Abstract
The stimulation of angiotensin II (Ang II), the effector peptide of renin–angiotensin system, has been reported to increase the expression of vascular endothelial growth factor (VEGF) through the activation of the Ang II type 1 receptor (AT1R). In this study, we investigated whether hyperglycemia (HG, 33 mM glucose) in ARPE-19 cells could promote the expression of VEGF independently of Ang II through prorenin receptor (PRR), via an NADPH oxidase (Nox)-dependent mechanism. ARPE-19 cells were treated with the angiotensin converting enzyme (ACE) inhibitor perindopril to block the synthesis of Ang II. Treatment with HG induced VEGF expression in ARPE-19 cells, which was attenuated by pretreatment with the inhibitors of Nox, but not those of nitric oxide synthase, xanthine oxidase and mitochondrial O2 synthesis. In addition, Nox-derived and H2O2 signaling in the regulation of VEGF was determined by using both polyethylene glycol (PEG)-catalase (CAT) and PEG-superoxide dismutase (SOD). We demonstrated that small interfering RNA (siRNA)-mediated knockdown of PRR, Nox2 and Nox4 significantly reduced the HG-induced stimulation of VEGF. On the other hand, Nox4 overexpression significantly potentiated PRR-induced stimulation of VEGF under hyperglycemia in ARPE-19 cells. Furthermore, Nox4 was shown to be associated with enhanced activities of ERK1/2 and NF-κB (p65), indicating their involvement in PRR-induced activation of VEGF under HG in ARPE-19 cells. Our results support the hypothesis that Nox4-derived reactive oxygen species (ROS) signaling is implicated in the hyperglycemia-induced increase of VEGF expression through PRR in ARPE-19 cells. However, further work is needed to evaluate the role of PRR and Nox-s in HG-induced stimulation of VEGF in vivo.
Keywords: Prorenin receptor, retina, NADPH oxidase (Nox), VEGF, renin–angiotensin system (RAS), diabetic retinopathy
Introduction
The classic renin–angiotensin-system (RAS) is involved in the regulation of blood pressure and electrolyte homeostasis [1]. All components of the RAS have been detected in the eye, including retinal pigment epithelium (RPE) [2,3], a tissue that underlies the photoreceptor cells. Angiotensin (Ang) II, the effector peptide hormone of RAS, is known to mediate its function through Ang II Type 1 (AT1R) and Type 2 (AT2R) receptors [4] and the stimulation of Ang II has been reported to increase the expression of vascular endothelial growth factor (VEGF) through the activation of the AT1R [5]. Oxidative stress is a key pathogenic factor in diabetic retinopathy (DR) [6]. Nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (Nox) isoforms Nox1 and Nox4 have been reported to be involved in vascular oxidative stress in response to Ang II [7] both in vitro as well as in vivo. Recently, much attention has been focused towards understanding the biological roles of prorenin, a precursor or renin, and its recently identified receptor named as ATPase, H+ transporting, lysosomal accessory protein 2 (ATP6AP2) or prorenin receptor (PRR) [8]. It is known that prorenin binds PRR three times more efficiently than renin [9]. Interestingly, it was shown that the soluble PRR protein level in vitreous fluids was higher in proliferative diabetic retinopathy (PDR) than in non-diabetic control eyes, and this correlated significantly with the levels of vitreous prorenin and VEGF [10].
Oxidative stress induced by diabetes and activated RAS may lead to both impaired antioxidant protection [11] and increased production of reactive oxygen species (ROS) [12,13]. Hyperglycemia (HG)-induced increase of ROS plays a crucial role in ocular diseases, including DR [14]; however, its underlying molecular mechanisms remain unknown. ROS such as superoxide inion radical ( ) and hydrogen peroxide (H2O2) function as signaling molecules in many aspects of growth factor-mediated physiological responses including angiogenesis [15]. The major source of ROS in the retina and endothelial cells has been reported to be Nox [7], where Nox4 as a major catalytic subunit plays an important role in endothelial angiogenesis [16] and diabetes-associated complications [17]. Nox4 activation in a diabetic db/db mouse model has been reported to upregulate VEGF and reduce the integrity of the endothelial blood–retinal barrier [18]. Diabetes-induced stimulation of VEGF expression, leukocyte adhesion and breakdown of the blood-retinal barrier are all reported to be associated with increase in ROS [19]. Also, Nox4-generated ROS in endothelial cells has been reported to contribute to the VEGF-induced angiogenesis [20].
In this study, we address the hypothesis that hyperglycemia promotes the expression of VEGF independently of Ang II through the PRR, via a Nox-dependent mechanism and investigate whether ROS derived from Nox functions as the signaling molecule in the stimulation of downstream factors such as VEGF and VEGF receptor (R). VEGF is known to induce angiogenesis primarily through the receptor tyrosine kinase VEGFR2 [21]. Understanding these mechanisms may provide insight into the Nox and redox signaling components as potential therapeutic targets for treatment of angiogenesis-dependent retinal diseases, including DR.
Materials and methods
Cell culture and treatment
ARPE-19 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). ARPE-19 cell line was authenticated by a short tandem repeat (STR) analysis as shown in Supplementary Appendix 1. ARPE-19 cells were grown in Dulbecco’s Modified Eagle’s medium (DMEM): F12 supplemented with 10% (v/v) fetal calf serum (FCS) and a mixture of streptomycin (100 µg/mL)-penicillin (100 IU/mL) (LONZA), and passaged twice a week. ARPE-19 cells were incubated at 37 °C, and 5% CO2 and 90% relative humidity. For glucose treatment, cells were exposed to 5.5 mM (normoglycemia, NG) as control or 33 mM d-glucose (hyperglycemia or high glucose, HG) and cultured for 48 h. To study HG-mediated induction of PRR signaling independently of Ang II, cells were treated with angiotensin converting enzyme (ACE) inhibitor perindopril (10 µmol/L, Sigma-Aldrich, St. Louis, MO, USA) for 24 h, followed by stimulation with human prorenin (Cayman Chemical, Ann Arbor, MI, USA, Item # 10007599) at a final concentration of 10 nmol/l and HG for 48 h. Specificity of ROS generation by Nox was assessed by treating hyperglycemic cells with the inhibitors of NADPH oxidase [diphenyleneiodonium, (DPI), 10 µM], nitric oxide synthase [NG-nitro-l-arginine methyl ester (l-NAME), 500 µM], mitochondrial O2 synthesis (Rotenone, 10 µM), and xanthine oxidase (Allopurinol, 100 µM) for 24 h.
