Abstract
Although the number of individuals reaching 80 who are considered to be healthy is increasing, the very elderly are likely to have long-term conditions, to report symptoms and/or be taking at least one regular medication. The impact of antihypertensive treatment has to be taken into account in this context. The treatment regimen in Hypertension in the Very Elderly Trial with a goal blood pressure of <150/80 mmHg has been shown to provide benefits in terms of a reduction in risk of total mortality, stroke, and cardiovascular events with potential benefits and no evidence of increased risk for fracture, dementia, depression, and quality-of-life outcomes. Questions remain as to the level of benefit that would be accrued in the frailer elderly and those at extreme age, for example, over 90.
Keywords: Hypertension, Antihypertensives, Aged, Elderly, Benefit, Risk
Introduction
The fastest growing sector of the population is the very elderly. In 1960, an estimated 1.4% of the European population was aged 80 and over, this rose to 3.0% in 2010 and is estimated to reach 8.4% in 2040 and still rising.1 With the rise in blood pressure (BP) associated with ageing, the prevalence of hypertension is also much higher in older age groups. Although epidemiological studies have suggested greater survival in those aged 80 or more who also have higher levels of BP, this may be due in part to those with low BP often having greater co-morbidity and being thus less, likely to survive. Hypertension even in late life has been shown to be associated with greater risk of cardiovascular mortality and morbidity. Despite the risks hypertension itself is often symptomless for the patient and despite the benefits medication has side effects and potentially dangerous drug–drug interactions in older adults who may be taking several drugs. The benefit associated with treatment may take time to accrue, whereas side effects may occur quickly. Therefore, in the every elderly (defined here as those aged 80 years or more) with an inevitably limited life expectancy, the balance between the risks and benefits of treatment needs careful assessment. There have been multiple double-blind placebo-controlled trials in younger hypertensive populations although the majority has not included those aged 80 and over. Meta-analyses with a focus on those aged 80 and over include subgroup data from just four double-blind trials in younger elderly and one trial designed specifically to include only those aged 80 and over, the Hypertension in the Very Elderly Trial (HYVET). Trial evidence on which the base clinical decision making in this age group is limited, despite substantial data having been accrued in the treatment of hypertension in those aged 65 and over in the past 15 years. The aim of this paper is to review the data relating to the benefits and risks associated with the use of antihypertensives in the very elderly, with a particular focus on the HYVET.
Cardiovascular benefit and all-cause mortality
Until 2008 there was uncertainty as to the benefit of antihypertensive treatment for the very elderly and guidelines were naturally cautious in their recommendations. In 1999, a meta-analysis of data in this age group had reported a significant reduction in stroke and major cardiovascular events with antihypertensive treatment but also observed a 14% (P = 0.05) increase in total mortality, when only double-blind trials were considered.2 This meta-analysis, included the results of seven intervention trials reported between 1986 and 1997 that had recruited octogenarians (n = 1670) with a range 1–650 of patients from the individual trials. During this time, as demographics changed with population ageing and the evidence for treating younger adults became clearer, it is likely that the characteristics of the very elderly population also changed.3 Additionally, the constituent double-blind trials used differing antihypertensive regimens and had a stronger focus on beta-blockers than would be recommended today4–8 (see Table 1).
Table 1.
Outcome for those aged 80 and over in intervention trials on the elderly and trial medication used
| EWPHE4 | STOP-H5 | SHEP6 | Syst-Eur7 | HYVET Pilot8 | |
|---|---|---|---|---|---|
| Trial Outcome for those aged 80 or more | No benefit | No benefit | Fall in non-fatal stroke, Not for fatal events | Fall in non-fatal stroke, Not for fatal events | Fall in stroke, not for fatal events |
| Medication Steps |
|
|
|
|
|
EWPHE, European Working Party on Hypertension in the Elderly; SHEP, The Systolic Hypertension in the Elderly Program; STOP, Swedish Trial in Old Patients with Hypertension; Syst-EuR, Systolic Hypertension in Europe trial (Syst-Eur); HYVET Pilot, Hypertension in the Very Elderly Trial Pilot study; ACE-I, angiotensin-converting enzyme inhibitor; Thz, thiazide; BB, beta-blocker; Dble, double; OL, open label; CCB, calcium-channel blocker.
In 2002, the Study on Cognition and Prognosis in the Elderly (SCOPE) trial and the Hypertension in the Very Elderly pilot trial (HYVET Pilot) reported results.8,9 SCOPE, a double-blind placebo-controlled trial of the Angiotensin Receptor Blocker (ARB) candesartan, recruited 4964 patients (70–89 years, mean age 76 and from 15 countries) of which 1053 were over 80. Unfortunately the SCOPE investigators were obliged to allow additional antihypertensive treatment during the trial resulting in only 16% of the placebo group remaining on placebo alone and a BP difference between the groups of just 3.2/1.6 mmHg.
