Abstract
Objective: To examine methylphenidate extended-release chewable tablets (MPH ERCT) dose patterns, attention-deficit/hyperactivity disorder (ADHD) symptom scores, and safety during the 6-week, open-label (OL) dose-optimization period of a phase 3, laboratory classroom study.
Methods: Boys and girls (6–12 years) diagnosed with ADHD were enrolled. MPH ERCT was initiated at 20 mg/day; participants were titrated in 10–20 mg/day increments weekly based on efficacy and tolerability (maximum dose, 60 mg/day). Dose-optimization period efficacy assessments included the ADHD Rating Scale (ADHD-RS-IV), analyzed by week in a post hoc analysis using a mixed-effects model for repeated measures with final optimized dose (20, 30/40, or 50/60 mg), visit, final optimized dose and visit interaction, and baseline score as terms. Adverse events (AEs) and concomitant medications were collected throughout the study.
Results: Mean MPH ERCT daily dose increased weekly from 29.4 mg/day after the first dose adjustment at week 1 (n = 90) to 42.8 mg/day after the final adjustment at week 5 (n = 86). Final optimized MPH ERCT dose ranged from 20 to 60 mg/day. Mean final optimized MPH ERCT dose ranged from 40.0 mg/day in 6–8 year-old participants to 44.8 mg/day for 11–12 year-old participants. There was a progressive decrease in mean (standard deviation) ADHD-RS-IV total score from 40.1 (8.72) at baseline to 12.4 (7.88) at OL week 5, with similar improvement patterns for hyperactivity/impulsivity and inattentiveness subscale scores. Participants optimized to MPH ERCT 50/60 mg/day had a significantly higher mean (standard error) ADHD-RS-IV score at baseline compared with participants optimized to MPH ERCT 20 mg/day (42.4 [1.34] vs. 35.1 [2.55]; p = 0.013). Treatment-emergent AEs were reported by 65/90 (72.2%) participants in the dose-optimization period.
Conclusions: Dose-optimization period results describing relationships between change in ADHD symptom scores and final optimized MPH ERCT dose will be valuable for clinicians optimizing MPH ERCT dose.
Keywords: : methylphenidate, pharmaceutical formulation, dose–response relationship, drug, attention-deficit/hyperactivity disorder, symptoms
Introduction
The American Academy of Pediatrics (AAP) strongly recommends pharmacotherapy and/or evidence-based behavior therapy (preferably both) for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children aged 6–11 years, and treatment with US Food and Drug Administration (FDA)-approved medication for ADHD is strongly recommended for adolescents (12–18 years) with ADHD (American Academy of Pediatrics 2011). Methylphenidate (MPH)- and amphetamine-based psychostimulants are considered the standard of care for ADHD pharmacotherapy (Pliszka 2007). Most clinicians who treat ADHD patients with MPH- and amphetamine-based medications prescribe a long-acting medication, alone or with an immediate-release formulation for afternoon dosing when coverage with an extended-release (ER) drug is not adequate (Fullerton et al. 2012; Lachaine et al. 2012; Briars and Todd 2016; Hauck et al. 2017).
An ER chewable tablet (ERCT) formulation of MPH (QuilliChew ER®) has been approved by the US FDA for the treatment of ADHD in patients aged 6 years and older (QuilliChew ER package insert 2016). The MPH ERCTs formulation offers a child-friendly alternative for patients or parents who are not satisfied with the available formulation options, and especially for those individuals who cannot or prefer not to swallow tablets or capsules. MPH ERCT is available in a range of dosage strengths for individualizing treatment, including 20-, 30-, and 40-mg tablets, and the 20- and 30-mg tablets are functionally scored for additional dosing options of 10 and 15 mg, respectively (QuilliChew ER package insert 2016).
As with other ADHD medications, the clinician should optimize MPH ERCT dose for the individual patient (Pliszka 2007; American Academy of Pediatrics 2011; QuilliChew ER package insert 2016). AAP treatment guidelines emphasize that although ADHD symptom improvements may be seen at lower doses, increasing dose may yield greater improvement in the individual patient (American Academy of Pediatrics 2011), as demonstrated in dose-response curves for ADHD symptoms (Greenhill et al. 1996). Adverse effects also increase with dose, however (Greenhill et al. 2001), and guidelines state that medication dose should be titrated to achieve maximum benefit with minimum adverse effects (American Academy of Pediatrics 2011). Each patient has their own therapeutic window and optimal dose (Pliszka 2007; Huss et al. 2014), and both ADHD symptoms and drug tolerability should be monitored over a titration period and throughout treatment (Pliszka 2007).
Efficacy and safety of MPH ERCT (20–60 mg) has been demonstrated in children with ADHD in a phase 3 study using the laboratory classroom design (Wigal et al. 2017), in which MPH ERCT treatment significantly improved ADHD symptoms compared with placebo based on average postdose Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Rating Scale-Combined scores (Wigal et al. 2017). The study design for that trial included a 6-week, open-label (OL), dose-optimization period followed by a double-blind laboratory classroom evaluation. Information collected during the dose-optimization period, including MPH ERCT dose and efficacy changes over time, may inform clinicians in making dosing decisions for this formulation. The objective of this analysis, therefore, was to examine MPH ERCT dose patterns, ADHD symptom scores, and safety outcomes during the OL dose-optimization period preceding the laboratory classroom evaluation.
