Abstract
RNA interference (RNAi)-based therapeutics are approaching clinical approval for genetically defined diseases. Current clinical success is a result of significant innovations in the development of chemical architectures that support sustained, multi-month efficacy in vivo following a single administration. Conjugate-mediated delivery has established itself as the most promising platform for safe and targeted small interfering RNA (siRNA) delivery. Lipophilic conjugates represent a major class of modifications that improve siRNA pharmacokinetics and enable efficacy in a broad range of tissues. Here, we review current literature and define key features and limitations of this approach for in vivo modulation of gene expression.
Keywords: : siRNA, lipid conjugation, delivery
Introduction
Oligonucleotide drugs hold tremendous promise to treat genetically defined diseases inaccessible to conventional small molecule and antibody-based therapies. The primary challenge facing the clinical development of small interfering RNAs (siRNA) has been overcoming barriers that impede in vivo delivery. siRNAs are large, polyanionic macromolecules with intrinsically poor pharmacological properties. Unmodified siRNAs have a half-life of less than 5 min in circulation, and they do not permeate intact cellular membranes. Both nanoscale and molecular-scale delivery strategies have been employed to surmount these limitations.
Lipid- and polymer-based nanoparticles prolong circulation time, stability, and bioavailability of siRNAs in vivo (reviewed in [1]). A lipid-formulated siRNA will likely be the first drug in this class to receive FDA approval (www.alnylam.com). However, nanoparticle-based delivery is typically limited to clearance organs with fenestrated or discontinuous endothelium (e.g., liver, spleen, and certain tumors) and may necessitate intravenous (IV) administration [2]. Molecular-scale delivery of siRNAs conjugated to small ligands, carbohydrates, cell-penetrating peptides, aptamers, or lipids offers a simple and effective alternative to carrier-based methods. The most clinically advanced siRNA conjugate is a trivalent N-acetylgalactosamine (GalNAc)-siRNA, which binds with high selectivity to the asialoglycoprotein receptor on hepatocytes. GalNAc-siRNAs trigger potent and durable (6–9 month) gene silencing in patients ([3], reviewed in [4]). The second widely used class of molecular siRNA conjugates are lipids, which greatly enhance circulation time and promote local and systemic delivery and efficacy. The goal of this review is to identify the key characteristics, factors, and limitations of lipophilic siRNAs as an approach for in vivo delivery.
Cholesterol conjugation promotes siRNA delivery and distribution in vivo
One of the most well-studied lipid moieties enabling efficient cellular and tissue delivery following direct oligonucleotide conjugation is cholesterol. Cholesterol constitutes 15%–30% of cellular membranes [5] and spontaneously intercalates into lipid membranes upon co-incubation with cells, fulfilling its biological role of supporting membrane structure and fluidity [6]. There are two primary mechanisms by which cholesterol conjugation promotes siRNA uptake. In the first, the cholesterol conjugate intercalates into the plasma membrane and oligonucleotide is internalized by endocytosis. In the second, the cholesterol conjugate binds circulating plasma lipoproteins and siRNA uptake is driven by interactions with lipoprotein receptors [7]. In vitro, cholesterol-conjugated siRNA is rapidly internalized—within seconds of exposure—by any cell type via EEA1-associated endocytosis [8]. Because internalization is rapid and only partially inhibited by serum, it is likely dominated by direct membrane incorporation. These features positioned cholesterol-conjugated siRNAs as ideal candidates for local in vivo delivery, particularly to the skin [9–11], eye [12,13], and brain parenchyma [14–16]. Rapid membrane association and uptake upon local administration in vivo typically leads to restricted distribution from the site of injection (e.g., >1 cm in mouse striatum) [16].
Cholesterol was the first reported conjugate to be used for systemic delivery of siRNA [17]. This study showed that cholesterol conjugation dramatically improved the pharmacokinetic parameters of siRNA, including half-life and bioavailability in serum. Cholesterol-conjugated siRNAs were hypothesized to target the liver due to its discontinuous, fenestrated endothelium, and to the high capacity for hepatocytes to internalize cholesterol in native lipid trafficking pathways [17]. However, cholesterol and other lipid-conjugated oligonucleotides were subsequently discovered to bind circulating lipoproteins, for example, low density lipoprotein (LDL), and their uptake by hepatocytes in vivo was found to depend on LDL receptor expression [18].
Several cholesterol-conjugated siRNAs have advanced to clinical evaluation. RXi Pharmaceuticals' cholesterol-conjugated siRNA RXI-109 was evaluated in several Phase II clinical studies (www.rxipharma.com/technology/rxi-109) for its ability to reduce hypertrophic scarring. RXI-109 targets connective tissue growth factor, a key regulator of cellular pathways related to fibrosis. RXI-109 was also evaluated in a Phase I/II trial in the eyes of age-related macular degeneration patients at risk for subretinal fibrosis. Arrowhead Pharmaceuticals used cholesterol-conjugated oligonucleotides to target the liver to treat hepatitis B. In this case, cholesterol-conjugated siRNAs were co-administrated with a polymeric carrier that enhanced endosomal release and in vivo potency [19]. This trial was later discontinued when the cationic formulation alone was found to cause toxicity in non-human primates. We have summarized the main published in vivo work on the local and systemic delivery of cholesterol-conjugate siRNAs in Table 1.
