Highlights
-
•
Virtual surgical planning (VSP) and rapid prototyping in complex chest wall resection and reconstruction are valuable tools to the surgical team.
-
•
VSP and 3D printing allow for rehearsal of the surgical plan with emphasis on technical details that are paramount to the success of the reconstruction.
-
•
Accessibility and cost remain the key limiting factors in the clinical use of this technology. Outcome-based studies and cost-analysis are needed to validate the efficacy of 3D printing in complex surgical care.
Keywords: Sternal reconstruction, Case report, Sternal sarcoma, Virtual surgical planning, Three-dimensional printing
Abstract
Introduction
Primary sarcomas of the sternum are extremely rare and present the surgical teams involved with unique challenges. Historically, local muscle flaps have been utilized to reconstruct the resulting defect. However, when the resulting oncologic defect is larger than anticipated, local tissues have been radiated, or when preservation of chest wall muscles is necessary to optimize function, local reconstructive options are unsuitable.
Presentation of case
Virtual surgical planning (VSP) and in house three-dimensional (3D) printing provides the platform for improved understanding of the anatomy of complex tumours, communication amongst surgeons, and meticulous pre-operative planning. We present the novel use of this technology in the multidisciplinary surgical care of a 35 year old male with primary sarcoma of the sternum. Emphasis on minimizing morbidity, maintaining function of chest wall muscles, and preservation of the internal mammary vessels for microvascular anastomosis are discussed.
Discussion
While the majority of patients at our institution receive local or regional flaps for reconstruction of thoracic defects, advances in microvascular surgery allow the reconstructive surgeon the latitude to choose other flap options if necessary. VSP and 3D printing allowed the surgical team involved to utilize free tissue transfer to reconstruct the defect with free tissue transfer from the thigh. Perseveration of the internal mammary vessels was paramount during tumor extirpation.
Conclusion
Virtual surgical planning and rapid prototyping is a useful adjunct to standard imaging in complex chest wall resection and reconstruction.
1. Introduction
Surgical management of locally advanced chest wall tumor necessitates a multidisciplinary team approach including thoracic surgery, plastic surgery, radiation oncologists, and pulmonary medicine. The primary goal of the oncologic surgeon is to obtain an R0 resection. To achieve this goal, oftentimes chest wall structures such as the pectoralis major muscles in proximity to the tumor may be involved in the resection margin. Local blood vessels, such as the internal mammary artery and vein may also be in proximity to the tumor and preservation of these vessels is paramount to allow microvascular free tissue transfer for reconstruction [1]. The primary goal of the plastic surgeon is to provide the patient with a durable reconstruction with minimal morbidity, preserve pulmonary function dynamics, and allow expedient healing should adjuvant therapies be required. Advances in thoracic imaging and virtual surgical planning over the past decade have allowed high-fidelity reproduction of chest wall tumours and structures involved in the ablative and reconstructive surgery. High-resolution computed tomography (CT), CT-angiography and magnetic resonance imaging (MRI) allows the reproduction of a three dimensional (3D) model of the tumor with pertinent anatomic structures and vasculature. This process also facilitates multidisciplinary virtual surgical planning (VSP) involving the thoracic and reconstructive surgeons. The discussion of anticipated margins for an R0 resection and the plan for soft tissue and skeletal reconstruction of the chest wall is had among the surgeons pre-operatively. Accordingly, identification of suitable flap options, such as the pectoralis major muscle, rectus abdominis, or latissimus dorsi muscle, is also planned prior to surgery. The patient is informed of the different options available for reconstruction and the implications of such flap options on post-operative recovery, morbidity and possible life-style changes. If free tissue transfer is planned, identification of suitable recipient vessels is planned ahead of the resection with the emphasis on preservation of recipient vessels. While this planning can be theoretically conceived using standard imaging, a high-fidelity 3D printed model allows the surgeons involved in exploring pertinent details of the surgical plans [2], [3]. As 3D printing technology continues to evolve, cost-effectiveness, accessibility and feasibility continue to increase. A recent move towards rapid prototyping in the hospital (in house) allows the surgeons close access to the radiologists and bioengineers. Complex surgical plans can be conceived and 3D printed models efficiently manufactured without the cost and time delay associated with outsourcing to third parties. This not only helps in cost-cutting, but also allows quick turnaround from surgical plan conception to 3D model production [4]. VSP and 3D printing technology has been utilized for a multitude of craniofacial, head and neck, orthopaedic, cardiac, vascular and general surgical applications [2], [5], [6]. Traditionally, outsourcing of the planning process and 3D printing to for-profit third party companies has been argued as a downside to the wide spread of this technology due to added expenses, increased labour requirement, time needed for planning and time inefficiency in prototype production. We have adopted this technology at our institution (in house) to facilitate VSP, rapid prototyping and delivery of the 3D model in less than 48 h. This has allowed for multiple services to utilize this technology in managing complex cases across multiple specialties [1], [7]. The work has been reported in line with the SCARE criteria [8].
