Abstract
Background
Vascularized composite allografts (VCA) are novel, life-enhancing forms of transplantation (Tx). However, host immune responses to the various VCA components, especially those involving skin, are complex and make selection of appropriate therapy challenging. Although the interplay between Foxp3+ Tregulatory (Treg) cells and CD4 and CD8 effector Tcells is of central importance in determining the acceptance or rejection of solid organ allografts, there is little information available concerning the contribution of Treg cells to VCA survival. In addition, the effects of therapeutic expansion in vivo of host Treg cell populations on VCA survival are unknown.
Methods
We established a fully major histocompatibility complex-disparate (BALB/c- > C57BL/6) murine orthotopic forelimb Tx model to explore the benefits of pre- and post-Tx IL-2/anti-IL-2 monoclonal antibody complex (IL-2C) administration to expand the host Treg cell population and thereby attempt to promote Treg cell–dependent VCA survival.
Results
Both strategies expanded the Treg cell population in vivo and prolonged VCA survival (P < 0.001), but IL-2C administration pre-Tx led to significantly longer survival compared with IL-2C administration post-Tx (P < 0.01). In addition, compared with post-Tx therapy, pre-Tx therapy resulted in an increased ratio of Treg cells to CD8+ T cells (P < 0.001), reduced proliferation of CD4 and CD8 effector Tcells, and reduced production of IFN-γ. Optimal effects were seen when combined with rapamycin therapy, whereas the combination of IL-2C therapy plus calcineurin inhibitor was counterproductive.
Conclusions
Our studies involving different IL-2C-mediated Treg cell expansion strategies demonstrate that pre-Tx IL-2C therapy may be a useful component for developing strategies to promote VCA survival.
An estimated 7 million people in the United States could be considered for some form of vascularized composite allotransplantation (VCA), because of surgical excision of tumors, trauma and accidents, and congenital malformations.1 Approximately 1.2 million patients with restorative needs after limb amputations, including military veterans, are included in this large cohort.1 However, after several cases in 1998 to 2000,2,3 only about 70 hand or forearm/hand transplantation (Tx), and approximately 150 VCA procedures of any type, have been performed worldwide,4–6 despite VCA offering a better quality of life, and functional and aesthetic results than conventional reconstructive methods.5 The basis for this huge shortfall is multifactorial, but the perceived need for maintenance calcineurin inhibitor-based immunosuppression to achieve this functional benefit is a major issue, and minimizing or eliminating the need for prolonged immunosuppression would likely alter risk/benefit calculations substantially.4–6 The relatively young age of military amputees and most other favorable VCA candidates necessitates prolonged exposure to immunosuppression, leading to significant cumulative risk of infection, nephrotoxicity, atherosclerotic disease, hypertension, diabetes, and tumor formation.7 All of the problems related to use of systemic immunosuppression in solid organ Tx have been witnessed in face and hand transplant recipients,4,7–9 and despite immunosuppression, limb allografts may still develop transplant vasculopathy and chronic rejection.10,11
As examples of the ongoing research efforts to avoid maintenance immunosuppression in VCA, some investigators have pursued strategies based on myeloablative regimens,12,13 or nonmyeloablative conditioning regimens that result in mixed chimerism.14 By contrast, our work has focused on the potential of Foxp3+ T regulatory (Treg) cells to facilitate VCA engraftment. Thus, although host CD4 and CD8 T-cell responses are central to the development of allograft rejection, their actions are opposed by Foxp3+ Treg cells. A defining characteristic of normal Foxp3+ Treg cells is their dependence on IL-2 production by adjacent T cells for their survival.15 This is due to Foxp3 binding to the IL-2 promoter and inhibiting IL-2 production by Treg cells themselves.16 As a result, exogenous IL-2 is crucial for the generation and expansion of Treg cells, and with relevance to their therapeutic manipulation, 3 injections of IL-2/anti-IL-2 complexes (IL-2C) can boost murine Treg cell numbers by 10-fold to 20-fold.17–19 Such expansion is sufficient to promote acceptance of murine islet allografts,19,20 and to suppress murine graft-versus-host disease,21 whereas IL-2C therapy was much less effective in preventing the rejection of skin allografts.20 Moreover, IL-2C administration can also promote the proliferation and differentiation of Tcells, especially CD8 Tcells,22 thereby potentially acting as a “double-edged sword.”23 Because there are currently no data as to the effects of IL-2C therapy in VCA models, we compared the effects of pre-and post-Tx administration of IL-2C in mice undergoing orthotopic forelimb VCA. The findings of these studies provide new insights into the functions of Treg cells in VCA recipients, and have implications for the ongoing development of strategies to induce long-term VCA survival without maintenance immunosuppression.
MATERIALS AND METHODS
Mice
Inbred male C57BL/6 (H-2Kb) and BALB/c (H-2Kd) mice were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions. All animals received adequate care per The Principles of Laboratory Animal Care of the University of Pennsylvania, and all studies were approved by our Institutional Animal Care and Use Committee (805405).