Table 1.
In Vivo Activity of Cholesterol-Conjugated Small Interfering RNAs
| Oligonucleotide chemistry | Target gene | Target tissue | Dose | mRNA silencing | Year | DOI |
|---|---|---|---|---|---|---|
| Cholesterol-conjugated siRNAs: systemic administration | ||||||
| Terminal 2′-O-methyl and PS modifications | Apolipoprotein B | Liver | Three doses of 50 mpk | 40%–60% (4 days) | 2004 | 10.1038/nature03121 |
| Jejunum | Three doses of 50 mpk | 50%–70% (4 days) | ||||
| 2′-fluoro pyrimidines, terminal dT, PS modifications | Apolipoprotein B | Liver | Single 50 mpk injection | 50% (7 days) | 2007 | 10.1038/nbt1339 |
| Terminal 2′-O-methyl and PS modifications | 12/15-lipoxygenase | Kidney | Biweekly 400 μg injection for 7 weeks | 50%–70% | 2008 | 10.1152/ajprenal.90268.2008 |
| Accell siRNA | Krüppel-like factor 6 | Tumor xenograft | Six doses of 3 mpk | 80% (30 days) | 2010 | 10.1158/0008-5472.CAN-08-4282 |
| Unmodified RNA nucleotides, terminal dT | Pokemon | Tumor xenograft | 25 μg | 0% silencing (14 days) | 2012 | 10.1016/j.biomaterials.2012.08.057 |
| 2′-fluoro pyrimidines, terminal dT, PS modifications | Plasma factor 7 | Liver | 5 mpk | 0% silencing (2 days) | 2012 | 10.1089/nat.2012.0389 |
| Accell siRNA | Mannan-binding lectin associated serine proteases 1,3 | Liver | Three doses of 8 μg | 58%–62% (7 days) | 2016 | 10.4049/jimmunol.1600719 |
| Complement factor D | Adipose tissue | Three doses of 8 μg | 53% (7 days) | |||
| Alternating 2′-fluoro and 2′-O-methyl, vP, PS modifications | Myostatin | Muscle | 50 mpk | 85%–95% (21 days) | 2016 | 10.1038/mtna.2016.55 |
| Alternating 2′-fluoro and 2′-O-methyl, vP, PS modifications, Cy3 | Cyclophilin B, Huntingtin | Liver | 10 mpk | 50%–70% (7 days) | 2017 | 10.1093/nar/gkx507 |
| Kidney | 20 mpk | 40%–60% (7 days) | ||||
| Heart | 20 mpk | 50%–55% (7 days) | ||||
| 2′-O-methyl modified at nuclease cleavage site | P-glycoprotein | Tumor xenograft | 10 mpk | 65% (6 days) | 2017 | 10.1016/j.omtn.2016.12.011 |
| Alternating 2′-fluoro and 2′-O-methyl, vP, PS modifications | Cyclophilin B | Liver | 20 mpk | 60% (7 days) | 2018 | * |
| Kidney | 20 mpk | 22% (7 days) | ||||
| Adrenal Gland | 20 mpk | 37% (7 days) | ||||
| Ovary | 20 mpk | 20% (7 days) | ||||
| Cholesterol-conjugated siRNAs: Local administration | ||||||
| Terminal 2′-O-methyl and PS modifications | Huntingtin | CNS | Single 20 nmol injection | 50%–60% (3 days; protein) | 2007 | 10.1073 pnas.0708285104 |
| Accell siRNA | Luciferase | Skin | Six doses of 120 ng | 25%–50% silencing (1 days) | 2010 | 10.1038/mt.2010.126 |
| Accell siRNA | MAP kinase-activated protein kinase 2 | Oral | 4 nmol/injection for 3–5 injections | 30%–50% silencing (9–16 days) | 2010 | 10.1124/jpet.110.172395 |
| Partially 2′-O-methyl, terminal dT | 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase | CNS | Infusion of 0.72 mg/day for 3–7 days | 60% | 2010 | 10.1016/j.jconrel.2010.02.011 |
| 2′-fluoro pyrimidines in guide strand, 2′-O-methyl pyrimidines in sense strand, PS modifications | Cyclophilin B, Mitogen-activated protein kinase 4 | Eye | 10 μg injection | 50% (2 days) | 2013 | 10.1089/jop.2013.0148 |
| Accell siRNA | Green fluorescent protein | Skin | Microinjection of 0.5 mg every other day 80% for 10 days | 80% | 2013 | 10.1038/mtna.2013.56 |
| Accell siRNA | Microtubule-associated protein tau | CNS | Bilateral intraparenchymal (0.4 nmol) | ∼50% (protein) 14 days | 2014 | 10.2174/156652321405140926160602 |
| Accell siRNA | DNMT1, 3a, and 3b | CNS (rat) | Intraparenchymal (0.1 nmol) | ∼30%–70% (2 days) | 2014 | 10.1111/ejn.12819 |
| Accell siRNA | Phosphoinositide 3-Kinase Gamma | CNS (rat) | ICV 500 nmol | ∼30% (1 days) | 2015 | 10.1523/JNEUROSCI.0546-15.2015 |
| Accell siRNA | Glyceradehyde-3-phosphate dehydrogenase (GAPDH) | CNS (rat) | ICV 5 μg | - | 2015 | 10.1074/jbc.M114.635607 |
| Alternating 2′-fluoro and 2′-O-methyl, PS modifications | Huntingtin | CNS | 2 nmol | 70% (7 days) | 2015 | 10.1038/mtna.2015.38 |
| - | Glyceraldehyde 3-phosphate dehydrogenase | Skin | 75 μg | 66% (1 days) | 2016 | 10.1038/srep21422 |
| - | Glyceraldehyde 3-phosphate dehydrogenase | Ear | 15 μg | ∼70% (1 days) | 2016 | 10.1038/srep21422 |
| Alternating 2′-fluoro and 2′-O-methyl, PS modifications | Luciferase | CNS (Tumor xenograft) | 5 nmol | 90% (7 days; protein) | 2018 | * |
‘-’ Data unavailable.