Methods
This was a secondary analysis of a phase 3, laboratory classroom study carried out at six U.S. sites (Wigal et al. 2017). The study was conducted in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice (International Council for Harmonisation 1998) and other applicable regulatory requirements. The protocol, consent and assent forms, and the investigator's brochure received institutional review board approval before initiation of the study. Parents/guardians provided written informed consent, and children gave assent, before participation and before any study procedures being performed.
Study design
The study included a 6-week OL, dose-optimization treatment period followed by a 1-week randomized, double-blind, placebo-controlled period. Participants received MPH ERCT at a starting dose of 20 mg once daily at baseline. The dose could be titrated up or down in 10–20 mg/day increments weekly at week 1 through 5 visits to optimize efficacy and tolerability. Dose adjustments were made at the weekly visit after efficacy and safety assessments were completed. No adjustments were made at the last OL dose-optimization period visit (week 6). Participants who could not tolerate the 20-mg dose were discontinued; the maximum dose was 60 mg/day. In the double-blind treatment period, participants were randomly assigned to receive 1 week of MPH ERCT at their final individually optimized dose or matching placebo, followed by a laboratory classroom evaluation.
Participants
Boys and girls 6–12 years of age diagnosed with ADHD, who were deemed to have the need for pharmacological treatment for ADHD in the judgment of the investigator, were eligible. Diagnosis was determined by a psychiatrist, developmental pediatrician, pediatrician, or licensed allied health professional, and confirmed at screening by Schedule for Affective Disorders and Schizophrenia for School Age Children (K-SADS) semistructured diagnostic interview (Kaufman et al. 1997). Eligible participants also had an investigator-administered Clinical Global Impressions-Severity (CGI-S) score of 3 (mildly ill) or greater at screening, and an ADHD Rating Scale (ADHD-RS-IV) home version score at or above 90th percentile for gender and age on hyperactive–impulsive subscale, inattentive subscale, and/or total score at screening or baseline.
Exclusion criteria and prohibited medications were described previously (Wigal et al. 2017). Briefly, they included presence of significant anxiety, depression, or other psychiatric disorder; substance abuse; personal or family history of Tourette's syndrome; seizure disorder; clinically significant or severe medical illness or condition; history of HIV or hepatitis B or C infections; use of psychotropic agents (sedative hypnotics at a stable dose for at least 30 days before baseline allowed as a sleep aid); history of hypersensitivity or lack of efficacy to MPH; or positive test for illicit drug use at screening. Individuals were also excluded from participating if they had severe hypertension, known structural cardiac disorders, serious cardiac conditions, serious arrhythmias, cardiomyopathy, coronary artery disease, or clinically significant abnormal electrocardiogram or abnormal cardiac findings on physical examination (including presence of a pathologic murmur); review and approval by the medical monitor were required for participation of any individual who had an immediate family history of sudden cardiac death. Pharmacologic treatment for ADHD, including stimulant medications for the control of ADHD (noninvestigational), was allowed until 24 hours before baseline measurements.
Assessments
The ADHD-RS-IV and CGI-S were administered by study investigators at screening, baseline, and weekly visits during the OL period, and the CGI-Improvement (CGI-I) scale was administered weeks 1 through 6. The Conners' Parent Rating Scale (CPRS) (Conners et al. 1998) was administered weekly by a parent or guardian. Endpoints derived from the ADHD-RS-IV included ADHD-RS-IV total score, hyperactivity/impulsivity and inattentiveness subscale scores, and treatment response (defined as ≥50% improvement from baseline in ADHD-RS-IV total score). CPRS endpoints included CPRS factor scores (oppositional, cognitive problems/inattention, hyperactivity, anxious-shy, perfectionism, social problems, and psychosomatic) and scale scores (ADHD index, Global index: restless-impulse, Global index: emotional lability, Global index total score, and Diagnostic and Statistical Manual for Mental Disorders, fourth edition [DSM-IV] inattentive, DSM-IV hyperactive/impulsive, and DSM-IV total score) (Conners 1998).
Safety assessments at weekly visits throughout the OL period included adverse event (AE) collection, vital sign measurements, and administration of the Columbia-Suicide Severity Rating Scale (C-SSRS). Height and weight were measured at screening, baseline, follow-up, and/or early termination. Physical examination, clinical laboratory tests, and electrocardiogram recordings were performed at screening.
Statistical analysis
The enrolled safety population was defined as all enrolled participants who received at least one dose of OL study medication and had at least one postbaseline safety assessment.
Final optimized MPH ERCT dose was defined as the assigned dose at the final dose-optimization period visit (week 6). Final optimized dose (in mg and in mg/kg) and number (%) of participants optimized at each dose level (20, 30, 40, 50, and 60 mg) were summarized overall and by sex, age (6–8, 9–10, 11–12 years), weight (<60 lb [27.2 kg], ≥60 lb) groups, and baseline ADHD-RS-IV total score using descriptive statistics. An ADHD-RS-IV total score of 42 was used as a cutoff for moderate (<42) versus marked (≥42) severity of symptoms at baseline based on an analysis linking ADHD-RS-IV total and CGI-S scores in children (6–12 years) with ADHD (Goodman et al. 2010). The number (%) of participants at each dose level and number (%) of participants whose MPH ERCT dose was increased, decreased, or maintained were summarized by time point.