Surgical Procedures and Peritransplant Therapies
Mouse unilateral forelimb orthotopic transplants were performed by a single surgeon (X.H.), using microsurgical techniques adapted from a previously developed rat model.24 Details of this new murine VCA model will be reported elsewhere. We used BALB/c donors and C57BL/6 recipients. IL-2C were prepared by mixing 2 μg of recombinant mouse IL-2 (Biolegend) with 10 μg of anti-IL-2 monoclonal antibody (mAb, JES6-1 from BioXcell) and incubating for 30 minutes at 37°C. Normal, untransplanted mice (n = 8) were injected intraperitoneally (i.p.) on days 0, 1 and 2 with IL-2C or PBS control, spleens were harvested and analyzed on days 3, 5, 10. In transplant studies (n = 6-8/group), IL-2C was injected at Tx (day 0) and on days 1 and 2 post-Tx. In mice that received IL-2C pre-Tx, IL-2C were injected on days −5, −4, −3, and spleens and allografts were harvested on POD 5. In additional groups, FK506 (1 mg/kg per day, i.p., 14 days), or rapamycin (RPM) (2 mg/kg per day) was delivered subcutaneously by Alzet osmotic pump (Fisher Scientific) for 28 days, beginning on the day of Tx.
Antibodies and Flow Cytometry
We purchased fluorochrome-conjugated mAbs directed against CD4 (RM 4-5), CD8 (53-6.7), Foxp3 (FJK-16 s), IL-10 (JES5-16E3), IFN-γ (XMG1.2) and Ki67 (SolA15) from eBioscience. Flow cytometry was performed on a CytoFLEX flow cytometer (Beckman Coulter), and data analyzed with FlowJo 8 software (Tree-Star).25
In Vitro Treg Cell Suppression Assays
Bead-purified CD4 + CD25+ Treg cells from C57BL/6 mice were added to 96-well plates and serially diluted in medium (RPMI-1640 and 10% FBS, plus penicillin/streptomycin). Equal numbers of CFSE-labeled CD4 + CD25- T cells and γ-irradiated antigen-presenting cells (APCs) were added, along with CD3 mAb (1 μg/mL), and cells were cultured for 3 days. Thereafter, cells were stained with CD4 mAb (Pacific blue), and CFSE- and CD4-positive T cell proliferation was determined, with data analysis using FlowJo software.25
Real-Time qPCR
RNA was isolated from allografts, including skin, fat and muscle tissues, using Trizol reagent (QIAGEN), and reverse transcribed to cDNA (Applied Biosystems). Gene expression was assessed by qPCR, using Taqman primer and probe sets. Data were normalized to endogenous 18s rRNA, and relative expression was determined by the formula 2−ΔΔCT.25
Western Blotting
Western blots were performed using mAbs to Foxp3 (eBioscience) and β actin (Cell Signaling Technology).25
Histopathology
Limb specimens were fixed in 10% neutral buffered formalin, decalcified in Formical-2000, and embedded in paraffin. Histologic sections (6 μM) were stained with hematoxylin and eosin, reviewed by a pathologist (W.W.H) blinded to conditions, and graded using Banff criteria.26
Statistics
Data were analyzed using GraphPad Prism 6.0 software, and are presented as mean ± SD unless specified otherwise. Measurements between 2 groups were performed with a 2-tailed Student t test if data were normally distributed, or by Mann-Whitney U unpaired test if the populations were not normally distributed. Groups of 3 or more were analyzed by 1-way ANOVA with corresponding Tukey multiple comparison test if normally distributed, or the Kruskal-Wallis with Dunn’s multiple comparison test if not. Graft survival was evaluated with Kaplan-Meier followed by log-rank test; P less than 0.05 was considered significant.
RESULTS
IL-2C Therapy Increases the Number But Not Function of Foxp3 CD4+ Treg Cells
To test the effects of JES6-1 mAb-based IL-2C administration on Foxp3+ Treg cells in normal mice, wild-type (WT) C57BL/6 mice were injected with PBS or IL-2C for 3 consecutive days. Compared with PBS injections, by 2 days after the last injection, IL-2C increased by fourfold to fivefold the proportion (P < 0.005) and by 10-fold the total number (P < 0.01) of splenic Foxp3 + CD4+ Treg cells (Figure 1A). The effects of IL-2C therapy were short-lived, as Foxp3+ Treg cells levels peaked at day 5 and gradually declined thereafter (Figure 1B). IL-2C therapy did not appear to affect the properties of Foxp3 Treg cells, because their production of the cytokine, IL-10 (Figure 1C), their suppressive function (Figures 1D and E), and their levels of Foxp3 protein were unchanged (Figure 1F), compared with Treg cells from PBS-treated control mice. Hence, IL-2C therapy appears to simply increase the total number of peripheral Foxp3+ Treg cells without modifying their function.
FIGURE 1.
