Abstract
Non-cystic fibrosis bronchiectasis (NCFBE) is a chronic inflammatory lung disease characterized by irreversible dilation of the bronchi, symptoms of persistent cough and expectoration, and recurrent infective exacerbations. The prevalence of NCFBE is on the increase in the United States and Europe, but no licensed therapies are currently available for its treatment. Although there are many similarities between NCFBE and cystic fibrosis (CF) in terms of respiratory symptoms, airway microbiology, and disease progression, there are key differences, for example, in response to treatment, suggesting differences in pathogenesis. This review discusses possible reasons underlying differences in response to inhaled antibiotics in people with CF and NCFBE. Pseudomonas aeruginosa infections are associated with the most severe forms of bronchiectasis. Suboptimal levels of antibiotics in the lung increase the mutation frequency of P. aeruginosa and lead to the development of mucoid strains characterized by formation of a protective polysaccharide biofilm. Mucoid strains of P. aeruginosa are associated with a chronic infection stage, requiring long-term antibiotic therapy. Inhaled antibiotics provide targeted delivery to the lung with minimal systemic toxicity and adverse events compared with oral/intravenous routes of administration, and they could be alternative treatment options to help address some of the treatment challenges in the management of severe cases of NCFBE. This review provides an overview of completed and ongoing trials that evaluated inhaled antibiotic therapy for NCFBE. Recently, several investigators conducted phase 3 randomized controlled trials with inhaled aztreonam and ciprofloxacin in patients with NCFBE. While the aztreonam trial results were not associated with significant clinical benefit in NCFBE, initial results reported from the inhaled ciprofloxacin (dry powder for inhalation and liposome-encapsulated/dual-release formulations) trials hold promise. A more targeted approach could identify specific populations of NCFBE patients who benefit from inhaled antibiotics.
Keywords: : aerosols, antibiotics, bronchiectasis, cystic fibrosis, inhalation therapy, nebulizer
Introduction
Non-cystic fibrosis bronchiectasis (NCFBE) is a chronic inflammatory lung disease characterized by irreversible dilation of the bronchi, symptoms of persistent cough and expectoration, and recurrent infective exacerbations. The incidence of NCFBE in the United States is estimated at 52 cases per 100,000 population.(1) Its prevalence is on the increase in the United States and Europe despite childhood vaccination programs and the widespread use of antibiotics to treat respiratory infections.(2–4) According to an analysis of the Medicare Part B database, the prevalence of bronchiectasis increased by 8.74% each year between 2000 and 2007.(5) This increase could be either due to a greater utilization of chest computed tomography (CT) scans resulting in an increase in diagnosis or due to unidentified etiologic factor(s) causing a real increase in prevalence in an aging population.(5)
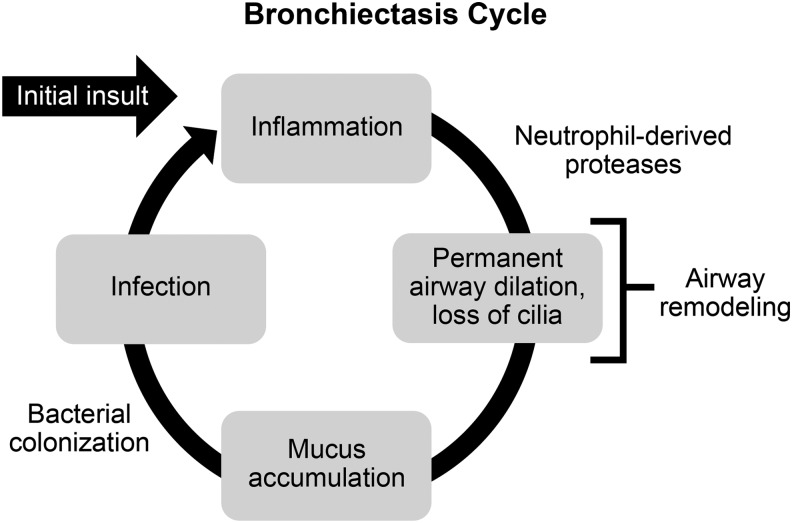
Progressive worsening of NCFBE is characterized by repeated cycles of bronchial bacterial infections that elicit a robust innate immune response, which leads to further airway damage and progressive loss of lung function (see “vicious circle” hypothesis developed by Cole(6) in Fig. 1). Loss of lung function in severe cases of NCFBE leads to frequent hospitalizations, reduced quality of life, and increased mortality rates.(7,8) Until recently, NCFBE received little attention because there are no approved drugs for its treatment. However, a recent revival of interest in this orphan disease has led to bronchiectasis registries being established in Europe(9) and in the United States.(10)
FIG. 1.
The “vicious circle” of bronchiectasis originally described by Cole.(6)
NCFBE most commonly presents with symptoms of chronic cough, sputum production, fatigue, and hemoptysis (“wet” or “productive bronchiectasis”); however, some patients exhibit minimal or no symptoms (“dry” bronchiectasis).(11,12) The reasons for a lack of symptoms in some patients with bronchiectasis could be due to the presence of localized or minimal disease and infrequent exacerbations due to adequate mucus drainage from the involved areas. The diagnosis of bronchiectasis is based on high-resolution CT scans of the chest showing that the internal diameter of the bronchus is larger than that of its accompanying vessel, or the bronchus fails to taper in the periphery of the chest.(13) Airway wall thickening is often present, but this radiographic finding is not diagnostic of bronchiectasis.
Frequent infections and exacerbations characterize the course of illness in NCFBE, with ∼40% of patients experiencing ≥2 exacerbations annually.(14) Exacerbations of NCFBE often require hospitalization and contribute to increased mortality.(1) In patients with NCFBE, mortality ranges from 10% to 16% over an approximate 4-year observation period,(15) and almost 30% over a 13-year follow-up period,(16) due primarily to bronchiectasis or related respiratory failure. Poor lung function and advanced dyspnea scores correlate with a higher mortality. Compared with other causes of NCFBE, patients with idiopathic bronchiectasis have a lower death rate.(17)
NCFBE and Cystic Fibrosis—Similarities and Differences
Although there are many similarities between NCFBE and the genetic disorder cystic fibrosis (CF) in terms of respiratory symptoms, airway microbiology, and disease progression, there are key differences, for example, in response to treatment,(18) suggesting differences in pathogenesis (Table 1). CF develops at a young age and is associated with genetic mutation in the CF transmembrane conductance regulator gene, whereas the etiology in patients with NCFBE is heterogeneous and the cause cannot be established in many patients (idiopathic bronchiectasis).(19) NCFBE is more prevalent than CF, its prevalence increases with advanced age, it is more frequent in women and the Asian population, and in contrast to CF occurs commonly in the lower lobe of the lungs, which makes the mucociliary clearance more difficult.(20)
Table 1.
Differences Between Cystic Fibrosis and Non-cystic Fibrosis Bronchiectasis
| Cystic fibrosis | Non-cystic fibrosis bronchiectasis | |
|---|---|---|
| Age | Young age | More common in older age |
| Sex | No gender difference in occurrence | More common in elderly women |
| Distribution | More common in upper lobes | More common in lower lobes |
| Etiology | Genetic mutation in CFTR gene complicated by infection | Generally postinfectious |
| Prevalence | Uncommon | Three to four times higher prevalence than CF; prevalence increases with age |
| Diagnosis | Sweat chloride level >60 mEq/L is diagnostic | Measurement of sweat chloride is not helpful for diagnosis |
| Comorbidities | Pancreatic insufficiency, sinusitis, airway hyper-responsiveness | Cardiovascular disease, COPD |
| Microbiology | Pseudomonas, Acinetobacter, Burkholderia | Pseudomonas, Haemophilus, Moraxella |
CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; COPD, chronic obstructive pulmonary disease.
NCFBE is often underdiagnosed because it may be associated with other comorbidities, particularly chronic obstructive pulmonary disease. There are also noteworthy differences in therapeutic responses to various interventions: inhaled antibiotics have the potential to clear or “eradicate” initial Pseudomonas aeruginosa (P. aeruginosa) infection, postpone the development of chronic infection, reduce the frequency of exacerbations, and improve lung function in patients with CF, but similar clinical success has not been achieved in patients with NCFBE.(7,21)
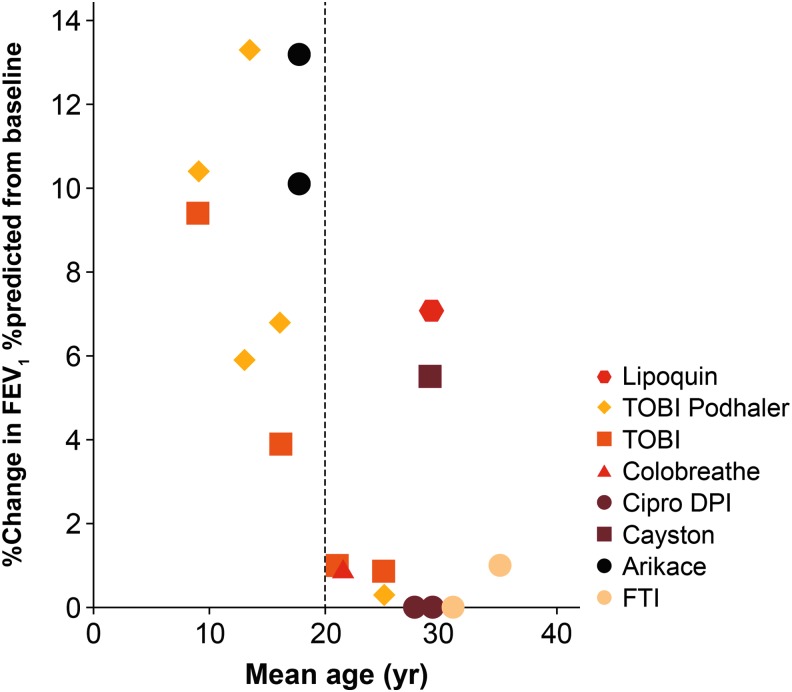
In young patients with CF, “early” chronic infection with P. aeruginosa could be successfully cleared by antibiotic treatment with favorable outcomes.(22) However, chronic infection with P. aeruginosa in patients with NCFBE is much more difficult to eradicate with antibiotics.(23) The reasons for disparate responses to inhaled therapies in CF bronchiectasis and NCFBE are not well understood, but may depend on differences in the age of patients with CF and NCFBE. Improvement in lung function with inhaled antibiotics in patients with CF decreases with patients' age (Fig. 2).(24,25) More advanced age of patients with NCFBE compared with the age of patients with CF who were included in clinical trials could explain the lack of improvement of lung function with inhaled antibiotics.
FIG. 2.
Improvements in lung function with inhaled antibiotics in patients with cystic fibrosis in relation to age. Reprinted from Adv Drug Deliv Rev 85, Cipolla et al., Comment on: Inhaled antimicrobial therapy—Barriers to effective treatment, e6–7, © 2015, with permission from Elsevier.143 DPI, dry powder for inhalation; FEV1, forced expiratory volume in 1 second; FTI, fosfomycin/tobramycin formulation; TOBI, tobramycin solution for inhalation.
In patients with CF, irreversible changes in the airways related to persistent inflammation over many decades, remodeling and fibrosis, and mucus plugs, as well as biofilm formation could result in poorly ventilated areas that impair delivery of antibiotics to airways that are heavily colonized with bacteria.(26) Moreover, antibiotics such as aminoglycosides and ciprofloxacin are less efficacious in anaerobic conditions that commonly exist in areas of poor ventilation, infection, and excessive mucus accumulation, whereas levofloxacin maintains its efficacy under such conditions.(27,28) The occurrence of chronic inflammation and airway wall damage in patients with NCFBE produces a similar milieu to that described above(29) and the efficacy of some inhaled antibiotics is likely to be impaired in such an environment.
