Abstract
Triclosan (TCS), an antibacterial, has been shown to be an endocrine disruptor in the rat. Previously, subchronic TCS treatment to female rats was found to advance puberty and potentiate the effect of ethinyl estradiol (EE) on uterine growth when EE and TCS were co-administered prior to weaning. In the pubertal study, a decrease in serum thyroxine (T4) concentrations with no significant change in serum thyroid-stimulating hormone (TSH) was also observed. The purpose of the present study was to further characterize the influence of TCS on the reproductive and thyroid axes of the female rat using a chronic exposure regimen. Female Wistar rats were exposed by oral gavage to vehicle control, EE (1 μg/kg), or TCS (2.35, 4.69, 9.375 or 37.5 mg/kg) for 8 months and estrous cyclicity monitored. Although a divergent pattern of reproductive senescence appeared to emerge from 5 to 11 months of age between controls and EE-treated females, no significant difference in cyclicity was noted between TCS-treated and control females. A higher % control females displayed persistent diestrus (PD) by the end of the study, whereas animals administered with positive control (EE) were predominately persistent estrus (PE). Thyroxine concentration was significantly decreased in TCS-administered 9.375 and 37.5 mg/kg groups, with no marked effects on TSH levels, thyroid tissue weight, or histology. Results demonstrate that a long-term exposure to TCS did not significantly alter estrous cyclicity or timing of reproductive senescence in females but suppressed T4 levels at a lower dose than previously observed.
Introduction
Triclosan (TCS; 2, 4, 4′-trichloro-2′-hydroxydiphenyl ether) is a halogenated phenol that has been used as an antimicrobial in personal care products since the 1960s. Although a new ruling by the FDA bans the use of TCS in body washes and soaps, this compound is still used in other products including cosmetics, kitchenware, toys, and other materials (FDA, 2016). Triclosan (TCS) was detected in human urine, serum, and breast milk, with urine concentrations ranging from approximately 2 to 4 μg/L according to the National Health and Nutrition Examination surveys (NHANES) (Calafat et al., 2008; CDC, 2013). One estimate of the daily intake of TCS was between 50 and 75 ug/kg/day based upon combined consumer product use (Rodricks et al., 2010). Previous studies demonstrated that TCS produced a decrease in thyroxine (T4) levels in rodents following acute or subacute oral exposures at doses of 18 mg/kg and higher in male and female rats (Crofton et al., 2007; Zorrilla et al., 2009; Stoker et al., 2010).
While several investigators indicated that TCS-induced estrogen receptor (ER) mediated reporter gene activity and promoted proliferation of human breast cancer (MCF-7) cells (Gee et al., 2008; Huang et al., 2014), Louis et al. (2013) found that TCS did not markedly alter ER transcriptional activation (TA) in the T47D-KDBluc breast cancer cells. However, it was found that TCS enhanced the estrogen-induced uterine responses (Louis et al., 2013; Stoker et al., 2010). Because this effect of TCS is not specific to the ER, such potentiation of the estrogenic response may involve reduced clearance of estradiol by competitive inhibition of sulfotransferases, which was demonstrated in sheep placental tissue (James et al., 2010). Other studies also reported a similar enhancement of an estrogenic response or inhibition of estrogen metabolism in both fish (Lange et al., 2015) and mammals (Wang et al., 2015, 2016; Pollock & Tang, 2014).
A number of studies provided evidence that exposure to estrogens or estrogen-like compounds disrupt estrous cyclicity in the rat by impairing hypothalamic–pituitary–gonadal (HPG) axis regulation. Prolonged exposure to estrogens altered ovarian cycling in adult females resulting in premature reproductive senescence typically identified by persistent vaginal estrus and polyfollicular or cystic ovaries (Cooper et al., 1984; Brawer et al., 1980, 1993; Felicio et al., 1984; Goldman et al., 2007; Shirwalkar et al., 2007). Estrogen exposure may erode the neuronal mechanisms controlling the ovulatory surge of luteinizing hormone (LH) to the extent that the female is no longer capable of achieving ovulation (Cooper et al., 1984). Although rats and humans differ somewhat in luteal phase and menses, the estrous cycle of both exhibit similar patterns based on hormone fluctuations and both exhibit erratic cycles prior to the onset of reproductive quiescence making rodents an acceptable model (Wu et al., 2005; Wise et al., 2002). Normally, reproductive senescence occurs in the rat by a change from 4-to 5-day regular cycles to an extension of diestrous days with some % persistent estrus at approximately 8–10 months of age progressing to complete persistent diestrus (LeFevre & McClintock, 1988). Our previous investigations demonstrated that two short-term exposures to TCS (4 or 21 days) in immature female rats enhanced uterine estrogenic activity and advanced puberty (Stoker et al., 2010; Louis et al., 2013). Therefore, it was postulated that chronic TCS exposure in the presence of endogenous estrogens may initiate changes in estrous cyclicity and/or early reproductive senescence. A positive control ethinyl estradiol (EE) was also included to identify changes that might occur with elevation of estrogen concentrations. As mentioned, TCS was also shown to alter serum thyroid hormones (TH) levels. Thyroid hormones (TH), which include triiodothyronine (T3) and thyroxine (T4), are essential regulators of numerous physiological processes including metabolism, neurodevelopment, and cardiac functions. Alterations in TH homeostasis by chemicals during gestation may lead to adverse health effects, including altered neurodevelopment (Porterfield & Hendrich, 1993; Brown et al., 2004; Carr & Patiño, 2011; Zoeller, 2010). Triclosan was found to decrease serum T4 levels following a short 3- to 4-day exposure and up to 31 days in the rat (Crofton et al., 2007; Zorrilla et al., 2009; Stoker et al., 2010). In most of these cases, no marked alterations were seen in serum for TSH concentrations or histopathology of the thyroid gland. To date, no apparent studies have determined the potential effects of longer term, oral exposure to TCS on thyroid hormone homeostasis in the rat.
The present study was designed to investigate the influence of chronic TCS exposure on the HPG and hypothalamic–pituitary–thyroid (HPT) axes in the female rat. Specifically, studies were undertaken to examine whether extended exposure to TCS might 1) affect estrous cyclicity in the adult, 2) to alter the age at reproductive senescence, and 3) to disrupt the regulation of the HPT axis.
