Abstract
Interfragmental ischaemia is a prerequisite for the initiation of the inflammatory and immunological response to fracturing of bone.
Intrafragmental ischaemia is inevitable: the extent of the initial ischaemic insult does not, however, directly relate to the outcome for healing of the fracture zones and avascular necrosis of the humeral head. The survival of distal regions of fragments with critical perfusion may be the result of a type of inosculation (blood vessel contact), which establishes reperfusion before either revascularization or neo-angiogenesis has occurred.
Periosteum has a poorly defined role in fracture healing in the proximal humerus. The metaphyseal periosteal perfusion may have a profound effect, as yet undefined, on the healing of most metaphyseal fractures of the proximal humerus, and may be disturbed further by inadvertent surgical manipulation.
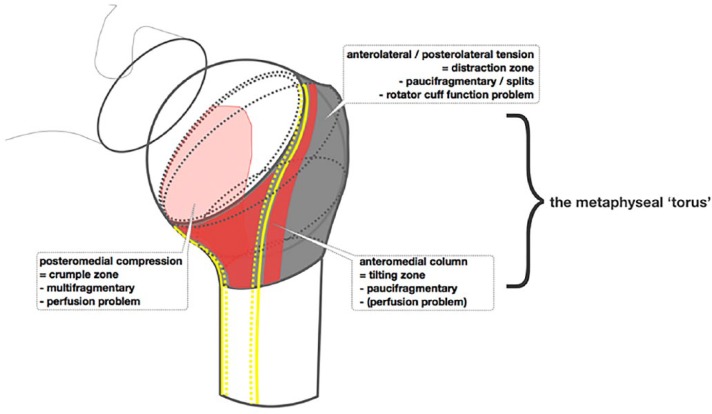
The metaphysis can be considered as a ‘torus’ or ring of bone, its surface covered by periosteum antero- and posterolaterally, through which the tuberosity segments gain perfusion and capsular reflections antero- and posteromedially, through which the humeral head (articular) fragment gains perfusion.
The torus is broken in relatively simple primary patterns: a fracture line at the upper surface of the torus is an anatomical ‘neck’ fracture; a fracture line at the lower surface of the torus is the surgical ‘neck’ fracture. Secondary fragmentation (through compression and/or distraction) of the torus itself creates complexity for analysis (classification), alters the capacity and outcome for healing (by variable interruption of the fragmental blood supply) and influences interfragmental stability.
Cite this article: EFORT Open Rev 2018;3 DOI: 10.1302/2058-5241.3.180005
Keywords: proximal humerus, periosteum, healing, ischaemia, outcomes
Introduction
The functional outcome of a proximal humeral fracture (PHF) is never that of a completely normal shoulder, however treated. Management of PHF, as for all fractures, combines an assessment of the mechanical and biological factors affecting fracture healing with a judgement about the likely prognosis for healing and function based on a multiplicity of intrinsic (shoulder) and extrinsic (comorbid) factors. For any displaced fracture treated nonoperatively, the outcome will be defined by the original displacement: the patient will inevitably heal with a malunion, and the functional value of the shoulder will reflect this. Clinicians who advocate nonoperative treatment for displaced PHF accept that they are relying on the patients’ tolerance of malunion at the completion of healing. Clinicians who advocate surgical intervention to restore the shape of the proximal humerus, with the intention of restoration of the centre of rotation of the glenohumeral joint for optimal deltoid function, accept that they will potentially interrupt, and possibly adversely affect, the cascade of healing processes, leading to complications of the intervention. A recent pragmatic trial concluded that there was no useful difference between intervention and nonoperative management for some displaced PHFs, provoking a debate about the quality of the trial methodology including, importantly, the methods of radiological assessment of the fractures, the methods of functional outcomes assessments and the expertise (judgement and surgical aptitude) of the treating clinicians. Radiological assessment (classification) is a surrogate for the assessment of the anticipated effect of a fracture line or zone on the perfusion of bone distal (in terms of perfusion) to the fracture, and an assessment of the stability of co-aptation of fracture fragments, i.e. the resistance to further interfragmental displacement with limb movement. The latter directly affects both the restoration of perfusion and the eventual shape of the bone on completion of healing, which largely defines the functional outcome. A poorly reproducible or, from the perspective of perfusion for fracture healing, a less relevant system of assessment, will inevitably lead to misinterpretation of prognosis. If a radiological assessment tool does not accurately forecast the likelihood of poor perfusion of a PHF, then incomplete decisions about management of PHF (to optimize healing in the optimal shape and form) will ensue. Radiographic assessment at intervals of healing inevitably reflect past cellular- and tissue-level events, and so may be unhelpful in predicting poor outcome at critical times for decisions to be made. Knowledge of the expected cascade of events, based on fracture morphology, must therefore be the basis for decision-making in individually specific PHF management. This review summarizes the current knowledge of disturbance of perfusion in healing of PHFs, and how this might translate into predictions about functional outcomes of PHFs. Definitions of terms used are given in Table 1: the surgical and radiological literature of PHF often conflates terms, and this set of definitions is designed to assist accuracy in discussion.
Table 1.