RNA interference (RNAi)
Following our previous protocol [22], transfection of small interfering RNA (siRNA) for targeting endogenous genes was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The pre-designed siRNAs from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA, cat# sc-41586 [Nox4]; and Ambion (Life Technologies, Grand Island, NY, USA, cat#. s19790) were used to knockdown endogenous Nox4 and PRR, respectively. The siRNA sequences used in the knockdown of the human PRR/Nox4gene were: Nox4-sense: 5′-AACGAAGGGGUUAAACACCUCtt-3′, antisense: 5′-GAGGUGU UUAACCCCUUCGUUtt-3′; PRR- sense: 5′-GGUCUGUUGUUUU CCGAAATT-3′, antisense: 5′-UUUCGGAAAACAACAGACCCT-3′. The scrambled siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA, sc-44230: Sense, 5′-CGAACUCACUGGUCUGACCtt-3′; Antisense, 5′-GGUCAGACCAGUGAGUUCGtt-3′) was used as the negative control (NC). ARPE-19 cells were grown to 80% confluence and transfected with 20 nM of PRR/Nox4 siRNA, or 20 nM of NC. The siRNAs were transfected using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were harvested for RNA analysis 48 h after siRNA transfection.
Nox4 overexpression
To determine the signaling role of Nox4 in the regulation of VEGF expression, hyperglycemic cells were transfected with pcDNA3.1 plasmid containing the coding sequence of human Nox4 (pcDNA3.1-Nox4, a kind gift from Dr. David Lambeth, Emory University School of Medicine, Atlanta, GA) or pcDNA3.1 empty vector (control) for 48 h and treated with 100 U/ml polyethylene glycol (PEG, Sigma, St. Louis, MO, USA, cat# 88440) alone, PEG-superoxide dismutase (SOD, Sigma, St. Louis, MO, USA, cat# S9549), or PEG-CAT (CAT, Sigma, St. Louis, MO, USA, cat# C4963) for the last 24 h before harvesting. Cells treated with 5.5 mM glucose, scramble siRNAs, and PEG alone were maintained as controls.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Following our previous protocol [23], cDNA synthesis on 200 ng of total RNA extracted from ARPE-19 cells using QIAzol lysis reagent and RNeasy Mini kit (cat# 74104) was carried out using a combination of oligo (dT) and random primers following the instruction of the QuantiTect Reverse Transcription Kit (Cat no. 205313; Qiagen Inc., Valencia, CA, USA). The mixture was incubated at 42 °C for 15 min followed by incubation at 95 °C for 5 min to inactivate reverse transcriptase. The qRT-PCR reactions were conducted in MyiQ Cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) in a 25 µL PCR master mix containing 2 µL cDNA, 1 × Quantifast SYBR Green PCR Master mix (Qiagen Inc., Valencia, CA, USA), and 300 nM gene-specific primers. The qRT-PCR amplification was performed in two-step cycling protocol, with a denaturation step at 95 °C and a combined annealing/extension step at 60 °C. Briefly, the protocols were as follows: 5 min at 95 °C, 40 cycles at 95 °C for 10 s (for denaturation) and 60 °C for 30 s (for combined annealing/ extension). The qRT-PCR data were normalized to the expression level of a reference gene, hypoxanthine-guanine phosphoribosyltransferase (HPRT). The primers were designed using the oligonucleotide properties calculator program (http://www.unc.edu/~cail/biotool/oligo/index.html) and the BLAST algorithm. All primer sequences and the amplicon sizes are shown in Table 1. Finally, the primers specificity was verified by performing a melt curve analysis and also confirmed by 1% agarose gel electrophoresis. The delta cycle threshold (ΔCt) values were used to analyze the expression levels of mRNA as follows: ΔCt = average mRNA Ct – average of housekeeping gene (HPRT) Ct. Change in gene expression was calculated according to the ΔΔCt, where ΔΔCt = ΔCt of the treated group – ΔCt of control group. The fold change for mRNA expression level normalized against HPRT was calculated using 2−ΔΔCt [24].
Table 1.
Primers used for quantitative real-time PCR.
| Gene | Primer sequence Amplicon size | (bp) |
|---|---|---|
| HPRT | Forward: 5′-ACA GGA CTG AAC GTC TTG CTC G-3′ | 87 |
| Reverse: 5′-TAT AGC CCC CCT TGA GCA CAC-3′ | ||
| AT1R | Forward: 5′-TGC AGA TAT TGT GGA CAC GGC C-3′ | 154 |
| Reverse: 5′-GTG GGA TTT GGC TTT TGG GGG-3′ | ||
| ATP6AP2 (PRR) | Forward: 5′-CAG ACG TGG CTG CAT TGT CC-3′ | 144 |
| Reverse: 5′-CTG GGG GTA GAG CCA GTT TGT T-3′ | ||
| Nox1 | Forward: 5′-GTC GCA ATC TGC TGT CCT TCC T-3′ | 113 |
| Reverse: 5′-GCA GAT CAT ATA GGC CAC CAG CT-3′ | ||
| Nox2 | Forward: 5′-GAA TGG TGT GTG AAT GCC CGA GT-3′ | 132 |
| Reverse: 5′-CAG GCC TCC TTC AGG GTT CTT TAT-3′ | ||
| Nox3 | Forward: 5′-AAC GAG AGC TAC CTC AAC CCT G-3′ | 140 |
| Reverse: 5′-CTG ATG AAC TCA GTT GAC GAG GTC-3′ | ||