Materials and Methods
Animals
One hundred and twenty female Wistar rats were delivered on postnatal day (PND) 60 from Charles River Laboratories (Raleigh, NC). Animals were housed in polycarbonate cages (2 per cage) with heat-treated pine shavings and housed under controlled conditions (20–24°C; 40–50% humidity) with a 14-hr light/10-hr dark cycles (lights on 0100hr). Animals were fed Purina Laboratory Rat Chow 5001 and had access to water ad libitum. After a week for acclimation to the 14:10-hr light schedule, estrous cyclicity of all animals was assessed daily by vaginal lavage and vaginal cytology for three weeks to identify females with regular cycles. Regular cycles were defined as having one day in proestrus, one or two days in estrus, and two days in diestrus (Cooper, 1999). Animals with regular cycles were weight-ranked and assigned to different treatment groups (as indicated below). Following this randomization, the mean body weight (BW) for all groups was similar. Animals were pair-housed, and BW and abnormal clinical signs (if any) were recorded daily throughout the study. All procedures were performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Dosing solutions
Animals were assigned to six different treatment groups, which consisted of vehicle control (corn oil; Sigma, St. Louis, MO), 4 doses of TCS (99.8% pure determined by high-performance liquid chromatography, Calbiochem-La Jolla, CA #647950) including 2.35, 4.69, 9.375, or 37.5 mg/kg/day (n=15 per group), and 1 μg/kg/day ethinyl estradiol (EE; [Sigma, St. Louis, MO #4876, >98% purity]) as positive control (n=8) for a total of 83 females. Chemicals were weighed, dissolved in corn oil, and stirred continuously during the dose administration period. Dosing solutions were prepared fresh weekly and stored in amber bottles at room temperature. Animals were weighed and dosed daily by oral gavage beginning on the first day of vaginal proestrus following PND 110 to synchronize the cycle day.
Analysis of estrous cyclicity
Estrous cycles were assessed daily between 0800 and 0900 by vaginal lavage for the first 3 months of treatment and collected on alternating two week cycles thereafter. Vaginal cytology was classified according to the following criteria: (1) diestrous smear containing mostly leukocytes; (2) proestrous smear containing mostly clumped nucleated vaginal epithelial cells; and (3) estrous smear with predominately large, irregular shaped, and cornified epithelial cells. Cycle data were analyzed in monthly segments after treatments began and classified as having regular (based on the criteria described above) or irregular cycles by blinded observers. Animals with irregular cycles were further classified as persistent diestrus (PD) (defined as 4 or more consecutive days in diestrus) or persistent estrus (PE) (defined as 3 or more consecutive days in estrus). Animals were also assessed weekly starting at 5 months of age for the presence of mammary tumors by palpitation. Mammary gland tumors may be indicative of changing hormonal concentrations, including estrogens and prolactin during reproductive senescence in the rat (Sinha et al., 1973).
Necropsy
After approximately 8 months of daily TCS administration, animals were necropsied between PND 346 and 349 at 1000–1200 hr. Animals that were still cycling were killed on the second day of diestrus for consistency. Animals were killed by decapitation, and trunk blood was collected, centrifuged at 3000 × g for 30 min and serum collected for subsequent hormone analyses. Anterior pituitary, uteri, ovaries, adrenal glands (paired), kidneys (paired), and whole liver were removed and weighed. Anterior pituitaries were immediately frozen on dry ice and stored at −80°C until further hormone analysis. Uteri, ovaries, and trachea with attached thyroid lobes were removed, weighed, and stored in 10% buffered formalin for 24 hr before transferring to 70% ethanol for subsequent histopathological analysis. The right lobe of the liver was immediately stored in RNAlater storage solution (Invitrogen, Waltham, MA) and kept at −80°C for subsequent RNA analysis. Animals were also visually assessed and palpated for evidence of mammary tumors on the day of necropsy.
Uterine histology
One cross section of each fixed uterine horn was taken, embedded in paraffin, sectioned (4–6 μm), and stained with hematoxylin and eosin (Experimental Pathology Laboratories, Inc., Durham, NC). Sections were examined at 40x magnification with an Olympus BH-2 microscope (Olympus, Center Valley, PA). The luminal epithelial cell height, an indicator for estrogenicity of xenobiotics (Padilla-Banks et al., 2001), was measured from the basement membrane to the apical surface and taken at three different locations of each sample, then averaged for each individual animal using a Moticam 2500 and Motic Images Plus software (Motic, Richmond, BC). Measurements were based on calibrations in an ocular micrometer on the microscope. The final sample size for uterine histology was 8 rats per group.
Hormone analysis
Frozen pituitaries were suspended in 1 ml 100 mM phosphate buffer (PB)/1% bovine serum albumin (BSA) solution and sonicated with a VC130PB ultrasonic processor (Sonics & Materials, Inc. Newtown, CT). The homogenized pituitary was subsequently diluted 1:30,000 in 100mM PB/1% BSA and stored at −80°C until further hormone analysis.
Serum samples from all females were assessed for the levels of estradiol (E2), progesterone (P4), T3, and total T4 with radioimmunoassay (RIA) Coat-a-Count kits (Siemens Corporation; Los Angeles, CA) and estrone (E1) using a kit from Beckman Coulter (Brea, CA) according to manufacturer’s protocol. Serum luteinizing hormone (LH) and TSH were measured in all females by RIA with reagents supplied by the National Hormone and Peptide Program through A. F. Parlow as described previously (Goldman et al., 1986). Pituitary LH was assessed in the same assay. Briefly, purified iodination preparations were radiolabeled with 125I (Perkin-Elmer Waltham, MA) using the chloramine-T method of Greenwood et al. (1963). Each radiolabeled antigen was separated from unreacted iodide by gel filtration chromatography as described previously (Goldman et al., 1986). Sample serum and pituitary homogenate (LH only) were diluted to a final volume of 500 μl in 100 mM phosphate buffer (PB) containing 1% BSA. About 200 μl primary antibody solution (100 mM PB, 50 mM EDTA, 1% BSA, 3% normal rabbit serum with primary antibody diluted 1:100) was added to each sample tubes, which was then vortexed and incubated overnight at 4°C. About 100 μl 125I radiolabeled antigen was added to each tube, which was then vortexed and incubated overnight at 4°C. Secondary antibody (goat anti-rabbit gamma globulin, GARGG, Calbiochem, San Diego, CA) diluted to 10U/ml was added, and the sample was vortexed and incubated overnight at 4°C. Samples were centrifuged at 1260g for 30 min and the supernatant aspirated. Pelleted sample was counted with a Wizard gamma counter (Perkin Elmer, MA) and hormone concentrations calculated. The assay sensitivities of LH and TSH were increased by an overnight incubation of sample and first antibody prior to the addition of the radiolabeled antigen.
