
Objectives
To assess the effects of ribavirin for treating people with Crimean Congo haemorrhagic fever.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; the Central Register of Controlled Trials (CENTRAL); MEDLINE (PubMed); Embase (OVID); Science Citation Index‐Expanded, Social Sciences Citation index, conference proceedings (Web of Science); and CINAHL (EBSCOHost). We also searched the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for trials in progress. We conducted all searches up to 16 October 2017. We also contacted experts in the field and obtained further studies from these sources.
Selection criteria
We evaluated studies assessing the use of ribavirin in people with suspected or confirmed Crimean Congo haemorrhagic fever. We included randomised control trials (RCTs); non‐randomised studies (NRSs) that included more than 10 participants designed as cohort studies with comparators; and case‐control studies.
Data collection and analysis
Two review authors assessed eligibility, risk of bias, and extracted data. For non‐randomized studies we used the ROBINS‐I tool to assess risk of bias. The main effects analysis included all studies where we judged the risk of bias to be low, moderate or high. We summarized dichotomous outcomes using risk ratios (RRs) and continuous outcomes using mean differences (MDs), and used meta‐analyses where appropriate. We carried out a subsidiary appraisal and analysis of studies with critical risk of bias for the primary outcome, as these are often cited to support using ribavirin.
Main results
For the main effects analysis, five studies met our inclusion criteria: one RCT with 136 participants and four non‐randomized studies with 612 participants. We excluded 18 non‐randomized studies with critical risk of bias, where none had attempted to control for confounding.
We do not know if ribavirin reduces mortality (1 RCT; RR 1.13, 95% confidence interval (CI) 0.29 to 4.32; 136 participants; very low‐certainty evidence; 3 non‐randomized studies; RR 0.72, 95% CI 0.41 to 1.28; 549 participants; very low‐certainty evidence). We do not know if ribavirin reduces the length of stay in hospital (1 RCT: mean difference (MD) 0.70 days, 95% CI ‐0.39 to 1.79; 136 participants; and 1 non‐randomized study: MD ‐0.80, 95% CI ‐2.70 to 1.10; 50 participants; very low‐certainty evidence). We do not know if it reduces the risk of patients needing platelet transfusions (1 RCT: RR 1.23, 95% CI 0.77 to 1.96; 136 participants; very low‐certainty evidence). For adverse effects (including haemolytic anaemia and a need to discontinue treatment), we do not know whether there is an increased risk with ribavirin in people with CCHF as data are insufficient.
We do not know if adding ribavirin to early supportive care improves outcomes. One non‐randomized study assessed mortality in people receiving ribavirin and supportive care within four days or less from symptom onset compared to after four days since symptom onset: mortality was lower in the group receiving early supportive care and ribavirin, but it is not possible to distinguish between the effects of ribavirin and early supportive medical care alone.
In the subsidiary analysis, 18 studies compared people receiving ribavirin with those not receiving ribavirin. All had a critical risk of bias due to confounding, reflected in the mortality point estimates favouring ribavirin.
Authors' conclusions
We do not know if ribavirin is effective for treating Crimean Congo haemorrhagic fever. Non‐randomized studies are often cited as evidence of an effect, but the risk of bias in these studies is high.
2 April 2019
Up to date
All studies incorporated from most recent search
Updated review: all eligible published studies found in the last search (16 Oct, 2017) were included
Plain language summary
Ribavirin for treating Crimean Congo haemorrhagic fever
What is the aim of this review?
The aim of this Cochrane review is to find out if ribavirin is an effective treatment for Crimean Congo haemorrhagic fever. Cochrane researchers collected and analysed all relevant studies to answer this question. We found 23 studies. We include five studies in this review that helped answer the question. We analysed the other 18 studies to help describe the limitations of the evidence.
Key messages
There is insufficient reliable evidence to show whether ribavirin is effective in treating Crimean Congo haemorrhagic fever. A randomised clinical trial could help answer this question.
What was studied in the review?
Crimean Congo haemorrhagic fever (CCHF) is an infection spread by tick bites. It has become more common in the last 15 years, particularly in Turkey and parts of Eastern Europe. CCHF can be life threatening. The most important way of caring for people who are seriously unwell with CCHF is to monitor them closely in hospital and give them any fluid or blood products they may need.
Ribavirin is an antiviral drug that some doctors use to treat CCHF. It is widely available and is normally taken by mouth. There is debate over whether ribavirin is needed to treat CCHF; some argue that it is an effective treatment, or helps if given early, whilst others say that it has no effect, in terms of the risk of death, the length of time needed in hospital, and the extent of harm from the drug itself.
Overall, the study designs did not take into account factors other than taking ribavirin that could result in better outcomes in the intervention group, including how ill the patient was when diagnosed, or when good supportive medical care was started. This made any association between ribavirin and lower mortality problematic.
We found five studies that took into account important factors that could confound the risk of dying with whether or not a patient received ribavirin. These include how sick the study participants were, what other care they received, and how long after they became sick they received medical care. All included studies were conducted in Turkey and Iran, and compared people with CCHF who received ribavirin and supportive care to those who received supportive care alone. We looked at five different outcomes relating to ribavirin use in CCHF, and found that there is insufficient reliable evidence to determine whether ribavirin is effective.
How up to date is the review?
The review authors searched for studies that had been published up to 16 October 2017.
Summary of findings
Background
Description of the condition
Crimean Congo haemorrhagic fever (CCHF) is a tick‐borne viral disease. The virus that causes CCHF is a Nairovirus, a member of the Bunyaviridiae family. The most common vectors of the disease are Hyalomma ticks, which spread the disease and also act as a disease reservoir. CCHF is found in Africa, Eastern Europe, the Middle East, and Asia, with further occasional cases in other European countries such as Spain and Greece (Hoogstraal 1979; Zapata 2014; García Rada 2016). In recent years, CCHF incidence has been increasing in several areas worldwide (Zapata 2014).
The disease starts with a headache, fever, abdominal pain, musculoskeletal pain, and nausea. Over the next few days this is followed by gastro‐intestinal symptoms, including vomiting, diarrhoea, and haemorrhagic rash. After three to five days, a few patients progress to severe microvascular instability and the haemorrhagic phase of the illness, which is usually manifested first by a petechial rash followed by ecchymosis and bleeding. As the disease progresses into the second week, bleeding can worsen and become more severe, resulting most commonly in haemorrhage under the skin and within the abdomen. Death rates in people infected can reach up to 50% (Hoogstraal 1979). In endemic areas where high‐quality supportive care and access to diagnostics are offered, death rates can be as low as 5% (Leblebicioglu 2016b).
Many infections occur without symptoms and some estimates suggest this occurs in most infections (Bodur 2012). CCHF severity varies in people who are clinically unwell. Different scores to assess severity are used but it remains unclear what proportion of all infections are severe (Swanepoel 1987; Dokuzoguz 2013).
Infection in people is usually due to a tick bite or by contact with infected bodily fluids from humans or animals (Ergönül 2006b). Those at highest risk of contracting the virus are people who work outdoors in CCHF‐endemic areas, those who work with large domestic animals, and healthcare workers (Whitehouse 2004). CCHF has been linked to reservoirs such as sheep, goats, hedgehogs, and hares (Causey 1970; Saluzzo 1985; Yen 1985; Shepherd 1987). Human‐to‐human transmission occurs within families and in healthcare settings, including nosocomial outbreaks. The greatest risk of nosocomial exposure is from splash exposures and needle stick injuries (Conger 2015; Leblebicioglu 2016a). Case series studies also suggest that in rare cases airborne transmission from ventilated patients may also occur (Pshenichnaya 2015). Case reports suggest possible sexual transmission, although there is no published evidence of the virus being present in seminal or vaginal fluid (Pshenichnaya 2016). The virus is also transmitted from person to person by infected bodily fluids, and is highly infectious.
The disease may become more important in future years because of changes to the habitat of the Hyalomma tick vector, which is due in part to changes in the rural landscape from large diffuse habitats to smaller habitats. This is shown to lead to densely‐populated habitats for the tick vector, which is associated with increasing incidence of the disease (Estrada‐Peña 2007). Given the high mortality of patients, the lack of a widely‐available viable vaccine (Dowall 2016), and an emerging pattern of spread with multiple countries reporting re‐emergence of epidemics or new cases (Messina 2015), CCHF should be considered a potential threat to public health.
Description of the intervention
Supportive medical care underpins CCHF treatment, and use of fluids, good nursing care, and blood products in response to changes in the blood's ability to clot are key components (Leblecioglu 2012). Previous attempts at therapeutic regimens have explored intravenous (IV) immunoglobulin (Ig) isolated from horses (Hoogstraal 1979), and from recovered patients (Vassilenko 1990), but these are not currently widely used.
Ribavirin is commonly used with interferon to treat people who have hepatitis C, and is used alone in treating people who have Lassa fever (Debing 2013). Ribavirin is also used in healthcare settings as a form of post‐exposure prophylaxis for those exposed to CCHF (Leblebicioglu 2016a). Non‐randomized studies show that it could be effective in treating cases of CCHF (Fisher‐Hoch 1995; Mardani 2003; Dokuzoguz 2013), although this has been debated (Kalin 2014; Leblebicioglu 2016a). One such idea is that administration of ribavirin early in the disease, when it appears to be at its most effective, may be a promising approach (Dokuzoguz 2013; Ozbey 2014). This fits with the known course of the disease, where the virus is most commonly only present in the blood within the first week following onset of CCHF symptoms (Bente 2013).
Ribavirin has adverse effects, and, as well as the questions about its efficacy, clinicians debate whether or not to risk using the drug (Ceylan 2013; Oflaz 2015). Some of the adverse effects include risks of haemolysis, arrhythmia, bone marrow suppression, and deranged liver function (EMA 2015). Two previous systematic reviews have shown no clear benefit of ribavirin in people with CCHF, although the available evidence is limited mainly to confounded non‐randomized data (Soares‐Weiser 2010; Ascioglu 2011).
No alternative therapy has been proposed as the mainstay of therapeutic treatment. Although newer drugs, such as favipiravir, have shown promise in vitro (Oestereich 2014), widespread adoption of new therapies is years away. Current treatment guidelines and case definitions vary from region to region and from country to country (DoH South Africa 2014; Kalin 2014).
How the intervention might work
Ribavirin is a synthetic nucleoside that is active against a broad spectrum of DNA and RNA viruses (Sidwell 1972). It is one of few drugs shown to be active against CCHF in vitro (Watts 1989). Ribavirin can be given in hospital settings either intravenously or orally, according to World Health Organization (WHO) recommendations (WHO 2015). National guidelines from countries such as South Africa (DoH South Africa 2014), India (NCDC 2011), and Pakistan (NIH 2013) recommend prompt treatment with ribavirin following diagnosis of CCHF. However, these recommendations for management are not based on a robust evidence base (Soares‐Weiser 2010). CCHF can be mild or more severe, and it is often not deemed necessary to treat mild cases of the disease (Ergönül 2004). Questions remain about the overall benefits of ribavirin, how long after the onset of symptoms it is most effective, and whether it is more or less effective in severe cases (Ergönül 2006b; Dokuzoguz 2013; Leblebicioglu 2016b).
Why it is important to do this review
The controversy surrounding ribavirin use and the benefits of a widely‐available treatment for CCHF mean an up‐to‐date review of the existing evidence is required. There are mixed views on whether to treat CCHF with ribavirin, given the uncertainty about the balance between potential but unproven benefit and known risks of the drug (Kalin 2014). It is therefore important to use the data available to address whether ribavirin reduces the number of deaths from a lethal disease, whilst assessing the possibility of harm from serious, life‐threatening adverse effects.
Objectives
To assess the effects of ribavirin for treating people with Crimean Congo haemorrhagic fever (CCHF).
Methods
Criteria for considering studies for this review
Types of studies
We included the following types of study:
Randomized controlled trials (RCTs), quasi‐RCTs, and non‐randomized controlled studies of ribavirin compared to no ribavirin; also studies comparing early versus late administration of ribavirin
Cohort studies with ribavirin compared to no ribavirin, and studies comparing early versus late administration of ribavirin (prospective and retrospective, with more than 10 participants).
Case‐control studies with ribavirin compared to no ribavirin, and studies comparing early versus late administration of ribavirin
Types of participants
Children or adults of any age with CCHF confirmed with a laboratory test (immunoglobulin (Ig) or polymerase chain reaction (PCR))
Types of interventions
Intervention
Ribavirin (intravenous (IV) or oral)
Early ribavirin (as defined in identified studies)
Control
Supportive care only
Late ribavirin (as defined by study authors)
We accepted co‐interventions as long as the indication for the co‐intervention was consistent between groups, for example, administration of platelets according to homeostatic need.
Types of outcome measures
Primary outcomes
Death (in hospital or 28 days post‐admission)
Secondary outcomes
Length of hospital stay (days)
Requirement for transfusion (any blood products, including platelets, fresh frozen plasma, packed red cells, or whole blood)
Withdrawal of treatment due to serious adverse events
Serious adverse events, as defined according to the accepted US Food and Drug Administration (FDA) definition of: "if in the view of the investigator or sponsor, the event results in any of the following outcomes: death, life threatening adverse event, inpatient hospitalizations, prolongation of existing hospitalisation, disability or permanent damage, congenital abnormality, required intervention to prevent permanent impairment or other serious medical events" (FDA 2016).
Search methods for identification of studies
We tried to identify all relevant trials, regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register (16 October 2017); the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 9 of 12, September 2017), published in the Cochrane Library; MEDLINE (PubMed, 1966 to 16 October 2017); Embase (OVID, 1947 to 16 October 2017); Science Citation Index‐Expanded, Social Sciences Citation index, conference proceedings (Web of Science, 1900 to 16 October 2017); and CINAHL (EBSCOHost (1982 to 16 October 2017). We also searched the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov/ct2/home) for trials in progress, up to 16 October 2017, using "ribavirin" and "Crimean Congo haemorrhagic fever" or "CCHF" as search terms.
Searching other resources
We searched the reference lists of any relevant systematic reviews. We contacted researchers in the field, requested information about grey literature and ongoing studies from the WHO, and checked reference lists of included studies.
Data collection and analysis
Selection of studies
Two review authors independently screened all citations and abstracts identified in the search according to predefined inclusion criteria. We obtained the full‐text reports of all potentially eligible studies or studies we were unclear about. Two review authors independently screened these full‐text articles, resolving any disagreements through discussion and if necessary consulting a third review author. We listed all studies excluded after full‐text assessment and their reasons for exclusion in the ‘Characteristics of excluded studies' table.
We included all unique studies in analyses; however, if there were any studies at critical risk of bias we excluded them from the main effects analyses. We included studies at critical risk of bias in a subsidiary descriptive analysis, using non‐overlapping samples as described in Appendix 2.
Data extraction and management
One review author extracted data using pre tested data extraction forms. A second review author cross‐checked the extracted data. We resolved any disagreements about data extraction by referring to the study report and through discussion. We attempted to contact the study authors where data were insufficient or missing.
We extracted data using a tool tailored for the inclusion criteria described above, including the following information:
Dose and method of administration (oral or IV)
Adult or child populations
Location
Setting
Design
Study size
Dates
Death
Length of hospital stay (days)
Transfusion of blood products
Serious adverse events
Time since onset of symptoms (days) to treatment with ribavirin or supportive care only
Assessment of risk of bias in included studies
Two review authors assessed the risks of bias of each included study, resolving any disagreements through discussion and consulting a third review author if necessary. For RCTs or quasi‐RCTs, we used the Cochrane ‘Risk of bias' tool for RCTs (Higgins 2011). For non‐randomized studies, we used the ROBINS‐I tool (Risk Of Bias In Non‐randomized Studies ‐ of Interventions) (Sterne 2016).
We assessed risks of bias through a hierarchy of domains, starting with critical then serious, moderate, and low. If any domain reached critical risk of bias we did not continue with the assessment, as further evaluation would not influence how we assess the certainty of the evidence.
As the risk of bias in the effect of an intervention may be different for different outcomes, we made a ‘Risk of bias' assessment for each outcome.
Our full methods for using ROBINS‐I are set out in Appendix 3. For assessment of confounding we considered length of time from onset of symptoms to receiving medical care or ribavirin, severity of disease, historical controls rather than contemporary controls, and quality of supportive care to be confounding domains. We made the decision to define these as confounding factors based on extensive debate in the literature (Ergonul 2009; Soares‐Weiser 2010; Kalin 2014), alongside consultation with clinicians with experience of treating viral haemorrhagic fever and CCHF. We listed co‐interventions that could differ between intervention groups impacting on outcomes as ‘quality of supportive care'.
The ROBINS‐I tool recommends only including non‐randomized studies that are not classified as having critical risk of bias. For our main effects analysis, we followed this approach. In addition, there was a further set of studies which met the inclusion criteria but which we classified as having critical risk of bias. As some of these studies have traditionally been used as part of the evidence base, we carried out a subsidiary descriptive analysis describing these studies and their estimates of effect. We established a non‐overlapping sample and performed meta‐analysis to describe the effect of confounding.
Measures of treatment effect
We analysed data using Review Manager 5 (RevMan 5) (RevMan 2014). For dichotomous outcomes, we presented analyses using risk ratios (RRs) with their 95% confidence intervals (CIs). For continuous data we used mean differences (MDs) with their 95% CIs.
Unit of analysis issues
We did not identify any studies that used a cluster‐randomized design or multiple interventions. For our subsidiary descriptive analysis of studies at critical risk of bias we established a non‐overlapping sample of studies using decision rules and methods set out in Appendix 2.
If we had identified studies of a cluster design we would have only used adjusted measures of effect. If the included study had not performed any adjustment for clustering, we would have adjusted the raw data ourselves, using an intracluster correlation coefficient (ICC).
We did not identify any studies with multiple intervention arms, but if we had we would have included data from these studies by either combining treatment arms, or by splitting the control group so that we only include participants once in the meta‐analysis.
Dealing with missing data
We attempted to contact the study authors to obtain missing data when the lack of reporting of necessary data restricted the use of the study.
We applied no imputation measures for missing data.
In one study there was an unclear amount of missing data from an analysis looking at the added benefit of corticosteroid use as well as ribavirin (Dokuzoguz 2013). This occurred because the number of participants included in the analysis did not tally with the explanation of how data were analysed in the text. These missing data may have affected the adjusted odds ratio (OR) presented in the study. We took this into account in the ‘Risk of bias' assessment, and it affected our decision not to present the adjusted estimate of effect; instead we presented a forest plot with the results stratified by severity of diease. As the missing data did not affect results relating to ribavirin and mortality, we did not classify the study as being at critical risk of bias.
Assessment of heterogeneity
We examined the included studies to determine whether there was heterogeneity in terms of co‐intervention, level of supportive care available, and risks of bias in the included studies.
We inspected forest plots to assess whether statistical heterogeneity was present. We deemed CIs that did not overlap as an indication of statistical heterogeneity. We also performed the Chi2 test using a cut‐off point of P < 0.10 to indicate statistical heterogeneity, and we used the I2 statistic to quantify the heterogeneity. We interpreted the I2 statistic value according to guidance in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
If applicable, we intended to use funnel plot analysis or statistical tests (such as the Egger regression test), or both, to assess for publication bias. We planned to perform funnel plot analysis if there were more than 10 studies in any meta‐analysis. As there were fewer than 10 studies included in any of the effects analyses we did not perform this test.
Data synthesis
In order to deal with non‐standard study designs we have presented our main effects analysis by study design. We separated study designs into different subgroups and did not pool results across randomised and non‐randomized subgroups. This included RCTs, retrospective cohort studies, matched cohort studies and historically‐controlled cohort studies.
We stratified studies by their risk of bias in the descriptive analysis. We did not include studies which we assessed as having critical risk of bias in the main effects analysis. We performed a meta‐analysis of studies using the random‐effects model; this was due to varying study type, differences in populations and supportive therapy available.
We examined those studies classified as being at critical risk of bias in a subsidiary descriptive analysis that assessed the degree of confounding. We assembled a non‐overlapping sample, as set out in Appendix 2.
