Abstract
PTEN loss has been associated with poorer prognosis in many solid tumors. However, such investigation in lymphomas is limited. In this study, PTEN cytoplasmic and nuclear expression, PTEN gene deletion, and PTEN mutations were evaluated in two independent cohorts of diffuse large B-cell lymphoma (DLBCL). Cytoplasmic PTEN expression was found in approximately 67% of total 747 DLBCL cases, more frequently in the activated B-cell–like subtype. Nuclear PTEN expression was less frequent and at lower levels, which significantly correlated with higher PTEN mRNA expression. Remarkably, loss of PTEN protein expression was associated with poorer survival only in DLBCL with AKT hyperactivation. In contrast, high PTEN expression was associated with Myc expression and poorer survival in cases without abnormal AKT activation. Genetic and epigenetic mechanisms for loss of PTEN expression were investigated. PTEN deletions (mostly heterozygous) were detected in 11.3% of DLBCL, and showed opposite prognostic effects in patients with AKT hyperactivation and in MYC rearranged DLBCL patients. PTEN mutations, detected in 10.6% of patients, were associated with upregulation of genes involved in central nervous system function, metabolism, and AKT/mTOR signaling regulation. Loss of PTEN cytoplasmic expression was also associated with TP53 mutations, higher PTEN-targeting microRNA expression, and lower PD-L1 expression. Remarkably, low PTEN mRNA expression was associated with down-regulation of a group of genes involved in immune responses and B-cell development/differentiation, and poorer survival in DLBCL independent of AKT activation. Collectively, multi-levels of PTEN abnormalities and dysregulation may play important roles in PTEN expression and loss, and that loss of PTEN tumor-suppressor function contributes to the poor survival of DLBCL patients with AKT hyperactivation.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common and heterogeneous type of B-cell lymphoma. Gene expression profiling (GEP) has classified DLBCL into two molecularly distinctive subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) types, with gene expression profiles resembling those of normal germinal center B cells and those of mitogenically activated blood B cells, respectively [1].
The current standard regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has clearly improved the outcome of DLBCL patients over the past decades [2], but because some patients with refractory disease or with early relapse still have worse outcomes [3], further clarification of disease subgroups with distinct pathology mechanisms is needed. Recent studies showed that the phosphatidylinositol-3 kinase (PI3K)-AKT pathway was constitutively activated in 25-52% of DLBCL [4], [5], which prompted us to study the significance of PTEN (phosphatase and tensin homologue), a major negative regulator of the PI3K/AKT signaling, in the pathogenesis of DLBCL. PTEN antagonizes PI3K signaling through dephosphorylation of phosphoinositide-3-phosphate (PIP3). PTEN deficiency leads to PIP3 accumulation and thereby de-repression of the PI3K/AKT pathway, which in turn promotes cell growth, proliferation, angiogenesis, and other cellular processes [6].
The phosphatase activities of PTEN in the plasma membrane are finely regulated by complex mechanisms. Dynamic PTEN binding to the plasma membrane, as a critical step for PI3K signaling inhibition by PTEN, is determined by local PIP2 and PIP3 gradients [7], [8] and PTEN conformation which is regulated by posttranslational modifications such as phosphorylation, ubiquitination, acetylation, and SUMOylation. Phosphorylation of the C-terminal tail prevents PTEN from membrane binding and keeps PTEN inactive in the cytoplasm [8], [9].
PTEN localizes not only to the cytoplasm but also to the nucleus and other subcellular compartments [8]. PTEN localized in the nucleus has tumor-suppressive functions in maintaining chromosomal stability by up-regulation of RAD51 and interaction with p53 promoting p300-mediated p53 acetylation, independent of its enzymatic activities against the PI3K/AKT pathway [10]. Several regulatory mechanisms for PTEN nuclear localization have been proposed, including passive diffusion, active transport mediated by major vault protein, nuclear localization signal, interaction with GTPase Ran, and monoubiquitination of PTEN [8], [11], [12].
Loss of PTEN function is significantly related to advanced disease, chemotherapy resistance, and poor survival in patients with prostate, breast, melanoma, colorectral, esophageal, and head and neck cancers [13], [14], [15], [16], [17], [18], [19], [20]. PTEN can be inactivated by genetic and epigenetic mechanisms. PTEN is one of the most frequently mutated genes, and PTEN gene alterations play critical roles in the pathogenesis of many human cancers [21], [22], [23], [24], [25]. In DLBCL, Lenz and colleagues found that PTEN gene deletion was associated with the GCB subtype [26]; Pfeifer et al demonstrated that absence of PTEN expression defines a PI3K/AKT-dependent GCB-DLBCL subtype in both cell lines and primary samples [27]. However, a few studies have suggested different prognostic effects of PTEN loss/expression in small DLBCL cohorts [28], [29], [30], [31]. Large-scale studies are needed to establish the clinical significance of PTEN expression/loss and genetic abnormalities in DLBCL.
In this study, we analyzed cytoplasmic and nuclear expression of PTEN protein, PTEN deletions, and PTEN mutations and their prognostic significance in a large number of patients with de novo DLBCL treated with R-CHOP, and explored the potential regulatory mechanisms for PTEN deficiency in DLBCL.
Materials and Methods
Patients
Patients were organized as a part of the International DLBCL Rituximab-CHOP Consortium Program study, and were selected according to the eligibility and exclusion criteria (fulfilling the DLBCL diagnostic criteria and treated with R-CHOP or R-CHOP–like therapy, and excluding patients with transformation from lower grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, primary cutaneous DLBCL, primary central nervous system DLBCL, or acquired immunodeficiency) which have been described previously [32], [33]. PTEN staining was achieved initially in 478 cases (training cohort) and additionally in 269 cases, a later assembled validation cohort. The institutional review boards of each participating center approved this study as being of minimal to no risk or as exempt. Nuclear expression of phospho-AKT-Ser473 (p-AKT, activated form of AKT) has been evaluated in the training cohort [34] and data were available in 461 cases. Cell-of-origin classification was according to GEP and/or immunohistochemistry (IHC) algorithms as described previously [32], [35].
PTEN and PD-L1 Immunohistochemistry
Hematoxylin and eosin–stained slides from DLBCL cases were reviewed, and representative areas of the formalin-fixed and paraffin-embedded (FFPE) tissue sections with the highest percentages of tumor cells were selected for tissue microarray construction and subject for IHC staining. PTEN expression was evaluated by IHC using a PTEN antibody (138G6, Cell Signaling). PTEN expression was analyzed for positive versus negative (i.e., loss of) expression status, as well as high versus low expression. The cutoff used for high cytoplasmic PTEN expression was >40% and the cutoff for high nuclear PTEN expression was >30% of tumor cells, which were determined by the X-tile software (Yale School of Medicine, New Haven, CT).
Expression of p-AKT, IL-6, PI3K [34], Myc [36], p-STAT3 [37], MDM2 [38], p21 [39], BLIMP-1 [40], IgA and IgG [41] had been assessed by previous studies; the cutoff for p-AKThigh expression (AKT hyperactivation) was ≥70% as described previously [34].
PD-L1 expression was assessed by IHC using a DAKO PD-L1 antibody. The IHC results were scored independently by three pathologists (J.K., Y.X, and K.H.Y), and final scores were based on consensus. The cutoff for PD-L1 positivity is ≥5% of tumor cells.
Fluorescence in situ Hybridization and Gene Sequencing
Fluorescence in situ hybridization (FISH) analysis was performed and data were available for 359 cases of the training cohort and 248 cases of the validation cohort. To evaluate PTEN gene (chromosome 17p13.1) deletions, a commercial PTEN probe was utilized (ZytoLight® SPEC PTEN/CEN 10 Dual Color Probe Z-2078-200; Zytovision, Bremerhaven, Germany). The ratio of PTEN signals (green) to CEP10 signals (red) was counted in 200 tumor cells. If this ratio was lower than 0.81, heterozygous PTEN deletion was considered to be present. Ratios lower than 0.46 were considered to be suggestive of homozygous deletions. The ratios were calculated as ratios below the mean plus three standard deviations of green to red signal ratios in reference cases (5 tonsils) and subtraction of tumor-infiltrating T cells, which accounted for 15% of undeleted alleles.
For PTEN sequencing, genomic DNA was extracted from FFPE tissues of 368 cases and then subjected to Sanger sequencing. The sequencing results were compared to the National Center for Biotechnology Information (NCBI) reference sequence NM_000314 (PTEN) to identify non-synonymous PTEN mutations. Single nucleotide polymorphisms documented by the NCBI dbSNP database (build 147) have been excluded.
Gene Expression Profiling and microRNA Profiling
Gene expression profiling was performed by using the Affymetrix GeneChip Human Genome HG-U133 Plus Version 2.0 Array as described previously (GSE31312) [32], [42]. Microarray data were normalized for further supervised clustering analysis. Multiple t-tests were used to identify differentially expressed genes between groups with and without PTEN abnormalities, and the P values obtained were corrected for the false discovery rate (FDR) using the beta-uniform mixture method.
microRNA (miRNA) profiling was performed by HTG Molecular Diagnostics Inc. (Tucson, AZ) using FFPE tissue sections (unpublished preliminary data). miRNAs targeting PTEN are according to the literature review [43] and TargetScan: http://www.targetscan.org).
Statistical Analysis
The clinical and pathological features of DLBCL patients were compared using the Fisher’s exact or chi-square test. The unpaired t-test (2-tailed) was used to compare mean expression levels of biomarkers between DLBCL groups. Overall survival (OS) was calculated from time of diagnosis to last follow-up or death due to any cause. Progression-free survival (PFS) was calculated from time of diagnosis to disease progression, relapse, or death from any cause. Patients who were alive and free of disease progression at last follow-up were censored. Survival analysis was performed using the Kaplan-Meier method with the Prism 5 program (GraphPad Software, San Diego, CA), and differences in survival were compared using the log-rank (Mantel-Cox) test. Multivariate survival analysis was performed using a Cox proportional hazards regression model with the SPSS software program (version 19.0; IBM Corporation, Armonk, NY). All differences with P ≤ .05 were considered statistically significant.
Results
PTEN is Expressed in Both Cytoplasm and Nucleus and the Cytoplasmic Expression is More Frequently Lost in GCB-DLBCL
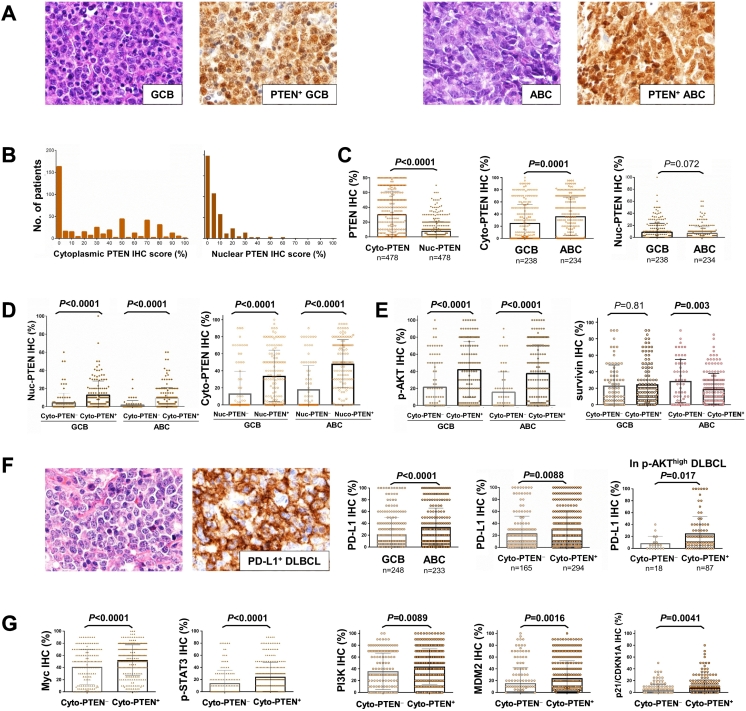
In view of PTEN’s distinct functions in the cytoplasm and nucleus, we evaluated PTEN expression in the cytoplasm and nucleus compartments separately. Representative PTEN+ IHC staining and the expression histogram for the training cohort are shown in Figure 1, A and B. We found cytoplasmic PTEN expression was significantly higher than that in the nuclei (Figure 1C). Expression of cytoplasmic PTEN (Cyto-PTEN+) was observed in 306 (64%) of 478 DLBCL in the training cohort, and showed significant differences between GCB and ABC subtypes: 57.6% (137/238) of GCB-DLBCL versus 70.5% (165/234) of ABC-DLBCL (P = .004, Table 1). The mean level of Cyto-PTEN expression for GCB-DLBCL was also significantly lower than that for ABC-DLBCL (Figure 1C). On the other hand, nuclear expression of PTEN (Nuc-PTEN+) was observed in 280 (58.6%) of 478 DLBCL, including 57.1% (136/238) of GCB-DLBCL and 59.8% (140/234) of ABC-DLBCL. In contrast with the higher cytoplasmic PTEN expression in ABC-DLBCL, there was a trend of higher nuclear PTEN expression in GCB than in ABC DLBCL (P = .072, Figure 1C), although nuclear PTEN expression significantly correlated with cytoplasmic PTEN expression (Table 1, Figure 1D). Regardless of the expression compartments, totally 129 (26.7%) of 478 DLBCL did not have any PTEN expression (Cyto-PTEN− and Nuc-PTEN−).