Definitions: for there to be a common language describing the events and processes involved in fracture healing a set of definitions is suggested, with comments
| Term | Definition |
|---|---|
| Ischaemia | A process characterized by deleterious cellular events resulting in aberrant or failing tissue homeostasis and impaired cellular function due to inadequate tissue perfusion. The effects of ischaemia can be irreversible and permanent, resulting in tissue death and (in bone) resorption at the site of injury and extending beyond it, or reversible, resulting in a spectrum of outcomes ranging from the restoration of healthy, functionally adequate bone to impaired bone formation at the zone of injury but not extending beyond it. The primary ischaemic injury may be occlusive, and its effects are (ischaemia) time-dependent, while a subsequent reperfusion injury may create a secondary ischaemia, so amplifying the primary ischaemic damage. Surgical manipulation of a proximal humeral fracture may generate a third phase of ischaemia, particularly if damaged vessels undergo further distortion and occlusion. Placement of a lateral humeral plate will potentially adversely affect periosteal perfusion at the critical period of healing when the lateral ‘tension band’ of subperiosteal bone formation is most beneficial to overall fracture stability. |
| Ischaemic penumbra | The region bounding a zone of ischaemia in which appropriately rapid restoration of perfusion may result in restoration of the potential for the tissue to heal. Conversely, if perfusion is not restored then cellular damage will continue and the zone of injury will increase in extent until oxygen delivery is sufficient for cell survival. The oxygen diffusion distance from a functioning capillary is approximately 150 μm to 200 μm. |
| (Neo-) angiogenesis | The development of new blood vessels. New vessels develop at approximately 5 μm/hr. |
| Revascularization | The re-establishment of continuity of microvasculature within pre-existing microvascular networks, sufficient for flow to be resumed. |
| Inosculation | The formation of interconnections between the pre-existing microvasculature of bone isolated from its blood supply and the host bone microvascular system. |
| Perfusion | The (re-) establishment of flow through the tissue microvasculature such that tissue oxygenation is sufficient for the maintenance or restoration of normal tissue homeostasis. Perfusion is related to the gradient between inflow (arteriolar) and outflow (venular) in the microvascular network: perfusion is reduced, and therefore oxygen availability diminishes and healing potential is adversely affected, if arterial inflow or venous outflow are reduced. |
| Necrosis | A term describing the microscopic features of tissues resulting from inadequate perfusion causing cell death with subsequent macrophage mobilization and activation. It is not a radiographic diagnosis. The radiological features appear late, and reflect the bone response to the healing, but mechanically insufficient, ischaemic penumbrae: features such as trabecular cysts, sclerotic margination, deformation, and collapse of the convex surfaces may be present. Haematopoietic cells are the most sensitive to hypoxia and die within 12 hrs of hypoperfusion. Osteocytes, osteoblasts and osteoclasts die within 12 to 48 hrs, and bone marrow fat cells die within five days. |
| Delayed union | A term used to describe bone healing over a prolonged (unexpected) period due to adverse but reversible mechanical and biological factors. Cessation of periosteal response before fracture bridging is characteristic.58 Interfragmental instability (causing critical interfragmental strain) and hypoperfusion are common cofactors. At a microscopic level increased apoptosis, decreased cell proliferation, chondrogenesis, particularly if poor vasculogenesis11 and fibrous and fatty deposition adjacent to the fracture zone characterize the delayed union.2 |
| Nonunion | A term used to describe failure of completion of bone healing in the expected period due to adverse mechanical and biological factors. Cessation of periosteal and endosteal responses without fracture bridging is characteristic.56 Interfragmental instability (adverse or supracritical interfragmental strain), poor perfusion and additional adverse factors (e.g. infection) are common cofactors. |
| Malunion | A term used to describe healing of bone in a shape and form different to the original model, usually with adverse mechanical (functional) outcomes. Intra-articular and juxta-articular malunions are associated with the greatest functional decrements. |
Ischaemia
Ischaemia of bone is both a prerequisite for initiation of the cascade of processes at cellular level that result in fracture healing1,2 and, at tissue level, a potential problem for the eventual healing, perfusion and therefore vitality of the fracture segments.
Microvascular injury in the fracture zone causes oedema and haematoma, therefore increasing the distance over which oxygen must diffuse (the normal oxygen diffusion distance is between 150 μm and 200 μm) and be available for cell survival. This focal hypoxia induces a systemic response as well as a local response which appears to be largely mediated by vascular endothelial growth factor (VEGF), the production of which is promoted by haematoma-derived transcription induction factors.3-5 Hypoxia and cellular and cytokine elements within the fracture haematoma appear to be essential for the initiation of the subsequent inflammatory response,6 in which immune cells appear to coordinate the timing, duration and extent of angiogenesis,7-9 the production of cytokine-induced endothelial and chondro- and osteo-progenitor cell lineages,10 and which is particularly sensitive to the prevailing mechanical conditions.11,12 Angiogenesis and osteogenesis are intimately dependent: without the first, the initiation of bone formation is often impaired,13,14 and the inflammatory response can be detrimental to haematoma differentiation if poorly controlled.15
This complex parallel processing produces the cell and matrix alterations which are grouped together under the term ‘ischaemic’. If ischaemia did not occur at a fracture zone then the hypoxia-induced inflammatory, immunological and haematological processes that set up the simultaneous removal of damaged cells and matrix and the organization of reparative cartilage and subsequent osteogenesis would be reduced in effect and duration.6 The timing of these events is critical, with defined and finite periods over which the cytokine effects are optimal, and outside which the same cytokines can be counter-productive rather than pro-inductive.15
Containment of the haematoma at the site of fracture provides enhanced stability and progression of the organizing cellular and humeral response. This is one of the many functions of the periosteum.16
Periosteum
The periosteum provides both mechanical support and biological resources vital for normal bone homeostasis and modelling.16-18 The tri-laminar structure19 comprises a highly trophic cambium layer juxtaposed with the Haversian system of normal cortical bone, with which it ‘communicates’ through cytokine20 and electromechanical mechanisms via the Sharpey fibre system,21 a middle highly vascular lamina in which lymphatics are also observed, and an outer connective tissue lamina, contiguous with the fibrous architecture of epimysium, tendon insertions and ligaments. Periosteum is densely adherent to the metaphyseal region of the long bones: in the case of the proximal humerus this is at the proximal extent of the tuberosities, where it is densely adherent and difficult to separate from the cristae of the bicipital sulcus and continuous with the entheses of the rotator cuff and capsular attachment. The periosteum therefore forms an asymmetric collar or ‘torus’ around the proximal humerus (Fig. 1). The torus is an asymmetric ring of bone including the calcar humerale and tuberosities. It is reinforced by two cortical columns from the diaphysis: one anteromedially, forming the medial bicipital crest in continuity with the anteromedial border of the humerus, the other forming the lateral bicipital crest. The calcar commonly fractures with an anteromedial metaphyseal extension from the articular segment and a corresponding posteromedial triangular extension from the diaphysis. The anterior capsular vessels (branches of the anterior circumflex humeral artery, ACHA) are associated with the former, while the posterior capsular vessels (branches of the posterior circumflex humeral artery, PCHA) are associated with the latter. If the former is more than 8 mm in length, or the articular segment is less than 5 mm offset with respect to the diaphysis, then perfusion of the articular segment is less likely to be at risk (Hertel criteria). Therefore, fragmentation of the medial part of the torus determines the risk of perfusion of the articular segment. Scant attention has been paid to the lateral toroidal periosteal perfusion (see Fig. 1), which may be a more important determinant of lateral segmental (tuberosity) healing. In this concept the ACHA and PCHA have vascular territories (angiosomes), which overlap lateral to the lateral bicipital crest: the PCHA is of more importance to articular segment and tuberosity perfusion than the ACHA.
Fig. 1.
Diagram to illustrate the concept of the metaphyseal torus.