Thyroid histology
Eight randomly selected thyroid glands per treatment group were prepared for histology and evaluated by Experimental Pathology Laboratories, Inc., Durham, NC. The right and left thyroid glands were examined for changes in central follicular epithelial height and colloid area within follicles using a combined 5-point grading scale (1– shortest/smallest; 5–tallest/largest) as shown in Stoker et al. (2006).
Determination of TCS residues in rat serum
A modified protocol from Ross and Filipov (2006) and Ross et al. (2009) with an Agilent 7890 A Gas Chromatograph equipped with an Agilent 7000 Triple Quadrupole Mass Spectrometer (GC-MS/MS) was used to determine TCS concentrations in three randomly selected serum samples from animals in all TCS treatment groups for a total of 15 samples run in triplicate (Analytical Chemistry Branch Laboratory, Office of Pesticide Programs, US EPA, Fort Meade, MD). The analytical method was validated for analysis of TCS in serum at 4 concentration levels including the limit of quantitation (LOQ = 3 ppb) with a standard of TCS, 99.7%, from Sigma-Aldrich (Buchs, Switzerland). The average recovery % obtained from fortified control serum was 105% with a relative standard deviation of 9.4%. The extraction method was similar to methods previously developed for the analysis of atrazine in rat tissue (Fraites et al., 2011). The gas chromatograph (GC) was attached to an HP 5ms ui (30m × 0.25 mm i.d) capillary column run with helium as carrier gas at a flow of 1 ml/min. The inlet temperature was set at 280°C, and the oven was programmed to run from 110°C for 1 min then raised by 20°C/min to 250°C and held for 7 min. The mass spectrometer’s quench gas was helium at 2.25 ml/min, and collision gas was nitrogen at 1.5 ml/min. Two precursors to product ion transitions were monitored for TCS in the electron impact (EI) mode at 70 eV. The ion transitions were as follows: m/z 288 to 217.8 at 20V collision energy and m/z 288 to 146 at 10V collision energy.
Expression of liver enzyme RNA
Liver samples from all females were homogenized in 500μl TRI Reagent (Sigma, Saint Louis, MO) with an Ultra-Turrax T-25 (IKA, Wilmington, NC). Total RNA was isolated via guanidine thiocyanate phenol–chloroform extraction, and concentrations were quantified with a NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE). Samples were diluted to 100ng/μl, and then, 1 ug RNA was used to produce cDNA with Applied Biosystems (ABI; Foster City, CA) High Capacity Reverse Transcription Kit according to manufacturer’s protocols. Using ABI TaqMan Gene Expression Assays, ABI TaqMan Gene Expression Master Mix, and ABI 7900HT Fast Real-Time PCR System, the expression of liver enzymes Cyp2b2 (Rn02786833_m1), Cyp3a23/3a1 (Rn03062228_m1), Ugt1a1 (Rn00754947_m1), Sult1b1 (Rn00673872_m1), and Sult 1c1/Sult1c3 (Rn00581955_m1) were assayed. Fold change of expression was calculated and normalized to β-actin (Rn00667869_m1) expression. These genes were previously shown to be affected by TCS treatment (Zorrilla et al., 2009; Paul et al., 2010).
Statistical analysis
Statistical significance for body weights, uterine weights, serum hormone measurements, and gene expression were analyzed by ANOVA and homogeneity of variance using Bartlett’s test using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA). When significant effects (p<0.05) were indicated by the ANOVA, Dunnett’s and Tukey’s multiple comparison post-tests were performed to compare treatment groups. For estrous cyclicity data, Fisher’s exact test of probability was used to analyze incidence data. Endpoint measurements were inclusive for all females (Control, N = 14:2.3 mg/kg TCS, N= 14; 4.69 mg/kg TCS, N= 15; 9.375 mg/kg, N = 13; 37.5 mg/kg TCS, N= 14; EE 1.0 ug/kg, N=8). For histological analysis of thyroid and uterus, the number included was 8 per group and 3 serum samples from each group were analyzed for TCS residues.
Results
All animals maintained a healthy appearance with no apparent abnormal clinical signs of toxicity or marked changes in body weight (BW) throughout the course of the study. Four females were euthanized during the study following confirmed tracheal gavage and esophageal perforations, and one female was euthanized following the development of a fluid-filled sebaceous cyst. The EE-treated animals displayed significantly lower BW than controls beginning at 15 weeks after treatment began, and this change persisted until termination with a 5% difference from controls (Figure 1). This decreased BW gain in EE females is consistent with rise in % PE females and in agreement with previous observations showing an anorexic effect of estrogens in female rats (weight gain at a slower rate than controls) (Cooper & Linnoila, 1977). The 37.5 mg/kg TCS group exhibited a significant decrease in mean BW from 30 to 32 weeks of exposure, with other time points demonstrating no marked difference from controls. In addition, the 2.35 and 9.375 mg/kg TCS groups showed a significant rise in BW beginning at 26 and 28 weeks of exposure, with elevation of 2 and 3%, respectively, over control BW on day of necropsy.
Figure 1.
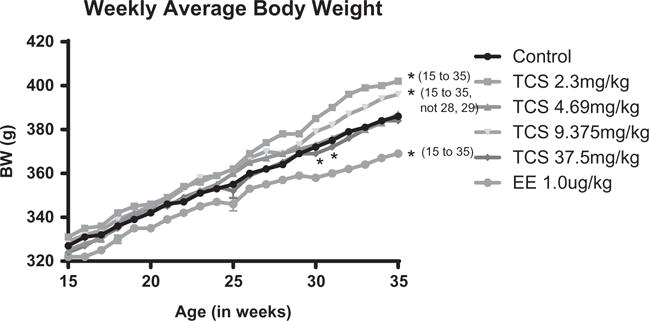
The average weekly body weights from 15 to 35 weeks of exposure to control, triclosan at concentrations of 2.3, 4.69, 9.375, and 37.5 mg/kg or 1 μg/kg EE. Beginning at 15 weeks of treatment, the mean BW of the EE group was significantly decreased from controls. In addition, the 2.35 and 9.375 TCS mg/kg groups were significantly increased above the control, except for the 28 and 29 weeks in the 9.375 group. The 37.5 mg/kg TCS group had a significant decrease as compared to controls from week 30 to 32. This significance was p < 0.05 as compared to control mean.