Figure 1.
Analysis of PTEN expression by immunohistochemistry (IHC). (A) Representative hematoxylin and eosin and immunohistochemistry of PTEN expression in GCB-DLBCL and ABC-DLBCL. (B and C) Histograms and comparison of cytoplasmic (Cyto-) and nuclear (Nuc-) PTEN expression in DLBCL and between GCB/ABC subtypes (training cohort). (D) Cytoplasmic PTEN expression was associated with higher nuclear PTEN expression in both GCB-DLBCL and ABC-DLBCL. (E) Cytoplasmic PTEN expression was associated with higher p-AKT expression in GCB-DLBCL and ABC-DLBCL, and inversely associated with survivin expression in ABC-DLBCL. (F) Representative hematoxylin and eosin and immunohistochemistry of PD-L1 expression in DLBCL. The ABC compared with the GCB subtype had a significantly higher mean level of PD-L1 expression. Cytoplasmic PTEN expression was associated with a higher mean level of PD-L1 expression in overall DLBCL and in cases with high p-AKT expression. (G) Cytoplasmic PTEN expression was associated with higher mean levels of Myc, p-STAT3, PI3K, MDM2, and p21 expression in DLBCL. Significant P values are in bold.
Table 1.
Comparison of clinical and molecular features of patients with diffuse large B-cell lymphoma (DLBCL) with and without PTEN cytoplasmic expression in the training cohort
| in DLBCL |
in GCB-DLBCL |
in ABC-DLBCL |
in p-AKThigh DLBCL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic PTEN+ |
Cytoplasmic PTEN¯ |
Cytoplasmic PTEN+ |
Cytoplasmic PTEN¯ |
Cytoplasmic PTEN+ |
Cytoplasmic PTEN¯ |
Cytoplasmic PTEN+ |
Cytoplasmic PTEN¯ |
|||||
| n=306 | n=172 | P | n=137 | n=101 | P | n=165 | n=69 | P | n=89 | n=18 | P | |
| GCB/ABC Subtype | ||||||||||||
| GCB | 137 | 101 | .004 | 41 | 12 | .13 | ||||||
| ABC | 165 | 69 | 48 | 6 | ||||||||
| Age, years | ||||||||||||
| < 60 | 128 | 76 | .63 | 70 | 52 | 1.0 | 55 | 22 | .88 | 44 | 9 | 1.0 |
| ≥ 60 | 178 | 96 | 67 | 49 | 110 | 47 | 45 | 9 | ||||
| Sex | ||||||||||||
| Male | 190 | 92 | .081 | 86 | 55 | .23 | 102 | 37 | .25 | 56 | 8 | .19 |
| Female | 116 | 80 | 51 | 46 | 63 | 32 | 33 | 10 | ||||
| Stage | ||||||||||||
| I - II | 134 | 85 | .21 | 72 | 56 | .42 | 60 | 27 | .88 | 39 | 5 | .41 |
| III - IV | 164 | 80 | 63 | 39 | 99 | 41 | 47 | 11 | ||||
| B-symptoms | ||||||||||||
| No | 190 | 103 | .92 | 95 | 61 | .38 | 92 | 40 | .88 | 57 | 9 | .57 |
| Yes | 103 | 57 | 37 | 31 | 65 | 26 | 29 | 7 | ||||
| LDH | ||||||||||||
| Normal | 109 | 51 | .12 | 52 | 31 | .26 | 56 | 20 | .29 | 30 | 4 | .28 |
| Elevated | 169 | 110 | 73 | 61 | 93 | 47 | 48 | 14 | ||||
| Extranodal sites | ||||||||||||
| 0 - 1 | 227 | 126 | .64 | 107 | 74 | 1.0 | 117 | 51 | .74 | 64 | 8 | .067 |
| ≥ 2 | 71 | 35 | 27 | 18 | 43 | 16 | 21 | 8 | ||||
| ECOG score | ||||||||||||
| 0 - 1 | 230 | 124 | .89 | 105 | 71 | 1.0 | 121 | 51 | .71 | 65 | 10 | .47 |
| ≥ 2 | 46 | 26 | 18 | 12 | 28 | 14 | 15 | 4 | ||||
| Tumor size | ||||||||||||
| < 5 cm | 135 | 67 | .73 | 62 | 40 | 1.0 | 71 | 26 | .73 | 35 | 2 | .035 |
| ≥ 5 cm | 95 | 51 | 43 | 29 | 52 | 22 | 23 | 8 | ||||
| IPI score | ||||||||||||
| 0 - 2 | 182 | 101 | .76 | 92 | 64 | 1.0 | 86 | 35 | .89 | 51 | 6 | .11 |
| > 2 | 118 | 61 | 43 | 29 | 75 | 32 | 36 | 11 | ||||
| Therapy response | ||||||||||||
| CR | 237 | 120 | .079 | 105 | 72 | .37 | 128 | 46 | .10 | 65 | 11 | .39 |
| PR | 35 | 29 | 12 | 16 | 23 | 13 | 13 | 4 | ||||
| SD | 11 | 11 | 7 | 6 | 4 | 5 | 7 | 0 | ||||
| PD | 23 | 12 | 13 | 7 | 10 | 5 | 4 | 3 | ||||
| Nuclear PTEN expression | ||||||||||||
| 0% | 69 | 129 | < .0001 | 30 | 72 | < .0001 | 39 | 55 | < .0001. | 25 | 13 | .0008 |
| > 0% | 237 | 43 | 107 | 29 | 126 | 14 | 64 | 5 | ||||
| TP53 mutations | ||||||||||||
| No | 214 | 115 | .044 | 90 | 67 | .41 | 121 | 46 | .065 | 62 | 13 | .81 |
| Yes | 51 | 44 | 28 | 27 | 23 | 17 | 12 | 3 | ||||
| MDM2 expression | ||||||||||||
| ≤ 10% | 169 | 119 | .001 | 82 | 72 | .03 | 86 | 47 | .022 | 53 | 10 | .75 |
| > 10% | 131 | 47 | 54 | 25 | 77 | 21 | 36 | 8 | ||||
| BCL6 expression | ||||||||||||
| ≤ 30% | 62 | 47 | .05 | 12 | 16 | .07 | 50 | 31 | .025 | 16 | 2 | .58 |
| > 30% | 237 | 116 | 124 | 80 | 113 | 36 | 72 | 14 | ||||
| BLIMP-1 expression | ||||||||||||
| < 5% | 173 | 116 | .033 | 94 | 77 | .24 | 78 | 39 | .32 | 44 | 15 | .017 |
| ≥ 5% | 118 | 51 | 37 | 21 | 80 | 30 | 43 | 3 | ||||
| IgA IHC | ||||||||||||
| 0% | 302 | 163 | .011 | 134 | 96 | .24 | 164 | 65 | .012 | 89 | 17 | .026 |
| 100% | 4 | 9 | 3 | 5 | 1 | 4 | 0 | 1 | ||||
| IgG IHC | ||||||||||||
| 0% | 282 | 146 | .013 | 126 | 88 | .22 | 152 | 56 | .015 | 82 | 17 | .73 |
| 100% | 24 | 26 | 11 | 13 | 13 | 13 | 7 | 1 | ||||
| PD-L1 IHC | ||||||||||||
| < 5% | 50 | 48 | .003 | 33 | 43 | .0045 | 17 | 5 | .62 | 14 | 7 | .047 |
| ≥ 5% | 244 | 117 | 99 | 56 | 142 | 60 | 73 | 11 | ||||
Abbreviations: LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; GCB, germinal center B-cell–like; ABC, activated B-cell–like. Significant P values are highlighted in bold.
To validate the results, we assembled an independent DLBCL cohort (n = 204). Compared with the training cohort, the validation cohort showed a similar pattern of PTEN expression, with a slightly lower frequency of Cyto-PTEN loss, whereas a higher frequency of Nuc-PTEN loss compared with the training cohort: 25% of DLBCLs were Cyto-PTEN−, and 69% of DLBCLs were Nuc-PTEN−; 11% of DLBCLs did not show either cytoplasmic or nuclear PTEN expression. Consistent with the results in the training cohort, in the validation cohort cytoplasmic expression is predominant and the cytoplasmic PTEN and nuclear PTEN expression are significantly correlated (Supplementary Figure S1A).
Surprisingly, PTEN expression (cytoplasmic and/or nuclear) was associated with a higher mean level of phospho-AKT-Ser473 protein (p-AKT) nuclear expression but not AKT1 mRNA expression (Figure 1E and Supplementary Figure S1A for the training and validation cohort, respectively). However, Cyto-PTEN+ (but not Nuc-PTEN+) expression was associated with significantly decreased survivin expression (a downstream target of the PI3K/AKT pathway [44]) in ABC-DLBCL (Figure 1E) independent of TP53 mutation status, which may suggest a correlation between PTEN expression and decreased AKT function.
Cyto-PTEN+ expression, but not p-AKThigh, PI3Khigh, or Nuc-PTEN+ expression, showed significant association with PD-L1 expression, which is considered as a tumor immune evasion mechanism of DLBCL [45] (Table 1, Figure 1F). Conversely, PD-L1+ cases had a higher mean level of PTEN expression than PD-L1− cases (P = .0015). Like Cyto-PTEN expression, PD-L1 expression was significantly higher in the ABC subtype (Figure 1F). Cyto-PTEN+ status was also associated with significantly higher mean levels of Myc, p-STAT3, PI3K, MDM2, and p21/CDKN1A expression (Figure 1G).
Absence of PTEN Expression is Associated with Unfavorable Clinical Features and Outcomes Only in DLBCL with AKT Hyperactivation
Clinical features for PTEN+ and PTEN− DLBCL groups are shown in Table 1 (cytoplasmic expression) and Supplementary Table S1 (nuclear expression). Cyto-PTEN− expression was not significantly associated with any clinical parameters (only trend of more female sex). Nuc-PTEN− status was associated with elevated serum lactate dehydrogenase (LDH) level (P < .0001). In the DLBCL subset with p-AKT hyperactivation (p-AKThigh) [34], Cyto-PTEN− status was associated with a larger tumor size (P = .035), and Nuc-PTEN− status was associated with elevated LDH, extranodal sites >1, ECOG performance status >1, tumor size ≥5cm, and International Prognostic Index (IPI) score >2.
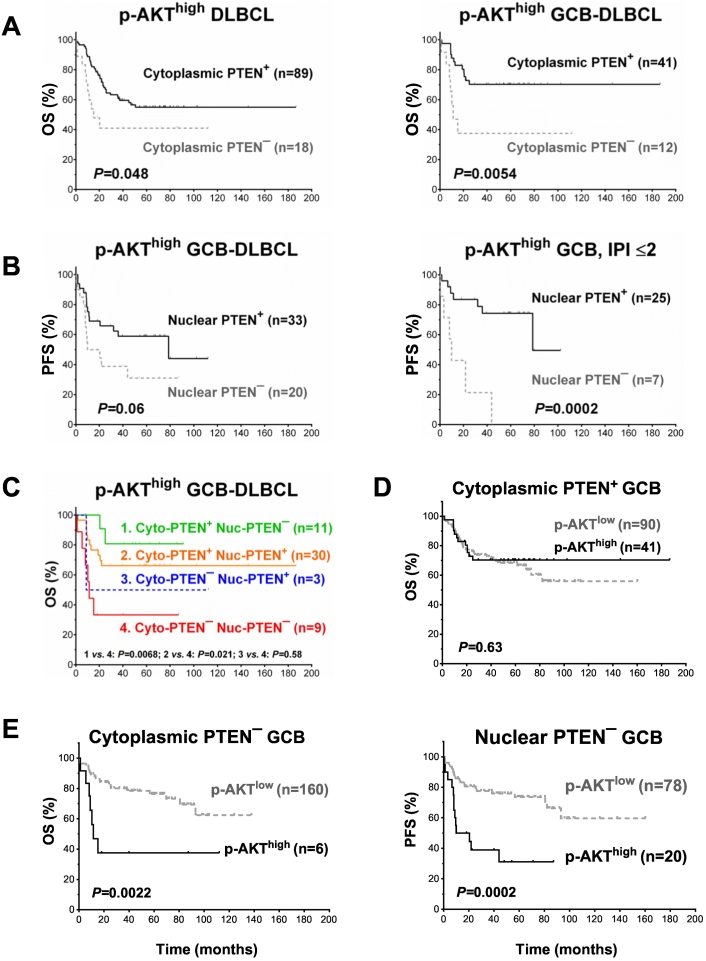
Neither cytoplasmic nor nuclear PTEN+ status showed significant prognostic impact in overall DLBCL. However, Cyto-PTEN− status was associated with a lower complete remission rate, with a trend of significance in the overall DLBCL cohort (P = .079), and significantly in the p-AKThigh ABC-DLBCL subset (P = .0007, Table 1). In p-AKThigh DLBCL, Cyto-PTEN− status was associated with lower mean levels of p-AKT (P = .042) and PD-L1 expression (P = .042, Figure 1F), but with higher frequency of survivin expression (26% vs. 8.9%, P = .031) and significantly poorer OS (P = .048), particularly in the GCB subtype (P = .0054) (Figure 2A). Moreover, in p-AKThigh GCB-DLBCL, loss of nuclear PTEN expression was associated with poorer PFS with borderline significance (P = .06, Figure 2B), although it was associated with significantly lower mean levels of antiapoptotic Bcl-2 (P = .0068) and MDM2 (P = .0011) expression.