One study (Dokuzoguz 2013) used a model to adjust for confounding of effect due to severity of disease. This resulted in an adjusted OR of 0.04 (95% CI 0.004 to 0.48). The small sample size, the size of the adjusted effect, missing data from the corticosteroid analysis, concerns about residual confounding due to time from onset of symptoms to presentation to hospital/administration of ribavirin, and the fact that the study analysed severely‐ill patients with gastro‐intestinal haemorrhage "per protocol" and not by intention‐to‐treat, meant that we took a conservative approach to synthesis. We presented non‐adjusted data from the study in the main effects analysis, and the stratified data in a separate analysis (Analysis 1.2).
1.2. Analysis.
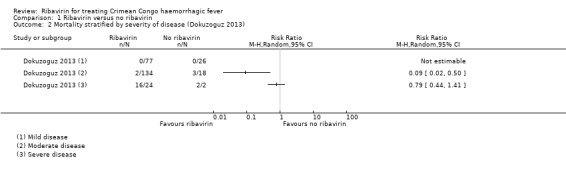
Comparison 1 Ribavirin versus no ribavirin, Outcome 2 Mortality stratified by severity of disease (Dokuzoguz 2013).
We used the GRADE approach to assess the certainty of the evidence, and created ‘Summary of findings' tables and Evidence Profiles (GRADEpro 2015). Data from observational studies started as low quality, but we intended to upgrade this to moderate or high quality if the pooled estimates revealed a large effect size, negligible concerns about confounders, or a strong dose‐response gradient.
Subgroup analysis and investigation of heterogeneity
If unexplained heterogeneity occurred we intended to perform subgroup analyses of the results, to assess whether the effect of ribavirin was influenced by any of the following factors:
Severity of symptoms: severe, moderate, mild
Duration of treatment, presence of severe gastro‐intestinal symptoms, and route of administration
Age (children versus adults). Children are defined as under 16 years of age.
We did not conduct subgroup analyses, because there were insufficient data to apply the prespecified subgroups in the primary effects analysis.
Sensitivity analysis
If we had estimated an ICC to adjust the results from cluster trials, we would have performed sensitivity analyses to investigate the robustness of our findings. We had intended to perform a sensitivity analysis, to consider excluding studies that were at high risk of bias according to the Cochrane ‘Risk of bias' tool for RCTs (Higgins 2011), and serious risk of bias according to ROBINS‐I tool for observational studies (Sterne 2016).
We performed no sensitivity analyses because we identified no cluster‐controlled studies, and because all studies included in the main effects analysis were at high risk of bias according to the Cochrane tool or at serious risk of bias according to ROBINS‐I.
Results
Description of studies
Results of the search
See PRISMA flow diagram Figure 1.
1.
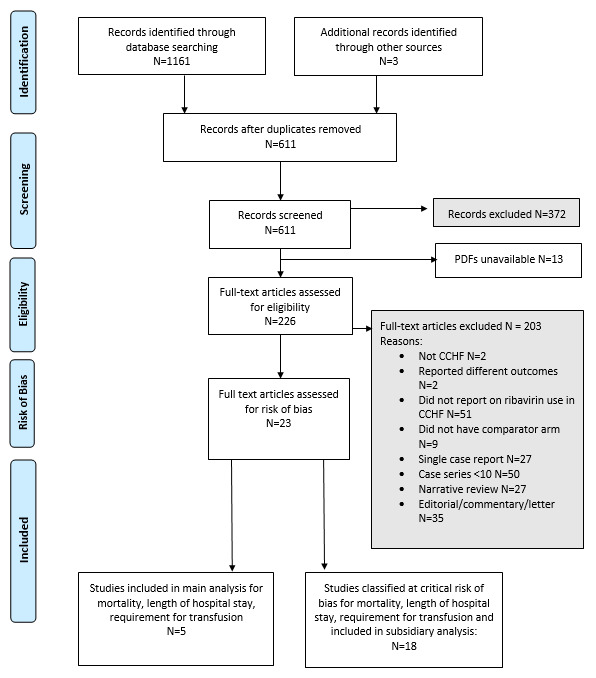
Flow of studies
We identified 1161 records, plus a further three references through contacting experts in the field. From these we identified 611 unique references after removing duplicates. We considered 372 references to be irrelevant for our review, and we were unable to obtain the articles for 13 of the references. We considered 226 full‐text articles for inclusion, of which we excluded 203. Two did not report on CCHF, and two reported on outcomes not included in our review. Fifty‐one studies did not report on ribavirin for treating CCHF, nine had no comparator arm and 27 were single‐case reports. Fifty were cohort studies with fewer than 10 participants, 27 were narrative reviews and 35 studies were editorial letters or comments on other studies. Twenty‐three studies met our inclusion criteria and are included in the review. No prospectively‐registered ongoing studies met the inclusion criteria.
Included studies
See Characteristics of included studies.
We include 23 studies that tested the use of ribavirin in people with CCHF, with the outcomes of mortality, length of hospital stay, and requirement for transfusion.
Main effects analysis
For the main effects analysis we include five studies that were not at critical risk of bias; one RCT of 136 participants (Koksal 2010) and four non‐randomized studies of 612 participants (Elaldi 2009; Izadi 2009a; Bodur 2011; Dokuzoguz 2013).
Study design
Of the five studies included in our main effects analysis, one study was an RCT (Koksal 2010), one was a matched cohort study (Bodur 2011), one was a cohort with a historical control (Elaldi 2009), one was a mixed prospective and retrospective cohort study (Dokuzoguz 2013), and one was a retrospective cohort (Izadi 2009a).
The design of the studies included in our primary analysis and how controls were selected varied. Elaldi 2009 used historical controls from a period when ribavirin was unavailable. Dokuzoguz 2013 selected controls based on clinical criteria including time from onset of symptoms to diagnosis or contraindication to oral ribavirin. Izadi 2009a compared administration of ribavirin given early in the disease to late in the disease. Bodur 2011 used a retrospective design that matched 10 participants who received ribavirin to 40 controls that did not, using various clinical and physiological parameters.
Setting
Four out of the five studies included in the main effects analysis were conducted in Turkey, and one was conducted in Iran (Izadi 2009a).
Participants
Most participants described in Dokuzoguz 2013 were adults or adolescents, with the youngest participant aged 16 years. Izadi 2009a described an age range of 11 to 75, with a median age of 29.2 years. It was not possible to ascertain the exact numbers of adolescents, as they were not described in the included studies. All of the studies included in our main effects analysis included confirmed cases only, using either Ig enzyme‐linked immunosorbent assay (ELISA) or PCR to verify.
Intervention
Doses of ribavirin differed between studies. Elaldi 2009 described weight‐based prescribing (30 mg/kg initial loading dose; 15 mg/kg 4 times daily for 4 days; 7.5 mg/kg 3 times daily for 6 days). Dokuzoguz 2013 and Bodur 2011 described standard doses in adults (4 g daily for 4 days, followed by 2.4 g daily for 6 days). Doses were broadly the same and full details are described in the ‘Characteristics of included studies' table. All studies in this analysis administered oral ribavirin.
Comparators
None of the participants in the comparator groups received ribavirin for the comparison of ribavirin versus no ribavirin. One study (Izadi 2009a) was included in the main effects analysis that offered a comparison of early versus late ribavirin; all those in the comparator arm received ribavirin after four days.
Length of follow‐up
No studies specified a length of follow‐up. Instead, they relied upon discharge from hospital or clinical care as the sole measure of follow‐up time.
Subsidiary descriptive analysis
For the subsidiary descriptive analysis we included 18 studies rated at critical risk of bias. These studies are frequently cited as evidence of benefit, so we appraised them against the primary outcome of mortality.
Study design
The rationale for ‘Risk of bias' assessments is set out in Table 3. They all failed to control for confounding due to severity of disease, time from the onset of symptoms to receiving medical care, or all of these. This critically affected the reliability of data collected for these studies. ROBINS‐I recommends studies at critical risk of bias are excluded from the review.
1. Table of studies at critical risk of bias: disease‐related outcomes.
| Studies at critical risk of bias outcomes: Death, timing of administration, length of stay in hospital, requirement for transfusion | ||
| Study | Bias due to Confounding | Comment |
| Alavi‐Nani 2006 | Critical | Confounders not controlled for. No information reported on care received in hospital. Variation in disease severity between ribavirin and control groups not measured. No discussion of potential confounding by severity of disease in paper. No control for time from onset of symptoms to administration of ribavirin. Small size of control group suggests clinical contraindication to ribavirin, a factor in selection into control group (although this is not expressly commented on) |
| Belet 2014 | Critical | Confounders not controlled for. Although criteria for administration of ribavirin reported, it is not clear whether recipients must fulfil all of these or only some Participants receiving ribavirin were more severe at baseline. There is no adjustment for severity on admission, and length of time between symptom onset and admission/ribavirin treatment |
| Cevik 2008 | Critical | Confounders not controlled for. Severe patients only included in case‐control study. No discussion of potential confounding in paper. Care provided during hospitalisation not described |
| Ergönül 2004 | Critical | Confounders not controlled for. Severe patients only included in retrospective cohort. Baseline severity of disease not established. Classification of severe disease is at any time point for 22 participants. Time from onset of symptoms not controlled for. No method for dealing with potential confounders. Patients were given preparations of erythrocytes, fresh frozen plasma, and total blood, depending on their homeostatic state ‐ disentangling the effect of this supportive care from that of ribavirin is not considered. Oral ribavirin was given to severe CCHF patients |
| Ergonul 2006 | Critical | Confounders not controlled for. Paper focuses on developing severity scoring system. Baseline characteristics not established between ribavirin and non‐ribavirin groups. Criteria for selection into control arm included clinical contraindication due to haematemesis. Time from onset of symptoms not controlled for. The authors developed specific criteria to identify severe cases |
| Ertugrul 2009 | Critical | Confounders not controlled for. No methods for controlling potential confounders are discussed. Authors stated in Discussion that no information was available to them on severity of cases. No information reported on care received by participants |
| Ertem 2016 | Critical | Confounders not controlled for. Controls for “time from onset of symptoms” for a comparison of early versus late ribavirin. However, not for the comparison of ribavirin versus no ribavirin. Rather than just comparing means, the authors should control for the confounders when comparing the groups. Mortality not reported |
| Gayretli Aydin 2015 | Critical | Confounders not controlled for. Paper focuses on bradycardia in paediatric patients No discussion of potential confounding in paper and no controlling for confounding factors such as severity of illness or time from onset of symptoms to administration of ribavirin |
| Kalin 2014 | Critical | Confounders not controlled for. Significant differences in baseline severity of disease and time from onset of symptoms. These confounders were measured but not controlled for by stratification or other method. Ribavirin group had more severe disease; confounding would reduce effect of ribavirin seen |
| Mardani 2003 | Critical | Confounders not controlled for. Baseline characteristics not established between ribavirin and no‐ribavirin groups. No method for dealing with potential confounders. Significant differences in arms of study ‐ suggests heterogeneous samples with no controlling for severity of disease. Time from disease onset to presentation/treatment not assessed. Historical control arm used supportive treatment likely to have differed substantially between intervention and control arms |
| Metanat 2005 | Critical | Confounders not controlled for. Conference abstract ‐ insufficient information reported by study authors about possible confounders such as severity of disease. No information provided on care received in hospital |
| Ozkurt 2006 | Critical | Confounders not controlled for. No methods for controlling potential confounders are discussed. Timing of administration of ribavirin is documented but severity of infection is not considered. Baseline characteristics not established between ribavirin and no‐ribavirin groups |
| Sannikova 2009 | Critical | Historical control group used. Study conducted from 1999‐2008, quality of supportive care likely to have changed significantly over this period of time. Control group originated during period before ribavirin was available. Substantial period of time from the start of follow up in historical control group to start of follow up in intervention group. |
| Sharifi‐Mood 2006 | Critical | Confounders not controlled for. No information reported on care received in hospital. Baseline characteristics not established. Variation in disease severity expected, although influence of this across the two groups unclear. No method of controlling for confounding by severity of disease. No discussion of potential confounding in paper. Timing of administration investigated, raw data not presented. |
| Sharifi‐Mood 2013a | Critical | Confounders not controlled for. Time from onset of symptoms adjusted for by stratification into early/late ribavirin. Baseline characteristics and severity of disease not assessed, measured or controlled for. No information provided on care received in hospital |
| Tasdelen Fisgin 2009 | Critical | Timing of administration of ribavirin controlled for by stratification. Baseline confounding due to severity of disease measured and not controlled for participants in no‐ribavirin group and late‐ribavirin group having more severe disease based on baseline biochemistry and haematology. At least one participant was included in no‐ribavirin group due to gastrointestinal haemorrhage and severe disease. Criteria for use of ribavirin changed during period and largely historical controls were used |
| Tezer 2016 | Critical | Confounders not controlled for. No methods for controlling potential confounders such as severity of disease and time since onset of symptoms are discussed. Authors recognize highly‐confounded data as limitation of their study |
| Tulek 2012 | Critical | Confounders not controlled for. Case‐control study with no information in abstract about how the controls were selected. Supportive care protocol was similar in both departments. No method for dealing with potential confounding by time since onset of symptoms. No matching for severity or time since onset of symptoms |
| Tuygun 2012 | Critical | Confounders not controlled for. No method for dealing with potential confounding by time since onset of symptoms, baseline characteristics were not established, no method for controlling for severity. The patients were given erythrocyte suspension, thrombocyte suspension and/or fresh frozen plasma based on their haemostasis status, and other supportive care when necessary ‐ disentangling the effect of this care from that of ribavirin is not considered. Oral ribavirin was given to the patients who were evaluated as severe or had bleeding symptoms, or both. Entirely unclear how the authors selected the 50 participants included from 202 confirmed cases. Also at critical risk of bias on selection of participants into the study |
All 18 studies at critical risk of bias were retrospective cohort studies. One study used a cohort of patients treated before the availability of ribavirin as a control arm. (Sannikova 2009).
Setting
In those studies included in the descriptive analysis 12 were conducted in Turkey, five were conducted in Iran and one study was set in Russia and was translated from Russian (Sannikova 2009). There were a number of studies that reported on populations that overlapped with each other. Our decisions on overlapping studies are outlined in Appendix 2.
Participants
In the subsidiary descriptive analysis for the comparison of ribavirin versus no ribavirin an additional 1214 participants in 10 studies at critical risk of bias were analysed.
In the subsidiary descriptive analysis for the comparison of early versus late ribavirin an additional 431 participants in 4 studies received either early or late ribavirin.
Intervention
For the comparison of ribavirin versus no ribavirin doses were broadly the same; we give full details in the ‘Characteristics of included studies' table. Most studies in this analysis administered oral ribavirin.
For the comparison of early versus late ribavirin, participants received ribavirin according to the study author's definitions of early versus late. Studies used different cut‐off time points for the definition of early care with ribavirin, either less than three days since onset of symptoms (Sharifi‐Mood 2006; Sharifi‐Mood 2013a), less than four days since onset of symptoms (Izadi 2009a) or less than five days since the onset of symptoms (Metanat 2005; Tasdelen Fisgin 2009). 114 participants received ribavirin less than three days from the onset of symptoms, 97 received ribavirin after 3 days since the onset of symptoms. One hundred and thirty participants received ribavirin less than five days from onset of symptoms with 90 participants receiving ribavirin after this time point.
Comparators
Of those studies covered by the descriptive analysis, four studies included children only (Sharifi‐Mood 2006; Tuygun 2012; Gayretli Aydin 2015; Tezer 2016). Three studies did not report the method used to confirm cases of CCHF (Metanat 2005; Tulek 2012; Sharifi‐Mood 2013a), all other studies used either Ig ELISA or PCR.
Length of follow‐up
No studies specified a length of follow‐up. Instead they relied upon discharge from hospital or clinical care as the sole measure of follow‐up time.
Excluded studies
We excluded 203 studies at the full‐text screening stage because they did not study CCHF, did not relate to a relevant CCHF topic, had fewer than 10 participants, or they were narrative reviews or commentaries. See the Excluded studies tables.
Risk of bias in included studies
Main effects analysis
Randomized controlled trials
We identified one randomised control trial (Koksal 2010), which we assessed using the Cochrane ‘Risk of bias' tool for RCTs (Higgins 2011). Methods for random sequence generation and allocation concealment were unclear in the single RCT. The trial authors did not report methods for this in the text. We judged the methods for blinding of participants and outcome assessments to be unclear; we identified no missing data.
There was no protocol available to assess selective reporting. We judged mortality and length of hospital stay as unlikely to be subject to reporting bias (Figure 2).
2.
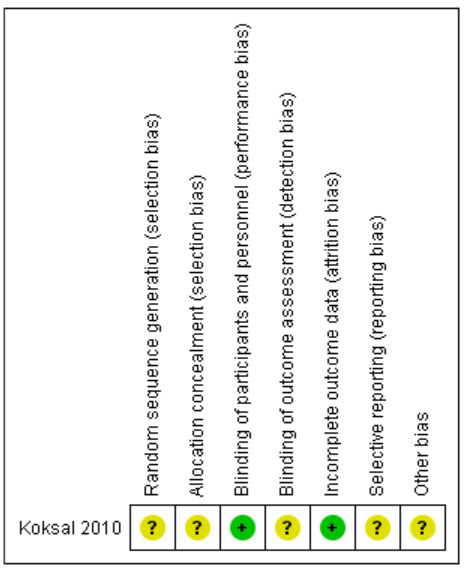
‘Risk of bias' assessment for all included trials
Non‐randomized studies
We identified 22 non‐randomized studies, which we assessed using the ROBINS‐I tool. Of these we classified 18 studies as being at critical risk of bias, and four studies at serious risk of bias.
Confounding
We have presented comprehensive ‘Risk of bias' assessments for non‐randomized studies included in the main effects analysis in Table 4; Table 5; Table 6; Table 7.
