Background
Neuromyelitis optica spectrum disorder (NMOSD) is a severe autoimmune disease of the optic nerve, spinal cord, and, less frequently, brain.1 Severity and degree of recovery from relapses are the factors that determine long-term visual and motor disability and mortality.1 Mortality rates in NMOSD worldwide range from 9% to 32%, depending on age, relapse rate, and recovery from attacks.2,3 In examining mortality data from 2 large, ethnically diverse NMOSD Centers in the Mid-Atlantic United States, we observed a striking race distribution: most deceased patients were of African ancestry. In this analysis, we focus on race as a risk factor for mortality in NMOSD.
Methods
This is a retrospective study of all patients with NMOSD seen at 2 large US-based clinics: Johns Hopkins Hospital (Baltimore, MD) and New York University (New York, NY). NMOSD was defined by the 2015 International Panel of NMO Diagnosis.4 Race was patient reported, whereas all other clinical and demographic factors, including the cause of death, were confirmed by site investigators. Patients not seen in the previous 12 months were called to verify living status. Time to diagnosis, frequency of clinic/hospital visits, and treatment regimen for relapses acted as surrogates of health care access. Institutional review boards from both institutions approved this study.
Results
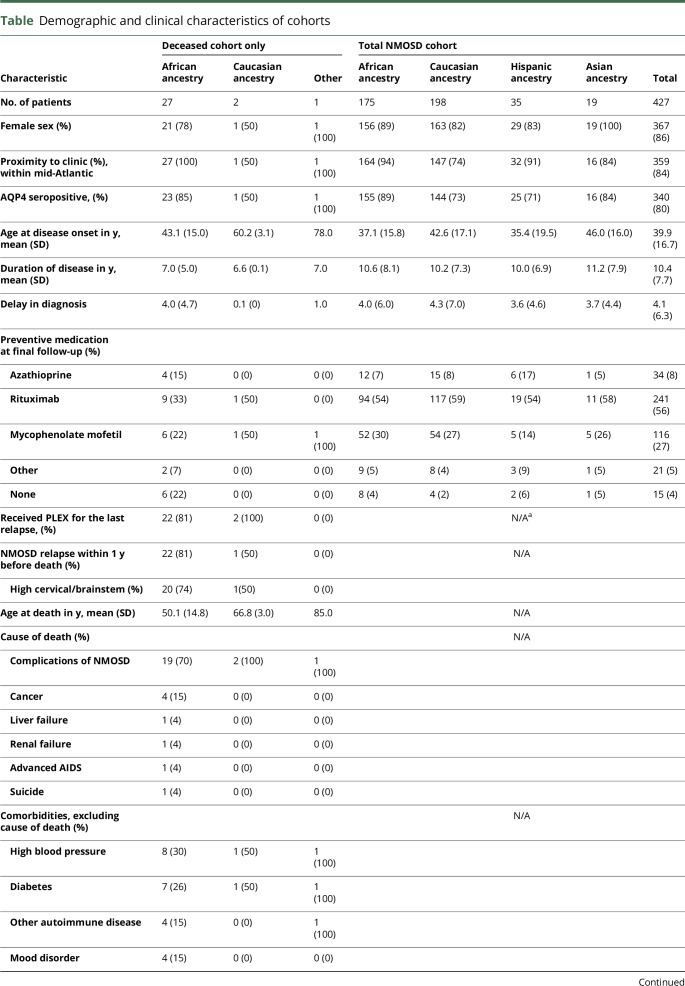
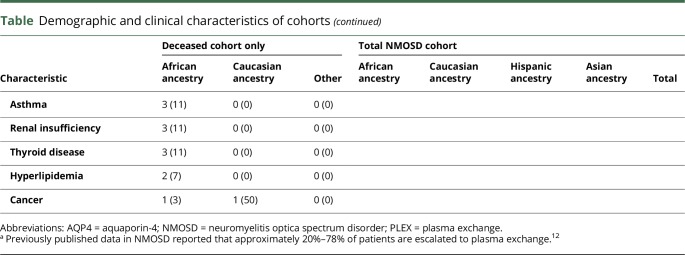
A total of 427 NMOSD patients were included in this analysis, 328 from Johns Hopkins Hospital and 99 from New York University. In total, 30 patients died during follow-up (table), with an annual mortality rate of 0.68 deaths per 100 patient-years. The mean disease duration at time of death was 6.9 years. Patients of African ancestry constituted 41% of our clinic population, but they comprised 90% of the deceased NMOSD patients, with average age at death of 52.3 years. The other 3 deceased patients included an Asian woman aged 85 years, a Caucasian man aged 69 years, and a Caucasian woman aged 65 years. The overall mortality rate in our total cohort was 7.0%, and among those of African ancestry was 15.4% (p < 0.0001).
Table.
Demographic and clinical characteristics of cohorts
Patients in each race group were similar regarding age, sex, aquaporin-4 serostatus, time to diagnosis, acute treatment care, and access to our clinics (table). Although more deceased patients were untreated at final follow-up (22% vs. 4%), there was no difference in treatment rates among the races.
In 22 of 30 deceased patients (73%), cause of death was related to NMOSD (table). Most deaths, 70%, were preceded by a relapse in the brainstem and/or upper cervical spinal cord within the previous 12 months despite preventive medications in 80% of patients at the time of the fatal relapse.