Figure 2.
Survival analysis for PTEN expression/loss in DLBCL with high phosphorylated-AKT expression (p-AKThigh, cutoff: ≥70%). (A) Loss of PTEN cytoplasmic expression was associated with significantly poorer overall survival rate (OS) in patients with high p-AKT expression, especially in GCB-DLBCL. (B) Loss of PTEN nuclear expression was associated with decreased progression-free survival rate (PFS) in GCB-DLBCL patients with high p-AKT expression. This effect was only significant in the group with an International Prognostic Index (IPI) score ≤2. (C) Survival analysis in respect to both cytoplasmic and nuclear PTEN+ status in patients with p-AKThigh GCB-DLBCL. (D) In GCB-DLBCL cases with cytoplasmic PTEN expression, p-AKT expression level was not prognostic. (E) In GCB-DLBCL patients without cytoplasmic/nuclear PTEN expression, p-AKThigh expression was associated with significantly poorer survival.
Notably, patients with Nuc-PTEN− GCB-DLBCL more frequently had IPI >2, extranodal sites >1, and elevated LDH (P = .008, .017, and .031, respectively) (Table 2). To eliminate the confounding effects by these unfavorable clinical factors, we further compared survival of Nuc-PTEN+ and Nuc-PTEN− patients with high and low IPI individually, and found that Nuc-PTEN− status was associated with markedly shorter PFS durations only for patients with an IPI ≤2 (P = .0002, Figure 2B).
Table 2.
Comparison of clinicopathologic features of patients with p-AKT overexpressing diffuse large B-cell lymphoma (DLBCL) respective to the status of cytoplasmic or nuclear PTEN expression, PTEN deletion, and PTEN mutation in the training cohort
| p-AKThigh GCB |
p-AKThigh GCB |
p-AKThigh DLBCL |
p-AKThigh DLBCL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyto-PTEN+ |
Cyto-PTEN¯ |
Nuc-PTEN+ |
Nuc-PTEN¯ |
PTEN deletion |
No PTEN deletion |
MUT-PTEN |
WT-PTEN |
|||||
| N (%) | N (%) | P | N (%) | N (%) | P | N (%) | N (%) | P | N (%) | N (%) | P | |
| Variables | 41 (100) | 12 (100) | 33 (100) | 20 (100) | 7 (100) | 75 (100) | 9 (100) | 74 (100) | ||||
| Age, years | ||||||||||||
| < 60 | 24 (58.5) | 8 (66.7) | .74 | 20 (60.6) | 12 (60.0) | 1.0 | 3 (42.9) | 32 (42.7) | 1.0 | 3 (33.3) | 40 (54.1) | .3 |
| ≥ 60 | 17 (41.5) | 4 (33.3) | 13 (39.4) | 8 (40.0) | 4 (57.1) | 43 (57.3) | 6 (66.7) | 34 (45.9) | ||||
| Sex | ||||||||||||
| Male | 29 (70.7) | 5 (41.7) | .09 | 24 (72.7) | 10 (50.0) | .14 | 4 (57.1) | 45 (60.0) | 1.0 | 7 (77.8) | 45 (60.8) | .47 |
| Female | 12 (29.3) | 7 (58.3) | 9 (27.3) | 10 (50.0) | 3 (42.9) | 30 (40.0) | 2 (22.2) | 29 (39.2) | ||||
| Stage | ||||||||||||
| I-II | 21 (52.5) | 3 (30.0) | .29 | 17 (53.1) | 7 (38.9) | .39 | 2 (28.6) | 27 (39.1) | .7 | 2 (25.0) | 26 (36.6) | .71 |
| III-IV | 19 (47.5) | 7 (70.0) | 15 (46.9) | 11 (61.1) | 5 (71.4) | 42 (60.9) | 6 (75.0) | 45 (63.4) | ||||
| B symptoms | ||||||||||||
| No | 33 (82.5) | 7 (70.0) | .4 | 28 (84.8) | 12 (70.6) | .28 | 4 (57.1) | 44 (62.0) | 1.0 | 5 (71.4) | 46 (64.8) | 1.0 |
| Yes | 7 (17.5) | 3 (30.0) | 5 (15.2) | 5 (29.4) | 3 (42.9) | 27 (38.0) | 2 (28.6) | 25 (35.2) | ||||
| LDH | ||||||||||||
| Normal | 14 (38.9) | 2 (16.7) | .29 | 13 (46.4) | 3 (15.0) | .031 | 3 (42.9) | 31 (47.7) | 1.0 | 2 (25.0) | 27 (42.2) | .46 |
| Elevated | 22 (61.1) | 10 (83.3) | 15 (53.6) | 17 (85.0) | 4 (57.1) | 34 (52.3) | 6 (75.0) | 37 (57.8) | ||||
| Extranodal sites | ||||||||||||
| 0 - 1 | 33 (82.5) | 5 (50.0) | .046 | 28 (87.5) | 10 (55.6) | .017 | 5 (71.4) | 45 (66.2) | 1.0 | 4 (44.4) | 48 (70.6) | .26 |
| ≥ 2 | 7 (17.5) | 5 (50.0) | 4 (12.5) | 8 (44.4) | 2 (28.6) | 23 (33.8) | 5 (55.6) | 20 (29.4) | ||||
| ECOG score | ||||||||||||
| 0 - 1 | 31 (83.8) | 6 (75.0) | .62 | 25 (89.3) | 12 (70.6) | .23 | 6 (85.7) | 50 (82.0) | 1.0 | 6 (85.7) | 52 (78.8) | 1.0 |
| ≥ 2 | 6 (16.2) | 2 (25.0) | 3 (10.7) | 5 (29.4) | 1 (14.3) | 11 (18.0) | 1 (14.3) | 14 (21.2) | ||||
| Tumor size | ||||||||||||
| < 5 cm | 14 (53.8) | 1 (16.7) | .18 | 12 (52.2) | 3 (33.3) | .44 | 3 (50.0) | 35 (58.3) | .69 | 2 (66.7) | 31 (60.8) | 1.0 |
| ≥ 5 cm | 12 (46.2) | 5 (83.3) | 11 (47.8) | 6 (66.7) | 3 (50.0) | 25 (41.7) | 1 (33.3) | 20 (39.2) | ||||
| IPI score | ||||||||||||
| 0 - 2 | 28 (68.3) | 4 (36.4) | .081 | 25 (75.8) | 7 (36.8) | .008 | 4 (57.1) | 38 (52.8) | 1.0 | 2 (25.0) | 42 (58.3) | .13 |
| 3 - 5 | 13 (31.7) | 7 (63.6) | 8 (24.2) | 12 (63.2) | 3 (42.9) | 34 (47.2) | 6 (75.0) | 30 (41.7) | ||||
| Therapy response | ||||||||||||
| CR | 29 (70.7) | 7 (58.3) | 1.0 | 24 (72.7) | 12 (60.0) | .38 | 7 (100) | 54 (72.0) | .18 | 4 (44.4) | 56 (75.7) | .11 |
| PR | 4 | 3 | 2 | 5 | 0 | 12 | 4 | 10 | ||||
| SD | 4 | 0 | 3 | 1 | 0 | 3 | 0 | 5 | ||||
| PD | 4 | 2 | 4 | 2 | 0 | 6 | 1 | 3 | ||||
| TP53 mutations | ||||||||||||
| No | 26 (78.8) | 8 (72.7) | .69 | 23 (79.3) | 11 (73.3) | .71 | 4 (57.1) | 64 (88.9) | .02 | 7 (77.8) | 59 (86.8) | .61 |
| Yes | 7 (21.2) | 3 (27.3) | 6 (20.7) | 4 (26.7) | 3 (42.9) | 8 (11.1) | 2 (22.2) | 9 (13.2) | ||||
| PD-L1 IHC | ||||||||||||
| < 5% | 9 (22.5) | 7 (58.3) | .031 | 10 (31.3) | 6 (30) | 1.0 | 0 (0) | 15 (22.7) | .33 | 13 (19.7) | 2 (22.2) | 1.0 |
| ≥ 5% | 31 (77.5) | 5 (41.7) | 22 (68.8) | 14 (70) | 6 (100) | 51 (77.3) | 53 (80.3) | 7 (77.8) | ||||
Abbreviations: Cyto-PTEN, cytoplasmic PTEN expression; Nuc-PTEN, nuclear PTEN expression; LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; GCB, germinal-center B-cell–like; MUT, mutated, WT, wild-type.
Incorporating both cytoplasmic and nuclear PTEN+ status in the survival analysis found Cyto-PTEN but not Nuc-PTEN expression had significant prognostic impact in p-AKThigh GCB-DLBCL patients (Figure 2C). However, the significance was lost in multivariate survival analysis adjusting clinical factors in p-AKThigh GCB-DLBCL. In contrast, in p-AKThigh ABC-DLBCL, Nuc-PTEN+ expression was an independent prognostic factor for better OS (P = .003; hazard ratio [HR] 0.16; 95% confidence interval [CI] 0.049-0.53) and PFS (P = .008; HR 0.22; 95% CI 0.07-0.67) after adjusting clinical factors (Table 3). In the validation cohort, loss of Cyto-PTEN expression was also associated with signficantly shorter PFS in p-AKThigh DLBCL (P = .029, Supplementary Figure S1B) but not in the overall DLBCL cohort. However, in the multivariate survival analysis adjusting for clinical factors, Cyto-PTEN− status lost signficance as an independent prognostic factor in the validation p-AKThigh DLBCL cohort (data not shown).
Table 3.
Multivariate analysis for PTEN expression (positive or high), PTEN deletion and PTEN mutations in overall DLBCL, cases with ≥70% p-AKT expression (p-AKThigh), and cases with ≤30% p-AKT expression (p-AKT−)
| OS |
PFS |
|||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P | HR | 95% CI | P |
| In p-AKThighABC-DLBCL | ||||||
| IPI >2 | 3.28 | 1.02-10.48 | .046 | 3.84 | 1.20-12.33 | .024 |
| Female | .27 | .096- .74 | .011 | .34 | .13- .89 | .028 |
| Tumor size >5cm | 1.91 | .67-5.49 | .23 | 1.94 | .71-5.28 | .19 |
| B-symptoms | 10.2 | 2.67-39.02 | .001 | 6.37 | 1.88-21.56 | .003 |
| *Nuclear PTEN+ | .16 | .049- .53 | .003 | .22 | .07- .67 | .008 |
| In p-AKThighABC-DLBCL | ||||||
| IPI >2 | 3.84 | 1.20-12.33 | .024 | 2.35 | .85-6.51 | .10 |
| Female | .19 | .063- .60 | .004 | .28 | .10- .79 | .016 |
| Tumor size >5cm | 2.10 | .73-6.09 | .17 | 2.12 | .79-5.71 | .14 |
| B-symptoms | 7.27 | 1.88-28.17 | .004 | 4.42 | 1.37-14.29 | .013 |
| *Cytoplasmic PTEN+ | .47 | .12-1.77 | .26 | .53 | .14-1.95 | .34 |
| In overall DLBCL | ||||||
| IPI >2 | 2.31 | 1.63-3.28 | < .001 | 2.28 | 1.64-3.18 | < .001 |
| Female | .75 | .52-1.07 | .12 | .72 | .51-1.02 | .064 |
| Tumor size >5cm | 1.15 | .80-1.64 | .45 | 1.12 | .79-1.57 | .53 |
| B-symptoms | 1.62 | 1.12-2.33 | .01 | 1.59 | 1.12-2.26 | .009 |
| *Nuclear PTENhigh | .39 | .16- .97 | .043 | .34 | .14- .84 | .02 |
| Mychigh | 2.15 | 1.49-3.09 | < .001 | 2.10 | 1.48-2.97 | < .001 |
| In overall DLBCL | ||||||
| IPI >2 | 2.38 | 1.68-3.38 | < .001 | 2.34 | 1.67-3.26 | < .001 |
| Female | .86 | .60-1.23 | .40 | .84 | .60-1.19 | .33 |
| Tumor size >5cm | 1.36 | .96-1.92 | .084 | 1.31 | .94-1.82 | .11 |
| B-symptoms | 1.41 | .98-2.03 | .063 | 1.39 | .98-1.98 | .061 |
| *Cytoplasmic PTENhigh | 1.19 | .84-1.69 | .33 | 1.42 | 1.02-1.98 | 0.036 |
| In p-AKT−DLBCL | ||||||
| IPI >2 | 2.59 | 1.65-4.05 | < .001 | 2.80 | 1.81-4.32 | < .001 |
| Female | .95 | .60-1.49 | .82 | .85 | .54-1.32 | .47 |
| Tumor size >5cm | 1.07 | .68-1.69 | .77 | 1.03 | .67-1.59 | .89 |
| B-symptoms | .97 | .61-1.56 | .90 | 1.00 | .64-1.57 | .99 |
| *Cytoplasmic PTENhigh | 1.33 | .84-2.09 | .22 | 1.62 | 1.05-2.50 | .03 |
| Mychigh | 1.71 | 1.05-2.79 | .03 | 1.63 | 1.02-2.61 | .041 |
| In p-AKThighDLBCL | ||||||
| IPI >2 | 4.80 | 1.78-12.98 | .002 | 3.47 | 1.51-7.96 | .003 |
| Female | .48 | .21-1.07 | .072 | .34 | .15- .78 | .011 |
| Tumor size >5cm | 1.38 | .63-3.02 | .43 | 1.72 | .80-3.71 | .17 |
| B-symptoms | 2.59 | 1.12-5.98 | .026 | 3.07 | 1.36-6.94 | .007 |
| *PTEN deletion | 4.53 | .98-20.89 | .052 | 5.30 | .99-28.33 | .051 |
| *PTEN mutation | 4.53 | .97-21.12 | .054 | 3.78 | 1.02-13.97 | .046 |
Abbreviations: OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; GCB, germinal center B-cell–like; ABC, activated B-cell–like; IPI, International Prognostic Index.