The contribution of the bronchial circulation to differences in responses between CF and NCFBE also needs further evaluation. The bronchial arteries show enlargement and tortuosity in bronchiectasis with increased bronchopulmonary anastomosis.(30,31) In patients with bronchiectasis, the principal source of blood supply is from the bronchial circulation; airway inflammation and remodeling contribute to an increase in the airway mucosal vasculature accompanied by new vessel growth from pre-existing vessels and dilatation of existing vessels.(32,33) Moreover, infection with P. aeruginosa induces vascular endothelial growth factor in vitro and in vivo.(34) The fenestrations in the endothelium of the bronchial capillaries,(35) especially in the newly formed vessels, facilitate drug penetration, and the presence of inflammation further enhances the permeability of the vessels.(36) The close proximity of the submucosal bronchial venous system to the airway lumen allows for rapid absorption of inhaled drugs.(37) Thus, the bronchial circulation could play a role in more rapid absorption of antibiotics, and rapid clearance of antibiotics after deposition could limit the duration of their effect at the site of infection unless formulations have adequate residual lung time. However, evaluation of bronchoalveolar lavage fluid found high levels of inflammatory markers in both patients with CF and NCFBE.(38) The levels of neutrophil elastase (and neutrophils), matrix metalloprotease (MMP)-2, and MMP-9 in patients with NCFBE were higher than those in healthy controls but were lower compared to patients with CF.(38) Notably, there are no reported differences in the bronchial circulation or bronchial blood flow to damaged airways that could explain the disparate response to inhaled therapies in patients with NCFBE compared with those with CF.(33)
In bronchiectasis, there is hypertrophy of the mucus-secreting glands, hyperplasia and metaplasia of goblet cells, and an increase in mucus production.(39) Disturbances in mucociliary clearance are central to the development of bronchiectasis and lead to mucus accumulation (Fig. 1) and plugging of small and large airways with mucus as seen on high-resolution chest CT scans. The nature of the mucus is also altered by the presence of chronic inflammation, and properties of the mucus in children with NCFBE differ from those observed in other respiratory diseases.(40) Airway clearance techniques are safe in patients with stable bronchiectasis, although their role in patients with acute exacerbations is not yet clearly established.(41)
In patients with CF, DNA and F-actin filaments released from apoptosis of neutrophils and from damaged epithelial cells are mainly responsible for the increase in viscosity of sputum, and rhDNase reduces the viscosity of sputum by depolymerizing DNA filaments.(42) In contrast, there is a paucity of DNA polymers in sputum of patients with NCFBE and clearance by cough is greater than sputum in CF patients. Moreover, inhalation of rhDNase may not be as effective in reducing sputum viscosity in adults with NCFBE.(43,44)
Alterations in the properties of mucus in patients with bronchiectasis may impair response to antibiotic therapy. The long and branching mucin glycoproteins form a mesh that impedes the transport of aminoglycosides, beta-lactam, and fluoroquinolone antibiotics in vitro,(45) especially for liposomal formulations of aminoglycosides.(46) Moreover, binding to certain large molecules in sputum could reduce antibiotic efficacy. Aminoglycosides bind to mucin, especially in the presence of free DNA, and in an acidic environment.(47–50) Mucus binding has not been observed with beta-lactam antibiotics(51) and further studies are needed to determine if similar interactions occur between mucus and fluoroquinolones or macrolide antibiotics.
Changes in the mucus and antibiotic binding may impede the penetration of antibiotics to the surface of epithelial cells. While high concentrations of intraluminal antibiotics achieved after inhalation are effective at clearing organisms in the sputum, they may not be effective in penetrating through the mucus barrier into the tissues, and suboptimal antibiotic concentrations at the site of infection could impair their ability to eradicate infection in the airways and lung parenchyma.
The response to mucoactive agents has been variable in patients with NCFBE. Their efficacy in addition to long-term antibiotics needs to be explored in future studies, particularly in view of the in vitro observation that prior deposition of mannitol delayed the transport of ciprofloxacin hydrochloride in a Calu-3 air–interface cell model.(52) The delay in ciprofloxacin hydrochloride transport across the epithelium could provide dual benefits by reducing the dosing frequency and by maintaining higher antibiotic concentrations at the site of infection for a longer period.
Role of Pseudomonas aeruginosa in NCFBE
Frequently identified bacterial pathogens in sputum isolates from patients with NCFBE include P. aeruginosa, Moraxella catarrhalis, and Haemophilus influenzae.(53) P. aeruginosa infections are reported in as many as one-fourth to one-half of patients and are associated with the most severe forms of bronchiectasis (defined as requiring hospital admission or characterized by the development of resistance to oral antibiotics), which are linked to higher morbidity and mortality.(7,8) Nontuberculous mycobacteria and gram-positive organisms (Streptococcus pneumoniae and Staphylococcus aureus) are also found.(54–57) Sputum studies with molecular techniques, instead of bacterial cultures, have identified conventional pathogens such as P. aeruginosa and H. influenzae as well as anaerobic organisms such as Prevotella and Veillonella organisms whose pathogenicity is not clearly established.(58)
Isolation of P. aeruginosa has been identified as an independent predictor of accelerated decline of lung function in patients with NCFBE.(59) Furthermore, patients with P. aeruginosa infections score higher than infections with other bacterial pathogens on the Bronchiectasis Severity Index (BSI), which accurately predicts lung function decline, mortality, hospital admissions, exacerbations, quality of life, and respiratory symptoms in patients with bronchiectasis.(60,61) P. aeruginosa is also a key determinant in another severity scoring system, the FACED score.(62) The BSI and FACED scores are multidimensional tools that are predictive of mortality for up to 15 years after diagnosis of NCFBE.(63)
Key strategies for the management of P. aeruginosa in patients with NCFBE cover three main stages: (1) eradication of the pathogen on first isolation; (2) treatment during acute exacerbations; and (3) management of chronic infections.(8) Data on the eradication of P. aeruginosa on first isolation are currently limited; however, a small, retrospective study investigating 30 patients with NCFBE found that eradication of the pathogen was initially successful in 80% of patients with a combination of intravenous, oral, or inhaled antibiotics, resulting in a reduced exacerbation rate following eradication.(22) Nevertheless, 46% of these patients were positive again for P. aeruginosa after a median follow-up of 6.2 months.(22)
Chronic infections with P. aeruginosa require long-term antibiotic therapy to reduce the bacterial load and therefore the frequency and severity of exacerbations.(8) The frequency of their acute exacerbations may determine whether a patient is considered a candidate for long-term antibiotic therapy. Three acute exacerbations per year were considered to be an appropriate threshold for therapy by the British Thoracic Society(64) and by 46% of respondents in a live electronic polling session that was conducted at an Expert Forum of the European Respiratory Society in 2014.(8)
The simplest method of administering long-term antibiotics is by the oral route. Commonly used oral macrolide antibiotics have immunomodulatory properties in addition to their antibacterial effects. Recently, three randomized, double-blind, placebo-controlled studies in patients with NCFBE reported that azithromycin or erythromycin administered orally for 6–12 months was generally well tolerated and led to a reduction in exacerbation rate, and a reduced rate of lung function decline (Table 2).(65–67)
Table 2.
Randomized, Double-Blind, Placebo-Controlled Trials of Oral Macrolides in Non-cystic Fibrosis Bronchiectasis
| EMBRACE65 | BLESS66 | BAT67 | ||||
|---|---|---|---|---|---|---|
| Placebo | Azithromycin | Placebo | Erythromycin | Placebo | Azithromycin | |
| Patient, n | 70 | 71 | 58 | 59 | 40 | 43 |
| Male/female, n | 20/50 | 23/48 | 25/33 | 21/38 | 12/28 | 18/25 |
| Age, years | 59.0 | 60.9 | 63.5 | 61.1 | 64.6 | 59.9 |
| Study duration | 6 months | 12 months | 12 months | |||
| FEV1% predicted at baseline | 67.3 | 67.1 | 70.1 | 66.9 | 82.7 | 77.7 |
| Change in FEV1 from baseline, L | −0.04 | 0 | −4.0 | −1.6 | −0.10a | 1.03a |
| SGRQ at baseline, total score | 36.6 | 31.9 | 38.1 | 36.7 | 40.2 | 40.6 |
| Change in SGRQ total score from baseline | −1.92 | −5.17 | −1.3 | −3.9 | −4.12 | −12.18 |
| Exacerbation rate in 12 months before trial | 3.93 | 3.34 | NRb | NRb | 4.0 | 5.0 |
| Total no. of exacerbations over 12 months | 178c | 109c | 114 | 76 | 78 | 39 |
| Annual exacerbation rate, patient/year | 2.73c | 1.58c | 1.97 | 1.27 | 1.95 | 0.91 |
| Patients with ≥1 exacerbation, n (%) | 58 (82.9) | 44 (62.0) | 42 (72.4) | 39 (66.1) | 32 (80.0) | 20 (46.5) |
| NNT to prevent one patient experiencing an exacerbation over 12 monthsd | 5 | 16 | 3 | |||
Data are means unless otherwise stated.
Reprinted from Respiratory Medicine, 108(10), Haworth CS, Bilton D, Elborn JS, Long-term macrolide maintenance therapy in non-CF bronchiectasis: Evidence and questions, 1397–1408, © 2014.144
Data change per visit (every 3 months), F1,78.8 = 4.085, p = 0.047.
BLESS study did not present exacerbation rate, but did present the number of patients with five or more exacerbations in the year preceding the trial (n = 20 and 22 for placebo and erythromycin, respectively).
EMBRACE was a 6-month study but presented annualized data for exacerbations.
Calculated as 1/absolute risk reduction (proportion with event [placebo] − proportion with event [intervention]). Values presented are the published NNT for BAT and estimates by the authors for EMBRACE and BLESS, based on the percentage of patients with exacerbation events.
BAT, Bronchiectasis and Long-term Azithromycin Treatment study; BLESS, Bronchiectasis and Low-dose Erythromycin study; EMBRACE, Effectiveness of Macrolides in patients with Bronchiectasis using Azithromycin to Control Exacerbations study; FEV1, forced expiratory volume in 1 second; NNT, number needed to treat; NR, not recorded; SGRQ, St. George's Respiratory Questionnaire.
Inhaled antibiotics in patients with NCFBE are primarily directed at gram-negative organisms, especially P. aeruginosa. Several classes of inhaled antibiotics, including aminoglycosides, cephalosporins, colistin, fluoroquinolones, and aztreonam, have been used in patients with NCFBE with the premise that airway and systemic inflammation are related to the bacterial load, and a decrease in the bacterial density in the airways could reduce airway inflammation and lung damage, leading to improved clinical outcomes.(68,69) Most of these antibiotics have a concentration-dependent killing effect and the rationale is to “hit hard and hit fast” to maximize efficacy while reducing the chances of development of resistance(54) and systemic toxicity. In patients with CF, the rationale for the 28-day on and 28-day off cycle of administration of inhaled antibiotics depends on the peak increase in lung function after 28 days of continuous antibiotic administration and the likelihood that the 28-day off period reduces the selective pressure for emergence of antibiotic-resistant organisms.(3,70)
Administration of intravenous antibiotics is an option for patients who are not responding to oral or inhaled therapy or if there is no suitable oral or inhaled alternative. Some centers use “pulsed intravenous antibiotics.” In this approach, regular intravenous antibiotics are self-administered at home or by the community health team for 7–14 days at set intervals of 6–8 weeks.(71) However, despite small benefits, including a small reduction in mean total exacerbation frequency and a significant reduction in hospital bed days, this approach has not been widely adopted. In addition to the route of administration and bacteriology, optimal therapy also needs to consider patient preferences and comorbidities. It may be more difficult for patients to have a high level of compliance needed for effective antibiotic therapy with nebulizers compared with oral administration of antibiotics.(72) The use of nebulized antibiotics requires close monitoring of adherence to treatment.