| Nox4 | Forward: 5′-TCA CTA CCT CCA CCA GAT GTT GG-3′ | 134 |
| Reverse: 5′-TCT GTG ATC CTC GGA GGT AAG C-3′ | ||
| Nox5 | Forward: 5′-GAG TCC TTC TTT GCA GAG CGA TTC-3′ | 152 |
| Reverse: 5′-TCG ATG TCA TAC ACC TGG AAG AGG-3′ | ||
| VEGF-A | Forward: 5′-TGC CAT CCA ATC GAG ACC CTG-3′ | 156 |
| Reverse: 5′-GGT GAT GTT GGA CTC CTC AGT G-3′ | ||
| VEGFR2 | Forward: 5′-CAG TCT GGG AGT GAG ATG AAG A-3′ | 138 |
| Reverse: 5′-ATG GAC CCT GAC AAA TGT GCT G-3′ |
Immunoblotting
ARPE-19 cells grown in 12-well plates were washed in ice-cold phosphate-buffered saline (PBS: 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.8) and lysed in RIPA buffer (50 mmol/L Tris–HCl (pH 8.0), 150 mmol/L NaCl, 100 µg/mL phenylmethylsulfonyl fluoride, 1% NP-40, 50 mmol/L NaF, 2 mmol/L EDTA) supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The cell lysates were centrifuged for 5 min at 14 000g and the protein concentration was determined by Lowry assay [25]. The aliquots containing equal amount of proteins (50 µg) were solubilized in XT sample buffer (Biorad, Hercules, CA, USA). Following our previous protocol [26], proteins were resolved on criterion (Biorad, Hercules, CA, USA) gels, and transferred to PVDF membranes (Millipore Corporation, Billerica, MA, USA) using Trans-Blot® semi-dry transfer cell (Bio-Rad, Hercules, CA, USA). After blocking the membrane with 5% fat-free milk at room temperature (~22 °C) for 2 h, the blot was incubated with specific primary antibodies: Nox4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, cat# sc-30141), p44/42 MAPK (Cell Signaling, Danvers, MA, USA, cat# 4695), phospho-p44/42 (Cell Signaling, Danvers, MA, USA, cat# 4370), p65 (NF-κB; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, 1:1000, sc-8008, a gift), and actin (Sigma, St. Louis, MO, USA, cat#A5441) overnight at 4 °C. Blots were washed three times with PBST (1 × PBS, 0.05% Tween-20), and incubated with a secondary antibody conjugated with horseradish peroxidases for 1 h at room temperature (~22 °C). Blots were washed again three times in 15 min intervals with PBST. The western image was captured with ChemiDocTM MP imager (Biorad, Hercules, CA, USA) using Luminata Forte Western HRP substrate (WBLUF0100, EMD Millipore). For repeated immunoblotting, membranes were stripped in the Restore Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
Data were analyzed using Sigmaplot (Systat Software, Inc., San Jose, CA, USA). Unless stated otherwise, data are presented as means ± standard error of the mean (SEM). Statistical differences between groups were evaluated using either a Student t-test or a one-way analysis of variance (ANOVA) with Student–Newman–Keuls multiple comparison test. Values of p < 0.05 were considered statistically significant.
Results
High glucose-induced VEGF expression in ARPE-19 cells is dependent on Nox signaling pathway
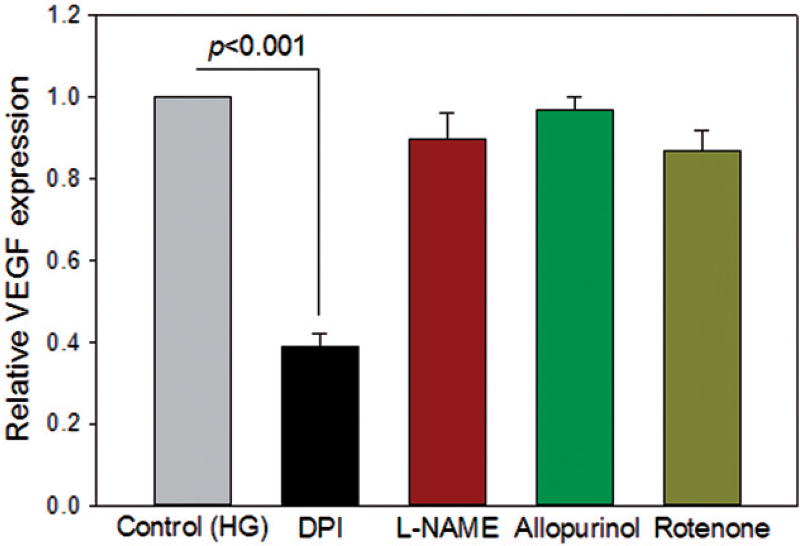
Incubation of ARPE-19 cells in high glucose (HG) increases the expression of VEGF [27,28]. Several enzymes including NADPH oxidase are known to generate low levels of ROS [6,29]. To investigate the role of ROS generating enzymes in the VEGF response to HG, we tested inhibitors of them on the HG-induced increase of VEGF expression. Hyperglycemic ARPE-19 cells were treated with the inhibitors of NADPH oxidase (10 µM DPI), nitric oxide synthase (500 µM l-NAME), mitochondrial respiratory chain complex I (10 µM rotenone), and xanthine oxidase (100 µM allopurinol). The NADPH oxidase inhibitor, DPI, abolished the HG-stimulated increase of VEGF transcript level in ARPE-19 cells (p < 0.001) (Figure 1). In contrast, inhibitors of nitric oxide synthase, xanthine oxidase, and mitochondrial respiratory chain complex I had no effect on the HG-mediated increase in VEGF expression (Figure 1).
Figure 1.
Nox is the major source of ROS signaling in HG-induced VEGF expression in ARPE-19 cells. As described in “Materials and Methods”, cells were treated with ACE inhibitor perindopril (10 µmol/L) for 24 h, followed by stimulation with human prorenin at a final concentration of 10 nmol/l and HG for 48 h. NADPH oxidase inhibitor [diphenyleneiodonium (DPI), 10 µM], NOS inhibitor [NG-nitro-l-arginine methyl ester (l-NAME), 500 µM], xanthine oxidase inhibitor (Allopurinol, 100 µM), or mitochondrial respiratory chain complex 1 inhibitor (Rotenone, 10 µM) were added for the last 24 h. Perindopril-treated cells incubated in HG alone were used as control. DPI, not other enzymes, significantly reduced the HG-mediated increase of VEGF expression. Values are presented as mean ± SEM; n = 4; p values vs. control (set to 1) by ANOVA.
