Abstract
This report describes the first confirmed case of isolated pyomyositis caused by a hypervirulent strain of Klebsiella pneumoniae. Pyomyositis is almost universally caused by gram-positive organisms and while the recent emergence of invasive disease due to hypervirulent K. pneumoniae has been well documented, the most common clinical manifestation reported is liver abscess. The K. pneumoniae isolate in our case had a hypermucousviscous phenotype as demonstrated by a positive string test and was confirmed to be hypervirulent with molecular testing. Documenting the extrahepatic manifestations of hypervirulent Klebsiella pneumoniae strains is important to increase clinical awareness and in guiding empiric antibiotic regimens.
Keywords: Klebsiella, Pyomyositis, Gastrocnemius, Virulence
Case presentation
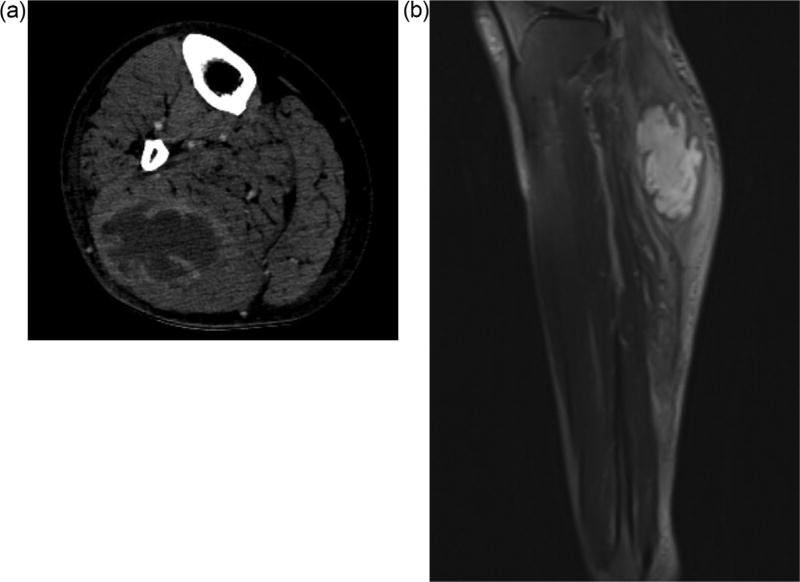
Our patient was a 49-year-old man with uncontrolled diabetes (hemoglobin A1c 12.5%) and hypertension who presented to our facility in Atlanta, Georgia, United States with two weeks of right calf pain and swelling. He reported no recent trauma, intravenous drug use, nor travel and reported moderate alcohol intake. He was born in Mexico but had not travelled outside the United States in the last 2 years. On presentation he was afebrile with a swollen, erythematous, and tender right calf. He had normal bilateral dorsalis pedis pulses. While the white blood cell count was normal, his c-reactive protein was >48 and ESR was 92. Blood cultures obtained were sterile. A computed tomography scan demonstrated a large multiloculated fluid collection with enhancing rim in the right gastrocnemius consistent with an abscess (Figure 1A). This was redemonstrated on MRI with gadolinium enhancement (Figure 1B). He was initially started on vancomycin and piper-acillin-tazobactam for presumed pyomyositis.
Figure 1.
A) Axial CT image of right leg showing intramuscular fluid collection with enhancing rim and internal septa in gastrocnemius. B) Sagittal T2 MRI image of right leg.