Challenges in treating Pseudomonas aeruginosa in the lung
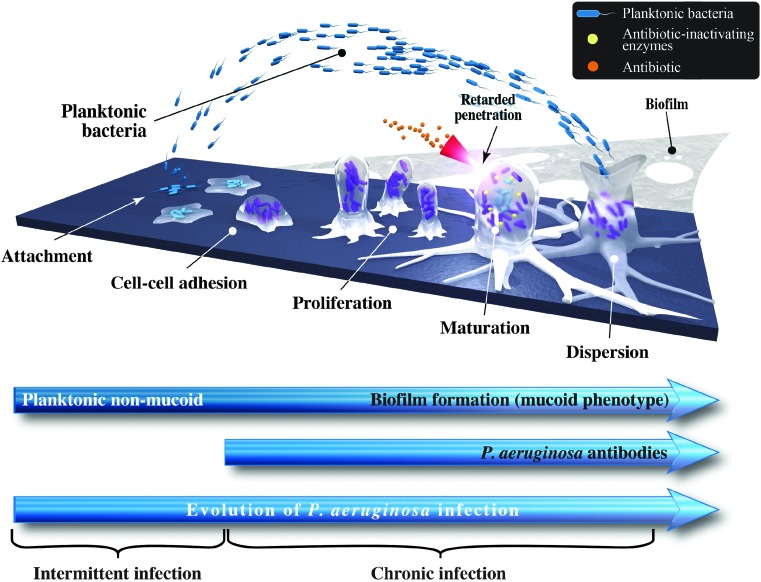
Systemically delivered antibiotics do not penetrate optimally into lung tissue.(73) In the initial stages, the nonmucoid phenotype of P. aeruginosa is present as planktonic organisms within sputum.(74–76) These nonmucoid strains do not form biofilm or induce antibody responses and do not cause much lung damage, and they could be treated with inhaled antibiotics or a combination of oral and intravenous antibiotics.(77) Suboptimal levels of antibiotics in the lung increase the mutation frequency of P. aeruginosa and lead to the formation of mucoid strains of P. aeruginosa that are characterized by a protective alginate, polysaccharide matrix known as a “biofilm.” These biofilms provide a physical and chemical barrier that allows P. aeruginosa to evade immune cells such as phagocytes and host antibodies, and makes it highly resistant to systemically administered antibiotics (Fig. 3).(56,59,78–82)
FIG. 3.
Evolution of Pseudomonas aeruginosa infection in the lung from intermittent to chronic infection.
Within biofilms, bacteria coordinate their biological activity and synchronize gene expression via quorum sensing.(83) In addition, the matrix of the biofilm prevents access of antibiotics to bacterial cells. In in vitro studies, alginate not only limits diffusion of aminoglycosides and beta-lactam antibiotics it also appears to bind aminoglycosides and polymyxin B.(27,84–87) In a preclinical study, a liposomal formulation of amikacin was shown to have greater ability to penetrate P. aeruginosa biofilms compared with the free drug.(88) Similarly, in vitro studies demonstrated that liposomal ciprofloxacin was able to penetrate biofilms from clinical isolates of P. aeruginosa with 99% reduction in cell viability at a concentration of 1 μg/mL.(2) Another phenomenon that limits the efficacy of antibiotics within biofilms is the presence of “persister cells.” This small fraction of bacteria within the colony is in a dormant or nongrowing state and antibiotics that typically target actively dividing cells are unable to kill them. Persister cells that remain after antibiotics kill the susceptible organisms are able to reconstitute the biofilm after the treatment has concluded.(89,90)
Failure to eradicate P. aeruginosa using antibiotic therapy and development of mucoid strains is associated with subsequent transition to a chronic infection stage, requiring long-term antibiotic therapy to control the infection.(61,62)
Chronic or frequent subtherapeutic exposure to antibiotics (i.e., levels lower than the minimum inhibitory concentration [MIC]) further increases the ability of P. aeruginosa to develop antibiotic resistance. Results from a meta-analysis revealed that the risk of antibiotic resistance in patients with bronchiectasis increases more than threefold with long-term (≥4 weeks) antibiotic therapy.(91) Furthermore, in a study of 89 patients with bronchiectasis, 10.1% of sputum isolates with P. aeruginosa showed antibiotic resistance after a mean 5.7 years of follow-up compared with 3.4% of sputum isolates at the initial assessment visit.(92) Treatment with a broad-spectrum antibiotic also may have a negative effect on the diversity of the lung microbiome, which may result in reinfection with P. aeruginosa, even in the presence of concurrent antipseudomonal antibiotics.(93)
Strategies to reduce development of resistance
Strategies to reduce the development of antibiotic resistance include alternating periods with antibiotic therapy with treatment-free periods; rotating use of different antipseudomonal antibiotics; using antibiotic combination therapy; and using antibiotic adjuvants such as gallium, antimicrobial peptides, and antibiofilm compounds, including alginate oligosaccharides that facilitate antibiotic penetration of the bacterial cell.(81,94–96) One of these alginate oligosaccharides (OligoG CF-5/20) was found to be safe for inhalation both in healthy and in chronically diseased lung patients and is currently undergoing phase IIb trials in CF patients.(97) Furthermore, a new family of compounds (“peptidomimetics”), mimicking antimicrobial peptides and showing activity against P. aeruginosa,(96,98) are currently being evaluated in preclinical studies.(95,96)
Other strategies to reduce the emergence of bacterial resistance include use of efflux pump inhibitors, suppression of hydroxyl-free radical generation, and pharmacokinetic/pharmacodynamic approaches. By achieving high levels of drug exposure in the respiratory tract, inhaled antibiotics may have the potential to reduce the occurrence of antibiotic resistance. A meta-analysis derived from seven trials (n = 445) that investigated the emergence of bacterial resistance in a pooled population with stable NCFBE found no statistically significant difference between the emergence of bacterial resistance in patients receiving inhaled antibiotic therapy (amikacin, aztreonam, ciprofloxacin, gentamicin, colistin, or tobramycin) compared with patients receiving either placebo or symptomatic treatment with oxygen, bronchodilators, and corticosteroids (7.8% vs. 3.5%, respectively; risk ratio [RR] [95% confidence interval (CI)] 1.68 [0.62–4.52]; p = 0.31).(99)
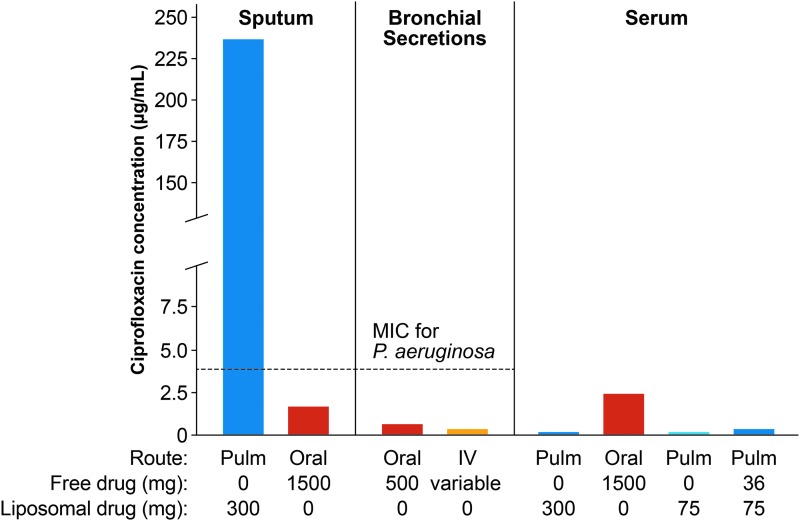
Inhaled antibiotic formulations are associated with minimal systemic toxicity and adverse events compared with oral/intravenous routes of administration.(18,54,88) This is illustrated in Figure 4, using the fluoroquinolone antibiotic ciprofloxacin as an example. Maximum oral doses of ciprofloxacin (750 mg twice a day, as recommended by the British Thoracic Society(100)) to patients with CF led to an antibiotic concentration in sputum that was lower than the MIC required for P. aeruginosa (Fig. 4). In contrast, inhaled ciprofloxacin resulted in drug concentrations in the sputum that were ≥50-fold greater than the MIC for P. aeruginosa.(2) At the same time, serum drug concentrations with inhaled ciprofloxacin were considerably lower than those achieved using oral administration, minimizing the possibility of systemic toxicity and adverse events (Fig. 4). It is worth noting that lung concentrations of inhaled antibiotics may vary depending on the particle size, ability to reach the smaller airways, disease state, formulation used, and occurrence of side effects such as bronchoconstriction and cough. Concerns about emergence of resistance with long-term use of inhaled antibiotics because of lower concentrations achieved in the distal airways and lung parenchyma due to mucus plugging and airway obstruction were not substantiated in clinical studies.(54,99)
FIG. 4.
The value of inhaled delivery using the antibiotic ciprofloxacin as an example. Reproduced from Cipolla et al.(2) IV, intravenous administration; MIC, minimum inhibitory concentration; Oral, oral administration; Pulm, inhaled administration.
Initially, drug solutions designed for intravenous antibiotic administration were used for inhalation. Later, specialized suspension formulations and, finally, liposomal formulations, such as amikacin and ciprofloxacin, were developed. Formulations that are suitable for inhalation should be sterile, preservative free, and nonpyrogenic. They also should be adjusted for the lung environment with a suitable pH (range 4.0–8.0, but preferably closer to neutral pH), osmolarity (150–1200 mOsm/L), and tonicity.(101,102) A specifically formulated solution for inhalation could minimize adverse effects, such as airway irritation, and increase delivery efficiency. More recently, dry powder formulations using PulmoSphere technology have the ability to carry a higher payload of antibiotics. The pulmonary deposition of the hollow porous particles within these powders is independent of the patient's inspiratory flow.(103,104) The ability to inhale at low inspiratory flow rates without compromising drug deposition could reduce the incidence of cough and bronchospasm in patients.(53,105)
Treatment failures due to lack of adequate concentration of antibiotics, inadequate time of retention in the lungs, and compliance issues with more than once-daily dosing could be ameliorated by use of sustained-release formulations, such as liposomes and those with solubility limited dissolution rate using salts of polylactic-co-glycolic acid (PLGA). By using novel inhaled antibiotic formulations, such as dry powder for inhalation (DPI) ciprofloxacin, nebulized liposomal amikacin, or nebulized dual-release liposomal ciprofloxacin, investigators have observed good efficacy and safety in clinical trials.(106) Clinical outcomes with inhalable PLGA formulations of tobramycin, clarithromycin, and ciprofloxacin have not been reported.(107)
In addition to the development of novel antibiotic formulations, rapid technological developments in portable aerosol delivery devices have contributed to the ability to achieve a higher efficiency of drug delivery to the lung.(108,109) With the jet nebulizer, the efficiency of tobramycin delivery is only 5%–15%.(110–112) The whole lung deposition of dry powder tobramycin with the PulmoSphere formulation was reported to be ∼34% in healthy volunteers(110) and the delivery time was only 2–3 minutes. Tobramycin solution for inhalation (Novartis, New York, NY) is administered twice a day with specific jet nebulizers, with each dose requiring 10–15 minutes to deliver. Aztreonam lysine inhalation solution (Cayston, Gilead, Foster City, CA) requires three times a day administration over 2–3 minutes using the Altera vibrating mesh nebulizer system (PARI Respiratory Equipment, Midlothian, VA). These newer delivery systems are portable and efficient for inhaled drug delivery. Other antibiotics are being delivered with delivery systems that are not specifically approved for use with these formulations, or have not been approved for clinical use.