Data for PTEN factors are highlighted in bold. Cutoffs for Nuclear PTENhigh and Cytoplasmic PTENhigh: >30% and >40%, respectively.
Consistent with the role of PTEN in suppressing AKT activation and activity, the adverse prognostic significance of p-AKThigh expression in GCB-DLBCL that we have reported previously [34] was only significant in the Cyto-PTEN− GCB-DLBCL (P = .0022 for OS and P = .0029 for PFS, respectively) and Nuc-PTEN− GCB-DLBCL subsets (P = .12 for OS and P = .0002 for PFS, respectively), but not in the Cyto-PTEN+ GCB-DLBCL (P = .63 for OS and P = .18 for PFS, respectively) or Nuc-PTEN+ GCB-DLBCL subset (P = .89 for OS and P = .50 for PFS, respectively) (Figure 2, D and E and Supplementary Figure S2).
High Cytoplasmic PTEN Expression is Associated with Poorer Survival Only in DLBCL Patients with Low AKT Activation
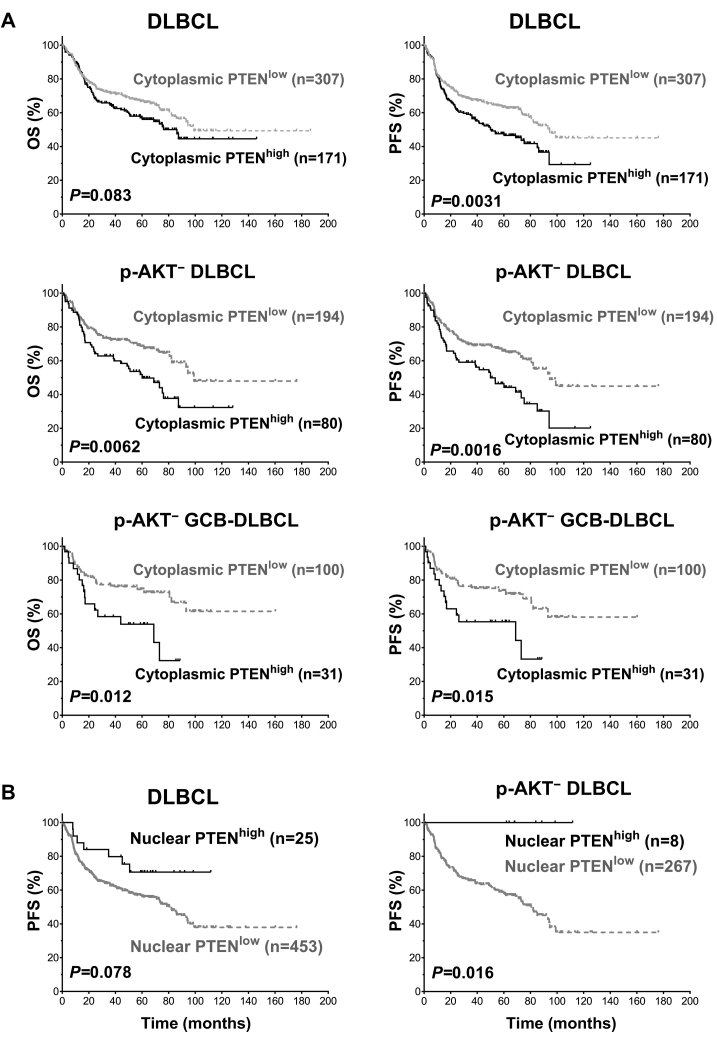
In contrast to the results above indicating that loss of PTEN expression was associated with unfavorable clinical outcomes only in DLBCL with AKT hyperactivation, in the p-AKT-deficient training subcohort (p-AKT−, cutoff: ≤30% which was approximate to the mean p-AKT expression level, 33%), high Cyto-PTEN expression (Cyto-PTENhigh, cutoff: >40%; frequency: 36%) was associated with inferior OS (P = .014) and PFS (P = .012), which was only significant in the GCB subtype (Figure 3A). In contrast, high Nuc-PTEN expression (Nuc-PTENhigh, cutoff: >30%%; frequency: 5.2%) was associated with better OS and PFS in p-AKT− DLBCL cases (Figure 3B), overall GCB-DLBCL cases, and the p-AKT− GCB-DLBCL subset.
Figure 3.
Survival analysis for high levels of PTEN expression in DLBCL. (A) DLBCL patients with high cytoplasmic PTEN+ expression (cutoff: >40%) had a significant poorer progression-free survival rate (PFS) compared with patients with low PTEN expression. The adverse prognostic effect was only significant in DLBCL with no or low p-AKT expression (p-AKT−, cutoff: ≤30%), and GCB-DLBCL with low p-AKT expression. (B) High nuclear PTEN+ expression (cutoff: >30%) was associated with trend of better PFS in DLBCL with no or low p-AKT expression. The favorable prognostic effect was only significant in patients with no or low p-AKT expression.
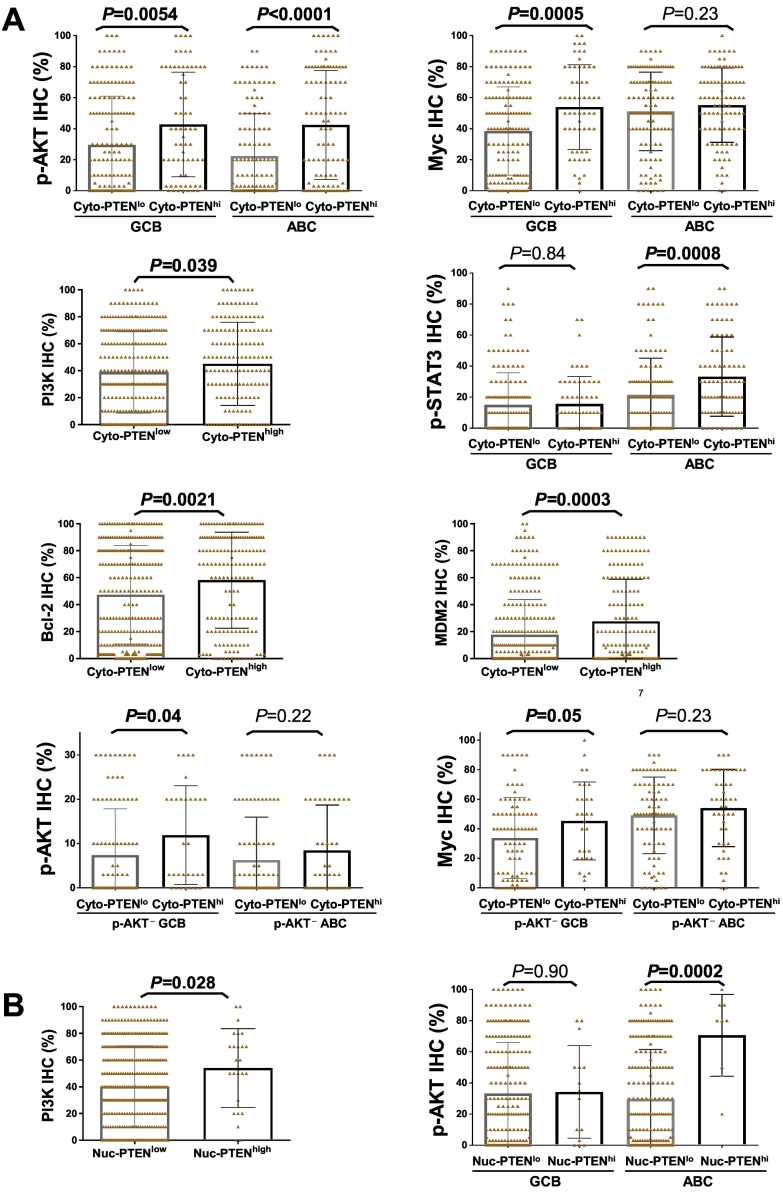
Notably, Cyto-PTENhigh expression was associated with higher mean levels of p-AKT (in both GCB and ABC), PI3K (P = .039), Myc (in GCB only), p21 (P = .0011), MDM2 (in both GCB and ABC), and p-STAT3 (in ABC only) expression at the protein level (Figure 4A) but not at the mRNA level, and associated with both Bcl-2 protein (P = .0021) and BCL2 mRNA (P = .0003) expression. Restricting the analysis in the p-AKT− DLBCL subset in which PTENhigh expression showed prognostic effect, Cyto-PTENhigh expression remained to be associated with high Myc (an unfavorable prognostic factor [36]) and p-AKT expression, significantly only in the GCB subtype (Figure 4A). Nuc-PTENhigh expression was associated with higher mean levels of p-AKT and PI3K but not Myc expression, and the association with p-AKT expression was significant only in the ABC subtype (Figure 4B).
Figure 4.
Biomarker expression analysis for high PTEN expression. (A) High cytoplasmic PTEN expression (>40%) was associated with higher mean levels of p-AKT, Myc (in GCB only), PI3K, p-STAT3 (in ABC only), Bcl-2, and MDM2 expression. Only in DLBCL with no or low p-AKT expression, high cytoplasmic PTEN expression was associated with higher mean levels of p-AKT and Myc expression. (B) High nuclear PTEN expression (>30%) was associated with higher mean levels of p-AKT (in ABC only) and PI3K expression. Significant P values are in bold.
In multivariate survival analysis adjusting for clinical parameters, Cyto-PTENhigh remained as an unfavorable factor for PFS in overall DLBCL and the p-AKT− DLBCL subcohort (P = .009; HR 1.77; 95% CI 1.15-2.72), whereas Nuc-PTENhigh was a favorable factor for PFS independent of clinical factors only in the overall cohort (P = .032; HR 0.37; 95% CI 0.15-0.92). After adding the factor of Mychigh in the Cox regression models, Cyto-PTENhigh remained as an independent factor for unfavorable PFS only in p-AKT− DLBCL cases but not in the overall cohort, whereas Nuc-PTENhigh was a favorable factor for both OS (P = .043; HR 0.39; 95% CI 0.16-0.97) and PFS in the overall cohort but not in the p-AKT− DLBCL subcohort (Table 3).
Compared with the training cohort, the validation cohort had a higher frequency of Cyto-PTENhigh expression (52%) and lower frequency of Nuc-PTENhigh expression (1.5%). As in the training cohort, in the validation cohort Cyto-PTENhigh expression was associated with higher mean levels of p-AKT and Myc expression (Supplementary Figure S2A). In p-AKT− cases (≤30% p-AKT expression), Cyto-PTENhigh expression was associated with trend of poorer survival, whereas Nuc-PTENhigh was associated with trend of better survival. In contrast, in p-AKT+ cases (>30% p-AKT expression), Cyto-PTENhigh expression was associated with significantly better PFS, whereas Nuc-PTENhigh (only two cases) was associated with poorer PFS (Supplementary Figure S2B). In the multivariate analysis, only Cyto-PTENhigh expression in the p-AKT+ subcohort showed trend toward being an independent factor for better PFS (P = .085; HR 0.30; 95% CI 0.076-1.18).
PTEN Gene Deletions and Mutations are Infrequent in DLBCL but are Independent Unfavorable Prognostic Factors in p-AKThigh DLBCL
To understand the mechanisms for PTEN deficiency in DLBCL, PTEN gene deletion status was assessed in a total of 607 cases of DLBCL (359 plus 248 from the training and validation cohorts, respectively), and PTEN mutation status was assessed in 368 cases from the training cohort.
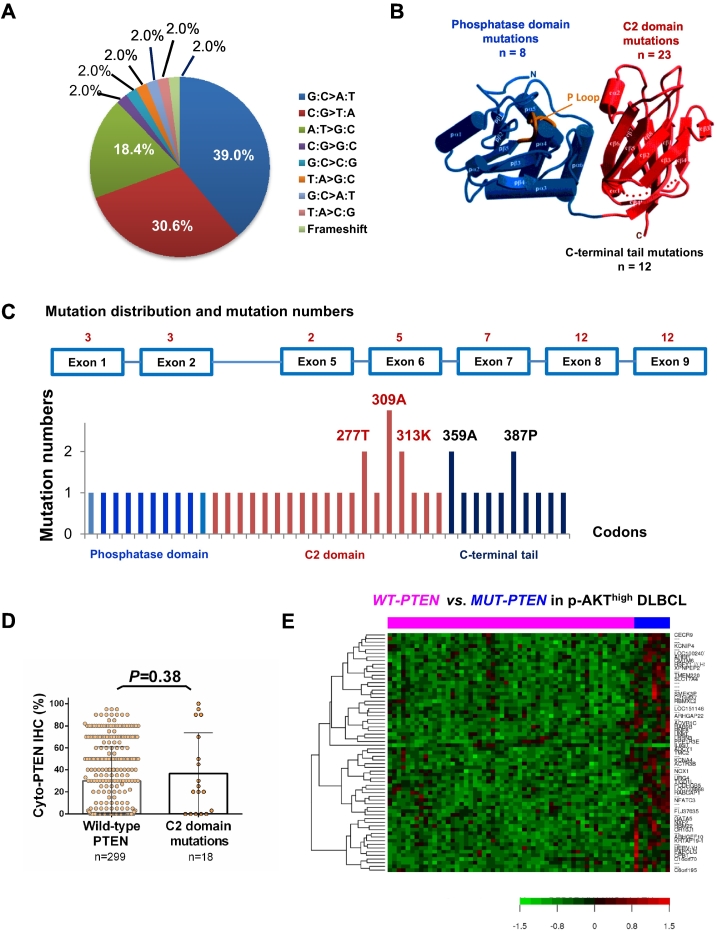
Totally 44 PTEN mutations were detected in 39 (10.6%) of 368 patients, including 23 (12.2%) patients with GCB-DLBCL and 16 (9.0%) patients with ABC-DLBCL. Of these, 8 (18%) mutations were in the regions encoding the phosphatase domain (corresponding to aa15-aa185, [46]) of the PTEN protein, 23 (52%) in the C2 domain (aa185-aa351), and 12 (27%) in the C-terminal tail (aa351-aa403) (Figure 5, A–C). No correlation between PTEN mutation and PTEN deletion or PTEN protein expression was observed, although the expression levels of PTEN with C2 domain mutations were slightly increased (P = .38, Figure 5D).