2. Presentation of case
We present a 35 year old mechanic with spindle cell sarcoma of the sternum who presented with a 3.7 × 3.1 × 7.0 cm sternal mass on chest CT (Fig. 1). The patient underwent neoadjuvant chemoradiation. The tumor had a mild response to neoadjuvant therapy. VSP and 3D printing of the tumor and anterior chest wall was performed at our facility. Oncological resection was planned to include enbloc sternal resection with portions of ribs 3–8 along with sternal origin of pectoralis major muscle. Given prior radiation of the anterior chest wall and physically demanding occupation of the patient, free tissue transfer of the antero-lateral thigh flap was planned in lieu of bilateral pectoralis major muscle flaps or rectus abdominis flap reconstruction to minimize morbidity to his chest wall and abdomen. After VSP, a 3D printed model was made in house, showing the relationship of the internal mammary vessels (IMV) to the tumor on both sides (Fig. 2). The tumor was centered on the right IMVs with anticipated involvement of the distal left IMVs. The 3D printed model facilitated outlining the surgical plan and communication between the thoracic and plastic surgeons pre-operatively with emphasis on preservation of the left IMVs for microvascular tissue transfer. A subtotal inferior sternectomy resulted in a large anterior chest wall defect measuring 13 × 8 cm resulted. Semi-rigid reconstruction of the sternum proceeded with Gore® DUALMESH® and the soft tissue defect in this radiated bed was reconstructed with an anterolateral thigh flap. (Fig. 3) The Gore-Tex mesh used was a standard mesh and not 3D printed for this specific case. However, the 3D model helps in estimating the size of the mesh needed and ensuring the appropriate size mesh is pre-ordered for the case.
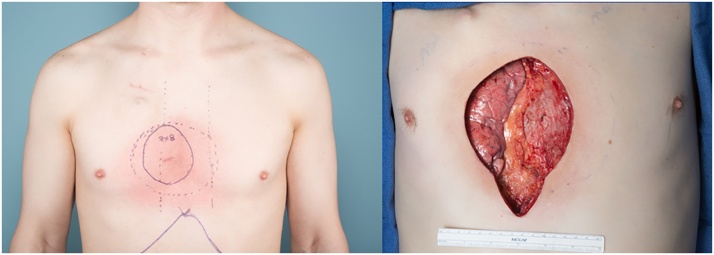
Fig. 1.
Left: Pre-operative photo of the patient showing the anticipated size of the defect. Note the radiation skin changes. Right: the surgical defect following the oncologic resection resulted in subtotal sternectomy, resection of ribs and sternal origins of bilateral pectoralis major muscles.