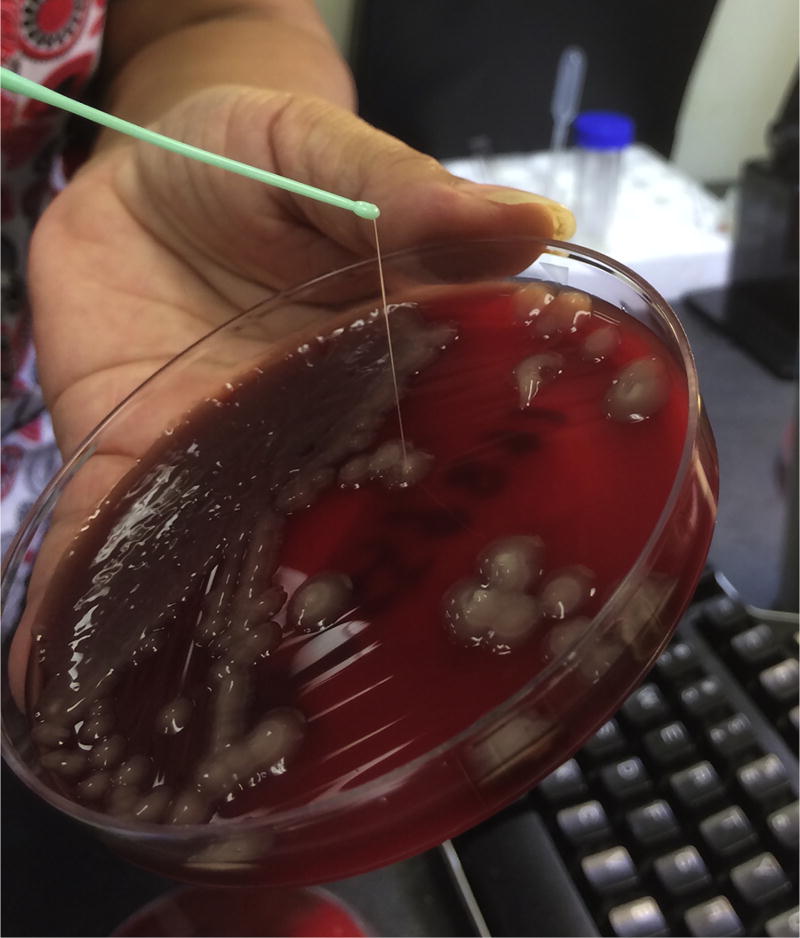
Surgical incision and drainage was performed, revealing a septated abscess with a large amount of purulence. Cultures grew a hypermucoviscous strain of K. pneumoniae as demonstrated by a positive string test (Figure 2). As has been shown for other strains of hypervirulent K. pneumoniae, the isolate was susceptible to all routinely tested antibiotics except ampicillin (Siu et al., 2012). Once drug susceptibility results were available the initial antibiotic regimen was changed to levofloxacin 750 mg by mouth daily. Ultimately, a drain remained in place for 2 weeks and the patient was treated with a total course of four weeks of antibiotic therapy, which resulted in a full and uncomplicated recovery.
Figure 2.
Culture of Klebsiella pneumoniae isolated from the muscle aspirate on sheep blood agar illustrating a positive string test (>5 mm in string length).
Additional work up included a normal abdominal ultrasound and negative stool and urine cultures performed after 5 days of antibiotics. Polymerase chain reaction was performed on the isolate and revealed presence of the wzy gene, indicating a K2 serotype. The isolate also harbored several genes (rmpA2, which encodes for increased capsular polysaccharide production, iutA, terW, and silS) indicating the presence of the pLVPK virulence plasmid.
Discussion
While the association of hypervirulent K. pneumoniae strains causing liver abscess has become a well-recognized syndrome in Asia, especially in Taiwan, an increasing number of cases are being reported in Europe and North America (Lee et al., 2010; Siu et al., 2012), although it remains a rare entity. It is unclear if people of Asian descent have unique genetic risk for this infection or if the organism is more prevalent in Asian countries. Diabetes mellitus, particularly with poor glycemic control, has been identified as one of the strongest risk factors for invasive disease. Gastrointestinal colonization with hypervirulent strains is hypothesized to lead to translocation and spread to the liver by the portal venous system. A rectal swab from our patient failed to grow the organism, however he had already been on antibiotics at the time of sampling. Along with liver abscess, other common manifesations of disease include bacteremia, endogenous endophthalmitis, CNS infection, osteomyelitis, and pneumonia (Mustafa and Aduriz-Lorenzo, 2016; Prokesch et al., 2016; Tang et al., 2015). Outbreaks of highly drug resistant hypervirulent K. pneumoniae have been reported, though our patient’s strain was pan-sensitive (Gu et al., 2017). This unique case of pyomyositis adds to the list of extrahepatic manifestations due to K. pneumoniae. A majority of cases of pyomyositis are caused by gram-positive organisms. K. pneumoniae has been reported as a rare cause as well, but no prior cases have been identified as a hypervirulent strain (Wang et al., 2001). Increased awareness of hypervirulent Klebsiella pneumoniae can both help the laboratory in being able to recognize hypermucoviscous strains and assist the clinician with choosing appropriate empiric antibiotic coverage for invasive infectious syndromes.