Osborn, Khvorova, unpublished data.
CNS, central nervous system; dT, deoxythymidine; ICV, intracerebroventricular; mpk, mg/kg; PS, phosphorothioate; siRNA, small interfering RNA; vP, vinylphosphonate.
Impact of siRNA chemical architecture on lipid conjugate-mediated delivery
Although cholesterol conjugation broadly promotes cellular internalization, the nucleic acid modifications also significantly affect the overall efficacy of siRNA in vivo. The relative potency, safety, and duration of conjugated siRNAs are predominantly dictated by the chemical architecture and siRNA stabilization strategy (Table 1). Because most early studies were performed using minimally modified siRNAs, high doses or repetitive/continuous administration was necessary to achieve silencing with a relatively limited duration of effect. Recently, fully chemically stabilized siRNA scaffolds that are compatible with Ago2 loading (and assembly of the RNA-induced silencing complex) have allowed durable silencing by a single, bolus injection of lipid-conjugated siRNAs.
Completely unmodified siRNAs are rapidly degraded in serum by endo- and exonucleases. A wide variety of chemical modifications to the phosphodiester backbone and ribose have been explored to protect siRNAs from nucleases (reviewed in [20]; Fig. 1). Early studies of lipid conjugate-mediated delivery in vivo used siRNA scaffolds that were minimally modified with terminal phosphorothioate (PS) and 2′-O-methyl modifications to shield the 3′ single-stranded overhang. In these studies, repetitive, high doses (cumulatively 150 mg/kg delivered over 3 days) were required to silence ApoB in liver and jejunum of mice [17], but limited potency blocked progression toward clinical development. Nevertheless, this work provided a critical proof-of-concept for the use of lipophilic conjugates for in vivo delivery. Subsequent research on lipid conjugation utilized siRNAs where all pyrimidine nucleotides were modified with 2′-O-methyl or 2′-fluoro [21]. In one case, a single high-dose injection (50 mg/kg) was sufficient to induce ∼50% gene silencing in the liver using either cholesterol or the saturated fatty acid docosanoic acid (DCA) as the conjugate [18].
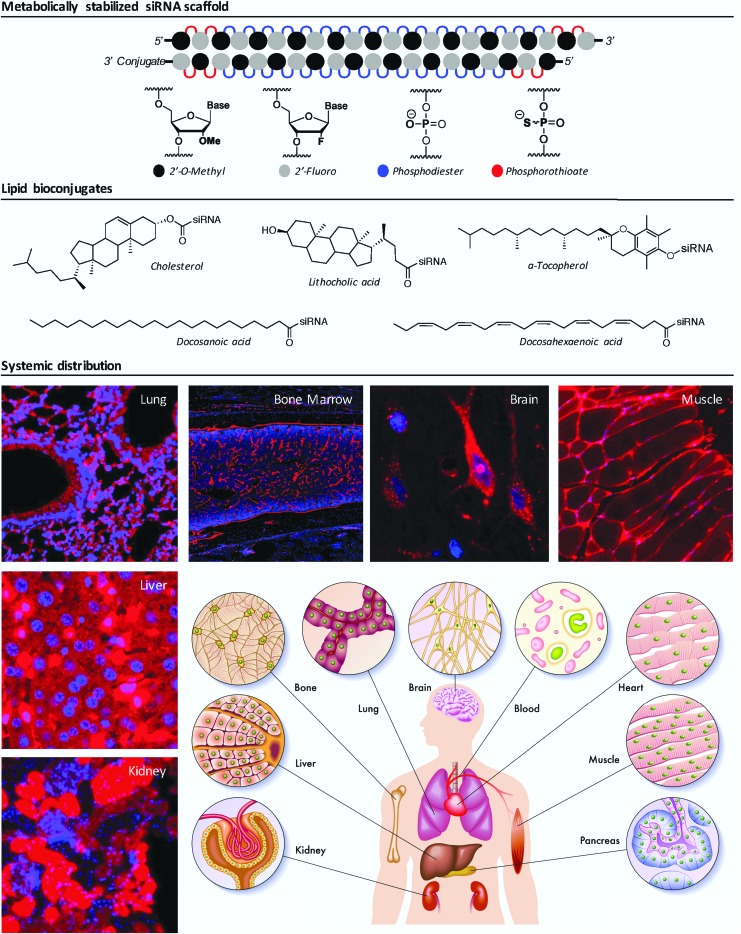
FIG. 1.
