Abstract
In human immunodeficiency virus (HIV)-negative individuals, a plasma metabolite profile, characterized by higher levels of branched-chain amino acids (BCAA), aromatic amino acids, and C3/C5 acylcarnitines, is associated with insulin resistance and increased risk of diabetes. We sought to characterize the metabolite profile accompanying insulin resistance in HIV-positive persons to assess whether the same or different bioenergetics pathways might be implicated. We performed an observational cohort study of 70 nondiabetic, HIV-positive individuals (50% with body mass index ≥30 kg/m2) on efavirenz, tenofovir, and emtricitabine with suppressed HIV-1 RNA levels (<50 copies/mL) for at least 2 years and a CD4+ count over 350 cells/μL. We measured fasting insulin resistance using the homeostatic model assessment 2, plasma free fatty acids (FFA) using gas chromatography, and amino acids, acylcarnitines, and organic acids using liquid chromatography/mass spectrometry. We assessed the relationship of plasma metabolites with insulin resistance using multivariable linear regression. The median age was 45 years, median CD4+ count was 701 cells/μL, and median hemoglobin A1c was 5.2%. Insulin resistance was associated with higher plasma C3 acylcarnitines (p = .01), but not BCAA or C5 acylcarnitines. However, insulin resistance was associated with lower plasma levels of C18, C16, C12, and C2 acylcarnitines (p ≤ .03 for all), and lower C18 and C16 acylcarnitine:FFA ratios (p = .002, and p = .03, respectively). In HIV-positive persons, lower levels of plasma acylcarnitines, including the C2 product of complete fatty acid oxidation, are a more prominent feature of insulin resistance than changes in BCAA, suggesting impaired fatty acid uptake and/or mitochondrial oxidation is a central aspect of glucose intolerance in this population.
Keywords: : metabolomics, HIV, insulin resistance, diabetes mellitus, lipid metabolism
Introduction
Metabolic abnormalities, including dyslipidemia, lipodystrophy, insulin resistance, and accelerated atherosclerosis, are increasingly prevalent in individuals infected with human immunodeficiency virus (HIV) on long-term antiretroviral therapy (ART).1–4 The incidence of diabetes mellitus in HIV-positive individuals is higher than age-matched controls,5 which contributes to morbidity and mortality in the HIV population and serves as a risk factor for cardiovascular disease.6 “Traditional” risk factors,2 the rising prevalence of obesity,7 and advanced age in the HIV population,8 in combination with exposure to older protease inhibitors and nucleoside reverse transcriptase inhibitors,2,9 chronic systemic inflammation,10 and impaired mitochondrial function, likely contribute to the development of insulin resistance in persons with HIV.11
Until recently, the bioenergetics pathways disrupted in insulin resistance and diabetes have not been well characterized. The use of metabolomics to measure potential biomarkers has provided new insights into the pathophysiology of metabolic, cardiovascular, and other human diseases.12,13 Several studies in cohorts of HIV-negative persons found associations between higher plasma concentrations of branched-chain amino acids (BCAA; leucine, isoleucine, and valine); the aromatic amino acids phenylalanine and tyrosine; and C3 and C5 acylcarnitines (BCAA degradation products) with contemporaneous insulin resistance or the risk of subsequently developing diabetes.13,14
A postulated mechanism underlying this finding is the toxic effect of high plasma BCAA on mitochondrial function in muscle and other tissues, which leads to cellular stress and reduced glucose uptake.15 In Framingham and other cohorts, elevated concentrations of BCAA, aromatic amino acids, and C3 and C5 acylcarnitines preceded the development of overt diabetes mellitus by more than a decade.14,16,17 However, studies have also demonstrated that increased circulating free fatty acids (FFA) are often present in diabetes, reflecting a dysregulation of the complex relationship between glucose and lipid metabolism.18,19
To date, few studies have examined plasma metabolite profiles in HIV-positive populations.20–22 HIV-positive women on long-term ART have higher insulin sensitivity and lower concentrations of BCAA, aromatic amino acids, and C3 and C5 acylcarnitines relative to HIV-positive males.23 Other studies found differences in plasma acylcarnitine levels associated with the magnitude of CD4+ T cell decline in persons with HIV,24 and altered in those exposed to ART.25 Given the prevalence of diabetes in the HIV population, we used metabolomics to characterize the changes in mitochondrial intermediary metabolism associated with insulin resistance in HIV-positive individuals by measuring fasting plasma levels of amino acids, acylcarnitines, organic acids, and FFA.