Figure 5.
PTEN mutation analysis in the DLBCL training cohort. (A) Proportions of classified point mutations. (B) PTEN mutation numbers in the phosphatase domain (blue), C2 domain (red) and C-terminal tail. PTEN crystal figure is edited from Lee et al 1999; reference [41]. (C) Distribution of mutation numbers according to PTEN exons and codons. (D) PTEN mutations in the C2 domain were associated with a trend of higher mean cytoplasmic PTEN level but it was not significant. (E) Genes significantly differently expressed between wild-type PTEN and mutated-PTEN groups in DLBCL with AKT hyperactivation.
A distinct GEP signature was identified for PTEN mutations (FDR < 0.25, Figure 5E). Seven genes were down-regulated and 43 were up-regulated in the mutated-PTEN DLBCL subgroup compared with the wild-type PTEN DLBCL subgroup (FDR < 0.05, Table 4). Notably, PDCD6 which encodes programmed cell death 6, a calcium-binding protein required for T-cell receptor-, Fas-, and glucocorticoid-induced cell death and having inhibitory function towards PI3K/AKT/mTOR signaling [47], was up-regulated in the mutated-PTEN DLBCL group. Many genes related to neural function (such as BOC, GLRA3, UNC5C, GPC4, GLRA3, and NLGN3) and protein degradation (such as CUL7, RNF7, USP46, and HERC6) were also up-regulated in the mutated-PTEN DLBCL group, whereas MYD88, which was recurrently mutated in primary central nervous sytem (CNS) lymphoma [48], was downregulated. We further compared mutated-PTEN DLBCL group with the wild-type PTEN DLBCL group in the p-AKThigh DLBCL subset. In the mutated PTEN subgroup, 43 genes were up-regulated whereas only IL6ST (interleukin 6 signal transducer, involved in STAT3 activation) was downregulated (Table 4). Up-regulated genes included CNS-related genes (ADCY1 and ARHGEF10), genes invovled in protein degradation, and PPP1R3E invovled in glycogen metabolism.
Table 4.
Gene expression profiling analysis
|
PTENlowvs. PTENnot low in GCB-DLBCL (FDR<0.01, fold >2) |
PTENlowvs. PTENnot low in ABC-DLBCL (FDR<0.01, fold >1.74) |
|||
|---|---|---|---|---|
| Up-regulated | Down-regulated | Up-regulated | Down-regulated | |
| Signaling, receptors, B-cell development and differentiation | PTEN, PTEN/PTENP1, STAP1, BLNK, FCRL1, KLHL6, LPAR5, RGS1, RGS13, FCRL5, BANK1 | PTEN, PKN2, RANBP9, MAP3K13, RGS13 | ||
| Transcriptional regulation, mRNA processing and regulation | INTS7, PABPC1, CBFB, EZH2, ZNF117, IGF2BP3, HNRNPD, MYBL1 | RFX7, ZEB1, HIF1A, TBL1XR1, OVOS/OVOS2, SMCHD1, MBD4, TCF4, PRDM2 | ||
| Cell cycle | NIPBL, CASC5 | GPSM2, DPY30/MEMO1, SMC1A, PTP4A1, C7orf11, ZYG11B, MARK4, SFI1 | ||
| Immune response, inflammation | HLA-DMA/HLA-DMB, HLA-DPA1, HLA-DOA, SERPINB9, HLA-DQB1, LYZ | POLR3E | ||
| Metabolism, ribosomes | AMD1, RPL15, PGK1, SAMM50, CIRH1A | C11orf54, AMD1, FUT8, RPL15, PDE7A, DERA, PNPLA8, C21orf57, SLC16A1 | ||
| Posttranslational modification, protein degradation, transport | IDE, LRMP, CSE1L, UBE2G1, FBXO6 | CCDC91, C18orf55, OSBPL8, USP1 | ||
| Actin, cytoskeleton, cell adhesion, extracellular matrix, motility | ANXA7, RABEP2, SYNE2, TMEM163, FGD6, ENPP2, POSTN | ANXA7, RABEP2, KIAA1217, DMD | ||
| Unknown function | ZDHHC11 | FAM82B | FAM82B, C12orf66, RP6-213H19.1, BAGE2/BAGE4 | |
|
MUT-PTEN vs. WT-PTEN in DLBCL (FDR< 0.05) |
MUT-PTEN vs. WT-PTEN in p-AKThigh DLBCL (FDR< 0.25) |
|||
|---|---|---|---|---|
| Upregulated | Downregulated | Upregulated | Downregulated | |
| Signaling, receptors | BOC, GPC4, GLRA3, PTPRF, C7orf16, ACVR1C, UNC5C | DENND4C | ACVR1C, ARHGAP22, OR10J1, CMTM6 | IL6ST |
| Transcriptional regulation | SOX10, DPPA4, TFAP2A | NFATC3, ARHGEF10, GATA5, HSFY1/HSFY2 | ||
| Cell growth and differentiation, development | ADCY1, URG4, RABGAP1, AHRR | |||
| Immune response, inflammation | PDCD6 | MYD88 | XPNPEP2 | |
| Metabolism | FAM19A5 | PGS1 | NOX1, CPB1, PPP1R3E | |
| mRNA processing and regulation, protein folding, posttranslational modification, degradation | C2orf30, AFF2, CUL7, RNF7, USP46, PSMG4, ELAVL4, HERC6 | ICMT | UNKL, PAPOLG, RBM22, NXF2, RNF8, RBMXL2 | |
| Transport, actin, cytoskeleton, motility | MYO1C, DYNLRB1, STARD13, TTLL2, RHO | KIF5B | RAB9B, SLC17A4, ACTR3B | |
| Cell adhesion, extracellular matrix, ion channels | PCDHGB5, NLGN3, GJA3, ATP4B | KCNA4, TMC2, PCDHGB5, KRTAP19-1, KCNIP4 | ||
| IncRNA, pseudogene, unknown function | ADCK2, PCA3, LOC283140, HYDIN/HYDIN2, PRAMEF12, LOC100129175, C20orf12, TMEM174, HEATR4, HSPC072/LINC00652, C7orf45, LOC219731, HMCN2, LOC404266 | LRPPRC, DCTN6 | C16orf70, LOC728868, LRRN3, LOC151146, C6orf195/LINC01600, HERV-V1, FLJ37035, CECR9, TIGD1L, LOC100240734, SMEK3P, C21orf37/LINC01549, TMEM220 | |
Abbreviations: MUT-PTEN, PTEN mutated genotype; WT-PTEN, PTEN wild-type genotype; FDR, false discovery rate.
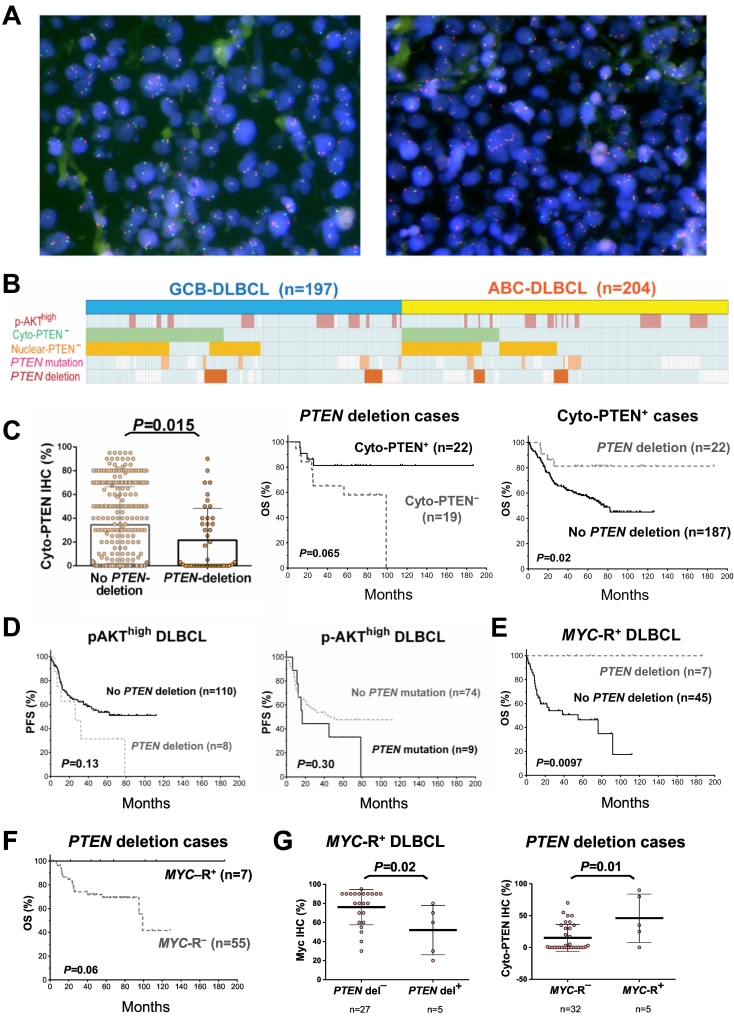
Heterozygous or homozygous PTEN deletion (Figure 6A) was only present in 44 (12.3%) of 359 patients in the training cohort, including 27 (61.4%) patients with GCB-DLBCL and 17 (38.6%) patients with ABC-DLBCL. Only two of these cases had homozygous deletion. Figure 6B depicted the case distribution of PTEN mutation and PTEN deletion in the training cohort. PTEN deletion cases overlapped with approximately 17.7% of Cyto-PTEN− DLBCL cases, and 14.6% of Nuc-PTEN− DLBCL cases. Similar frequency of PTEN deletion (heterozygous or homozygous) was observed in the validation cohort (10.1%, 25 of 248 patients, including 4 patients [1.6%] who had homozygous deletion), which overlapped with approximately 13.6% of the Cyto-PTEN− cases, and 15.9% of the Nuc-PTEN− cases (Supplementary Figure S1C).
Figure 6.
PTEN deletion and PTEN mutation analysis in the DLBCL training cohort. (A) Representative FISH results for normal (left) and PTEN deletion (right). Red signals: centromere 10; green signals: PTEN gene. (B) Distribution of PTEN deletions and mutations in GCB-DLBCL and ABC-DLBCL cases, and their correlations with PTEN expression deficiency and p-AKT overexpression. Each column represents one patient; cases with PTEN deletion, mutation, PTEN loss, and p-AKT overexpression are highlighted in corresponding colors; cases without indicated abnormalities are shown in light blue or white color (for negative or unknown status, respectively). (C) The mean level of cytoplasmic PTEN expression was significantly lower in patients with PTEN gene deletion than that in patients without PTEN gene deletion. Among patients with heterozygous or homozygous PTEN deletion, patients with cytoplasmic PTEN expression had trend of better overall survival rate (OS) in the training cohort. Among patients with positive PTEN cytoplasmic expression, PTEN deletion was associated with significantly better OS. (D) PTEN deletion/mutation showed trends towards decreased progression-free survival (PFS) rates in DLBCL cases with p-AKT overexpression. (E) In combined training and validation cohort, PTEN deletion was associated with significantly better OS in DLBCL cases with MYC gene rearrangement. (F) In DLBCL cases with PTEN deletion, MYC gene rearrangement was associated with better OS with borderline significance. (G) In MYC rearranged DLBCL cases (training cohort), PTEN deletion was associated with a significantly lower mean level of Myc expression. In DLBCL cases with PTEN deletion, MYC rearrangement was associated with a significantly higher mean level of PTEN cytoplasmic expression.
PTEN deletion was associated with lower mean levels of Cyto-PTEN expression in both the training and validation cohorts (P = .015 and P = .013, respectively; Figure 6C, and Supplementary Figure S1D). Only a trend of decrease in Nuc-PTEN expression was associated with PTEN deletion (P = .24) likely due to the low nuclear PTEN expression and small number of positive cases. Among cases with PTEN deletion, Cyto-PTEN+ expression status was associated with trend of better OS in the training cohort (P = .065, Figure 6C), and significant better OS in the combined training and validation cohort (P = .031). Conversely, among Cyto-PTEN+ cases, PTEN deletion was associated significantly better OS (P = .02 in the training cohort and P = .006 in the combined cohort), despite the association with decreased Cyto-PTEN expression (P = .008 in the combined cohort).
