Abstract
Prostate cancer (PCa) progression involves a shift from endocrine to paracrine and eventually autocrine control resulting from alterations in molecular mechanisms in the cells. Deregulation of RNA translation is crucial for tumor cells to grow and proliferate; therefore, overactivation of the translation machinery is often observed in cancer. The two most important signal transduction pathways regulating PCa progression are PI3K/Akt/mTOR and Ras/MAPK. These two pathways converge on the eukaryotic translation initiation factor 4E (eIF4E) which binds to the protein scaffold eIF4G upon mechanistic target of rapamycin (mTOR) activation and is phosphorylated by the mitogen-activated protein kinase (MAPK) interacting protein kinases (Mnk1/2). This review describes the role of eIF4E in mRNA translation initiation mediated by its binding to the methylated 5′ terminal structure (m7G-cap) of many mRNAs, and the ability of many tumor cells to bypass this mechanism. Hormonal therapy and chemotherapy are two of the most prevalent therapies used in patients with advanced PCa, and studies have implicated a role for eIF4E phosphorylation in promoting resistance to both these therapies. It appears that eIF4E phosphorylation enhances the rate of translation of oncogene mRNAs to increase tumorigenicity.
Abbreviations: eIF4E, eukaryotic translation initiation factor 4E; mTOR, mammalian target of rapamycin; PCa, prostate cancer; Mnk, mitogen activated protein kinase interacting protein kinase; ADT, androgen deprivation therapy; MAPK, mitogen-activated protein kinase; CRPC, castration resistant prostate cancer; PTEN, phosphatase and tensin homolog; EGFR, epidermal growth factor receptor; PI3K, phosphoinositide 3-kinase; eIF, eukaryotic initiation factor; IRES, internal ribosome entry site; ITAFs, IRES trans-acting factors; RAPTOR, regulatory associated protein or mTOR; PRAS40, 40 kDa pro-rich Akt substrate; RICTOR, rapamycin insensitive companion of mTOR; PROTOR, protein observer of RICTOR; mSIN1, mammalian stress-activated map kinase-interacting protein 1; Rheb, Ras homolog enriched in brain; 4EBP1, eukaryotic translation initiation factor 4E binding protein 1; PIN, prostate intraepithelial neoplasia; MEK, mitogen-activated protein kinase kinase; SRPK, Ser/Arg (SR)-rich protein kinase; BPH, benign prostate hyperplasia; TOP, 5′-Terminal OligoPyrimidine; LARP1, La-related protein 1; MTA1, metastasis associated protein; HSP, heat shock protein; FKBP12, FK506 binding protein 12; MTC, medullary thyroid carcinoma; EMT, epithelial mesenchymal transition; CYP17A, cytochrome P450 17A1
Introduction
Prostate cancer (PCa) development, growth and metastasis depends initially on androgens, therefore androgen deprivation therapy (ADT) is the first line of treatment for metastatic PCa. However, despite initial response the majority of these patients eventually relapse, giving rise to castration resistant prostate cancer (CRPC) [1]. Many factors play different roles in PCa progression to CRPC including: (i) chromosomal aberrations, with deletion of chromosomal segments and some amplifications [2], (ii) inactivating mutations in tumor suppressors, including the phosphatase and tensin homolog (PTEN) [3] and the p53 gene at around 30% of the cases [4], (iii) overexpression of oncogenes (or proto-oncogenes) such as epidermal growth factor receptor (EGFR) or MYC [5] and (iv) activation of cancer specific pathways decreasing apoptosis, increasing proliferation and affecting differentiation, such as those downstream of phosphatidylinositol 3-kinase (PI3K) and Ras [6].
There are three main causes for the increased expression of certain factors with PCa progression – (i) increased transcription, (ii) increased translation and (iii) decreased internalization and degradation. Among the various factors that contribute to the progression of PCa, one in particular shows increasing relevance, which is the deregulation of protein synthesis control [7]. Protein overexpression is commonly observed in cancer, conferring its ability to increase proliferation or decrease apoptosis rapidly. Expressions of several proteins have been linked with oncogenesis, such as Myc, Ras and Cyclin D1. To increase protein expression, cancer cells alter the cellular translational machinery, a case in point is ErbB3, a member of the EGFR family of receptor tyrosine kinases (RTK), which shows no change at the mRNA level between normal prostate and prostate cancer, but display significantly higher protein expression in PCa compared to normal prostate [8]. In this review, we will discuss the role of mRNA translation mechanisms in the progression of prostate cancer to a castration resistant state.
Mechanisms of mRNA Translation Initiation
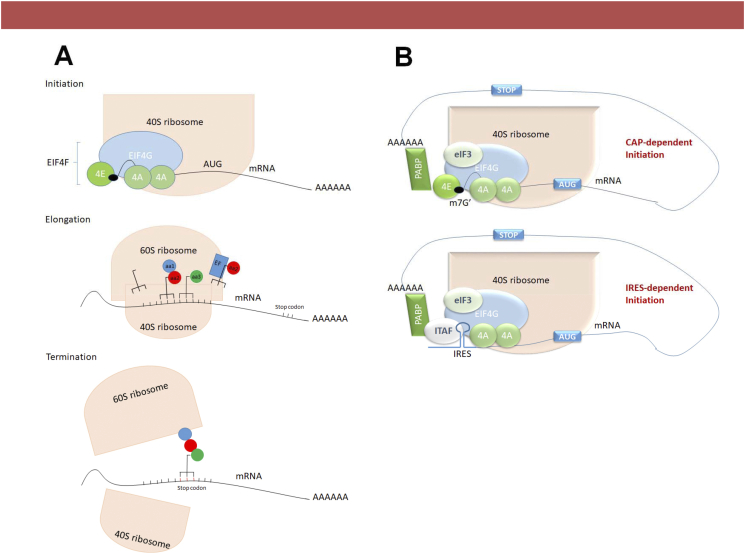
Translation of proteins in eukaryotes occurs in three phases: initiation, elongation and termination. Initiation is usually the phase implicated in cancer development and progression [9]. During initiation, several eukaryotic initiation factors (eIFs) bring together the first transfer RNA (tRNA), the small ribosomal subunit (40S) and the mRNA. This pre-initiation complex scans the 5’ untranslated region (5’UTR) in the 5′ to 3′ direction of the mRNA with the methionyl tRNA specialized for initiation (Met-tRNAi) in search of the startcodon, usually (but not always) AUG [9]. Once the start codon is recognized, the eIFs are separated from the complex and the large ribosomal subunit (60S) joins the complex to form the elongation competent 80S ribosome.
After the reading frame for the protein is determined, the elongation phase starts recruiting aminoacylated tRNAs to the first binding site, adding amino acids in a chain regulated by eukaryotic elongation factors (eEFs), binding them by peptide bonds until a stop codon is recognized and the synthesis is terminated, releasing the 60S ribosome from the complex and dissociating it into its subunits to be recycled into another round of protein synthesis (Figure 1A). All steps of protein synthesis are strictly regulated but a large part of the translational control is observed in the initiation step [10].
Figure 1.
(A) Steps of eukaryotic protein translation. At initiation, the eukaryotic translation initiation complex eIF4F, bound to the small ribosomal subunit 40S scans the messenger RNA (mRNA) for the start codon, when then the big ribosomal 60S subunit binds the complex to initiate translation. At Elongation, amynoacylated transfer RNAs (tRNA) bring together amino acids to be bound together by eukaryotic elongation factors (eEFs) through peptide bonds forming a chain. In the termination phase, the stop codon is read by the complex, releasing the large ribosomal subunit and terminating synthesis. (B) Protein translation initiation mechanisms. Cap-dependent initiation takes place with binding of the eIF4E to the m7G 5′ PCa of the mRNA and bringing together all proteins to form the eIF4F protein translation initiation complex. Alternatively, Cap-independent translation, or translation initiation by internal ribosomal entry site (IRES) does not need the mRNA PCa binding to eIF4E, utilizing a group of proteins named IRES trans-activating factors (ITAFs).