Change from baseline in ADHD-RS-IV total score, ADHD-RS-IV hyperactivity/impulsivity and inattentiveness subscale scores, and CGI-S score was summarized by time point using descriptive statistics; CGI-I score was summarized by time point. In a post hoc analysis, ADHD-RS-IV total score was analyzed by week using a mixed-effects model for repeated measures with terms for final optimized dose (using three groups: 20, 30/40, or 50/60 mg), visit, interaction of final optimized dose and visit, and baseline score. CPRS factor and scale scores and the proportion of responders based on ADHD-RS-IV total score were determined for the final OL visit (week 6).
Results
Ninety participants were enrolled in the study and 85 completed dose optimization. Of the five participants who discontinued during the OL period, one discontinued due to an AE (dysgeusia, bad taste of medicine [last assigned dose, 40 mg]), three withdrew consent (one each: unhappy with treatment/dose [last assigned dose, 50 mg], inability to attend weekly appointments [last assigned dose, 30 mg], and lack of clinical benefit [last assigned dose, 50 mg]), and one was lost to follow-up (last assigned dose, 50 mg). No children were discontinued due to an inability to tolerate the 20-mg dose. The mean (standard deviation [SD]) age of enrolled participants was 9.5 (1.73) years; 55 participants were boys (Table 1). In the enrolled safety population, 25.6% of patients were diagnosed as inattentive type ADHD, 0% as hyperactive/impulsive, and 74.4% as combined.
Table 1.
Baseline Demographics and Clinical Characteristics, Enrolled Safety Population
| MPH ERCT (n = 90) | |
|---|---|
| Gender, n (%) | |
| Male | 55 (61.1) |
| Female | 35 (38.9) |
| Mean ± SD age, years | 9.5 (1.73) |
| Age categories, (%) | |
| 6–7 years | 16 (17.8) |
| 8–10 years | 47 (52.2) |
| 11–12 years | 27 (30.0) |
| Race, n (%) | |
| White | 52 (57.8) |
| Black/African American | 32 (35.6) |
| Asian | 1 (1.1) |
| Other | 5 (5.6) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 13 (14.4) |
| Non-Hispanic/Latino | 77 (85.6) |
| ADHD type, n (%) | |
| Inattentive | 23 (25.6) |
| Hyperactive/impulsive | 0 |
| Combined | 67 (74.4) |
ADHD, attention-deficit/hyperactivity disorder; ADHD-RS-IV, attention-deficit/hyperactivity disorder rating scale; MPH ERCT, methylphenidate extended-release chewable tablets; SD, standard deviation.
Dose optimization
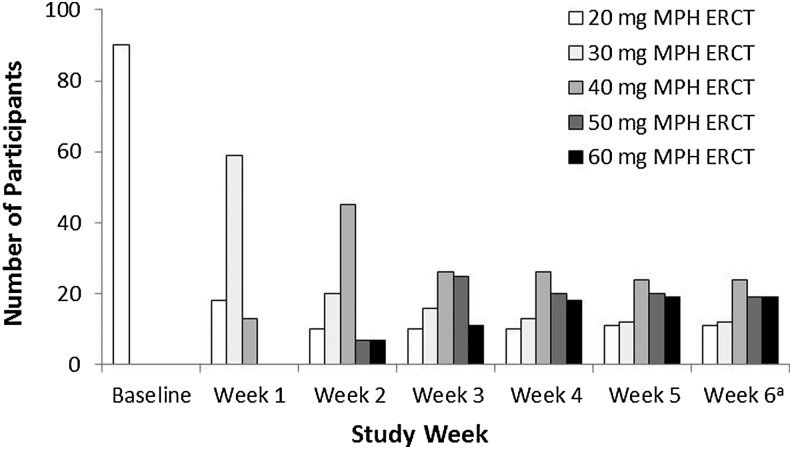
All participants received MPH ERCT 20 mg/day through the first study week. Mean MPH ERCT daily dose then increased weekly, from 29.4 mg/day (median, 30 mg/day) after the first postassessment adjustment at week 1 (n = 90) to 42.8 mg/day (median, 40 mg/day) after the final adjustment at week 5 (n = 86); at week 6, the mean optimized dose for the 85 subjects who completed the OL period was 42.7 mg/day (median, 40 mg/day). Dose shifts over the course of the optimization period are summarized in Table 2, and the distribution of the individual participants' MPH ERCT daily dose is shown by week in Figure 1. The first adjustments to doses greater than 30 mg/day were made later in the optimization period for participants aged 6–8 compared with the 9–10 and 11–12 year-old groups. Some of the participants in the older two groups had their dose titrated up to 40 mg after 1 week of treatment (at the week 1 visit), and then to 60 mg after 2 weeks. The earliest any child in the 6–8 year-old group received the 40-mg dose was after 2 weeks of treatment. No children in the youngest age group had their dose adjusted to 50 mg/day before 3 weeks of treatment had been completed or to 60 mg/day dose before 4 weeks were completed.
Table 2.
Dose Titration During the Open-Label Period; Adjustments by Weeka
| Titration of MPH ERCT during OL period | ||||
|---|---|---|---|---|
| OL week | Increased n (%) | Decreased n (%) | Maintained n (%) | Total n |
| 1 | 72 (80.0) | 0 | 18 (20.0) | 90 |
| 2 | 64 (71.9) | 0 | 25 (28.1) | 89 |
| 3 | 31 (35.2) | 2 (2.3) | 55 (62.5) | 88 |
| 4 | 18 (20.7) | 5 (5.7) | 64 (73.6) | 87 |
| 5 | 3 (3.5) | 2 (2.3) | 81 (94.2) | 86 |
| 6 | 0 | 0 | 85 (100) | 85 |
Dose adjustments were made following efficacy assessment; adjustment was not allowed at the week 6 visit.