2. ROBINS‐I assessment: Bodur 2011.
| ROBINS‐I assessment | |||
| Reference: Bodur 2011 | |||
| Risk of bias domain | Assessments by outcome | Comment | Conclusion |
| Bias due to confounding | Mortality, Length of hospital stay, transfusion, withdrawal of treatment: serious risk of bias due to baseline confounding | Matching controls were included in the study in order to increase the study's power. Baseline characteristics established and are similar between groups for severity of disease and time from onset of symptoms to admission. No significant differences in baseline laboratory findings. Differences occur between groups in rates of splenomegaly (1 case in each arm), petechiae, haematemesis (2/10 in ribavirin group, 3/40 in control), melena. However, limited information reported on how the controls or baseline characteristics were selected.Given the differences in clinical symptoms serious risk of bias was attributed |
Serious |
| Bias in selection of participants into the study | Mortality, length of hospital stay, transfusion, withdrawal of treatment: serious risk of bias. Direction: would show increased effect of ribavirin |
Selection into study did not appear to be related to intervention, outcome or any prognostic factor. Limited information reported on how the controls were selected. Controls selected “at random” that matched baseline characteristics |
Moderate |
| Bias in classification of interventions | All outcomes | Interventions well defined | Low |
| Bias due to deviations from intended interventions | All outcomes | No information on deviation from intended intervention, as would be the case in usual practice | Low |
| Bias due to missing data | All outcomes: serious | All data appear to be reported | Low |
| Bias in selection of the reported result | All outcomes | No outcomes of interest to study authors are specified. No protocol available, no prespecified outcomes in Methods section | Serious |
3. ROBINS‐I assessment Dokuzoguz 2013.
| ROBINS‐I assessment | |||
| Reference:Dokuzoguz 2013 | |||
| Domain | Assessments by outcome | Comment | Conclusion |
| Bias due to confounding | Mortality, length of hospital stay, transfusion, withdrawal of treatment: Serious risk of bias due to baseline confounding | Time from onset of symptoms not adequately controlled or adjusted for. All participants with time from onset of symptoms to diagnosis < 7 days received ribavirin unless contraindicated Both time from onset of symptoms and clinical contraindication are prognostic factors that predict whether the individual receives the intervention |
Serious |
| Bias in selection of participants into the study | Mortality, length of hospital stay, transfusion, withdrawal of treatment: serious risk of bias | Control group selected by including patients with time from onset of symptoms to diagnosis > 7 days and clinical contraindication to ribavirin Both time from onset of symptoms and clinical contraindication are prognostic factors that predict whether the individual receives the intervention Analysis was performed per protocol (2 participants in control group were intended to be treated with ribavirin but due to gastrointestinal bleeding were unable to receive oral medication) |
Serious |
| Bias in classification of interventions | All outcomes | Interventions well‐defined in Methods section | Low |
| Bias due to deviations from intended interventions | All outcomes | No deviation from intervention not expected in normal practice | Low |
| Bias due to missing data | All outcomes | Some missing outcome data not dealt with in text. This is related to numbers of participants receiving co‐administration of corticosteroids with ribavirin Unbalanced across groups | Serious |
| Bias in selection of the reported result | All outcomes | Analysis was performed per protocol. (2 participants in control group were intended to be treated with ribavirin but due to gastrointestinal bleeding were unable to receive oral medication). Effect of ribavirin as measured will be overestimated compared to intention‐to‐treat analysis | Serious |
4. ROBINS‐I assessment: Elaldi 2009.
| ROBINS‐I assessment | |||
| Reference: Elaldi 2009 | |||
| Risk of bias domain | Assessments by outcome | Comment | Conclusion |
| Bias due to confounding | Mortality, length of hospital stay, transfusion, withdrawal of treatment: Serious risk of bias due to baseline confounding | Baseline characteristics established and are similar between groups for severity of disease and time from onset of symptoms to admission Differences occur between groups in rates of maculopapular rash, hepatomegaly and lactate dehydrogenase. None of these are markers of disease severity unless petechiae were misclassified as maculopapular rash Use of historical control arm at the onset of an epidemic establishes a difference in the quality of supportive care between groups. As the time elapsed was only one year we classified this as serious and not critical confounding No participants diagnosed received ribavirin in the historical control group All participants diagnosed received ribavirin in the intervention group No method to adjust for potential confounders reported |
Serious |
| Bias in selection of participants into the study | Mortality, length of hospital stay, transfusion, withdrawal of treatment | Selection into study was not related to intervention, outcome or any prognostic factor Historical control group may confound results as set out above It appears that all potential participants for the specified study years have been included in the studies for the particular treatment groups |
Serious |
| Bias in classification of interventions | All outcomes | Interventions well‐defined in Methods section | Low |
| Bias due to deviations from intended interventions | All outcomes | No information reported on adherence of participants to ribavirin treatment schedule. For supportive care: "Same proportions of patients received ES (12%) and FFP (39%) in treated and untreated groups. On the other hand, more patients in the treated group were infused with PS (52%) than those in the untreated group (42%)." No other information provided about co‐interventions | Low |
| Bias due to missing data | All outcomes: serious | All data appear to be reported | Low |
| Bias in selection of the reported result | All outcomes | Unclear selection criteria for establishing baseline similarities between groups. PT/APTT may be missing from baseline characteristics Reported results are in keeping with those specified in the study methods |
Low |
5. ROBINS‐I assessment: Izadi 2009.
| ROBINS‐I assessment | |||
| Reference: Izadi 2009a | |||
| Risk of bias domain | Assessments by outcome | Comment | Conclusion |
| Bias due to confounding | Mortality, length of hospital stay, transfusion, withdrawal of treatment: serious risk of bias due to baseline confounding | Time from onset of symptoms adjusted for by stratification into early/late ribavirin Baseline characteristics and severity of disease are not assessed, measured or controlled for Multiple regression models used to identify factors predictive of mortality. Regression does not adjust for severity and prognostic factors for the efficacy of ribavirin Adjusted estimates of effect not included in analysis due to linear regression not outlined clearly, although it is unlikely to control for confounding of the effect of ribavirin |
Serious |
| Bias in selection of participants into the study | Mortality, length of hospital stay, transfusion, withdrawal of treatment: low risk of bias | Unclear if selection into the study was based on participant's characteristics observed after the start of the study; retrospective design | Moderate |
| Bias in classification of interventions | All outcomes | Not recorded | Moderate |
| Bias due to deviations from intended interventions | All outcomes | No deviation from intended intervention, as would be the case in usual practice. Most participants received a transfusion ‐ Table 3 shows the proportions of participants who received a transfusion of platelet concentrates, and in some cases fresh frozen plasma and packed erythrocytes. No other aspects of care or co‐interventions are discussed No information on adhering to ribavirin treatment | Low |
| Bias due to missing data | All outcomes: serious | Outcome data (mortality or cured) reported for all 63 participants according to treatment group | Low |
| Bias in selection of the reported result | All outcomes | Although no protocol available or prespecified outcomes, the authors state that they attempted to assess the effect of ribavirin in reducing mortality | Moderate |
One study (Bodur 2011) controlled for confounding by matching baseline characteristics of 10 cases with 40 controls (Table 4). One mixed retrospective/prospective cohort study (Dokuzoguz 2013) established a severity scoring index and stratified results using this as a way of controlling for confounding by severity. Time from onset of symptoms to diagnosis was addressed by not prescribing ribavirin to anyone with more than seven days history of symptoms (Table 5). One historically‐controlled cohort study (Elaldi 2009) established similar baseline characteristics between cohorts. The use of a historical control arm at the onset of an epidemic establishes a difference in the quality of supportive care between groups. The time period elapsed was only one year, so we classified this as serious and not critical confounding (Table 6). One retrospective cohort study (Izadi 2009a) stratified mortality outcome by time from onset of symptoms to administration of ribavirin (Table 7). This study performed a regression analysis, although this was designed to identify predictive factors for mortality and not to control for confounding. We classified all four non‐randomized studies included in the main effects analysis as being at serious risk of bias for the domain of confounding.
Bias in selection of participants into the study
In Bodur 2011 (Table 4), participants were matched by their baseline severity according to clinical presentation and laboratory values. The trial authors did not adequately describe the matching process, although stated the controls were selected "at random". The lack of clarity meant that we classified this study as being at serious risk of bias in this domain.
In Dokuzoguz 2013 (Table 5), participants in the control group were selected based on time from onset of symptoms (more than seven days) and clinical contraindication. Both are prognostic factors that predict whether the individual receives the intervention. We therefore judged this as being at serious risk of bias for this domain. We did not judge this domain as critical, because baseline severity was established, measured, and sufficiently comparable to garner useful data from the study.
In Elaldi 2009 (Table 6), selection of participants was not related to the intervention, outcome, or any prognostic factor; the historical control group may have confounded results. We therefore judged this domain to be at serious risk of bias. We did not judge this domain as critical, because baseline severity was established, measured, and sufficiently comparable to garner useful data from the study.
In Izadi 2009a (Table 7), it was unclear if selection into the study was based on participants' characteristics observed after the start of the retrospective study design.
Bias in classification of interventions
We judged all studies to be at low risk of bias, as the doses and methods of administration of ribavirin were well‐defined.
Bias due to deviations from intended interventions
We judged all studies to be at low risk of bias. In none of the studies were there deviations from the intended intervention other than what would be expected in normal practice.
Bias due to missing data
In Dokuzoguz 2013 there were some missing outcome data not described in the text. The missing data were related to numbers of participants receiving co‐administration of corticosteroids with ribavirin. We judged the balance of missing data across groups as unclear, as there was insufficient documentation explaining this. We therefore classed this study as being at serious risk of bias in this domain.
Bias in measurement of outcomes
We judged all studies to be at low risk of bias. Whilst investigators will have been aware of the intervention status of the participants (if they received ribavirin or not), none of the measured outcomes were subjective and thus prone to bias.
Bias in the selection of reported result
Bodur 2011 and Elaldi 2009 used unclear criteria to establish similar baseline characteristics between arms. Most of the expected clinical and laboratory criteria were included, but a severity score would be more comprehensive. Whilst severity indices have been developed since the publication of these papers, an accepted severity index was available at the time (Swanepoel 1987). We therefore classed both studies as being at moderate risk of bias in this domain.
Subsidiary descriptive analysis
We classified all 18 studies included in the descriptive analysis as being at critical risk of bias due to confounding, as described in Table 3. All of these studies were retrospective cohorts by design. The main reason for this was the failure to control for baseline confounding due to severity of disease. Most studies did not describe important baseline characteristics in intervention and control groups.
Effects of interventions
Summary of findings for the main comparison. Ribavirin versus no ribavirin for Crimean Congo haemorrhagic fever.
| Ribavirin compared to no ribavirin for Crimean Congo haemorrhagic fever | ||||||
| Patient or population: people diagnosed with suspected or confirmed Crimean Congo haemorrhagic fever Setting: global Intervention: ribavirin Comparison: no ribavirin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no ribavirin | Risk with ribavirin | |||||
| Mortality | 56 per 1000 | 63 per 1000 (16 to 240) | RR 1.13 (0.29 to 4.32) | 136 (1 RCT)1 | ⊕⊝⊝⊝ VERY LOW2,3 | ‐ |
| Length of hospital stay (days) | The mean length of hospital stay in 1 RCT was 0.7 days longer in the experimental group (0.39 days fewer to 1.79 days longer) | ‐ | 136 (1 RCT)4 | ⊕⊝⊝⊝ VERY LOW2,3 | ‐ | |
| Requirement for transfusion (platelets) | 306 per 1000 | 376 per 1000 (235 to 599) | RR 1.23 (0.77 to 1.96) | 136 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,3 | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1In addition there were three non‐randomized studies (mixed retrospective and prospective cohort; single arm cohort with historical control; matched case series) with serious risk of bias (ROBINS‐I), providing an estimate of RR 0.59, 95% CI 0.27 to 1.27; 549 participants; very low‐certainty evidence). 2Downgraded one level for risk of bias: one RCT with no description of randomisation or concealment of allocation. 3Downgraded two levels for imprecision. Few events and wide CI containing appreciable benefit and harm. 4In addition one non‐randomized study (matched case series) with serious risk of bias (ROBINS‐I) providing an estimate of 0.8 days fewer in the experimental group (2.7 days fewer to 1.1 days longer); very low‐certainty evidence.
Summary of findings 2. Early versus late supportive care plus ribavirin for Crimean Congo haemorrhagic fever.
| Early versus late supportive care plus ribavirin for Crimean Congo haemorrhagic fever | ||||||
| Patient or population: people diagnosed with suspected or confirmed Crimean Congo haemorrhagic fever Setting: global Intervention: early supportive care plus ribavirin1 Comparison: late supportive care plus ribavirin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Late ribavirin | Risk with Early ribavirin | |||||
| Mortality in early versus late supportive care plus ribavirin | 400 per 1000 | 156 per 1000 (64 to 380) | RR 0.39 (0.16 to 0.95) | 63 (1 non‐randomised study) | ⊕⊝⊝⊝ VERY LOW2,3 | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Early defined according to that reported in the included study (< 4 days since onset of symptoms) 2Downgraded one level for risk of bias: all studies at serious risk of bias. 3Downgraded two levels for imprecision: few events and wide CIs.
Our main effects analysis included one RCT and four non‐randomized studies. The remaining 18 studies, which we assessed as being at critical risk of bias, are used in a subsidiary descriptive analysis for our primary outcome of mortality.
Ribavirin versus no ribavirin
Mortality
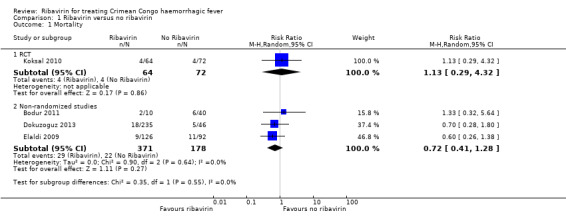
One RCT and three non‐randomized studies were included that compared the effect on mortality of ribavirin and no ribavirin in participants with CCHF (Figure 3).
3.
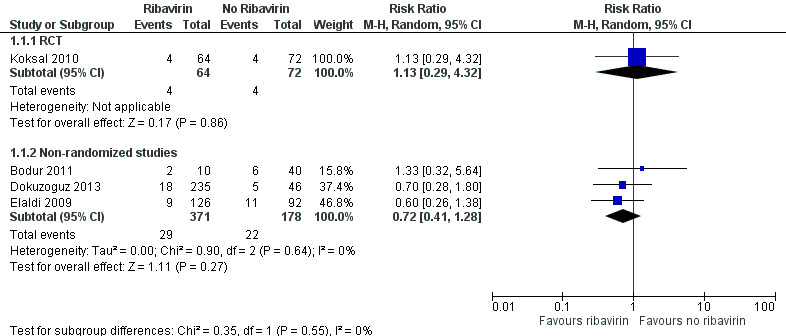
Forest plot of Ribavirin versus no ribavirin, outcome: mortality.
RCT
One RCT of 136 participants (Koksal 2010) found no statistically significant effect in favour of either ribavirin or no ribavirin (RR 1.13, 95% CI 0.29 to 4.32; Analysis 1.1).
1.1. Analysis.
Comparison 1 Ribavirin versus no ribavirin, Outcome 1 Mortality.
Non‐randomized studies
One mixed retrospective and prospective cohort study of 281 participants stratified risk of death by severity of disease (Dokuzoguz 2013). No deaths occurred in 103 mild cases and risk ratios were therefore not calculable. In 152 moderate cases (subgroup 2) ribavirin reduced mortality (RR 0.09, 95% CI 0.02 to 0.50). In 26 severe patients no effect of ribavirin on mortality was seen (RR 0.79, 95% CI 0.44 to 1.41; Analysis 1.2, Figure 4 ). The two participants in the severe disease strata control group were unable to take oral ribavirin due to gastro‐intestinal bleeding, despite an intention to treat them with ribavirin.
4.
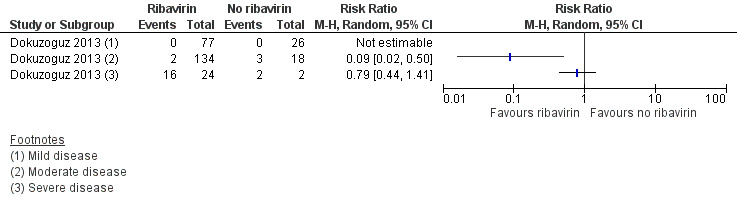
Forest plot of comparison: 1 Ribavirin versus no ribavirin, outcome: 1.2 Mortality stratified by severity of disease (Dokuzoguz 2013).
One cohort study with a historical control arm of 218 participants had similar baseline characteristics in terms of severity of disease and time from onset of symptoms (Elaldi 2009). This study showed no statistically significant benefit of ribavirin on mortality (RR 0.60, 95% CI 0.26 to 1.38; Analysis 1.1)
One retrospective matched cohort study of 50 participants used a matched design where those who received ribavirin were randomly matched to a control group with similar baseline characteristics (Bodur 2011). In this study no statistically significant effect was seen (RR 1.33, 95% CI 0.32 to 5.64; Analysis 1.1).
In a pooled analysis of these three non‐randomized studies we found no statistically significant effect (RR 0.72, 95% CI 0.41 to 1.28; 549 participants; Analysis 1.1; Figure 3). With few events and wide CIs containing clinically appreciable benefit and harm, it is not possible to draw a conclusion of benefit or of no effect from the available evidence. Given the concerns over the internal validity of the studies, this further decreases our confidence in the effect estimate.
In summary, it is uncertain whether ribavirin reduces mortality, because the certainty of the evidence is very low from both the RCT and the non‐randomized studies (Table 1).
Length of hospital stay
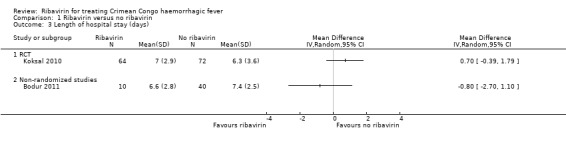
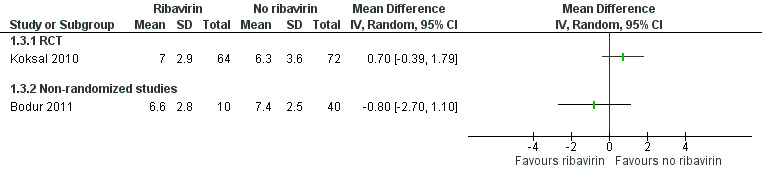
One non‐randomized study (retrospective matched cohort design) and one RCT met our inclusion criteria and evaluated the effect of ribavirin on length of hospital stay in participants with CCHF receiving ribavirin or not (Koksal 2010; Bodur 2011; Analysis 1.3; Figure 5).
1.3. Analysis.
Comparison 1 Ribavirin versus no ribavirin, Outcome 3 Length of hospital stay (days).
5.
Forest plot of ribavirin versus no ribavirin, outcome: length of hospital stay (days).
RCT
Koksal 2010 showed no effect of ribavirin on the length of hospital stay in days (MD 0.70, 95% CI ‐0.39 to 1.79; 136 participants; Analysis 1.3).
Non‐randomized studies
Bodur 2011 showed no effect of ribavirin on the length of hospital stay in days (MD ‐0.80, 95% CI ‐2.70 to 1.10; 50 participants; Analysis 1.3).
In summary, we do not know if ribavirin reduces the length of stay in hospital, as the certainty of the evidence is very low (Table 1).
Requirement for transfusion
One included RCT compared the effect of ribavirin with no ribavirin on the need for transfusion of blood products in participants with CCHF (Koksal 2010). There was no statistically significant difference in requirement for transfusion of platelets between treated and untreated participants in the RCT (RR 1.23, 95% CI 0.77 to 1.96; 136 participants; Analysis 1.4).
1.4. Analysis.
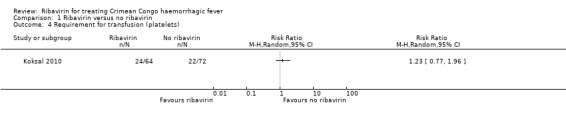
Comparison 1 Ribavirin versus no ribavirin, Outcome 4 Requirement for transfusion (platelets).
Withdrawal of treatment due to adverse events
One study included in the primary analysis reported on adverse events leading to discontinuation of treatment. One participant among 44 who received ribavirin and corticosteroids discontinued ribavirin due to elevated amylase levels (Dokuzoguz 2013).
Serious adverse events
No studies in the primary analysis reported on adverse events.
Timing of administration of ribavirin: early versus late ribavirin
Mortality
One non‐randomized study (retrospective cohort) was included that addressed the timing of administration of ribavirin alongside supportive care and mortality (Izadi 2009a).
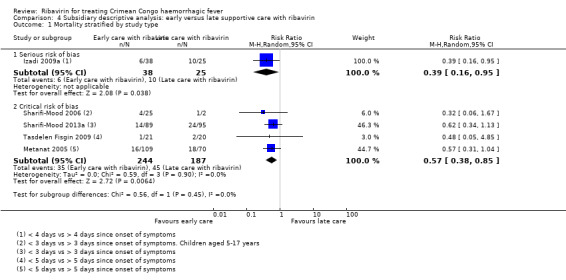
Izadi 2009a outlined an association between reduced mortality in those who received supportive care and ribavirin less than four days since the onset of any symptoms compared to those receiving supportive care and ribavirin after four days (RR 0.39, 95% CI 0.16 to 0.95; 63 participants; Analysis 2.1; Figure 6).
2.1. Analysis.
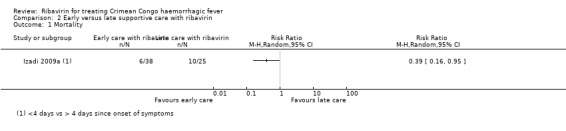
Comparison 2 Early versus late supportive care with ribavirin, Outcome 1 Mortality.
6.
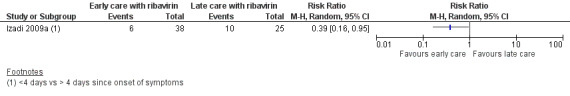
Forest plot early versus late ribavirin, outcome: mortality in early versus late ribavirin.