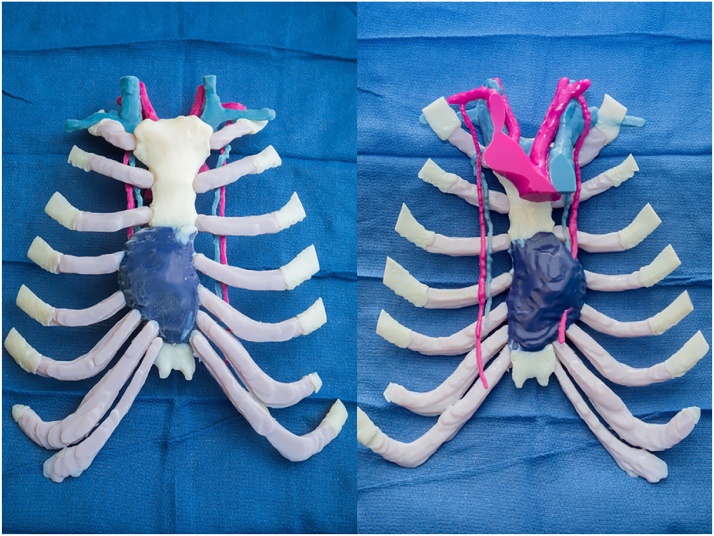
Fig. 2.
Left: In house 3D-printed model of the sternal tumor. Right: Posterior view showing the relationship of the tumor to the internal mammary vessels (IMV) on both sides. The tumor was centered on the right IMVs. The left proximal IMVs were used for microvascular reconstruction.
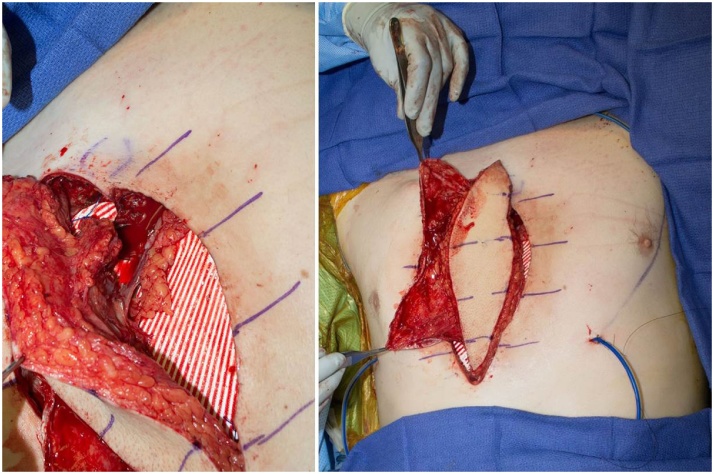
Fig. 3.
Left: The microvascular anastomoses are shown prior to flap inset over mesh (white).Right: The ALT flap fascia was used as an additional vascular layer to cover the Gore-Tex mesh (white).
3. Discussion
Our pathway for reconstructing patients with large chest wall defects begins with preoperative imaging using a high-resolution CT scan. The majority of patients at our institution receive local or regional flaps for reconstruction. Advances in microvascular surgery allow the reconstructive surgeon to choose other flap options if necessary. Microsurgery is more technically demanding and complex than local flap reconstruction. To adopt microvascular surgery as an option for such complex reconstruction, communication and meticulous pre-operative planning between the different surgical specialities involved is a must. At our institution, 3D printing is pursued in selective patients based on the complexity of the case and anticipated surgery. High resolution CT scans of the chest were imported into Mimics software (Materialise, Belgium) and the sternal images were segmented. The segmented 3D volume was then transferred to 3-Matic (Materialise, Belgium) where an .stl file was created. This file was then transferred into Pro-plan (Materialise, Belgium) where the virtual surgical planning took place. The models were 3D printed using material jetting on an objet 500 Connex3 (Stratasys, Minnesota). Data processing and segmentation is performed by a bio-engineering specialist and radiologist. Virtual surgical planning is performed by a bio-engineering specialist and the surgeon. The entire process takes an average of 4–6 h depending on anatomical complexity. The 3D model printing takes an average of 5–40 h depending on the size of the model (i.e. anterior chest wall vs. entire thoracic cage). The 3D model consists of the bony skeleton of the chest, the tumor, relevant flap anatomy, and the recipient or other pertinent blood vessels. Since the entire process is done in-house, direct communication between the surgeons and the radiologist is easy and enables more efficient communication regarding the surgical guides and model. The 3D printed model is then used for patient education and counselling regarding tumor resection and flap options pre-operatively. Furthermore, the 3D model helps in planning of the resection margins, and the use of cutting guides, when needed, improve precision in bony osteotomies. Having a physical model to inspect prior to surgery helps the surgeons in delineating greater details regarding involved structures, vasculature, anticipated size of the defect, estimating the size of the mesh needed and in reviewing the reconstructive requirements, which