MPH ERCT, methylphenidate extended-release chewable tablets; OL, open label.
FIG. 1.
Distribution of individual MPH ERCT daily dose by week. aDose adjustment was not allowed at week 6. MPH ERCT, methylphenidate extended-release chewable tablets.
The final daily MPH ERCT dose at the end of dose optimization was 20 mg for 11 participants, 30 mg for 14 participants, 40 mg for 25 participants, 50 mg for 21 participants, and 60 mg for 19 participants (mean final dose, 42.6 mg; n = 90). Mean (SD) final optimized dose in mg/kg/day was 1.19 (0.506), ranging from 0.38 to 2.45 mg/kg for all participants. Mean final optimized daily dose is shown by baseline demographic characteristics in Table 3. Mean dose at the end of the OL period was numerically highest for children aged 11–12 years (44.8 mg/day) and lowest for those aged 6–8 years (40.0 mg/day), with mean dose for 9–10-year olds in between (42.8 mg/day); the median final dose was 40 mg for all 3 age groups. Mean final dose was similar for participants weighing less than 60 lb (n = 19; 42.1 mg [1.76 mg/kg]) compared with participants weighing 60 lb or greater (n = 71; 42.7 mg [1.04 mg/kg]). Participants with baseline ADHD-RS-IV total scores of 42 or greater (n = 45) had a mean (SD) final optimized MPH ERCT dose of 45.8 (12.52) mg/day, while those with baseline ADHD-RS-IV total scores less than 42 had a mean (SD) of 39.3 (12.68) mg/day (n = 45).
Table 3.
Final Optimized Methylphenidate Extended-Release Chewable Tablets Dosea
| MPH ERCT dose in mg | MPH ERCT dose in mg/kg | ||||
|---|---|---|---|---|---|
| N | Mean (SD) | Median (min, max) | Mean (SD) | Median (min, max) | |
| Overall | 90 | 42.6 (12.94) | 40 (20, 60) | 1.19 (0.506) | 1.07 (0.38, 2.45) |
| Sex | |||||
| Male | 55 | 42.7 (13.53) | 40 (20, 60) | 1.20 (0.571) | 1.01 (0.38, 2.45) |
| Female | 35 | 42.3 (12.15) | 40 (20, 60) | 1.19 (0.393) | 1.09 (0.52, 2.01) |
| Age | |||||
| 6–8 years | 27 | 40.0 (13.87) | 40 (20, 60) | 1.41 (0.519) | 1.38 (0.67, 2.45) |
| 9–10 years | 36 | 42.8 (12.79) | 40 (20, 60) | 1.18 (0.544) | 1.06 (0.38, 2.35) |
| 11–12 years | 27 | 44.8 (12.21) | 40 (20, 60) | 0.99 (0.342) | 0.94 (0.54, 1.65) |
| Weight | |||||
| <60 lb | 19 | 42.1 (15.48) | 50 (20, 60) | 1.76 (0.577) | 1.90 (0.74, 2.45) |
| ≥60 lb | 71 | 42.7 (12.30) | 40 (20, 60) | 1.04 (0.361) | 1.01 (0.38, 2.01) |
| Baseline ADHD-RS-IV scoreb | |||||
| <42 | 45 | 39.3 (12.68) | 40 (20, 60) | 1.15 (0.478) | 1.04 (0.38, 2.30) |
| ≥42 | 45 | 45.8 (12.52) | 50 (20, 60) | 1.24 (0.536) | 1.09 (0.56, 2.45) |
At OL week 6 or last assessment.
Based on the third quartile of children who were moderately ill (CGI-S = 4) in Goodman et al. 2010.
CGI-S, Clinical Global Impressions-Severity scale; MPH ERCT, methylphenidate extended-release chewable tablets; ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale; SD, standard deviation; OL, open label.