Effects on estrous cyclicity
Over the 8 months of daily TCS administration, the % animals cycling regularly declined as rats aged in all dose groups (Figure 2). These data are presented as number of animals with regular cycles out of the total number of rats in each group for each month of treatment (dosing began at approximately 110 days of age). Although there were no significant effects on estrous cyclicity when comparing % regular cycles with irregular cycles between controls and treated females in any of the groups, a distinct pattern emerged with the type of irregular cycles was observed between groups by the end of the study. Upon closer examination of animals with irregular cycles after 2 months of exposure (approximately 5 months of age), approximately half of the females in both vehicle control and EE displayed irregular cycles in PD and the other half in PE (Figure 3a). However, by 11 months of age, 71% of vehicle control animals were in PD, whereas only 43% of EE-treated animals were in PD. Conversely, a greater number of EE-treated animals were PE at this age compared with controls (57 and 9%, respectively; Figure 3b). This distribution was significantly different between control and EE females at 11 months of age. On the other hand, females in TCS treatment groups with irregular cycles displayed proportionally more PD than PE after 2 months of treatment (particularly in the 4.69 mg/kg group), but there were no marked differences compared to control females due to the small number of irregular cyclers in each group (Figure 3a). However, following 8 months of treatment, there was a distinct shift in the pattern of the two highest TCS groups (9.375 and 37.5 mg/kg) with more females displaying PE smears as compared to number of females undergoing PE in the control group (Figure 3b). This nonsignificant change in irregular cyclicity appeared to be shifting toward the type of smear observed in the EE-treated females at this time with the lower two groups of TCS females following a pattern more similar to controls.
Figure 2.
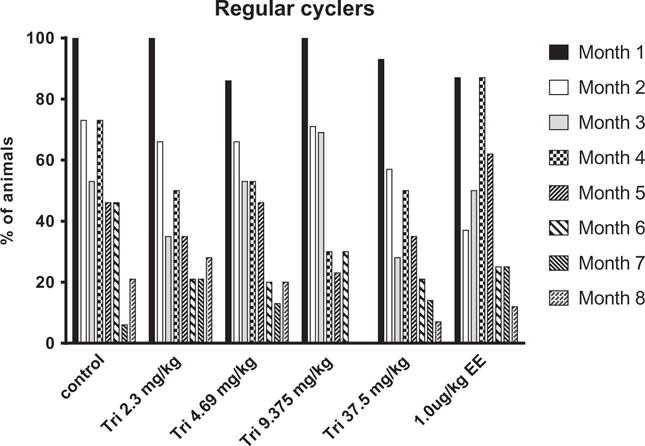
Percentage of animals in each treatment group with regular cycles during months 1 to 8 of exposure from 4 to 11 months of age). See methods for description of regular cyclicity. No significant effect was observed between controls and TCS or EE groups.
Figure 3.
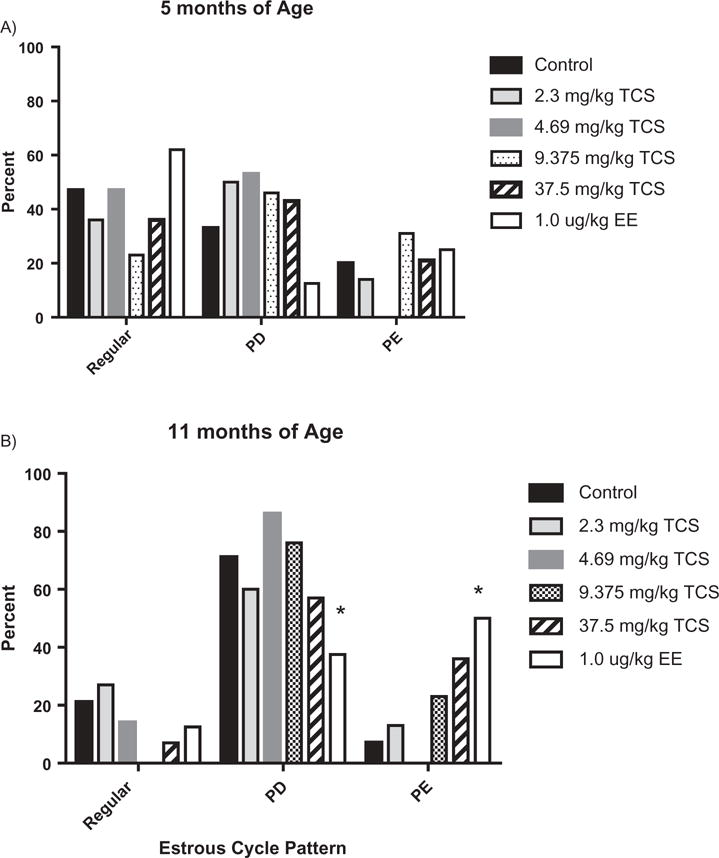
Percentage of animals within each estrous cycle pattern (categorized by Regular, PD or PE) at A) 5 months and B) 11 months of age in control, TCS or EE females. * significantly different from control, p < 0.05.
Effects on tissue weights and morphology
Mean uterine weight (wet and blotted) was not significantly different between EE- and TCS-treated females and vehicle controls (included as supplemental data). One uterus from the TCS 9.375 mg/kg group was omitted from analysis because it was filled with inflammatory cells and necrotic debris. Mean pituitary, ovary, kidneys, adrenal glands, and whole liver weight (absolute or relative to BW) were not markedly different between controls and EE or TCS administered (included in supplemental file). When uteri were examined morphologically, no marked changes in epithelial cell height were observed between TCS-treated and control rats. TCS-treated animals categorized as PD showed no significant alterations in epithelial cell height compared with PD in vehicle- or EE-treated groups (data not shown). Animals in PE also displayed no marked differences in epithelial cell height between treatment and control females.
Effects on reproductive hormones
Mean serum and pituitary hormones were measured in serum collected at the study termination (approximately 11 months of age) (Figure 4). The levels of P4 were significantly greater in PD than PE animals for every dose group, with the exception of the 2.3 and 4.69 mg/kg TCS, as only one female in the 2.35 mg/kg and no females in the 4.69 mg/kg group were PE at 11 months of age (Figure 4a). None of the treated groups were significantly different from controls for P4. Serum LH levels were not markedly different between PD and PE females (Figure 4b). A dose-dependent change in P4 or LH was not observed in either cycle pattern compared to controls. No marked differences were detected between PD and PE groups for the mean serum E2 and E1 concentrations or between control and treated groups (Figure 4 c & 4d). However, there was a significant difference between 4.69 and 37.5 mg/kg rat mean for serum E2 levels in the PD group. Further, the anterior pituitary LH concentrations in TCS-treated animals were not significantly different from controls at necropsy (data not shown).