Whilst an association was seen between early supportive care and ribavirin and reduced mortality in one included study at serious risk of bias, we are uncertain if early ribavirin is more effective than late ribavirin in treating CCHF. Separating the effect of early presentation to hospital, early diagnosis and early supportive care from the effect of early ribavirin treatment is very difficult without an adequately‐powered randomised study.
Subsidiary descriptive analyses
Ribavirin versus no ribavirin
In the subsidiary descriptive analysis we explored the effect of confounding on the effect estimates for ribavirin versus no ribavirin. We included 10 studies at critical risk of bias that reported mortality outcomes. We established a non‐overlapping sample using the methods described in Appendix 2 and present these in a forest plot alongside the single RCT and cohort studies at serious risk of bias (Analysis 3.1; Figure 7). In these studies with a critical risk of bias, the point estimates shows an effect skewed towards benefit for ribavirin (1 RCT; RR 1.13, 95% CI 0.29 to 4.32; 136 participants; 3 non‐randomized studies at serious risk of bias; RR 0.72, 95% CI 0.42 to 1.28; 549 participants; 10 non‐randomized studies at critical risk of bias RR 0.43, 95% CI 0.22 to 0.86; 1214 participants). There was also increasing heterogeneity (NRS serious risk of bias I2 statistic = 0%; NRS critical risk of bias I2 statistic = 58%). This supports the conclusions of a previous meta‐analysis (Soares‐Weiser 2010) that the effect seen is likely to be attributable to confounding and that no evidence of benefit could be drawn. Secondly, our descriptive analysis demonstrates that a critical failure to control for confounding is associated with an increase in heterogeneity and inconsistency between studies.
3.1. Analysis.
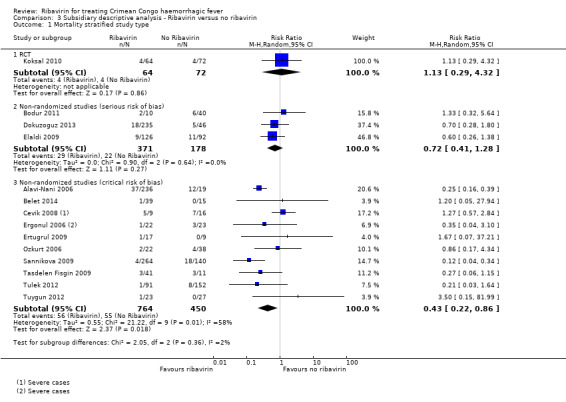
Comparison 3 Subsidiary descriptive analysis ‐ Ribavirin versus no ribavirin, Outcome 1 Mortality stratified study type.
7.
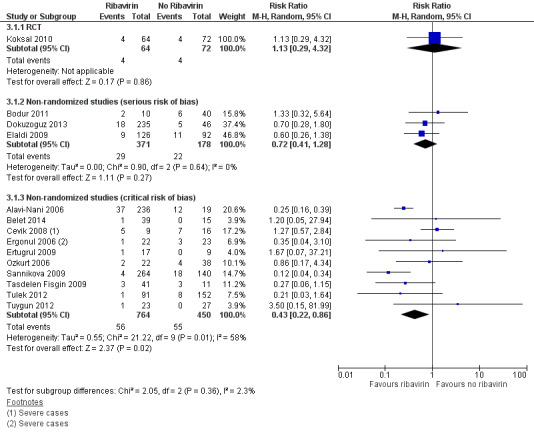
Forest plot of subsidiary descriptive analysis: ribavirin versus no ribavirin, outcome: mortality.
Early versus late supportive care with ribavirin
In the subsidiary descriptive analysis of early versus late ribavirin, we explored the effect of confounding of the effect estimates. We included four studies at critical risk of bias and present these in a forest plot alongside the single non‐randomized study at serious risk of bias (Analysis 4.1; Figure 8).
4.1. Analysis.
Comparison 4 Subsidiary descriptive analysis: early versus late supportive care with ribavirin, Outcome 1 Mortality stratified by study type.
8.
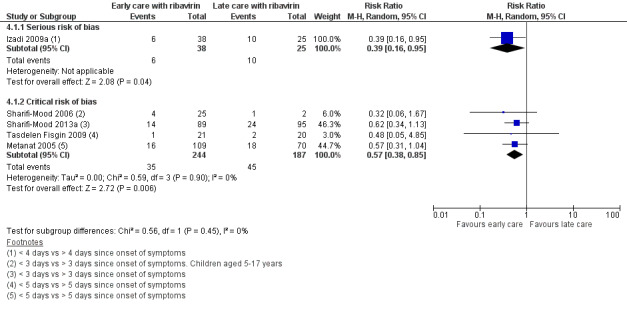
Forest plot of comparison: 4 Subsidiary descriptive analysis: early versus late supportive care with ribavirin, outcome: 4.1 Mortality stratified by study type.
Our subsidiary descriptive analysis showed an association between early supportive care with ribavirin and a reduction in mortality in studies with critical risk of bias (4 NRS; RR 0.57, 95% CI 0.38 to 0.85; 431 participants), there was no difference in effect compared to the study at serious risk of bias (1 NRS; RR 0.39, 95% CI 0.16 to 0.95; 63 participants; I2 statistic = 0%).
Discussion
Summary of main results
Five studies met the inclusion criteria for our main effects analysis. These was one RCT with 136 participants and four non‐randomized studies with 612 participants. We judged all four non‐randomized studies to have serious risk of bias by ROBINS‐I. There were a further 18 non‐randomized studies classified at critical risk of bias which we included in a subsidiary descriptive analysis. None of these studies attempted to control for confounding.
We do not know if ribavirin reduces mortality (very low‐certainty evidence).
We do not know if ribavirin is more effective when given early with supportive care rather than late with supportive care (very low‐certainty evidence), and we do not know if ribavirin reduces the length of stay in hospital (very low‐certainty evidence).
In terms of possible adverse effects, we do not know if it reduces the risk of patients needing platelet transfusions (very low‐certainty evidence), and we do not know what the adverse effects of treating CCHF with ribavirin are, because there is a lack of data for this outcome.
In the subsidiary descriptive analysis of studies with a critical risk of bias, the point estimates show an effect skewed towards benefit for ribavirin, as well as increasing heterogeneity.
Overall completeness and applicability of evidence
This review includes a single RCT and 22 non‐randomized studies from multiple countries in Europe and Asia. We found no studies from Africa, where CCHF is also endemic.
There is insufficient reliable evidence to be confident of the effects of ribavirin on mortality, length of hospital stay or the need for transfusion of blood products. There is insufficient high‐quality evidence to draw conclusions about the likelihood of serious adverse events occurring when administering ribavirin to people infected with CCHF. Ribavirin is frequently used in the treatment of hepatitis C and the side‐effect profile is well established (Brok 2009). However, given different dosing schedules and the differences in the length of use of ribavirin, we do not think this evidence is sufficiently generalizable to CCHF.
We wondered whether the non‐randomized studies would be sufficient to show a benefit for ribavirin if indeed they had a very large effect on mortality and were of sufficient quality. However, all but four of the studies were at critical risk of bias, which means drawing inferences from these studies is not possible (Sterne 2016). In those non‐randomized studies not at critical risk of bias, the evidence base is not of sufficient robustness to draw conclusions about benefit or harm, given our concerns about the internal validity of the studies and imprecision of the effect estimates.
Certainty of the evidence
The overall certainty of the evidence for all outcomes was very low. Any estimate of effect is highly uncertain and is likely to change with further research on the treatment of CCHF. Most research done in this area is of non‐randomized designs and is critically compromised by uncontrolled confounding and small sample sizes. Because of this, we are unable to reach any conclusions on the efficacy of ribavirin for treating CCHF.
For mortality, the single RCT, which was the study with the most reliable internal validity and which we felt provided the most reliable effect estimate, was at high risk of bias and underpowered to show an effect, with few events. As a result we downgraded it to very low‐certainty evidence for the outcome of mortality.
For mortality in early versus late ribavirin, all studies were of a non‐randomized design at serious risk of bias. The pooled effect estimate included few events and broad CIs, which meant we downgraded the evidence to very low certainty.
For length of hospital stay, the single RCT was at high risk of bias and underpowered to show an effect, with few events. We therefore downgraded it to very low‐certainty evidence for this outcome.
For the requirement for transfusion of blood products, the single RCT was at high risk of bias and underpowered to show an effect, with few events. We therefore downgraded it to very low certainty evidence for this outcome.
Potential biases in the review process
We have minimized the effect of confounding bias on the effect estimates in the non‐randomized studies by only presenting those at serious, moderate, high, low or unclear risk of bias in the main analysis. To describe the effect of confounding we conducted a subsidiary analysis only including those studies at critical risk of bias. We used the latest tools in assessing risk of bias in non‐randomized studies. We sought guidance from specialist methodologists developing the ROBINS‐I tool to aid our processes. Despite these efforts, we included no studies in this review with a low risk of bias, which means that confounding is still likely to bias any estimates in the main effects analysis.
The included studies populations largely came from Turkey and Iran, with little evidence available from other countries, although we attempted to include a broad range of geographic locations by searching extensively for literature and by including a PhD thesis from Russia (Sannikova 2009).
Agreements and disagreements with other studies or reviews
A previous systematic review (Soares‐Weiser 2010) concluded that there was no clear evidence of benefit from the data then available, as non‐randomized studies were heavily confounded. In our review we have tried to stratify analysis by different degrees of confounding in the studies. This analysis agrees with the opinion of the authors of the Soares‐Weiser review that the effect seen in their meta‐analysis was likely to have been due to confounding in non‐randomized studies.
Soares‐Weiser 2010 included two further studies not included in our review. We excluded these studies because of a sample size of less than 10 participants (Jamil 2005), and the lack of a comparator arm (Nadeem 2003). See the Excluded studies section.
We agree with the assessment of the authors of the Ascioglu 2011 review about the internal validity of the included studies and the effect of systematic bias on the effect estimate. We further agree that the results of a meta‐analysis of flawed studies cannot be used as evidence of an effect, and that a randomised controlled trial is needed and ethically justified, given the ambiguity of observational studies.
All studies included in the Ascioglu 2011 systematic review are included in our review.
We agree with the two previous systematic reviews on this topic (Soares‐Weiser 2010; Ascioglu 2011). We cannot draw conclusions about the efficacy of ribavirin for treating Crimean Congo haemorrhagic fever using the data currently available. This is largely attributable to too few studies that adequately control for confounding and the lack of a reliable RCT. Any estimate of effect based on currently available data is very uncertain.
Research in outbreaks
In a broader sense, the current status of the evidence for ribavirin in CCHF highlights the difficulties when non‐randomized studies or consensus is used to establish a treatment in the absence of reliable evidence. Once established as standard practice, clinicians feel uneasy about the ethics of conducting a placebo controlled trial whether reliable evidence of efficacy exists or not. This is made more acute because of a previous lack of preparedness for experimental research therapeutics in outbreak situations. In 2016 WHO issued guidance on managing ethical issues in infectious disease outbreaks which highlights the need to learn as much as possible as quickly as possible and that in such situations where no proven treatment exists research should be conducted using rigorous methodology that is capable of providing valid results (WHO 2016).
Whilst monitored use of experimental or unproven therapies can be ethically justifiable in outbreak situations provided; 1) no proven effective treatment exists; 2) it is not possible to initiate clinical studies immediately; 3) data providing preliminary support of the intervention’s efficacy and safety are available; 4) the relevant country authorities, as well as an appropriately qualified ethics committee, have approved such use; 5) adequate resources are available to ensure that risks can be minimized; 6) the patient’s informed consent is obtained; and 7) the emergency use of the intervention is monitored and the results are documented and shared in a timely manner with the wider medical and scientific community (WHO 2016). It is important always to be clear that no harm is likely, considering the potential for causing harm is important as sometimes there is a perception that any intervention will help because of the high mortality, however, a harmful intervention could push case fatality rates even higher as well as potentially costing valuable time and resources.
As this review demonstrates, establishing efficacy of a therapeutic in acute infectious diseases using observational or non‐randomised data is difficult and results can be unreliable. As such the ability to conduct methodologically rigorous research that is able to demonstrate efficacy should be a requirement of any use of unproven therapeutics in outbreak situations.
Authors' conclusions
Implications for practice.
We do not know from the current literature if ribavirin is an effective treatment for CCHF. Most research on this question is of a non‐randomized design and is critically confounded. Any estimates of effect based on the existing literature is highly uncertain and likely to change with further methodologically rigorous research.
Implications for research.
This review improves on the previous systematic reviews by including all the relevant information from observational studies and assessing them systematically. We have used the latest methods examining confounding and other important methodological aspects important in assessing the findings of non randomised studies looking at the effects of ribavirin in CCHF.
There remains considerable controversy on the effects of ribavirin in CCHF and whether to use it, reflecting true uncertainty in the field, with some strong advocates (Ergonul 2006); and others recommend supportive care only (Kalin 2014). These clear variations in practice and viewpoints and the lack of any clear message from this independent systematic review of the of the evidence point us in the direction of a randomised clinical trial to establish or disprove the efficacy of ribavirin, as has been suggested previously (Soares‐Weiser 2010; Ascioglu 2011).
Acknowledgements
Dr Gerry Davies was the Academic Editor for this review.
We acknowledge the contribution made by Cochrane Response in the work carried out for this review and the comments and contribution made by the Expert Networks in the Department of Infectious Disease Hazard Management, WHO, in the development of the research question studied in this review.
We thank those who reviewed the manuscript and Gerry Davies, Contact Editor, for their comprehensive assessments of this review.
We thank Paul Garner for his insight and guidance throughout this review in his role as Co‐ordinating Editor.
We thank Karla Soares Weiser, who contributed to planning and conducting this review.
We also thank Marty Richardson for providing statistical support in the analysis.
We are grateful to the ROBINS‐I development team for methodological advice in using the ROBINS‐I tool.
We thank Vittoria Lutje, Information Specialist of the Cochrane Infectious Diseases Group (CIDG), for kindly conducting searches for us.
We thank Dr Natalia Pshenichnaya for providing access to Sannikova 2009 and for translating this text originally written in Russian.
We thank Dr Tina Bani for her help in translating texts written in Farsi.
Samuel Johnson and the editorial base of the Cochrane Infectious Diseases Group are supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
This work was supported through a grant from World Health Organization (WHO) Crimean‐Congo Hemorrhagic Fever Clinical Practice Guidelines Agreement for Performance of Work (APW) Grant 2017 (number 702828)
Appendices
Appendix 1. Search strategies
MEDLINE (PubMed)
#1 Search "Hemorrhagic Fever, Crimean"[Mesh] OR "Hemorrhagic Fever Virus, Crimean‐Congo"[Mesh]
#2 Search CCHF [Title/Abstract] OR "Crimean Congo hemorrhagic fever" [Title/Abstract] or "Crimean Congo haemorrhagic fever" [Title/Abstract]
#3 Search #1) or #2
#4 Search "ribavirin"[MeSH Terms] OR "ribavirin" [Title/Abstract]
#5 Search #3) and #4
Cochrane Library
Cochrane Central Register of Controlled Trials
ID Search Hits
#1 MeSH descriptor: [Hemorrhagic Fever Virus, Crimean‐Congo] explode all trees
#2 MeSH descriptor: [Hemorrhagic Fever, Crimean] explode all trees
#3 Crimean‐Congo hemorrhagic fever
#4 Crimean‐Congo haemorrhagic fever
#5 #1 or #2 or #3 or #4
Embase 1947‐Present, updated daily
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Crimean Congo hemorrhagic fever/
2 Crimean‐Congo hemorrhagic fever.mp.
3 Crimean congo hemorrhagic fever virus.mp. or Crimean‐Congo hemorrhagic fever virus/
4 CCHF.mp.
5 1 or 2 or 3 or 4
6 ribavirin/ or ribavirin.mp.
7 5 and 6
(from Web of Science Core Collection)
You searched for: TOPIC: ("Crimean Congo hemorrhagic fever" or CCHF) ANDTOPIC: (ribavirin or treatment)
Timespan: All years. Indexes: SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, IC.
Interface ‐ EBSCOhost Research Databases
CINAHL
| # | Query |
| S4 | S2 AND S3 |
| S3 | TX ribavirin OR MH ribavirin |
| S2 | "Crimean Congo hemorrhagic fever OR Crimean‐Congo haemorrhagic fever" |
| S1 | "Crimean Congo hemorrhagic fever OR Crimean‐Congo haemorrhagic fever" |
Appendix 2. Subsidiary descriptive analysis methods
Methods
We conducted a subsidiary descriptive analysis to quantify and help explain the effect of confounding on the effect estimate for the outcome of mortality. We included all non‐randomized studies at critical risk of bias in this descriptive analysis.
We established the sample from non‐overlapping studies as set out below . We made the following decisions on overlapping samples based on the following decision rules:
We considered populations to be overlapping if they were conducted in:
the same country, region and/or hospital; and
the same or overlapping time periods; and
the same population characteristics (for example, age); and
the same intervention (for example, route of administration).
We did not consider author names as indicators of potentially overlapping populations.
Where studies' populations potentially overlapped, we contacted study authors for confirmation.
We selected the study with the largest number of participants that most directly assessed the review question
Risk of bias
We classified all studies as being at critical risk of bias due to confounding. None of the studies controlled for confounding due to severity of disease or length of time from onset of symptoms to administration of ribavirin. These confounding factors were set out a priori and full details are given in Table 3.
Results of sensitivity analysis
We included 10 studies at critical risk of bias (Alavi‐Nani 2006; Ergonul 2006; Ozkurt 2006; Cevik 2008; Ertugrul 2009; Sannikova 2009; Tasdelen Fisgin 2009; Tulek 2012; Tuygun 2012; Belet 2014), that did not have overlapping populations using the methods outlined above.
The studies considered and deemed to be overlapping are set out below*.
| Ribavirin versus no ribavirin: mortality | |||||
| Alavi‐Nani 2006 | 1999‐2004 | Iran | Boo‐Ali Hospital | 255 | Children |
|
Sharifi‐Mood 2006 (overlaps with Alavi‐Nani 2006) |
1999‐2006 | Iran | Boo‐Ali Hospital | 25 | Children |
|
Mardani 2003 (overlaps with Alavi‐Nani 2006) |
1999‐2001 | Iran | Multi‐region | 187 | Includes Sistan‐Baluchestan province, where Boo‐Ali hospital is located. Although age range not reported for this study, 69% < 33 years, and substantial overlap with Alavi‐Naini is suspected |
| Ergonul 2006 | 2002‐2004 | Turkey | Ankara | 45 | Severe cases only |
| Ergönül 2004 (overlaps with Ergonul 2006) | 2002‐2003 | Turkey | Ankara | 35 | ‐ |
| Tuygun 2012 | 2005‐2010 | Turkey | Ankara | 50 | Children |
|
Gayretli Aydin 2015 (overlaps with Tuygun 2012) |
2005‐2013 | Turkey | Ankara | 26 | Children |
|
Tezer 2016 (overlaps with Tuygun 2012) |
2009‐2014 | Turkey | Ankara | 46 | Children |
| Cevik 2008 | 2006 | Turkey | Ankara | 25 | IV RBV only (all other studies were oral or not reported) |
| Tulek 2012 | 2007‐2012 | Turkey | Ankara | 243 | Mean age 51 years (SD 18) and 48 years (SD 17), so most participants suspected to be adults, and not to overlap substantially withTuygun 2012 |
|
Ertem 2016 (overlaps with Tulek 2012) |
2007‐2010 | Turkey | Ankara | 100 | ‐ |
|
Kalin 2014 (overlaps with Tulek 2012) |
2007‐2010 | Turkey | Ankara | 81 | ‐ |
| Ertugrul 2009 | 2007‐2008 | Turkey | Aydin | 26 | ‐ |
| Ozkurt 2006 | 2002‐2004 | Turkey | Erzurum | 60 | ‐ |
| Tasdelen Fisgin 2009 | 2004‐2007 | Turkey | Samsun | 52 | ‐ |
| Belet 2014 | 2008‐2011 | Turkey | Samsun | 54 | Children |
| Sannikova 2009 | 1999‐2008 | Russia | Stavropol | 404 | ‐ |
*Studies in bold included in descriptive analysis.