ADHD symptoms
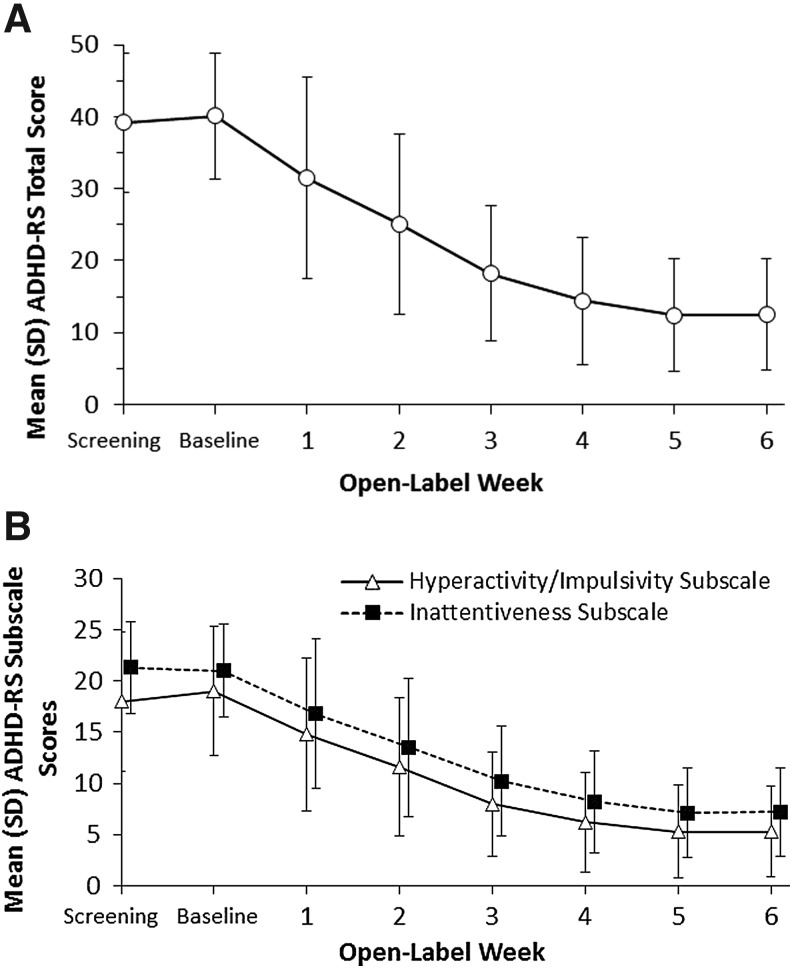
Over the course of MPH ERCT dose optimization, there was a progressive decrease in mean (SD) ADHD-RS-IV total score from 40.1 (8.72) at baseline visit to 12.4 (7.88) at week 5 (Fig. 2A). There was little change in ADHD-RS-IV total score between week 5 and the last dose of OL treatment at week 6 (12.5 [7.80]), when few dose adjustments were made (dosage was maintained in 81/86 [94%] participants). Mean (SD) change from baseline in ADHD-RS-IV total score at week 6 was −27.5 (9.69). Improvement in ADHD-RS-IV hyperactivity/impulsivity and inattentiveness subscale scores showed a similar pattern of progressive improvement over the dose-optimization period (Fig. 2B); at week 6, mean (SD) change from baseline in hyperactivity/impulsivity subscale score was −13.7 (5.92) and in inattentiveness subscale score was −13.8 (5.36). A total of 74/85 (87.1%) participants had achieved ADHD-RS-IV response (≥50% improvement from baseline) at the final dose-optimization period visit, week 6. The pattern of improvement in ADHD symptoms during dose optimization was also reflected in a shift in the distribution of CGI-S and CGI-I scores over the 6-week period (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertpub.com/cap). Changes from baseline to week 6 in CPRS factor and scale scores are reported in Supplementary Table S1.
FIG. 2.
Mean (SD) ADHD-RS-IV scores by dose-optimization period open-label week, intent-to-treat population. (A) ADHD-RS-IV total score. (B) ADHD-RS-IV subscale scores. ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale; SD, standard deviation.
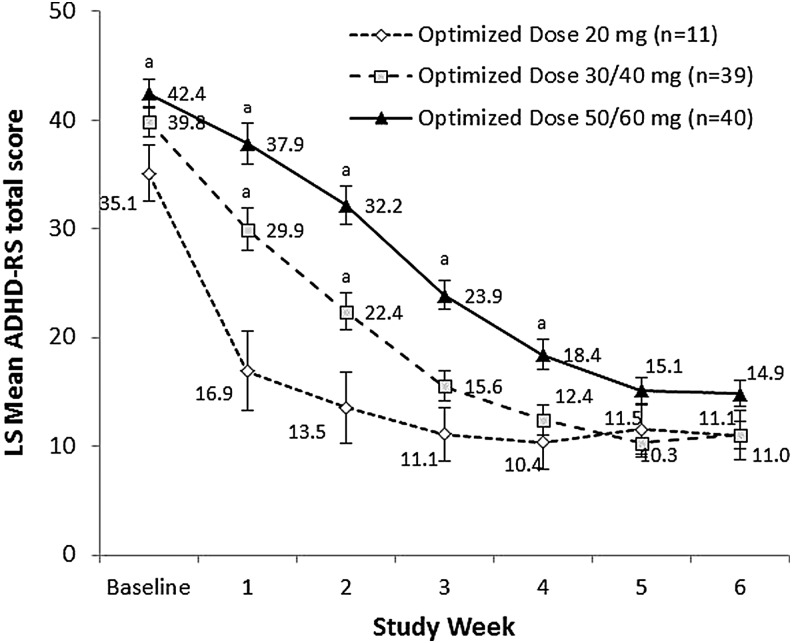
In the analysis of ADHD-RS-IV total score by visit, there was a significant interaction between final optimized dose and study visit (p < 0.001), indicating that the pattern of improvement in symptoms over the course of the dose-optimization period differed for participants optimized to final daily doses of 20, 30/40, and 50/60 mg MPH ERCT (Fig. 3). Least squares mean (standard error) ADHD-RS-IV total score at baseline was significantly higher for participants with a final optimized MPH ERCT dose of 50/60 mg/day (42.4 [1.34]) compared with those optimized to the 20 mg/day dose (35.1 [2.55]; p = 0.013). The 30/40 mg/day (39.8 [1.36]) and 20 mg/day final optimized dose groups did not differ significantly at baseline. Differences in mean ADHD-RS-IV total score were observed between final optimized dose groups during the optimization period (weeks 1–4); however, all dosage groups reach a similar mean ADHD-RS-IV total score by the end of the dose optimization period (week 5; Fig. 3).
FIG. 3.
Mean (SD) ADHD-RS-IV total score by dose-optimization period open-label week, by final optimized dose. ADHD-RS scores were analyzed using a mixed-effects model for repeated measures with terms for week, final optimized dose (20, 30/40, and 50/60 mg), and the interaction between visit and final optimized dose. ap < 0.05 versus 20 mg final optimized dose.