are done prior to the procedure. We find VSP and the 3D model especially useful in planning uncommon complex multidisciplinary surgical cases. Flap options (local vs. reginal vs. free flap) are discussed with the patient considering their professional requirements, hobbies and sports. Localization of suitable recipient blood vessels and their proximity to tumor are outlined. The patient recovered well from the operation and achieved a durable reconstruction with low morbidity (Fig. 4). Resection and reconstruction of large chest wall defects is challenging and requires a multidisciplinary team approach. Intra-operative findings may necessitate wider resection margins than expected and local reconstructive options may be limited. In our case, the patient was a very active mechanic who lifted 20–30 pound machinery routinely. To preserve chest and abdominal strength, a free tissue transfer from the lateral thigh was deemed less morbid and planned for coverage of the Gore-Tex mesh and reconstruction of the defect. Furthermore, neoadjuvant radiation of the anterior chest wall made local flap options and skin quality less optimal for local flap advancement. The main challenge in this case was locating suitable vessels for flap inset. The IMVs are the most suitable option. Other options include intercostal perforators, which are less reliable, thoracoacromial vessels, or neck vessels. The further the defect is from recipient vessels, the more likelihood it is for vein grafts. This adds to the complexity of the microvascular reconstruction. In this particular patient, VSP and 3D modeling of the tumor at our facility allowed precise surgical planning and the identification of potential recipient vessels based on tumor location and anticipated resection margins. A multidisciplinary approach allowed careful dissection of the tumor and preservation of the IMV for microvascular tissue transfer. The IM artery diameter measured 1.5 mm and the IM vein measured 3.5 mm. The ALT flap was elevated simultaneously with the ablative procedure, which translated to shorter operative time. An end to end hand-sewn arterial anastomosis was performed between the IMA and the flap artery (2.5 mm) and a single venous anastomosis was performed between the IMV and one of the vena comitantes with a 3.0 coupler. Arterial size mismatch between the IMA and ALT pedicle was managed by performing an oblique fish-mouth anasotmosis. When uncertainty exists as to the extent of resection or reconstruction plan, the benefits of VSP and 3D modeling are magnified. It allows the surgeons to rehearse the procedure and consider various options of reconstruction based on anticipated scenarios [9], [10].
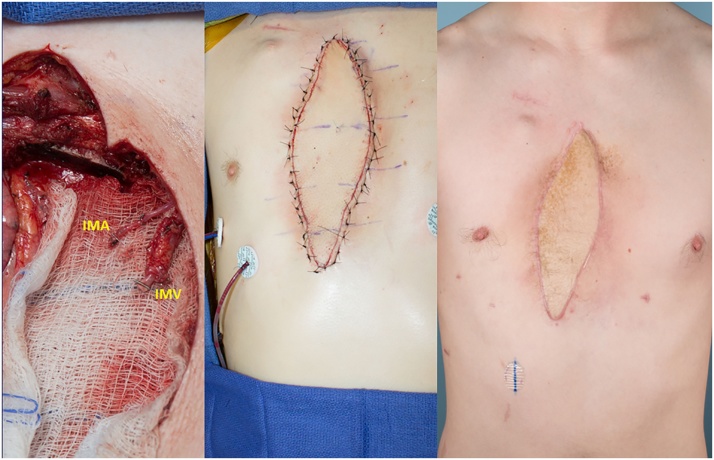
Fig. 4.
Left: The left proximal internal mammary artery (IMA) and internal mammary vein (IMV) were dissected by the plastic surgery team during oncologic resection. Center: A right anterolateral thigh flap was harvested and transferred to the chest. Right: Post-operative photo showing flap healing at six months after surgery.