Materials and Methods
Study enrollment
We enrolled 70 HIV-positive adults on ART for a minimum of 2 years from the Vanderbilt Comprehensive Care Clinic (VCCC), the HIV clinic affiliated with Vanderbilt University Medical Center (VUMC), between April 2013 and September 2014. The patients were evenly distributed between nonobese [body mass index (BMI) <30 kg/m2] and obese (BMI ≥30 kg/m2), with a similar number of men and women in each group. All patients were on fixed-dose efavirenz, tenofovir, and emtricitabine (EFV/TDF/FTC; Atripla®) for at least 6 months before enrollment in the study, had been on ART and maintained an HIV-1 RNA concentration of <50 copies/mL for at least 2 years, and had a CD4+ T cell count of more than 350 cells/μL.
EFV/TDF/FTC was selected as the sole regimen for this study because it was commonly prescribed during the enrollment period, and limiting enrollment to a single regimen avoided potential confounding effects of different antiretroviral agents on metabolic parameters. Patients were excluded if they were on either antidiabetic agents or HMG-CoA reductase inhibitors (statins) within the previous 6 months, had self-reported heavy alcohol use (>14 drinks/week), had self-reported current use of cocaine or amphetamines, had an active infection other than HIV and hepatitis C virus (HCV), or had a previous diagnosis of diabetes mellitus, cardiovascular disease, or rheumatological disease documented in the medical record.
Clinical assessment
We obtained information on demographics, ART regimen, duration on ART, other medications or comorbidities, HIV-1 RNA concentration, CD4+ T cell counts, HCV antibody status, and any previous history of thymidine analog [i.e., zidovudine (AZT) and stavudine (d4T)] exposure from the medical record. Smoking status was self-reported. All research subjects underwent an assessment in the Vanderbilt Clinical Research Center after fasting (except water) and abstaining from smoking or the use of electronic cigarettes for a minimum of 8 h overnight (all visits occurred between 8 and 11 AM).
Fasting blood (10 mL) was collected in an ethylenediamine tetraacetic acid (EDTA) vacutainer, immediately centrifuged for 10 min at 4°C, and the plasma was removed and frozen at −80°C for metabolomics profiling. Fasting plasma glucose and insulin were measured and insulin resistance was calculated using the Homeostasis Model Assessment 2 of insulin resistance (HOMA2-IR) equation (www.dtu.ox.ac.uk/homacalculator).26 Weight was measured using an electronic scale, height was determined with a stadiometer fixed to the wall, and waist circumference was measured using a flexible tape measure parallel to the floor at a point one inch above the navel.
A full-body, dual-energy X-ray absorptiometry (DEXA) scan was performed using a GE Healthcare Lunar iDXA and analyzed using enCORE software version 13.6 (GE Healthcare Lunar, Madison, WI). Total fat mass was used to calculate fat mass index (FMI; defined as DEXA total fat in kilograms divided by height in meters squared), which compensates for the nonlinear relationship between fat-free mass and height, and provides a more accurate estimation of relative adiposity compared with BMI or percent body fat.27,28
Measurements of serum metabolites by liquid chromatography/mass spectrometry
Cryopreserved fasting plasma samples were thawed and amino acids, acylcarnitines, and organic acids were extracted and derivatized before liquid chromatography/mass spectrometry analysis as previously described.23 Quantification of derivatized amino acids and acylcarnitines was performed using multiple reaction monitoring on an Agilent 1290 Infinity HPLC/6490 triple quadrupole mass spectrometer (Agilent, Wilmington, DE), and quantification of derivatized organic acids was performed using multiple reaction monitoring on a Dionex UltiMate 3000 HPLC/Thermo Scientific Quantiva Triple Quadrupole Mass Spectrometer (Thermo Scientific, San Jose, CA), as previously described.23 Standard calibration curves were prepared using commercially available reagents as previously described.23 FFA were extracted and analyzed by the Vanderbilt Lipid Sub-Core using gas chromatography.29 Briefly, FFA were extracted from plasma using heptane/isopropanol and separated by thin-layer chromatography on silica gel plates. Once separated, the FFA band was methylated with BF3/methanol. Quantification of derivatized FFA methyl esters was performed using an Agilent 7890 gas chromatograph and known standards.