Eukaryotic mRNA translation initiation starts with the binding of the eukaryotic initiation factor 4E (eIF4E) to the mRNA 5′-cap structure, which consists of a 7-methyl-guanosine triphosphate (m7G) moiety linked to the first nucleotide via a 5′-5′ triphosphate bridge. Subsequently, eIF4E-mRNA binds to the scaffolding protein eIF4G and the helicase eIF4A forming the eIF4F translational complex, facilitating the approximation of the small ribosomal subunit, completing the formation of the 48S pre-initiation complex [11].
Another component that allows cap-independent translation initiation is the internal ribosome entry site (IRES), a mechanism that recruits the 40S ribosomal subunit to initiate translation without the necessity of the cap-binding protein eIF4E. This process is facilitated by a group of proteins named IRES trans-acting factors (ITAFs) that do not participate in the canonical cap-dependent translation initiation. This mechanism is better understood and extensively studied in viral organisms, and is still a matter of debate and controversy in cellular translation [12], but there are lines of evidence where highly 5′ UTR structured mRNAs containing IRES promotes IRES translation in stress conditions when cap-dependent is decreased (Figure 1B) [12], [13].
The mTOR Pathway
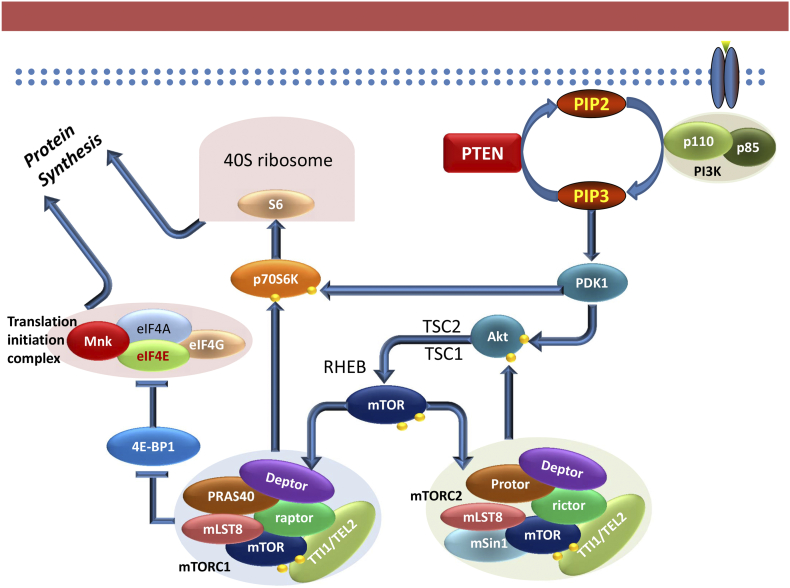
mTOR is an atypical serine/threonine kinase and a member of the phosphatidylinositol 3-kinase-related kinase (PIKK) family that acts downstream of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. MTOR partakes in various functions as part of two complexes, mTORC1 and mTORC2, depending on its binding proteins (Figure 2). Both complexes share the mTOR protein, mLST8/G-protein β-subunit-like protein (GβL), the Tti1/Tel2 complex which likely stabilizes the complex [14] and the Disheveled, EGL-10 and pleckstrin (DEP) domain-containing mTOR-interacting protein (DEPTOR). DEPTOR is thought to be an inhibitor of mTORC1 [15], although the mechanism by which it inhibits this complex is unknown. mTORC1 has additionally the regulatory associated protein or mTOR (RAPTOR) and a 40kDa pro-rich Akt substrate (PRAS40) whereas mTORC2 consists of the rapamycin insensitive companion of mTOR (RICTOR), the mammalian stress-activated map kinase-interacting protein 1 (mSIN1) and the protein observer of RICTOR (PROTOR).
Figure 2.
Scheme of PI3K/Akt/mTOR pathway depicting both complexes mTOR protein takes part in, mTORC1 and mTORC2. MTORC1 increases protein translation through phosphorylation of its two direct targets, 4EBP1 and P70S6K. While P70S6K induces protein translation through activation of the S6 ribosomal protein, 4EBP1 phosphorylation promotes protein synthesis by releasing eIF4E that can then bind to eIF4G to form, along with other proteins, the initiation complex eIF4F.
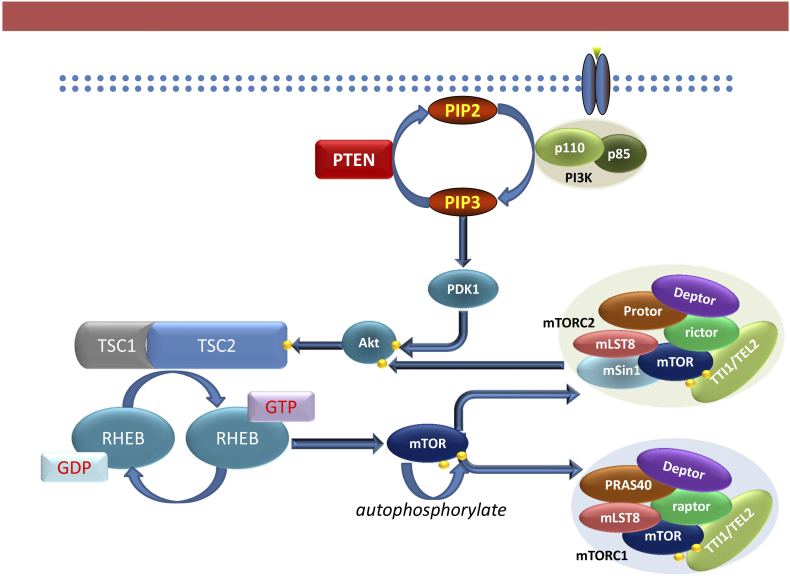
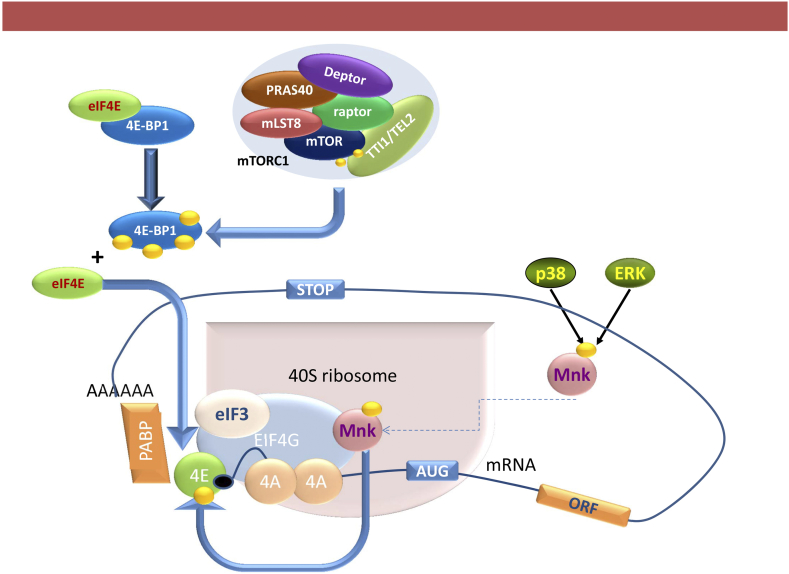
The mTOR complexes have distinct functionalities in the cell. While mTORC2 is implicated in cytoskeletal organization, glucose and lipid metabolism [16], mTORC1 affects cellular proliferation, growth, autophagy and protein synthesis [17]. mTORC1 is activated by the PI3K/AKT pathway where activated AKT disrupt the tuberous sclerosis protein (TSC) 1/2 complex formation (Figure 3), a repressor of the small GTPase Rheb, which phosphorylates mTORC1 at Ser2448 [18]. Upon activation, mTORC1 increases mRNA translation by phosphorylating its downstream molecules p70S6 kinase (p70S6K) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1). The phosphorylation of p70S6K enhances translation of mRNAs encoding ribosomal proteins, elongation factors and insulin growth factor 2 [19]. 4EBP1 phosphorylation causes its dissociation from the eukaryotic translation initiation factor 4E (eIF4E), allowing the latter to bind to the scaffolding protein eIF4G, that along with the RNA helicase eIF4A forms the translation initiation complex eIF4F (Figure 4). EIF4E is significantly less abundant than the other proteins that form the eIF4F complex, hence it's expression and activation are rate-limiting factors in translation initiation [20].