Mechanical stability of the torus defines the orientation of the articular segment with the diaphysis and final shape of the healed PHF. The lateral periosteum acts as a lateral tension band if elevated by haematoma (Fig. 2), providing the periosteum remains in continuity. The periosteum of elderly patients is thinner, inelastic, and more readily ruptured.22,23 Hence, fractures creating discontinuity in the lateral part of the torus, whether by compression (valgus) or distraction (varus), will be associated with fracture instability and displacement in the elderly, where a similar type of fracture in a younger patient may not. The anchoring Sharpey’s fibre network degenerates from a 3D network in young bone comprising tangential, oblique and perpendicular fibres (which penetrate to the endosteum), to fewer, stiffer perpendicular and oblique fibres in old bone,24 rendering it more susceptible to damage by distraction or torsion. The cambial lamina of the periosteum generates bone when the tension placed on it is reduced.25 Therefore, when a fracture occurs in the subcapital region of the proximal humerus, endochondral bone formation within the subperiosteal haematoma is promoted: hypoxia within the haematoma is highly angiogenic (mediated via VEGF),26 while the reduced tension within the periosteum (caused by fragmentation of the fracture zone and collapse of the bone structure) promotes cambial activity.27 If the periosteum is ruptured then, as in the experimental condition of periosteal ablation,27 osteogenesis is delayed both by the direct effect of injury to the cambial lamina and through dispersal of the haematoma. The periosteum has been characterized as a ‘smart’ tissue, with properties that influence the function and activity of the endosteum.28,29 The periosteal-endosteal synergy is diminished in periosteum-deficient bone, and endosteal neo-osteogenesis is delayed if the periosteum is injured. The corollary for the proximal humerus is that as interfragmentary displacement (translation) in the fracture increases, so the likelihood of periosteal rupture increases and endosteal signaling is delayed. The periosteum contains the cellular subsets30 and molecular mechanisms31 required for bone remodelling. The collagen orientation of the healing periosteum responds to local mechanical stimuli.32 From these basic principles of periosteal biology it appears logical that assessment, and preservation or restoration, of the integrity of the periosteum might play a large part in the evolution of bone healing. Periosteal ‘stripping’ or rupture may have a significant role in failure to heal (either in time or completion), especially as endosteal vessels will also be disrupted by the fracture.33
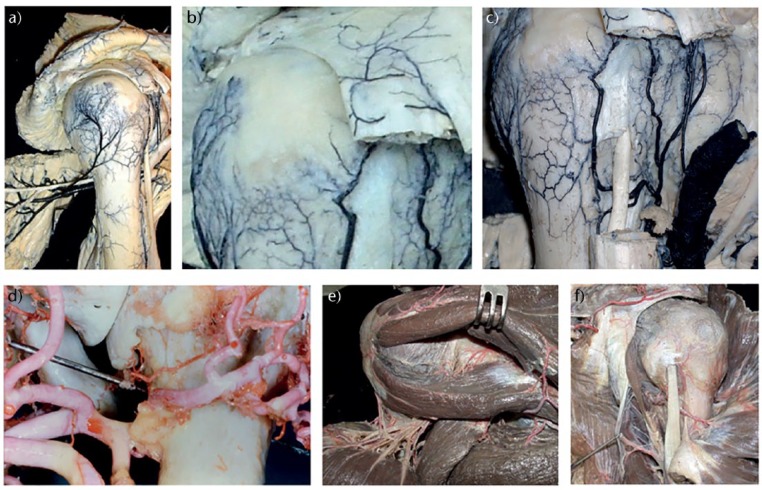
Fig. 2.
The blood supply to the proximal humerus. Dissections and photographs (a-c, e,f) courtesy of Dr C. Zaidenberg; dissection and photograph (d) courtesy of Mr J.I.L. Bayley.
Periosteal blood supply
Most of the periosteal blood supply of the proximal humerus derives from the posterior circumflex humeral artery (PCHA) (Fig. 2) as a network arborizing across the lateral half of the metaphyseal torus. Figure 2a shows that there is a substantial branch (or branches) from the posterior circumflex humeral artery (PCHA) distal to the quadrilateral space and the branches of that vessel to the posterior deltoid and teres minor. The humeral branch ramifies over the majority of the greater tuberosity, terminating as a fine network of vessels which penetrate the tuberosity immediately distal to the insertion footprints of the rotator cuff tendons, as shown in Figure 2b. There are fine anastomoses with the terminal lateral branches of the ascending branch of the anterior circumflex artery, which can be seen in Figures 2b and 2c. These anastomoses lie over the junction of the posterior fragment of a complex proximal humeral fracture (PHF) and the corresponding anterior fragment or, if the anterior fragment is complex, the lateral margin of the shield fragment. Therefore, fractures through the lateral aspect of the humeral metaphyseal torus may create regions of poorly perfused periosteum. Since periosteal vessels are the major contributor to perfusion of the non-articular fragments of a PHF, such fragments may become ischaemic and fail to heal or necrose. This appears to be a more logical concept for the apparent disappearance of the posterior fragment in some PHF than loss of traction through the rotator cuff leading to stress-shielding of the posterior fragment. The PCHA contributes small articular segment branches rather more posteriorly than medially (as seen in Fig. 2d) and continues as a muscular artery of large calibre to the deltoid muscle. The contribution from the rotator cuff to proximal humeral perfusion is minimal (Fig. 2e), with only small and few vessels reaching the capsule from the suprascapular artery. The anterior and posterior circumflex arteries form a metaphyseal arterial ring at the level of the insertion of the upper margin of the teres major (Fig. 2f): if periosteal continuity is preserved at this level in a PHF, then perfusion of the posterior fragment can be maintained or re-established rapidly, providing this is respected if surgical treatment is utilised. A lateral plate will contribute to the risk of deficits of periosteal perfusion, and so may contribute to a small but definite risk of failure to heal the lateral torus. The PCHA supplies multiple small branches as capsular vessels which penetrate the posteromedial capsule at the reflection of the capsule at the calcar humerale (Fig. 2d) as well as the bulk of the blood supply to the posterior and middle parts of the deltoid muscle. The structures supplied by the artery comprise the vascular territory or angiosome34 of the PCHA. Anastomoses with the lateral longitudinal branches of the anterior circumflex humeral artery (ACHA) occur lateral to the lateral bicipital crest, but these are small. The ACHA therefore provides relatively little periosteal perfusion (Fig. 2c). The corresponding angiosome of the ACHA comprises the terminal