Statistical analyses
Median values and interquartile ranges (IQR) or percentages were calculated for demographic and clinical characteristics, and plasma levels of amino acids, acylcarnitines, organic acids, and FFA separately for nonobese and obese subjects. We assessed the association of plasma metabolites with insulin resistance (log-transformed HOMA2-IR) using multivariable linear regression, adjusted for age, sex, CD4+ T cell count at enrollment (square root transformed), duration of ART, FMI, and smoking status. Model diagnostics were assessed using residual/predictor plots. An interaction term between each metabolite and obesity status (i.e., BMI <30 or ≥30 kg/m2) was included in the models to assess whether obesity modified the relationship between the particular metabolite and insulin resistance. The term was removed from the final models because the p-value for interaction was not significant (p > .05) for any metabolites, except threonine (p = .02). C5 valeryl acylcarnitine was excluded from the multivariable analyses since 29 subjects (41%) had a level below the 0.0025 μM limit of detection. Separate sensitivity analyses were performed in which FMI was replaced with BMI, or prior thymidine analog exposure or HCV status was included in the model. Analyses were conducted using SPSS 22.0.0 (IBM, Armonk, NY) and R Statistical Software, Version 3.3.2 (www.R-project.org). The study was reviewed and approved by the Vanderbilt University Institutional Review Board. All study participants provided written informed consent.
Results
Study cohort characteristics
The clinical and demographic characteristics of the 70 HIV-positive subjects are shown in Table 1. The median BMI of participants in this study was 30.3 kg/m2 (IQR 23.9–35.7 kg/m2), reflecting inclusion of subjects across a range of BMI. Age, sex, race, smoking status, HCV infection, and CD4+ T cell counts were not statistically different between obese and nonobese groups. Hemoglobin A1c was not different between obese and nonobese subjects, but obese subjects had significantly higher HOMA2 insulin resistance and higher HOMA2 beta cell output (Table 1). Table 2 shows median values of selected metabolites that have been associated with insulin resistance and/or diabetes in HIV-negative cohorts, according to obesity status. Notably, plasma concentrations of BCAA and their degradation products (C3/C5 acylcarnitines) were not significantly higher in the obese subjects despite this group having significantly greater insulin resistance. Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/aid) shows the median values for all amino acids, acylcarnitines, and organic acids analyzed in this study. Plasma tyrosine (p = .03) and glutamic acid (p = .004) concentrations were significantly higher in obese subjects compared with nonobese subjects.
Table 1.
Clinical Characteristics and Body Composition
| Baseline variables | Obese (n = 35) | Nonobese (n = 35) | p |
|---|---|---|---|
| Age, years | 46 (39–50) | 45 (38–49) | .61 |
| Female, n (%) | 16 (45.7) | 14 (40) | .63 |
| Non-white, n (%) | 20 (57.1) | 18 (51.4) | .63 |
| Smoking, n (%) | 13 (37.1) | 12 (34.3) | .80 |
| Hepatitis C infection, n (%) | 4 (11.4) | 4 (11.4) | 1.00 |
| Body composition | |||
| BMI, kg/m2 | 35.6 (33.3–40.1) | 24.0 (22.0–26.5) | <.001 |
| Fat mass index, kg/m2 | 14.8 (13.1–18.7) | 7.1 (5.0–9.3) | <.001 |
| HIV characteristics | |||
| CD4+ at enrollment, cells/μL | 758 (618–962) | 621 (514–873) | .08 |
| CD4+ at ART initiation, cells/μL | 262 (141–392) | 250 (156–304) | .59 |
| Duration of ART, years | 6.7 (4.7–10.8) | 6.0 (4.3–9.6) | .58 |
| Measurements of glucose tolerance | |||
| Fasting glucose, mg/dL | 88.0 (77.5–93.5) | 82 (76.5–87.0) | .39 |
| Plasma insulin, μU/mL | 13.3 (8.6–19.4) | 6.1 (4.2–10.6) | <.001 |
| Hemoglobin A1c, % | 5.2 (5.0–5.6) | 5.1 (4.9–5.5) | .14 |
| HOMA2 insulin resistancea | 1.72 (1.14–2.46) | 0.77 (0.53–1.26) | <.001 |
| HOMA2 beta cell function,a % | 172 (119–209) | 100 (82–127) | <.001 |
| Plasma lipids | |||
| Total cholesterol, mg/dL | 177 (155–200) | 174 (152–203) | .59 |
| High-density lipoprotein, mg/dL | 44 (39–49) | 46 (35–64) | .28 |
| Low-density lipoprotein, mg/dL | 111 (88–129) | 101 (85–122) | .50 |
| Triglycerides, mg/dL | 104 (85–152) | 94 (66–131) | .12 |
Continuous variables are shown as median values with IQR. p-Values <.05 are shown in bold.
The HOMA2 estimates steady state insulin release (beta cell function) and insulin resistance.
ART, antiretroviral therapy; BMI, body mass index; HOMA, homeostasis model assessment; HIV, human immunodeficiency virus; IQR, interquartile range.