Architecture, chemical modifications, and systemic distribution of metabolically stabilized lipid-siRNA conjugates. Upper panel: a fully chemically modified siRNA scaffold, depicting a 2′ overhang at the 3′ end of the antisense (guide) strand and the lipid conjugate at the 3′ end of the sense (passenger) strand. Middle panel: chemical structures of biologically occurring lipids commonly used as siRNA conjugates in vivo. Lower panel: representative fluorescence microscopy of Cy3-labeled, lipid-conjugated siRNA accumulation in a variety of tissues following a single SC (for kidney, liver, lung, bone marrow, muscle; 20 mg/kg) or intracerebroventricular (for brain; 40 nmol) injection in wild-type female mice (Osborn, Khvorova, unpublished data). SC, subcutaneous; siRNA, small interfering RNA.
It is now clear that the defining factor that limits siRNA potency—regardless of the nature of the conjugate—is siRNA stability. Indeed, a direct comparison of fully modified (all ribose 2′-OH substituted) versus partially modified (∼60% of ribose 2′-OH substituted) siRNA revealed that total 2′-OH substitution improved oligonucleotide stability, tissue accumulation, and efficacy by several orders of magnitude following a single 10 mg/kg dose [22]. Furthermore, a fully stabilized siRNA scaffold permitted extrahepatic siRNA delivery, resulting in moderate levels of accumulation in the kidney, spleen, and heart, among other tissues [23]. These levels of accumulation reach the threshold required for silencing activity. For instance, administration of 50 mg/kg of a cholesterol-conjugated, metabolically stabilized siRNA resulted in >90% myostatin silencing in the muscle that persisted for 3 weeks [24]. Similar durations of effect have been noted for liver and kidney [25]. In contrast, the efficacy of partially modified siRNAs in vivo is typically limited to several days postinjection (Table 1).
Iterative improvements continue to be made to the siRNA architecture to enhance siRNA efficacy and reduce immunogenicity. Based on structural analysis of siRNA-loaded Ago2 and the requirement for a 5′-phosphate to anchor the siRNA guide strand in Ago2 [26], the 5′-phosphate of siRNA guide has been replaced with the stable analog, 5′-vinylphosphonate. This modification enhances siRNA accumulation, silencing activity, and duration of effect in multiple organs, including heart, where systemically administered siRNAs had not been detected [25, 27–30]. Combining modification strategies—for example, complete elimination of the ribose 2′-OH, stabilization of the phosphodiester backbone, and incorporation of 5′ guide strand phosphate analogs—will likely become a prerequisite for future clinical development of lipophilic siRNA drugs [25,29,30].
Another interesting consideration is oligonucleotide structure. To date, lipophilic conjugates have been used for delivery of both single- and double-stranded RNAs, including siRNAs (reviewed in Table 1), Dicer substrates [31,32], single-stranded siRNAs [33], antisense oligonucleotides (ASOs) [34], and asymmetric siRNAs [16,25,35,36]. Single-stranded RNAs are inherently less stable than duplex RNAs. Substituting the phosphodiester linkage with PS is the simplest modification to resist hydrolysis and increase oligonucleotide half-life both in vitro and in vivo [37]. PS modifications have therefore become an essential chemistry in oligonucleotide delivery [38]. Indeed, they are perhaps most responsible for in vivo efficacy of ASOs [39]. For unconjugated ASOs, a minimum of ∼14 PS linkages are believed necessary to promote plasma protein binding and liver deposition [33]. In contrast, uniformly PS-modified siRNAs show reduced activity compared to unmodified siRNAs, presumably due to interference with Ago2 loading [40,41]. However, as few as 6–8 terminal PS linkages are sufficient for increased siRNA stability, uptake, and efficacy in tissues such as the kidney (Osborn, Khvorova, unpublished data). To date, most published studies reporting the distribution and efficacy of lipophilic siRNAs use scaffolds that contain multiple PS bonds, either in the context of single-stranded or base-paired regions (Table 1).
In addition to enhancing siRNA stability, PS modifications likely contribute to oligonucleotide delivery by providing an additional anchor for membrane binding and cellular internalization (ref). How PS linkages promote uptake are unknown, but binding to serum proteins or cell surface receptors (e.g., ASGPR) presumably contribute to cellular internalization [42]. Stabilizing the backbone and 5′ phosphate of GalNAc-siRNAs (via PS bonds and 5′-vinylphosphonate) together significantly enhances siRNA retention in the liver [28]. We recently compared the systemic distribution of PS-modified siRNAs with different structures. As a general rule, the presence of PS modifications markedly enhances lipid-conjugated siRNA accumulation in tissues. For instance, we observe that in the presence of a PS-modified, single-stranded overhang, lipid-conjugated siRNA uptake into liver hepatocytes is increased >10-fold (Osborn et al., 2018, manuscript submitted). A detailed understanding of how PS linkages and RNA structure promote uptake will allow optimization of conjugate-mediated delivery.