Clinical Evidence Supporting Use of Inhaled Antibiotics
The majority of the clinical evidence on the use of inhaled antibiotics comes from studies investigating pseudomonal infections in CF and nosocomial pneumonia in ventilated patients. The greatest success of use of inhaled antibiotics has been for the treatment of pseudomonal infections in CF.(3,4,113,114) For this indication, inhaled forms of tobramycin(115) and aztreonam(20) are approved in the United States and Europe, and colistimethate(116) in Europe. In patients with CF, inhaled antibiotics slow the rate of decline of lung function, delay onset of chronic infection with P. aeruginosa, reduce the rate of exacerbations and hospitalizations, and improve quality of life.(3,4,113,114) Some formulations that have been tested in patients with CF but not in those with NCFBE include levofloxacin nebulizer solution,(117) DPI colistin,(118) and fosfomycin/tobramycin nebulizer solution.(119)
Macrolides have positive immunomodulatory effects, including reduced airway inflammation and airway damage, with decrease in mucus hypersecretion and less biofilm formation.(57,120) Long-term use of oral macrolides in patients with NCFBE improves respiratory symptoms and quality of life, while reducing the decline in lung function and the frequency of acute exacerbations (Table 2).(121) Some inhaled macrolide formulations have not been clinically tested.(122–126) Concerns with long-term use of macrolides include the potential for macrolide resistance and emergence of new pathogens.(127) Increasing use of macrolides, especially azithromycin, is associated with a consistent increase in macrolide resistance at the population level.(25) Increasing macrolide-resistant strains in the community could influence the clinical outcomes of macrolide therapy, especially when a large number of patients use macrolides on a long-term basis and become carriers of macrolide-resistant organisms.(25)
Long-term intermittent therapy with ciprofloxacin DPI could reduce the frequency of acute exacerbations in patients with NCFBE colonized with respiratory bacterial pathogens.(53,128,129) Patients inhale ciprofloxacin inhalation powder from one capsule of ciprofloxacin DPI 32.5 mg twice daily using a pocket-sized, T-326 breath-actuated inhaler. The proposed regimen for ciprofloxacin DPI is to use cycles of 14 days on drug and 14 days off drug (or 28 days on/28 days off). The DPI facilitates lung deposition and achieves a high local concentration of ciprofloxacin in the lung with a longer half-life than ciprofloxacin hydrochloride.(105) A recent scintigraphic study with the ciprofloxacin DPI reported mean lung deposition of 53% in patients with NCFBE, 51% in patients with chronic obstructive pulmonary disease, and 51% to 53% in healthy volunteers. After inhalation of ciprofloxacin, there were no episodes of bronchospasm or clinically significant changes in lung function, and systemic exposure to ciprofloxacin was low.(130)
As previously mentioned, although pathogens are similar in CF and NCFBE, the rate of progression and extent of changes in the lung are not the same, suggesting that there are differences in pathogenesis (Table 1). Therefore, outcomes of inhaled antibiotic therapy should not be assumed to be the same in CF and NCFBE.(18)
Clinical trials evaluating the use of inhaled antibiotic therapy in NCFBE
There are currently no drugs that are specifically approved for treatment of patients with NCFBE. In NCFBE patients with P. aeruginosa infections, the British Thoracic Society recommends monotherapy with oral ciprofloxacin (500–750 mg twice a day) as first-line treatment.(100) However, the British Thoracic Society guidelines do not currently make any recommendations with regard to the use of inhaled ciprofloxacin as monotherapy for NCFBE,(100) until further evidence is available.
Table 3 provides an overview of completed and ongoing clinical trials that have evaluated inhaled antibiotic therapy for NCFBE. The efficacy and tolerability profiles of some commercially available inhaled antibiotics in patients with NCFBE do not appear to be as promising as those in patients with CF. Inhaled tobramycin resulted in a statistically significant reduction in P. aeruginosa bacterial load; however, a high proportion of patients (50%) had respiratory adverse events.(95) A randomized, placebo-controlled trial of colistin in 144 patients did not meet the primary endpoint of time to exacerbation.(72) Two large, randomized, double-blind, phase 3 trials that evaluated aztreonam lysinate in a total of 540 patients reported no difference versus placebo across outcome measures.(20) There was no significant difference in quality-of-life scores, and treatment-related adverse events were more common in the aztreonam-treated group compared with the placebo group.(19)
Table 3.
Overview of Clinical Trials Investigating Inhaled Antibiotic Therapy for Non-cystic Fibrosis Bronchiectasis
| Study | Study design | No. of randomized patients | Intervention | Key outcome measures | Efficacy results | Safety results |
|---|---|---|---|---|---|---|
| Barker et al.(20) | Two randomized, placebo-controlled, phase 3 trials | AIR-BX1: n = 266 | Aztreonam for inhalation | Change in QOL-B respiratory symptom scores to week 4 | No difference versus placebo across outcome measures | Treatment-related AEs were more common in the aztreonam group versus placebo |
| AIR-BX2: n = 274 | ||||||
| Drobnic et al.(141) | Randomized, placebo-controlled, two-period, crossover trial | n = 30 | Inhaled tobramycin BID in two cycles, each for 6 months | Number of exacerbations, number of hospital admissions and number of hospital admission days | No difference in number of exacerbations (p = 0.330); significant reduction in hospital admissions (p = 0.038) and days in hospital (p = 0.047) in treatment group | Inhaled tobramycin was associated with bronchospasm in 10% of patients |
| Wilson et al.(53) (Clinicaltrials.gov identifier: NCT00930982) | Randomized, placebo-controlled, phase 2 trial | n = 124 | Ciprofloxacin DPI 32.5 mg or placebo BID 28 days on/28 days off | Effect on total bacterial density of predefined pathogens in sputum on day 28 | Ciprofloxacin DPI resulted in a statistically significant reduction in total bacterial load on day 28 (p < 0.001) | No significant differences between the ciprofloxacin DPI and placebo arms; the incidence of bronchospasm was low |
| RESPIRE-1(132) (Clinicaltrials.gov identifier: NCT01764841) | Randomized (2:1), placebo-controlled, phase 3 trial | n = 416 | Ciprofloxacin DPI 32.5 mg or placebo BID 28 days on/28 days off or 14 days on/14 days off over 48 weeks | Time to first pulmonary exacerbation versus pooled placebo and frequency of exacerbation versus matched placebo | Ciprofloxacin DPI significantly reduced number of exacerbations (p = 0.0005) and exacerbation frequency (p = 0.0061) versus placebo with the 14-day on/14-day off regimen. No significant changes versus placebo in exacerbations or exacerbation frequency were observed with the 28-day on/28-day off regimen | No difference in serious treatment-emergent AEs with the 14-day regimen (16.9%), the 28-day regimen (19.9%), or placebo (23.4%). Rates of discontinuation because of respiratory AEs were similar |
| RESPIRE-2(133) (Clinicaltrials.gov identifier: NCT02106832) | Randomized (2:1), placebo-controlled, phase 3 trial | n = 521 | Ciprofloxacin DPI 32.5 mg BID 28 days on/28 days off or 14 days on/14 days off over 48 weeks | Time to first pulmonary exacerbation versus pooled placebo and frequency of exacerbation versus matched placebo | Ciprofloxacin DPI did not significantly prolong time to first exacerbation or reduce exacerbation frequency to predefined significance thresholds | Ciprofloxacin DPI was well tolerated in both regimens |
| ORBIT-1(2,59) (Clinicaltrials.gov identifier: NCT00889967) | Randomized, placebo-controlled, double-blind trial | n = 96 | Ciprofloxacin for inhalation (150 or 100 mg once daily) for one cycle of 28 days on and 28 days off | Mean change in Pseudomonas aeruginosa density in sputum (log10) CFU/g of sputum from baseline to day 28 | Significant mean decreases from baseline in P. aeruginosa CFU at day 28 after 150 mg dose of 3.5 log10 (p < 0.001) and after 100 mg dose of 4.0 log10 units (p < 0.001) | Treatment was well tolerated, with no statistically significant differences between active treatment and placebo groups in the number of patients experiencing ≥1 respiratory treatment-emergent event |
| Serisier et al.(131) ORBIT-2 | Randomized, placebo-controlled, phase 2 trial | n = 42 | Dual-release liposomal ciprofloxacin for inhalation (150 mg) and free ciprofloxacin (60 mg) versus placebo 28 days on/28 days off in three cycles | Mean change in sputum P. aeruginosa density from baseline to day 28 (first treatment cycle) | At day 28, dual-release ciprofloxacin resulted in a reduction from baseline of mean (SD) −4.2 (3.7) log10 CFU/g in sputum P. aeruginosa density versus a change from baseline of −0.08 (3.8) log10 CFU/g in the placebo group (p = 0.002) | Incidence of systemic AEs similar between two arms; there were fewer pulmonary AEs in the ciprofloxacin arm versus Placebo |
| ORBIT-3/ORBIT-4(134–136) (Clinicaltrials.gov identifier: NCT01515007, NCT02104245) | Randomized (2:1), placebo-controlled, phase 3 trials | Five hundred eighty-two patients were enrolled (ORBIT-3, n = 278; ORBIT-4, n = 304) | Dual-release ciprofloxacin for inhalation (ARD-3150; liposome-encapsulated ciprofloxacin [150 mg/3 mL] and free ciprofloxacin [60 mg/3 mL]) for 28 days on/28 days off for six cycles | Time to first pulmonary exacerbation, frequency of all and severe pulmonary exacerbations | ARD-3150 was associated with an increased median time to first exacerbation >2 months versus placebo; the result was significant for ORBIT-4, but not for ORBIT-3. Significant reductions in frequency of all and severe exacerbations were observed for ARD-3150 versus placebo in ORBIT-4, but not ORBIT-3. In the pooled analysis of the two trials, ARD-3150 led to a significant increase versus placebo in median time to first exacerbation that required antibiotics and a significant reduction in PE frequency; it also significantly reduced PA sputum density during each on-treatment period | Rates of TEAEs and serious TEAEs were similar in both treatment groups |
| Murray et al.(88) | Randomized, controlled trial | Sixty-five patients with NCFBE and chronically infected sputum | Nebulized gentamicin 80 mg BID or placebo (0.9% saline) BID for 12 months | Sputum bacterial density reduction of at least 1 log unit, sputum purulence, exacerbation rate, time to first exacerbation | Baseline sputum bacterial density was 8.02 log10 CFU/g. Gentamicin treatment significantly reduced sputum bacterial density to 2.96 log10 CFU/g versus 7.67 log10 CFU/g in the placebo group (p < 0.0001) | Gentamicin was well tolerated, 7 (22%) patients reported bronchospasm but 5 continued treatment with albuterol use as a bronchodilator. Only two patients withdrew owing to poor tolerability, the same as in the placebo arm |
| Gentamicin treatment reduced purulence, exacerbation rates, and increased time to first exacerbation. However, 3 months post-treatment, there was no difference between treatment and placebo groups in any of these parameters | ||||||
| Barker et al.(142) | Randomized, placebo controlled trial | Randomized 74 patients with chronic P. aeruginosa colonization | Nebulized tobramycin 300 mg BID or placebo (quinine/saline) BID for 28 days | P. aeruginosa density in sputum, medical condition, and emergence of resistance | Tobramycin significantly reduced P. aeruginosa density by 4.54 log10 CFU/g sputum compared with a mean increase of 0.02 log10 CFU/g sputum in placebo patients (p < 0.01) Tobramycin improved medical condition at week 6. No significant increase in tobramycin resistance was observed |
Tobramycin was associated with significantly increased dyspnea, chest pain, and wheezing compared with the placebo group |
| Haworth et al(72) | Multicenter, randomized, controlled trial | Bronchiectasis patients with chronic P. aeruginosa infection (n = 144) | Nebulized colistimethate sodium 1,000,000 U BID or placebo (0.45% saline) BID for 6 months using an INeb device or until first exacerbation. Patients receiving oral macrolides at the start of the trial were continued on therapy | Time to first exacerbation, emergence of resistant organisms, P. aeruginosa CFUs | No significant difference between treatment and placebo in time to first exacerbation was observed (p = 0.11). Analysis of patients with ≥80% compliance showed that median time to first exacerbation was significantly increased; no emergence of resistant organisms. P. aeruginosa CFUs were reduced at weeks 4 and 12 | Bronchoconstriction after the first dose in 7.5% of colistin patients versus 1.4% in patients receiving placebo. No significant difference in AEs (143 in 47 colistin patients versus 108 in 38 placebo patients, p = 0.25) |
AE, adverse events; BID, twice a day; CFU, colony-forming units; DPI, dry powder for inhalation; EOT, end of treatment; NCFBE, non-cystic fibrosis bronchiectasis; PE, pulmonary exacerbation; QOL, quality of life; SD, standard deviation; TEAEs, treatment-emergent adverse events.