Table 2.
Unadjusted Comparison of Selected Plasma Metabolites Between Obese and Nonobese HIV-Positive Subjects
| Variable | Obese (n = 35) | Nonobese (n = 35) | p |
|---|---|---|---|
| BCAA (μM) | |||
| Isoleucine | 55.7 (50.8–64.0) | 51.7 (46.6–63.3) | .26 |
| Leucine | 105.3 (87.8–115.6) | 95.2 (84.0–113.8) | .42 |
| Valine | 185.6 (160.7–210.0) | 171.7 (142.1–204.6) | .10 |
| Aromatic amino acids (μM) | |||
| Phenylalanine | 41.7 (38.0–46.7) | 40.5 (34.5–46.0) | .32 |
| Tyrosine | 49.6 (43.0–55.0) | 42.9 (34.0–52.6) | .03 |
| BCAA metabolism acylcarnitines (μM) | |||
| Propionyl (C3) | 0.32 (0.24–0.40) | 0.29 (0.22–0.33) | .45 |
| Isovaleryl (C5) | 0.069 (0.050–0.087) | 0.051 (0.040–0.077) | .10 |
| 2-Methylbutyryl (C5) | 0.034 (0.025–0.042) | 0.027 (0.023–0.035) | .12 |
Metabolites are shown as median values with IQR. p-Values <.05 are shown in bold.
BCAA, branched-chain amino acids.
Plasma metabolites associated with insulin resistance
After adjusting for age, sex, CD4+ T cell count at enrollment, duration of ART, FMI, and smoking status, higher concentrations of plasma C3 acylcarnitine, a degradation product of BCAA, were associated with insulin resistance (p = .01) (Table 3). Furthermore, higher levels of plasma tyrosine (p < .001) and glutamic acid (p = .001) were also associated with insulin resistance. However, none of the BCAA (isoleucine, leucine, valine), or C5 acylcarnitine, were significantly associated with insulin resistance.
Table 3.
Multivariable Linear Regression Model for the Relationship of Insulin Resistance with Selected Plasma Metabolites in HIV-Positive Subjects
| Outcome variable | Regression coefficient (95% CI) | p |
|---|---|---|
| BCAA | ||
| Isoleucine | 0.07 (−0.02 to 0.16) | .13 |
| Leucine | 0.04 (−0.05 to 0.14) | .36 |
| Valine | 0.05 (−0.04 to 0.15) | .29 |
| Aromatic amino acids | ||
| Phenylalanine | 0.04 (−0.02 to 0.11) | .19 |
| Tyrosine | 0.12 (0.06 to 0.18) | <.001 |
| Gluconeogenic amino acids | ||
| Alanine | 0.0008 (−0.07 to 0.07) | .98 |
| Glutamine | −0.01 (−0.09 to 0.06) | .69 |
| Glutamic acid | 0.13 (0.05 to 0.21) | .001 |
| BCAA metabolism acylcarnitinesa | ||
| Propionyl (C3) | 0.12 (0.03 to 0.21) | .01 |
| Isovaleryl (C5) | 0.08 (−0.02 to 0.18) | .12 |
| 2-Methylbutyryl (C5) | 0.009 (−0.07 to 0.09) | .83 |
| Acetyl-carnitine (fully oxidized degradation product) | ||
| C2 | −0.06 (−0.12 to −0.006) | .03 |
| C2:C3+C5 ratio | −0.11 (−0.19 to −0.03) | .006 |
| Medium and long-chain acylcarnitines | ||
| C10 | −0.05 (−0.10 to −0.004) | .04 |
| C10-OH | −0.07 (−0.12 to −0.03) | .003 |
| C12 | −0.05 (−0.10 to −0.004) | .03 |
| C14 | −0.06 (−0.14 to 0.009) | .08 |
| C14:1 | −0.06 (−0.12 to 0.008) | .03 |
| C14:2 | −0.05 (−0.11 to 0.004) | .07 |
| C16 | −0.08 (−0.14 to −0.009) | .03 |
| C16:2 | −0.003 (−0.06 to 0.05) | .91 |
| C18 | −0.10 (−0.18 to −0.02) | .01 |
| C18:1 | −0.11 (−0.17 to −0.04) | .002 |
| C18:2 | −0.06 (−0.14 to 0.02) | .16 |
| Acylcarnitine-to-FFA ratio | ||
| C14 acylcarnitine:C14FFA | −0.02 (−0.09 to 0.05) | .61 |
| C16 acylcarnitine:C16 FFA | −0.09 (−0.17 to −0.008) | .03 |
| C18 acylcarnitine:C18FFA | −0.09 (−0.15 to −0.04) | .002 |
Multivariable model adjusted for age, sex, CD4+ count at enrollment (square root transformed), duration of antiretroviral treatment, smoking status, and fat mass index. The dependent variable (HOMA2) was log-transformed. Regression coefficients represent the change in log(HOMA2) from the 25th to the 75th percentile of the metabolite. p-Values <.05 are shown in bold.