Impact of lipid conjugation on siRNA distribution, efficacy, and safety
Although cholesterol has been extensively explored as a ligand for promoting oligonucleotide delivery, recent studies have shown that a wide variety of lipids affect siRNA pharmacokinetics, many with improved safety profiles (Biscans et al., 2018, manuscript submitted). The chemical compositions of lipophilic conjugates dramatically influence siRNA clearance kinetics [43]. Compared to unconjugated siRNA, for example, a diacyl lipid conjugate resulted in six to ninefold increases in circulation half-life and siRNA bioavailability [44]. A direct comparison between cholesterol (more hydrophobic) and phosphatidylcholine-docosahexanoic acid (DHA; less hydrophobic) revealed that cholesterol-modified siRNAs have approximately twofold longer terminal elimination half-life following subcutaneous (SC) or IV injection. However, after SC administration, the time to maximum blood concentration was much faster for phosphatidylcholine-DHA (15 min) than cholesterol (2–3 h). This is likely due to cholesterol-siRNA accumulation in the skin around the injection site [43].
Altering the nature of the lipid conjugate also profoundly affects tissue distribution [18,33]. One rationale for this observation is that the structure and physicochemical properties of the lipid conjugate largely define protein binding capacity in circulation. For instance, in the context of lipid-conjugated asymmetric siRNAs, highly hydrophobic lipids (e.g., cholesterol, DCA) promote association with LDL, while less hydrophobic lipids (e.g., DHA) predominantly associate with high-density lipoprotein (HDL) or albumin (Osborn et al., 2018, manuscript submitted). Protein or lipoprotein association drives tissue-specific internalization via recognition by cell surface receptors. It is important to note that although lipid conjugation increases siRNA-albumin binding in vitro, albumin association may not persist in vivo in the presence of higher affinity, competing chaperones, such as LDL and HDL.
While lipid conjugation enables siRNA uptake by a variety of tissues, the concentration required to induce RNA interference (RNAi)-mediated gene silencing is tissue- and conjugate-specific. As much as 50–100 ng/mg siRNA is necessary for efficacy in the kidney, while as little as 1–2 ng/mg in sufficient in muscle and fat (Biscans et al., 2018, manuscript submitted). This supports the hypothesis that both “productive” and “non-productive” oligonucleotide uptake pathways exist, although the molecular players in each pathway have yet to be defined [42]. For instance, α-tocopherol-siRNA was active against ApoB in the liver at a concentration as low as 2 mg/kg, yet cholesterol-siRNA was inactive at that dose [45]. Similarly, following equimolar (10 mg/kg) administration of GalNAc- or cholesterol-conjugated siRNA, cholesterol-siRNA accumulated to higher levels in the liver, but GalNAc-siRNA was more potent (Osborn, Khvorova, unpublished data). While the underlying mechanism is still under investigation, the nature of the conjugate clearly determines siRNA bioavailability.
In addition to driving siRNA clearance kinetics and tissue tropism, lipid conjugates directly impact oligonucleotide safety profile. Acute toxicity following local administration of highly hydrophobic lipid-siRNA conjugates has been reported in the central nervous system [35]. Whereas intraparenchymal injection of up to 200 μg (limit of solubility) fully chemically stabilized DHA-conjugated siRNAs showed no evidence of neuronal toxicity or innate immune activation, as little as 25 μg cholesterol-conjugated siRNA induced significant neuronal death. In the brain, exceedingly high local concentrations of cholesterol-siRNA likely disturb membrane potential and activate glial cells. RNA stability also contributed to the observed toxicity of the fully stabilized cholesterol-conjugated siRNA: when cholesterol is conjugated to a partially modified siRNA, a 25 μg dose is safe and well tolerated [16].
The maximum tolerated dose and therapeutic index for DHA-conjugated siRNAs is also much higher than that of cholesterol-conjugated siRNA when administered systemically (Turanov et al., 2018, manuscript in preparation). Systemic administration of 100–200 mg/kg DHA-conjugated siRNA were well tolerated, but at the same dose, cholesterol-conjugated siRNA triggered detectable cytokine activation. How and why do cholesterol-siRNA conjugates stimulate an innate immune response? One theory may relate to the deposition of cholesterol-conjugated siRNAs in monocytes that reside in spleen and bone marrow (Coles, Khvorova, unpublished data). Indeed, lipid conjugates that show poor uptake by monocytes in vivo exhibit lower levels of cytokine activation (Turanov et al., 2018, manuscript in preparation).
Future of lipid conjugate mediated siRNA delivery
GalNAc conjugation, when used in conjunction with a fully chemically stabilized siRNA scaffold, has solved the quandary of targeted delivery to liver hepatocytes [22]. The clinical development of GalNAc-siRNA conjugates has been reviewed extensively, and as low as 2–5 mg/kg is sufficient to silence liver-expressed genes for up to 6 months in the clinic [3,46]. There are over 15 clinical trials relying on this delivery concept for a variety of nucleic acid drugs (ASOs, siRNAs, and microRNAs). Though highly potent and clinically convenient (defined by ease of manufacturing, SC administration, long duration of effect, saline formulation, and long shelf life), the GalNAc-siRNA platform is limited to one cell type—hepatocytes. Lipid conjugation supports a much broader therapeutic distribution, and co-optimization of the siRNA scaffold and lipid conjugate has enabled functional delivery to liver, kidneys, lung, heart, muscle, spleen, and adipose tissue after a single injection.