The trajectory of symptom improvement observed over the course of dose optimization for age, sex, weight, and treatment/naive versus experienced subgroups is shown in Supplementary Figure S2.
Safety
Treatment-emergent AEs (TEAEs) were reported by 65/90 (72.2%) participants in the OL period. OL period TEAEs reported by at least 5% of participants in either the ADHD medication-naive or previous ADHD medication group are listed in Table 4.
Table 4.
Treatment-Emergent Adverse Events With an Incidence ≥5% in the Dose-Optimization Period by Previous Attention-Deficit/Hyperactivity Disorder Medication Treatment; Safety Population, n (%)
| Treatment naive | |||
|---|---|---|---|
| TEAE, n (%) | Yes (n = 56) | No (n = 34) | Total (N = 90) |
| Any TEAE | 40 (71.4) | 25 (73.5) | 65 (72.2) |
| Decreased appetite | 22 (39.3) | 11 (32.4) | 33 (36.7) |
| Upper abdominal pain | 8 (14.3) | 5 (14.7) | 13 (14.4) |
| Irritability | 8 (14.3) | 4 (11.8) | 12 (13.3) |
| Mood swings | 9 (16.1) | 3 (8.8) | 12 (13.3) |
| Insomnia | 7 (12.5) | 3 (8.8) | 10 (11.1) |
| Upper respiratory tract infection | 7 (12.5) | 3 (8.8) | 10 (11.1) |
| Dysgeusia | 6 (10.7) | 2 (5.9) | 8 (8.9) |
| Headache | 5 (8.9) | 3 (8.8) | 8 (8.9) |
| Initial insomnia | 4 (7.1) | 0 | 4 (4.4) |
| Vomiting | 4 (7.1) | 0 | 4 (4.4) |
| Viral infection | 3 (5.4) | 1 (2.9) | 4 (4.4) |
| Nausea | 3 (5.4) | 0 | 3 (3.3) |
| Gastroenteritis | 1 (1.8) | 2 (5.9) | 3 (3.3) |
| Tic | 1 (1.8) | 2 (5.9) | 3 (3.3) |
| Excoriation | 0 | 3 (8.8) | 3 (3.3) |
| Contusion | 0 | 2 (5.9) | 2 (2.2) |
TEAE, treatment-emergent adverse event.
The greatest severity of TEAEs reported was mild (19/90 [21.1%] participants) or moderate (46/90 [51.1%]); no severe TEAEs were reported during the dose-optimization period (or at any time during the study). Participants with cardiovascular-related TEAEs reported during the dose-optimization period included one child with syncope (moderate) and tachycardia (mild) and a second child with tachycardia (mild), both treatment naive; increased systolic blood pressure (114 mm Hg, +20 mm Hg from baseline) was reported as a TEAE in one participant whose prior medications included mixed amphetamine salts (resolved by following visit). No serious AEs were reported at any time during the study. Based on the C-SSRS, no suicidal thoughts or behaviors were reported during the OL period.
Changes from baseline in vital signs at week 6 are summarized in Table 5. A total of 42/90 participants had at least one blood pressure value during the dose-optimization period considered by the sponsor to be potentially clinically significant; 20/90 participants had 1 or more potentially clinically significant pulse rate values during the dose-optimization period (Table 5).
Table 5.
Vital Sign Measures and Potentially Clinically Significant Results
| Systolic BP, mm Hg | Diastolic BP, mm Hg | Pulse rate, bpm | |
|---|---|---|---|
| Baseline, mean (SD) | 103.6 (9.08) | 63.6 (6.19) | 82.6 (11.02) |
| Week 6, mean (SD) | 105.7 (10.28) | 65.6 (7.39) | 81.8 (9.89) |
| Change from baseline, mean (SD) | 1.9 (8.97) | 2.0 (7.76) | –0.7 (12.12) |
| PCS value,an/N | 13/90 | 8/90 | 5/90 |
| PCS increase,bn/N | 6/90 | 36/90 | 15/90 |
PCS values defined by sponsor: blood pressure value greater than the 95th percentile for age and sex or pulse rate greater than 110 bpm.
Systolic BP increase ≥20 mm Hg, diastolic BP increase ≥10 mm Hg, or pulse rate increase ≥25 bpm.
BP, blood pressure; PCS, potentially clinically significant.
Discussion
Although ADHD treatment guidelines emphasize the importance of optimizing stimulant medication dose (Pliszka 2007; American Academy of Pediatrics 2011), there are few published reports assessing response to ADHD medication during dose titration in clinical trials (Greenhill et al. 1996, 2001; Huss et al. 2014). The MPH ERCT dose and ADHD symptom data collected over the course of the initial 6-week OL period of a laboratory classroom study provide insight into how investigators adjusted ADHD medication dosage during optimization at treatment onset. Overall, the final optimized daily MPH ERCT dose for the enrolled population ranged from 20 to 60 mg (the minimum and maximum doses allowed in the study), with a mean of 42.6 mg (1.19 mg/kg; n = 90). Previous laboratory classroom studies of stimulants for the treatment of children with ADHD that used dose optimization have similarly shown that the range of doses to which individual patients were optimized included all available study doses (Wigal et al. 2009, 2013; Murray et al. 2011; Wigal et al. 2014; Childress et al. 2015, 2017).