The results for all measured metabolites are shown in Supplementary Table S2.
C5 valeryl was excluded from the multivariable analyses as 29 subjects (41%) had a level below the 0.0025 μM limit of detection.
CI, confidence interval; FFA, free fatty acid.
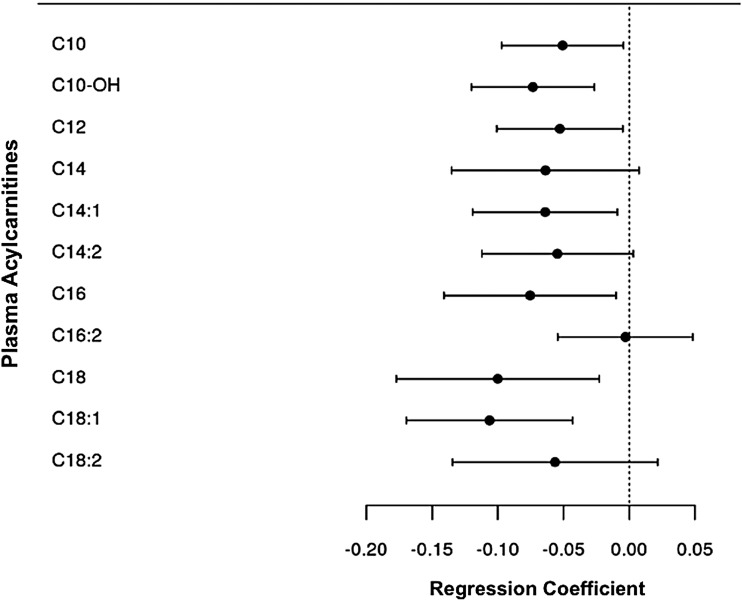
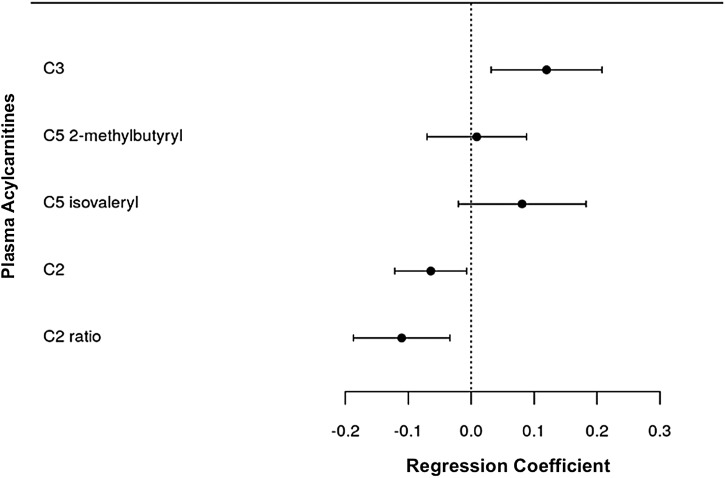
Lower plasma concentrations of C10 (p = .04), C12 (p = .03), C14:1 (p = .03), C16 (p = .03), C18 (p = .01), and C18:1 acylcarnitines (p = .002) were associated with insulin resistance, whereas lower concentrations of C14 acylcarnitine trended toward significance (Table 3 and Fig. 1). Lower plasma C2 acylcarnitine concentrations, the end degradation product of acylcarnitine catabolism, were also significantly associated with insulin resistance (p = .03), as was the C2:C3+C5 acylcarnitine ratio (p = .006) (Fig. 2). None of the measured organic acids, including lactate, pyruvate, and α-ketoglutarate, was significantly associated with insulin resistance in the model. Regression models for all metabolites included in the study are shown in Supplementary Table S2.
FIG. 1.
Forest plot of multivariable regression coefficients for medium and long-chain acylcarnitines and insulin resistance. Model adjusted for age, sex, CD4+ count at enrollment (square root transformed), duration of antiretroviral treatment, smoking status, and fat mass index. The dependent variable (HOMA2) was log-transformed. HOMA, Homeostasis Model Assessment.
FIG. 2.