Several phase 2 studies have evaluated the use of inhaled formulations of ciprofloxacin. A phase 2 trial of ciprofloxacin DPI at a dose of 32.5 mg twice a day showed a significant 3.6 log reduction in total sputum bacterial load versus placebo (p < 0.001) at the end of treatment, but no significant difference in exacerbation rates (p = 0.605; Table 3).(53) ORBIT-1 evaluated ciprofloxacin for inhalation (150 mg of ciprofloxacin in 3 mL or 100 mg ciprofloxacin in 2 mL), while ORBIT-2 investigated a dual-release formulation of ciprofloxacin combining liposomal ciprofloxacin for inhalation (150 mg in 3 mL) with free ciprofloxacin (60 mg in 3 mL). Both trials demonstrated potent antipseudomonal activity (4 log reduction in density of P. aeruginosa; Table 3) and, in ORBIT-2, there was an increased median time to first exacerbation (by 76 days) in the per-protocol group compared with placebo (p = 0.046).(59,131) Safety results from ORBIT-2 showed a similar incidence of overall adverse events for subjects who received the dual-release ciprofloxacin formulation and placebo.(131)
Both the dual-release (ORBIT-3 and ORBIT-4) and DPI formulations of ciprofloxacin (RESPIRE-1 and RESPIRE-2) have been tested in phase 3 trials; the results were recently presented but not published at the time of submission of this article.
RESPIRE-1 included adults with a positive sputum culture for predefined bacteria and a history of treatment of at least two exacerbations in the previous 12 months. Patients (N = 416) randomly received inhaled ciprofloxacin DPI (32.5 mg) or placebo twice daily administered in either 12 cycles of 14 days on/14 days off, or in 6 cycles of 28 days on/28 days off, for 48 weeks. The investigators used a 2:1 active therapy versus placebo randomization schedule. Primary outcome measures were time to first exacerbation and frequency of exacerbation. Exacerbations were strictly predefined as the worsening of at least three respiratory symptoms (dyspnea, wheezing, cough, increased sputum volume over 24 hours, or increased sputum purulence) plus fever or malaise/fatigue, and systemic antibiotic use. Both primary endpoints were met with the 14-day on/off regimen. There was a 39% reduction in the number of exacerbations with the 14-day regimen over placebo (adjusted incidence rate ratio [IRR] 0.61; p = 0.0061); furthermore, active treatment significantly prolonged time to first exacerbation (adjusted hazard ratio [HR] 0.53; p = 0.0005), with a mean time to first exacerbation >336 days with active treatment versus 186 days in the two placebo arms (which were pooled for analysis). The 28-day regimen also reduced the number of exacerbations versus placebo, but the difference was not statistically significant (adjusted IRR 0.98, p = 0.89). Furthermore, there was a trend for a delay in the time to first exacerbation for those randomized to inhaled ciprofloxacin versus placebo on the 28-day on/off schedule (adjusted HR 0.73; p = 0.065). Serious treatment-emergent adverse events were similar with the 14-day regimen (16.9%), the 28-day regimen (19.9%), and placebo (23.4%).(132)
In RESPIRE-2, 521 patients were randomized to either ciprofloxacin DPI (32.5 mg) or placebo BID in a 2:1 ratio, using either a 14-day on/off or a 28-day on/off regimen over 48 weeks. Endpoints were evaluated using analyses with different alpha levels. The primary endpoints were time to first exacerbation versus pooled placebo and frequency of exacerbation versus matched placebo. Ciprofloxacin DPI did not significantly prolong time to first exacerbation versus pooled placebo using either of the two regimens (HR = 0.87, p = 0.40 and HR = 0.71, p = 0.05, respectively). A reduction in exacerbation frequency versus matched placebo did not reach predefined significance thresholds. Ciprofloxacin DPI was well tolerated in both regimens.(133)
A once-a-day inhaled formulation of liposome-encapsulated ciprofloxacin (150 mg/3 mL) and free ciprofloxacin (60 mg/3 mL, ARD-3150; Aradigm Corp., Hayward, CA) was recently evaluated in two double-blind, placebo-controlled phase 3 trials (ORBIT-3; n = 278 and ORBIT-4; n = 304) over 48 weeks in NCFBE patients chronically infected with P. aeruginosa. The trials consisted of six cycles of 28 days on and 28 days off treatment. The key efficacy endpoints were time to first pulmonary exacerbation (protocol defined) and the frequency of all and severe pulmonary exacerbations (defined as requiring treatment with IV antibiotics and/or hospitalization). Recently presented findings of pooled data showed that ARD-3150 increased median time to first exacerbation and reduced the frequency of protocol-defined pulmonary exacerbations and severe pulmonary exacerbations compared to placebo.(134–136) In addition, ARD-3150 reduced sputum P. aeruginosa density without attenuation of ciprofloxacin activity and P. aeruginosa MIC, which remained stable throughout each on-treatment period. Rates of treatment-emergent adverse events and serious adverse events were similar across treatment arms in both studies.(134–136)
Some of the studies cited above(2,53,59,131,134,136) provide evidence that sputum bacterial density was reduced with inhaled ciprofloxacin. A significant decrease in P. aeruginosa bacterial load was associated with trends for reduction in the risk and frequency of exacerbations.(134,136) Serious adverse events were similar between active treatment and control groups.(2,53,59,131,134–136) To achieve the maximum benefits from inhaled antibiotic therapy, further research could help to identify optimal drug regimens in specific groups of patients with NCFBE.
Safety of inhaled antibiotics
A meta-analysis of eight trials involving 590 patients reported an acceptable safety profile for inhaled antibiotics (amikacin, aztreonam lysinate, ciprofloxacin, gentamicin, colistin, or tobramycin), with a withdrawal rate for inhaled therapy that was similar to that of the control group.(99) The most frequent adverse event was bronchospasm, which occurred in 10% of patients receiving inhaled antibiotic therapy versus 2.3% in the control group (RR 2.96 [95% CI 1.30–6.73]; p = 0.01).(99) Patients who received inhaled aminoglycosides were five times more likely to develop bronchospasm than those in the placebo group or those in the symptom-based therapy group (RR 4.78 [95% CI 1.55–14.76], p = 0.007). In contrast, the risk of bronchospasm was not significantly increased with inhaled ciprofloxacin (RR 1.07 [95% CI 0.25–4.56], p = 0.93) or colistin (RR 4.86 [95% CI 0.58–40.59], p = 0.14).(96) Systemic absorption of inhaled antibiotics is variable but there is a potential for development of systemic side effects.(137,138)
Limitations of inhaled antibiotic therapy in NCFBE
Most clinical trials investigating the use of inhaled antibiotic therapy in NCFBE only involved a small number of patients and were of short duration. Compliance with inhaled antibiotic therapy was not ideal in these studies, and there is currently no clear consensus on the endpoints to evaluate in clinical trials (microbiological vs. clinical efficacy). The full results of the recently completed phase 3 trials of ciprofloxacin in different formulations, DPI and liposome-encapsulated/dual-release, with a higher number of patients and of longer duration should advance our knowledge of choosing appropriate patient populations and different endpoints in the future. Overall, the benefits of inhaled antibiotics in NCFBE have been modest, but favorable trends should be further explored.
Oral/intravenous antibiotics in conjunction with inhaled antibiotics could lead to early eradication of P. aeruginosa in both CF and NCFBE patients, and this may reduce the frequency of exacerbations and improve quality of life.(22,139) However, the value of adding an inhaled antibiotic for treatment of an acute exacerbation has not been established.(95) In a retrospective study, 31 patients with NCFBE who were successfully treated with chronic inhaled antibiotics were compared with an age- and sex-matched cohort of 60 patients who were not treated.(140) The study cohort had a greater number of exacerbations in the year before start of inhaled antibiotics, had a significantly lower lung function at baseline, and had higher scores on the BSI. However, in the year after initiation of inhaled antibiotics, the study group had significantly fewer exacerbations (p = 0.003) compared with the control cohort.(140) The authors proposed using inhaled antibiotics in patients with NCFBE who suffered repeated exacerbations despite appropriate use of chronic oral macrolide therapy, aerosolized hypertonic saline, and airway clearance therapies. Selected patients with NCFBE may benefit from inhaled antibiotic therapy, and therapy may be more successful if targeted to specific patient groups.
Conclusions
NCFBE is an underdiagnosed chronic inflammatory lung disease that is associated with significant morbidity and mortality, for which no licensed therapies are currently available. The most severe forms of NCFBE are associated with chronic P. aeruginosa infections and often require long-term antibiotic therapy that may increase the risk of antibiotic resistance. Inhaled antibiotics could have a role in treatment of initial infection with P. aeruginosa with the aim of eradicating the infection. Chronic infection with P. aeruginosa is much more difficult to eradicate, and the goal of antibiotic therapy is to reduce the bacterial load, reduce airway inflammation, and prevent exacerbations. Long-term therapy with oral macrolides is effective in this respect and supplementation with inhaled antibiotics could augment their efficacy in patients with severe illness or in patients infected with multidrug-resistant organisms. Evaluation of the liposomal dual-release formulation of ciprofloxacin in phase 3 clinical trials reduces the P. aeruginosa bacterial load, with a trend toward reduced risk of exacerbations. Inhaled antibiotics have antipseudomonal activity, have fewer systemic side effects, and they could provide alternative treatment options to address some of the treatment challenges that exist in the management of severe cases of NCFBE.