parts of the subscapularis muscle and tendon, a proportion of the humeral head, the biceps tendon and its synovial ensheathment and the lesser tuberosity. Both PCHA and ACHA contribute to endosteal perfusion of the humeral head (see below). In many patterns of proximal humeral fracturing, such as fracture-dislocation in the elderly, the lateral periosteal–greater tuberosity–rotator cuff (P-GT-RC) continuum remains intact and the injury heals rapidly once the dislocated humeral head has been relocated: the tension in the P-GT-RC tissue is restored and the whole lesion is thus stabilized, while the detachment of the periosteum from cortical bone (and therefore disruption of the Sharpey’s fibres) signals rapid periosteal bone formation (Fig. 3). By the criteria described by Hertel, the articular segment of the fracture shown in Figure 3 should remain sufficiently perfused: treatment should not be guided by articular segment survival, but by the projected eventual shape of the proximal humerus, which will determine the functional outcome. Offset, rotation and inclination are all relevant parameters to discuss, but this fracture is essentially a compression injury of the metaphyseal torus, with regions of fragmentation determined by the stronger cortical regions of the metaphysis (see Fig. 3). There is a low-intensity stripe in continuity bounding the fragments and ‘containing’ material isodense with muscle (and therefore haematoma, Figure 3 c-f). The CT images shown in Figure 3 demonstrate the fracture to be a haematoma-contained, therefore periosteum likely intact, fracture. The periosteal perfusion should be maintained, and the tension-band effect of the periosteum, enhanced by the contained haematoma, should contribute to fracture stability. There should therefore be a good prognosis for healing and modelling respectively, without intervention. Subsequent radiographs (Fig. 3g and h) taken at 4 weeks, and at six weeks (Fig. 3i and j) show the rapid and contained development of the lateral periosteal mineralised callus response. Maintenance of the upper extremity in flexion at the shoulder with internal rotation support by a simple sling has contributed to restoration of the head-neck relationship in both sagittal and frontal planes. In the case of a displaced transverse subcapital fracture through the surgical neck of the humerus, in particular where the diaphysis is displaced laterally with respect to the humeral head, as in a varus displacement, the periosteum may be ruptured: the periosteal blood supply to the fractured zone is disrupted, and tension in the periosteum is completely lost. Delayed union and nonunion in this specific group of fractures is relatively common.35,36 In contrast, periosteal continuity is more common in the valgus-displacement fractures and therefore nonunion is rare, while the fate of the humeral head is determined by factors that reflect more proximal vascular injury.
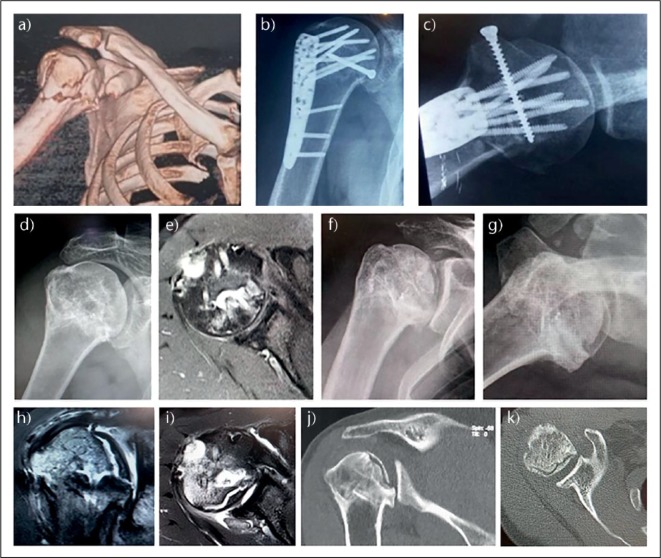
Fig. 3.
Evolution of healing of a complex proximal humeral fracture (PHF). 55-year-old male; office-based profession; dominant arm; fall from standing height; no local or distal neurovascular impairment. Anterior posterior (a) and lateral scapular (b) radiographs of the right shoulder at initial presentation. Axial CT images of the superior (c), equatorial (d), inferior humeral head (e), and (f) the proximal diaphysis immediately distal to the distal extent of the anterior fragment. Radiographs taken at 4 weeks (g, h) and at six weeks (i, j).
Endosteal blood supply
The ACHA and its terminal branches has received the most interest,37 since it is the most readily seen at dissection for fracture. However, the ACHA is approximately three times smaller than the PCHA, which is a much more muscular vessel (Figs 1, 2, 3 and 4). The flow in a blood vessel, approximated by the principle of Poiseuille, is proportional to the radius to the fourth power and inversely proportional to the length of the vessel: the flow in the PCHA is therefore substantially greater than that in the ACHA, which makes sense given the relevance of the perfusion of the majority of the deltoid muscle by the PCHA. The perfusion of the articular segment (humeral head) was considered to be largely reliant on the ACHA through its ascending and arcuate terminal branches,37 but these become irrelevant in fractures involving cleavage through the anterior humeral head (a common observation in so-called three- and four-part fractures) or through the shield fragment.38 The PCHA vessels are equally relevant to perfusion of the humeral head but contribute to a greater volume of bone.39 The posterior capsular branches of the PCHA are several and short, lying closely opposed to the calcar humerale while ‘protected’ by the reflection of the capsule and periosteum up to the articular margin. If this region of the bone is fractured, then these vessels are at risk of rupture or distortion sufficient to impede perfusion of the humeral head. The contribution of vessels derived from the rotator cuff perfusion is minimal: fragments of greater tuberosity attached to rotator cuff tendons still undergo involution and failure to heal with secondary displacement if the lateral periosteal vasculature has been disrupted and not restored (Fig. 2), a mechanism perhaps relevant to the survival of the tuberosities in fractures for which humeral head replacement or reversed total shoulder arthroplasty is performed. The humeral head can survive if vascularly isolated on the deltoid artery, which anastomoses with the ACHA distally.40 This has two implications: (1) the surgical approach to the proximal humerus in cases in which there is a possibility of rupture of the axillary artery or its circumflex branches should respect the integrity of the deltoid artery, a branch of the thoraco-acromial trunk, and there is a sufficient endosteal vascular supply to the humeral head for survival of the head segment without the contribution from the circumflex vessels; (2) inosculation may permit the head segment to survive if there is a fracture at the anatomical neck.41
Fig. 4.