Hyperglycemia induces PRR and Nox signaling independent of Ang II
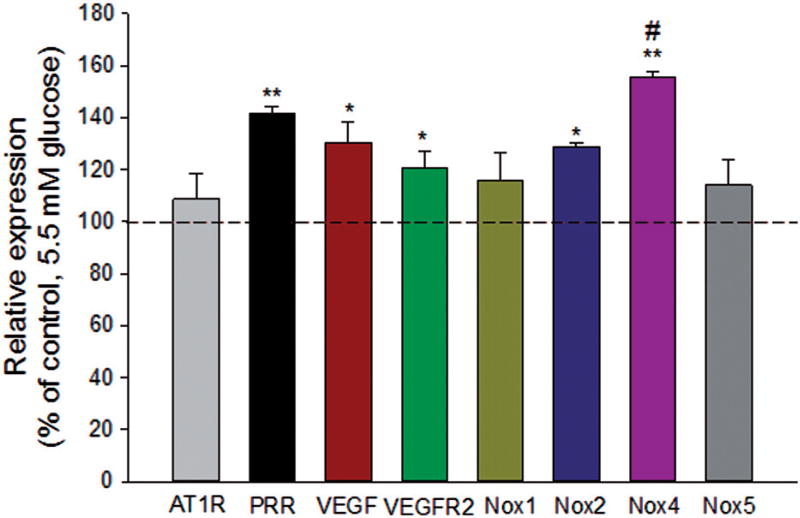
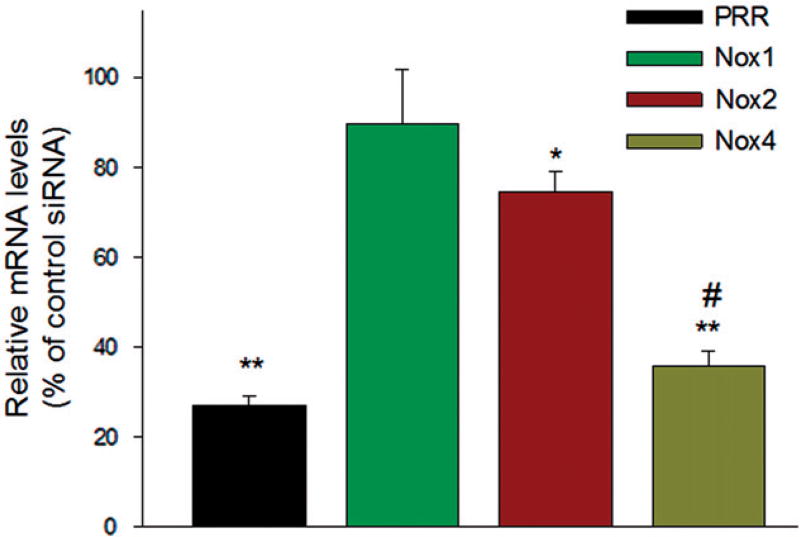
In perindopril-treated ARPE-19 cells, HG significantly upregulated the transcript levels of PRR (p < 0.001), VEGF (p < 0.01), VEGFR2 (p < 0.01), Nox2 (p < 0.05) and Nox4 (p < 0.001), as compared to NG (Figure 2). The high expression level of Nox4 suggests that this isoform is the major catalytic component of NADPH oxidase in ARPE-19 cells. The Nox2 homolog Nox3 was not detected in ARPE-19 cells. Also, hyperglycemia did not increase mRNA expression of AT1R in perindopril-treated cells, indicative of the involvement of PRR signaling. In order to examine if HG-induced PRR signaling is linked to Nox4 induction, perindopril-treated ARPE-19 cells were transfected with scrambled control siRNA (Scr) or PRR siRNA in presence of HG. Compared to scrambled control, siRNA-mediated silencing of PRR significantly reduced the expression of Nox2 (p < 0.05) and Nox4 (p < 0.001), but not Nox1, indicative of the involvement of PRR in the regulation of Nox2 and Nox4 expression (Figure 3). The reduction of Nox4 expression was significantly greater (p < 0.001) than that of Nox2 (Figure 3).
Figure 2.
Effect of high glucose on AT1R, PRR, VEGF, VEGFR2 and Nox expression in ARPE-19 cells. Perindopril (10 µM) treated cells were incubated with 33 mM glucose (HG) for 48 h. Cells incubated in 5.5 mM glucose were used as controls. Both the control and HG groups were treated with prorenin (10 nmol/l) as described in “Materials and Methods”. Cells were harvested for total RNA and each mRNA was measured by qRT-PCR. The levels of each transcript are normalized to that of HPRT. Compared to control, high glucose significantly expressed the transcript level of PRR, VEGF, VEGFR2, Nox2, and Nox4, but not the expression of AT1R, Nox1, and Nox5. The ratio is normalized to that of NG and is plotted as a percentage of control. Values are presented as mean ± SEM; n = 4; *p < 0.05, **p < 0.001 vs. control (set to 100%) by ANOVA; #p < 0.05 compared with Nox2.
Figure 3.
PRR mediated signal transduction under HG condition is linked to Nox4 induction. Perindopril (10 µM)-treated cells were transfected with 20 nM of scramble control or PRR siRNA for 24 h, followed by stimulation with prorenin (10 nmol/l) and high glucose for 24 h. The mRNA levels were measured by qRTPCR. The levels of each transcript are normalized to that of HPRT. Compared to control, PRR-specific siRNA significantly attenuated the expression of Nox2 and Nox4, but not Nox1. Values are presented as mean ± SEM; n = 3, mean ± SEM. *p < 0.05, **p < 0.001 compared with control-siRNA group, #p < 0.001 vs. Nox2 by ANOVA.
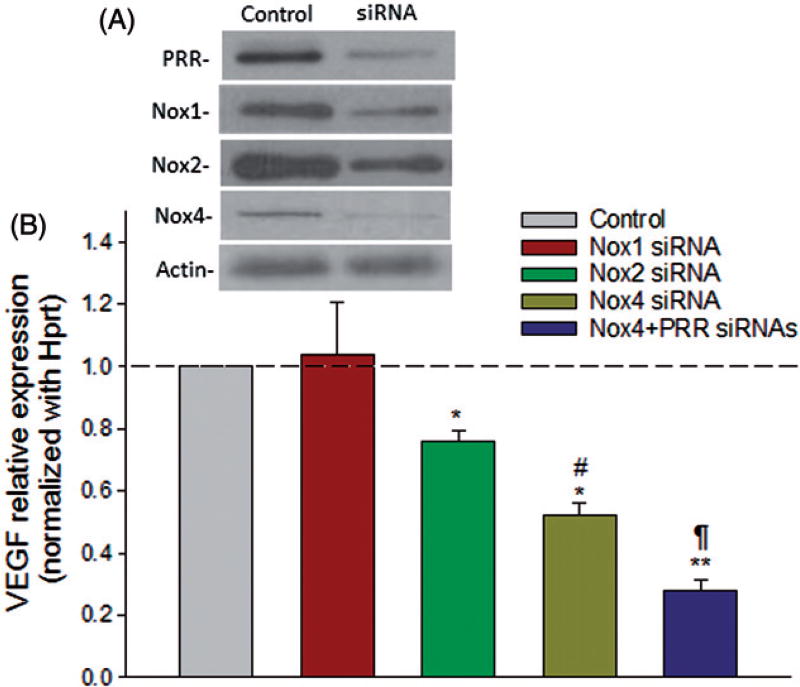
Reactive oxygen species function as signaling molecules to mediate various biological responses such as gene expression, cell proliferation, migration, angiogenesis, apoptosis and senescence. In order to investigate if PRR-mediated induction of VEGF under diabetic conditions occurs via Nox-derived ROS signaling, ARPE-19 cells were transfected with Nox1, Nox2 or Nox4 siRNAs. Cells transfected with scrambled siRNA were used as control. In ARPE-19 cells exposed to HG, selective siRNA-mediated knockdown of Nox2 and Nox4, but not Nox1, led to a significant reduction in VEGF transcript level compared to cells treated with scrambled siRNAs (Figure 4). Moreover, the expression of VEGF was reduced further (p < 0.001) in cells transfected with both Nox4 and PRR siRNAs, indicating the involvement of both PRR and Nox4 in the HG-induced activation of these molecules (Figure 4).