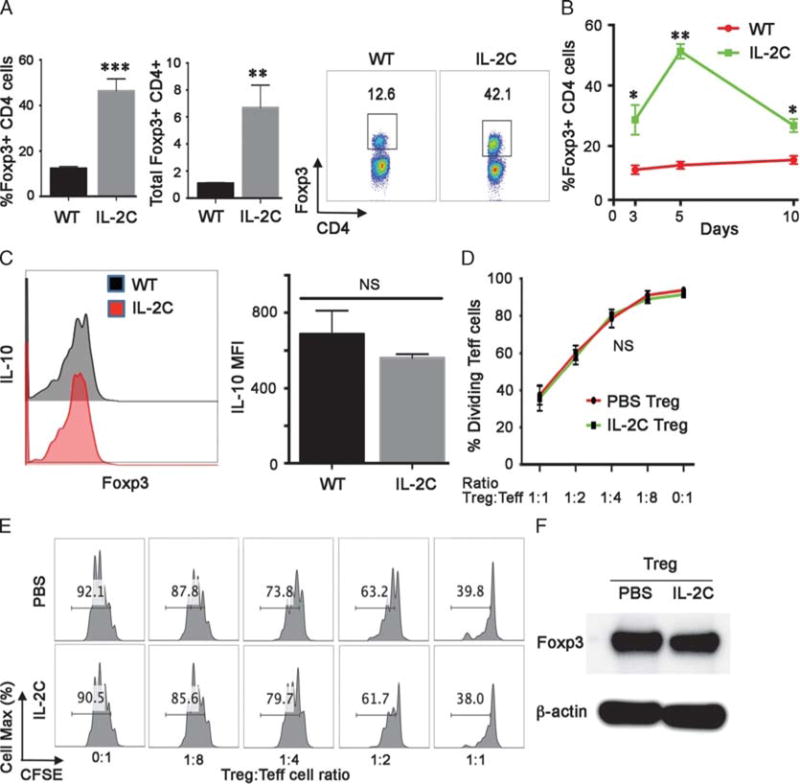
IL-2C administration increases Treg cell numbers. A, IL-2C significantly increases the percentage of Foxp3+ Treg cells within the splenic CD4+ T fraction, and total Foxp3+ Treg cell numbers (106 cells/spleen); data (mean ± SD) are from 4 animals/group/time-point, **P < 0.01, ***P < 0.005. A representative flow plot is shown at right with the percentage of Foxp3 + CD4+ Treg cells indicated. B, IL-2C administration on days 0, 1 and 2 led to a peak in the percentage of Foxp3 + CD4+ Treg cells on day 5, with a decline thereafter towards baseline; data (mean ± SD) are from 4 animals/group/time-point, *P < 0.05, **P < 0.01. C, IL-2C administration did not affect expression of IL-10 by Treg cells harvested at 5 days after beginning IL-2C therapy; a representative flow plot is shown at left, and cumulative MFI data (mean ± SD, n = 4/group, NS, not significant) are shown at right. D, IL-2C administration did not affect Treg cell suppressive function as assessed by in vitro assays (mean ± SD, n = 4/group) using cells analyzed at day 5. E, An in vitro suppression assay, representative of 4 studies, with the proportion of dividing T effector cells shown in each panel. E, Western blots of Foxp3 protein expression in Treg cells isolated from mice treated with IL-2C or PBS (representative of 3 experiments). MFI, mean fluorescent intensity.
Pre- or Post-Tx IL-2C Therapy Alone or Plus RPM Prolongs VCA Survival
A mouse unilateral forelimb orthotopic Tx model was established during this research and used to test IL-2C-based therapy during the pre-Tx or post-Tx period. In the initial studies (Figure 2A), we tested the effects of combining post-Tx IL-2C therapy with administration of FK506 (1 mg/kg per day, i.p.) for 14 days from the time of Tx. We found that post-Tx IL-2C therapy alone significantly prolonged VCA survival compared with the 3 other treatment groups (P < 0.01); that is, FK506 at this dose was ineffective in prolonging survival compared with untreated controls, and its combination with IL-2C therapy revoked the efficacy of the IL-2C regimen.
FIGURE 2.
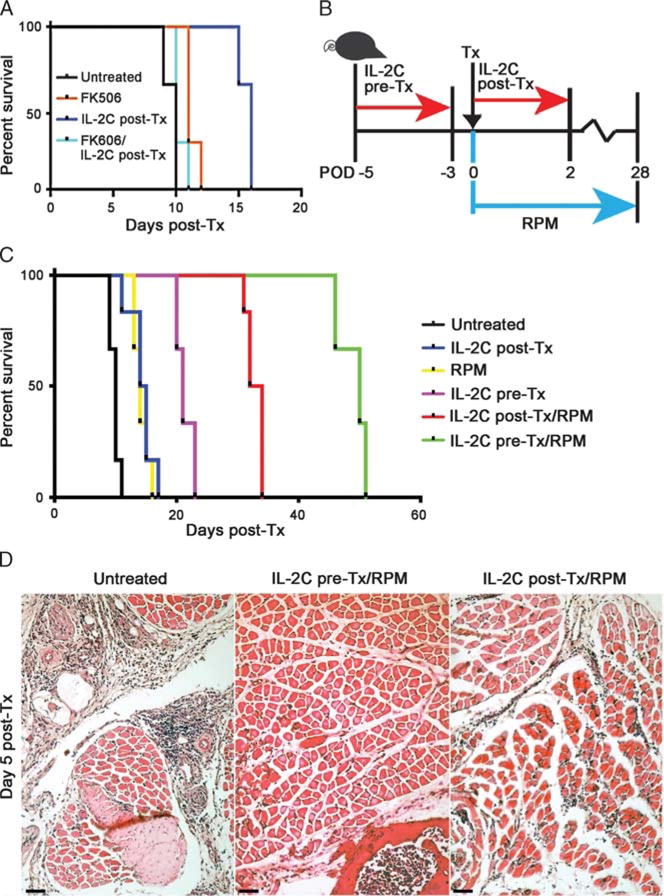
Both pre- and post-Tx IL-2C treatment prolonged murine forelimb VCA survival. A, Post-Tx IL-2C therapy alone was significantly better (P < 0.01) at extending VCA survival than FK506 alone (1 mg/kg per day, i.p. for 14 days), or FK506 plus IL-2C therapy. B, Diagram sum-marizing use of pre-Tx and post-Tx IL-2C administration, as well as use of RPM in a subset of recipients. C, Kaplan-Meier plots of forelimb VCA survival for the different experimental groups (n = 6 allografts/group). D, Representative histopathology at day 5 post-Tx (H&E-stained paraffin sections, scale bar = 100 μ). H&E, hematoxylin and eosin.