Approximately 70%–80% of participants had MPH ERCT dose increases after efficacy and safety assessments at the week 1 and 2 visits. The majority of participants were judged by investigators to have reached their individual optimal dose by the end of week 3; 63% and 74% of patients had no dose increased at the end of weeks 3 and 4, respectively. Few dose reductions were reported during the dose-optimization period. Adjustments to the highest allowable doses were made more slowly for younger children (6–8 years) compared with older children (9–10 and 11–12 years); shifts to the 60-mg dose were observed as early as the end of week 2 in older children, but not until the end of week 4 for those 6–8 years of age. Mean final optimized daily dose of MPH ERCT generally increased with age: numerically lowest for children aged 6–8 years and highest for those aged 11–12 years, with the mean dose for 9–10-year olds in between. However, the median dose was the same across age groups (40 mg).
Overall, the severity of ADHD symptoms, based on mean ADHD-RS-IV total score, diminished progressively over the course of dose optimization as mean MPH ERCT dose increased, in line with previous reports showing dose-optimization period results or dose–response relationships for ADHD patients treated in clinical trials (Stein et al. 2003; Childress et al. 2015). The week-by-week improvement found in both hyperactive–impulsive and inattentive subscale scores was similar to that observed for ADHD-RS-IV total score. The pattern of improvement in ADHD-RS-IV total score during dose optimization was generally similar between age, sex, weight, and previous treatment experience subgroups.
Differences in pattern of symptom improvement over the course of the dose-optimization period were observed between participants who were optimized to high (50/60 mg) versus low (20 mg) MPH ERCT final daily doses. However, by the end of the dose-optimization period, mean ADHD-RS-IV total scores were similar for participants at each final optimized dose (20, 30–40, and 50–60 mg/day). On average, participants with more severe symptoms at baseline, based on an ADHD-RS-IV total score of 42 or greater, were optimized to a numerically higher mean final MPH ERCT dose compared with participants with less severe symptoms, consistent with the significantly higher mean baseline ADHD-RS-IV total score observed for participants who were optimized to highest (50/60 mg) versus lowest (20 mg) daily doses of MPH ERCT.
There were several limitations of the analysis. Measures of clinical response used in this analysis were secondary efficacy endpoints in the original study protocol. The original study was designed to examine the efficacy of MPH ERCT primarily in the double-blind, placebo-controlled laboratory classroom evaluation. In addition, the dose-optimization period was OL, which could have biased ADHD-RS-IV, CGI, and CPRS scoring, and did not include a placebo control. Dose was not randomly assigned in this study, and therefore dose–response inferences cannot be made based on these results. The objective of this analysis was to present descriptively the MPH ERCT dose pattern during the optimization period. Finally, restriction of participation in the study to children without significant comorbid psychiatric or medical conditions may limit the generalizability of these findings.
Conclusions
Because each individual has their own therapeutic window for efficacy and safety of ADHD medication and a therapeutic plasma or blood range for MPH has not been established, dose optimization is an important step in dose selection both in the clinic and in ADHD trials (Kimko et al. 1999; Swanson and Volkow 2002; Pliszka 2007; American Academy of Pediatrics 2011). A dose-optimization period preceding a randomized, double-blind testing period (for example, in laboratory classroom studies) is a commonly used study design for ADHD clinical trials (McGough et al. 2006; Wigal et al. 2009, 2013; Murray et al. 2011; Wigal et al. 2014; Childress et al. 2015, 2017). Several studies have reported efficacy, safety, or dosing data collected during dose optimization (Huss et al. 2014; Childress et al. 2015, 2017). However, the current analysis is one of the few analyses that have explored in detail how ADHD medication dose was adjusted over the optimization period, together with changes in ADHD symptoms as dose was optimized. The results of this analysis provide information about the relationship between change in ADHD symptom scores and final optimized MPH ERCT dose and patient subgroup comparisons, which will be valuable for clinicians optimizing MPH ERCT dose in practice.
Clinical Significance
In the 6-week, OL dose-optimization period of this laboratory classroom study, ADHD symptom severity progressively decreased as MPH ERCT dose increased from an initial dose of 20 mg/day to a mean of 42.8 mg/day after final adjustment at week 5. Children optimized to higher doses had a significantly higher mean ADHD-RS-IV score at baseline compared with those optimized to the lowest MPH ERCT dose. Understanding relationships between change in ADHD symptom scores and final optimized MPH ERCT dose overall and in subgroups in this study will help clinicians optimize MPH ERCT dose in their own patients.
Supplementary Material
Acknowledgments
This research was sponsored by NextWave Pharmaceuticals, a wholly owned subsidiary of Pfizer Inc. Medical writing support was provided by Kathleen M. Dorries, PhD, Peloton Advantage, and was funded by Pfizer Inc.
Author Contributions
Study design: S.A.B. and S.B.W.; Study investigator: S.B.W.; Enrolled patients: S.B.W.; Collection and assembly of data: S.B.W.; Data analysis: D.B.W.; Data interpretation: all authors; Article preparation: S.B.W. and D.B.W.; Article review and revisions: all authors; Final approval of article: all authors.