Figure 3.
Mechanism of PI3K activation of mTOR proteins. Upon activation by transmembrane cell receptors, PI3K produces phosphatidylinositol trisphosphate (PIP3) from phosphatidylinositol biphosphate (PIP2), which PTEN reverses. PIP3 recruits proteins with pleckstrin homology domain such as PDK1 which phosphorylates Akt at Thr308. Akt phosphorylates and disrupts the complex Tuberous sclerosis complex 1/2 (TSC1-TSC2) which activates the GTPase activity of Rheb and phosphorylate and activate mTOR.
Figure 4.
Formation of the translation initiation complex eIF4F. Upon phosphorylation, 4EBP1 releases eIF4E, which binds to the 5′ PCa of the mRNA and brings to the scaffolding protein eIF4G that along with the mRNA helicase eIF4A forms the initiation complex eIF4F. Within this complex, the MAP kinase-interacting kinase (Mnk), which is phosphorylated by the P38 MAPK and ERK, is brought to proximity to its substrate eIF4E, phosphorylating it at Ser209.
mTOR is activated by different extracellular stimuli such as nutrients, growth factors, hormone availability and intracellular activation of signaling pathways like the PI3K/ATK and Ras-related small GTP-binding proteins [21]. The mTOR pathway has been implicated in the drive and maintenance of cell survival and proliferation of different types of cancer cells [22], [23] and in epithelial derived cancers such as PCa, the PI3K/mTOR pathway is notoriously hyperactive [24]. Many factors contribute to the aberrant activation of mTOR pathway elements, such as PI3K amplification, PTEN loss of function or deletion and AKT, S6K1, 4EBP1 and eIF4E overexpression [18]. In a murine model of prostate cancer, Akt overexpression led to activation of p70S6K, increase in prostate epithelial cell size and development of prostatic intraepithelial neoplasia (PIN), a precursor of prostatic carcinoma [25]. PTEN conditional knockout in mice also decrease latency for PIN formation, and progression to invasive adenocarcinoma with subsequent formation of metastatic prostate cancer [26]. mTOR protein itself is also essential for PTEN null prostate cancer and conditional deletion of mTOR decrease tumor formation in this mouse model [27]. A recent report showed that in PTEN deficient tumors, hypoxia prevents 4EBP1 dephosphorylation and increases 4E-BP1 binding to eIF4E, which suppresses translation [28]. Taken together, these reports indicate an important role for the PTEN/PI3K/Akt pathway in mRNA translation.
Sitting at the translational end of the mTOR pathway, eIF4E plays an important role in cap-dependent translation. Elevated levels of eIF4E enhances translation of mRNA with lengthy and high structured 5’ untranslated regions, such as c-myc, cyclin-D1 and survivin [29], and is also related to poor prognosis in prostate cancer [30]. Thus, eIF4E is likely to be an important player in the regulation of prostate cancer progression.
Mechanism of eIF4E phosphorylation by Mnk
As the least abundant protein that form the initiation complex eIF4F, eIF4E is considered to be the rate limiting component in binding and cap-dependent translation of certain mRNAs [20]. Phosphorylation of eIF4E occurs by only two known kinases, MAP kinase-interacting kinases (Mnk1 and Mnk2), and is observed when both Mnk and eIF4E are bound respectively to the C and N-terminal portion of the scaffolding protein eIF4G facilitating the approximation of these two proteins [31]. The phosphorylation of eIF4E by Mnks was initially thought to increase its affinity to the m7G cap [32]; however, this idea was refuted when it was demonstrated that phosphorylated eIF4E binds with lower affinity to the cap [33]. Corresponding investigations in plants also showed that phosphorylation of plant eIF4E (eIFiso4E) decreased the kinetic rate (2-fold) and increased the dissociation rate (2-fold) of binding to the m7G cap as compared to non-phosphorylated eIFiso4E binding at 22°C [34]. As early as 2002, it had been proposed that eIF4E phosphorylation likely enables a conformational change in the molecule which prevents it from binding to the m7G cap [35]. However, this may not limit the level of translation, but may change the mode of translation from a cap-dependent to a cap-independent one. The Mnks are activated by phosphorylation of two threonine residues. Mnk1 displays low activity in resting cells and is highly responsive to agents that activate its upstream kinases ERK or p38 MAPK. In contrast, Mnk2 has a high basal activity, but is less responsive to ERK/p38 MAPK activating agents [36]. P38 MAPK and ERK mediated phosphorylation of Mnk1 not only activates its kinase activity but also moderates the interaction of Mnk1 with the scaffolding protein eIF4G [37].
There has been previous evidence that MAPKs activate Mnk1 to induce phosphorylation of eIF4E, whereas Mnk2 mainly contributes to eIF4E's basal, constitutive phosphorylation [38]. On the other hand, it is suggested by different reports that Mnk2 knock down, but not Mnk1, decreased rapamycin mediated eIF4E phosphorylation suggesting that this phosphorylation is dependent on Mnk2 [39], [40] but neither of the Mnks are essential for development as double Mnk1/2 knockout mice have normal phenotype [38].
Mnks are known to have functions outside of their action in phosphorylation of eIF4E. In fact, activation of the Raf-MEK-ERK1/2 signals induced IRES-mediated translation that is dependent on MNK1/2 that was independent of either eIF4G or eIF4E [41]. In contrast, MNK enabled IRES-mediated translation through negative regulation of the Ser/Arg (SR)-rich protein kinase (SRPK) [41] via mTORC2 and AKT. The resulting suppression of AKT signaling attenuates SRPK activity to enhance IRES-dependent translation [42]. Although these observations were made in viral systems, there is ample evidence that they may be observed in mammalian systems as well. MNK also can sustain mTORC1 activity upon rapamycin treatment and contribute to mTORC1 signaling following T cell activation and growth stimuli in cancer cells [43]. MNK engages in a complex with mTORC1, promotes mTORC1 association with TEL2, and facilitates mTORC1 binding to its substrate [43]. Thus, Mnks can regulate mTORC1 enzymatic activity.
EIF4E Phosphorylation in Prostate and Prostate Cancer
Increased eIF4E and its Phosphorylation in Prostate Cancer
Early studies indicated that eIF4E levels were significantly overexpressed in prostate cancer (78%) [44]. eIF4E levels were strongly correlated with VEGF and cyclin D1 in the prostate (Spearman's r=0.46 and 0.54, p<0.005), suggesting a role for eIF4E in translational regulation of proteins related to angiogenesis and growth [44]. Immunohistochemical analysis of 148 tissues from 89 PCa patients showed that eIF4E expression is increased in advanced PCa compared to benign prostatic hyperplasia (BPH) [45]. Increased eIF4E expression was also significantly related to reduced patient survival, and is related to increased eIF4E activation [45]. Increased phosphorylation of eIF4E correlate with disease progression in PCa patients [30]. We recently showed that eIF4E phosphorylation in localized PCa samples strongly correlated with the cell proliferation marker Ki67, which supports a role for eIF4E phosphorylation in this disease [46]. These studies indicate a strong role for eIF4E and eIF4E phosphorylation in human PCa progression.