Most hypervirulent K. pneumoniae isolates have been shown to express the pLVPK virulence plasmid. The pLVPK is a 219-kb virulence plasmid with 251 open reading frames (ORFs) which encodes for capsular polysaccharide synthesis regulator p-rmpA and its homolog p-rmpA2 (Chen et al., 2004). P-rmpA/A2 is present in most hypervirulent isolates and was found in more than 91% of spontaneous invasive K. pneumoniae infections with the HV phenotype in a cross-sectional study in Taiwan (Lee et al., 2010). The presence of this plasmid is thought to increase polysaccharide synthesis but the actual correlation between increased polysaccharide synthesis and increased virulence remains unclear. In a recent study, K1 and K2 serotypes were found to be more virulent than non-K1/K2 isolates because the former lack mannose sequences recognized by macrophages, thus protecting them from lectinophagocytosis (Kabha et al., 1995).
Conclusion
There has been increasing prevalence of infections caused by strains of hypervirulent K. pneumoniae, initially in Asia, and more recently on other continents. The geographic spread of this syndrome has been accompanied by an expanding spectrum of reported clinical manifestations. Multiple virulence genes encoded on the pLVPK plasmid are thought to be responsible for the invasive nature of these isolates. Our report of isolated pyomyositis due to hypervirulent K. pneumoniae provides an example of how this unique virulence mechanism can result in a diverse range of clinical presentations.
Acknowledgments
We would like to add an acknowledgement: The authors acknowledge Barbara Everette for her laboratory assistance in specimen testing and processing.
Financial support
DSW is supported by a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award. RRK is supported in part by the National Institutes of Allergy and Infectious Diseases Award (K23AI103044).
Footnotes
Contribution
All authors had access to all of the data and all were involved in the production of this manuscript.
Potential conflicts of interest
The authors declare no competing financial interests.
Ethical approval
The authors have read the policy on ethical consent and the standards of animal care and this manuscript complies with those policies. DPS is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene. 2004;337:189–98. doi: 10.1016/j.gene.2004.05.008. doi: http://dx.doi.org/10.1016/j.gene.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30489-9. doi: http://dx.doi.org/10.1016/S1473-3099(17)30489-9 pii: S1473-3099(17)30489-9. [Epub ahead of print]. [DOI] [PubMed]
- Kabha K, Nissimov L, Athamna A, Keisari Y, Parolis H, Parolis LA, et al. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun. 1995;63(3):847–52. doi: 10.1128/iai.63.3.847-852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Liu JW, Su LH, Chien CC, Li CC, Yang KD. Hypermucoviscosity associated with Klebsiella pneumoniae-mediated invasive syndrome: a prospective cross-sectional study in Taiwan. Int J Infect Dis. 2010;14(8):e688–92. doi: 10.1016/j.ijid.2010.01.007. doi: http://dx.doi.org/10.1016/j.ijid.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Mustafa OM, Aduriz-Lorenzo PM. Images in clinical medicine. Hypopyon and Klebsiella sepsis. N Engl J Med. 2016;374(26):e33. doi: 10.1056/NEJMicm1509932. doi: http://dx.doi.org/10.1056/NEJMicm1509932. [DOI] [PubMed] [Google Scholar]
- Prokesch BC, TeKippe M, Kim J, Raj P, TeKippe EM, Greenberg DE. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. 2016;16(9):e190–5. doi: 10.1016/S1473-3099(16)30021-4. doi: http://dx.doi.org/10.1016/S1473-3099(16)30021-4. [DOI] [PubMed] [Google Scholar]
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–7. doi: 10.1016/S1473-3099(12)70205-0. doi: http://dx.doi.org/10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- Tang HL, Lai YC, Chiou CS, Liu PY, Weng LL, Hou W, et al. Liver abscess caused by Klebsiella pneumoniae in a red-footed tortoise. J Microbiol Immunol Infect. 2015;48(3):347–9. doi: 10.1016/j.jmii.2013.12.004. doi: http://dx.doi.org/10.1016/j.jmii.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Wang TK, Wong SS, Woo PC. Two cases of pyomyositis caused by Klebsiella pneumoniae and review of the literature. Eur J Clin Microbiol Infect Dis. 2001;20(8):576–80. doi: 10.1007/s100960100556. [DOI] [PubMed] [Google Scholar]