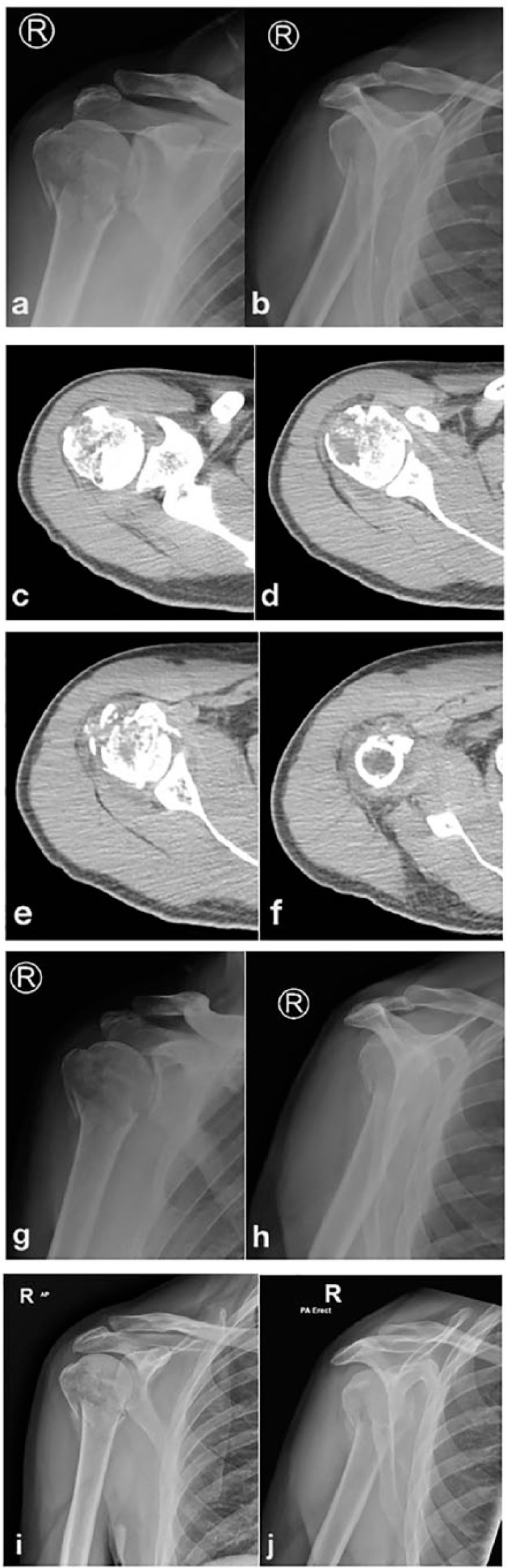
Evolution of avascular necrosis in a complex proximal humeral fracture (PHF). 40-year-old male; surgeon; dominant arm; high speed fall from above standing height; no local or distal neurovascular impairment. (a) 3D-CT reconstruction showing a relatively simple fracture pattern with articular segment, posterior segment and shield segment relatively undisplaced with respect to each other; (b) anteroposterior and (c) axial radiographs; (d) anteroposterior radiograph at 4 months; (e) representative axial MR at the same period; (f) anteroposterior and (g) axial radiographs; (h) coronal oblique MR; (i) axial MR; (j) coronal 2D-CT.
The circumflex arterial angiosomes and classification of PHF
By considering these bone injuries in the context of the angiosomal distribution of the PCHA and ACHA, and considering the medial, short branches (to the humeral articular segment via the calcar humerale capsular reflection) and the lateral, long branches (to the periosteally-dependent bone of the metaphyseal torus) from each artery, it can be seen that classifications which describe or refer to displacement through fracture cleavage planes are, in effect, describing two related aspects: the proximo-distal disruption of the angiosome, and the intrinsic mechanical (in-)stability of the fragmented region. The first concept includes two pathomechanisms: the risk of ischaemia to survival of bone fragments by proximal arterial occlusion or disruption (not only of the humeral head or articular segment); and the relative risk to healing through the haematoma-angiogenesis-osteogenesis cascade (at a distal tissue level). The second concept relates to the relative risk of adverse movement between fragments, which may cause healing in a ‘fracture-stable’ state of malunion, or complete failure to heal (nonunion). The pattern of fracturing through the metaphyseal region of the proximal humerus (the metaphyseal ‘torus’) therefore determines both the outcome for survival of the humeral head fragment and the stability (and subsequent shape and form) of the proximal humerus as a whole (Fig. 1).
Criteria of ischaemia
These concepts form the basis of the work of Hertel et al,42 in which factors relevant to the risk of ischaemia of the humeral head were identified after intra-operative assessment of humeral head perfusion. The factors which appeared most relevant to the relative risk of ischaemia were: the length of the metaphyseal segment (i.e. the length of the calcar humerale which remained attached to the humeral head) and the integrity of the medial hinge (of capsule-periosteum) as judged by the distance or offset of the humeral head from the shaft segment, whether medial or lateral (Table 2). Both, of course, reflect the risk to the proximal PCHA and ACHA. Displacement (angular or linear) of the fracture fragments and the number of fragments, as described by the Neer criteria,43 were not as accurate in defining the risk of ischaemia to the humeral head; these factors are descriptors of the more distal risk of perfusion at the fracture cleavage zones. Since the humeral head may survive (presumably by both inosculation in the short-term and neo-angiogenesis in the longer term) in the absence of proximal flow in the PCHA and ACHA branches,40 the Hertel criteria must be used carefully when defining the risk to fracture healing in individual cases, and then using this summated risk as a prognostic predictor with consequent clinical decision-making. The value of the Hertel criteria to decision-making has been questioned,44 and the risk for eventual necrosis of the humeral head is not predicted by the initial humeral head ischaemia.45 This is very important if clinical decisions are based on criteria for the relative risk of ischaemia based on imaging of the initial fracture, which are only relevant for an appreciation of the risk to healing and therefore assist the decision whether to intervene to optimize both the likelihood of healing (the biological aspect) and the eventual shape of the healed bone (the mechanical aspect), rather than the vitality of the humeral head segment.
Table 2.