Cell line and animal studies also support an important role for eIF4E and eIF4E phosphorylation in this disease. PTEN is a commonly deleted gene in prostate cancer. In PCa cells with intact PTEN, which can effectively counter the PI3K/AKT/mTOR pathway, Mnk-dependent phosphorylation of eIF4E is elevated whereas it is low in cells that express a mutant PTEN and constitutively active Akt/mTOR pathway [47]. It is likely that this effect of PTEN reflects the convergence of the PI3K/Akt and the Ras/MAPK pathways on eIF4E and the balance between the two pathways. Therefore, in PTEN-positive tumors where the PI3K/Akt pathway is suppressed, the Ras/MAPK pathway that activates Mnk phosphorylation plays a more important role, resulting in higher levels of eIF4E phosphorylation, despite lower availability of eIF4G bound eIF4E due to lower 4E-BP1 phosphorylation.
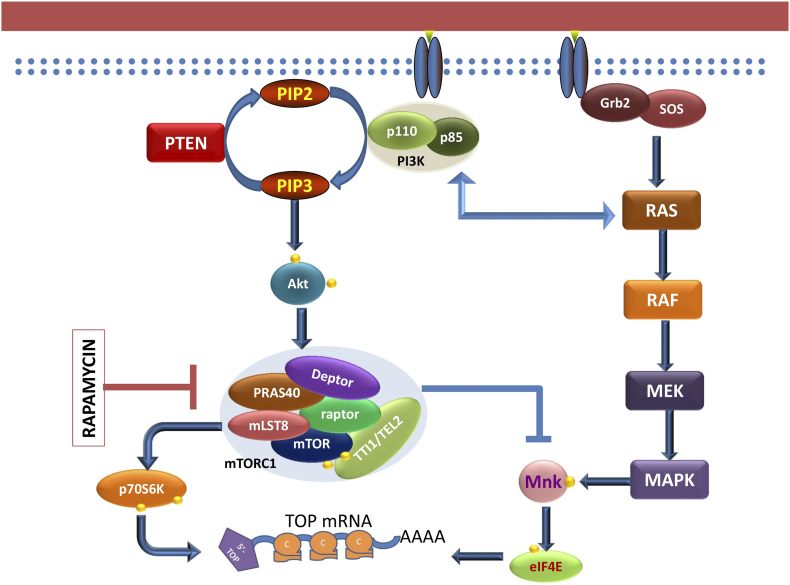
mRNAs that encode components of the translational apparatus carry a common cis-regulatory element, the 5′-Terminal OligoPyrimidine motif (5′TOP); these mRNAs are referred to as TOP mRNAs. They are identified by a sequence of 6–12 pyrimidines at the 5′ end and are characterized by a growth-associated translational regulation. All vertebrate genes for the 80 ribosomal proteins are TOP genes. Remarkably, inhibition of Mnk and therefore eIF4E phosphorylation strongly reduced the polysomal recruitment of terminal oligopyrimidine messenger RNAs (TOP mRNAs), which are known targets of mTOR-dependent translational control [47]. This indicates a necessary role of eIF4E phosphorylation in translation mechanisms downstream of mTOR in the translation of the TOP mRNAs (Figure 5). Recent studies have also implicated LA-related protein 1 (LARP1), which competes with eIF4F for binding to mRNA 5' ends [48], [49], [50]. In addition to TOP mRNAs, some other mRNAs regulating important functions, such as some cell cycle proteins are similarly regulated. Microarray analysis showed that Mnk affected translation of mRNAs involved in cell cycle progression and inhibition of both mTOR and Mnk was needed to fully inhibit mRNA translation into protein, suggesting that the two pathways had parallel and perhaps competing roles in the synthesis of these proteins (Figure 5) [47].
Figure 5.
Both mTOR and eIF4E phosphorylation are needed for polysomal recruitment of terminal oligopyrimidine messenger RNAs (TOP mRNA). Inhibition of mTOR by rapamycin prevents polysomal recruitment of TOP mRNA. However, rapamycin also induced the phosphorylation of eIF4E at S209, which was also needed for TOP mRNA recruitment. This shows that inhibition of the PI3K/Akt/mTOR pathway moved signal transduction towards a Ras/MAPK pathway, resulting in an increase in eIF4E phosphorylation. Therefore, both rapamycin and an Mnk inhibitor were needed to completely inhibit protein translation.
In a series of elegant experiments, Furic et al. [30] developed a knock-in mouse expressing a nonphosphorylatable form of eIF4E. This knock-in mouse that could not be phosphorylated was resistant to tumorigenesis by Ras overexpression or PTEN loss in an animal model of PCa progression, while control mice that did express phosphorylated eIF4E developed tumors. These studies unquestionably demonstrated the tumorigenic potential of eIF4E phosphorylation. However, abrogating eIF4E phosphorylation alone did not reduce cell proliferation rates demonstrating that eIF4E phosphorylation needs to cooperate with other oncogenic processes to promote tumorigenicity. They also show a significant role of mTOR in regulating eIF4E phosphorylation. Genome-wide analysis of translated mRNAs showed that the phosphorylation of eIF4E is required for translational of several proteins implicated in tumorigenesis, and that eIF4E phosphorylation increases the efficiency of translation of the mRNAs associated with these proteins [30]. This article sheds considerable light on the mechanism by which eIF4E phosphorylation works.
Other commonly upregulated oncogenes in advanced prostate cancer are c-Myc and Akt. It has been shown that cells expressing the oncogenes Myc and myristoylated AKT can initiate heterogeneous tumors in a prostate cancer model [51]. The resulting tumors were acinar-type adenocarcinoma with elevated eIF4E-driven mRNA translation, indicating a close relationship between c-myc and Akt activation, and increased eIF4E [51]. These tumors also expressed the metastasis associated protein MTA1, a transcriptional co-regulator that is part of the histone-deacetylase multiprotein complex, and Sox2, a transcription factor that controls self-renewal of stem cells. These two transcription regulators are known targets of eIF4E-driven cap-dependent translation that are normally expressed in self-renewing basal cells but not benign luminal cells. These findings suggest that eIF4E may promote cell survival and self-renewal in conjunction with c-myc and myristoylated Akt [51]. While these studies show myc-regulation of eIF4E, other studies have shown that c-Myc protein expression is also regulated by eIF4E expression and hence by cap-dependent translation, although not in prostate cancer [52]. Elevated protein levels of c-Myc due to increased eIF4E activity is also observed in genetically defined human mammary epithelial cells that are made resistant to the mTOR/PI3K dual inhibitor BEZ235 [53]. Together, these studies show a positive correlation between c-myc and eIF4E in prostate cancer progression. Based on the studies noted above, it appears that eIF4E phosphorylation enhances the rate of translation of oncogenic mRNAs, thereby increasing tumorigenicity.
Relationship Between eIF4E Phosphorylation and Androgen Receptor Activation
PCa progression is strongly regulated by the androgen receptor (AR), which is activated by binding of its ligands, the androgens [54]. Studies revealed that increase of eIF4E was linked to progression to castration resistance in experimental models [45]. We showed that activation of the AR suppresses eIF4E phosphorylation whereas inhibition of AR with anti-androgens and AR inhibitors stimulated eIF4E phosphorylation [46]. Knockdown of eIF4E by siRNA or Mnk inhibitors sensitized CRPC cells to bicalutamide, whereas eIF4E overexpression induced resistance to this anti-androgen. Downstream targets of eIF4E-mediated translation, such as survivin, showed that eIF4E(S209) phosphorylation increased cap-independent translation, whereas its inhibition restored cap-dependent translation. Our results demonstrate three main points: (i) inhibition of AR increased eIF4E phosphorylation (ii) the increase in eIF4E phosphorylation at S209 as a result of AR inhibition induced cap-independent translation and (iii) simultaneous inhibition of eIF4E phosphorylation and mTOR activation was effective in suppressing tumor growth by inhibiting both cap-dependent and –independent translation caused by progression to CRPC.