The clinical features of patients with and without PTEN deletion/mutation in the training cohort are shown in Supplementary Tables S2 and S3. PTEN deletion was associated with age <60 years (P = .024) in ABC-DLBCL. PTEN mutation tended to be associated with age ≥60 years (P = .078) in GCB-DLBCL and elevated LDH levels (P = .051) in ABC-DLBCL. Different from PTEN− expression status, PTEN deletion/mutation was not associated with decreased p-AKT, PI3K, survivin, Myc, p-STAT3, p21, or PD-L1 expression. However, PTEN deletion was associated with lower mean levels of MDM2 and BLIMP-1 expression (P = .01 and .027, respectively). We have shown previously that BLIMP-1 expression was associated with the ABC subtype [40].
Similarly with the prognostic effects associated with PTEN protein loss, PTEN deletion and PTEN mutation were associated with trends towards poorer survival in p-AKThigh DLBCL despite the small case numbers (Figure 6D), although not in overall DLBCL (data not shown). The effect of PTEN deletion was stronger in the GCB compared with the ABC subtype. Moreover, multivariate analysis adjusting for clinical factors showed that both PTEN mutation and deletion were independent prognostic factors for poorer OS and PFS in p-AKThigh DLBCL (Table 3).
Opposite to this observation in p-AKThigh DLBCL, in MYC-rearranged DLBCL cases, seven cases had PTEN deletion (heterozygous or homozygous) and significantly better survival (in the training cohort, P = .033 for OS and P = .064 for PFS; in the combined traning and validation cohorts, P = .0097 for OS and P = .025 for PFS; Figure 6E). Similar favorable effect of PTEN deletion was also shown in GCB-DLBCL with BCL2 rearrangement (P = .08 for OS in the training cohort and P = .048 for OS in the combined cohorts). Conversely, among DLBCL cases harboring PTEN deletion, MYC rearrangement was associated with trend of better OS (P = .096 in the training cohort, P = .06 in the combined training and validation cohort; Figure 6F). However, multivariate analysis indicated that PTEN deletion was not a prognostic factor independent of clinical factors in MYC-rearranged patients. The better survival may be attributable to the decreased Myc protein expression in these MYC-rearranged cases harboring PTEN deletion (P = .02 in the training cohort); we have shown previously that MYC-rearranged DLBCL cases without Myc overexpression had superior survival [36]. In addition, PTEN expression was positive in five of seven MYC-rearranged cases with PTEN deletion. Among cases with PTEN deletion, MYC rearrangement was associated with increased cytoplasmic PTEN expression (P = .01, Figure 6G), which is similar to the association of Myc overexpression with cytoplasmic PTEN expression (P = .0022, figure not shown).
Both Transcriptional and Post-transcriptional Mechanisms are Involved in Nuclear and Cytoplasmic PTEN Expression Regulation
The above data showed that PTEN genetic lesions only contributed to a small proportion of DLBCL with PTEN deficiency. We further correlated PTEN expression to biologic data from our previous studies [32], [33], [38] and found that loss of PTEN expression was associated with TP53 mutation and IgA/IgG positive immunophenotypes (Table 1, Supplementary Table S1). Notably, previous studies have shown that wild-type but not mutated p53 transactivates PTEN [49], [50].
At the transcription level, we found that Nuc-PTEN negativity was associated with significantly lower PTEN mRNA expression (P = .0054), more signficant in GCB-DLBCL (P = 0.0092) than in ABC-DLBCL (P = 0.081). Comparably, the association between PTEN downregulation and Cyto-PTEN− status was not significant (P = 0.065), with a stronger trend in ABC-DLBCL (P = 0.087) than in GCB-DLBCL (P = 0.36).
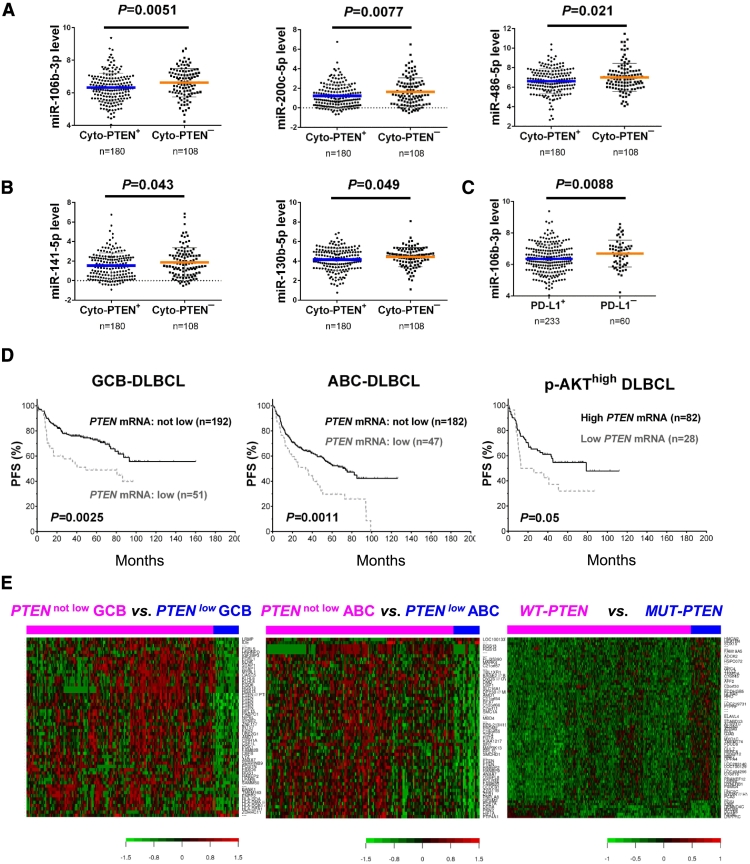
The lack of significant association of Cyto-PTEN expression with PTEN mRNA expression may suggest the important role of posttranscriptional regulation in Cyto-PTEN expression. We further extracted PTEN-targeting miRNA from miRNA profiling data and found that Cyto-PTEN− status was associated with significantly higher expression of several PTEN-targeting miRNAs, including miR-106b-3p, miR-200c-5p, miR-486-5p, miR-141-5p, and miR-130b-5p (Figure 7, A and B). When further analyzed in GCB/ABC subtypes, Cyto-PTEN negativity was associated with higher miR-486, miR-130b, and miR-106b expression in GCB-DLBCL, and with higher miR-200c and miR-222 in ABC-DLBCL. In comparison, loss of Nuc-PTEN expression did not show correlations with expression of most PTEN-targeting miRNAs except for higher miR-106b-3p expression (P = .042, figure not shown). Interestingly, absence of PD-L1 expression was also associated with significantly higher levels of miR-106b-3p (P = .0088, Figure 7C) and miR-130b-5p (P = .036, figure not shown) expression. These data may suggest that posttranscriptional regulation including miRNA-mediated epigenetic mechanism played a significant role in regulating cytoplasmic PTEN expression, whereas nuclear PTEN expression was mainly regulated at the transcription level in GCB-DLBCL.
Figure 7.
miRNA profiling and gene expression profiling analysis in the training cohort. (A-B) Loss of cytoplasmic PTEN expression was associated with significantly higher levels of miR-106b-3p, miR-200c-5p, miR-486-5p, miR-141-5p, and miR-130b-5p expression in DLBCL. (C) Absence of PD-L1 expression was associated with significantly higher miR-106b-3p expression. (D) Low PTEN mRNA expression was associated with significantly worse progression-free survival (PFS) in GCB-DLBCL, ABC-DLBCL, and the p-AKThigh DLBCL subset. (E) Genes significantly differently expressed between DLBCL groups with low PTEN mRNA expression and other cases (designated as PTENlow and PTENnot low, respectively), and between DLBCL patients with wild-type PTEN (WT-PTEN) and mutated PTEN (MUT-PTEN).
Striking Prognostic Effect and Gene Expression Signatures Associated with Low PTEN mRNA Expression
PTEN mRNA expression showed much greater prognostic effect than PTEN protein expression. Low PTEN mRNA levels (PTEN-mRNAlow) was associated with significantly poorer OS and PFS in overall DLBCL and GCB-DLBCL, ABC-DLBCL, p-AKTlow, and p-AKThigh subsets with multiple cutoffs (Figure 7D, with a cutoff at 21st percentile).
Moreover, distinct GEP signatures were identified for low PTEN mRNA expression, but not for Cyto- or Nuc-PTEN protein negativity. In GCB-DLBCL, up to 11,556 transcripts were up- or down-regulated in PTEN-mRNAlow patients compared with PTEN-mRNAnot low patients with a FDR threshold of 0.01. In ABC-DLBCL, 2,358 transcripts were differentially expressed between PTEN-mRNAlow and PTEN-mRNAnot low groups (FDR < 0.01). When use another cutoff at 50th percentile (median) for PTEN-mRNAhigh, a greater number of significant transcripts were differentially expressed between PTEN-mRNAhigh ABC-DLBCL and other ABC-DLBCL patients (n = 10,361, FDR<0.01, data not shown). The spectrum of PTEN-mRNAlow genes in ABC-DLBCL was similar with that in GCB-DLBCL, and both showed downregulation of genes involved in immune responses, B-cell receptor (BCR) signaling, gene expression, and metabolism, such as downregulation of HLA-DRB4, CD58, MS4A1/CD20, FCRL3, CSE1L, RPL15, and HNRNPA1. Notably, GEP analysis for AKT hyperactivation also demonstrated downregulation of many genes involved in immune responses, microenvironment, and metabolism in p-AKThigh GCB-DLBCL patients [34]. Two genes regulating mRNA turnover (PABPC1 and IGF2BP3) were downregulated in both GCB and ABC subtypes of PTEN-mRNAlow DLBCL, including IGF2BP3 which protects mRNAs against miRNA-mediated degradation [51]. PTEN-mRNAlow gene signatures in GCB-DLBCL and in ABC-DLBCL with >2-fold and >1.74-fold differences, respectively, are shown in Figure 7E and Table 4.
Discussion
In two large cohorts of DLBCL, PTEN expression was observed mainly in the cytoplasmic compartments of the tumor cells (64–75% of cases); PTEN expression in the nucleus was less frequent and at lower levels. PTEN cytoplasmic expression was more frequent and higher (by mean level) in the ABC compared with GCB subtype. The frequency of loss of cytoplasmic PTEN expression observed in this study (25–36%) is comparable to those by other studies in DLBCL (31-37%) [28], [30]. Complete loss of both cytoplasmic and nuclear PTEN expression was observed in 27% of the training cohort and 11% of the validation cohort, which was comparable to the frequency of complete loss of PTEN expression reported in melanoma (25%) [25], and lower than those in some solid tumors, such as hepatocellular (57%), prostate (52%), colorectal (48%) [25], glioblastoma (53%) [52], and triple-negative breast cancer (48%) [19]. Loss of cytoplasmic and/or nuclear PTEN expression was associated with poorer clinical outcomes only in DLBCL with high p-AKT (Ser473) nuclear expression, which were mainly manifested in the GCB subtype by univariate survival analysis but were retained only in the ABC subtype by multivariate analysis adjusting for clinical parameters. In contrast, in patients without abnormal AKT activities, high cytoplasmic PTEN expression was associated with poorer survival, which is also only significant in the GCB subtype.
These findings may explain the inconsistent prognostic results in DLBCL by previous studies, and strongly suggest that the tumor-suppressor function of PTEN is limited to the negative regulation of the AKT signaling pathway. Supportingly, recent studies demonstrated that the dependence of GCB-DLBCL on surface BCR density and signaling is only in the presence of PTEN [53], and that most AKT inhibitor-sensitive DLBCL models did not express PTEN and were of GCB subtype; in contrast, PI3K inhibitor is selectively effective in ABC-DLBCL through NF-κB inhibition [54]. These findings are consistent with that PTEN inhibits BCR-induced AKT activation in DLBCL [55], [56], and intracellular PTEN levels determine whether BCR signaling promotes cell death or cell survival via differential regulation of PI3K/AKT and NF-κB pathways [57]; loss of the PTEN gene was preferentially detected in GCB-DLBCL, and loss of PTEN expression defined a PI3K/AKT-dependent GCB-DLBCL [26], [27], [54]. On the other hand, studies also showed that besides the well-known inhibition of PI3K/AKT via lipid phosphatase activity, PTEN has many other functions including those in the nucleus [8], [58], [59], [60], negative regulation of central B-cell tolerance checkpoints [61], and roles in B-cell homeostasis in the immune system [62]. Paradoxically, PTEN is required for both initiation and maintenance of pre-B acute lymphoblastic leukemia cells, and loss of PTEN causes rapid cell death of transformed pre-B leukemia cells [61]. Such multi-directional functions of PTEN may explain the opposite prognostic effects of PTEN expression in AKT-hyperactive DLBCL and p-AKT− DLBCL cases, the lack of synergy between PTEN deletion and MYC rearrangement, and lack of distinct GEP signatures for PTEN expression and PTEN deletion.