Acknowledgments
Fiona Nitsche, PhD, CMPP, and Susan Sutch, PharmD, CMPP, provided medical writing and editorial assistance, and this was funded by Grifols.
Author Disclosure Statement
Dr. R. Dhand is a consultant for Astra-Zeneca, Bayer, and Cipla (India) and has received honoraria from Astra-Zeneca, Cipla (India), and Sunovion. The author received no financial support for the writing of this article.
Note Added After Online Publication
Since online publication of the final accepted version of this article, there have been a couple of important updates regarding the evidence for the use of inhaled antibiotics as therapy for bronchiectasis. First, in September 2017, the European Respiratory Society (ERS) published guidelines for the management of adult bronchiectasis.(145) Despite no approved inhaled antibiotic for noncystic fibrosis bronchiectasis (NCFBE) being available, they suggest offering long-term inhaled antibiotic treatment for adults with bronchiectasis and chronic Pseudomonas aeruginosa infection with three or more exacerbations per year. They consider it a conditional recommendation based on moderate-quality evidence. In addition, they suggest long-term treatment with an inhaled antibiotic for patients not infected with P. aeruginosa infection with three or more exacerbations per year that do not respond to or tolerate oral antibiotic prophylaxis (conditional recommendation, low quality of evidence).
The ERS recommendations also include the use of antibiotics, including inhaled formulations, as an option for eradication treatment in patients with a first/new isolation of P. aeruginosa; however, this is based on very low-quality evidence. Nonetheless, this review is timely in light of these recommendations.
Second, in this review of various studies evaluating use of inhaled antibiotics in NCFBE, the promise of large clinical trial programs for inhaled ciprofloxacin has been alluded to, including the RESPIRE trials (now published in full(146,147)) with twice-daily ciprofloxacin DPI, and the ORBIT trials with nebulized once-daily dual-release liposomal ciprofloxacin.(148,149) There was much anticipation that one or both of these products would result in the first Food and Drug Administration (FDA)-approved inhaled antibiotic to offer to patients with advanced NCFBE. However, this does not appear to be imminent, as the FDA's Antimicrobial Drugs Advisory Committee recently voted against a recommendation for approval of both products.(150,151)
In a press release on January 29, 2018, Aradigm Corporation announced that the FDA did not approve ARD-3150 “in its present form” and provided recommendations for resubmission of the application. Bayer has not disclosed any details of the FDA's complete response letter pertaining to the new drug application for ciprofloxacin DPI. Both companies are expected to submit applications for their inhaled antibiotic formulations to the European Medicines Agency (EMA) for approval in Europe.
These recent developments highlight key challenges for this therapeutic field, including the importance of establishing consensus regarding the most meaningful clinical endpoint for NCFBE in future trial designs. For the FDA submissions, the primary endpoint in both the RESPIRE(146,147) and the ORBIT(148,149) trials was time to first pulmonary exacerbation over 48 weeks. This endpoint has limitations, since it is unclear whether a treatment-mediated delay in time to first exacerbation over 48 weeks may be clinically meaningful to patients who are likely to be on this treatment for a considerable length of time. Reducing exacerbation frequency (the primary endpoint for any potential EMA application) may be more clinically meaningful for long-term treatment. In this context, it is worth noting that a meta-analysis of pooled data from each RESPIRE-1/RESIRE-2(152) and from ORBIT-3/ORBIT-4(148) evaluating frequency of exacerbations significantly favored the respective ciprofloxacin formulation over placebo.
Given the aforementioned pooled trial data and the favorable safety/tolerability profile of inhaled ciprofloxacin formulations, inhaled antibiotics are of benefit in some patient groups. More stringent inclusion criteria for future trials based on more clearly defined subgroups of patients and better methods of distinguishing these subgroups may be the way forward in the fight to find a licensed treatment for this debilitating orphan disease. At present, the lack of an approved therapy limits implementation of the ERS recommendations, and clinicians are awaiting clearance of one or more inhaled antibiotics as an additional therapeutic option for selected patients with NCFBE.
References
- 1.Weycker D, Edelsberg J, Oster G, and Tino G: Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12:205–209 [Google Scholar]
- 2.Cipolla D, Blanchard J, and Gonda I: Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics. 2016;8:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, Marshall S, and Smith AL: Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30 [DOI] [PubMed] [Google Scholar]
- 4.Hodson ME, Gallagher CG, and Govan JR: A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 2002;20:658–664 [DOI] [PubMed] [Google Scholar]
- 5.Seitz AE, Olivier KN, Adjemian J, Holland SM, and Prevots R: Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole PJ: Inflammation: A two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:61–65 [PubMed] [Google Scholar]
- 7.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, and Chalmers JD: A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611 [DOI] [PubMed] [Google Scholar]
- 8.Wilson R, Aksamit T, Aliberti S, De Soyza A, Elborn JS, Goeminne P, Hill AT, Menendez R, and Polverino E: Challenges in managing Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respir Med. 2016;117:179–189 [DOI] [PubMed] [Google Scholar]
- 9.Chalmers JD, Aliberti S, Polverino E, Vendrell M, Crichton M, Loebinger M, Dimakou K, Clifton I, van der Eerden M, Rohde G, Murris-Espin M, Masefield S, Gerada E, Shteinberg M, Ringshausen F, Haworth C, Boersma W, Rademacher J, Hill AT, Aksamit T, O'Donnell A, Morgan L, Milenkovic B, Tramma L, Neves J, Menendez R, Paggiaro P, Botnaru V, Skrgat S, Wilson R, Goeminne P, De Soyza A, Welte T, Torres A, Elborn JS, Blasi F, on behalf of EMBARC: The EMBARC European Bronchiectasis Registry: Protocol for an international observational study. ERJ Open Res. 2016;2 [Epub ahead of print]; DOI: 10.1183/23120541.00081-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COPD Foundation. Bronchiectasis and NTM Initiative: COPD foundation website. http://copdfoundation.org/Research/Bronchiectasis-Research-Registry/Learn-More.aspx (2017). Last accessed October19, 2017
- 11.O'Donnell AE: Bronchiectasis. Chest. 2008;134:815–823 [DOI] [PubMed] [Google Scholar]
- 12.Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, Fardon TC, Rutherford R, Pesci A, Restrepo MI, Sotgiu G, and Chalmers JD: Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016;47:1113–1122 [DOI] [PubMed] [Google Scholar]
- 13.McGuinness G, and Naidich DP: CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002;40:1–19 [DOI] [PubMed] [Google Scholar]
- 14.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, Poppelwell L, Salih W, Pesci A, Dupont LJ, Fardon TC, De Soyza A, and Hill AT: The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onen ZP, Gulbay BE, Sen E, Yildiz ÖA, Saryal S, Acican T, and Karabiyikoglu G: Analysis of the factors related to mortality in patients with bronchiectasis. Respir Med. 2007;101:1390–1397 [DOI] [PubMed] [Google Scholar]
- 16.Loebinger MR, Wells AU, Hansell DM, Chinyanganya N, Devaraj A, Meister M, and Wilson R: Mortality in bronchiectasis: A long-term study assessing the factors influencing survival. Eur Respir J. 2009;34:843–849 [DOI] [PubMed] [Google Scholar]
- 17.Goeminne PC, Nawrot TS, Ruttens D, Seys S, and Dupont LJ: Mortality in non-cystic fibrosis bronchiectasis: A prospective cohort analysis. Respir Med. 2014;108:287–296 [DOI] [PubMed] [Google Scholar]
- 18.Tay GT, Reid DW, and Bell SC: Inhaled antibiotics in cystic fibrosis (CF) and non-CF bronchiectasis. Semin Respir Crit Care Med. 2015;36:267–286 [DOI] [PubMed] [Google Scholar]
- 19.Chalmers JD, Loebinger M, and Aliberti S: Challenges in the development of new therapies for bronchiectasis. Expert Opin Pharmacother. 2015;16:833–850 [DOI] [PubMed] [Google Scholar]
- 20.Barker AF, O'Donnell AE, Flume P, Thompson PJ, Ruzi JD, de Gracia J, Boersma WG, De Soyza A, Shao L, Zhang J, Haas L, Lewis SA, Leitzinger S, Montgomery AB, McKevitt MT, Gossage D, Quittner AL, and O'Riordan TG: Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): Two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med. 2014;2:738–749 [DOI] [PubMed] [Google Scholar]
- 21.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, Lubsch L, Matsui J, Oermann CM, Ratjen F, Rosenfeld M, Simon RH, Hazle L, Sabadosa K, and Marshall BC: Cystic Fibrosis Foundation pulmonary guideline. Pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc. 2014;11:1640–1650 [DOI] [PubMed] [Google Scholar]
- 22.White L, Mirrani G, Grover M, Rollason J, Malin A, and Suntharalingam J: Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med. 2012;106:356–360 [DOI] [PubMed] [Google Scholar]
- 23.Schelstraete P, Haerynck F, Van daele S, Deseyne S, and De Baets F: Eradication therapy for Pseudomonas aeruginosa colonization episodes in cystic fibrosis patients not chronically colonized by P. aeruginosa. J Cyst Fibros. 2013;12:1–8 [DOI] [PubMed] [Google Scholar]
- 24.Weers J: Inhaled antimicrobial therapy—barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43 [DOI] [PubMed] [Google Scholar]
- 25.Serisier DJ: Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. 2013;1:262–274 [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay S, Staddon GE, Eastman C, Palmer M, Davies ER, and Carswell F: The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir Med. 1994;88:203–211 [DOI] [PubMed] [Google Scholar]
- 27.Hill D, Rose B, Pajkos A, Robinson M, Bye P, Bell S, Elkins M, Thompson B, Macleod C, Aaron SD, and Harbour C: Antibiotic susceptibilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J Clin Microbiol. 2005;43:5085–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King P, Citron DM, Griffith DC, Lomovskaya O, and Dudley MN: Effect of oxygen limitation on the in vitro activity of levofloxacin and other antibiotics administered by the aerosol route against Pseudomonas aeruginosa from cystic fibrosis patients. Diagn Microbiol Infect Dis. 2010;66:181–186 [DOI] [PubMed] [Google Scholar]
- 29.Moulton BC, and Barker AF: Pathogenesis of bronchiectasis. Clin Chest Med. 2012;33:211–217 [DOI] [PubMed] [Google Scholar]
- 30.Liebow AA, Hales MR, and Lindskog GE: Enlargement of the bronchial arteries, and their anastomoses with the pulmonary arteries in bronchiectasis. Am J Pathol. 1949;25:211–231 [PMC free article] [PubMed] [Google Scholar]
- 31.Pump KK: Distribution of bronchial arteries in the human lung. Chest. 1972;62:447–451 [DOI] [PubMed] [Google Scholar]
- 32.Charan NB, Baile EM, and Pare PD: Bronchial vascular congestion and angiogenesis. Eur Respir J. 1997;10:1173–1180 [DOI] [PubMed] [Google Scholar]
- 33.Paredi P, and Barnes PJ: The airway vasculature: Recent advances and clinical implications. Thorax. 2009;64:444–450 [DOI] [PubMed] [Google Scholar]