Our results do not elucidate the mechanism by which AR inhibition may lead to an increase in eIF4E phosphorylation, but there are other published reports that may fill in these details. Heat shock protein 27 (Hsp27) is a small heat shock protein that acts as a molecular chaperone to maintain denatured proteins in a folding-competent state [55], [56]. Reports show that Hsp27 plays a significant role in the regulation of AR transcriptional activity in prostate cancer cells [57], [58]. This effect of Hsp27 on the AR may be mediated by PKD1 [59]. It has been shown that both androgen withdrawal and chemotherapy causes an increase in Hsp27, and this causes a simultaneous increase in eIF4E protein but not mRNA levels [60]. This effect is mediated by cytoprotection provided by Hsp27 which binds to eIF4E and prevents its degradation [60]. Therefore, bicalutamide may cause an increase in eIF4E phosphorylation by inducing an increase in Hsp27, which in turn causes an increase in eIF4E phosphorylation simply by preventing its dephosphorylation.
Various laboratories have taken advantage of the significant increase in eIF4E phosphorylation upon AR inhibition and have developed various drugs that dually target both the AR and Mnk, in an effort to prevent the increase in eIF4E phosphorylation caused by AR inhibition. In androgen-dependent LNCaP cells, the dual AR/Mnk inhibitor VNLG-152 was shown to simultaneously inhibit both eIF4E phosphorylation and AR transcriptional activity by promoting post-translational degradation of these proteins [61]. Other dual AR/Mnk degration inducers VNHM-1-81, VNHM-1-66 and VNHM-1-73 suppressed Mnk activation and induced degradation of both full-length AR and AR splice variants [62]. Therefore, these classes of drugs appear to be poised to generate traction regarding the possible role of dual AR/Mnk inhibitors in preventing PCa progression.
EIF4E-Driven Resistance to mTOR Inhibitors in Prostate and Other Cancers
mTOR deregulation is observed in multiple cancers; therefore mTOR inhibitors have been used in clinical trials in many of them [63], [64], [65]. The first mTOR inhibitor was rapamycin (sirolimus), a macrolide compound primary used as antifungal agent until its immunosuppressive and anti-proliferative properties were discovered [66]. Due to poor bioavailability of rapamycin, rapamycin analogs (rapalogs) have been developed, such as temsirolimus (CCI-779), everolimus (RAD001) and ridaforolimus (AP-23573) [67]. Both everolimus and temsirolimus are FDA-approved for advanced renal carcinoma, but other tumors where mTOR inhibitors have been tried as single agents have displayed significant resistance to their use. One major player in this resistance is overexpression or increased phosphorylation of eIF4E by Mnk1/2. Recent clinical studies using the dual mTORC1/mTORC2 inhibitor MLN128 failed to affect eIF4E activity and offered limited clinical efficacy at tolerable toxicity levels [28].
Rapamycin binds to the FK506 binding protein 12 (FKBP12) and the complex then binds to and inhibits mTORC1 [68], but not mTORC2 [69]. Rapamycin is known to inhibit cell growth in multiple prostate cancer cell lines and affect the expression of various signaling proteins, including eIF4E [70], [71]. Despite downregulating the phosphorylation of the mTOR substrates p70S6K and 4E-BP1, rapamycin nevertheless upregulates the phosphorylation of both Akt (at S473) and eIF4E (at S209) [71]. Rapamycin-induced eIF4E phosphorylation is dependent on the expression of mTOR protein and it's binding with RAPTOR in the mTORC1 complex but not RICTOR in the mTORC2 complex (Figure 5) [40]. Although 4EBP1 levels limit the availability of eIF4E to bind and form the protein translational complex eIF4F, rapalog-mediated eIF4E phosphorylation is independent of 4EBP1 [71].
PI3K, which does not directly interact with eIF4E, was also demonstrated to be critical for its phosphorylation, as its knock down prevents the effects of rapamycin on eIF4E [72]. The increase in eIF4E (S209) phosphorylation stimulated cell growth, but this effects of eIF4E phosphorylation could be overcome by the PI3K inhibitor LY294002 [71]. eIF4E related resistance is also reported in other cancers such as small-cell lung carcinoma, in which rapalog-insensitive strains express high levels of eIF4E and MYC [73]. Also, in colorectal cancer cells, increased expression and phosphorylation of eIF4E is necessary and sufficient for acquired resistance to the mTOR inhibitor AZD8055, showing augmented cap-dependent translation even in the presence of the drug [74].
Unlike PI3K, Akt, a downstream target of PI3K, was ruled out as a mediator of rapamycin induced eIF4E phosphorylation as its knock down increased eIF4E phosphorylation, proving it to be a negative regulator of eIF4E phosphorylation [72]. This supports the hypothesis put forth in Figure 5, that inhibitors of the Akt/mTOR pathway shift the balance toward Ras/MAPK activation downstream of PI3K. However, eIF4E phosphorylation due to rapamycin treatment is also independent of MAPK pathway, since neither the MEK inhibitor U0126, the p38 inhibitor SB203580 nor the Rsk1 inhibitor BI-D1870 prevented the increase in Ser209 phosphorylation upon rapamycin treatment, despite the fact that eIF4E phosphorylation is regulated by Mnk which itself is phosphorylated by the MAPKs [72]. Since Mnk is the only kinase known to phosphorylate eIF4E at S209, these seemingly contradictory reports can be reconciled if rapamycin induced Mnk phosphorylation by a mechanism that is independent of MAPK activation.
Increased Mnk-eIF4E Activity Results in Chemoresistance of Prostate and Other Cancers
The involvement of Mnk in eIF4E phosphorylation and its implications in cancer are elegantly reviewed recently [75], but there are several other reports that demonstrate the Mnk-eIF4E axis role in acquired resistance to specific targeted therapies. Various drugs used or tested in prostate cancer has shown significant dependence of their response on eIF4E phosphorylation status. Similar to androgen withdrawal, resistance to chemotherapy was also traced to elevated levels of eIF4E expression, also by a mechanism mediated by Hsp27 [60]. A recent study demonstrated that eIF4E interacts with the C-terminal part of Hsp27 [76]. Loss of this interaction increased chemosensitivity of prostate cancer cells and the phenazine derivative 14 as the compound able to efficiently interfere with this protein/protein interaction [76]. In addition, chemotherapy agents such as temozolomide (TMZ), used in medullary thyroid carcinoma (MTC), were shown to increase Mnk phosphorylation of eIF4E, altering mRNA translation regulation and ultimately conferring resistance to treatment [77].
In pancreatic cancer, repopulation of surviving tumor cells after irradiation is one of the major reasons for recurrence of the disease [78]. It was shown that eIF4e phosphorylation is related to resistance to the chemotherapeutic agent Gemcitabine [79] and also to promote epithelial-mesenchymal transition (EMT), which can be reverted by inhibition of Mnk [80]. Taken together, these articles suggest significant implications of eIF4E increased expression and phosphorylation in the development of resistance to chemotherapeutic agents.