Figure 4.
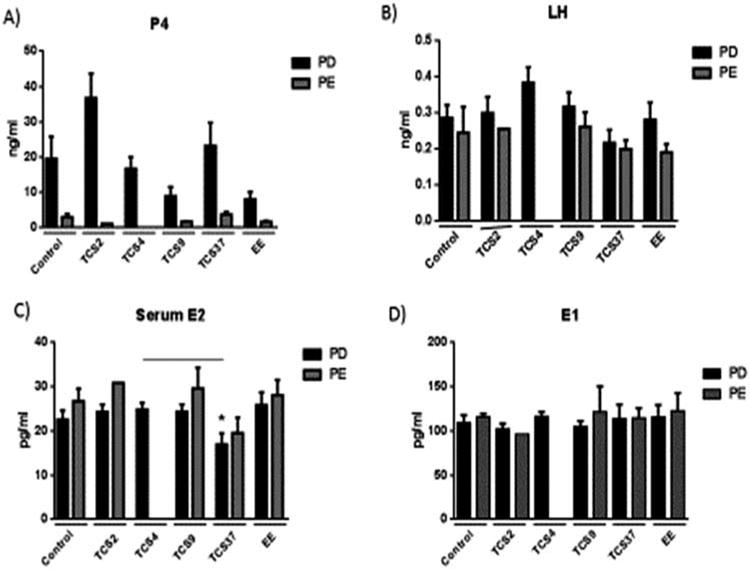
Serum hormone concentrations of females at 11 months of age categorized by persistent diestrum (PD) and persistent estrus (PE). A) progesterone (P4), B) luteinizing hormone (LH), C) estradiol (E2), and D) estrone (E1). Overall, the mean P4 concentrations in the PD groups were significantly higher than the PE group in each treatment (p < .05). However, the 4.69 mg/kg PE group could not be compared because there were no PE females in that group.
TCS residues
There was a dose-dependent increase in amounts of TCS (ppm) residues in serum taken from TCS-administered females on the last day of exposure. Control, 2.35, 4.69, 9.375, and 37.5 mg/kg groups had levels of 0.01 +/− 0.005, 3.97 +/− 2.24, 9.2 +/−5.31, 30.07 +/− 17.36, and 38.62 +/− 22.3 ppm, respectively. The serum levels appear to be dose related and comparable to TCS dose administered. Although 2.35 mg/kg TCS dose is similar to baseline TCS levels in human plasma, the other doses are approximately 100-fold higher than concentrations found in human plasma following a variety of exposures of single to multiple days of exposure using oral mouth wash or toothpaste with 0.3% TCS (Allmyr et al., 2008, 2009; Sandborgh-Englund et al., 2006).
Influence on thyroid function
Levels of serum T3 and TSH were not markedly affected by TCS treatment (Figure 5a & 5b). Further, relative thyroid gland weight was not significantly different between dose groups compared to controls (Figure 5c). The mean serum concentrations of T4 were significantly decreased in females following 8 months of treatment with 9.375 or 37.5 mg/kg of TCS compared to controls by approximately 17 and 35%, respectively (Figure 5d). Histopathology of thyroid glands was similar among groups for peripheral and central follicular height (FH) and colloid volume (CV) (Table 1).
Figure 5.
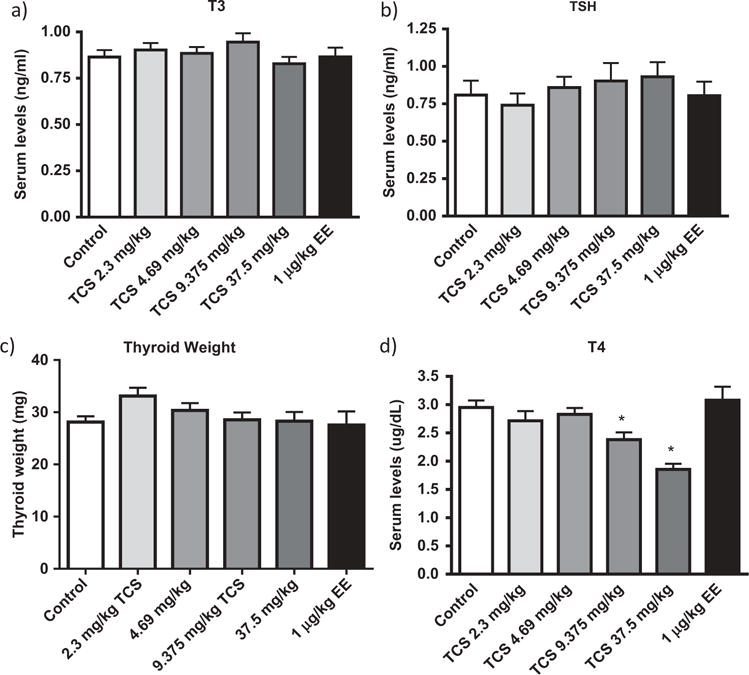
Mean serum thyroid hormone levels and mean thyroid weight of control, EE, or TCS. Mean +/− SEM. * p< 0.05, significantly different from Control mean.
Table 1.
Mean thyroid histology scores of colloid volume and follicular cell heights in females following a chronic daily oral exposure to triclosan in the rat.
| Control | 2.3 mg/kg | 4.69 mg/kg | 9.375 mg/kg | 37.5 mg/kg | |
|---|---|---|---|---|---|
| Follicle Ht | 1.75 ± 0.163 | 1.875 ± 0.125 | 1.375 ± 0.182 | 1.25 ± 0.163 | 1.625 ± 0.182 |
| Colloid Vol. | 4.375 ± 0.182 | 4.75 ± 0.163 | 4.625 ± 0.263 | 4.75 ± 0.163 | 4.5 ± 0.267 |
Effects on hepatic enzyme gene expression
There was a dose-dependent 2.2-fold increase in gene expression of Cyp2b2 at 37.5 mg/kg TCS (Figure 6B to 6E). The other 4 genes, Cyp3a23/3a1, Sult1c1/1c3, Sult1b1, and Ugt1a1, were not significantly different from control liver gene expression (Figure 6B to 6E), although there did appear to be a nonsignificant 1.5–2.3-fold rise in Sult1c1/1c3 expression at 4.69 and 37.5 mg/kg TCS. However, enzyme levels and activity were not measured to confirm that altered gene expression might initiate changes in enzyme activity following TCS exposure.