As we have discussed in the previous review [43], loss/deficiency of PTEN expression can be attributed to genetic alterations and transcriptional, translational, and post-translational dysregulations. PTEN deletion (mostly heterozygous) and mutation as genetic mechanisms for PTEN deficiency and inactivation were observed in only approximately 11.3% and 10.6% of DLBCLs, respectively. These frequencies of PTEN gene alterations are much lower than those in some solid tumors (homozygous deletion, up to 42.5%; mutation, up to 44%) [20], [21], [63], which is consistent with previous studies in DLBCL and high-grade non-Hodgkin lymphoma [31], [64]. Distinct GEP signatures were identified for low PTEN mRNA expression and PTEN mutations, which suggest that BCR signaling and B cell differentiation regulate PTEN expression at the transcription level, and that PTEN is involved in CNS and immune response regulation in addition to its function in AKT/mTOR signaling. It has been reported that PTEN loss was associated with brain metastasis in melanoma patients [16].
We further explored the biological correlations and regulation mechanisms of PTEN expression. We surprisingly found that p-AKT (Ser473) nuclear expression and PTEN cytoplasmic expression were positively correlated in both training and validation cohorts, although no correlations were found between PTEN protein expression and AKT1 mRNA, nor between p-AKT protein expression and PTEN mRNA. As this was opposite to what one would expect (PTEN loss should correlate with increased p-AKT expression), we stained a separate set of FFPE tissue samples for the entire DLBCL training and validation cohorts using another PTEN monoclonal antibody from DAKO (clone 6H2.1). However, again we found that PTEN positivity and high expression were associated with p-AKT expression in DLBCL samples (data not shown). Such surprising positive (instead of negative) correlation between AKT and PTEN expression was also found in breast cancer, melanomas, and urinary bladder cancer by other studies [65], [66], [67]. Although paradoxical at the first glance, these results may reflect the complex regulation network of PI3K/AKT/PTEN with divergent activating and inactivating [68] mechanisms as demonstrated by previous studies [54]. It is known that phosphorylation at the Ser473 residue of AKT is mainly regulated by mTORC2 [69], [70]; AKT activation in GCB-DLBCL is the principal consequence of tonic BCR signaling but AKT activation must not depend soly on the BCR signaling [53]. Notably, our results [34] showed that p-AKT (Ser473) expression was primarily associated with Myc and Bcl-2 expression (targets of mTORC2 and BCR signaling) in GCB-DLBCL (P < 0.0001), and with IL-6 expression in ABC-DLBCL (P = 0.0005), whereas the association with PI3K was rather weak (P = 0.019 in the overall DLBCL cohort only). It is possible that the inhibitory effect of PTEN on AKT activation did not dominate the divergent mechanisms activating p-AKT (Ser473) expression among DLBCL cases; these divergent mechanisms may also indirectly up-regulate PTEN expression, since PTEN mRNA expression showed correlation with BCR signaling gene signatures, and Cyto-PTEN expression was associated with the ABC subtype, whereas loss of PTEN was associated with IgA/IgG expression. However, as cytoplasmic PTEN expression was associated with significantly decreased survivin expression (an indicator of AKT function in antiapoptosis) in ABC-DLBCL (Figure 1E), and we did not examine the p-AKT (Thr308) expression, PTEN may still have a significant role in repressing AKT function in DLBCL.
Moreover, the complexity between PTEN stability and function by posttranslational modifications may also contributes to the positive correlation between p-AKT and PTEN IHC results. Earlier studies indicated that phosphorylation of the PTEN’s C-terminal tail causes a conformation change that stabilizes PTEN but at the same time inhibits its phosphatase activity and binding to the plasma membrane [9]; PD-1 inhibits this stabilizing/inactivating phosphorylation [71]. Since the antibody we used detected total PTEN, it is possible that the observed PTEN positivity also included stabilized phosphorylated PTEN (which has no tumor suppressor function), and PTEN expression levels were not linearly correlated with PTEN function; common mechanisms for the phosphorylation modification of PTEN and AKT could exists. Notably, although PTEN mRNA expression showed significantly favorable prognostic effect and striking GEP signatures, such effect and distinct GEP signatures were lacking for PTEN protein expression in overall DLBCL. Therefore, the effect and interpretation based on PTEN mRNA expression in DLBCL by previous studies may deserve precaution.
In our DLBCL cohort, p-AKT expression was significantly associated with both PTEN and Myc expression; accordingly, Myc expression also showed positive association with Cyto-PTEN expression. MYC rearrangement and PTEN deletion showed antagonistic rather than synergistic prognostic effect, but the case number was small. Whether the antagonistic effect resulted from MYC/PTEN gene structures is unknown; comparably lower Myc expression and increased PTEN expression in these cases with concurrent MYC/PTEN abnormalities (Figure 6G) could be relevant. Notably, earlier functional studies demonstrated that Myc transcriptionally activates PTEN expression [72]; on the other hand, Myc negatively regulates PTEN expression posttranscriptionally through miRNAs [73], [74]. Conversely, PTEN represses Myc expression by inhibiting PI3K/AKT signaling and transcriptional modulation [27], [75]; scenario that PTEN deletion did not cooperate with Myc activation in tumorigenesis was also reported [76]. Again, these findings suggested the complexity of PTEN-involved molecular network.
In this study, loss of cytoplasmic PTEN expression was also associated with TP53 mutations and increased miRNA expression. Among the PTEN-targeting miRNAs showing negative correlations with Cyto-PTEN expression, miR-106b, miR-222, miR-200c, and miR-130b have been associated with poor prognosis [77], [78], [79], [80]. Targeting these overexpressing miRNAs could be a feasible strategy to increase PTEN expression in DLBCL.
Interestingly, we found that loss of Cyto-PTEN expression was associated with a lower mean level of PD-L1 expression in DLBCL, whereas PTEN deletion/mutation and expression of p-AKT, PI3K, or Nuc-PTEN had no association with PD-L1 expression. There was no correlation between PTEN and PD-L1 (CD274) mRNA expression. These data did not support the finding in vitro that loss of PTEN function was associated with increased PD-L1 expression [81]. However, in vivo studies found that PTEN loss through PTEN deletion and mutation did not increase PD-L1 expression in several mouse models. Because our data showed that both PD-L1 and Cyto-PTEN expression were associated with the ABC subtype and decreased miR-106b-3p and miR-130b-5p expression, which have been shown to target PD-L1 expression in cancer cells [82], [83], and PTEN-targeting miR-200c [73] is a key regulators of PD-L1 expression in acute myeloid leukemia [84], we speculated that common regulators of PD-L1 surface expression and PTEN cytoplasmic expression possibly underlie the positive correlation between Cyto-PTEN and membrane PD-L1 expression in this DLBCL cohort. Furthermore, because PD-L1 expression was often associated with greater likelihood of response to PD-1 blockade [85], our results may suggest that Cyto-PTEN− DLBCL cases with lower PD-L1 expression would be less likely to respond to PD-1 blockade, which is consistent with the observation that PTEN loss was associated with inferior outcomes in patients with metastatic melanoma who received PD-1 inhibitor therapy [86].
Conclusions
In summary, the prognostic significance of PTEN loss and high expression in de novo DLBCL treated with R-CHOP depends on AKT activities. PTEN deletion and mutation may have limited significance for poorer clinical outcome in DLBCL. PTEN protein and PTEN mRNA expression showed totally different prognostic effects and gene signatures in DLBCL. Our data suggest that the PI3K/PTEN/AKT and Myc signaling pathways are divergent rather than linear. Epigenetic and posttranslational mechanisms may play important roles in PTEN and PD-L1 expression.
Conflict of Interest Disclosure
The authors declare no conflict of interest.
Funding
This work was supported by National Cancer Institute/National Institutes of Health grants R01CA138688, R01CA187415 and 1RC1CA146299 to YL and KHY, and was also partially supported by National Cancer Institute and National Institutes of Health grants P50CA136411 and P50CA142509. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672. KHY is also supported by The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, a Gundersen Lutheran Medical Foundation Award, the University Cancer Foundation via the Sister institution network Fund at The University of Texas MD Anderson Cancer Center. KHY receives research support from Roche Molecular System, Gilead Sciences Pharmaceutical, Seattle Genetics, Dai Sanyo Pharmaceutical, Adaptive Biotechnology, Incyte Pharmaceutical, HTG and Perfectgen Molecular Diagnostics.
Author Contributions
X.W., X.C., R.S., Z.Y.X-M., A.T., Y. L., and K.H.Y designed the study, conducted the research, and performed the statistical analysis. X.W., X.C., R.S., C.T., A.T., J. Z., G.C.M., M. X., Y.M., K.J., X.T., Y.P., C.V., Y.X., K.D., A.C., A.O., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., J.H.K., J.H., M.P., A.J.M.F., M.B.M., B.M.P., J.N.W., M.A.P., S.L., R.N.M., L.J.M., Y.L., Z.Y.X-M., and K.H.Y. contributed vital new reagents, resources, technology, and analytical tools. X.W., A.T., C.V., W.C., K.D., A.C., A.O., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., J.H.K., J.H., M.P., A.J.M.F., M.B.M., B.M.P., J.N.W., M.A.P., Y.L., Z.Y.X-M., and K.H.Y. collected clinical and follow-up data under approval by the institutional review boards and the material transfer agreement. X.W., X.C., R.S., Z.Y.X-M., L.J.M., and K.H.Y. edited the manuscript. All authors contributed vital strategies, participated in discussions, and provided scientific input.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2018.03.002.
Appendix A. Supplementary data
Supplementary material
References
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JG, Sabet H, Tran T, Yu X. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli JJ, Sutherland J. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 3.Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H. Role of phosphatidylinositol 3 '-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–4186. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 5.Hasselblom S, Hansson U, Olsson M, Toren L, Bergstrom A, Nilsson-Ehle H, Andersson PO. High immunohistochemical expression of p-AKT predicts inferior survival in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Br J Haematol. 2010;149:560–568. doi: 10.1111/j.1365-2141.2010.08123.x. [DOI] [PubMed] [Google Scholar]
- 6.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfern RE, Redfern D, Furgason MLM, Munson M, Ross AH, Gericke A. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–2171. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- 8.Bononi A, Pinton P. Study of PTEN subcellular localization. Methods. 2015;77-78:92–103. doi: 10.1016/j.ymeth.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 10.Shen WH, Balajee AS, Wang JL, Wu H, Eng C, Pandolfi PP, Yin YX. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Trotman LC, Wang XJ, Alimonti A, Chen ZB, Teruya-Feldstein J, Yang HJ, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung JH, Ginn-Pease ME, Eng C. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res. 2005;65:4108–4116. doi: 10.1158/0008-5472.CAN-05-0124. [DOI] [PubMed] [Google Scholar]
- 13.Mithal P, Allott E, Gerber L, Reid J, Welbourn W, Tikishvili E, Park J, Younus A, Sangale Z, Lanchbury JS. PTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancer. Int J Urol. 2014;21:1209–1214. doi: 10.1111/iju.12571. [DOI] [PubMed] [Google Scholar]
- 14.Nagata Y, Lan KH, Zhou XY, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 15.da Costa AABA, Costa FD, Ribeiro AR, Guimaraes AP, Chinen LT, Lopes CAP, de Lima VCC. Low PTEN expression is associated with worse overall survival in head and neck squamous cell carcinoma patients treated with chemotherapy and cetuximab. Int J Clin Oncol. 2015;20:282–289. doi: 10.1007/s10147-014-0707-1. [DOI] [PubMed] [Google Scholar]
- 16.Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, Fox P, Bassett R, Hwu P, Gershenwald JE. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20:5527–5536. doi: 10.1158/1078-0432.CCR-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana M, Shibakita M, Ohno S, Kinugasa S, Yoshimura H, Ueda S, Fujii T, Rahman MA, Dhar DK, Nagasue N. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:1955–1960. doi: 10.1002/cncr.0678. [DOI] [PubMed] [Google Scholar]
- 18.Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y, Wakasugi T, Takahashi H, Funahashi H, Sato M, Takeyama H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008;8:56. doi: 10.1186/1471-230X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean SJ, Perks CM, Holly JM, Bhoo-Pathy N, Looi LM, Mohammed NA, Mun KS, Teo SH, Koobotse MO, Yip CH. Loss of PTEN expression is associated with IGFBP2 expression, younger age, and late stage in triple-negative breast cancer. Am J Clin Pathol. 2014;141:323–333. doi: 10.1309/AJCPR11DEAYPTUSL. [DOI] [PubMed] [Google Scholar]