EIF4E Targeted Therapies
As mentioned above, since eIF4E overexpression and phosphorylation is linked to resistance to many targeted therapies, several approaches to decrease eIF4E protein synthesis, coupling with eIF4G or its phosphorylation have been tested in pre-clinical models. In addition to Mnk inhibitors that suppress eIF4E phosphorylation, other attempts to develop eIF4E inhibitors have yielded considerable results. Galeterone (gal), or TOK-001, is a potent AR and CYP17A1 inhibitor, that in LNCaP, CWR22Rv1 and PC-3 prostate cancer cells have been shown to decrease phosphorylation of eIF4E, mainly because of its action in depleting Mnk protein expression [81]. Prostate cancer cells treated with gal and its analog VNPT55 also affected migration, invasion and expression of EMT factors. Downstream targets of eIF4E such as Cox-2, Mcl-1 and Cyclin D1 were also suppressed in gal/VNPT55 treated LNCaP [81]. Galeterone (gal) was designed to inhibit proliferation of AR-dependent prostate cancer cell in vitro and in vivo and is currently in phase III clinical development [81]. Preclinical studies with gal show that it exhibits strong antiproliferative activities against AR-negative prostate cancer cells and tumors through a mechanism involving phosphorylation of eIF2α. Gal also inhibit migration and invasion of prostate cancer cells via inhibition of Mnk-eIF4E [81]. Thus a new class of compound is emerging that will be able to effectively inhibit eIF4E in addition to AR transcriptional activity, so as to avoid negative feedback (Table 1).
Table 1.
Drugs that affect the functioning of eIF4E.
| Drug | Target/action | Mechanism of inhibition of eIF4E |
|---|---|---|
| Galeterone | AR and CYP17A1 | Depletes Mnk protein expression causing suppression of eIF4E phosphorylation |
| VNPT55 | Galeterone analog | Depletes Mnk protein expression causing suppression of eIF4E phosphorylation |
| VNLG-152 | Dual AR/Mnk inhibitor | Prevents eIF4E phosphorylation |
| VNHM-1-81, VNHM-1-66 VNHM-1-73 | AR/Mnk degration inducers | Depletes Mnk protein expression causing suppression of eIF4E phosphorylation |
| Ribavarin | Guanosine analog | Inhibits eIF4E binding to m7G cap |
| 4EGI-1 | eIF4E/eIF4G interaction | Prevents eIF4E binding to eIF4G |
| Retinamides | Retinoic acid metabolism | Induce Mnk1/2 degradation |
| Ligustrazine | Anti-angiogenic | Decreases eIF4E phosphorylation, reduced eIF4E levels |
| C-KβBA | Anti-proliferative, pro-apoptotic | Inhibits cap-dependent translation |
| eFT508 | Mnk1/2 | Inhibits Mnk1/2 phosphorylation of eIF4E |
| BAY1143269 | Mnk1/2 | Inhibits Mnk1/2 phosphorylation of eIF4E |
Ribavirin is a guanosine analog that stops viral RNA synthesis and interfere with viral replication therefore has a broad spectrum activity against RNA and DNA viruses [82] This well-known anti-virus drug is usually used to treat hepatitis-C infections, but presents wide range effects on mTOR and MEK pathways. Ribavirin mimics the mRNA m7G cap-binding site competing with eIF4E binding and decreasing translational potential of phosphorylated eIF4E in the eIF4F complex therefore it has been used as an anti-oncogenic drug, demonstrating decrease in onco-proteins such as Mcl-1 [82].
4EGI-1 (eIF4E/eIF4G interaction inhibitor) is a direct competitor of eIF4E to the eIF4G binding site that prevents formation of the translational initiation complex [83]. By disrupting eIF4F formation, 4EGI-1 decreases eIF4E phosphorylation by Mnk, since this phosphorylation occurs once both are bound to the scaffolding protein eIF4G, consequently reducing the amount of weak mRNAs that are translated by a cap-dependent mechanism such as c-MYC and Bcl-xL, and the epithelial-mesenchymal transition (EMT) factor Snail [84]. Also, 4EGI-1 is thought to have higher effect in tumorous cells than in normal tissues because of the dependence of cancer cells on cap-dependent translation. [83]
C-4 heteroaryl 13-cis-retinamides (Novel Retinamides, NRs) are a series of Retinoic acid metabolism blocking agents, which were demonstrated to have potent anticancer and growth inhibitory effects in human breast and PCa xenograft models. [62]. In prostate cancer cell lines, the compounds induce Mnk1/2 degradation and suppress eIF4E phosphorylation, while also inducing the degradation of both full-length AR and a splice variant (AR-V7) [62]. Mbatia and colleagues demonstrated that this class of compounds mainly decrease eIF4E phosphorylation by ubiquitin-dependent degradation of Mnks in breast cancer and also decrease AR transcriptional levels in PCa cells [62]. Variants of these NRs compounds significantly decrease growth of castration-resistant prostate cancer CWR22Rv1 xenografts and induced apoptosis in vivo [62].
Ligustrazine (Tetramethylpyrazine or TMP) is a plant extracted compound used in traditional Chinese medicine for the treatment of neurovascular and cardiovascular diseases [85]. TMP carries anti-angiogenic properties and has been tested for its antitumor activity showing promising results in prostate cancer cells with little effect on normal prostate cells [86]. Although its mechanism of action is not fully understood, TMP was shown to decrease in a dose dependent fashion the phosphorylation of mTOR and its downstream proteins 4EBP1 and P70S6K, affecting amounts of free eIF4E and decreasing cap-dependent translation. Contrary to some mTOR inhibitors, eIF4E phosphorylation at Ser209 was decreased in TMP treated PC-3 cells, and overexpression of either eIF4E or its kinase Mnk renders resistance to Ligustrazine, demonstrating that cap-dependent translation inhibition is crucial for TMP effect in these cells [86]. Ligustrazine (TMP) dose- and time-dependently inhibited the growth of CRPC cells by reducing their proliferation and promoting apoptosis, by inhibition of the activation of mTOR and downstream targets critical for cell growth [86]. Pull-down assays with 7-methyl- GTP Sepharose 4B beads indicated that Ligustrazine reduced eIF4E levels, and inhibited cap-dependent translation. Ligustrazine-induced inhibition of proliferation and induction of apoptosis could be overcome by overexpression of eIF4E in these cells [86].
A semisynthetic triterpenoid derivative, 3-cinnamoyl-11-keto-β-boswellic acid (C-KβBA) exhibits antiproliferative and proapoptotic effects in PC-3 prostate cancer xenografts in vivo [87]. This compound was found to inhibits the cap-dependent translation machinery, decreases expression of eIF4E and cyclin D1, and induces G(1) cell-cycle arrest [87]. In contrast to conventional mTOR inhibitors, C-KβBA does not activate Akt and extracellular signal-regulated kinase pathways. It binds to the FKBP12-rapamycin-binding domain of mTOR with high affinity [87].
Graff et al. presented data on an eIF4E-specific antisense oligonucleotides (ASOs) that binds and target eIF4E mRNA for degradation, decreasing by 50% the amount of mRNA and effectively reducing protein levels by 80%. In pre-clinical models, ASOs demonstrated significant decrease of eIF4E targeted onco-proteins translation [88] but failed to achieve tumor response in phase I/II clinical trials [89]. However, some of the other drugs targeting eIF4E mentioned above are now in clinical development and may be of more widespread use in the future.
Finally, two Mnk selective inhibitors are in clinical tests for advanced solid tumors: Bayer's BAY1143269 and eFFECTOR's eFT508, an orally available Mnk1/2 inhibitor that is being tested in phase II clinical trials for hematological tumors and phase I solid tumors including prostate cancer.
Concluding Remarks
This review describes the mechanisms by which eIF4E expression and phosphorylation mediates the resistance of prostate cancer and related diseases to various therapies, including hormonal therapy, chemotherapy and mTOR inhibitors. The studies referenced above demonstrate that inhibition of eIF4E by itself is unlikely to be of therapeutic value in prostate cancer or for that matter, other cancers as well; but when in combination with inhibitors of other pathways, drugs that prevent the activation of eIF4E, whether by inhibiting its phosphorylation, or its binding to the scaffolding protein eIF4G or to the m7G cap of target mRNAs, are likely to be of significance in preventing the progression of prostate cancer to a castration resistant state.