Discussion
Our study involved a very large patient sample—427 patients from 2 large specialized NMO centers. The overall mortality rate was 7.0% (30 of 427 patients). This rate is slightly lower than in contemporary studies (9%–13%)3,5 and considerably improved from older landmark studies (22%–32%).2,6,7 The decrease in mortality over time is likely due to earlier diagnosis, use of plasmapheresis for acute relapses, and preventive immunotherapies, which have been shown to decrease relapse rates in observational studies.8 It is important that the definition of NMOSD has changed over the past 2 decades, allowing for milder cases to be diagnosed. Thus, the decrease in mortality could also be in part a technical artifact (“Will Rogers effect”).
The most striking finding of this study is the observation that nearly all the deceased patients in our combined cohort were of African ancestry. Patients of African ancestry make up 41% of the total cohort, but account for 90% of the mortality. This is unlikely due to chance (p < 0.0001, Fisher test) or to differences in delay in diagnosis, clinic access, or treatment (table). This is also unlikely to be due to differences in death rates among races: according the CDC, mortality rate among those of African race was 0.8% and 0.7% among Caucasians (2009–2014). One other study has identified African ancestry as a strong predictor of mortality in NMOSD. In Brazil, where the estimated mortality rate among all patients with NMOSD is 23%, the mortality rate among Afro-Brazilians is a staggering 58%.9 Two European studies did not implicate African race as a risk factor for mortality, but the proportion of African patients in their cohorts was much lower than in the Eastern US and Brazilian NMOSD cohorts.2,10,11 Our results have important implications for management of patients of African ancestry with NMOSD. Further studies, especially prospective studies assessing factors that affect the severity of relapses, may shed light on the high risk of death among patients of African ancestry with NMOSD.
Author contributions
M. Levy, R.A. Kessler, M.A. Mealy, G. Cutter, and I. Kister contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting of the manuscript.
Study funding
This study was funded by the NIH, K08 NS078555 (M. Levy).
Disclosure
M.A. Mealy received speaker honoraria from the Consortium of Multiple Sclerosis Centers and research support from the NIH/NCATS. R.A. Kessler, Z. Rimler, A. Reid, and L. Totonis report no disclosures. G. Cutter served on the scientific advisory board of AMO Pharmaceuticals, Apotek, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck, Merck/Pfizer, Opko Biologics, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva, NHLBI, and NICHD; received speaker honoraria from the Consortium of Multiple Sclerosis Centers and, Teva; served on the editorial board of Multiple Sclerosis, JASN, and Alzheimer's & Dementia: Translational Research & Clinical Interventions; is president of and holds stock in Pythagoras; consulted for Argenx BVBA, Atara Biotherapeutics, Consortium of Multiple Sclerosis Centers, Genzyme, Genentech, Innate Therapeutics, Janssen Pharmaceuticals, Klein-Buendel Incorporated, MedImmune, Medday, Nivalis, Novartis, Opexa Therapeutics, Roche, Savara Inc, Somahlution, Teva, Transparency Life Sciences, and TG Therapeutics; participates in the NARCOMS MS Patient Registry funded by the CMSC; received research support from the NIH/NINDS, NIH/NIAID, UAB Center for AIDS Research, NIH/NHLBI, NIH/NICHD, NIH/NIA, US Department of Defense, NIH/MIAMS Children's Hospital (Boston), Consortium of MS Centers, and Myasthenia Gravis Foundation of America; and reviewed statistical data for Galderma Litigation. I. Kister received served on the scientific advisory board of Biogen IDEC MS Franchise Data Generation and Genentech; consulted for Biogen Idec; and received research support from Biogen Idec, Serono, Novartis, Genzyme, Guthy-Jackson Charitable Foundation, and National Multiple Sclerosis Society. M. Levy served on the scientific advisory board of Asterias, Chugai, and Alexion; is on the editorial board of Multiple Sclerosis and Related Disorders; holds patents for Aquaporin-4 sequence that elicits pathogenic T cell response in animal model of neuromyelitis optica and Use of peptide for diagnostic and therapeutic developments; consulted for Guidepoint Global, Gerson Lehrman Group, and Cowen Group; and received research support from ViroPharma/Shire, Acorda, AcoPharma, Sanofi, Genzyme, Alnylan, Alexion, Terumo BCT, and NINDS. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol 2012;69:1176–1180. [DOI] [PubMed] [Google Scholar]
- 2.Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012;135:1834–1849. [DOI] [PubMed] [Google Scholar]
- 3.Collongues N, Marignier R, Jacob A, et al. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler J 2014;20:1086–1094. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabre P, González-Quevedo AG, Bonnan M, et al. Relapsing neuromyelitis optica: long term history and clinical predictors of death. J Neurol Neurosurg Psychiatry 2008;80:1162–1164. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107–1114. [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology 2003;60:848–853. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol 2016;18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papais-Alvarenga RM, Carellos SC, Alvarenga MP, Holander C, Bichara RP, Thuler LC. Clinical course of optic neuritis in patients with relapsing neuromyelitis optica. Arch Ophthalmol 2008;126:12–16. [DOI] [PubMed] [Google Scholar]
- 10.Chan KH, Lee R, Lee JCY, et al. Central nervous system inflammatory demyelinating disorders among Hong Kong Chinese. J Neuroimmunol 2013;262:100–105. [DOI] [PubMed] [Google Scholar]
- 11.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 2012;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler 2016;22:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]