Features of proximal humeral fractures predictive of ischaemia
| Criterion | Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| Three fragments | 0.36 | 0.40 | 0.38 | 0.43 | 0.34 |
| Four fragments | 0.62 | 0.73 | 0.67 | 0.74 | 0.61 |
| Anatomic neck involvement | 0.76 | 0.62 | 0.70 | 0.71 | 0.68 |
| Calcar segment (< 8 mm) | 1.00 | 0.64 | 0.84 | 0.77 | 1.00 |
| Disrupted medial hinge (> 2 mm) | 0.78 | 0.80 | 0.79 | 0.83 | 0.75 |
| Tuberosity displacement (>10 mm) | 0.69 | 0.51 | 0.61 | 0.63 | 0.58 |
| Glenohumeral joint dislocation | 0.22 | 0.82 | 0.49 | 0.60 | 0.46 |
| Head-split | 0.20 | 0.84 | 0.49 | 0.61 | 0.46 |
| Anatomic neck + calcar < 8 mm | 0.76 | 0.82 | 0.79 | 0.84 | 0.74 |
| Anatomic neck + calcar < 8 mm + disrupted medial hinge | 0.58 | 0.98 | 0.76 | 0.97 | 0.66 |
Note: Created using data from Hertel et al42
In summary
Hypoxia at the cellular level is the prerequisite for the initiation of the bone (and cartilage) healing cascade;
hypoxia induces angiogenesis which is coupled with the initiation and progression of osteogenesis;
inosculation plays an undefined but probably important role in the restoration of perfusion of bone in the ischaemic penumbra in the early phase of healing;
revascularization may be more important for the perfusion of the bone (and associated soft tissues) at the proximal periphery of the fracture fragments, while neo-angiogenesis leading to endochondral ossification and creeping substitution are more important for the more distal or central regions of the fracture fragments (‘proximal’ and ‘distal’ are expressed here with respect to the peripheral blood supply, see below);
reperfusion injury can occur at cellular level in bone, and may lead to delayed healing;
prolonged ischaemia (i.e. time-dependent insufficiency of perfusion) is associated with failure to heal fractures;
periosteum, the ‘bounding membrane’ of bone28 plays a vital role in the initiation, containment and progression of the cellular, immunological and vascular response to fracturing of bone;
consideration of the vascular angiosomes of the PCHA and ACHA may reveal the potential risks to segment and intersegmental ischaemia leading to failure to survive and failure to heal, respectively.
Mechanical factors related to blood supply and healing of PHFs
As has been extensively discussed in recent and controversial literature46 concerning the management of PHFs, the optimal management and rehabilitation of these fractures is incompletely understood. Meta-analyses and pragmatic trials have confounded the difficulty of understanding PHFs better by combining fractures with inherently different biology and biomechanical attributes, and drawing generalizations from the great heterogeneity of the material through statistical manipulations. The more valuable literature includes those of specific fracture patterns47,48 and specific treatments,49-51 which accords with the conclusions of another meta-analysis,52 and from which guidance about the management of specific fractures can be inferred. If the underlying biology (the vascular basis for healing) was better realized for individual fractures then perhaps the management of specific fractures would be better developed. As an example of where this concept might lead, there is little or no literature specifically addressing the integrity or otherwise of the lateral periosteum of the proximal humerus; the condition of the periosteum is inferred from radiographs and cross-sectional imaging (Fig. 2). For instance, if the integrity of the periosteum and thus the containment of the fracture haematoma was known, and the lateral tension-band effect of the periosteum assessed by ultrasound examination, there would be a firm and logical basis for the active clinical decision to manage certain PHFs nonoperatively, and yet others (similar in overall broad category) by operative intervention of a minimally-invasive nature. Figure 4 shows 3D-CT reconstruction of a relatively simple fracture pattern with articular segment, posterior segment and shield segment relatively undisplaced with respect to each other. These segments are displaced with respect to the anterior segment, which is itself displaced with respect to the diaphysis, and the fracture line between the anterior segment and the diaphysis is at the articular margin (not seen on the CT, but clear on subsequent imaging). This defines a relatively poor prognosis for ischaemia of the articular segment. The fracture type is a shearing in the oblique horizontal plane. The fracture has been fixed anatomically, with restoration of the torus form and shape (Fig. 4b,c). This was undertaken using a lateral plate and thus the lateral periosteal perfusion was also at risk. The fracture has an intrinsic hypovascularity due to the fracture, and an iatropathic hypovascularity due to the choice of internal fixation. The plate was removed at three months, at the patient’s request and not for biological or implant-related reasons. The torus healed, and the articular segment appeared to have survived (Fig. 4d, e). However, the shoulder remained stiff and there was pain, independent of activity. By 6 months there was a clear subarticular fracture with arthritis, although the joint space was relatively preserved (Fig. 4 f,g). By 8 months the necrotic segment had clearly demarcated, and bone necrosis had evolved with no evidence of healing (Fig. 4 h-k). There was collapse and established arthritis, but with excellent healing of the metaphysis with sufficiently normal architecture to consider a short-stemmed hemiarthroplasty in this young patient. Waiting for the evolution of the avascular necrosis permitted time for the torus to heal (thus restoring the relationship between the rotator cuff and centre of rotation of the joint) while the glenoid was preserved (by earlier removal of the plate), and thus the need for a long-stemmed hemiarthroplasty was avoided, while glenoid surface replacement is not mandated by surface damage.
The extensive literature of the management of PHFs permits some summary points:
There is a clinically-relevant and prognostically-important direct relationship between fracture perfusion and interfragmental stability;
conversely, restoration of interfragmental stability generally contributes to optimization of fractures healing through stabilizing the vascular response;
failure of initial perfusion is tolerated well, even if the humeral head segment undergoes necrosis and involution, provided the torus of the proximal humerus is restored to near-normal anatomy;
failure of mechanical stability (through bone healing) is not tolerated well: partial and complete nonunion can be predicted from the pattern of fracturing and the evolution of early fracture healing;
surgical treatment that aims to restore interfragmental stability so that optimal perfusion of the fracture fragments can be established must take into account specific regions of the fracture: the medial and posteromedial cortical region, the lateral cortical region, and the shield region.
Treatments that risk further injury to these regions can impede healing: surgical treatment that aims to restore optimal shape to the proximal humerus must take into account the same parameters, and the supporting columns (Fig. 1). Treatment that fails to gain sufficient implant-bone stability due to insufficient quality or quantity of bone53,54 risks failure of mechanical stability, and subsequent failure to heal with secondary displacement or nonunion;
the lack of understanding about the timing and sequence of fracture healing leads to uncertainty about the value and type of rehabilitation required for optimal outcomes following PHF whether treated surgically or non-surgically. Inappropriate early rehabilitation in specific fracture types (example: the displaced transverse subcapital humeral fracture with lateral periosteal rupture) may contribute to a greater risk of nonunion;
malalignment of the articular segment at healing is tolerated for personal and domestic level function within an ‘inner cone’ of movement of the shoulder providing the torus segment has healed anatomically, but not for function ranging into an ‘outer cone’ for social functions;
conversely, malunion (defined rather broadly in the literature, but generally taken to be healing within the distances and angles given by the Neer criteria for fracture segment displacement) of the torus segments is not well tolerated for personal and domestic level functions;
nonunion occurs in those fractures involving the torus segments (due to periosteal rupture), particularly if mechanical stability is not gained early;55
cross-sectional imaging reveals incomplete interfragmentary healing in the majority of fractures imaged for the management of complications; this is a potent and probable cause for persistent fracture-related pain, even in apparently well-healed fractures, since articular segment excision and hemi-arthroplasty usually results in less pain, even if movement is not improved;
avascular necrosis is relatively rare, is tolerated well if the torus is anatomical after healing, and is managed according to the extent of necrosis and sequestration56,57 (Table 3).