Forest plot of multivariable regression coefficients for C2, C3, and C5 acylcarnitines and insulin resistance. Model adjusted for age, sex, CD4+ count at enrollment (square root transformed), duration of antiretroviral treatment, smoking status, and fat mass index. The dependent variable (HOMA2) was log-transformed.
Lastly, we calculated the acylcarnitine-to-FFA ratio for C14, C16, and C18 carbon length chains as an indirect measurement of fatty acid oxidation. In multivariable models, lower C16 and C18 acylcarnitine:FFA ratio was associated with insulin resistance (p = .03 and p = .002, respectively), whereas the C14 ratio did not meet significance (Table 3). These findings suggest that reduced conversion of FFA to acylcarnitines, either due to lower uptake and/or β-oxidation of available FFA, is a feature of insulin resistance in HIV-positive persons.
In sensitivity analyses, results were similar when BMI was replaced with FMI or when thymidine analog exposure or HCV status were included in the multivariable regression models. Furthermore, while statin use was an exclusion criterion, four subjects were receiving omega-3 fish oil supplements or fenofibrate; results were similar when models were adjusted for exposure to these medications.
Discussion
While there has been a significant reduction in AIDS-related illness with the introduction of combination ART, noncommunicable diseases, including diabetes mellitus, have emerged as an important source of morbidity and mortality in the HIV population. Although several studies have examined the unique metabolite profile that accompanies insulin resistance in HIV-negative cohorts, few have investigated similar profiles in HIV-positive populations. Our study found that lower concentrations of plasma medium and long-chain acylcarnitines are a consistent feature of insulin resistance in persons with HIV. However, we did not observe an association with higher circulating BCAA concentrations or C5 acylcarnitines, as previously demonstrated in multiple HIV-negative cohorts, suggesting that different perturbations of bioenergetic pathways may contribute to insulin resistance in HIV-positive persons.
Multiple studies have shown a strong association between elevated concentrations of circulating BCAA and insulin resistance in both animal models and HIV-negative humans.13,30,31 These underlying changes in amino acid levels can precede the development of diabetes mellitus by several years and improve the predictive model over standard clinical factors alone.14,16 Infusion of amino acids into healthy individuals induces insulin resistance, likely mediated by inhibition of glucose transportation and/or phosphorylation resulting in decreased glycogen synthesis in skeletal muscle.32 Newgard et al. found that rats fed a high-fat diet and BCAA were more insulin resistant and had higher concentrations of BCAA-derived C3 and C5 acylcarnitines in skeletal muscle than standard chow or high-fat diet alone.13 However, BCAA supplementation did not induce insulin resistance in absence of high-fat intake, suggesting that excessive fat intake contributes to changes in BCAA metabolism and interference with downstream signaling.13
While altered lipid metabolism is common in diabetes and has been implicated in glucose dysregulation and development of insulin resistance,19 the role of acylcarnitines in the pathogenesis of insulin resistance is an area of continued study. Plasma concentrations of medium and long-chain acylcarnitine species in HIV-negative diabetic individuals are elevated relative to controls.33,34 Acylcarnitines remain elevated in insulin-resistant and diabetic individuals even with exposure to glucose and insulin,34 which is thought to arise from reduced capacity of skeletal muscle to efficiently switch from predominantly fatty acid oxidation during fasting to predominantly glucose oxidation after feeding and in the presence of insulin, a term coined “metabolic inflexibility.”35 In one postulated model linking BCAA and insulin resistance, decreased adipose tissue uptake of BCAA in the setting of overnutrition leads to higher concentrations of circulating BCAA and subsequent uptake in skeletal muscle and liver, resulting in catabolic intermediates that reduce the efficiency of lipid metabolism with accumulation of incompletely oxidized fatty acid products which promote mitochondrial stress and subsequent insulin resistance.15 This is supported by studies demonstrating increased but incomplete beta oxidation in the presence of high circulating FFA in insulin-resistant persons and animal models.34,36 Another possible mechanism is through direct BCAA activation of the mTOR pathway that phosphorylates insulin receptor substrate-1 and insulin receptor substrate-2, resulting in peripheral tissue insulin resistance.37 This in turn increases insulin demand to maintain glucose homeostasis and over time, along with accumulation of toxic metabolites, has deleterious effects on the pancreatic β cells leading to exhaustion.37 Finally, BCAA may act directly on β cells to promote hyperinsulinemia that over time, contributes to β cell stress.13