In subsequent studies, we tested the effects of IL-2C therapy alone or in conjunction with RPM therapy (2 mg/kg per day) delivered via 28-day Alzet pumps that were implanted beginning at the time of VCA engraftment. The experimental design is summarized in Figure 2B, and comparisons between groups were undertaken at day 5 post-Tx. This point was selected given the onset of limb swelling and erythema by day 5 in untreated recipients. Rejection occurred by 10 days post-Tx in 50% of untreated recipients, and all allografts were rejected by day 12 post-Tx (Figure 2C). Administration of IL-2C alone prolonged VCA survival, compared with untreated recipients, using both pre- and post-Tx protocols (P < 0.05) (Figure 2C), and administration of IL-2C post-Tx for longer periods, for example, 5 days rather than 3 days had no additional benefit on VCA survival.
Use of RPM monotherapy was about as effective as post-Tx IL-2C in prolonging survival (P < 0.05, Figure 2C). Co-administration of IL-2C and post-Tx RPM had additional benefits, with pre-Tx IL-C plus RPM causing a fivefold increase in survival, and post-Tx IL-2C plus RPM causing a threefold increase in survival, compared with untreated VCA recipients (Figure 2). Comparison of intragraft events at day 5 post-Tx showed dense mononuclear cell infiltrates within the skin and muscle of grafts from untreated controls, along with areas of focal muscle necrosis (grade III rejection, Figure 2D). Infiltrates were absent in recipients receiving pre-Tx IL-2C plus post-Tx RPM (grade 0, Figure 2D), and were mainly confined to perivascular areas, without epidermal involvement or muscle necrosis, in recipients treated with post-Tx IL-2C plus RPM (grade I, Figure 2D). The results of statistical comparisons of survival data for the various groups are shown in Table 1. We conclude from these data that although each therapy tested had some benefit for graft survival, combinations of IL-2C plus RPM therapy were better, and pre-Tx IL-2C plus RPM resulted in the best overall prolongation of VCA survival and initial preservation of graft histology.
TABLE 1.
Kaplan-Meier analysis of allograft survival in the various experimental groupsa
| Group | Control | Post-Tx IL-2C | Post-Tx IL-2C + RPM |
|---|---|---|---|
| RPM alone | P < 0.001 | P = 0.502 | P < 0.001 |
| Post-Tx IL-2C | P = 0.002 | N/A | P < 0.001 |
| Post-Tx & IL-2C + RPM | P < 0.001 | P < 0.001 | N/A |
| Pre-Tx IL-2C | P < 0.001 | P = 0.010 | P = 0.002 |
| Pre-Tx IL-2C + RPM | P < 0.001 | P < 0.001 | P = 0.010 |
Comparison of survival curves (log-rank test, P value) using six to eight allografts/group.
Differing Effects of Pre- or Post-Tx IL-2C Therapy on Host Treg and Teff Cells
At day 5 post-Tx, the proportions of Foxp3+ CD4+ Treg cells in recipients treated with IL-2C alone, or IL-2C plus RPM, were about fourfold higher than in untreated allograft recipients (P < 0.05), and about twofold higher than in mice treated with RPM alone (Figure 3A). Mice treated pre-Tx with IL-2C (P < 0.05) ± post-Tx RPM (P > 0.05 vs. RPM alone) had lesser increases in Treg cells (Figure 3A). However, at day 5 post-Tx, Treg cells isolated from all 6 groups of engrafted mice showed comparable levels of IL-10 (Figure 3B), glucocorticoid-induced TNFR-related protein, inducible costimulator, and TGF-β, and comparable levels of cell proliferation (Ki67 expression) (Figure 3C).
FIGURE 3.