Though lipid conjugation is a potentially transformative strategy for functional genomics and therapeutic intervention, the approach has several major caveats. Delivery is not tissue- or cell type-specific, and a significant fraction of the injected dose will be delivered to primary clearance tissues, including liver, kidney, and spleen. Thus, for clinical utility, target selection is a critical parameter. Ideally, lipid-conjugated siRNAs should be generated against disease-causing genes whose expression is limited to a targetable disease tissue or whose silencing in normal tissue is well tolerated. This is not an insurmountable task. For example, lipid-conjugated siRNAs efficiently reach the placenta (>8% of injected dose) to silence soluble fms-like tyrosine kinase 1 (sFlt1), the primary cause of preeclampsia. As the placenta is responsible for sFlt1 production in pregnant animals, sFlt1 silencing in the liver and kidney is well tolerated and does not contribute to disease progression (Turanov et al., 2018, under review). There are other targets that are only expressed in the affected tissues, including double homeobox 4 (DUX4) in muscular dystrophy [47]. Thus, lipophilic conjugates can be broadly used to modulate gene expression, provided that the target and clinical indication are carefully considered and matched to the lipid conjugate pharmacological and safety profiles.
Another interesting area of application for lipophilic conjugates is tumor delivery following local or systemic injection. Despite being a major limitation for use in neurodegenerative disorders, the restricted diffusion and resultant toxicity of cholesterol-siRNAs could be an advantage for local tumor treatment. Cholesterol-conjugated siRNAs broadly penetrate and induce >90% gene silencing in orthotopic glioma brain tumors (Osborn, Coles et al., 2018, in press). Lipid-modified siRNAs and ASOs have also been successfully used to reach implanted tumors after systemic administration [36,44]. Cancer immunotherapy is a burgeoning field that may benefit greatly from advances in lipid-siRNA conjugation and tumor delivery.
For lipid nanoparticle-based siRNA delivery, a considerable and concerted effort over several years yielded chemical formulations that improved efficacy by several orders of magnitude [2]. We predict that a similar degree of combinatorial chemistry will be required to achieve the same results for lipid-conjugated oligonucleotides. Naturally, overall distribution will be driven by protein binding properties and restricted to currently targetable tissues. However, further chemical engineering will likely improve the efficiency of endosomal escape, the primary factor limiting siRNA bioavailability [48].
Lipid conjugate-mediated delivery of other classes of oligonucleotides
We have focused this review on the use of lipophilic modification (i.e., “hydrophobization”) to improve siRNA delivery in vivo. Although we have provided extensive evidence that the chemical architecture of the nucleic acid in question contributes to pharmacokinetic behavior, general trends remain consistent. Therefore, the concept of lipid conjugation for nucleic acid delivery represents an area worth further exploration. Indeed, ligand-conjugated antisense (LICA) technology comprises the next frontier of ASO development, with conjugates ranging from sugars (e.g., GalNAc, [42,49]) to peptides [50–52], and lipids [33,53]. While our current understanding of the impact of lipid conjugation on nucleic acid delivery is limited to siRNAs and ASOs, these fundamental discoveries will likely be applicable during future clinical development of other oligonucleotide classes, including aptamers, messenger RNAs, and CRISPR components.
Acknowledgments
The authors thank Annabelle Biscans and Julia Alterman for help preparing the figures and NIH F32 NS095508-03 and GM108803 for funding.
Author Disclosure Statement
M.F.O. has no competing financial interests to disclose. A.K. owns stock of RXi Pharmaceuticals and Advirna.
References
- 1.Schroeder A, Levins CG, Cortez C, Langer R. and Anderson DG. (2010). Lipid-based nanotherapeutics for siRNA delivery. J Intern Med 267:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zatsepin TS, Kotelevtsev YV. and Koteliansky V. (2016). Lipid nanoparticles for targeted siRNA delivery—going from bench to bedside. Int J Nanomedicine 11:3077–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, et al. (2017). A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med 376:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y. (2017). Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol Ther Nucleic Acids 6:116–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meer G. and de Kroon AI. (2011). Lipid map of the mammalian cell. J Cell Sci 124:5. [DOI] [PubMed] [Google Scholar]