Table 3.
Classification of proximal humeral avascular necrosis, following Ficat and Arlet classification of femoral head necrosis59
| Stage | Description of imaging, clinical picture and possible treatment options |
|---|---|
| 1 | Normal radiograph; changes on MRI; bone pain/‘disproportionate' stiffness; core decompression considered |
| 2 | Sclerosis (wedged, mottled); osteopaenia; bone and surface, movement-related pain, core decompression and arthroscopic debridement considered |
| 3 | Crescent sign, indicating a subchondral fracture; loose bodies; synovitis; bone, surface and articular/synovial pain at rest; resurfacing, partial or hemiarthroplasty considered, depending on metaphyseal bone quality |
| 4 | Flattening and collapse; possibly less pain at rest, crepitation and movement-related pain; resurfacing, partial or hemiarthroplasty considered |
| 5 | Degenerative changes involve the glenoid surface; rest, movement-related, synovial and bone pain; consider total shoulder arthroplasty |
Conclusions
Decisions about the management of PHFs should be based on optimization of the vascular integrity of the angiosomes of the PCHA and ACHA (for healing) and optimization of shape for optimal function, rather than on the risk of avascular necrosis of the humeral head.
Necrosis, chondromalacia, segmental collapse and secondary arthritis of the glenohumeral joint (the inner gliding plane) are tolerated well if the shape of the proximal humerus has been restored and relative movement between the tuberosities and coraco-acromio-deltoid arch (the outer gliding plane) has been restored. Secondary, planned surgical intervention for the articular degeneration is facilitated by restoration of the shape of the proximal humerus. Therefore, if it is possible (using the criteria described) that the humeral head segment might necrose, then the aim of PHF management must be to optimize the healing and shape of the metaphyseal torus: preservation or restoration of periosteal continuity has an important, yet undefined, role in this. When avascular necrosis of the humeral head segment does occur, management depends on the extent of the subsequent infarction and sequestrum formation (Table 3 and Fig. 4).
Footnotes
ICMJE Conflict of interest statement: S. Lambert declares board membership of the Upper Extremity Expert Group, AO Foundation; consultancy for Mathys and Depuy; grants/grants pending from RCS England; payment for development of educational presentations from University College London; travel/accommodation/meeting expenses from AO Foundation, activities outside the submitted work.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999;5:434-438. [DOI] [PubMed] [Google Scholar]
- 2. Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res 2007;25:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci 2000;113:59-69. [DOI] [PubMed] [Google Scholar]
- 4. Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J 2010;6:85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 1999;5:623-628. [DOI] [PubMed] [Google Scholar]
- 6. Kolar P, Schmidt-Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 2010;16:427-434. [DOI] [PubMed] [Google Scholar]
- 7. Bragdon B, Lam S, Aly S, et al. Earliest phases of chondrogenesis are dependent upon angiogenesis during ectopic bone formation in mice. Bone 2017;101:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prisby RD. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J Endocrinol 2017;235:R77-R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiss S, Zimmermann G, Pufe T, Varoga D, Henle P. The systemic angiogenic response during bone healing. Arch Orthop Trauma Surg 2009;129:989-997. [DOI] [PubMed] [Google Scholar]
- 10. Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 2003;18:1584-1592. [DOI] [PubMed] [Google Scholar]
- 11. Carter DR, Beaupré GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res 1998;355(suppl):S41-S55. [DOI] [PubMed] [Google Scholar]
- 12. Klein P, Schell H, Streitparth F, et al. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res 2003;21:662-669. [DOI] [PubMed] [Google Scholar]
- 13. Towler DA. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr Osteoporos Rep 2008;6:67-71. [DOI] [PubMed] [Google Scholar]
- 14. Grellier M, Bordenave L, Amédée J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol 2009;27:562-571. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Engineering: Part B. Volume. 2015;21:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol 2010;39:319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chanavaz M. Anatomy and histophysiology of the periosteum: quantification of the periosteal blood supply to the adjacent bone with 85Sr and gamma spectrometry. J Oral Implantol 1995;21:214-219. [PubMed] [Google Scholar]
- 18. Kitaoka K, Furman B, Saha S, Tomita K. The mechanical role of the periosteum on the impact resistance of bone. 45th Annual Meeting, Orthopaedic Research Society Abstracts, February 1-4, 1999, Anaheim, California, p. 760. [Google Scholar]
- 19. Augustin G, Antabak A, Davila S. Review. The periosteum. Part 1: Anatomy, histology and molecular biology. Int J Care Injured 2007;38:1115-1130. [DOI] [PubMed] [Google Scholar]
- 20. Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res 2012;30:1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aaron JE. Periosteal Sharpey’s fibers: a novel bone matrix regulatory system? Front Endocrinol (Lausanne) 2012;3:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross Crawford Fan W., Xiao Yin. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone 2008;42:81-89. [DOI] [PubMed] [Google Scholar]
- 23. Fan W, Bouwense SAW, Crawford R, Xiao Y. Structural and cellular features in metaphyseal and diaphyseal periosteum of osteoporotic rats. J Mol Histol 2010;41:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Qtaitat A, Shore RC, Aaron JE. Structural changes in the ageing periosteum using collagen III immuno-staining and chromium labelling as indicators. J Musculoskelet Neuronal Interact 2010;10:112-123. [PubMed] [Google Scholar]