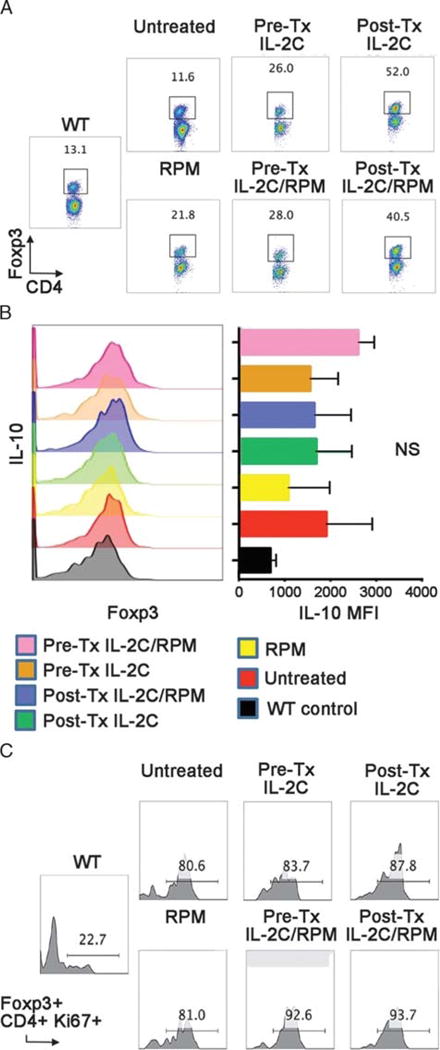
Comparison of Treg cell populations at day 5 post-Tx (4 mice/group). A, Percentages of Foxp3+ cells within the CD4+ Tcells of Tx groups and WT mice are shown. B, Treg cell intracellular expression of IL-10, as determined by flow cytometry (MFI), did not significantly differ across groups. C, The percentages of proliferating (Ki67+) Foxp3+ CD4+ Treg cells were comparable across groups but markedly increased compared with untransplanted WT control mice.
Flow cytometric analysis of conventional CD4 and CD8 T cells at day 5 post-Tx showed comparable proportions of CD4 cells in untreated recipients and those receiving pre-Tx IL-2C ± RPM (Figure 4A). However, allograft recipients receiving post-Tx IL-2C ± RPM showed a threefold to fourfold expansion of the CD8 population (Figure 4A). Analysis of Ki67 expression showed increased proliferation of CD4 (Figure 4B) and especially CD8 T cells (Figure 4C) in all allograft groups compared with WT controls. This increase in proliferating CD8 T cells was most marked in recipients receiving post-Tx IL-2C, and in contrast to the other groups receiving RPM, was not diminished by post-Tx RPM therapy (Figure 4C). Analysis of IFN-γ production by CD4 (Figure 4D) and CD8 T cells (Figure 4E) showed increases in all groups compared with WT controls, but was greatest in the case of recipients receiving post-Tx IL-2C and was diminished but not abolished by concomitant RPM therapy (Figure 4E). Flow cytometric comparisons of the ratios of proliferating Treg cells to CD4 or CD8 T cells at day 5 post-Tx (Figure 4F) showed that the pre-Tx IL-2C/RPM protocol was especially effective at facilitating Treg cell expansion while curtailing CD4 and CD8 alloproliferation. In contrast, the groups receiving post-Tx IL-2C ± RPM showed particularly low Treg cell to CD8 T cell ratios. These data are indicate important differences in the levels of alloreactive CD8 T cells in VCA recipients receiving the post-Tx IL-2C, regardless of added RPM therapy, compared with pre-Tx IL-2C usage.
FIGURE 4.
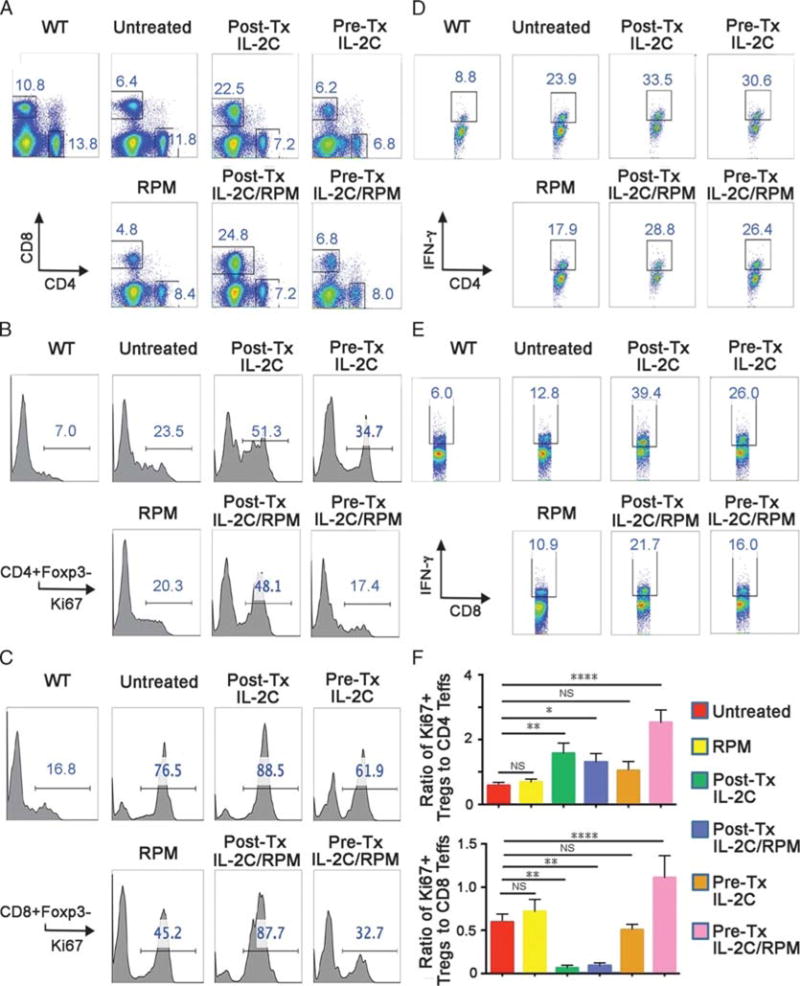
Effects of IL-2C on non-Treg cell populations at day 5 post-Tx (4 mice/group). A, Increased percentage of CD8 T cells in allograft recipients treated with IL-2C post-Tx. B, Increased proliferation of CD4+ T cells in allograft recipients treated with IL-2C post-Tx. C, Increased proliferation of CD8+ Tcells in allograft recipients treated with IL-2C post-Tx. D, Increased production of IFN-γ by CD4+ Tcells in allograft recipients. E, Increased production of IFN-γ by CD8+ Tcells in allograft recipients. F, Ratio of Ki67+ Treg cells to CD4 + Foxp3- or CD8 + Foxp3- Tcells; higher ratios were associated with longer allograft survival.