4. Conclusion
Virtual surgical planning and rapid prototyping in complex chest wall resection and reconstruction is a versatile tool which provides the surgical teams an excellent adjunct to standard imaging. The 3D model allows for rehearsal of pertinent details to the surgical plan with emphasis on areas of high interest for the surgical teams involved. VSP and rapid prototyping has the potential to improve surgical outcomes by decrease operative times, having a well-thought out plan prior to complex multidisciplinary surgery. Accessibility to this technology and associated costs remain the key limiting factors in its widespread use. Future outcome-based studies and cost-analyses are needed to validate the efficacy of 3D modeling and printing in complex surgical care.
Conflicts of interest
None.
Funding
None.
Ethical approval
This is a clinical case report and is exempt from ethical approval at our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors contribution
Basel Sharaf: concept, paper writing.
Aparna Vijayasekaran: paper writing.
Diya Sabbagh: data collection, figures.
Mark Allen: Paper writing.
Registration of research studies
This is a clinical case report and not an experimental study.
Guarantor
Basel Sharaf, MD, FACS.
Contributor Information
Basel Sharaf, Email: sharaf.basel@mayo.edu.
M. Diya Sabbagh, Email: sabbagh.mohamed@mayo.edu.
Aparna Vijayasekaran, Email: appudoc@gmail.com.
Mark Allen, Email: allen.mark@mayo.edu.
Jane Matsumoto, Email: Matsumoto.Jane@mayo.edu.
References
- 1.Gillaspie E.A., Matsumoto J.S., Morris N.E., Downey R.J., Shen K.R., Allen M.S. From 3-dimensional printing to 5-dimensional printing: enhancing thoracic surgical planning and resection of complex tumors. Ann. Thorac. Surg. 2016;101(5):1958–1962. doi: 10.1016/j.athoracsur.2015.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauermeister A.J., Zuriarrain A., Newman M.I. Three-dimensional printing in plastic and reconstructive surgery: a systematic review. Ann. Plast. Surg. 2016;77(November (5)):569–576. doi: 10.1097/SAP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 3.Tan H., Yang K., Wei P., Zhang G., Dimitriou D., Xu L. A novel preoperative planning technique using a combination of CT angiography and three-dimensional printing for complex toe-to-hand reconstruction. J. Reconstr. Microsurg. 2015;31(5):369–377. doi: 10.1055/s-0035-1546419. [DOI] [PubMed] [Google Scholar]
- 4.Mendez B.M., Chiodo M.V., Patel P.A. Customized in-office three-dimensional printing for virtual surgical planning in craniofacial surgery. J. Craniofac. Surg. 2015;26(5):1584–1586. doi: 10.1097/SCS.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 5.Kurenov S.N., Ionita C., Sammons D., Demmy T.L. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J. Thorac. Cardiovasc. Surg. 2015;149(4):973–979. doi: 10.1016/j.jtcvs.2014.12.059. (e1) [DOI] [PubMed] [Google Scholar]
- 6.Zerr J., Chatzinoff Y., Chopra R., Estrera K., Chhabra A. Three-dimensional printing for preoperative planning of total hip arthroplasty revision: case report. Skeletal Radiol. 2016;45(10):1431–1435. doi: 10.1007/s00256-016-2444-1. [DOI] [PubMed] [Google Scholar]
- 7.Sharaf B., Levine J.P., Hirsch D.L., Bastidas J.A., Schiff B.A., Garfein E.S. Importance of computer-aided design and manufacturing technology in the multidisciplinary approach to head and neck reconstruction. J. Craniofac. Surg. 2010;21(4):1277–1280. doi: 10.1097/SCS.0b013e3181e1b5d8. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S. Orgill DP, for the SCARE group. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34(October):180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Gerstle T.L., Ibrahim A.M., Kim P.S., Lee B.T., Lin S.J. A plastic surgery application in evolution: three-dimensional printing. Plast. Reconstr. Surg. 2014;133(2):446–451. doi: 10.1097/01.prs.0000436844.92623.d3. [DOI] [PubMed] [Google Scholar]
- 10.Shui W., Zhou M., Chen S., Pan Z., Deng Q., Yao Y. The production of digital and printed resources from multiple modalities using visualization and three-dimensional printing techniques. In. J. Comput. Assist. Radiol. Surg. 2017;12(1):13–23. doi: 10.1007/s11548-016-1461-9. [DOI] [PubMed] [Google Scholar]