- 25. Wang T, Zhang X, Bikle DD. Osteogenic differentiation of periosteal cells during fracture healing. J Cell Physiol 2017;232:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Epari DR, Lienau J, Schell H, Witt F, Duda GN. Pressure, oxygen tension and temperature in the periosteal callus during bone healing-an in vivo study in sheep. Bone 2008;43:734-739. [DOI] [PubMed] [Google Scholar]
- 27. Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci 2000;5:64-70. [DOI] [PubMed] [Google Scholar]
- 28. Evans SF, Parent JB, Lasko CE, et al. Periosteum, bone’s “smart” bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res 2013;28:608-617. [DOI] [PubMed] [Google Scholar]
- 29. Evans SF, Chang H, Knothe Tate ML. Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng Part B Rev 2013;19:147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alexander KA, Raggatt L-J, Millard S, et al. Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration. Immunol Cell Biol 2017;95:7-16. [DOI] [PubMed] [Google Scholar]
- 31. Malizos KN, Papatheodorou LK. The healing potential of the periosteum molecular aspects. Injury 2005;36(suppl 3):S13-S19. [DOI] [PubMed] [Google Scholar]
- 32. Foolen J, van Donkelaar C, Nowlan N, et al. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J Orthop Res 2008;26:1263-1268. [DOI] [PubMed] [Google Scholar]
- 33. Whiteside LA, Ogata K, Lesker P, Reynolds FC. The acute effects of periosteal stripping and medullary reaming on regional bone blood flow. Clin Orthop Relat Res 1978;131:266-272. [PubMed] [Google Scholar]
- 34. Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical application. Br J Plast Surg 1987;40:113-118. [DOI] [PubMed] [Google Scholar]
- 35. Papakonstantinou MK, Hart MJ, Farrugia R, et al. Prevalence of non-union and delayed union in proximal humeral fractures. ANZ J Surg 2017;87:55-59. [DOI] [PubMed] [Google Scholar]
- 36. Valencia M, Barco R, Antuna SA. Review article. Pseudoarthrosis and proximal humeral malunions. Pseudoartrosis y maluniones de humero proximal. Rev Esp Cir Ortop Traumatol 2011;55:405-412. [Google Scholar]
- 37. Brooks CH, Revell WJ, Healey FW. Vascularity of the humeral head after proximal humeral fractures. An anatomical study. J Bone Joint Surg [Br] 1993;75-B:l32-l36. [DOI] [PubMed] [Google Scholar]
- 38. Edelson G, Kelly I, Vigder F, Reis ND. A three-dimensional classification for fractures of the proximal humerus. J Bone Joint Surg [Br] 2004;86-B:413-425. [DOI] [PubMed] [Google Scholar]
- 39. Hettrich CM, Boraiah S, Dyke JP, et al. Quantitative assessment of the vascularity of the proximal part of the humerus. J Bone Joint Surg [Am] 2010;92-A:943-948. [DOI] [PubMed] [Google Scholar]
- 40. Gerber C, Lambert SM, Hoogewoud HM. Absence of avascular necrosis of the humeral head after post-traumatic rupture of the anterior and posterior humeral circumflex arteries. A case report. J Bone Joint Surg [Am] 1996;78-A:1256-1259. [DOI] [PubMed] [Google Scholar]
- 41. Laschke MW, Vollmar B, Menger MD. Inosculation: connecting the life-sustaining pipelines. Tissue Engineering: Part B 2009;15:455-465. [DOI] [PubMed] [Google Scholar]
- 42. Hertel R, Hempfing A, Stiehler M, Leunig M. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg 2004;13:427-433. [DOI] [PubMed] [Google Scholar]
- 43. Neer CS., II Displaced proximal humeral fractures. Part I. Classification and evaluation. J Bone Joint Surg [Am] 1970;52-A:1077-1089. [PubMed] [Google Scholar]
- 44. Campochiaro G, Rebuzzi M, Baudi P, Catani F. Complex proximal humerus fractures: Hertel’s criteria reliability to predict head necrosis. Musculoskeletal Surg 2015;99(suppl. Milan):9-15. [DOI] [PubMed] [Google Scholar]
- 45. Bastian JD, Hertel R. Initial post-fracture humeral head ischemia does not predict development of necrosis. J Shoulder Elbow Surg 2008;17:2-8. [DOI] [PubMed] [Google Scholar]
- 46. Rangan A, Handoll H, Brealey S, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA 2015;313:1037-1047. [DOI] [PubMed] [Google Scholar]
- 47. Court-Brow CM, Garg A, McQueen MM. The translated two-part fracture of the proximal humerus. Epidemiology and outcome in the older patient. J Bone Joint Surg [Br] 2001;83-B:799-804. [DOI] [PubMed] [Google Scholar]
- 48. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72:365-371. [DOI] [PubMed] [Google Scholar]
- 49. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg [Br] 2002;84-B:504-508. [DOI] [PubMed] [Google Scholar]
- 50. Blonna D, Rossi R, Fantino G, et al. The impacted varus (A2.2) proximal humeral fracture in elderly patients: Is minimal fixation justified? A case control study. J Shoulder Elbow Surg 2009;18:545-552. [DOI] [PubMed] [Google Scholar]
- 51. Court-Brown C, McQueen M. The impacted varus (A2.2) proximal humeral fracture Prediction of outcome and results of nonoperative treatment in 99 patients. Acta Orthop Scand 2004;75:736-740. [DOI] [PubMed] [Google Scholar]
- 52. Sabharwal S, Patel NK, Griffiths D, et al. Trials based on specific fracture configuration and surgical procedures likely to be more relevant for decision making in the management of fractures of the proximal humerus: findings of a meta-analysis. Bone Joint Res 2016;5:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siebenbürger G, Van Delden D, Helfen T, et al. Timing of surgery for open reduction and internal fixation of displaced proximal humeral fractures. Injury 2015;46(suppl 4):S58-S62. [DOI] [PubMed] [Google Scholar]
- 54. Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing – an update. Injury 2007;38(suppl 1):S3-S10. [DOI] [PubMed] [Google Scholar]
- 55. Court-Brown CM, McQueen MM. Nonunions of the proximal humerus: their prevalence and functional outcome. J Trauma 2008;64:1517-1521. [DOI] [PubMed] [Google Scholar]
- 56. Lee CK, Hansen HR. Post-traumatic avascular necrosis of the humeral head in displaced proximal humeral fractures. J Trauma 1981;21:788-791. [DOI] [PubMed] [Google Scholar]
- 57. Patel S, Colaco HB, Elvey ME, Lee MH. Post-traumatic osteonecrosis of the proximal humerus. Injury 2015;46:1878-1884. [DOI] [PubMed] [Google Scholar]
- 58. Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res 1998;355S(355)(suppl):S22-S30. [DOI] [PubMed] [Google Scholar]
- 59. Cruess RL. Osteonecrosis of bone. Current concepts as to etiology and pathogenesis. Clin Orthop Relat Res 1986;208:30-39. [PubMed] [Google Scholar]