Acknowledgments
Acknowledgments
The authors would like to thank the reviewers of a previous version of this manuscript for their careful scrutiny that helped significantly improve this manuscript. The work reported here does not represent the views or opinions of the Department of Veteran Affairs or the United States Government. This work was supported by a Biomedical Laboratory Research & Development (BLRD) Merit Award (I01BX000400, PMG) from the Department of Veterans Affairs, and by Awards R01CA133209 (PMG) and R01CA185509 (PMG) from the National Institutes of Health.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Funding: This work was supported by a Biomedical Laboratory Research & Development (BLRD) Merit Award (I01BX000400, PMG) from the Department of Veterans Affairs, and by Awards R01CA133209 (PMG) and R01CA185509 (PMG) from the National Institutes of Health.
Declaration of interest: none
References
- 1.Diaz M, Patterson SG. Management of androgen-independent prostate cancer. Cancer Control. 2004;11(6):364–373. doi: 10.1177/107327480401100604. [DOI] [PubMed] [Google Scholar]
- 2.Nupponen N, Visakorpi T. Molecular biology of progression of prostate cancer. Eur Urol. 1999;35(5-6):351–354. doi: 10.1159/000019907. [DOI] [PubMed] [Google Scholar]
- 3.Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2006;97(3):433–447. doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]
- 4.Ecke TH, Schlechte HH, Hubsch A, Lenk SV, Schiemenz K, Rudolph BD, Miller K. TP53 mutation in prostate needle biopsies--comparison with patients follow-up. Anticancer Res. 2007;27(6B):4143–4148. [PubMed] [Google Scholar]
- 5.Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57(3):524–531. [PubMed] [Google Scholar]
- 6.Doucet L, Terrisse S, Gauthier H, Pouessel D, Le Maignan C, Teixeira L, Culine S. Molecular biology of castration-resistant prostate cancer. Bull Cancer. 2015;102(6):497–500. doi: 10.1016/j.bulcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Rosenwald IB. The role of translation in neoplastic transformation from a pathologist's point of view. Oncogene. 2004;23(18):3230–3247. doi: 10.1038/sj.onc.1207552. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Siddiqui S, Bose S, Mooso B, Asuncion A, Bedolla RG, Vinall R, Tepper CG, Gandour-Edwards R, Shi X. Nrdp1-mediated regulation of ErbB3 expression by the androgen receptor in androgen-dependent but not castrate-resistant prostate cancer cells. Cancer Res. 2010;70(14):5994–6003. doi: 10.1158/0008-5472.CAN-09-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11(2):113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 12.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle. 2011;10(2):229–240. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin X, Sarnow P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J Biol Chem. 2004;279(14):13721–13728. doi: 10.1074/jbc.M312854200. [DOI] [PubMed] [Google Scholar]
- 14.Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, Takehana K, Iemura S, Natsume T, Mizushima N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285(26):20109–20116. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10(14):2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 20.Duncan R, Milburn SC, Hershey JW. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262(1):380–388. [PubMed] [Google Scholar]
- 21.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 24.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl. 2):S43–S51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100(13):7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 27.Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. 2009;2(55):ra2. doi: 10.1126/scisignal.2000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham L, Banda K, Torres A, Carver BS, Chen Y, Pisano K, Shelkey G, Curley T, Scher HI, Lotan TL. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Investig New Drugs. 2018 doi: 10.1007/s10637-018-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene. 2004;23(18):3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 30.Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A. 2010;107(32):14134–14139. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18(1):270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minich WB, Balasta ML, Goss DJ, Rhoads RE. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci U S A. 1994;91(16):7668–7672. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277(5):3303–3309. doi: 10.1074/jbc.M103607200. [DOI] [PubMed] [Google Scholar]
- 34.Khan MA, Goss DJ. Kinetic analyses of phosphorylated and non-phosphorylated eIFiso4E binding to mRNA cap analogues. Int J Biol Macromol. 2018;106:387–395. doi: 10.1016/j.ijbiomac.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 35.Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269(22):5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16(8):1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shveygert M, Kaiser C, Bradrick SS, Gromeier M. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1- eIF4G interaction. Mol Cell Biol. 2010;30(21):5160–5167. doi: 10.1128/MCB.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24(15):6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stead RL, Proud CG. Rapamycin enhances eIF4E phosphorylation by activating MAP kinase- interacting kinase 2a (Mnk2a) FEBS Lett. 2013;587(16):2623–2628. doi: 10.1016/j.febslet.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 40.Eckerdt F, Beauchamp E, Bell J, Iqbal A, Su B, Fukunaga R, Lulla RR, Goldman S, Platanias LC. Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells. Oncotarget. 2014;5(18):8442–8451. doi: 10.18632/oncotarget.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown MC, Bryant JD, Dobrikova EY, Shveygert M, Bradrick SS, Chandramohan V, Bigner DD, Gromeier M. Induction of viral, 7-methyl-guanosine cap-independent translation and oncolysis by mitogen-activated protein kinase-interacting kinase-mediated effects on the serine/arginine-rich protein kinase. J Virol. 2014;88(22):13135–13148. doi: 10.1128/JVI.01883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MC, Dobrikov MI, Gromeier M. Mitogen-activated protein kinase-interacting kinase regulates mTOR/AKT signaling and controls the serine/arginine-rich protein kinase-responsive type 1 internal ribosome entry site-mediated translation and viral oncolysis. J Virol. 2014;88(22):13149–13160. doi: 10.1128/JVI.01884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown MC, Gromeier M. MNK Controls mTORC1:Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep. 2017;18(6):1444–1457. doi: 10.1016/j.celrep.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang SX, Hewitt SM, Steinberg SM, Liewehr DJ, Swain SM. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol Rep. 2007;17(2):281–287. [PubMed] [Google Scholar]
- 45.Graff JR, Konicek BW, Lynch RL, Dumstorf CA, Dowless MS, McNulty AM, Parsons SH, Brail LH, Colligan BM, Koop JW. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 2009;69(9):3866–3873. doi: 10.1158/0008-5472.CAN-08-3472. [DOI] [PubMed] [Google Scholar]
- 46.D’Abronzo LS, Bose S, Crapuchettes ME, Beggs RE, Vinall RL, Tepper CG, Siddiqui S, Mudryj M, Melgoza FU, Durbin-Johnson BP. The Androgen Receptor is a negative regulator of eIF4E Phosphorylation at S209: Implications for the use of mTOR inhibitors in advanced prostate cancer. Oncogene. 2017;36(46):6359–6373. doi: 10.1038/onc.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchini A, Loiarro M, Bielli P, Busa R, Paronetto MP, Loreni F, Geremia R, Sette C. Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells. Carcinogenesis. 2008;29(12):2279–2288. doi: 10.1093/carcin/bgn221. [DOI] [PubMed] [Google Scholar]