The effects are described and discussed in the main text.
The effect observed was largely driven by two studies (Alavi‐Nani 2006; Sannikova 2009).
We considered Alavi‐Nani 2006 to be at critical risk of bias, as there was no control for severity of disease as a confounder. The intervention and control groups were highly unbalanced and we therefore had concerns that selection of participants into the two arms was closely related to severity of disease. This is important in the context of CCHF, given the likelihood of patients developing gastro‐intestinal bleeding and therefore being unable to receive oral ribavirin (Ergonul 2009).
Sannikova 2009 is a retrospective cohort study using historical controls. The control group was selected from a period of several years before ribavirin was available in Stavropol. We rated this design as critically confounded, as there will have been variable supportive care over this period, and given the emerging nature of the CCHF epidemic as physicians gained more experience in treating this disease and supportive care will have improved outcomes over time.
Appendix 3. ROBINS‐I methods
Risk of bias: ROBINS‐I
Target trial
A designated target trial was theoretically specified to aid in assessment of risk of bias.(Sterne 2016)
Design: individually randomised
Participants: patients with confirmed CCHF
Experimental intervention: oral or parenteral ribavirin at WHO recommended dose.
Comparator: placebo control group with similar supportive care setting and protocols established, for example, for administration of blood products.
The aim of the study is to assess the effect of assignment to the intervention.
We applied an interpretation of domain‐level and overall risk of bias according to ROBINS‐I guidance. We made all judgements based on the guidance provided by signalling questions and reaching risk of bias judgements in ROBINS‐I (Table 12; Sterne 2016).
6. ROBINS‐I Interpretation of domain level and overall risk of bias judgements.
| Judgement | Within each domain | Across domains | Criterion |
| Low risk of bias | The study is comparable to a well‐performed randomised trial with regard to this domain | The study is comparable to a well‐performed randomised trial | The study is judged to be at low risk of bias for all domains |
| Moderate risk of bias | The study is sound for a non‐randomized study with regard to this domain but cannot be considered comparable to a well‐performed randomised trial | The study provides sound evidence for a non‐randomized study but cannot be considered comparable to a well‐performed randomised trial | The study is judged to be at low or moderate risk of bias for all domains |
| Serious risk of bias | the study has some important problems in this domain | The study has some important problems | The study is judged to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain |
| Critical risk of bias | the study is too problematic in this domain to provide any useful evidence on the effects of intervention | The study is too problematic to provide any useful evidence and should not be included in any synthesis | The study is judged to be at critical risk of bias in at least one domain |
| No information | No information on which to base a judgement about risk of bias for this domain | No information on which to base a judgement about risk of bias | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in one or more key domains of bias (a judgement is required for this) |
Reproduced from Sterne 2016.
Criteria used to assess risk of bias within ROBINS‐I tool
Death, death in those with early or late administration of ribavirin, length of hospital stay and requirement for transfusion were considered sufficiently related to have similar confounding factors associated with them. We considered classification of risk of bias for these outcomes using the following domains. We gave separate consideration to serious adverse events and withdrawal of treatment.
Listing of confounding domains
Controlling for baseline confounding is an issue that affects most non‐randomized studies of interventions (NRSIs). Baseline confounding occurs when one or more prognostic variables (factors that predict the outcome of interest) also predict the intervention received at baseline (Sterne 2016). As part of the ROBINS‐I assessment, we considered the length of time from onset of symptoms to start of ribavirin and severity of disease as confounding domains. We made the decisions to include these as confounding domains based on extensive debate in the literature (Ergonul 2009). We listed co‐interventions that could differ between intervention groups impacting on outcomes as quality of supportive care.
For the purposes of assessing confounding we considered all except one of our outcomes sufficiently related to have common confounding factors and so they were assessed in a group, as in the ROBINS‐I guidance. (Sterne 2016) The only outcome assessed separately was serious adverse events.
Length of time from onset of symptoms
The pathology of CCHF presents in multiple stages with viraemia labile and typically present early in the disease. Supportive treatment early in the condition is therefore important and any antiviral effect is likely to be more effective if antivirals are initiated earlier in the disease course.
We considered any study that did not take into account the length of time since onset of symptoms before starting ribavirin to be at risk of bias.
Severity of disease
CCHF can present at a variety of severities. If ribavirin were only to be prescribed to patients with severe disease, any analysis would be biased toward ribavirin being harmful. Furthermore, treatment of all CCHF cases with ribavirin except for those in whom it was contraindicated (for example, patients with haematemesis, other significant co‐morbidities or late presentation to medical care) would bias results in showing a false efficacy of ribavirin. Documentation of disease severity and indication for not receiving ribavirin where it is standard practice is therefore critical for any balanced analysis.
Historical controls and quality of supportive care
In any emerging epidemic, it is necessary for medical professionals and institutions to develop and constantly improve the way they treat patients. Given supportive care is of critical importance to the management of CCHF, it follows that improving standards of supportive and nursing care and development of local expertise and management protocols would cause mortality to decline. As such any use of a historical control arm was considered methodologically confounded. The degree to which any use of a historical control arm confounds the estimate of effect and whether this placed a study at serious or critical risk of bias was assessed on a study by study basis.
Bias in selection of participants into the study
Retrospective cohort Studies
Information describing how participants were selected into any analysis must be included in any analysis. Failure to do so renders any study at critical risk of bias, due to the risk of selection bias.
Bias in classification of intervention
Studies were required to define the route of ribavirin which was administered.
Bias due to deviations from intended interventions
Varying length of ribavirin administration may impact outcomes. Studies were required to give information on the duration of treatment. We judged this against WHO‐recommended treatment regimens (WHO 2015).
Bias due to missing data
Data from all participants should be included in any analysis.
Bias in measurement of outcomes
Outcomes must be well defined within any analysis, for example mortality, or directly measurable, for example laboratory results. Serious adverse events must have an analysis of the degree to which any serious adverse event was attributable to the intervention. This is particularly difficult in ribavirin for CCHF, given the overlap between side effects and the disease process, as previously outlined.
Bias in selection of the reported result
Reporting of outcomes should be in keeping with a prespecified study protocol. If this was not feasible prespecified outcomes should be made clear in the Methods section.
Data and analyses
Comparison 1. Ribavirin versus no ribavirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RCT | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.29, 4.32] |
| 1.2 Non‐randomized studies | 3 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| 2 Mortality stratified by severity of disease (Dokuzoguz 2013) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Length of hospital stay (days) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 RCT | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Non‐randomized studies | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Requirement for transfusion (platelets) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Early versus late supportive care with ribavirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Subsidiary descriptive analysis ‐ Ribavirin versus no ribavirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality stratified study type | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RCT | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.29, 4.32] |
| 1.2 Non‐randomized studies (serious risk of bias) | 3 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| 1.3 Non‐randomized studies (critical risk of bias) | 10 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.86] |
Comparison 4. Subsidiary descriptive analysis: early versus late supportive care with ribavirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality stratified by study type | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Serious risk of bias | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.16, 0.95] |
| 1.2 Critical risk of bias | 4 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alavi‐Nani 2006.
| Methods | Retrospective cohort study | |
| Participants | 255/155 confirmed cases Age NR, Gender 22.4% female | |
| Interventions | Ribavirin 30 mg/kg initial loading dose; 15 mg/kg 4 x daily for 4 days; 7.5 mg/kg 3 x daily for 6 days oral | |
| Outcomes | Mortality | |
| Location and dates | Boo‐Ali Hospital, Zahedan + Zabol: Sistan‐Baloochestan province, Iran Unclear ‐ whether patients treated at hospital that is source of study June 1999 ‐ February 2004 | |
| Number of days since onset of symptoms (mean/SD) | Mean incubation period 4.4 days (SD = 2.6, range 1 ‐ 14) (whole study population) | |
| Supportive therapy | NR | |
| Notes | ||
Belet 2014.
| Methods | Retrospective cohort study | |
| Participants | 167 suspected, 54 confirmed cases, mean age 12.8 years (SD = 3.3) | |
| Interventions | Ribavirin 30 mg/kg initial loading dose; 15 mg/kg 4 x daily for 4 days; 7.5 mg/kg 3 x daily for 6 days oral | |
| Outcomes | Mortality, bradycardia | |
| Location and dates | University Faculty of Medicine, Samsun, Turkey; Tertiary care centre CCHF reference centre May 2008 ‐ September 2011 | |
| Number of days since onset of symptoms (mean/SD) | Mean: 3.6 (SD = 2.4) (range = 1 ‐ 15) | |
| Supportive therapy | FFP, thrombocyte suspension or erythrocyte suspension | |
| Notes | Children | |
Bodur 2011.
| Methods | Matched retrospective cohort study | |
| Participants | 50 confirmed cases | |
| Interventions | Ribavirin 4 g/day for 4 days and then 2.4 g/day for 6 days oral | |
| Outcomes | Mortality, length of hospitalisation, requirement for transfusion (PRC), requirement for transfusion (FFP), requirement for transfusion (platelets) ‐ RBV versus no RBV | |
| Location and dates | Ankara Numune Education and Research Hospital, Ankara, Turkey 2006 ‐ 2008 | |
| Number of days since onset of symptoms (mean/SD) | Ribavirin 4.3 ± 1.4 (to hospitalisation); Control group 4.4 ± 1.4 (to hospitalisation) | |
| Supportive therapy | Erythrocytes, platelets, FFP, or hydration according to homeostatic status | |
| Notes | ||
Cevik 2008.
| Methods | Case‐control study | |
| Participants | 25 confirmed cases Age NR, Sex NR | |
| Interventions | Ribavirin loading dosage of 17 mg/kg IV, then 17 mg/kg every 6 h for 4 days, and then 8 mg/kg every 8 h for 6 days Intravenous administration | |
| Outcomes | Mortality, SAEs, length of hospitalisation, treatment discontinuation, requirement for transfusion (PRC, FFP, platelets) | |
| Location and dates | Ankara Numune Education and Research Hospital and Sivas Cumhuriyet University Hospital, Ankara, Turkey May ‐ August 2006 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | NR | |
| Notes | ||
Dokuzoguz 2013.
| Methods | Prospective and retrospective cohort study | |
| Participants | 281 confirmed cases, mean age 47 (SD = 16) (range = 16 ‐ 86), 49% women | |
| Interventions | Ribavirin with or without corticosteroids, Ribavirin: 4 g daily for 4 days, followed by 2.4 g daily for 6 days; Corticosteroids: 10 mg/m2 dexamethasone; oral ribavirin, corticosteroids route NR. Unclear proportions of patients received steroids |
|
| Outcomes | Severity scoring index, mortality | |
| Location and dates | Ankara Numune Education and Research Hospital, Ankara, Turkey tertiary centre 2004 ‐ 2011 | |
| Number of days since onset of symptoms (mean/SD) | All patients < 7 days | |
| Supportive therapy | Erythrocytes, platelets,and total blood according to homeostasis needs | |
| Notes | ||
Elaldi 2009.
| Methods | Historical control study | |
| Participants | 218 confirmed cases, Mean age: ribavirin group mean 44.4 (SD = 19.1); No‐ribavirin group mean 40.9 (SD = 16.7), 50% women |
|
| Interventions | Ribavirin 30 mg/kg initial loading dose; 15 mg/kg 4 x daily for 4 days; 7.5 mg/kg 3 x daily for 6 days; Oral (nasogastric tube if oral not possible) | |
| Outcomes | Mortality, length of hospital stay, requirement for transfusion (PRC, FFP, platelets) | |
| Location and dates | Cumhuriyet University; Ankara Numune Training Hospital, Ataturk University Research Hospital; Ondokuz Mayis University, Sivas; Ankara; Erzurum; Samsun, Turkey tertiary centres 2004 | |
| Number of days since onset of symptoms (mean/SD) | Median 5 (range = 1 ‐ 11) | |
| Supportive therapy | Erythrocyte suspensions,platelet suspensions, FFP and other supportive as required | |
| Notes | ||
Ergonul 2006.
| Methods | Retrospective cohort study | |
| Participants | 54 confirmed cases | |
| Interventions | Ribavirin 4 g 4 x daily for 4 days, and 2.4 g 4 x daily for 6 days; oral | |
| Outcomes | Mortality | |
| Location and dates | Ankara Numune Education and Research Hospital, Ankara, Turkey tertiary centre 2002 ‐ 2004 | |
| Number of days since onset of symptoms (mean/SD) | 5.5 | |
| Supportive therapy | Erythrocytes, platelets, and total blood according to homeostasis needs | |
| Notes | ||
Ergönül 2004.
| Methods | Prospective cohort study | |
| Participants | 35 confirmed cases Mean age 43 (SD = 17), 51% women | |
| Interventions | Ribavirin 4 g 4 x daily for 4 days, and 2.4 g 4 x daily for 6 days; oral | |
| Outcomes | Mortality (severe CCHF cases only) | |
| Location and dates | Ankara Numune Education and Research Hospital, Ankara, Turkey tertiary centre 2002 ‐ 2003 | |
| Number of days since onset of symptoms (mean/SD) | 5.5 (SD = 1.7) | |
| Supportive therapy | Erythrocytes, platelets, and total blood according to homeostasis needs | |
| Notes | ||
Ertem 2016.
| Methods | Retrospective cohort study | |
| Participants | 56 confirmed cases | |
| Interventions | Ribavirin 2 g as an initial loading dose, then 1 g 4 x daily for 4 days, and then 0.5 g 4 x daily for 6 days Oral ribavirin | |
| Outcomes | Mortality, length of hospitalisation, requirement for transfusion (FFP), requirement for transfusion (platelets), SAEs | |
| Location and dates | Ankara Training and Research Hospital in Central Anatolia, Ankara, Turkey tertiary centre 2007 ‐ 2010 | |
| Number of days since onset of symptoms (mean/SD) | Early ribavirin, median 2 (range = 1 ‐ 5 days); Late ribavirin, median 5 (range = 4 ‐ 8 days); No ribavirin, median 3 (range 1 ‐ 10 days) | |
| Supportive therapy | Erythrocytes, platelets, FFP, or hydration as needed | |
| Notes | Comparator ‐ late ribavirin | |
Ertugrul 2009.
| Methods | Retrospective cohort study | |
| Participants | 61 cases, 26 confirmed | |
| Interventions | Ribavirin ‐ route and dose NR | |
| Outcomes | Mortality | |
| Location and dates | Adnan Menderes, University Medical Faculty, Aydin, Turkey, Hospital/community:18/26 cases admitted to hospitals April 2007 ‐ Jun 2008 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | NR | |
| Notes | ||
Gayretli Aydin 2015.
| Methods | Retrospective cohort study | |
| Participants | 26 confirmed cases Age 10 years ± 2, sex 30.7% female |
|
| Interventions | Ribavirin 30 mg/kg as an initial loading dose, followed by 15 mg/kg every 6 h for 4 days, and then 7.5 mg/kg every 8 h for 6 days; oral | |
| Outcomes | Mortality | |
| Location and dates | Maternity and Children’s Research and Education Hospital, Ankara, Turkey, tertiary hospital 2005 ‐ 2013 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | Replacement of fluid and electrolytes, and administration of platelet suspension, FFP and erythrocyte suspension | |
| Notes | Study only included children | |
Izadi 2009a.
| Methods | Retrospective cohort study | |
| Participants | 63 confirmed cases Mean age 29.2 years (range = 11 ‐ 75 years), sex NR | |
| Interventions | Early ribavirin (< 4 days), late ribavirin (> 4 days) For adults, 2 g of ribavirin had been prescribed initially as a loading dose, followed by 1 g every 6 h for 4 days and then 500 mg every 8 h for 6 days. For children, a 30 mg/kg bolus was initially administered, followed by 15 mg/kg every 6 h for 4 days; oral |
|
| Outcomes | Mortality | |
| Location and dates | Boo‐Ali Educational Hospital, Zahedan, Iran, tertiary centre, 2000 ‐ 2006 | |
| Number of days since onset of symptoms (mean/SD) | Mean 5.0 (SD = 1.6) | |
| Supportive therapy | Blood products | |
| Notes | ||
Kalin 2014.
| Methods | Retrospective cohort study | |
| Participants | 81 confirmed cases Mean age: ribavirin group 54 ± 14.98; no‐ribavirin group 42.81 ± 16.50 | |
| Interventions | Ribavirin 2 g loading then 4 g/day maintenance; oral ribavirin | |
| Outcomes | Mortality, requirement for transfusion (FFP, PRC, platelets) | |
| Location and dates | Erciyes University Hospital and Yozgat State Hospital, Kayseri and Yozgat, Turkey, tertiary centre. January 2007 ‐ December 2010 | |
| Number of days since onset of symptoms (mean/SD) | Ribavirin: median = 5 days, No ribavirin: median = 7 days | |
| Supportive therapy | Erythrocytes, platelets, FFP, or hydration according to homeostatic status | |
| Notes | Severity assessed according to Swanepoel and Ergonul criteria. | |
Koksal 2010.
| Methods | RCT | |
| Participants | 136 confirmed cases Mean age 49.2 | |
| Interventions | Ribavirin 30 mg/kg initial loading dose; 15 mg/kg 4 x daily for 4 days; 7.5 mg/kg 3 x daily for 6 days; Oral ribavirin | |
| Outcomes | Mortality, length of hospital stay, requirement for transfusion. | |
| Location and dates | Karadeniz Hospital, Trabzon, Turkey, tertiary centre; June 2004 ‐ August 2007 | |
| Number of days since onset of symptoms (mean/SD) | Ribavirin: mean 4.5 (SD = 2.5); No ribavirin: mean 3.9 (SD = 2.4) | |
| Supportive therapy | Supportive care and fluid, platelet, FFP, blood products as necessary | |
| Notes | ||
Mardani 2003.
| Methods | Retrospective cohort study | |
| Participants | 139 suspected, 69 confirmed cases Age: 68.9% < 33 years of age |
|
| Interventions | Ribavirin 30 mg/kg initial loading dose; 15 mg/kg 4 x daily for 4 days; 7.5 mg/kg 3 x daily for 6 days; Oral (nasogastric tube of oral not possible) | |
| Outcomes | Mortality | |
| Location and dates | Shahid Beheshti University and regional hospitals, Tehran and regions: Sistan Balouchestan, Esfahan, Golestan, Iran Patients treated at "local hospitals where they had presented"; June 1999 ‐ September 2001 | |
| Number of days since onset of symptoms (mean/SD) | Mean 4 days | |
| Supportive therapy | NR | |
| Notes | ||
Metanat 2005.
| Methods | Retrospective cohort study | |
| Participants | 179 cases Age; NR | |
| Interventions | Oral ribavirin, dose NR; early intervention < 5 days since onset of symptoms | |
| Outcomes | Mortality | |
| Location and dates | Boo‐Ali Hospital in Zahedan, Zahedan, Iran tertiary centre; Dates NR | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | NR | |
| Notes | Conference abstract | |
Ozkurt 2006.
| Methods | Retrospective cohort study | |
| Participants | 60 confirmed cases Mean age: 40 ± 17 (range = 15 – 76) years |
|
| Interventions | Ribavirin 2000 mg orally initial loading dose, then 1000 mg every 6 h for 4 days, and then 500 mg every 6 h for 6 days; Oral | |
| Outcomes | Mortality, duration of hospitalisation, SAEs, requirement for transfusion (PRC), requirement for transfusion (FFP), requirement for transfusion (platelets) | |
| Location and dates | Ataturk University Research Hospital, Eastern Turkey, Turkey tertiary centre 2002 ‐ 2004 | |
| Number of days since onset of symptoms (mean/SD) | Ribavirin group 6 (SD = 2.27); No‐ribavirin group 6.5 (SD = 3.46) | |
| Supportive therapy | Fluid, platelet, blood, or components were replaced if necessary | |
| Notes | ||
Sannikova 2009.