- 6.Razin S. (1975). Cholesterol incorporation into bacterial membranes. J Bacteriol 124:570–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaibelet G, Allart S, Tercé F, Azalbert V, Bertrand-Michel J, Hamdi S, Collet X. and Orlowski. S. (2015). Specific cellular incorporation of a pyrene-labelled cholesterol: lipoprotein-mediated delivery toward ordered intracellular membranes. PLoS One 10:e0121563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ly S, Navaroli DM, Didiot MC, Cardia J, Pandarinathan L, Alterman JF, Fogarty K, Standley C, Lifshitz LM, et al. (2017). Visualization of self-delivering hydrophobically modified siRNA cellular internalization. Nucleic Acids Res 45:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickerson RP, Wey WC, Rimm DL, Speaker T, Suh S, Flores MA, Gonzalez-Gonzalez E, Leake D, Contag CH. and Kaspar RL. (2013). Gene silencing in skin after deposition of self-delivery siRNA with a motorized microneedle array device. Mol Ther Nucleic Acids 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong RH, Gonzalez-Gonzalez E, Lara MF, Speaker TJ, Contag CH, Kaspar RL, Coulman SA, Hargest R. and Birchall JC. (2013). Gene silencing following siRNA delivery to skin via coated steel microneedles: in vitro and in vivo proof-of-concept. J Control Release 166:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, Chen J, Zhao Y, Yan X, Zhang L, Choy K, Hu J, Sant HJ, Gale BK. and Tang. T. (2016). Transdermal delivery of siRNA through microneedle array. Sci Rep 6:21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne M, Tzekov R, Wang Y, Rodgers A, Cardia J, Ford G, Holton K, Pandarinathan L, Lapierre J, et al. (2013). Novel hydrophobically modified asymmetric RNAi compounds (sd-rxRNA) demonstrate robust efficacy in the eye. J Ocul Pharmacol Ther 29:855–864 [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Haasdijk R, Tempel D, van de Kamp EHM, Herpers R, Bos F, Den Dekker WK, Blonden LAJ, de Jong R, et al. (2012). Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation 125:3142–3158 [DOI] [PubMed] [Google Scholar]
- 14.DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, et al. (2007). Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A 104:17204–17209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Butler D, Querbes W, Pandey RK, Ge P, Maier MA, Zhang L, Rajeev KG, Nechev L, et al. (2010). Lipophilic siRNAs mediate efficient gene silencing in oligodendrocytes with direct CNS delivery. J Control Release 144:227–232 [DOI] [PubMed] [Google Scholar]
- 16.Alterman JF, Hall LM, Coles AH, Hassler MR, Didiot MC, Chase K, Abraham J, Sottosanti E, Johnson E, et al. (2015). Hydrophobically modified siRNAs silence huntingtin mRNA in primary neurons and mouse brain. Mol Ther Nucleic Acids 4:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, et al. (2004). Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432:173–178 [DOI] [PubMed] [Google Scholar]
- 18.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, et al. (2007). Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotech 25:1149–1157 [DOI] [PubMed] [Google Scholar]
- 19.Wong SC, Klein JJ, Hamilton HL, Chu Q, Frey CL, Trubetskoy VS, Hegge J, Wakefield D, Rozema DB. and Lewis DL. (2012). Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther 22:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khvorova A. and Watts JK. (2017). The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol 35:238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, Wanders L, Griffey RH, Swayze EE. and Bhat B. (2005). Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem 48:901–904 [DOI] [PubMed] [Google Scholar]
- 22.Nair JK, Willoughby JLS, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel'in AV, et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136:16958–16961 [DOI] [PubMed] [Google Scholar]
- 23.Hassler MR, Turanov AA, Alterman JF, Haraszti RA, Coles AH, Osborn MF, Echeverria D, Nikan M, Salomon WE, et al. (2018). Comparison of partially and fully chemically-modified siRNA in conjugate-mediated delivery in vivo. Nucleic Acids Res 46:2185–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan T, Weber H, DiMuzio J, Matter A, Dogdas B, Shah T, Thankappan A, Disa J, Jadhav V, et al. (2016). Silencing myostatin using cholesterol-conjugated siRNAs induces muscle growth. Mol Ther Nucleic Acids 5:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haraszti RA, Roux L, Coles AH, Turanov AA, Alterman JF, Echeverria D, Godinho B, Aronin N. and Khvorova A. (2017). 5-Vinylphosphonate improves tissue accumulation and efficacy of conjugated siRNAs in vivo. Nucleic Acids Res 45:7581–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schirle NT, Kinberger GA, Murray HF, Lima WF, Prakash TP. and MacRae IJ. (2016). Structural analysis of human Argonaute-2 bound to a modified siRNA guide. J Am Chem Soc 138:8694–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parmar RG, Brown CR, Matsuda S, Willoughby JLS, Theile CS, Charisse K, Foster DJ, Zlatev I, Jadhav V, et al. (2018). Facile synthesis, geometry, and 2′-substituent-dependent in vivo activity of 5′-(E)- and 5′-(Z)-vinylphosphonate-modified siRNA conjugates. J Med Chem 61:734–744 [DOI] [PubMed] [Google Scholar]
- 28.Prakash TP, Kinberger GA, Murray HM, Chappell A, Riney S, Graham MJ, Lima WF, Swayze EE. and Seth PP. (2016). Synergistic effect of phosphorothioate, 5′-vinylphosphonate and GalNAc modifications for enhancing activity of synthetic siRNA. Bioorg Med Chem Lett 26:2817–2820 [DOI] [PubMed] [Google Scholar]
- 29.Parmar R, Willoughby JL, Liu J, Foster DJ, Brigham B, Theile CS, Charisse K, Akinc A, Guidry E, et al. (2016). 5′-(E)-vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. Chembiochem 17:985–989 [DOI] [PubMed] [Google Scholar]