- 34.Martin C, Thevenot G, Danel S, Chapron J, Tazi A, Macey J, Dusser DJ, Fajac I, and Burgel PR: Pseudomonas aeruginosa induces vascular endothelial growth factor synthesis in airway epithelium in vitro and in vivo. Eur Respir J. 2011;38:939–946 [DOI] [PubMed] [Google Scholar]
- 35.Staehelin LA: Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283 [DOI] [PubMed] [Google Scholar]
- 36.Honeybourne D, and Baldwin DR: The site concentrations of antimicrobial agents in the lung. J Antimicrob Chemother. 1992;30:249–260 [DOI] [PubMed] [Google Scholar]
- 37.Deffebach ME, Charan NB, Lakshminarayan S, and Butler J: The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135:463–481 [DOI] [PubMed] [Google Scholar]
- 38.Bergin DA, Hurley K, Mehta A, Cox S, Ryan D, O'Neill SJ, Reeves EP, and McElvaney NG: Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis. J Inflamm Res. 2013;6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daviskas E, and Anderson SD: Hyperosmolar agents and clearance of mucus in the diseased airway. J Aerosol Med. 2006;19:100–109 [DOI] [PubMed] [Google Scholar]
- 40.Redding GJ, Kishioka C, Martinez P, and Rubin BK: Physical and transport properties of sputum from children with idiopathic bronchiectasis. Chest. 2008;134:1129–1134 [DOI] [PubMed] [Google Scholar]
- 41.Lee AL, Burge AT, and Holland AE: Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2015:CD008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King M, and Rubin BK: Pharmacological approaches to discovery and development of new mucolytic agents. Adv Drug Deliv Rev. 2002;54:1475–1490 [DOI] [PubMed] [Google Scholar]
- 43.Wills PJ, Wodehouse T, Corkery K, Mallon K, Wilson R, and Cole PJ: Short-term recombinant human DNase in bronchiectasis. Effect on clinical state and in vitro sputum transportability. Am J Respir Crit Care Med. 1996;154:413–417 [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell AE, Barker AF, Ilowite JS, Fick RB, and rhDNase Study Group: Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest. 1998;113:1329–1334 [DOI] [PubMed] [Google Scholar]
- 45.Bhat PG, Flanagan DR, and Donovan MD: Drug diffusion through cystic fibrotic mucus: Steady-state permeation, rheologic properties, and glycoprotein morphology. J Pharm Sci. 1996;85:624–630 [DOI] [PubMed] [Google Scholar]
- 46.Alipour M, Suntres ZE, and Omri A: Importance of DNase and alginate lyase for enhancing free and liposome encapsulated aminoglycoside activity against Pseudomonas aeruginosa. J Antimicrob Chemother. 2009;64:317–325 [DOI] [PubMed] [Google Scholar]
- 47.Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, and Davis RL: Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis. 1985;132:761–765 [DOI] [PubMed] [Google Scholar]
- 48.Hunt BE, Weber A, Berger A, Ramsey B, and Smith AL: Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bataillon V, Lhermitte M, Lafitte JJ, Pommery J, and Roussel P: The binding of amikacin to macromolecules from the sputum of patients suffering from respiratory diseases. J Antimicrob Chemother. 1992;29:499–508 [DOI] [PubMed] [Google Scholar]
- 50.Levy J, Smith AL, Kenny MA, Ramsey B, and Schoenknecht FD: Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: Comparison with activity in serum. J Infect Dis. 1983;148:1069–1076 [DOI] [PubMed] [Google Scholar]
- 51.Ramphal R, Lhermitte M, Filliat M, and Roussel P: The binding of anti-pseudomonal antibiotics to macromolecules from cystic fibrosis sputum. J Antimicrob Chemother. 1988;22:483–490 [DOI] [PubMed] [Google Scholar]
- 52.Ong HX, Traini D, Salama R, Anderson SD, Daviskas E, and Young PM: The effects of mannitol on the transport of ciprofloxacin across respiratory epithelia. Mol Pharm. 2013;10:2915–2924 [DOI] [PubMed] [Google Scholar]
- 53.Wilson R, Welte T, Polverino E, De Soyza A, Greville H, O'Donnell A, Alder J, Reimnitz P, and Hampel B: Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: A phase II randomised study. Eur Respir J. 2013;41:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin BK, and Williams RW: Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. Respiration. 2014;88:177–184 [DOI] [PubMed] [Google Scholar]
- 55.Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, and Sadikot RT: Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17:e1000–e1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalmers JD, and Hill AT: Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27–34 [DOI] [PubMed] [Google Scholar]
- 57.McShane PJ, Naureckas ET, Tino G, and Strek ME: Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–656 [DOI] [PubMed] [Google Scholar]
- 58.Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, Ennis M, Boucher RC, Wolfgang MC, and Elborn JS: Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilton D, Serisier DJ, De Soyza A, Wolfe R, and Bruinenberg P: Multicenter, randomized, double-blind, placebo-controlled study (ORBIT 1) to evaluate the efficacy, safety, and tolerability of once daily ciprofloxacin for inhalation in the management of Pseudomonas aeruginosa infections in patients with non-cystic fibrosis bronchiectasis. [Abstract]. Eur Respir J. 2011;38:1925 [Google Scholar]
- 60.McDonnell MJ, Aliberti S, Goeminne PC, Dimakou K, Zucchetti SC, Davidson J, Ward C, Laffey JG, Finch S, Pesci A, Dupont LJ, Fardon TC, Skrbic D, Obradovic D, Cowman S, Loebinger MR, Rutherford RM, De Soyza A, and Chalmers JD: Multidimensional severity assessment in bronchiectasis: An analysis of seven European cohorts. Thorax. 2016;71:1110–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer-Hamblett N, Rosenfeld M, Gibson RL, Ramsey BW, Kulasekara HD, Retsch-Bogart GZ, Morgan W, Wolter DJ, Pope CE, Houston LS, Kulasekara BR, Khan U, Burns JL, Miller SI, and Hoffman LR: Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med. 2014;190:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayer-Hamblett N, Ramsey BW, Kulasekara HD, Wolter DJ, Houston LS, Pope CE, Kulasekara BR, Armbruster CR, Burns JL, Retsch-Bogart G, Rosenfeld M, Gibson RL, Miller SI, Khan U, and Hoffman LR: Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin Infect Dis. 2014;59:624–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellis HC, Cowman S, Fernandes M, Wilson R, and Loebinger MR: Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: A 19-year cohort study. Eur Respir J. 2016;47:482–489 [DOI] [PubMed] [Google Scholar]
- 64.Hill AT, Pasteur M, Cornford C, Welham S, and Bilton D: Primary care summary of the British Thoracic Society Guideline on the management of non-cystic fibrosis bronchiectasis. Prim Care Respir J. 2011;20:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, Milne D, Fergusson W, Tuffery C, Sexton P, Storey L, and Ashton T: Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): A randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:660–667 [DOI] [PubMed] [Google Scholar]
- 66.Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, Biga S, Schlebusch S, Dash P, and Bowler SD: Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: The BLESS randomized controlled trial. JAMA. 2013;309:1260–1267 [DOI] [PubMed] [Google Scholar]
- 67.Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, van der Werf TS, and Boersma WG: Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: The BAT randomized controlled trial. JAMA. 2013;309:1251–1259 [DOI] [PubMed] [Google Scholar]
- 68.Angrill J, Agustí C, De Celis R, Filella X, Rañó A, Elena M, De La Bellacasa JP, Xaubet A, and Torres A: Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–1632 [DOI] [PubMed] [Google Scholar]
- 69.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, and Hill AT: Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665 [DOI] [PubMed] [Google Scholar]
- 70.Cipolla D, Froehlich J, and Gonda I: Emerging opportunities for inhaled antibiotic therapy. J Antimicrob Agents. 2015;1:104 [Google Scholar]
- 71.Mandal P, Sidhu MK, Donaldson LS, Chalmers JD, Smith MP, Turnbull K, Scott J, and Hill AT: Eight-weekly intravenous antibiotics is beneficial in severe bronchiectasis. QJM. 2013;106:27–33 [DOI] [PubMed] [Google Scholar]
- 72.Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, and Bilton D: Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2014;189:975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abu-Salah T, and Dhand R: Inhaled antibiotic therapy for ventilator-associated tracheobronchitis and ventilator-associated pneumonia: An update. Adv Ther. 2011;28:728–747 [DOI] [PubMed] [Google Scholar]
- 74.Döring G, Flume P, Heijerman H, and Elborn JS: Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J Cyst Fibros. 2012;11:461–479 [DOI] [PubMed] [Google Scholar]
- 75.Høiby N, Ciofu O, and Bjarnsholt T: Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674 [DOI] [PubMed] [Google Scholar]
- 76.Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, and James G: Biofilms, the customized microniche. J Bacteriol. 1994;176:2137–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ratjen F, Munck A, Kho P, Angyalosi G, for the ELITE Study Group: Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: The ELITE trial. Thorax. 2010;65:286–291 [DOI] [PubMed] [Google Scholar]
- 78.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, and O'Toole GA: A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310 [DOI] [PubMed] [Google Scholar]
- 79.Parks QM, Young RL, Poch KR, Malcolm KC, Vasil ML, and Nick JA: Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: Human F-actin and DNA as targets for therapy. J Med Microbiol. 2009;58:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, and Splaingard ML: Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–588 [DOI] [PubMed] [Google Scholar]
- 81.Waters V, and Smyth A: Cystic fibrosis microbiology: Advances in antimicrobial therapy. J Cyst Fibros. 2015;14:551–560 [DOI] [PubMed] [Google Scholar]
- 82.Xiong MH, Bao Y, Yang XZ, Zhu YH, and Wang J: Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76 [DOI] [PubMed] [Google Scholar]
- 83.del Pozo JL, and Patel R: The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82:204–209 [DOI] [PubMed] [Google Scholar]
- 84.Nichols WW, Dorrington SM, Slack MP, and Walmsley HL: Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988;32:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slack MP, and Nichols WW: The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981;318:502–503 [DOI] [PubMed] [Google Scholar]
- 86.Gordon CA, Hodges NA, and Marriott C: Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother. 1988;22:667–674 [DOI] [PubMed] [Google Scholar]
- 87.Hatch RA, and Schiller NL: Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, Greening AP, Haslett C, and Hill AT: A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2011;183:491–499 [DOI] [PubMed] [Google Scholar]
- 89.Costerton JW, Stewart PS, and Greenberg EP: Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 90.O'Toole G, Kaplan HB, and Kolter R: Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79 [DOI] [PubMed] [Google Scholar]
- 91.Hnin K, Nguyen C, Carson KV, Evans DJ, Greenstone M, and Smith BJ: Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst Rev. 2015:CD001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King PT, Holdsworth SR, Freezer NJ, Villanueva E, and Holmes PW: Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638 [DOI] [PubMed] [Google Scholar]
- 93.Dickson RP, Erb-Downward JR, and Huffnagle GB: The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7:245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antoniu S: Novel inhaled combined antibiotic formulations in the treatment of Pseudomonas aeruginosa airways infections in cystic fibrosis. Expert Rev Anti Infect Ther. 2015;13:897–905 [DOI] [PubMed] [Google Scholar]