Disclosures
S.B.W. receives or has received research support, acted as a consultant, been an advisory board member, and/or served on a speakers bureau for Addrenex Pharmaceuticals, Akili, Arbor, Eli Lilly and Company, Forest Pharmaceuticals, Ironshore, McNeil Consumer and Specialty Pharmaceuticals, Neos, Neurovance, NextWave Pharmaceuticals, NIMH, NLS, Noven, NuTec Pharma, Otsuka, Pfizer, Purdue, Quintiles, Rho, Rhodes Pharmaceuticals, Shionogi, Shire US, Sunovion, Taisho Pharmaceutical, and Tris Pharma. A.C. receives or has received research support, acted as a consultant, been an advisory board member, and/or served as a speaker for Shire Pharmaceuticals, Pfizer, Noven, NextWave Pharmaceuticals, Lilly USA, Forest Research Institute, Otsuka, Sunovion, Ironshore, Rhodes, Theravance, Neurovance, Neos, Arbor, Tris Pharma, Purdue, Lundbeck, Pearson, and Alcobra. S.A.B. and H.W.B. were paid consultants for Pfizer Inc. in connection with the development of this article. P.N. is a former employee of Pfizer Inc. P.C., D.B.W., R.A., and D.P. are employees of Pfizer Inc. P.C. and D.B.W. hold Pfizer stock and stock options.
References
- American Academy of Pediatrics: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briars L, Todd T: A review of pharmacological management of attention-deficit/hyperactivity disorder. J Pediatr Pharmacol Ther 21:192–206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AC, Brams M, Cutler AJ, Kollins SH, Northcutt J, Padilla A, Turnbow JM: The efficacy and safety of Evekeo, racemic amphetamine sulfate, for treatment of attention-deficit/hyperactivity disorder symptoms: A multicenter, dose-optimized, double-blind, randomized, placebo-controlled crossover laboratory classroom study. J Child Adolesc Psychopharmacol 25:402–414, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AC, Kollins SH, Cutler AJ, Marraffino A, Sikes CR: Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6–12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. J Child Adolesc Psychopharmacol 27:66–74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK: Rating scales in attention-deficit/hyperactivity disorder: Use in assessment and treatment monitoring. J Clin Psychiatry 59 Suppl 7:24–30, 1998 [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN: The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268, 1998 [DOI] [PubMed] [Google Scholar]
- Fullerton CA, Epstein AM, Frank RG, Normand SL, Fu CX, Mcguire TG: Medication use and spending trends among children with ADHD in Florida's medicaid program, 1996–2005. Psychiatr Serv 63:115–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Faraone SV, Adler LA, Dirks B, Hamdani M, Weisler R: Interpreting ADHD rating scale scores: Linking ADHD rating scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim Psychiatry 17:44–52, 2010 [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, Elliott G, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, March JS, Newcorn J, Pelham WE, Severe JB, Swanson JM, Vitiello B, Wells K: Medication treatment strategies in the MTA Study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 35:1304–1313, 1996 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, Arnold LE, Abikoff HB, Bukstein OG, Conners CK, Elliott GR, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Severe JB, Wells K, Wigal T: Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry 40:180–187, 2001 [DOI] [PubMed] [Google Scholar]
- Hauck TS, Lau C, Wing LL, Kurdyak P, Tu K: ADHD Treatment in primary care: demographic factors, medication trends, and treatment predictors. Can J Psychiatry 62:393–402, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss M, Ginsberg Y, Arngrim T, Philipsen A, Carter K, Chen CW, Gandhi P, Kumar V: Open-label dose optimization of methylphenidate modified release long acting (MPH-LA): A post hoc analysis of real-life titration from a 40-week randomized trial. Clin Drug Investig 34:639–649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Council for Harmonisation: ICH harmonised tripartite guideline: Statistical principles for clinical trials E9. International Conference on Harmonisation 1998; www.ich.org/products/guidelines/efficacy/efficacy-single/article/statistical-principles-for-clinical-trials.html (accessed April16, 2017)
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kimko HC, Cross JT, Abernethy DR: Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet 37:457–470, 1999 [DOI] [PubMed] [Google Scholar]
- Lachaine J, Beauchemin C, Sasane R, Hodgkins PS: Treatment patterns, adherence, and persistence in ADHD: A Canadian perspective. Postgrad Med 124:139–148, 2012 [DOI] [PubMed] [Google Scholar]
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E: A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord 9:476–485, 2006 [DOI] [PubMed] [Google Scholar]
- Murray DW, Childress A, Giblin J, Williamson D, Armstrong R, Starr HL: Effects of OROS methylphenidate on academic, behavioral, and cognitive tasks in children 9 to 12 years of age with attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 50:308–320, 2011 [DOI] [PubMed] [Google Scholar]
- Pliszka S: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921, 2007 [DOI] [PubMed] [Google Scholar]
- QuilliChew ER. [package insert]: New York, NY, NextWave Pharmaceuticals, Inc., 2016 [Google Scholar]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, Black DO, Seymour KE, Newcorn JH: A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 112:e404–e413, 2003 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND: Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behav Brain Res 130:73–78, 2002 [DOI] [PubMed] [Google Scholar]
- Wigal S, Childress A, Berry S, Belden H, Walters F, Chappell P, Sherman N, Orazem J, Palumbo D: Efficacy and safety of a chewable methylphenidate extended-release tablet in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 27:690–699, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Childress AC, Belden HW, Berry SA: NWP06, an extended-release oral suspension of methylphenidate, improved attention-deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. J Child Adolesc Psychopharmacol 23:3–10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Greenhill LL, Nordbrock E, Connor DF, Kollins SH, Adjei A, Childress A, Stehli A, Kupper RJ: A randomized placebo-controlled double-blind study evaluating the time course of response to methylphenidate hydrochloride extended-release capsules in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24:562–569, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Kollins SH, Childress AC, Squires L: A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health 3:17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.