Figure 6.
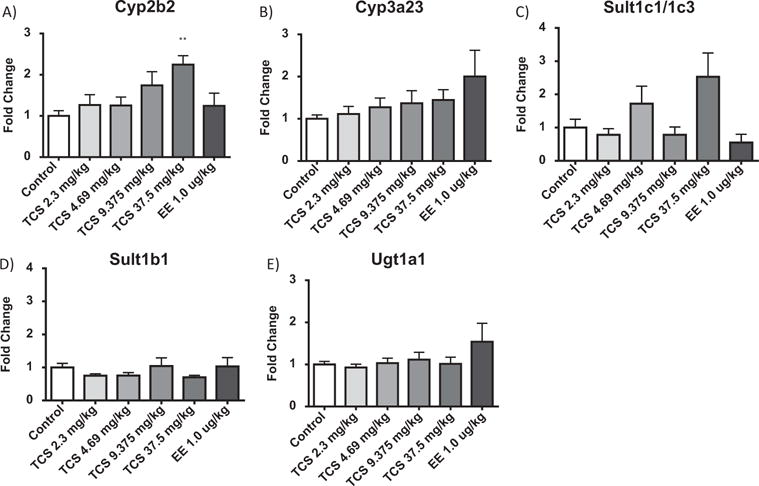
Effect of TCS on relative liver mRNA expression of A) Cyp2b2, B) Cyp3a23, C) Sult 1c11/1c3, D) Sult1b1, and E) Ugt1a1 in the female rat following a chronic oral exposure. Data are plotted as the mean (± SE) fold change (2−ΔΔCT) from the control.
*significantly different from vehicle control, p < 0.05.
Discussion
Previously, Stoker et al. (2010) reported that acute exposures to TCS potentiated estrogenic effects in the weanling and pubertal female rat. In pubertal female, TCS resulted in an advancement in puberty and age of first estrus. In the weanling female (prior to the production of endogenous estrogens), TCS alone did not markedly affect uterine growth after a 3-day exposure. However, following administration of exogenous E2 in the weanling study, TCS enhanced the uterotrophic response at doses of 4.69 mg/kg and higher. It was therefore postulated that chronic TCS exposure might enhance the potency of endogenous estrogen in the adult female and result in an advancement in reproductive senescence. Data in this study demonstrated that extended exposures to TCS (ranging from 2.35 to 37.5 mg/kg) in adult rat did not induce significant changes in estrous cyclicity, reproductive hormone levels, or uterine tissue morphology compared to control females. However, the two higher TCS doses displayed a trend of increasing numbers in PE after 8 months of dosing compared to control females, a direction that was observed in the EE group to a more pronounced and significant extent.
Vehicle control females transitioned from extended days in diestrus and estrus to an almost complete shift to reproductive senescence in permanent diestrus by 11 months of age. This agrees with previous rat studies examining shifts in reproductive states advancing into early reproductive senescence by approximately one year of age (LeFevre & McClintock, 1988), with variations among many of the rat strains. The EE females shifted from this pattern with fewer females transitioning into the PD state and more into the PE state at 11 months of age. The nonsignificant effect on reproductive senescence in TCS females may be partially attributed to compensatory mechanisms. James et al. (2010) previously showed that TCS is an inhibitor of E2 sulfonation in sheep placenta, which indicates that shorter exposure to TCS may enhance the availability of estrogen by decreasing its clearance. The chronic exposure used here may allow time for acclimation and compensation by hepatic sulfonation, such that the transient effect of elevated estrogen availability is overcome with continued exposure. In fact, a difference in serum estradiol or estrone concentrations was not observed in the terminal serum collected at 11 months of age, demonstrating compensatory acclimation following such a long exposure period.
It is well known that estrogen is anorexigenic (Brown & Clegg, 2010; Santollo et al., 2010). An anorexigenic effect was noted in the present study in the EE group, with a significant reduction in BW beginning at 15 weeks of exposure and persisting throughout the study. There was a fall in the 37.5 mg/kg TCS group at 30–32 weeks of exposure, but no other time points were significant and none of the other TCS groups displayed reduced BW following exposure. However, animals exposed to 2.35 and 9.375 mg/kg TCS demonstrated a significantly increased mean BW compared to controls by 15 weeks and 26 weeks, respectively, which persisted until the end of the study. This may be due to more of the TCS-administered females displaying a PD pattern of cyclicity, which is typically associated with increased progesterone and elevated growth in the rat (Cooper & Linnoila, 1977), although this does not hold true for the 4.69 mg/kg data in this study. Results showed that the levels of P4 were lower in PE than PD, which might be expected because PE animals were found in earlier studies to be polyfollicular and anovulatory while PD females typically possess more corpora lutea for P4 production (Harrison et al., 1987).
In our previous study in weanling and pubertal rats, TCS suppressed the levels of serum T4 following a 3-, 21-, or 31-day oral exposure, with no change in TSH and no marked effect on thyroid follicular cell height or colloid area. In the present study, chronic TCS exposure (at doses of 9.375 and 37.5 mg/kg) decreased T4 levels in the adult female rat. Our lowest observed effect level (LOEL) was 9.375, and a no observed effect level (NOEL) was 4.69 mg/kg. These effect levels are lower than our previous findings in the weanling female rats, which indicated a NOEL of 9.375 and LOEL of 18.75 mg/kg (Louis et al., 2013; Stoker et al., 2010). A significant effect was not noted in serum T3 or TSH in the current study, which agrees with previous observations on TCS (Paul et al., 2010; Zorrilla et al., 2009). Similar to previous studies, no marked changes in thyroid follicular histopathology (follicular cell height or colloid area) were found following this chronic exposure. Other chemicals such as polychlorinated biphenyls, tetrabromobisphenol A (TBBPA), and 3-methylcholanthrene were also shown to lower T4 levels without effects on subsequent TSH (Hood et al., 1999; Liu et al., 1995; Cope et al., 2015). The reason for this lack of rebounding TSH is not well understood, but has been postulated to be due to a lack of induction of T3 glucuronidation activity in rodents (Barter & Klaassen, 1994; Hood & Klaassen, 2000; Lai et al., 2015).