| Methods | Retrospective cohort study [PhD thesis] | |
| Participants | 404 confirmed cases | |
| Interventions | Ribavirin: 1200 mg if > 75 kg, 1000 mg if < 75 kg | |
| Outcomes | Mortality | |
| Location and dates | Stavropol' State Medical Academy, Russia 1999‐2008 | |
| Number of days since onset of symptoms (mean/SD) | Early ribavirin 1 ‐ 3 days; Late ribavirin 2 ‐ 6 days | |
| Supportive therapy | Blood products and fluids as indicated | |
| Notes | ||
Sharifi‐Mood 2006.
| Methods | Retrospective cohort study | |
| Participants | 29 confirmed cases Age 5 ‐ 17 years | |
| Interventions | Early ribavirin (< 3 days) 30 mg/kg as an initial dose, then 15 mg/kg every 6 h for 4 days, then 7.5 mg/kg every 8 h for 6 days; Late ribavirin (> 3 days); Oral ribavirin | |
| Outcomes | Mortality | |
| Location and dates | Departments of Infectious Diseases, Boo‐Ali Hospital and Iman‐Ali Hospital in Zabol, Province: Sistan and Baluchistan (south‐east Iran), Province: Sistan and Baluchistan (south‐east Iran) tertiary centre June 1999 to February 2006 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | NR | |
| Notes | Study conducted only in children | |
Sharifi‐Mood 2013a.
| Methods | Retrospective cohort study | |
| Participants | 184 cases, Age NR |
|
| Interventions | Early ribavirin (< 3 days) 30 mg/kg of body weight as an initial dose and then 15 mg/kg every 6 h for 4 days, and thereafter 7.5 mg/kg for 6 days; Oral Comparator: Late ribavirin (> 3 days) Same regimen as early ribavirin; Oral | |
| Outcomes | Mortality | |
| Location and dates | Boo‐Ali Hospital, Sistan and Baluchestan, in Southeast of Iran, Iran tertiary centre January 2000 ‐ September 2005 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | NR | |
| Notes | ||
Tasdelen Fisgin 2009.
| Methods | Retrospective cohort study | |
| Participants | 52 cases (unknown if confirmed) | |
| Interventions | Ribavirin versus no ribavirin; dose and route not recorded | |
| Outcomes | Mortality, requirement for transfusion (platelets) | |
| Location and dates | Ondokuz Mayis University Faculty of Medicine, Samsun, Turkey tertiary centre 2004 ‐ 2007 | |
| Number of days since onset of symptoms (mean/SD) | Early ribavirin 1 ‐ 4 days, late ribavirin 5 or more days | |
| Supportive therapy | ||
| Notes | ||
Tezer 2016.
| Methods | Retrospective cohort study | |
| Participants | 46 confirmed cases Mean age: ribavirin 11.6; no ribavirin 7.3 | |
| Interventions | Ribavirin, route and dose NR | |
| Outcomes | Mortality, length of hospital stay, requirement for transfusion | |
| Location and dates | Ankara Hematology Oncology Children’s Training and Research Hospital, Ankara, Turkey tertiary centre January 2009 ‐ Novenber 2014 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | Erythrocytes, FFP | |
| Notes | Children only | |
Tulek 2012.
| Methods | Retrospective cohort study | |
| Participants | 243 cases (unclear if suspected or confirmed) Average age NR |
|
| Interventions | Ribavirin, route and dose NR | |
| Outcomes | Mortality | |
| Location and dates | Ankara Hospital, Ankara, Turkey tertiary centre, 2007 ‐ 2011 | |
| Number of days since onset of symptoms (mean/SD) | NR | |
| Supportive therapy | Similar supportive care in 2 departments involved in study ‐ no further information provided | |
| Notes | ||
Tuygun 2012.
| Methods | Retrospective cohort study | |
| Participants | 50 confirmed cases | |
| Interventions | Oral ribavirin 30 mg/kg as an initial loading dose, then 15 mg/kg every 6 h for 4 days, and then 7.5 mg/kg every 8 h for 6 days | |
| Outcomes | Mortality | |
| Location and dates | Dr Sami Ulus Maternity and Children’s Health and Diseases Training and Research Hospital in Ankara, Turkey; tertiary centre 2005 ‐ 2010 | |
| Number of days since onset of symptoms (mean/SD) | 3.5 ± 2.1 (range = 1.0 to 9.0) | |
| Supportive therapy | Erythrocyte/thrombocyte suspension, FFP based on homeostatic status and other supportive care | |
| Notes | ||
Abbreviations: FFP = fresh frozen plasma; h = hours; NR: not reported; PRC: packed red cells; RBV: ribavirin; SAE: serious adverse event; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abuova 2012 | This was a case series with fewer than 10 participants |
| Ajazaj 2013 | This was a case series with fewer than 10 participants |
| Alavi‐Naini 2004 | This is a single case report |
| Ali 2010 | This was a case series with fewer than 10 participants |
| Anon 1984 | This was a survey and therefore was a different study design from our inclusion criteria |
| Ardalan 2006 | This was a single case report |
| Athar 2003 | This was a case series with fewer than 10 participants |
| Athar 2005 | This was a case series with fewer than 10 participants |
| Barr 2013 | This was a single case report |
| Canpolat 2011 | This was a single case report |
| Caylan 2010 | This was a single case report |
| Ceri 2013 | This was a single case report |
| Chinikar 2013 | This was a case series with fewer than 10 participants |
| Dilber 2010 | This was a case series with fewer than 10 participants. |
| Drosten 2002 | This was a single case report and discussion |
| El Bahnasawy 2015 | This was a survey and therefore did not meet inclusion criteria; it was based on a different study design |
| Elata 2011 | This was a case report of a single nosocomial transmission. |
| Ergonul 2009 | This was a commentary on an included study |
| Ergonul 2014 | This was a case series with fewer than 10 participants. |
| Ergonul 2017 | This study compared individuals with CCHF to healthy individuals |
| Fazlalipour 2016 | This was a case series with fewer than 10 participants. |
| Gonen 2014 | This was a case series with fewer than 10 cases |
| Gozel 2013 | This was a case series with fewer than 10 cases |
| Guner 2014 | This was a case series with fewer than 10 participants |
| Gursoy 2014 | Translated from Turkish. This was an editorial letter that reported a single case |
| Hasan 2013 | This was a single case report |
| Izadi 2009b | This is an editorial letter written as a reply to comments on an included study |
| Jabbari 2006 | This was a case series with fewer than 10 participants |
| Jamil 2005 | This was a case series with fewer than 10 participants. |
| Joubert 1985 | This was a case series with fewer than 10 participants. |
| Kadanali 2012 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Kader 2011 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Kleib 2016 | This was a single case report |
| Kubar 2011 | This reported the effects of administration of hyperimmunoglobulin, not ribavirin. |
| Kunchev 2008 | This was a case series with fewer than 10 participants. |
| Leblebicioglu 2016a | This reported on the use of ribavirin for prophylaxis but did not report on ribavirin used as treatment for disease |
| Makwana 2015 | This was a single case report |
| Mardani 2009 | This was case series of fewer than 10 participants |
| Mardani 2013 | This was a case series with fewer than 10 participants |
| Midilli 2007 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Mishra 2011 | This was a single case report |
| MMWR 1984 | This was a case series with fewer than 10 participants |
| Mohamed 2016 | This was a single case report |
| Nabeth 2004 | This was a single case report |
| Naderi 2011 | This was a case series with fewer than 10 participants. |
| Naderi 2013 | This was a case series with fewer than 10 participants. |
| NCT00992693 | This ongoing study did not include a comparator group where no ribavirin is given. |
| Oflaz 2013 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Ozbey 2014 | This did not compare use of ribavirin to supportive care only. It did not report mortality as an outcome in a useable way, reporting only a case fatality ratio in those who were transferred to tertiary centres or not transferred. As such this study did not meet our inclusion criteria. |
| Ozsoy 2015 | This was a case series with fewer than 10 participants |
| Papa 2008 | This was a single case report |
| Pourahmad 2011 | This was a single case report |
| Pshenichnaya 2015 | This was a case series with fewer than 10 participants |
| Raoofi 2012 | This was a case series with fewer than 10 participants |
| Richards 2015 | This was a case series with fewer than 10 participants |
| Sahin 2016 | This was a single case report |
| Saluzzo 1985b | This was a single case report |
| Schwarz 1995 | This was a single case report |
| Scrimgeour 1996 | This was a case series with fewer than 10 participants |
| Sefikotullari 2013 | This case series reported different outcomes from those in our review |
| Sharifi‐Mood 2008 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Sharifi‐Mood 2009 | This was an overlapping study reporting the same data as an included study (Sharifi‐Mood 2006) |
| Sharifi‐Mood 2013b | This was a quasi‐RCT that did not report on ribavirin compared to supportive care only ‐ all participants received ribavirin with or without corticosteroids |
| Sheikh 2005 | This was a cohort study that did not compare ribavirin to supportive care only ‐ no comparator arm |
| Sheikh, 2004 | This was a case series with fewer than 10 participants |
| Smego 2004 | This was a case series with fewer than 10 participants |
| Suleiman 1980 | This was a case series with fewer than 10 participants |
| Sunbul 2016 | This was a single case report |
| Tall 2009a | This was a single case report |
| Tall 2009b | This was a case series with fewer than 10 participants |
| Tatar 2005 | This was a case series with fewer than 10 participants |
| Tezer 2014 | This was a case series with fewer than 10 participants. |
| Tulek 2010 | This was a case series with fewer than 10 participants |
| Tutuncu 2009 | This was a case series with fewer than 10 participants |
| Ugurlu 2013 | This was a single case report |
| Unlusoy 2014 | This was a single case report |
| Uysal 2012 | This was a single case report |
| Van Eeden 1985a | This was a case series with fewer than 10 participants |
| Van Eeden 1985b | This was a case series with fewer than 10 participants |
| Weber 2001 | This was a case series with fewer than 10 participants |
| Yadav 2013 | This did not report on ribavirin use in CCHF |
| Yadav 2016 | This did not report on ribavirin use in CCHF |
| Yesilyurt 2011 | This did not report on ribavirin use in CCHF |
| Yildirmak 2016 | This was a case series with fewer than 10 participants |
| Yilmaz 2009a | This did not report on ribavirin use in CCHF |
| Yilmaz 2009b | This was a case series with fewer than 10 participants |
| Yolcu 2014 | This was a survey that did not report on ribavirin use for treatment of CCHF |
| Zakhashvili 2010 | This was a single case report |
| Öztürk 2012 | This was a case series with fewer than 10 participants |
RCT: randomized controlled trial
Differences between protocol and review
One additional review author joined the review author team (Nicholas Henschke).
We restructured the Background to the review to add clarity.
We also included studies that compared early to late administration of ribavirin. This is an important part of the debate around the effectiveness of ribavirin as a treatment. We allowed definitions of ‘early' and ‘late' to be determined by the study authors.
We did not include any cohort studies without comparators.
We allowed studies that had co‐interventions other than ribavirin, as long as the indications were the same across all arms of the study.
We clarified that the outcome of death amongst those receiving ribavirin early versus late was a separate comparison, but mortality was still the primary outcome.
We amended the prespecified subgroup analyses. We removed early versus late ribavirin as a subgroup and instead included it as a comparison. This was because it required different study designs.
In our protocol we stated that wherever possible we would combine adjusted measures of effect for non‐randomized studies. One study, Dokuzoguz 2013, used a model to control for confounding of effect due to severity of disease. This resulted in an adjusted OR of 0.04 (0.004 to 0.48), which is an extremely large effect. The small sample size, the size of the adjusted effect, missing data from the corticosteroid analysis, concerns about unmeasured confounding factors such as time from onset of symptoms, and the fact that the study analysed severe patients with gastro‐intestinal haemorrhage per protocol and not by intention‐to‐treat meant that we took a conservative approach to synthesis and presented the non‐adjusted stratified data in a forest plot instead of the adjusted estimate.
We also only included cohort studies if they had more than 10 participants.
Contributions of authors
SJ drafted the protocol, extracted data, assessed risk of bias and analysed results. He drafted the final review.
NH extracted data, assessed risk of bias, and helped draft the final review.
NM, IM, AK, and BSB screened, extracted data, and assessed risk of bias.
RM helped draft the protocol, coordinated all aspects of protocol production, screening, data extraction, and analysis of results.
All authors reviewed and commented on the final review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
Cochrane Response, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
-
World Health Organization (WHO), Switzerland.
WHO Crimean‐Congo Hemorrhagic Fever Clinical Practice Guidelines Agreement for Performance of Work (APW) Grant 2017 (number 702828)
Declarations of interest
SJ is supported by the Effective Health Care Research Consortium, which is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). This review was partially funded by WHO as part of a wider project on clinical management of CCHF. Sam attended a WHO Guideline Development Group meeting on CCHF in Berlin in April 2017, and the expenses for this meeting were paid for by the WHO. NH: since June 2016 I have been employed by Cochrane Response, an evidence services unit operated by Cochrane. Cochrane Response was contracted by the WHO to produce this review. Nicholas attended a WHO Guideline Development Group meeting on CCHF in Berlin in April 2017. NM previously worked for Enhanced Reviews Ltd, a company that conducts systematic reviews mostly for the public sector. Since June 2016 I have been employed by Cochrane Response, an evidence services unit operated by Cochrane. Cochrane Response was contracted by the WHO to produce this review. IM: from August 2016 to June 2017 I was employed by Cochrane Response, an evidence services unit operated by Cochrane. Cochrane Response was contracted by the WHO to produce this review. AK has no known conflicts of interest. BSB has no known conflicts of interest. RM was employed by Cochrane Response, and as part of her employment she was involved in the writing of a systematic review for the WHO on CCHF. The review was paid for by the WHO. Some of the work from that systematic review has been included within this Cochrane Review. Rachel also attended a WHO Guideline Development Group meeting on CCHF in Berlin in April 2017, to present the results of the full systematic review. The expenses for this meeting were paid for by the WHO.