Differing Effects of Pre- or Post-Tx IL-2C Therapy on Intragraft Gene Expression
Analysis of intragraft gene expression at day 5 post-Tx showed that, compared with pre-Tx IL-2C therapy, post-Tx IL-2C usage increased intragraft CD8, IFN-γ and granzyme B expression (Figure 5). Addition of RPM decreased expression of CD8, IFN-γ and granzyme B in the post-Tx IL-C group, but was especially effective in decreasing expression of these genes in recipients treated with IL-2C in the pre-Tx period. Foxp3 and IL-10 gene expression were increased in all groups receiving IL-2C therapy, and levels were only modestly decreased by RPM therapy. These data suggest that at the level of the graft, as with events in secondary lymphoid tissues, post-Tx IL-2C therapy was less effective than pre-Tx therapy in controlling alloreactive CD8 T cell responses.
FIGURE 5.
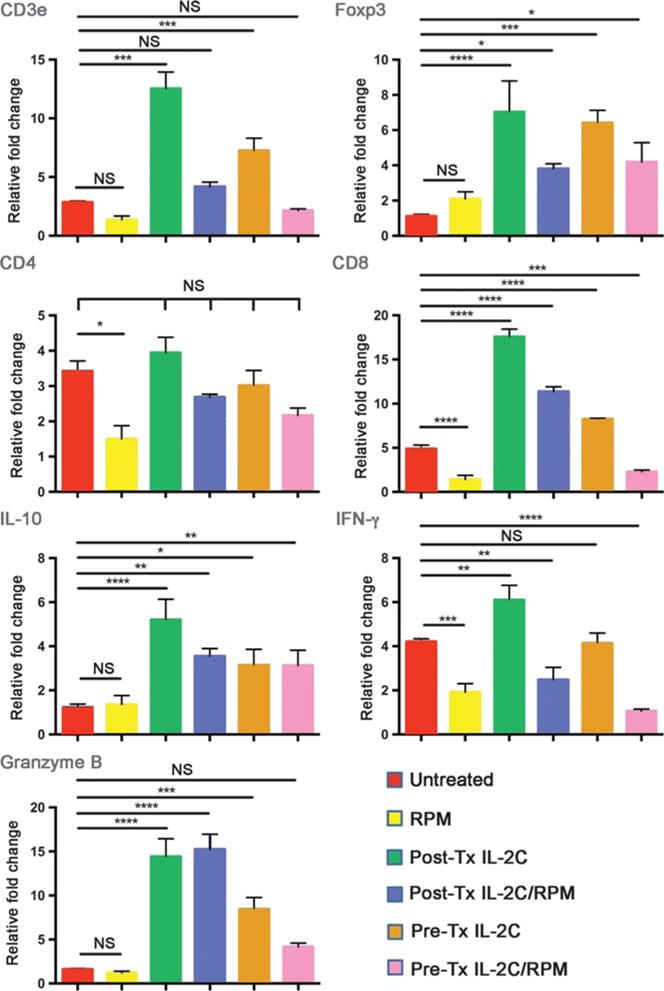
Real-time qPCR analysis of gene expression within allografts at day 5 post-Tx (4 mice/group). IL-2C therapy pre-Tx was accompanied by increases in expression of Cd3e, Foxp3, Cd8, IL10, and Gzmb, but not Ifng, consistent with a Treg cell-dominant effect. The Cd8 increase was muted by concomitant RPM use. IL-2C therapy post-Tx was accompanied by increases in Cd3e, Foxp3, Cd8, IL10, Gzmb and Ifng, consistent with a prominent CD8 T-cell response occurring in addition to positive effects on Treg cells. The increases in Cd8 and Ifng were muted by concomitant RPM.
DISCUSSION
Alternatives to maintenance immunosuppression are important to the development and more widespread use of VCA. Treg cell–based strategies are a promising approach in organ Tx, and various clinical trials of FOXP3+ Treg cell therapy are underway in liver and kidney allograft recipients, though we are unaware of any application as yet in VCA recipients. Treg cell therapy has various shortcomings, including its expense and requirement of individualized preparation of Treg cells. By comparison with cell therapy, pharmacologic modulation of Treg cell numbers and/or suppressive function has considerable advantages, including far lower costs, and titratable responses without the need for individualized therapy. One pharmacologic option is the expansion of Treg cells using IL-2C therapy.