Several reports attempted to identify the mechanisms underlying the effects of TCS on serum T4. One study reported that TCS increased catabolism and elimination of T4 via enhanced expression of Phase I and Phase II hepatic enzymes involved in TH metabolism (Paul et al., 2010). Specifically, TCS induced increased activity of the hepatic enzymes involved in TH metabolism, including ethoxyresorufin O-deethylase (EROD), PROD, and cytochrome P450 2b1/2 (Hanioka et al., 1996; Jinno et al., 1997) but at excessively higher doses. In addition, iodothyronine sulfotransferase (SULF) activity in hepatic microsomes, which is an important transporter protein, was found to be inhibited by TCS (Schuur et al., 1998; Wang et al., 2004). However, in the current study, no marked change in SULT 1b1 or 1c3 gene expression was observed.
Interestingly, TCS was also shown to activate pregnane-X receptors (PXR) (Jacobs et al., 2005), which may increase expression of metabolic enzymes that catabolize and transport T4, including uridine diphosphoglucuronyl transferases (UGTs) (Visser et al., 1996; Chen et al., 2003; Mackenzie et al., 2005; Zhou et al., 2005). The 2.2-fold rise in expression of Cyp2b2 in the current study agrees with data that suggest that TCS may activate nuclear receptors, such as CAR (Paul et al., 2013). Previous studies also indicate that TCS elevated PROD activity in the rat, which would increase Cyp2b2 (Hanioka et al., 1996; Jinno et al., 1997; Zorrilla et al., 2009), further supporting activation of CAR/PXR in response to TCS. Paul et al. (2010) found enhanced expression of both Cyp2b2 (2.2-fold) and Cyp3a1 (4-fold), at a higher dose (300 mg/kg) than the current study following short-term exposure in the weanling female rat.
The serum concentrations associated with TH alterations in the current study are over 100-fold higher than human TCS plasma concentrations following short-term exposure in toothpaste or oral rinse (Allmyr et al., 2009; Sandborgh-Englund et al., 2006) and over 1000-fold higher than reported baseline TCS human plasmas levels (Allmyr et al., 2008). Therefore, such differences in exposure and life stage of such exposures need to be considered for human relevance derived from this study.
In conclusion, the current study expanded on our earlier findings describing estrogen-induced uterine responses, advanced puberty, and altered serum TH to help elucidate whether such effects on the endocrine system might still be observed following chronic exposure to TCS. The effects of TCS on female reproductive function appear to be minimal following chronic exposure, as no significant effects were observed when estrous cycles were compared to controls over the exposure period. However, chronic exposure to TCS did extend our earlier observations on the influence of this compound on TH concentrations in the rat, as this extended exposure resulted in a lower NOEL (4.69 mg/kg) and LOEL (9.375 mg/kg) for suppression of serum T4 levels as compared to shorter exposures in the rodent. Importantly, our data confirmed that no marked changes in TSH, thyroid weight, or histology alterations occur following chronic exposure, which is similar to the profile observed following shorter exposures.
Supplementary Material
Acknowledgments
We would like to thank Angela Buckalew, Deborah Best, and Ashley Murr for their technical assistance with this project. The animal husbandry and dosing support provided by Alfred Moore was also greatly appreciated.
Funding
This research was supported in part by an appointment to the Research Participation Program for the United States Environmental Protection Agency, Office of Research and Development, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US EPA.
Footnotes
Conflict of interest statement
The authors do not have any conflicts to disclose.
Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Allmyr M, Harden F, Toms LMI, Mueller JF, McLachlan MS, Adolfsson-Erici M, Sandborgh-Englund G. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci Total Environ. 2008;393:162–167. doi: 10.1016/j.scitotenv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic Clin Pharmacol Toxicol. 2009;105:339–344. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- Barter RA, Klaassen CD. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol Appl Pharmacol. 1994;128:9–17. doi: 10.1006/taap.1994.1174. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Beaudet ALAIN, Desjardins GC, Schipper HM. Pathologic effect of estradiol on the hypothalamus. Biol Reprod. 1993;49:647–652. doi: 10.1095/biolreprod49.4.647. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Schipper H, Naftolin F. Ovary-dependent degeneration in the hypothalamic arcuate nucleus. Endocrinology. 1980;107:274–279. doi: 10.1210/endo-107-1-274. [DOI] [PubMed] [Google Scholar]
- Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleost fish thyroid. Environ Toxicol Chem. 2004;23:1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. Population: 2003–2004. Environ Health Persp. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, Patiño R. The hypothalamus–pituitary– thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen Comp Endocrinol. 2011;170:299–312. doi: 10.1016/j.ygcen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- CDC. Report. 2013 Available at https://wwwn.cdc.gov/Nchs/Nhanes/Search/nhanes13_14.aspx (accessed April 20, 2016)
- Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregnane X receptor, is required for induction of UDP-glucuronosyltransferases in mouse liver by pregnenolone-16α-carbonitrile. Drug Metab Disposit. 2003;31:908–915. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- Cooper RL. Vaginal cytology. In: Daston G, Kimmel C, editors. An Evaluation and Interpretation of Reproductive Endpoints for Human Health Risk Assessment. Vol. 9. Washington: ILSI Press; 1999. pp. 32–56. [Google Scholar]
- Cooper RL, Linnoila M. Sexual behavior in aged, non-cycling female rats. Physiol Behav. 1977;18:573–576. doi: 10.1016/0031-9384(77)90054-3. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Roberts B, Rogers DC, Seay SG, Conn PM. Endocrine status versus chronologic age as predictors of altered luteinizing hormone secretion in the “aging” rat. Endocrinology. 1984;114:391–396. doi: 10.1210/endo-114-2-391. [DOI] [PubMed] [Google Scholar]
- Cope RB, Kacew S, Dourson M. A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague-Dawley rats. Toxicology. 2015;329:49–59. doi: 10.1016/j.tox.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Paul KB, DeVito MJ, Hedge JM. Short-term in-vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 2007;24:194–197. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Available at http://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm519798.pdf (accessed 2016)