In contrast to previous studies in HIV-negative populations and animal models, we observed far more consistent associations between insulin resistance and low medium- and long-chain acylcarnitines in our HIV-positive subjects as compared with plasma BCAA and BCAA oxidation products. Based on our findings, we do not discount that an association between insulin resistance and BCAA or BCAA oxidation products would have emerged if our cohort had been larger or had more severe glucose intolerance. However, we hypothesize that impaired FFA uptake and/or fatty acid oxidation, resulting in low concentrations of plasma acylcarnitines, is either an early or more prominent feature of insulin resistance in HIV-positive persons. The reduced acylcarnitine:FFA ratio in insulin-resistant subjects supports this hypothesis and a prior study examining lipid metabolism in subjects with HIV on ART found that compared with HIV-seronegative individuals, individuals with HIV had lower increases of lipolysis during exercise with decreased fatty acid oxidation.38 Of note, our findings are similar to a metabolomics study performed in HIV-negative obese adolescents, which found that lower concentrations of plasma fatty acid oxidation byproducts were associated with insulin resistance.30
In the HIV literature, there are few studies at present to compare our results. Infants exposed to ART had increased levels of some plasma acylcarnitines relative to unexposed infants,25 and a more recent study of HIV-exposed uninfected infants showed significant abnormalities in plasma acylcarnitines.39 A recent study found plasma C3 and C8-acylcarnitines were decreased in HIV-positive rapid progressors and correlated with both CD4+ T cell counts and mycobacterium acellular infections, suggesting that multiple factors could affect acylcarnitine levels in the HIV population.24 We postulate that several risk factors unique to this population, including chronic systemic inflammation,10 alteration in lipid metabolism with prolonged exposure to ART,9 and changes in adipose tissue inflammation and T cell profiles in HIV infection,40–42 could explain the different metabolite profile accompanying insulin resistance in HIV-negative versus HIV-positive persons.
Our study had several limitations. The small sample size may have limited the power to detect changes in circulating metabolite concentrations between insulin-sensitive and insulin-resistant individuals. This could explain why we did not detect an association of circulating BCAA concentrations with insulin resistance, but did find an association with C3 acylcarnitine, a common catabolic intermediate. Second, subjects in this study were on EFV/TDF/FTC, which may limit the generalization of the results to other regimens. A sensitivity analysis adjusting for previous thymidine analog exposure did not change the main results of this study. Third, we used HOMA2-IR as a surrogate measure for insulin sensitivity rather than employing a euglycemic–hyperinsulinemic clamp, although, studies have validated the use of HOMA2 for clinical studies.43 Fourth, we measured plasma metabolite concentrations, which may not accurately reflect substrate utilization at the cellular level in tissues important for insulin resistance. Fifth, lack of an HIV-negative control population in our study kept us from directly comparing differences in metabolite associations between HIV-positive and HIV-negative individuals. However, several large trials of HIV-negative individuals, including the Framingham cohort, have consistently found an association of BCAA with insulin resistance, a finding that appears well before the onset of diabetes mellitus and is one of the earliest signs of insulin resistance. Our methodology used targeted mass spectrometry with a reference standard, making spurious metabolite values unlikely compared with a nontargeted approach. Lastly, we did not adjust for multiple comparisons, which may have led to a rejection of the null hypothesis by chance. However, all of the medium and long-chain acylcarnitines were either below p < .05 or near significance suggesting a clear trend that would make spurious findings unlikely.
To our knowledge, this is the first study to characterize the association of metabolite profiles in HIV-positive individuals with insulin resistance. We found that contrary to studies in HIV-negative individuals, lower concentrations of circulating acylcarnitines were associated with insulin resistance, but not higher concentrations of plasma BCAA. While further studies are needed, these findings suggest metabolomics could be used to identify biomarkers of insulin resistance in people with HIV before the onset of hyperglycemia, and potentially lead to new therapeutic targets unique to this population. As persons with HIV can now survive decades on ART, understanding the pathophysiology and unique aspects of comorbid diseases in the HIV population will be central for preventative treatment and management of complications to improve long-term health outcomes.