IL-2C can be produced using recombinant IL-2 and various anti-IL-2 mAbs, though the choice of mAb is important in determining the immune cells that are targeted. The IL-2 receptor is heterotrimeric, consisting of 3 chains that can be differentially expressed by immune cells.27 In the normal state, only Foxp3+ Treg cells express the nonsignaling low affinity alpha chain (CD25), whereas memory CD8 T cells and NK cells express the intermediate affinity beta (CD122) and gamma (CD132) chains that signal via MAP kinase, PI3K and JAK-STAT pathways.27 IL-2 initially binds the alpha-chain, followed by recruitment of beta and gamma chains to form the high affinity IL-2R. Empirically, treatment of mice with an anti-IL-2 mAb, S4B6, was found to prevent the interaction of IL-2 with the CD25, whereas binding to CD122 and CD132 was unaffected, leading to the rapid and greater than 100-fold expansion of memory CD8 T cells and >20-fold expansion of NK cells.17 In contrast, treatment with the anti-IL-2 mAb, JES6-1, inhibited the binding of IL-2 to CD122 but did not affect its binding to CD25.17 These studies provide the context for our exploration of the effects of iL-2C therapy in VCA, and help explain some of our own findings.
As anticipated, we observed a transient expansion of the Foxp3+ Treg cell population in naïve mice, and when this was coupled with Tx at the time of peak Treg cell numbers, VCA survival was prolonged about threefold. Sole use of a subtherapeutic regimen of the mTOR inhibitor, RPM, in the month post-Tx had no effect on the tempo of rejection, but combined with pre-Tx IL-2C therapy prolonged VCA survival by sevenfold. In contrast to conventional T cells, Treg cells do not require mTOR for activation and proliferation. As a result, RPM promotes the selective expansion of murine and human Foxp3 + CD4 + Treg cells in vitro and in vivo, and these cells maintain their suppressive functions.28–30 Although the effects of RPM on post-Tx Treg cell numbers, longevity and function were not analyzed in the current study, RPM can also promote Treg cell resistance to apoptosis though upregulation of Bcl-2 and Bcl-xL.31 Because IL-2C treatment did not alter Treg cell suppressive function in our analysis, the efficacy of pre-Tx IL-2C plus post-Tx RPM likely reflects the suppressive effects of above normal numbers of Treg cells on host alloresponses. However, the rapid decline of Treg cell numbers once IL-2C administration ended did not result in long-term suppression of alloresponses. The sevenfold prolongation of survival seen in our study is different to the indefinite survival (>100 days) of islet allografts in mice receiving IL-2C therapy,20 but better than the very limited effects of IL-2C administration alone, or in combination with lymphodepletion, on skin allograft survival.32 Lymphodepletion in conjunction with Treg cell therapy was initially thought to favorably shift the balance towards Treg cell-dominant responses, and showed long-term efficacy in semi-allogeneic33 or nonstringent cardiac transplant models.34 However, after lymphodepletion, populations of residual memory and naïve T cells reconstitute through homeostatic proliferation and are more resistant than normal T cells to Treg cell suppression, leading to less than expected prolongation of survival of strongly immunogenic tissues such as skin grafts.32
Post-Tx IL-2C therapy was considerably less effective than pre-Tx therapy, with a 2.5-fold prolongation of survival, when coupled with post-RPM, compared with use of RPM alone. In addition to increasing the Foxp3+ Treg cell population, post-Tx therapy was accompanied by expansion of host T cells, especially CD8+ effector T cells producing IFN-γ and granzyme B. CD8 responses in mice receiving post-Tx IL-2C were greater than in untreated controls, indicating effects of IL-2C beyond simply targeting Treg cells. This difference with pre-Tx therapy may reflect the point that IL-2C formation with JES6-1 mAb is not completely selective for Treg cells over conventional T cells,17 or that as T cells undergo alloactivation, they upregulate CD25 and can thereby be rapidly expanded by concomitant IL-2C therapy. An inability of IL-2C therapy to control already-developing T-cell immune responses was noted in other contexts, too, including in the NOD mouse model of type I diabetes, and in experimental allergic encephalomyelitis.19,35 Efforts to further increase the efficacy of post-Tx IL-2C therapy by extending the duration of IL-2C treatment did not improve outcomes, consistent with data from other models.19,20 Efforts to promote the efficacy of post-Tx IL-2C therapy by coadministration of FK506 revoked the effects of IL-2C and led to rejection with a tempo similar to that of untreated recipients or those treated with FK506 alone. This finding is consistent with knowledge that calcineurin inhibitor use impairs calcineurin activation and translocation of NFAT, and that NFAT translocation is required for the optimal development and function of Foxp3+ Treg cells.30,36,37
In summary, our studies show that pre-Tx expansion of host Treg cell numbers can significantly prolong VCA survival, especially if coupled with post-Tx RPM. Such short-term use of IL-2C therapy is unlikely to increase risk of infections or cancer and appears to warrant further investigation in VCA models in which Treg cell-dependent immunoregulation has considerable potential.
Acknowledgments
This work was supported by grants from the Department of Defense (W81XWH-13-2-0058 RT150100) and by funding from the Wyss Foundation.
Footnotes
The authors declare no conflicts of interest.