- 20.McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer. 2008;99:1296–1301. doi: 10.1038/sj.bjc.6604680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- 22.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 23.Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res. 1998;4:811–815. [PubMed] [Google Scholar]
- 24.Cairns P, Evron E, Okami K, Halachmi N, Esteller M, Herman JG, Bose S, Wang SI, Parsons R, Sidransky D. Point mutation and homozygous deletion of PTEN/MMAC1 in primary bladder cancers. Oncogene. 1998;16:3215–3218. doi: 10.1038/sj.onc.1201855. [DOI] [PubMed] [Google Scholar]
- 25.Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19784 Diverse Solid Tumors. JAMA Oncol. 2016;2:1565–1573. doi: 10.1001/jamaoncol.2016.0891. [DOI] [PubMed] [Google Scholar]
- 26.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer M, Grau M, Lenze D, Wenzel SS, Wolf A, Wollert-Wulf B, Dietze K, Nogai H, Storek B, Madle H. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:12420–12425. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YY, Yao SN, Zhao Y, Yao ZH, Ma J, Xia QX, Fu K, Yang SJ. PTEN tumor suppressor plays less prognostic role than P53 tumor suppressor in diffuse large B-cell lymphoma. Leuk Lymphoma. 2010;51:1692–1698. doi: 10.3109/10428194.2010.502584. [DOI] [PubMed] [Google Scholar]
- 29.Fridberg M, Servin A, Anagnostaki L, Linderoth J, Berglund M, Soderberg O, Enblad G, Rosen A, Mustelin T, Jerkeman M. Protein expression and cellular localization in two prognostic subgroups of diffuse large B-cell lymphoma: Higher expression of ZAP70 and PKC-beta II in the non-germinal center group and poor survival in patients deficient in nuclear PTEN. Leuk Lymphoma. 2007;48:2221–2232. doi: 10.1080/10428190701636443. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Zhang P, Gao Y, Fan H, Zhang M, Wu J. Evaluation of AKT phosphorylation and PTEN loss and their correlation with the resistance of rituximab in DLBCL. Int J Clin Exp Pathol. 2015;8:14875–14884. [PMC free article] [PubMed] [Google Scholar]
- 31.Cui W, Ma M, Zheng S, Ma Z, Su L, Zhang W. PIK3CA amplification and PTEN loss in diffused large B-cell lymphoma. Oncotarget. 2017;8:66237–66247. doi: 10.18632/oncotarget.19889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visco C, Li Y, Xu-Monette ZY, Miranda RN, Green TM, Li Y, Tzankov A, Wen W, Liu WM, Kahl BS. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia. 2012;26:2103–2113. doi: 10.1038/leu.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120:3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Xu-Monette ZY, Jabbar KJ, Shen Q, Manyam GC, Tzankov A, Visco C, Wang J, Montes-Moreno S, Dybkaer K. AKT Hyperactivation and the Potential of AKT-Targeted Therapy in Diffuse Large B-Cell Lymphoma. Am J Pathol. 2017;187:1700–1716. doi: 10.1016/j.ajpath.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 36.Xu-Monette ZY, Dabaja BS, Wang X, Tu M, Manyam GC, Tzankov A, Xia Y, Zhang L, Sun R, Visco C. Clinical features, tumor biology, and prognosis associated with MYC rearrangement and Myc overexpression in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2015;28:1555–1573. doi: 10.1038/modpathol.2015.118. [DOI] [PubMed] [Google Scholar]
- 37.Ok CY, Chen J, Xu-Monette ZY, Tzankov A, Manyam GC, Li L, Visco C, Montes-Moreno S, Dybkaer K, Chiu A. Clinical Implications of Phosphorylated STAT3 Expression in De Novo Diffuse Large B-cell Lymphoma. Clin Cancer Res. 2014;20:5113–5123. doi: 10.1158/1078-0432.CCR-14-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu-Monette ZY, Moller MB, Tzankov A, Montes-Moreno S, Hu W, Manyam GC, Kristensen L, Fan L, Visco C, Dybkaer K. MDM2 phenotypic and genotypic profiling, respective to TP53 genetic status, in diffuse large B-cell lymphoma patients treated with rituximab-CHOP immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;122:2630–2640. doi: 10.1182/blood-2012-12-473702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu-Monette ZY, Zhang S, Li X, Manyam GC, Wang XX, Xia Y, Visco C, Tzankov A, Zhang L, Montes-Moreno S. p63 expression confers significantly better survival outcomes in high-risk diffuse large B-cell lymphoma and demonstrates p53-like and p53-independent tumor suppressor function. Aging (Albany NY) 2016;8:345–365. doi: 10.18632/aging.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Xu-Monette ZY, Tzankov A, Li X, Manyam GC, Murty V, Bhagat G, Zhang S, Pasqualucci L, Visco C. Loss of PRDM1/BLIMP-1 function contributes to poor prognosis of activated B-cell-like diffuse large B-cell lymphoma. Leukemia. 2017;31:625–636. doi: 10.1038/leu.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu-Monette ZY, Li L, Byrd JC, Jabbar KJ, Manyam GC, Maria de Winde C, van den Brand M, Tzankov A, Visco C, Wang J. Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. 2016;128:3083–3100. doi: 10.1182/blood-2016-05-715094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu WM, Li R, Sun JZ, Wang J, Tsai J, Wen W, Kohlmann A, Williams PM. PQN and DQN: algorithms for expression microarrays. J Theor Biol. 2006;243:273–278. doi: 10.1016/j.jtbi.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Huang H, Young KH. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging (Albany NY) 2015;7:1032–1049. doi: 10.18632/aging.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Xu-Monette ZY, Cao X, Manyam GC, Wang X, Tzankov A, Xia Y, Li X, Visco C, Sun R. Prognostic and biological significance of survivin expression in patients with diffuse large B-cell lymphoma treated with rituximab-CHOP therapy. Mod Pathol. 2015;28:1297–1314. doi: 10.1038/modpathol.2015.94. [DOI] [PubMed] [Google Scholar]
- 45.Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 47.Rho SB, Song YJ, Lim MC, Lee SH, Kim BR, Park SY. Programmed cell death 6 (PDCD6) inhibits angiogenesis through PI3K/mTOR/p70S6K pathway by interacting of VEGFR-2. Cell Signal. 2012;24:131–139. doi: 10.1016/j.cellsig.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A, Bruno A, Jouvet A, Polivka M, Adam C. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18:5203–5211. doi: 10.1158/1078-0432.CCR-12-0845. [DOI] [PubMed] [Google Scholar]
- 49.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 50.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 51.Ennajdaoui H, Howard JM, Sterne-Weiler T, Jahanbani F, Coyne DJ, Uren PJ, Dargyte M, Katzman S, Draper JM, Wallace A. IGF2BP3 Modulates the Interaction of Invasion-Associated Transcripts with RISC. Cell Rep. 2016;15:1876–1883. doi: 10.1016/j.celrep.2016.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carico C, Nuno M, Mukherjee D, Elramsisy A, Dantis J, Hu J, Rudnick J, Yu JS, Black KL, Bannykh SI. Loss of PTEN is not associated with poor survival in newly diagnosed glioblastoma patients of the temozolomide era. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Havranek O, Xu J, Kohrer S, Wang Z, Becker L, Comer JM, Henderson J, Ma W, Man Chun Ma J, Westin JR. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood. 2017;130:995–1006. doi: 10.1182/blood-2016-10-747303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erdmann T, Klener P, Lynch JT, Grau M, Vockova P, Molinsky J, Tuskova D, Hudson K, Polanska UM, Grondine M. Sensitivity to PI3K and AKT inhibitors is mediated by divergent molecular mechanisms in subtypes of DLBCL. Blood. 2017;130:310–322. doi: 10.1182/blood-2016-12-758599. [DOI] [PubMed] [Google Scholar]
- 55.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94:193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 56.Young RM, Shaffer AL, Phelan JD, Staudt LM. B-Cell Receptor Signaling in Diffuse Large B-Cell lymphoma. Semin Hematol. 2015;52:77–85. doi: 10.1053/j.seminhematol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng SH, Hsia CY, Feng B, Liou ML, Fang XY, Pandolfi PP, Liou HC. BCR-mediated apoptosis associated with negative selection of immature B cells is selectively dependent on Pten. Cell Res. 2009;19:196–207. doi: 10.1038/cr.2008.284. [DOI] [PubMed] [Google Scholar]
- 58.Chang CJ, Mulholland DJ, Valamehr B, Mosessian S, Sellers WR, Wu H. PTEN nuclear localization is regulated by oxidative stress and mediates p53-dependent tumor suppression. Mol Cell Biol. 2008;28:3281–3289. doi: 10.1128/MCB.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gil A, Andres-Pons A, Pulido R. Nuclear PTEN: a tale of many tails. Cell Death Differ. 2007;14:395–399. doi: 10.1038/sj.cdd.4402073. [DOI] [PubMed] [Google Scholar]
- 60.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 61.Shojaee S, Chan LN, Buchner M, Cazzaniga V, Cosgun KN, Geng H, Qiu YH, von Minden MD, Ernst T, Hochhaus A. PTEN opposes negative selection and enables oncogenic transformation of pre-B cells. Nat Med. 2016;22:379–387. doi: 10.1038/nm.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srividya MR, Thota B, Shailaja BC, Arivazhagan A, Thennarasu K, Chandramouli BA, Hegde AS, Santosh V. Homozygous 10q23/PTEN deletion and its impact on outcome in glioblastoma: a prospective translational study on a uniformly treated cohort of adult patients. Neuropathology. 2011;31:376–383. doi: 10.1111/j.1440-1789.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- 64.Sakai A, Thieblemont C, Wellmann A, Jaffe ES, Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–3415. [PubMed] [Google Scholar]
- 65.Panigrahi AR, Pinder SE, Chan SY, Paish EC, Robertson JF, Ellis IO. The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol. 2004;204:93–100. doi: 10.1002/path.1611. [DOI] [PubMed] [Google Scholar]
- 66.Slipicevic A, Holm R, Nguyen MT, Bohler PJ, Davidson B, Florenes VA. Expression of activated Akt and PTEN in malignant melanomas: relationship with clinical outcome. Am J Clin Pathol. 2005;124:528–536. doi: 10.1309/YT58WWMTA6YR1PRV. [DOI] [PubMed] [Google Scholar]
- 67.Harris LD, De La Cerda J, Tuziak T, Rosen D, Xiao L, Shen Y, Sabichi AL, Czerniak B, Grossman HB. Analysis of the expression of biomarkers in urinary bladder cancer using a tissue microarray. Mol Carcinog. 2008;47:678–685. doi: 10.1002/mc.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Davies MA. Regulation, role, and targeting of Akt in cancer. J Clin Oncol. 2011;29:4715–4717. doi: 10.1200/JCO.2011.37.4751. [DOI] [PubMed] [Google Scholar]
- 70.Robbins HL, Hague A. The PI3K/Akt Pathway in Tumors of Endocrine Tissues. Front Endocrinol (Lausanne) 2015;6:188. doi: 10.3389/fendo.2015.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patsoukis N, Li L, Sari D, Petkova V, Boussiotis VA. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol. 2013;33:3091–3098. doi: 10.1128/MCB.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur M, Cole MD. MYC acts via the PTEN tumor suppressor to elicit autoregulation and genome-wide gene repression by activation of the Ezh2 methyltransferase. Cancer Res. 2013;73:695–705. doi: 10.1158/0008-5472.CAN-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen P, Guo X, Zhang L, Zhang W, Zhou Q, Tian Z, Zheng Y, Liao Q, Wang H, Li G. MiR-200c is a cMyc-activated miRNA that promotes nasopharyngeal carcinoma by downregulating PTEN. Oncotarget. 2017;8:5206–5218. doi: 10.18632/oncotarget.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benhamou D, Labi V, Novak R, Dai I, Shafir-Alon S, Weiss A, Gaujoux R, Arnold R, Shen-Orr SS, Rajewsky K. A c-Myc/miR17-92/Pten Axis Controls PI3K-Mediated Positive and Negative Selection in B Cell Development and Reconstitutes CD19 Deficiency. Cell Rep. 2016;16:419–431. doi: 10.1016/j.celrep.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh AK, Grigorieva I, Steele R, Hoover RG, Ray RB. PTEN transcriptionally modulates c-myc gene expression in human breast carcinoma cells and is involved in cell growth regulation. Gene. 1999;235:85–91. doi: 10.1016/s0378-1119(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 76.Radziszewska A, Choi D, Nguyen KT, Schroer SA, Tajmir P, Wang L, Suzuki A, Mak TW, Evan GI, Woo M. PTEN deletion and concomitant c-Myc activation do not lead to tumor formation in pancreatic beta cells. J Biol Chem. 2009;284:2917–2922. doi: 10.1074/jbc.M805183200. [DOI] [PubMed] [Google Scholar]
- 77.Battistella M, Romero M, Castro-Vega LJ, Gapihan G, Bouhidel F, Bagot M, Feugeas JP, Janin A. The High Expression of the microRNA 17-92 Cluster and its Paralogs, and the Down-Regulation of the Target Gene PTEN are Associated with Primary Cutaneous B-cell Lymphoma Progression. J Invest Dermatol. 2015 doi: 10.1038/jid.2015.27. [DOI] [PubMed] [Google Scholar]
- 78.Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Tejero R, Navarro A, Campayo M, Vinolas N, Marrades RM, Cordeiro A, Ruiz-Martinez M, Santasusagna S, Molins L, Ramirez J. miR-141 and miR-200c as markers of overall survival in early stage non-small cell lung cancer adenocarcinoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malumbres R, Sarosiek KA, Cubedo E, Ruiz JW, Jiang XY, Gascoyne RD, Tibshirani R, Lossos IS. Differentiation stage-specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood. 2009;113:3754–3764. doi: 10.1182/blood-2008-10-184077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 82.Cioffi M, Trabulo SM, Vallespinos M, Raj D, Kheir TB, Lin ML, Begum J, Baker AM, Amgheib A, Saif J. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget. 2017;8:21609–21625. doi: 10.18632/oncotarget.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y, Zhou H, Li R, Wang K, Wang W. MiR-20b, -21, and -130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol. 2014;75:348–353. doi: 10.1016/j.humimm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Pyzer AR, Stroopinsky D, Rosenblatt J, Anastasiadou E, Rajabi H, Washington A, Tagde A, Chu JH, Coll M, Jiao AL. MUC1 inhibition leads to decrease in PD-L1 levels via upregulation of miRNAs. Leukemia. 2017 doi: 10.1038/leu.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material