Figure 4.
Nox4-mediated signaling is linked to the stimulation of VEGF expression in ARPE-19 cells under HG condition. Perindopril (10 µM)-treated cells were transfected with 20 nM of control siRNA or Nox siRNA/PRR siRNAs for 24 h, followed by stimulation with prorenin (10 nmol/l) and high glucose for 24 h. The mRNA levels were measured by qRT-PCR. The levels of each transcript are normalized to that of HPRT. The mRNA levels of the samples treated with HG + scramble siRNA were arbitrarily set at 1. A representative western blot (A) shows the efficiency of the target-specific siRNAs. Compared to control, Nox2/ Nox4 siRNA significantly reduced the expression of PRR at the transcript level, which was further reduced when both Nox4 and PRR were knocked down together (B). Values are presented as mean ± SEM; n = 4; *p < 0.05, **p < 0.001 vs. control siRNA; #p < 0.05 vs. Nox2; ¶p < 0.05 vs. Nox4 siRNA by ANOVA.
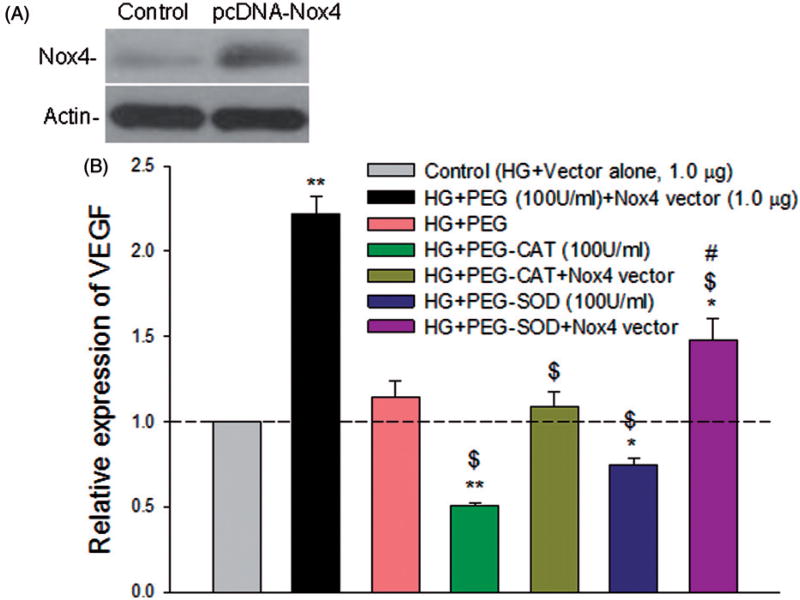
Nox4 overexpression potentiates PRR-induced stimulation of VEGF
To determine the relative role of Nox4-derived oxidant signaling species, and H2O2, in the regulation of VEGF in ARPE-19 cells, hyperglycemic cells were transfected with pcDNA3.1 plasmid containing the coding sequence of human Nox4 or pcDNA3.1 empty vector and treated with 100 U/ml PEG alone, PEG-superoxide dismutase (SOD), or PEG-CAT for the last 24 h before harvesting. Cells treated with pcDNA3.1 empty vector alone under HG were maintained as control. Nox4 overexpression (Figure 5(A)) potentiated the HG-induced increase of VEGF mRNA in ARPE-19 cells (p < 0.001; Figure 5(B)). However, both PEG-CAT and PEG-SOD, but not PEG alone, attenuated the HG-mediated induction of VEGF and suppressed the Nox4-induced upregulation of VEGF mRNA under HG condition (p < 0.001), implicating Nox4-derived and H2O2 signaling in the regulation of VEGF (Figure 5(B)).
Figure 5.
Nox4 overexpression potentiates PRR-induced stimulation of VEGF in ARPE-19 cells. Perindopril (10 µM)-treated cells were transfected with pcDNA3.1-Nox4 (1.0 µg) or pcDNA3.1 vector alone (1.0 µg), followed by stimulation with prorenin (10 nmol/l) and high glucose for 48 h. Cells were treated with 100 U/ml PEG, PEG-CAT or PEG-SOD for last 24 h before harvesting. (A) A representative western blot showing vector-mediated overexpression of Nox4 protein. Actin was used as an internal control. (B) The mRNA levels were measured by qRT-PCR. The levels of each transcript are normalized to that of HPRT. The mRNA levels of the samples treated with HG + vector alone were used as control and arbitrarily set at 1. Compared to control, both PEG-CAT and PEG-SOD significantly attenuated the high glucose-mediated induction of VEGF and also significantly reduced the Nox4-induced upregulation of VEGF at the transcript level. Values are presented as mean ± SEM; n = 4; *p < 0.05, **p < 0.001 vs. control; $p < 0.001 vs. col. HG + PEG + Nox4 vector; #p < 0.05 vs. HG + PEG-SOD + Nox4, by ANOVA.