H.X., L.W., L.Q., M.H.L., W.W.H., L.S.L. conceived and designed the experiments. H.X. performed transplantations. H.X., S.D., T.A., T.Z., R.H., L.Q., L.W., W.W.H. performed the experiments and analyzed data. H.X., M.H.L., W.W.H., L.S.L. wrote and edited the article. H.X., L.W., T.A., S.D., Y.Z., M.H.L., W.W.H., L.S.L. discussed and reviewed the article.
References
- 1.Gander B, Brown CS, Vasilic D, et al. Composite tissue allotransplantation of the hand and face: a new frontier in transplant and reconstructive surgery. Transpl Int. 2006;19:868–880. doi: 10.1111/j.1432-2277.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N Engl J Med. 2000;343:468–473. doi: 10.1056/NEJM200008173430704. [DOI] [PubMed] [Google Scholar]
- 3.Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery. 2000;20:360–371. doi: 10.1002/1098-2752(2000)20:8<360::aid-micr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Petruzzo P, Dubernard JM, Lanzetta M. International Registry on Hand and Composite Tissue Transplantation. https://wwwhandregistrycom/. Updated 2017.
- 5.Gupta A, Kumer S, Kaplan B. Novel immunosuppressive strategies for composite tissue allografts. Curr Opin Organ Transplant. 2014;19:552–557. doi: 10.1097/MOT.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 6.Siemionow M, Gharb BB, Rampazzo A. Successes and lessons learned after more than a decade of upper extremity and face transplantation. Curr Opin Organ Transplant. 2013;18:633–639. doi: 10.1097/MOT.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 8.Khalifian S, Brazio PS, Mohan R, et al. Facial transplantation: the first 9 years. Lancet. 2014;384:2153–2163. doi: 10.1016/S0140-6736(13)62632-X. [DOI] [PubMed] [Google Scholar]
- 9.Petruzzo P, Gazarian A, Kanitakis J, et al. Outcomes after bilateral hand allotransplantation: a risk/benefit ratio analysis. Ann Surg. 2014;261:213–220. doi: 10.1097/SLA.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman CL, Ouseph R, Blair B, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12:1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 11.Mundinger GS, Drachenberg CB. Chronic rejection in vascularized composite allografts. Curr Opin Organ Transplant. 2014;19:309–314. doi: 10.1097/MOT.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 12.Mathes DW, Chang J, Hwang B, et al. Simultaneous transplantation of hematopoietic stem cells and a vascularized composite allograft leads to tolerance. Transplantation. 2014;98:131–138. doi: 10.1097/TP.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang J, Graves SS, Butts-Miwongtum T, et al. Long-term tolerance toward haploidentical vascularized composite allograft transplantation in a canine model using bone marrow or mobilized stem cells. Transplantation. 2016;100:e120–e127. doi: 10.1097/TP.0000000000001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madariaga ML, Shanmugarajah K, Michel SG, et al. Immunomodulatory strategies directed toward tolerance of vascularized composite allografts. Transplantation. 2015;99:1590–1597. doi: 10.1097/TP.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 16.Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 17.Boyman O, Kovar M, Rubinstein MP, et al. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 18.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7:280rv282. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyman O, Krieg C, Letourneau S, et al. Selectively expanding subsets of T cells in mice by injection of interleukin-2/antibody complexes: implications for transplantation tolerance. Transplant Proc. 2012;44:1032–1034. doi: 10.1016/j.transproceed.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 21.Shin HJ, Baker J, Leveson-Gower DB, et al. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4 + CD25 + Foxp3+ regulatory T cells. Blood. 2011;118:2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith C, Martinez M, Peet J, et al. Differential outcome of IL-2/anti-IL-2 complex therapy on effector and memory CD8+ Tcells following vaccination with an adenoviral vector encoding EBV epitopes. J Immunol. 2011;186:5784–5790. doi: 10.4049/jimmunol.1003394. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Xie F, Luo X, et al. Orthotopic forelimb allotransplantation in the rat model. Microsurgery. 2015;36:672–675. doi: 10.1002/micr.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 26.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 27.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4 + CD25 + FOXP3+ regulatory Tcells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia M, Stabilini A, Migliavacca B, et al. Rapamycin promotes expansion of functional CD4 + CD25 + FOXP3+ regulatory Tcells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 30.Akimova T, Kamath BM, Goebel JW, et al. Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant. 2012;12:3449–3461. doi: 10.1111/j.1600-6143.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss L, Czystowska M, Szajnik M, et al. Differential responses of human regulatory Tcells (Treg) and effector Tcells to rapamycin. PLoS One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YH, Koo SK, Kim Y, et al. Effect of in vitroexpanded CD4(+)CD25(+) Foxp3(+) regulatory T cell therapy combined with lymphodepletion in murine skin allotransplantation. Clin Immunol. 2010;135:43–54. doi: 10.1016/j.clim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Golshayan D, Jiang S, Tsang J, et al. In vitro-expanded donor alloantigen-specific CD4 + CD25+ regulatory Tcells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 34.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4 + CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008;8:298–306. doi: 10.1111/j.1600-6143.2007.02088.x. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaeth M, Schliesser U, Muller G, et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2012;109:16258–16263. doi: 10.1073/pnas.1203870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory Tcell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]