- 48.Philippe L, Vasseur JJ, Debart F, Thoreen CC. La-related protein 1 (LARP1) repression of TOP mRNA translation is mediated through its cap-binding domain and controlled by an adjacent regulatory region. Nucleic Acids Res. 2018;46(3):1457–1469. doi: 10.1093/nar/gkx1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahr RM, Fonseca BD, Ciotti GE, Al-Ashtal HA, Jia JJ, Niklaus MR, Blagden SP, Alain T, Berman AJ. La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs. Elife. 2017;6 doi: 10.7554/eLife.24146. [pii: e24146] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fonseca BD, Zakaria C, Jia JJ, Graber TE, Svitkin Y, Tahmasebi S, Healy D, Hoang HD, Jensen JM, Diao IT. La-related Protein 1 (LARP1) Represses Terminal Oligopyrimidine (TOP) mRNA Translation Downstream of mTOR Complex 1 (mTORC1) J Biol Chem. 2015;290(26):15996–16020. doi: 10.1074/jbc.M114.621730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoyanova T, Cooper AR, Drake JM, Liu X, Armstrong AJ, Pienta KJ, Zhang H, Kohn DB, Huang J, Witte ON. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc Natl Acad Sci U S A. 2013;110(50):20111–20116. doi: 10.1073/pnas.1320565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10(5):484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 53.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci U S A. 2011;108(37):E699–E708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamont KR, Tindall DJ. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol. 2011;25(6):897–907. doi: 10.1210/me.2010-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 56.Almeida-Souza L, Goethals S, de Winter V, Dierick I, Gallardo R, Van Durme J, Irobi J, Gettemans J, Rousseau F, Schymkowitz J. Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem. 2010;285(17):12778–12786. doi: 10.1074/jbc.M109.082644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67(21):10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 58.Stope MB, Schubert T, Staar D, Ronnau C, Streitborger A, Kroeger N, Kubisch C, Zimmermann U, Walther R, Burchardt M. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J Urol. 2012;30(3):327–331. doi: 10.1007/s00345-012-0843-z. [DOI] [PubMed] [Google Scholar]
- 59.Hassan S, Biswas MH, Zhang C, Du C, Balaji KC. Heat shock protein 27 mediates repression of androgen receptor function by protein kinase D1 in prostate cancer cells. Oncogene. 2009;28(49):4386–4396. doi: 10.1038/onc.2009.291. [DOI] [PubMed] [Google Scholar]
- 60.Andrieu C, Taieb D, Baylot V, Ettinger S, Soubeyran P, De-Thonel A, Nelson C, Garrido C, So A, Fazli L. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene. 2010;29(13):1883–1896. doi: 10.1038/onc.2009.479. [DOI] [PubMed] [Google Scholar]
- 61.Ramamurthy VP, Ramalingam S, Gediya L, Kwegyir-Afful AK, Njar VC. Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines. Oncotarget. 2015;6(5):3195–3210. doi: 10.18632/oncotarget.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mbatia HW, Ramalingam S, Ramamurthy VP, Martin MS, Kwegyir-Afful AK, Njar VC. Novel C- 4 heteroaryl 13-cis-retinamide Mnk/AR degrading agents inhibit cell proliferation and migration and induce apoptosis in human breast and prostate cancer cells and suppress growth of MDA-MB-231 human breast and CWR22Rv1 human prostate tumor xenografts in mice. J Med Chem. 2015;58(4):1900–1914. doi: 10.1021/jm501792c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8(8):647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Pinillos A, Ferrari AC. mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol Oncol Clin North Am. 2012;26(3):483–505, vii. doi: 10.1016/j.hoc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319(1):1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Francavilla A, Carr BI, Starzl TE, Azzarone A, Carrieri G, Zeng QH. Effects of rapamycin on cultured hepatocyte proliferation and gene expression. Hepatology. 1992;15(5):871–877. doi: 10.1002/hep.1840150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121(4):1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 69.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor- independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 70.van der Poel HG, Hanrahan C, Zhong H, Simons JW. Rapamycin induces Smad activity in prostate cancer cell lines. Urol Res. 2003;30(6):380–386. doi: 10.1007/s00240-002-0282-1. [DOI] [PubMed] [Google Scholar]
- 71.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Yue P, Chan CB, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun SY. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase- dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation. Mol Cell Biol. 2007;27(21):7405–7413. doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumoto M, Seike M, Noro R, Soeno C, Sugano T, Takeuchi S, Miyanaga A, Kitamura K, Kubota K, Gemma A. Control of the MYC-eIF4E axis plus mTOR inhibitor treatment in small cell lung cancer. BMC Cancer. 2015;15:241. doi: 10.1186/s12885-015-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cope CL, Gilley R, Balmanno K, Sale MJ, Howarth KD, Hampson M, Smith PD, Guichard SM, Cook SJ. Adaptation to mTOR kinase inhibitors by amplification of eIF4E to maintain cap-dependent translation. J Cell Sci. 2014;127(Pt 4):788–800. doi: 10.1242/jcs.137588. [DOI] [PubMed] [Google Scholar]
- 75.Proud CG. Mnks, eIF4E phosphorylation and cancer. Biochim Biophys Acta. 2015;1849(7):766–773. doi: 10.1016/j.bbagrm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Hajer Z, Claudia A, Erik L, Sara K, Maurizio F, Ridha O, David T, Michel C, Olivier S, Sabrina P. Targeting Hsp27/eIF4E interaction with phenazine compound: a promising alternative for castration-resistant prostate cancer treatment. Oncotarget. 2017;8(44):77317–77329. doi: 10.18632/oncotarget.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grzmil M, Seebacher J, Hess D, Behe M, Schibli R, Moncayo G, Frank S, Hemmings BA. Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy. Cell Signal. 2016;28(9):1412–1421. doi: 10.1016/j.cellsig.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Yu Y, Tian L, Feng X, Cheng J, Gong Y, Liu X, Zhang Z, Yang X, He S, Li CY. eIF4E- phosphorylation-mediated Sox2 upregulation promotes pancreatic tumor cell repopulation after irradiation. Cancer Lett. 2016;375(1):31–38. doi: 10.1016/j.canlet.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 79.Adesso L, Calabretta S, Barbagallo F, Capurso G, Pilozzi E, Geremia R, Delle Fave G, Sette C. Gemcitabine triggers a pro-survival response in pancreatic cancer cells through activation of the MNK2/eIF4E pathway. Oncogene. 2013;32(23):2848–2857. doi: 10.1038/onc.2012.306. [DOI] [PubMed] [Google Scholar]
- 80.Kumar K, Chow CR, Ebine K, Arslan AD, Kwok B, Bentrem DJ, Eckerdt FD, Platanias LC, Munshi HG. Differential regulation of ZEB1 and EMT by MAPK-interacting protein kinases (MNK) and eIF4E in pancreatic cancer. Mol Cancer Res. 2016;14(2):216–227. doi: 10.1158/1541-7786.MCR-15-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwegyir-Afful AK, Bruno RD, Purushottamachar P, Murigi FN, Njar VC. Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion. FEBS J. 2016;283(21):3898–3918. doi: 10.1111/febs.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177(4050):705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 83.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, Gross JD, Degterev A, Yuan J, Chorev M. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128(2):257–267. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 84.Cai W, Ye Q, She QB. Loss of 4E-BP1 function induces EMT and promotes cancer cell migration and invasion via cap-dependent translational activation of snail. Oncotarget. 2014;5(15):6015–6027. doi: 10.18632/oncotarget.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27(6):297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Han J, Song J, Li X, Zhu M, Guo W, Xing W, Zhao R, He X, Liu X, Wang S. Ligustrazine suppresses the growth of HRPC cells through the inhibition of cap-dependent translation via both the mTOR and the MEK/ERK pathways. Anti Cancer Agents Med Chem. 2015;15(6):764–772. doi: 10.2174/1871520615666150305112120. [DOI] [PubMed] [Google Scholar]
- 87.Morad SA, Schmid M, Buchele B, Siehl HU, El Gafaary M, Lunov O, Syrovets T, Simmet T. A novel semisynthetic inhibitor of the FRB domain of mammalian target of rapamycin blocks proliferation and triggers apoptosis in chemoresistant prostate cancer cells. Mol Pharmacol. 2013;83(2):531–541. doi: 10.1124/mol.112.081349. [DOI] [PubMed] [Google Scholar]
- 88.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, Schwier P, Capen A, Goode RL, Dowless MS. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117(9):2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong DS, Kurzrock R, Oh Y, Wheler J, Naing A, Brail L, Callies S, Andre V, Kadam SK, Nasir A. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin Cancer Res. 2011;17(20):6582–6591. doi: 10.1158/1078-0432.CCR-11-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]