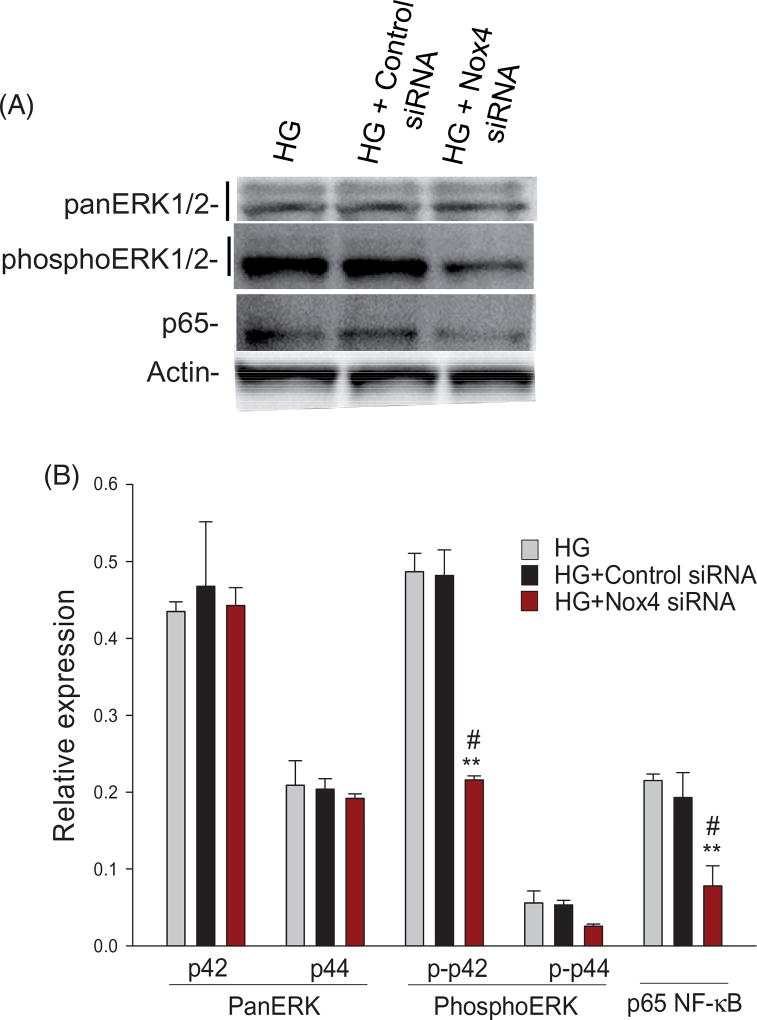
Nox4 regulates ERK1/2 activities and NF-jB p65 protein levels in ARPE-19 cells
We next examined whether Nox4 in ARPE-19 cells under HG was associated with the enhanced phosphorylation of ERK1/2. With the use of a specific antibody to p44/p42 (ERK1/2), Western blot analysis of cell lysates showed that siRNA-mediated Nox4 downregulation under HG condition significantly (p < 0.001) decreased the p42 phosphorylation state when compared to HG alone or HG-control siRNA. However, Nox4 inhibition did not affect the total protein level of p44/p42. Also, NF-κB protein level was affected with a significant decrease (p < 0.001) of the p65 subunit in cells transfected with Nox4 siRNA, when compared to HG alone or HG-control siRNA (Figure 6(A,B)).
Figure 6.
Nox4-mediated signaling is linked to the activation of ERK1/2 and p65 activities in ARPE-19 cells under HG condition. Perindopril (10 µM)-treated cells were transfected with 20 nM of control siRNA or Nox4 siRNA for 24 h, followed by stimulation with prorenin (10 nmol) and high glucose for 24 h. The protein levels and phosphorylation state were measured by immunoblotting. Compared to HG/HG-control siRNA, a representative western blot (A) and protein analysis (B) showed a Nox4 siRNA-mediated downregulation of p42 phosphorylation, but not the total p44/p42 protein levels. The siRNA-mediated Nox4 inhibition also significantly decreased the level of p65 as compared to control. The levels of each protein are normalized to that of Actin. Values are presented as mean ± SEM; n = 3; **p < 0.001 vs. HG; #p < 0.001 vs. HG + Control siRNA, by ANOVA.
Discussion
Oxidative injury to RPE and retinal photoreceptors is considered as one of the critical factors involved in the etiology of age-related macular degeneration (AMD) [30,31] and DR [32]. It is known that the RPE is at high risk for oxidative stress because it is exposed to high levels of phototoxic blue light and high oxygen tension [33]. Nox, which catalyzes the production of superoxide ( ) by the one-electron reduction of oxygen using NADPH as the reducing agent [34], is one of the major sources of ROS in many cell types and tissues, including the retina [35]. In the present study, DPI, an inhibitor of Nox, significantly prevented the HG stimulation of VEGF expression, indicating that Nox-mediated ROS production is involved in the regulation of VEGF in ARPE-19 cells.
We have examined the effect of high glucose on the expression of five subtypes of NADPH oxidase (Nox1–Nox5). Nox2 and Nox4, but not Nox1 or 5, expression have been found to be significantly induced by HG in ARPE-19 cells as compared to NG, with Nox4 being highly expressed. Nox3 was not detected in ARPE-19 cells. Consistent with our findings, Nox4 has been reported to be significantly overexpressed in human embryonic kidney cells (HEK) cells after a 24-h exposure to renin or prorenin, as compared to other Nox subtypes [36]. Increased activity and expression of Nox2 and Nox4 have also been found in the retinas of type 1 and type 2 diabetic animal models, and have been related to increased oxidative stress in the diabetic retina [18,20,37,38]. Accumulating evidence suggests that the increased production of ROS and subsequent pathological angiogenesis are critical factors in retinopathy [12,39].
The RPE maintains the choriocapillaris (CC) in the normal eye [40]. VEGF is produced by differentiated human RPE cells in vitro and in vivo and also involved in paracrine signaling between the RPE and the CC [40]. Hyperglycemia has been reported to induce the level of VEGF in the retina [41], RPE [42] and retinal endothelial cells [43]. Also, an increased level of VEGF mRNA in retina has been detected in DR and ROP [44]. In our current study, high glucose in perindopril-treated ARPE-19 cells also significantly induced the expression of PRR, VEGF and VEGFR2 as compared to NG. However, blockade of Ang II production by perindopril did not induce the expression of AT1R in hyperglycemic ARPE-19 cells. For the first time we are demonstrating in perindopril-treated ARPE-19 cells that silencing of PRR significantly blocked the HG-mediated induction of Nox2 and Nox4 indicating that the PRR-mediated signal transduction is linked to both Nox2 and Nox4, with Nox4 being the major candidate of PRR’s linkage to ROS production. Nox4 shares 39% sequence similarity with Nox2; however, unlike other isoforms of Nox, Nox4 has been reported to constitutively produce H2O2 rather than [45]. It is well known that Ang II influences the expression of Nox subunits, such as Nox1 [46], Nox2 [47], Nox4 [48] and Nox5 [49], and contribute to ROS-mediated damage in a number of pathologies, including hypertension and cardiovascular disease [50]. Using AT1R- and PRR-targeted siRNAs, we showed previously a much larger reduction in VEGF, VEGFR2 and TGFβ1 expressions upon knockdown of both receptors together as compared to the separate knockdown of AT1R or PRR, indicative of the role of PRR signaling in the regulation of VEGF, VEGFR2 and TGFβ1 expressions in hRECs [43].