- 30.Elkayam E, Parmar R, Brown CR, Willoughby JL, Theile CS, Manoharan M. and Joshua-Tor L. (2017). siRNA carrying an (E)-vinylphosphonate moiety at the 5 end of the guide strand augments gene silencing by enhanced binding to human Argonaute-2. Nucleic Acids Res 45:3528–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubo T, Takei Y, Mihara K, Yanagihara K. and Seyama T. (2012). Amino-modified and lipid-conjugated dicer-substrate siRNA enhances RNAi efficacy. Bioconjug Chem 23:164–173 [DOI] [PubMed] [Google Scholar]
- 32.Kubo T, Yanagihara K, Takei Y, Mihara K, Sato Y. and Seyama T. (2012). Lipid-conjugated 27-nucleotide double-stranded RNAs with dicer-substrate potency enhance RNAi-mediated gene silencing. Mol Pharm 9:1374–1383 [DOI] [PubMed] [Google Scholar]
- 33.Lima WF, Prakash TP, Murray HM, Kinberger GA, Li W, Chappell AE, Li CS, Murray SF, Gaus H, et al. (2012). Single-stranded siRNAs activate RNAi in animals. Cell 150:883–894 [DOI] [PubMed] [Google Scholar]
- 34.Karaki S, Benizri S, Mejias R, Baylot V, Branger N, Nguyen T, Vialet B, Oumzil K, Barthelemy P. and Rocchi P. (2017). Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J Control Release 258:1–9 [DOI] [PubMed] [Google Scholar]
- 35.Nikan M, Osborn MF, Coles AH, Godinho BM, Hall LM, Haraszti RA, Hassler MR, Echeverria D, Aronin N. and Khvorova. A. (2016). Docosahexaenoic acid conjugation enhances distribution and safety of siRNA upon local administration in mouse brain. Mol Ther Nucleic Acids 5:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikan M, Osborn MF, Coles AH, Biscans A, Godinho B, Haraszti RA, Sapp E, Echeverria D, DiFiglia M, et al. (2017). Synthesis and evaluation of parenchymal retention and efficacy of a metabolically stable O-phosphocholine-N-docosahexaenoyl-l-serine siRNA conjugate in mouse brain. Bioconjug Chem 28:1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein F. and Gish G. (1989). Phosphorothioates in molecular biology. Trends Biochem Sci 14:97–100 [DOI] [PubMed] [Google Scholar]
- 38.Eckstein F. (2014). Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther 24:374–387 [DOI] [PubMed] [Google Scholar]
- 39.Geary RS, Norris D, Yu R. and Bennett CF. (2015). Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87:46–51 [DOI] [PubMed] [Google Scholar]
- 40.Jahns H, Roos M, Imig J, Baumann F, Wang Y, Gilmour R. and Hall. J. (2015). Stereochemical bias introduced during RNA synthesis modulates the activity of phosphorothioate siRNAs. Nat Commun 6:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braasch DA, Paroo Z, Constantinescu A, Ren G, Oz OK, Mason RP. and Corey DR. (2004). Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett 14:1139–1143 [DOI] [PubMed] [Google Scholar]
- 42.Tanowitz M, Hettrick L, Revenko A, Kinberger GA, Prakash TP. and Seth PP. (2017). Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res 45:12388–12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godinho B, Gilbert JW, Haraszti RA, Coles AH, Biscans A, Roux L, Nikan M, Echeverria D, Hassler M. and Khvorova A. (2017). Pharmacokinetic profiling of conjugated therapeutic oligonucleotides: a high-throughput method based upon serial blood microsampling coupled to peptide nucleic acid hybridization assay. Nucleic Acid Ther 27:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarett SM, Werfel TA, Lee L, Jackson MA, Kilchrist KV, Brantley-Sieders D. and Duvall CL. (2017). Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci U S A 114:E6490–E6497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H. and Yokota T. (2008). Efficient in vivo delivery of siRNA to the liver by conjugation of [alpha]-tocopherol. Mol Ther 16:734–740 [DOI] [PubMed] [Google Scholar]
- 46.Khvorova A. (2017). Oligonucleotide therapeutics—a new class of cholesterol-lowering drugs. N Engl J Med 376:4–7 [DOI] [PubMed] [Google Scholar]
- 47.Ansseau E, Vanderplanck C, Wauters A, Harper SQ, Coppee F. and Belayew. A. (2017). Antisense oligonucleotides used to target the DUX4 mRNA as therapeutic approaches in faciosscapulohumeral muscular dystrophy (FSHD). Genes (Basel) 8:E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dowdy SF. (2017). Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol 35:222–229 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt K, Prakash TP, Donner AJ, Kinberger GA, Gaus HJ, Low A, Ostergaard ME, Bell M, Swayze EE. and Seth PP. (2017). Characterizing the effect of GalNAc and phosphorothioate backbone on binding of antisense oligonucleotides to the asialoglycoprotein receptor. Nucleic Acids Res 45:2294–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henke E, Perk J, Vider J, de Candia P, Chin Y, Solit DB, Ponomarev V, Cartegni L, Manova K, et al. (2008). Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat Biotechnol 26:91–100 [DOI] [PubMed] [Google Scholar]
- 51.Jarver P, Coursindel T, Andaloussi SE, Godfrey C, Wood MJ. and Gait. MJ. (2012). Peptide-mediated cell and in vivo delivery of antisense oligonucleotides and siRNA. Mol Ther Nucleic Acids 1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P. and Wood MJ. (2008). Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 17:3909–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishina T, Numata J, Nishina K, Yoshida-Tanaka K, Nitta K, Piao W, Iwata R, Ito S, Kuwahara H, et al. (2015). Chimeric antisense oligonucleotide conjugated to alpha-tocopherol. Mol Ther Nucleic Acids 4:e220. [DOI] [PMC free article] [PubMed] [Google Scholar]