- 95.Bilton D, Henig N, Morrissey B, and Gotfried M: Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest. 2006;130:1503–1510 [DOI] [PubMed] [Google Scholar]
- 96.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RL, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Kach A, Eberl L, Riedel K, DeMarco SJ, and Robinson JA: Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327:1010–1013 [DOI] [PubMed] [Google Scholar]
- 97.Pritchard MF, Powell LC, Menzies GE, Lewis PD, Hawkins K, Wright C, Doull I, Walsh TR, Onsoyen E, Dessen A, Myrvold R, Rye PD, Myrset AH, Stevens HN, Hodges LA, MacGregor G, Neilly JB, Hill KE, and Thomas DW: A new class of safe oligosaccharide polymer therapy to modify the mucus barrier of chronic respiratory disease. Mol Pharm. 2016;13:863–872 [DOI] [PubMed] [Google Scholar]
- 98.Bakka TA, Strom MB, Andersen JH, and Gautun OR: Methyl propiolate and 3-butynone: Starting points for synthesis of amphiphilic 1,2,3-triazole peptidomimetics for antimicrobial evaluation. Bioorg Med Chem. 2017;pii:S0968-0896(17)31284-1. [DOI] [PubMed] [Google Scholar]
- 99.Brodt AM, Stovold E, and Zhang L: Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: A systematic review. Eur Respir J. 2014;44:382–393 [DOI] [PubMed] [Google Scholar]
- 100.Pasteur MC, Bilton D, Hill AT, on behalf of the British Thoracic Society Bronchiectasis (non-CF) Guideline Group: British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1–i58 [DOI] [PubMed] [Google Scholar]
- 101.Dhand R: The role of aerosolized antimicrobials in the treatment of ventilator-associated pneumonia. Respir Care. 2007;52:866–884 [PubMed] [Google Scholar]
- 102.Bassetti M, Luyt CE, Nicolau DP, and Pugin J: Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care. 2016;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weers J, and Tarara T: The PulmoSphere™ platform for pulmonary drug delivery. Ther Deliv. 2014;5:277–295 [DOI] [PubMed] [Google Scholar]
- 104.Weers JG, Bell J, Chan HK, Cipolla D, Dunbar C, Hickey AJ, and Smith IJ: Pulmonary formulations: What remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 2:S5–S23 [DOI] [PubMed] [Google Scholar]
- 105.Stass H, Nagelschmitz J, Willmann S, Delesen H, Gupta A, and Baumann S: Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: A phase I study. Clin Drug Investig. 2013;33:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loira-Pastoriza C, Todoroff J, and Vanbever R: Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91 [DOI] [PubMed] [Google Scholar]
- 107.Ungaro F, d'Angelo I, Coletta C, d'Emmanuele di Villa Bianca R, Sorrentino R, Perfetto B, Tufano MA, Miro A, La Rotonda MI, and Quaglia F: Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release. 2012;157:149–159 [DOI] [PubMed] [Google Scholar]
- 108.Dolovich MB, and Dhand R: Aerosol drug delivery: Developments in device design and clinical use. Lancet. 2011;377:1032–1045 [DOI] [PubMed] [Google Scholar]
- 109.Nikander K, Prince I, Coughlin S, Warren S, and Taylor G: Mode of breathing-tidal or slow and deep-through the I-neb adaptive aerosol delivery (AAD) system affects lung deposition of 99mTc-DTPA. J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 1:S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Newhouse MT, Hirst PH, Duddu SP, Walter YH, Tarara TE, Clark AR, and Weers JG: Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest. 2003;124:360–366 [DOI] [PubMed] [Google Scholar]
- 111.Coates AL, Dinh L, MacNeish CF, Rollin T, Gagnon S, Ho SL, and Lands LC: Accounting for radioactivity before and after nebulization of tobramycin to insure accuracy of quantification of lung deposition. J Aerosol Med. 2000;13:169–178 [DOI] [PubMed] [Google Scholar]
- 112.Lenney W, Edenborough F, Kho P, and Kovarik JM: Lung deposition of inhaled tobramycin with eFlow rapid/LC plus jet nebuliser in healthy and cystic fibrosis subjects. J Cyst Fibros. 2011;10:9–14 [DOI] [PubMed] [Google Scholar]
- 113.MacLusky IB, Gold R, Corey M, and Levison H: Long-term effects of inhaled tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Pediatr Pulmonol. 1989;7:42–48 [DOI] [PubMed] [Google Scholar]
- 114.Carswell F, Ward C, Cook DA, and Speller DC: A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the outpatient management of children with cystic fibrosis. Br J Dis Chest. 1987;81:356–360 [DOI] [PubMed] [Google Scholar]
- 115.Food and Drug Administration: FDA approves TOBI Podhaler to treat a type of bacterial lung infection in cystic fibrosis patients [press release]. http://fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345123.htm (March 22, 2013)
- 116.European Medicines Agency: Colobreathe: EPAR product information. http://ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001225/human_med_001507.jsp&mid=WC0b01ac058001d124 (2012). Last accessed on October19, 2017
- 117.Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ, for the Mpex 204 Study Group: Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;183:1510–1516 [DOI] [PubMed] [Google Scholar]
- 118.Schuster A, Haliburn C, Döring G, Goldman MH, for the Freedom Study Group: Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax. 2013;68:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, Moorehead L, Montgomery AB, and Geller DE: Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with Pseudomonas airway infection. Am J Respir Crit Care Med. 2012;185:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanoh S, and Rubin BK: Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Q, Shen W, Cheng H, and Zhou X: Long-term macrolides for non-cystic fibrosis bronchiectasis: A systematic review and meta-analysis. Respirology. 2014;19:321–329 [DOI] [PubMed] [Google Scholar]
- 122.Togami K, Chono S, and Morimoto K: Aerosol-based efficient delivery of clarithromycin, a macrolide antimicrobial agent, to lung epithelial lining fluid and alveolar macrophages for treatment of respiratory infections. J Aerosol Med Pulm Drug Deliv. 2012;25:110–115 [DOI] [PubMed] [Google Scholar]
- 123.Togami K, Chono S, Seki T, and Morimoto K: Aerosol-based efficient delivery of telithromycin, a ketolide antimicrobial agent, to lung epithelial lining fluid and alveolar macrophages for treatment of respiratory infections. Drug Dev Ind Pharm. 2010;36:861–866 [DOI] [PubMed] [Google Scholar]
- 124.Hickey AJ, Lu D, Ashley ED, and Stout J: Inhaled azithromycin therapy. J Aerosol Med. 2006;19:54–60 [DOI] [PubMed] [Google Scholar]
- 125.Siekmeier R, Hofmann T, and Scheuch G: Inhalation of macrolides: A novel approach to treatment of pulmonary infections. Adv Exp Med Biol. 2015;839:13–24 [DOI] [PubMed] [Google Scholar]
- 126.Hadipour Moghaddam P, Ramezani V, Esfandi E, Vatanara A, Nabi-Meibodi M, Darabi M, Gilani K, and Najafabadi AR: Development of a nano-micro carrier system for sustained pulmonary delivery of clarithromycin. Powder Technol. 2013;239:478–483 [Google Scholar]
- 127.Elborn JS, and Tunney MM: Macrolides and bronchiectasis: Clinical benefit with a resistance price. JAMA. 2013;309:1295–1296 [DOI] [PubMed] [Google Scholar]
- 128.Stass H, Weimann B, Nagelschmitz J, Rolinck-Werninghaus C, and Staab D: Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: A phase I, randomized, dose-escalation study. Clin Ther. 2013;35:1571–1581 [DOI] [PubMed] [Google Scholar]
- 129.Stass H, Delesen H, Nagelschmitz J, and Staab D: Safety and pharmacokinetics of ciprofloxacin dry powder for inhalation in cystic fibrosis: A phase I, randomized, single-dose, dose-escalation study. J Aerosol Med Pulm Drug Deliv. 2015;28:106–115 [DOI] [PubMed] [Google Scholar]
- 130.Stass H, Nagelschmitz J, Kappeler D, Sommerer K, Kietzig C, and Weimann B: Ciprofloxacin dry powder for inhalation in patients with noncystic fibrosis bronchiectasis or chronic obstructive pulmonary disease, and in healthy volunteers. J Aerosol Med Pulm Drug Deliv. 2016;30:53–63 [DOI] [PubMed] [Google Scholar]
- 131.Serisier DJ, Bilton D, De Soyza A, Thompson PJ, Kolbe J, Greville HW, Cipolla D, Bruinenberg P, and Gonda I: Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): A randomised, double-blind, placebo-controlled trial. ORBIT-2 investigators. Thorax. 2013;68:812–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Soyza A, Aksamit T, Bandel TJ, Criollo M, Elborn JS, Krahn U, Lau M, Operschall E, Polverino E, Winthrop K, and Wilson R: Efficacy and tolerability of ciprofloxacin dry powder for inhalation (ciprofloxacin DPI) in bronchiectasis (non-CF etiology): Results from the phase III RESPIRE 1 study. [Abstract]. Chest. 2016;150:1315A [Google Scholar]
- 133.Aksamit TR, Tiemo-Joerg B, Criollo M, Elborn JS, Lau M, Operschall E, Polverino E, Montegriffo E, De Soyza A, Winthrop KL, and Wilson R: RESPIRE 2: Ciproflaxacin DPI 32.5 mg b.i.d. administered 14 days on/off or 28 days on/off vs placebo for 48 weeks in patients with non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2017;195:A7642 [Google Scholar]
- 134.Haworth C, Wanner A, Froehlich J, O'Neil T, Davis A, Gonda L, and O'Donnell A: Inhaled liposomal ciprofloxacin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection: Results from two parallel phase III trials (ORBIT-3 and -4). Am J Respir Crit Care Med. 2017;195:A7604 [Google Scholar]
- 135.Haworth C, Wanner A, Froehlich J, O'Neil T, Davis A, and O'Donnell A: Inhaled liposomal ciprofloxacin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection: Results from two parallel phase III trials (ORBIT-3 and ORBIT-4). 2nd World Bronchiectasis Conference Milan, Italy, 2017 [Google Scholar]
- 136.Haworth C, Wanner A, Froehlich J, O'Neil T, Davis A, Gonda I, and O'Donnell A: Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis (NCFBE) and chronic Pseudomonas aeruginosa (PA) infection (NCFBEPA+). J Aerosol Med Pulm Drug Deliv. 2017;30:A-1–A-38 [Google Scholar]
- 137.Gibson RL, Retsch-Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, Ahrens R, Leon K, Cohen M, McNamara S, Callahan TL, Markus R, and Burns JL: Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41:656–665 [DOI] [PubMed] [Google Scholar]
- 138.Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit JS, and Dudley MN: Pharmacokinetics and safety of MP-376 (levofloxacin inhalation solution) in cystic fibrosis subjects. Antimicrob Agents Chemother. 2011;55:2636–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Langton Hewer SC, and Smyth AR: Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2014:CD004197. [DOI] [PubMed] [Google Scholar]
- 140.Nadig TR, and Flume PA: Aerosolized antibiotics for patients with bronchiectasis. Am J Respir Crit Care Med. 2016;193:808–810 [DOI] [PubMed] [Google Scholar]
- 141.Drobnic ME, Suñé P, Montoro JB, Ferrer A, and Orriols R: Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa. Ann Pharmacother. 2005;39:39–44 [DOI] [PubMed] [Google Scholar]
- 142.Barker AF, Couch L, Fiel SB, Gotfried MH, Ilowite J, Meyer KC, O'Donnell A, Sahn SA, Smith LJ, Stewart JO, Abuan T, Tully H, Van Dalfsen J, Wells CD, and Quan J: Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162:481–485 [DOI] [PubMed] [Google Scholar]
- 143.Cipolla D, Froehlich J, and Gonda I: Comment on: Inhaled antimicrobial therapy—barriers to effective treatment, by J. Weers, inhaled antimicrobial therapy—barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:e6–e7 [DOI] [PubMed] [Google Scholar]
- 144.Haworth CS, Bilton D, Elborn JS: Long-term macrolide maintenance therapy in non-CF bronchiectasis: Evidence and questions. Respir Med. 2014;108:1397–1408 [DOI] [PubMed] [Google Scholar]