In this study, we demonstrated that siRNA-mediated knockdown of Nox2 or Nox4, but not of Nox1, significantly inhibited HG-induced VEGF activation. However, the inhibition of VEGF by Nox4 siRNA was significantly greater than that of Nox2. The expression of VEGF was further reduced when ARPE-19 cells were transfected with both Nox4 and PRR siRNAs, thus indicating that PRR-mediated signaling is linked to the Nox4-induced stimulation of VEGF expression under hyperglycemic condition in ARPE-19 cells.
Also, as compared to the pcDNA3.1 vector alone, Nox4 overexpression potentiated HG-induced stimulation of VEGF in ARPE-19 cells that further confirmed the role of Nox4 in the regulation of VEGF. Nox4 overexpression has also been reported to promote endothelial cell proliferation, migration and tubulogenesis [16,51,52]. Diabetes is associated with upregulation of Nox4, a major isoform of NADPH oxidase in retinal endothelial cells, and the knockdown of Nox4 has been reported to ameliorate BRB breakdown and retinal vascular leakage in diabetic animals through a VEGF-dependent mechanism [53]. In vivo, Nox4 (−/−) mice exhibited attenuated angiogenesis and PEG-CAT treatment in control mice showed a similar effect [54]. Also, Nox4 (−/−) mice undergo less tubulogenesis in vitro and restored by low concentrations of H2O2 whereas PEG-CAT attenuated the tube formation in controls [54]. In ARPE-19 cells, we demonstrated here that Nox4-induced VEGF expression was attenuated by both PEG-SOD and PEG-CAT, suggesting the function of Nox4 as a source of both and H2O2 for the regulation of VEGF. Consistent with our results, overexpression of Nox4 in hRECs has been shown to significantly increase H2O2 generation, resulting in intensified VEGF and VEGFR2 activation [53]. In contrast, silencing Nox4 expression or scavenging H2O2 by PEG-CAT inhibited endothelial migration, tube formation, and VEGF-induced activation of VEGFR2 signaling [53]. Ang II-induced activation of AT1R has been reported to increase Nox4 expression and H2O2 production in rat renal medulla, whereas PEG-CAT reduced these events, indicative of H2O2 as the key mediator enhancing intrarenal RAS activation and renal medullary dysfunction [55]. For the first time, we are showing in ARPE-19 cells that HG-induced activation of PRR upregulates VEGF through Nox4-derived H2O2 and .
Oxidative stress responses are more complex and the molecular mechanisms underlying this phenomenon are not clearly understood. However, a growing body of evidence suggests that oxygen-derived radicals such as superoxide anions and hydrogen peroxide can act as intracellular signaling molecules for activation of diverse signaling pathways by oxidation of reactive cysteine on target molecules including kinases, phosphatases [56–58], and redox sensitive transcription factors [59], including nuclear factor (NF)-κB [60]. Overexpression of hNox4 transgene in mice is reported to activate NF-κB signaling by enhancing phosphorylation of p65 [61]. Also, this study demonstrated Nox4-mediated ROS enhances ERK1/2 phosphorylation and p65 levels in the HG-induced PRR signaling pathway in ARPE-19 cells. PRR/ Nox-induced ROS may prolong the activation of ERK-p65 and thereby enhance the downstream signaling including VEGF synthesis under hyperglycemic condition.
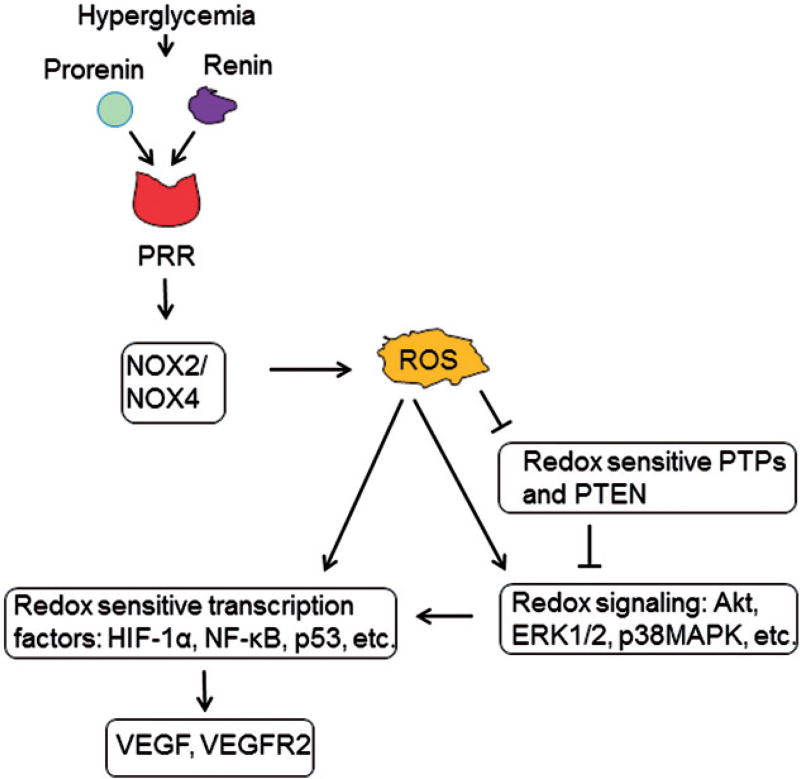
In summary, Nox2/4-derived ROS signaling is implicated in the hyperglycemia-induced increase of VEGF expression through PRR in ARPE-19 cells (Figure 7). Further work is needed to evaluate the role of PRR and Nox-s in high glucose-induced stimulation of VEGF and VEGF receptor, and to identify more downstream targets to develop therapeutic strategies in vivo.
Figure 7.
Schematic representation showing PRR-induced Nox signaling for the regulation of VEGF under hyperglycemic condition. The intrinsic link between PRR and Nox-mediated induction of VEGF under hyperglycemic condition in ARPE-19 cells was investigated. Hyperglycemia stimulates PRR and NADPH oxidase, thereby promoting activation of redox signaling events and induction of redox sensitive transcription factors that induce the expression of VEGF and VEGFR2. Arrows indicate activation and T’s indicates inhibition.
Supplementary Material
Acknowledgments
The authors thank Dr. David Lambeth, Emory University School of Medicine, for a gift of human Nox4 plasmid and Dr. Purnachandra Ganji (Emory University) for donating NF-κB (p65) antibody. The authors also thank Jane Abey, Curran S. Sidhu, and Julien Brock for technical assistance. The STR allele(s) analysis for ARPE-19 cell line authentication was supported in part by the Emory Integrated Genomics Core (EIGC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities.
Funding
The research was supported by Research to Prevent Blindness (RPB), R01 EY004864 and P30 EY006360.