- Fraites MJ, Narotsky MG, Best DS, Stoker TE, Davis LK, Goldman JM, Hotchkiss MG, Klinefelter GR, Kamel A, Qian Y, Podhorniak L. Gestational atrazine exposure: effects on male reproductive development and metabolite distribution in the dam, fetus, and neonate. Reprod Toxicol. 2011;32:52–63. doi: 10.1016/j.reprotox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Cooper RL, Rehnberg GL, Hein JF, McElroy WK, Gray LE., Jr Effects of low subchronic doses of methoxychlor on the rat hypothalamic-pituitary reproductive axis. Toxicol Appl Pharmacol. 1986;86:474–483. doi: 10.1016/0041-008x(86)90375-3. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B: Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Greenwood FC, Hunter WM, Glover T. The preparation of 131I-labeled human growth hormone of high specific activity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, Kenny N, Niswender GD. Progesterone production, LH receptors, and oxytocin secretion by ovine luteal cell types on days 6, 10 and 15 of the oestrous cycle and day 25 of pregnancy. J Reprod Fertil. 1987;79:539–548. doi: 10.1530/jrf.0.0790539. [DOI] [PubMed] [Google Scholar]
- Hood A, Hashmi R, Klaassen CD. Effects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol Appl Pharmacol. 1999;160:163–170. doi: 10.1006/taap.1999.8752. [DOI] [PubMed] [Google Scholar]
- Hood A, Klaassen CD. Effects of microsomal enzyme inducers on outer-ring deiodinase activity toward thyroid hormones in various rat tissues. Toxicol Appl Pharmacol. 2000;163:240–248. doi: 10.1006/taap.1999.8883. [DOI] [PubMed] [Google Scholar]
- Huang H, Du G, Zhang W, Hu J, Wu D, Song L, Xia Y, Wang X. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol. 2014;34:1060–1067. doi: 10.1002/jat.3012. [DOI] [PubMed] [Google Scholar]
- James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int. 2010;36:942–949. doi: 10.1016/j.envint.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno H, Hanioka N, Onodera S, Nishimura T, Ando M. Irgasan® DP 300 (5-chloro-2-(2, 4-dichlorophenoxy) phenol) induces cytochrome P450s and inhibits haembiosynthesis in rat hepatocytes cultured on Matrigel. Xenobiotica. 1997;27:681–692. doi: 10.1080/004982597240271. [DOI] [PubMed] [Google Scholar]
- Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Omae E, Nishimura T, Jinno H, Onodera S, Yoda R, Ando M. Interaction of 2, 4, 4′-trichloro-2′-hydroxydiphenyl ether with microsomal cytochrome P450-dependent monooxygenases in rat liver. Chemosphere. 1996;33:265–276. doi: 10.1016/0045-6535(96)00169-5. [DOI] [PubMed] [Google Scholar]
- Lai DY, Kacew S, Dekant W. TetrabromobisphenolA (TBBPA): possible modes of action of toxicity and carcinogenicity in rodents. Food Chem Toxicol. 2015;80:206–214. doi: 10.1016/j.fct.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Lange A, Sebire M, Rostkowski P, Mizutani T, Miyagawa S, Iguchi T, Hill EM, Tyler CR. Environmental chemicals active as human antiandrogens do not activate a stickleback androgen receptor but enhance a feminising effect of oestrogen in roach. Aquat Toxicol. 2015;168:48–59. doi: 10.1016/j.aquatox.2015.09.014. [DOI] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Stoker TE. The effect of triclosan on the uterotrophic response to extended doses of ethinyl estradiol in the weanling rat. Reprod Toxicol. 2013;36:71–77. doi: 10.1016/j.reprotox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Barter RA, Klaassen CD. Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther. 1995;273:977–985. [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro SI, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) Pharmacogen Gen. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- Padilla-Banks E, Jefferson WN, Newbold RR. The immature mouse is a suitable model for detection of estrogenicity in the uterotropic bioassay. Environ Health Persp. 2001;109:821. doi: 10.1289/ehp.01109821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300:31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young Long-Evans rats. Toxicol Sci. 2010;113:367–379. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Thompson JT, Simmons SO, Heuvel JPV, Crofton KM. Evidence for triclosan-induced activation of human and rodent xenobiotic nuclear receptors. Toxicol in Vitro. 2013;27:2049–2060. doi: 10.1016/j.tiv.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Pollock T, Tang B. Triclosan exacerbates the presence of 14 C-bisphenol A in tissues of female and male mice. Toxicol Appl Pharmacol. 2014;278:116–123. doi: 10.1016/j.taap.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development —current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Ross MK, Filipov NM. Determination of atrazine and its metabolites in mouse urine and plasma by LC–MS analysis. Anal Biochem. 2006;351:161–173. doi: 10.1016/j.ab.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Ross MK, Jones TL, Filipov NM. Disposition of the herbicide 2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine (Atrazine) and its major metabolites in mice: a liquid chromatography/mass spectrometry analysis of urine, plasma, and tissue levels. Drug Metab Dispos. 2009;37:776–786. doi: 10.1124/dmd.108.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERα is necessary for estradiol’s anorexigenic effect in female rats. Horm Behav. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur AG, Legger FF, van Meeteren ME, Moonen MJ, van Leeuwen-Bol I, Bergman Å, Visser TJ, Brouwer A. In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons. Chem Res Toxicol. 1998;11:1075–1081. doi: 10.1021/tx9800046. [DOI] [PubMed] [Google Scholar]
- Shirwalkar H, Modi DN, Maitra A. Exposure of adult rats to estradiol valerate induces ovarian cyst with early senescence of follicles. Mol Cell Endocrinol. 2007;272:22–37. doi: 10.1016/j.mce.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Sinha D, Cooper D, Dao TL. The nature of estrogen and prolactin effect on mammary tumorigenesis. Cancer Res. 1973;33:411–414. [PubMed] [Google Scholar]
- Stoker TE, Ferrell JM, Laws SC, Cooper RL, Buckalew A. Evaluation of ammonium perchlorate in the endocrine disruptor screening and testing program’s male pubertal protocol: ability to detect effects on thyroid endpoints. Toxicology. 2006;228:58–65. doi: 10.1016/j.tox.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci. 2010;117:45–53. doi: 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, Gijzel A, de Herder WW, Cannon ML, Bonthuis F, de Greef WJ. Effects of thyroid status and thyrostatic drugs on hepatic glucuronidation of lodothyronines and other substrates in rats. Endocrine. 1996;4:79–85. doi: 10.1007/BF02738878. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Chen L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci Rep. 2015;5 doi: 10.1038/srep18252. Article number 18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32:162–169. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Li J, Hao FR, Yuan Y, Li JY, Lu W, Zhou TY. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta Pharmacol Sinica. 2016;37:845–856. doi: 10.1038/aps.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Bottner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Rec Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med. 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang J, Xie W. Xenobiotic nuclear receptor-mediated regulation of UDP-glucuronosyltransferases. Curr Drug Metab. 2005;6:289–298. doi: 10.2174/1389200054633853. [DOI] [PubMed] [Google Scholar]
- Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens) 2010;9:28–40. doi: 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