Unchanged
References
References to studies included in this review
Alavi‐Nani 2006 {published data only}
- Alavi‐Naini R, Moghtaderi A, Koohpayeh HR, Sharifi‐Mood B, Naderi M, Metanat M, et al. Crimean‐Congo hemorrhagic fever in Southeast of Iran. Journal of Infection 2006;52(5):378‐82. [DOI] [PubMed] [Google Scholar]
Belet 2014 {published data only}
- Belet N, Top A, Terzi O, Arslan HN, Baysal K, Sensoy G. Evaluation of children with Crimean‐Congo hemorrhagic fever in the central Blacksea region. Pediatric Infectious Disease Journal 2014;33(8):e194‐7. [DOI] [PubMed] [Google Scholar]
Bodur 2011 {published data only}
- Bodur H, Erbay A, Akıncı E, Öngürü P, Bayazıt N, Eren SS, et al. Effect of oral ribavirin treatment on the viral load and disease progression in Crimean‐Congo hemorrhagic fever. International Journal of Infectious Diseases 2011;15(1):e44‐7. [DOI] [PubMed] [Google Scholar]
Cevik 2008 {published data only}
- Cevik MA, Elaldi N, Akinci E, Ongürü P, Erbay A, Buzgan T, et al. A preliminary study to evaluate the effect of intravenous ribavirin treatment on survival rates in Crimean‐Congo hemorrhagic fever. Journal of Infection 2008;57(4):350‐1. [DOI] [PubMed] [Google Scholar]
Dokuzoguz 2013 {published data only}
- Dokuzoguz B, Celikbas AK, Gök ŞE, Baykam N, Eroglu MN, Ergönül Ö. Severity scoring index for Crimean‐Congo hemorrhagic fever and the impact of ribavirin and corticosteroids on fatality. Clinical Infectious Diseases 2013;57(9):1270‐4. [DOI] [PubMed] [Google Scholar]
Elaldi 2009 {published data only}
- Elaldi N, Bodur H, Ascioglu S, Celikbas A, Ozkurt Z, Vahaboglu H, et al. Efficacy of oral ribavirin treatment in Crimean‐Congo haemorrhagic fever: a quasi‐experimental study from Turkey. Journal of Infection 2009;58(3):238‐44. [DOI] [PubMed] [Google Scholar]
Ergönül 2004 {published data only}
- Ergönül O, Celikbaş A, Dokuzoguz B, Eren S, Baykam N, Esener H. Characteristics of patients with Crimean‐Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clinical Infectious Diseases 2004;39(2):284‐7. [DOI] [PubMed] [Google Scholar]
Ergonul 2006 {published data only}
- Ergonul O, Celikbas A, Baykam N, Eren S, Dokuzoguz B. Analysis of risk‐factors among patients with Crimean‐Congo haemorrhagic fever virus infection: severity criteria revisited. Clinical Microbiology and Infection 2006;12(6):551‐4. [DOI] [PubMed] [Google Scholar]
Ertem 2016 {published data only}
- Ertem G, Sönmezer MÇ, Temoçin F, Ataman Hatipoğlu Ç, Tülek N, Oral B. The efficacy of oral ribavirin on clinical and laboratory parameters in Crimean‐Congo hemorrhagic fever: an observational study from Turkey. Turkish Journal of Medical Sciences 2016;46(5):1407‐14. [DOI] [PubMed] [Google Scholar]
Ertugrul 2009 {published data only}
- Ertugrul B, Uyar Y, Yavas K, Turan C, Oncu S, Saylak O, et al. An outbreak of Crimean‐Congo hemorrhagic fever in western Anatolia, Turkey. International Journal of Infectious Diseases 2009;13(6):431‐6. [DOI] [PubMed] [Google Scholar]
Gayretli Aydin 2015 {published data only}
- Gayretli Aydin ZG, Tanir G, Metin O, Aydin Teke T, Bayhan GI, Oz FN, et al. Transient sinus bradycardia during the course of Crimean‐Congo hemorrhagic fever in children. Ticks and Tick‐borne Diseases 2015;6(2):185‐8. [DOI] [PubMed] [Google Scholar]
Izadi 2009a {published data only}
- Izadi S, Salehi M. Evaluation of the efficacy of ribavirin therapy on survival of Crimean‐Congo hemorrhagic fever patients: a case‐control study. Japanese Journal of Infectious Diseases 2009;62(1):11‐5. [PubMed] [Google Scholar]
Kalin 2014 {published data only}
- Kalın G, Metan G, Demiraslan H, Doganay M. Do we really need ribavirin in the treatment of Crimean‐Congo hemorrhagic fever?. Journal of Chemotherapy 2014;26(3):146‐9. [DOI] [PubMed] [Google Scholar]
Koksal 2010 {published data only}
- Koksal I, Yilmaz G, Aksoy F, Aydin H, Yavuz I, Iskender S, et al. The efficacy of ribavirin in the treatment of Crimean‐Congo hemorrhagic fever in Eastern Black Sea region in Turkey. Journal of Clinical Virology 2010;47(1):65‐8. [DOI] [PubMed] [Google Scholar]
Mardani 2003 {published data only}
- Mardani M, Jahromi MK, Naieni KH, Zeinali M. The efficacy of oral ribavirin in the treatment of Crimean‐Congo hemorrhagic fever in Iran. Clinical Infectious Diseases 2003;36(12):1613‐8. [DOI] [PubMed] [Google Scholar]
Metanat 2005 {published data only}
- Metanat M, Sharifi‐Mood B, Salehi M. A comparative study of the efficacy of oral ribavirin for Crimean‐Congo hemorrhagic fever in patients treated during the initial 5 days vs after 5 days of the disease, Southeast Iran. International Journal of Antimicrobial Agents 2005;26:S85. [Google Scholar]
Ozkurt 2006 {published data only}
- Ozkurt Z, Kiki I, Erol S, Erdem F, Yilmaz N, Parlak M, et al. Crimean‐Congo hemorrhagic fever in Eastern Turkey: clinical features, risk factors and efficacy of ribavirin therapy. Journal of Infection 2006;52(3):207‐15. [DOI] [PubMed] [Google Scholar]
Sannikova 2009 {unpublished data only}
- Sannikova IV. [КРЫМСКАЯ‐КОНГО ГЕМОРРАГИЧЕСКАЯ ЛИХОРАДКА:КЛИНИКО‐ПАТОГЕНЕТИЧЕСКИЕ АСПЕКТЫ И ОПТИМИЗАЦИЯЛЕЧЕНИЯ]. Crimean‐Congo Haemorrhagic Fever: Clinico‐pathogenic Aspects and Optimisation of Treatment [Dissertation for degree of Doctor of Medical Sciences]. Moscow: Stavropol' State Medical Academy of the Federal Agency of Public Health and Social Development, 2009. [Google Scholar]
Sharifi‐Mood 2006 {published data only}
- Sharifi‐Mood B, Alavi‐Naini R, Metanat M, Rakhshani F. Ribavirin: an effective drug treatment of children with Crimean‐Congo haemorrhagic fever: a seven years experience. Pakistan Journal of Biological Sciences 2006;9(8):1598‐600. [Google Scholar]
Sharifi‐Mood 2013a {published data only}
- Sharifi‐Mood B, Alavi‐Naini R, Metanat M, Mohammadi M, Shakeri A, Amjadi A. Efficacy of high‐dose methylprednisolone in patients with Crimean‐Congo haemorrhagic fever and severe thrombocytopenia. Tropical Doctor 2013;43(2):49‐53. [DOI] [PubMed] [Google Scholar]
Tasdelen Fisgin 2009 {published data only}
- Tasdelen Fisgin N, Tanyel E, Doganci L, Tulek N. Risk factors for fatality in patients with Crimean‐Congo haemorrhagic fever. Tropical Doctor 2009;39(3):158‐60. [DOI] [PubMed] [Google Scholar]
Tezer 2016 {published data only}
- Tezer H, Ozkaya‐Parlakay A, Gulhan B, Kanik‐Yuksek S. Ribavirin use in paediatric patients with Crimean Congo Hemorrhagic Fever: is it really necessary?. Brazilian Journal of Infectious Diseases 2016;20(2):222‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulek 2012 {published data only}
- Tulek N, Ozturk B, Bulut C, Tuncer Ertem G, Erdinc FS, Altun S, et al. The evaluation of ribavirin use in patients with Crimean‐Congo haemorrhagic fever. Clinical Microbiology and Infection 2012;18:579‐80. [Google Scholar]
Tuygun 2012 {published data only}
- Tuygun N, Tanir G, Caglayik DY, Uyar Y, Korukluoglu G, Cenesiz F. Pediatric cases of Crimean‐Congo hemorrhagic fever in Turkey. Pediatric International 2012;54(3):402‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abuova 2012 {published data only}
- Abuova G, Pshenichnaya N, Irsimbetova N, Apsatarov Z. Clinical and epidemiological aspects of Crimean‐Congo hemorrhagic fever in pregnant women in South Kazakhstan. International Journal of Infectious Diseases 2012;16(Suppl 1):e66. [Google Scholar]
Ajazaj 2013 {published data only}
- Ajazaj Berisha L, Ahmeti S, Dreshaj S, Namani S, Qehaja‐Buqaj E, Vishaj A, et al. Nosocomial infection of Crimean‐Congo hemorrhagic fever in Kosovo. European Journal of Internal Medicine 2013;24(1):e207. [Google Scholar]
Alavi‐Naini 2004 {published data only}
- Alavi‐Naini R, Moghtaderi A, Metanat M. An unusual intracerebral haemorrhage. Canadian Journal of Infectious Diseases & Medical Microbiology 2004;15(3):175‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2010 {published data only}
- Ali F, Saleem T, Khalid U, Mehmood SF, Jamil B. Crimean‐Congo hemorrhagic fever in a dengue‐endemic region: lessons for the future. Journal of Infection in Developing Countries 2010;4(7):459‐63. [DOI] [PubMed] [Google Scholar]
Anon 1984 {published data only}
- Anonymous. Viral hemorrhagic fever. Initial management of suspected and confirmed cases. Annals of Internal Medicine 1984;101(1):73‐81. [Google Scholar]
Ardalan 2006 {published data only}
- Ardalan MR, Tubbs RS, Chinikar S, Shoja MM. Crimean‐Congo haemorrhagic fever presenting as thrombotic microangiopathy and acute renal failure. Nephrology, Dialysis, Transplantation 2006;21(8):2304‐7. [DOI] [PubMed] [Google Scholar]
Athar 2003 {published data only}
- Athar MN, Baqai HZ, Ahmad M, Khalid MA, Bashir N, Ahmad AM, et al. Short report: Crimean‐Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002. American Journal of Tropical Medicine and Hygiene 2003;69(3):284‐7. [PubMed] [Google Scholar]
Athar 2005 {published data only}
- Athar MN, Khalid MA, Ahmad AM, Bashir N, Baqai HZ, Ahmad M, et al. Crimean‐Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002: contact tracing and risk assessment. American Journal of Tropical Medicine and Hygiene 2005;72(4):471‐3. [PubMed] [Google Scholar]
Barr 2013 {published data only}
- Barr DA, Aitken C, Bell DJ, Brown CS, Cropley I, Dawood N, et al. First confirmed case of Crimean‐Congo haemorrhagic fever in the UK. Lancet 2013;382(9902):1458. [DOI] [PubMed] [Google Scholar]
Canpolat 2011 {published data only}
- Canpolat G, Kocak Tufan Z, Bulut C, Pekcan Demiroz A. EDTA‐dependent pseudothrombocytopenia and unnecessary transfusion in a patient with Crimean‐Congo haemorrhagic fever. Klimik Dergisi 2011;24(1):184‐6. [Google Scholar]
Caylan 2010 {published data only}
- Caylan R, Yapar D, Keske S, Hasanoglu I, Tasyaran MA. Nosocomial transmission of Crimean‐Congo haemorrhagic fever. Clinical Microbiology and Infection 2010;16:S700. [Google Scholar]
Ceri 2013 {published data only}
- Ceri M. An unusual cause of perirenal haemorrhage. Renal Failure 2013;35(3):430‐1. [DOI] [PubMed] [Google Scholar]
Chinikar 2013 {published data only}
- Chinikar S, Shayesteh M, Khakifirouz S, Jalali T, Rasi Varaie FS, Rafigh M, et al. Nosocomial infection of Crimean‐Congo haemorrhagic fever in eastern Iran: case report. Travel Medicine and Infectious Disease 2013;11(4):252‐5. [DOI] [PubMed] [Google Scholar]
Dilber 2010 {published data only}
- Dilber E, Cakir M, Erduran E, Koksal I, Bahat E, Mutlu M, et al. High‐dose methylprednisolone in children with Crimean‐Congo haemorrhagic fever. Tropical Doctor 2010;40(1):27‐30. [DOI] [PubMed] [Google Scholar]
Drosten 2002 {published data only}
- Drosten C, Minnak D, Emmerich P, Schmitz H, Reinicke T. Crimean‐Congo hemorrhagic fever in Kosovo. Journal of Clinical Microbiology 2002;40(3):1122‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elata 2011 {published data only}
- Elata AT, Karsany MS, Elageb RM, Hussain MA, Eltom KH, Elbashir MI, et al. A nosocomial transmission of Crimean‐Congo hemorrhagic fever to an attending physician in north Kordufan, Sudan. Virology Journal 2011;8:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
El Bahnasawy 2015 {published data only}
- Bahnasawy MM, Sabah AA, Morsy TA. Crimean‐Congo Hemorrhagic Fever cases in Egypt: Is it a warning sign for other viral haemorrhagic fever disasters?. Tropical Medicine and International Health 2015;20:204‐5. [Google Scholar]
Ergonul 2009 {published data only}
- Ergonul O. DEBATE (see Elaldi N et al, Efficacy of oral ribavirin treatment in Crimean‐Congo haemorrhagic fever: a quasi‐experimental study from Turkey. Journal of Infection 2009; 58: 238‐244): Biases and misinterpretation in the assessment of the efficacy of oral ribavirin in the treatment of Crimean‐Congo hemorrhagic fever. Journal of Infection 2009;59(4):284‐6; author reply 286‐9. [DOI] [PubMed] [Google Scholar]
Ergonul 2014 {published data only}
- Ergonul O, Battal I. Potential sexual transmission of Crimean‐Congo hemorrhagic fever infection. Japanese Journal of Infectious Diseases 2014;67(2):137‐8. [DOI] [PubMed] [Google Scholar]
Ergonul 2017 {published data only}
- Ergonul O, Seref C, Eren S, Celikbas A, Baykam N, Dokuzoguz B, et al. Cytokine response in Crimean‐Congo hemorrhagic fever virus infection. Journal of Medical Virology 2017;89(10):1707‐13. [DOI] [PubMed] [Google Scholar]
Fazlalipour 2016 {published data only}
- Fazlalipour M, Baniasadi V, Mirghiasi SM, Jalali T, Khakifirouz S, Azad‐Manjiri S, et al. Crimean‐congo hemorrhagic fever due to consumption of raw meat: case reports from east‐north of Iran. Japanese Journal of Infectious Diseases 2016;69(3):270‐1. [DOI] [PubMed] [Google Scholar]
Gonen 2014 {published data only}
- Gonen I, Ermis F. Crimean‐Congo hemorrhagic fever presenting with gastrointestinal manifestations: two cases. Turkish Journal of Gastroenterology 2014;25(1):120‐1. [DOI] [PubMed] [Google Scholar]
Gozel 2013 {published data only}
- Gozel MG, EngIn A, Elaldi N, Bakir M, Dokmetas I, Uyar Y. First cases of hemorrhagic fever with renal syndrome from the middle Anatolia region of Turkey and the first case of hantavirus and Crimean‐congo hemorrhagic fever virus co‐infection in a patient. Turkiye Klinikleri Journal of Medical Sciences 2013;33(1):224‐8. [Google Scholar]
Guner 2014 {published data only}
- Guner R, Hasanoglu I, Tasyaran MA, Yapar D, Keske S, Guven T, et al. Is ribavirin prophylaxis effective for nosocomial transmission of Crimean‐Congo hemorrhagic fever?. Vector Borne and Zoonotic Diseases 2014;14(8):601‐5. [DOI] [PubMed] [Google Scholar]
Gursoy 2014 {published data only}
- Gursoy B. Crimean‐congo haemorrhagic fever: The first case in Sanliurfa, Turkey. Klimik Dergisi 2014;27(1):36. [Google Scholar]
Hasan 2013 {published data only}
- Hasan Z, Mahmood F, Jamil B, Atkinson B, Mohammed M, Samreen A, et al. Crimean‐Congo hemorrhagic fever nosocomial infection in a immunosuppressed patient, Pakistan: case report and virological investigation. Journal of Medical Virology 2013;85(3):501‐4. [DOI] [PubMed] [Google Scholar]
Izadi 2009b {published data only}
- Izadi S, Salehi M. Ribavirin treatment for Crimean‐Congo hemorrhagic fever: reply. Japanese Journal of Infectious Diseases 2009;62(6):486. [PubMed] [Google Scholar]
Jabbari 2006 {published data only}
- Jabbari A, Besharat S, Abbasi A, Abdollah M, Khodaberdi K. Crimean‐Congo hemorrhagic fever: case series from a medical centre in Golestan province, Northeast of Iran (2004). Indian Journal of Medical Science 2006;60(8):327‐9. [PubMed] [Google Scholar]
Jamil 2005 {published data only}
- Jamil B, Hasan RS, Sarwari AR, Burton J, Hewson R, Clegg C. Crimean‐Congo hemorrhagic fever: Experience at a tertiary care hospital in Karachi, Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(8):577‐84. [DOI] [PubMed] [Google Scholar]
Joubert 1985 {published data only}
- Joubert JR, King JB, Rossouw DJ, Cooper R. A nosocomial outbreak of Crimean‐Congo haemorrhagic fever at Tygerberg Hospital. Part III. Clinical pathology and pathogenesis. South African Medical Journal 1985;68(10):722‐8. [PubMed] [Google Scholar]
Kadanali 2012 {published data only}
- Kadanali A, Ozden K, Erol S. Crimean‐Congo hemorrhagic fever virus infection: clinical and laboratory observations and predictors of fatality. Turkiye Klinikleri Tip Bilimleri Dergisi 2012;32(2):432‐7. [Google Scholar]
Kader 2011 {published data only}
- Kader C, Erbay A, Bakir Ozbey S. Crimean‐Congo haemorrhagic fever: six‐year experience of a secondary care hospital in the epidemic region. Clinical Microbiology and Infection 2011;17:S258. [Google Scholar]
Kleib 2016 {published data only}
- Kleib AS, Salihy SM, Ghaber SM, Sidiel BW, Sidiya KC, Bettar ES. Crimean‐Congo hemorrhagic fever with acute subdural hematoma, Mauritania, 2012. Emerging Infectious Diseases 2016;22(7):1305‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubar 2011 {published data only}
- Kubar A, Haciomeroglu M, Ozkul A, Bagriacik U, Akinci E, Sener K, et al. Prompt administration of Crimean‐Congo hemorrhagic fever (CCHF) virus hyperimmunoglobulin in patients diagnosed with CCHF and viral load monitorization by reverse transcriptase‐PCR. Japanese Journal of Infectious Diseases 2011;64(5):439‐43. [PubMed] [Google Scholar]
Kunchev 2008 {published data only}
- Kunchev A, Kojouharova M. Probable cases of Crimean‐Congo‐haemorrhagic fever in Bulgaria: a preliminary report. Euro Surveillance 2008;13(17):18845. [PubMed] [Google Scholar]
Leblebicioglu 2016a {published data only}
- Leblebicioglu H, Sunbul M, Guner R, Bodur H, Bulut C, Duygu F, et al. Healthcare‐associated Crimean‐Congo haemorrhagic fever in Turkey, 2002‐2014: a multi‐centre retrospective cross‐sectional study. Clinical Microbiology and Infections 2016;22(4):387 e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makwana 2015 {published data only}
- Makwana D, Yadav PD, Kelaiya A, Mourya D. First confirmed case of Crimean‐Congo haemorrhagic fever from Sirohi district in Rajasthan State, India. Indian Journal of Medical Research 2015;142(October):489‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mardani 2009 {published data only}
- Mardani M, Keshtkar‐Jahromi M, Ataie B, Adibi P. Crimean‐Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. American Journal of Tropical Medicine and Hygiene 2009;81(4):675‐8. [DOI] [PubMed] [Google Scholar]
Mardani 2013 {published data only}
- Mardani M, Namazee N. Close contact precautions could prevent an outbreak of Crimean‐Congo hemorrhagic Fever: a case series report from southern part of Tehran. International Journal of Preventive Medicine 2013;4(6):715‐9. [PMC free article] [PubMed] [Google Scholar]
Midilli 2007 {published data only}
- Midilli K, Gargili A, Ergonul O, Sengoz G, Ozturk R, Bakara M, et al. Imported Crimean‐Congo hemorrhagic fever cases in Istanbul. BMC Infectious Diseases 2007;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2011 {published data only}
- Mishra AC, Mehta M, Mourya DT, Gandhi S. Crimean‐Congo haemorrhagic fever in India. Lancet 2011;378(9788):372. [DOI] [PubMed] [Google Scholar]
MMWR 1984 {published data only}
- Centers for Disease Control. MMWR Congo‐Crimean hemorrhagic fever‐‐Republic of South Africa. JAMA 1984;252(18):2533, 2537. [PubMed] [Google Scholar]
Mohamed 2016 {published data only}
- Mohamed ADL, Rahimi Shahmirzadi MR, Baderldin S, Abro A, Zaki A, Dessi Z, et al. Crimean‐Congo hemorrhagic fever in Dubai, United Arab Emirates, 2010: case report. Iranian Red Crescent Medical Journal 2016;18(8):e38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabeth 2004 {published data only}
- Nabeth P, Thior M, Faye O, Simon F. Human Crimean‐Congo hemorrhagic fever, Senegal. Emerging Infectious Diseases 2004;10(10):1881‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naderi 2011 {published data only}
- Naderi HR, Sarvghad MR, Bojdy A, Hadizadeh MR, Sadeghi R, Sheybani F. Short report: Nosocomial outbreak of Crimean‐Congo haemorrhagic fever. Epidemiology and Infection 2011;139(6):862‐6. [DOI] [PubMed] [Google Scholar]
Naderi 2013 {published data only}
- Naderi HR, Sheybani F, Bojdi A, Khosravi N, Mostafavi I. Fatal nosocomial spread of Crimean‐Congo hemorrhagic fever with very short incubation period. American Journal of Tropical Medicine and Hygiene 2013;88(3):469‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00992693 {published data only}
- NCT00992693. Treatment of viral hemorrhagic fevers with intravenous ribavirin in military treatment facilities. clinicaltrials.gov/ct2/show/NCT00992693 (first posted 9 October 2009).