Supplementary Material
Acknowledgments
The authors thank the participants in the Adiposity and Immune Activation Cohort study (AIAC study). This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) grants K23 AI100700 and K24 AI65298, and the Tennessee Center for AIDS Research grant P30 AI110527, the Vanderbilt Clinical and Translational Science grant UL1TR000445 from the National Center for Advancing Translational Sciences, and the Southeast Center for Integrated Metabolomics grant U24DK097209, and grant R01DK112262, from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
These results were presented in part at the 77th American Diabetes Association Scientific Session, June 9–13, 2017, San Diego, CA, Abstract No. 1730-P.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Hadigan C, Meigs JB, Corcoran C, et al. : Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis 2001;32:130–139 [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, Furrer H, Rickenbach M, et al. : Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–119 [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon S, Carr A: Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352:48–62 [DOI] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, et al. : Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–1608 [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, et al. : Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med 2005;165:1179–1184 [DOI] [PubMed] [Google Scholar]
- 6.Holmberg SD, Moorman AC, Williamson JM, et al. : Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002;360:1747–1748 [DOI] [PubMed] [Google Scholar]
- 7.Herrin M, Tate JP, Akgün KM, et al. : Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016;73:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. : HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr A, Samaras K, Chisholm DJ, Cooper DA: Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 1998;351:1881–1883 [DOI] [PubMed] [Google Scholar]
- 10.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA: Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010;33:2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takemoto JK, Miller TL, Wang J, et al. : Insulin resistance in HIV-infected youth is associated with decreased mitochondrial respiration. AIDS 2017;31:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SH, Bain JR, Muehlbauer MJ, et al. : Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010;3:207–214 [DOI] [PubMed] [Google Scholar]
- 13.Newgard CB, An J, Bain JR, et al. : A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Vasan RS, et al. : Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newgard CB: Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Würtz P, Soininen P, Kangas AJ, et al. : Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013;36:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tillin T, Hughes AD, Wang Q, et al. : Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015;58:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randle PJ: Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998;14:263–283 [DOI] [PubMed] [Google Scholar]
- 19.McGarry JD: Banting lecutre 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 20.Scarpellini B, Zanoni M, Sucupira MCA, et al. : Plasma metabolomics biosignature according to HIV stage of infection, pace of disease progression, viremia level and immunological response to treatment. PLoS One 2016;11:e0161920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickens AM, Anthony DC, Deutsch R, et al. : Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS 2015;29:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D: Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 2013;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koethe JR, Jenkins CA, Petucci C, Culver J, Shepherd BE, Sterling TR: Superior glucose tolerance and metabolomic profiles, independent of adiposity, in HIV-infected women compared with men on antiretroviral therapy. Medicine 2016;95:e3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waagsbø B, Svardal A, Ueland T, et al. : Low levels of short- and medium-chain acylcarnitines in HIV-infected patients. Eur J Clin Invest 2016;46:408–417 [DOI] [PubMed] [Google Scholar]
- 25.Kirmse B, Hobbs CV, Peter I, et al. : Abnormal newborn screens and acylcarnitines in HIV-exposed and ARV-exposed infants. Pediatr Infect Dis J 2013;32:146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy JC, Matthews DR, Hermans MP: Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 27.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA: Height-normalized indices of the body's fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am J Clin Nutr 1990;52:953–959 [DOI] [PubMed] [Google Scholar]
- 28.Wells JC, Cole TJ; ALSPAC Study Team: Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 2002;26:947–952 [DOI] [PubMed] [Google Scholar]
- 29.Ko H, Royer ME: A gas-liquid chromatographic assay for plasma free fatty acids. J Chromatogr 1974;88:253–263 [DOI] [PubMed] [Google Scholar]
- 30.Newbern D, Balikcioglu PG, Balikcioglu M, et al. : Sex differences in biomarkers associated with insulin resistance in obese adolescents: Metabolomic profiling and principal components analysis. J Clin Endocrinol Metab 2014;99:4730–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffman KM, Shah SH, Stevens RD, et al. : Relationships between circulating metabolic intermediates and insulin action in overweight to obese, intact men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krebs M, Krssak M, Bernroider E, et al. : Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002;51:599–605 [DOI] [PubMed] [Google Scholar]
- 33.Adams SH, Hoppel CL, Lok KH, et al. : Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihalik SJ, Goodpaster BH, Kelley DE, et al. : Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley DE, Mandarino LJ: Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 36.Koves TR, Ussher JR, Noland RC, et al. : Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 37.Lynch CJ, Adams SH: Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cade WT, Reeds DN, Mittendorfer B, et al. : Blunted lipolysis and fatty acid oxidation during moderate exercise in HIV-infected subjects taking HAART. Am J Physiol Endocrinol Metab 2007;292:E812–E819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirmse B, Yao TJ, Hofherr S, et al. : Acylcarnitine profiles in HIV-exposed, uninfected neonates in the United States. AIDS Res Hum Retroviruses 2016;32:339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couturier J, Suliburk JW, Brown JM, et al. : Human adipose tissue as a reservoir for memory CD4 T cells and HIV. AIDS 2015;29:667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal N, Iyer D, Patel SG, et al. : HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci Transl Med 2013;5:213ra164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damouche A, Lazure T, Avettand-Fènoël V, et al. : Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 2015;11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonora E, Targher G, Alberiche M, et al. : Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.