Footnotes
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Lavoie JL, Sigmund CD. Minireview: overview of the renin–angiotensin system – an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 2.Alcazar O, Cousins SW, Striker GE, et al. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res. 2009;89:638–647. doi: 10.1016/j.exer.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson-Berka JL, Miller AG, Fletcher EL. Prorenin and the (pro)-renin receptor: do they have a pathogenic role in the retina? Front Biosci. 2010;2:1054–1064. doi: 10.2741/e163. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. J Neurogastroenterol Motil. 2007;292:26. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Tamarat R, Silvestre JS, Durie M, et al. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest. 2002;82:747–756. doi: 10.1097/01.lab.0000017372.76297.eb. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson-Berka JL, Rana I, Armani R, et al. Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clin Sci. 2013;124:597–615. doi: 10.1042/CS20120212. [DOI] [PubMed] [Google Scholar]
- 7.Wingler K, Wunsch S, Kreutz R, et al. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G. Renin (pro)renin and receptor: an update. Clin Sci. 2011;120:169–178. doi: 10.1042/CS20100432. [DOI] [PubMed] [Google Scholar]
- 9.Batenburg WW, Krop M, Garrelds IM, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 10.Kanda A, Noda K, Saito W, et al. (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia. 2012;55:3104–3113. doi: 10.1007/s00125-012-2702-2. [DOI] [PubMed] [Google Scholar]
- 11.Obrosova IG, Drel VR, Kumagai AK, et al. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49:2525–2533. doi: 10.1007/s00125-006-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Luther JM, Brown NJ. The renin–angiotensin–aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–739. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi G. Diabetic retinopathy. Minerva Med. 2003;94:419–435. [PubMed] [Google Scholar]
- 15.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;349:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 16.Craige SM, Chen K, Pei Y, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedeek M, Callera G, Montezano A, et al. Critical role of Nox4- based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:14. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wang JJ, Yu Q, et al. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood–retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Yang Z, Jiang Y, et al. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol Vis. 2014;20:231–241. [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 22.Haque R, Chong NW, Ali F, et al. Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem. 2011;119:6–17. doi: 10.1111/j.1471-4159.2011.07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque R, Chun E, Howell JC, et al. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS One. 2012;7:6. doi: 10.1371/journal.pone.0042542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Haque R, Ali FG, Biscoglia R, et al. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–1306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grigsby J, Betts B, Vidro-Kotchan E, et al. A possible role of acrolein in diabetic retinopathy: involvement of a VEGF/TGFbeta signaling pathway of the retinal pigment epithelium in hyperglycemia. Curr Eye Res. 2012;37:1045–1053. doi: 10.3109/02713683.2012.713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis EA, Guberski DL, Somogyi-Mann M, et al. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28:91–101. doi: 10.1016/s0891-5849(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 29.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 30.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 31.Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci. 2010;51:1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 32.Madsen-Bouterse SA, Mohammad G, Kanwar M, et al. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010;13:797–805. doi: 10.1089/ars.2009.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006;51:461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Babior BM. NADPH oxidase: an update. Blood. 1999;9:1464–1476. [PubMed] [Google Scholar]
- 35.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 36.Clavreul N, Sansilvestri-Morel P, Magard D, et al. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:16. doi: 10.1152/ajprenal.00119.2010. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He M, Pan H, Xiao C, et al. Roles for redox signaling by NADPH oxidase in hyperglycemia-induced heme oxygenase-1 expression in the diabetic retina. Invest Ophthalmol Vis Sci. 2013;54:4092–4101. doi: 10.1167/iovs.13-12004. [DOI] [PubMed] [Google Scholar]
- 39.Kowluru RA, Koppolu P, Chakrabarti S, et al. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 40.Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennis MD, Kimball SR, Fort PE, et al. Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J Biol Chem. 2015;2:3865–3874. doi: 10.1074/jbc.M114.623058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai Y, Li X, Wang YS, et al. Hyperglycemia promotes vasculogenesis in choroidal neovascularization in diabetic mice by stimulating VEGF and SDF-1 expression in retinal pigment epithelial cells. Exp Eye Res. 2014;123:87–96. doi: 10.1016/j.exer.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Haque R, Hur EH, Farrell AN, et al. MicroRNA-152 represses VEGF and TGFbeta1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015;21:224–235. [PMC free article] [PubMed] [Google Scholar]
- 44.Young TL, Anthony DC, Pierce E, et al. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J Aapos. 1997;1:105–110. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 45.Takac I, Schroder K, Zhang L, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yogi A, Mercure C, Touyz J, et al. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51:500–506. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- 47.Fu Y, Zhang R, Lu D, et al. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol. 2010;298:6. doi: 10.1152/ajpregu.00762.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fazeli G, Stopper H, Schinzel R, et al. Angiotensin II induces DNA damage via AT1 receptor and NADPH oxidase isoform Nox4. Mutagenesis. 2012;27:673–681. doi: 10.1093/mutage/ges033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montezano AC, Burger D, Paravicini TM, et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ago T, Kuroda J, Kamouchi M, et al. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. Circ J. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 51.Petry A, Djordjevic T, Weitnauer M, et al. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 52.Datla SR, Peshavariya H, Dusting GJ, et al. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Wang JJ, Zhang SX. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J Diabetes Res. 2015;963289:18. doi: 10.1155/2015/963289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroder K, Zhang M, Benkhoff S, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 55.Sousa T, Oliveira S, Afonso J, et al. Role of H(2)O(2) in hypertension, renin-angiotensin system activation and renal medullary disfunction caused by angiotensin II. Br J Pharmacol. 2012;166:2386–2401. doi: 10.1111/j.1476-5381.2012.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhee SG, Bae YS, Lee SR, et al. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;53:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 57.Lee SR, Kwon KS, Kim SR, et al. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 58.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 59.Storz G, Polla BS. Transcriptional regulators of oxidative stress-inducible genes in prokaryotes and eukaryotes. Exs. 1996;77:239–254. doi: 10.1007/978-3-0348-9088-5_16. [DOI] [PubMed] [Google Scholar]
- 60.Brar SS, Kennedy TP, Quinn M, et al. Redox signaling of NFkappaB by membrane NAD(P)H oxidases in normal and malignant cells. Protoplasma. 2003;221:117–127. doi: 10.1007/s00709-002-0059-y. [DOI] [PubMed] [Google Scholar]
- 61.Zhao QD, Viswanadhapalli S, Williams P, et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation. 2015;131:643–655. doi: 10.1161/CIRCULATIONAHA.114.011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.