Oflaz 2013 {published data only}
- Oflaz MB, Kucukdurmaz Z, Guven AS, Karapinar H, Kaya A, Sancakdar E, et al. Bradycardia seen in children with Crimean‐Congo hemorrhagic fever. Vector Borne and Zoonotic Diseases 2013;13(11):807‐11. [DOI] [PubMed] [Google Scholar]
Ozbey 2014 {published data only}
- Ozbey SB, Kader Ç, Erbay A, Ergönül Ö. Early use of ribavirin is beneficial in Crimean‐Congo hemorrhagic fever. Vector Borne and Zoonotic Diseases 2014;14(4):300‐2. [DOI] [PubMed] [Google Scholar]
Ozsoy 2015 {published data only}
- Ozsoy S, Gokmen A, Ozdemir M, Akduman B, Korkusuz I, Javan GT. Medical examiners and Crimean‐Congo hemorrhagic fever contamination risk. Journal of Forensic and Legal Medicine 2015;36:32‐6. [DOI] [PubMed] [Google Scholar]
Öztürk 2012 {published data only}
- Öztürk DB, Sencan I, Gürbuz Y, Kuscu F, Tutuncu EE. People to people transmission of CCHF among society: four cases. Konuralp Medical Journal / Konuralp Tip Dergisi 2012;4(1):28‐31. [Google Scholar]
Papa 2008 {published data only}
- Papa A, Maltezou HC, Tsiodras S, Dalla VG, Papadimitriou T, Pierroutsakos I, et al. A case of Crimean‐Congo haemorrhagic fever in Greece, June 2008. Euro Surveillance 2008;13(33):18952. [DOI] [PubMed] [Google Scholar]
Pourahmad 2011 {published data only}
- Pourahmad M, Raoofi R, Chinikar S, Ghiasi SM, Ghalyanchi‐Langeroudi A. Nosocomial transmission of Crimean‐Congo hemorrhagic fever in a health care worker, Fars province, Iran. Iranian Journal of Clinical Infectious Diseases 2011;6(1):47‐50. [Google Scholar]
Pshenichnaya 2015 {published data only}
- Pshenichnaya NY, Nenadskaya SA. Probable Crimean‐Congo hemorrhagic fever virus transmission occurred after aerosol‐generating medical procedures in Russia: nosocomial cluster. International Journal of Infectious Diseases 2015;33:120‐2. [DOI] [PubMed] [Google Scholar]
Raoofi 2012 {published data only}
- Raoofi R, Pourahamad M, Nazer MR, Pournia Y, Chinikar S. Case series of Crimean‐Congo disease: An outbreak in south of Fars, Iran. [Persian]. Journal of Babol University of Medical Sciences 2012;14(5):96‐100. [Google Scholar]
Richards 2015 {published data only}
- Richards GA. Nososcomial transmission of viral haemorrhagic fever in South Africa. South African Medical Journal 2015;105(9):709‐12. [DOI] [PubMed] [Google Scholar]
Sahin 2016 {published data only}
- Sahin IO, Guven AS, Kaya A, Guney C, Cevit O, Arslan M. A child with an unusual complication of Crimean‐Congo hemorrhagic fever: Hemorrhagic pleural effusion. Journal of Vector‐Borne Diseases 2016;53(1):87‐9. [PubMed] [Google Scholar]
Saluzzo 1985b {published data only}
- Saluzzo JF, Aubry P, McCormick J, Digoutte JP. Haemorrhagic fever caused by Crimean Congo haemorrhagic fever virus in Mauritania. Transactions of the Royal Society of Tropical Medicine and Hygiene 1985;79(2):268. [DOI] [PubMed] [Google Scholar]
Schwarz 1995 {published data only}
- Schwarz TF, Nitschko H, Jager G, Nsanze H, Longson M, Pugh R, et al. Crimean‐Congo haemorrhagic fever in Oman. Lancet 1995;346(8984):1230. [DOI] [PubMed] [Google Scholar]
Scrimgeour 1996 {published data only}
- Scrimgeour EM, Zaki A, Mehta FR, Abraham AK, Al‐Busaidy S, El‐Khatim H, et al. Crimean‐Congo haemorrhagic fever in Oman. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(3):290‐1. [DOI] [PubMed] [Google Scholar]
Sefikotullari 2013 {published data only}
- Sefikotullari M, Kaya A, Aydin H, Sancakder E, Celik VK, Bagci G. The role of VEGF and HIF‐1 in the pathogenesis of Crimean‐Congo haemorrhagic fever. Turkish Journal of Biochemistry 2013;38:not stated. [Google Scholar]
Sharifi‐Mood 2008 {published data only}
- Sharifi‐Mood B, Mardani M, Keshtkar‐Jahromi M, Rahnavardi M, Hatami H, Metanat M. Clinical and epidemiologic features of Crimean‐Congo hemorrhagic fever among children and adolescents from Southeastern Iran. Pediatric Infectious Disease Journal 2008;27 (6):561‐3. [DOI] [PubMed] [Google Scholar]
Sharifi‐Mood 2009 {published data only}
- Sharifi‐Mood B, Metanat M, Ghorbani‐Vaghei A, Fayyaz‐Jahani F, Akrami E. The outcome of patients with Crimean‐Congo hemorrhagic fever in Zahedan, southeast of Iran: a comparative study. Archives of Iranian Medicine 2009;12(2):151‐3. [PubMed] [Google Scholar]
Sharifi‐Mood 2013b {published data only}
- Sharifi‐Mood B, Alavi‐Naini R, Metanat M, Mohammadi M, Shakeri A, Amjadi A. Efficacy of high‐dose methylprednisolone in patients with Crimean‐Congo haemorrhagic fever and severe thrombocytopenia. Tropical Doctor 2013;43(2):49‐53. [DOI] [PubMed] [Google Scholar]
Sheikh, 2004 {published data only}
- Sheikh NS, Sheikh AS, Sheikh AA. Knowledge, attitude and practices regarding Crimean‐Congo haemorrhagic fever among healthcare workers in Balochistan. Journla of the Ayub Medical College Abbottabad 2004;16(3):39‐42. [PubMed] [Google Scholar]
Sheikh 2005 {published data only}
- Sheikh AS, Sheikh AA, Sheikh NS, U‐Shan R, Asif M, Afridi F, et al. Bi‐annual surge of Crimean‐Congo haemorrhagic fever (CCHF): a five‐year experience. International Journla of Infectious Diseases 2005;9(1):37‐42. [DOI] [PubMed] [Google Scholar]
Smego 2004 {published data only}
- Smego RA, Sarwari AR, Siddiqui AR. Crimean‐Congo hemorrhagic fever: Prevention and control limitations in a resource‐poor country. Clinical Infectious Diseases 2004;38(12):1731‐5. [DOI] [PubMed] [Google Scholar]
Suleiman 1980 {published data only}
- Suleiman MN, Muscat‐Baron JM, Harries JR, Satti AG, Platt GS, Bowen ET, Simpson DI. Congo/Crimean haemorrhagic fever in Dubai. An outbreak at the Rashid Hospital. Lancet 1980;2(8201):939‐41. [PubMed] [Google Scholar]
Sunbul 2016 {published data only}
- Sunbul M, Esen S, Fletcher TE, Dilek A, Guler N, Beeching NJ, Leblebicioglu H. A fatal case of healthcare associated Crimean‐Congo haemorrhagic fever with severe disease and multi‐organ failure. Journal of Infection 2016;72(2):253‐5. [DOI] [PubMed] [Google Scholar]
Tall 2009a {published data only}
- Tall A, Diallo M, Faye O, Diab H, Diatta B, Sall AA. Crimean‐Congo hemorrhagic fever in Senegal. Medicine Tropicale (Mars) 2009;69(1):18. [PubMed] [Google Scholar]
Tall 2009b {published data only}
- Tall A, Sall AA, Faye O, Diatta B, Sylla R, Faye J, et al. Two cases of Crimean‐Congo haemorrhagic fever (CCHF) in two tourists in Senegal in 2004. Bulletin de la Societé de Pathologie Exotique 2009;102(3):159‐61. [PubMed] [Google Scholar]
Tatar 2005 {published data only}
- Tatar A, Ozkurt Z, Kiki I. Genotoxic effect of ribavirin in patients with Crimean‐Congo hemorrhagic fever. Japanese Journla of Infectious Diseases 2005;58(5):313‐5. [PubMed] [Google Scholar]
Tezer 2014 {published data only}
- Tezer H, Ozkaya Parlakay A, Gulhan B, Ilker C. Bradycardia related to ribavirin in four paediatric patients with Crimean‐Congo hemorrhagic fever. Vector Borne and Zoonotic Diseases 2014;14(6):464‐5. [DOI] [PubMed] [Google Scholar]
Tulek 2010 {published data only}
- Tulek N, Ozturk B, Bulut C, Tuncer Ertem G, Erdinc FS, Altun S. Unusual nosocomial transmission of Crimean‐Congo haemorrhagic fever; two cases report from Turkey. Clinical Microbiology and Infection 2010;16:S701. [Google Scholar]
Tutuncu 2009 {published data only}
- Tütüncü EE, Gurbuz Y, Ozturk B, Kuscu F, Sencan I. Crimean Congo haemorrhagic fever, precautions and ribavirin prophylaxis: a case report. Scandinavian Journal of Infectious Diseases 2009;41(5):378‐80. [DOI] [PubMed] [Google Scholar]
Ugurlu 2013 {published data only}
- Ugurlu GK, Ugurlu M, Caykoylu A. The emergence of obsessive compulsive and compulsive buying symptomatology after acute stress and short‐term use of ribavirin: Case reports. Therapeutic Advances in Psychopharmacology 2013;3(4):246‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unlusoy 2014 {published data only}
- Unlusoy Aksu A, Havali C, Tapisiz A, F. Aktas, Ezgu F. Crimean‐congo haemorrhagic fever in pregnancy and in newborn: a case with a unique clinical course. Journal of Obstetrics and Gynaecology 2014;34(4):360. [DOI] [PubMed] [Google Scholar]
Uysal 2012 {published data only}
- Uysal B, Metan G. Bradycardia in a patient with Crimean‐Congo hemorrhagic fever related to ribavirin treatment. Journla of Vector‐Borne Diseases 2012;49(3):193‐4. [PubMed] [Google Scholar]
Van Eeden 1985a {published data only}
- Eeden PJ, Joubert JR, Wal BW, King JB, Kock A, Groenewald JH. A nosocomial outbreak of Crimean‐Congo haemorrhagic fever at Tygerberg Hospital. Part I. Clinical features. South African Medical Journal 1985;68(10):711‐7. [PubMed] [Google Scholar]
Van Eeden 1985b {published data only}
- Eeden PJ, Eeden SF, Joubert JR, King JB, Wal BW, Michell WL. A nosocomial outbreak of Crimean‐Congo haemorrhagic fever at Tygerberg Hospital. Part II. Management of patients. South African Medical Journal 1985;68(10):718‐21. [PubMed] [Google Scholar]
Weber 2001 {published data only}
- Weber DJ, Rutala WA. Risks and prevention of nosocomial transmission of rare zoonotic diseases. Clinical Infectious Diseases 2001;32(3):446‐56. [DOI] [PubMed] [Google Scholar]
Yadav 2013 {published data only}
- Yadav PD, Raut CG, Mourya DT. Re‐occurrence of Crimean‐Congo haemorrhagic fever in Ahmedabad, Gujarat, India (2012): a fatal case report. Indian Journal of Medical Research 2013;138(6):1027‐8. [PMC free article] [PubMed] [Google Scholar]
Yadav 2016 {published data only}
- Yadav PD, Patil DY, Shete AM, Kokate P, Goyal P, Jadhav S. Nosocomial infection of CCHF among health care workers in Rajasthan, India. BMC Infectious Diseases 2016;16:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yesilyurt 2011 {published data only}
- Yesilyurt M, Gul S, Ozturk B, Kayhan BC, Celik M, Uyar C, et al. The early prediction of fatality in Crimean Congo hemorrhagic fever patients. Saudi Medical Journal 2011;32(7):742‐3. [PubMed] [Google Scholar]
Yildirmak 2016 {published data only}
- Yildirmak T, Tulek N, Bulut C. Crimean‐Congo haemorrhagic fever: transmission to visitors and healthcare workers. Infection 2016;44(5):687‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yilmaz 2009a {published data only}
- Yilmaz GR, Buzgan T, Irmak H, Safran A, Uzun R, Cevik MA, et al. The epidemiology of Crimean‐Congo hemorrhagic fever in Turkey, 2002‐2007. International Journal of Infectious Diseases 2009;13(3):380‐6. [DOI] [PubMed] [Google Scholar]
Yilmaz 2009b {published data only}
- Yilmaz R, Kundak AA, Ozer S, Esmeray H. Successful treatment of severe Crimean‐Congo hemorrhagic fever with supportive measures without ribavirin and hypothermia. Journal of Clinical Virology 2009;44(2):181‐2. [DOI] [PubMed] [Google Scholar]
Yolcu 2014 {published data only}
- Yolcu S, Kader C, Kayipmaz AE, Ozbay S, Erbay A. Knowledge levels regarding Crimean‐Congo hemorrhagic fever among emergency healthcare workers in an endemic region. Journal of Clinical Medical Research 2014;6(3):197‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zakhashvili 2010 {published data only}
- Zakhashvili K, Tsertsvadze N, Chikviladze T, Jghenti E, Bekaia M, Kuchuloria T, et al. Crimean‐Congo hemorrhagic fever in man, Republic of Georgia, 2009. Emerging Infectious Diseases 2010;16(8):1326‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ascioglu 2011
- Ascioglu S, Leblebicioglu H, Vahaboglu H, Chan KA. Ribavirin for patients with Crimean‐Congo haemorrhagic fever: a systematic review and meta‐analysis. Journal of Antimicrobial Chemotherapy 2011;66(6):1215‐22. [DOI: 10.1093/jac/dkr136] [DOI] [PubMed] [Google Scholar]
Bente 2013
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean‐Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Research 2013;100(1):159‐89. [DOI] [PubMed] [Google Scholar]
Bodur 2012
- Bodur H, Akinci E, Ascioglu S, Öngürü P, Uyar Y. Subclinical infections with Crimean‐Congo hemorrhagic fever virus, Turkey. Emerging Infectious Diseases 2012;18(4):640‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Gluud LL, Gluud C. Ribavirin monotherapy for chronic hepatitis C. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005527.pub2] [DOI] [PubMed] [Google Scholar]
Causey 1970
- Causey OR, Kemp GE, Madbouly MH, David‐West TS. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. American Journal of Tropical Medicine and Hygiene 1970;19(5):846‐50. [DOI] [PubMed] [Google Scholar]
Ceylan 2013
- Ceylan B, Calica A, Ak O, Akkoyunlu Y, Turhan V. Ribavirin is not effective against Crimean‐Congo hemorrhagic fever: observations from the Turkish experience.. International Journal of Infectious Diseases 2013;17(10):e799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debing 2013
- Debing Y, Jochmans D, Neyts J. Intervention strategies for emerging viruses: use of antivirals. Current Opinion in Virology 2013;3(2):217‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
DoH South Africa 2014
- Department of Health, South Africa. National Guidelines for Recognition and Management of Viral Haemorrhagic Fevers 2014. www.caa.co.za/Aviation%20Medicine%20General%20Information/National%20Guidelines%20for%20Viral%20Haemorrhagic%20Fevers.pdf (accessed 9 January 2017).
Dowall 2016
- Dowall SD, Buttigieg KR, Findlay‐Wilson SJD, Rayner E, Pearson G, Miloszewska A, et al. A Crimean‐Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Human Vaccines & Immunotherapeutics 2016;12(2):519‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2015
- European Medicines Agency. Rebetol. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000246/human_med_001017.jsp&mid=WC0b01ac058001d124 (accessed 9 January 2017).
Ergönül 2006b
- Ergönül O. Crimean‐Congo haemorrhagic fever. Lancet Infectious Diseases 2006;6(4):203‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Estrada‐Peña 2007
- Estrada‐Peña A, Venzal JM. Climate niches of tick species in the Mediterranean region: modelling of occurrence data, distributional constraints, and impact of climate change. Journal of Medical Entomology 2007;44(6):1130‐8. [DOI: 10.1603/0022-2585(2007)44[1130:CNOTSI]2.0.CO;2] [DOI] [PubMed] [Google Scholar]
FDA 2016
- US Food, Drug Administration (FDA). Code of Federal Regulations Title 21. April 2016. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32 (accessed 9 March 2017).
Fisher‐Hoch 1995
- Fisher‐Hoch SP, Khan JA, Rehman S, Mirza S, Khurshid M, McCormick JB. Crimean Congo‐haemorrhagic fever treated with oral ribavirin. Lancet 1995;346(8973):472‐5. [DOI] [PubMed] [Google Scholar]
García Rada 2016
- García Rada A. First outbreak of Crimean‐Congo haemorrhagic fever in western Europe kills one man in Spain. BMJ 2016;354:i4891. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 9 January 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoogstraal 1979
- Hoogstraal H. The epidemiology of tick‐borne Crimean‐Congo hemorrhagic fever in Asia, Europe, and Africa. Journal of Medical Entomology 1979;15(4):307‐417. [DOI: 10.1093/jmedent/15.4.307] [DOI] [PubMed] [Google Scholar]
Leblebicioglu 2016b
- Leblebicioglu H, Ozaras R, Irmak H, Sencan I. Crimean‐Congo hemorrhagic fever in Turkey: Current status and future challenges. Antiviral Research 2016;126:21‐34. [DOI] [PubMed] [Google Scholar]
Leblecioglu 2012
- Leblebicioglu H, Bodur H, Dokuzoguz B, Elaldi N, Guner R, Koksal I, et al. Case management and supportive treatment for patients with Crimean‐Congo hemorrhagic fever. Vector Borne and Zoonotic Diseases 2012;12(9):805–11. [DOI: 10.1089/vbz.2011.0896] [DOI] [PubMed] [Google Scholar]
Messina 2015
- Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean‐Congo hemorrhagic fever. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;109(8):503‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadeem 2003
- Nadeem M, Ali N, Anwar M, Hussain I, Mohammad T, Hayee A. A comparison of clinical diagnosis & serological diagnosis in an epidemic of Crimean‐Congo Haemorrhagic Fever. Pakistan Journal of Medical Sciences 2003;19(4):247‐52. [Google Scholar]
NCDC 2011
- National Centre for Disease Control, Directorate General of Health Services, Government of India. Crimean Congo Haemorrhagic Fever. CD Alert, Monthly newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India. January 2011. www.ncdc.gov.in/writereaddata/linkimages/January7434567273.pdf (accessed 9 January 2017).
NIH 2013
- National Institute of Health Islamabad, World Health Organization. Guidelines for Crimean Congo Haemorrhagic Fever (CCHF). Islamabad: National Institute of Health, September 2013. [Google Scholar]
Oestereich 2014
- Oestereich L, Rieger T, Neumann M, Bernreuther C, Lehmann M, Krasemann S, et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T‐705 (favipiravir) in a mouse model for Crimean‐Congo hemorrhagic fever. PLoS Neglected Tropical Diseases 2014;8(5):e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oflaz 2015
- Oflaz MB, Kucukdurmaz Z. Bradycardia with ribavirin therapy in Crimean‐Congo hemorrhagic fever. Pediatric Infectious Disease Journal 2015;34(4):460–1. [DOI: 10.1097/INF.0000000000000613] [DOI] [PubMed] [Google Scholar]
Pshenichnaya 2016
- Pshenichnaya NY, Sydenko IS, Klinovaya EP, Romanova EB, Zhuravlev AS. Possible sexual transmission of Crimean‐Congo hemorrhagic fever. International Journal of Infectious Diseases 2016;45:109‐11. [DOI: 10.1016/j.ijid.2016.02.1008] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saluzzo 1985
- Saluzzo JF, Digoutte JP, Camicas JL, Chauvancy G. Crimean‐Congo haemorrhagic fever and Rift Valley fever in south‐eastern Mauritania. Lancet 1985;1(8420):116. [DOI] [PubMed] [Google Scholar]
Shepherd 1987
- Shepherd AJ, Swanepoel R, Leman PA, Shepherd SP. Field and laboratory investigation of Crimean‐Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(6):1004‐7. [DOI: 10.1016/0035-9203(87)90379-8] [DOI] [PubMed] [Google Scholar]
Sidwell 1972
- Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad‐spectrum antiviral activity of virazole: 1‐f8‐ D‐Ribofuranosyl‐ 1,2,4‐triazole‐ 3‐carboxamide. Science 1972;177(4050):705‐6. [DOI] [PubMed] [Google Scholar]
Soares‐Weiser 2010
- Soares‐Weiser K, Thomas S, Thomson G, Garner P. Ribavirin for Crimean‐Congo hemorrhagic fever: systematic review and meta‐analysis. BMC Infectious Diseases 2010;10:207. [DOI: 10.1186/1471-2334-10-207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanepoel 1987
- Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, McGillivray GM, Erasmus MJ, et al. Epidemiologic and clinical features of Crimean‐Congo hemorrhagic fever in southern Africa. American Journal of Tropical Medicine and Hygiene 1987;36(1):120‐32. [DOI] [PubMed] [Google Scholar]
Vassilenko 1990
- Vassilenko SM, Vassilev TL, Bozadjiev LG, Bineva IL, Kazarov GZ. Specific intravenous immunoglobulin for Crimean‐Congo haemorrhagic fever. Lancet 1990;335(8692):791‐2. [DOI] [PubMed] [Google Scholar]
Watts 1989
- Watts DM, Ussery MA, Nash D, Peters CJ. Inhibition of Crimean‐Congo hemorrhagic fever viral infectivity yields in vitro by ribavirin. American Journal of Tropical Medicine and Hygiene 1989;41(5):581‐5. [DOI] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. 19th WHO Model List of Essential Medicines. April 2015. www.who.int/medicines/publications/essentialmedicines/EML2015_8‐May‐15.pdf (accessed 9 January 2017).
WHO 2016
- World Health Organization. Guidance For Managing Ethical Issues In Infectious Disease Outbreaks. http://apps.who.int/iris/bitstream/handle/10665/250580/9789241549837‐eng.pdf?sequence=1 2016, issue ISBN 978 92 4 154983 7.
Yen 1985
- Yen YC, Kong LX, Lee L, Zhang YQ, Li F, Cai BJ, et al. Characteristics of Crimean‐Congo hemorrhagic fever virus (Xinjiang strain) in China. American Journal of Tropical Medicine and Hygiene 1985;34(6):1179‐82. [PubMed] [Google Scholar]
Zapata 2014
- Zapata JC, Cox D, Salvato MS. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Neglected Tropical Diseases 2014;8(6):e2858. [DOI: 10.1371/journal.pntd.0002858] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Johnson 2017
- Johnson S, Maayan N, Mills I, Buckley BS, Kakourou A, Marshall R. Ribavirin for treating Crimean Congo haemorrhagic fever. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012713] [DOI] [PMC free article] [PubMed] [Google Scholar]