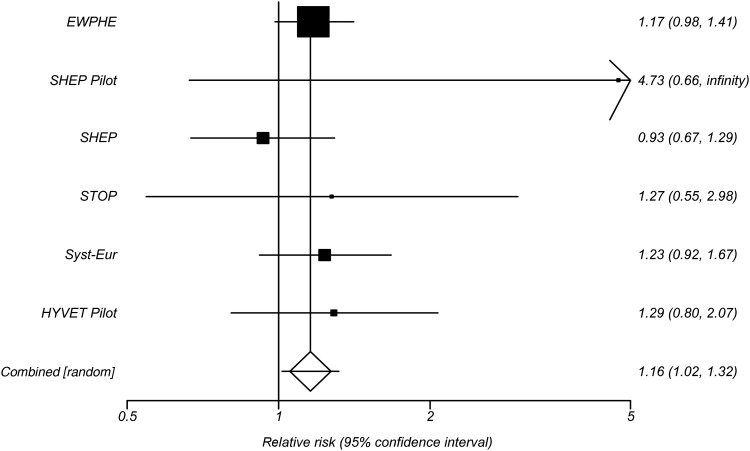
The HYVET Pilot recruited only those aged 80 and over. It employed a Prospective Randomized Open Blinded Endpoints (PROBE) design, randomizing 1283, mean age 83.8 (just over 60% being female) from 10 countries with an entry requirement for BP of 160-219/90-109 mmHg. Participants were randomized to treatment with a diuretic or an angiotensin converting enzyme inhibitor (ACE-I) or to no treatment. The results were in general agreement with the previous meta-analysis in that for every stroke saved with active treatment there was one additional death. Figure 1 shows the trials from the meta-analysis selecting out the double-blind trials only and the results of the HYVET Pilot for the outcome of total mortality, reinforcing the possible increase with treatment. Other trials of BP lowering followed, but without a focus on the very elderly and at times their exclusion.
Figure 1.
Forest plot of results for total mortality outcomes from the HYVET alongside trials from the 1999 meta-analysis.1 Relative risk with 95% confidence limits.
In 2008, the main HYVET concluded.10 Similar to the pilot only those aged 80 and over were enrolled, but it was a double-blind placebo-controlled design. Hypertension in the Very Elderly Trial randomized 3845 with an entry BP of ≥160 mmHg from centres in Eastern and Western Europe, China, and Australasia. An unexpected and significant 21% reduction in total mortality [hazard ratio (HR) 0.79 (95% confidence intervals (CI) 0.65–0.95) P = 0.02] was reported and when late arriving data were taken into account a 32% reduction in stroke [HR 0.68, 95% CI 0.47–0.98, P = 0.04].11 Cardiovascular events (fatal and non-fatal stroke, fatal and non-fatal myocardial infarction, and fatal and non-fatal heart failure) and heart failure events were also reduced, HR0.66 (95% CI 0.53–0.82) and HR0.36 (95% CI 0.22–0.58), respectively. Recent results from HYVET which reported on a 1-year open-label extension from the end of the double-blind phase has suggested that the cardiovascular benefit is observed within 1 year of initiating treatment, although reduction in mortality takes longer.11
Subsequent to these results a Cochrane review has combined the HYVET data with data from the HYVET Pilot, the Systolic Hypertension in the Elderly Program (SHEP), SHEP Pilot, Swedish Trial in Old Patients with Hypertension (STOP), Systolic Hypertension in Europe (Syst-Eur) trial, the Coope and Warrender trial and the European working party on Hypertension in the Elderly (EWPHE) reporting on outcomes in those aged 80 or over.12 A non-significant combined risk ratio of 0.98 (95% CI 0.87–1.10) for total mortality was seen, although treatment significantly reduced cardiovascular mortality and morbidity combined, risk ratio 0.75 (95% CI 0.65–0.87). The combining of data from HYVET with earlier trials may not be ideal. The HYVET results are unusual with regard to total mortality and it may be that this is due to differences in trial population/selection criteria and or drug regimen and level of BP lowering compared with earlier trials. The drug regimen in particular differed between the trials including that of the HYVET Pilot (see Tables 1 and 2). Certainly, the baseline risk of a cardiovascular event would have been different among the different trial populations.
Table 2.
Characteristics of trials recruiting individuals aged 80 and over
| EWPHE4 | STOP5 | SHEP6 | Syst-Eur6 | HYVET10 | |
|---|---|---|---|---|---|
| BP at entry (mmHg) | 182/101 | 195/102 | 179/77 | 174/85 | 174/96 |
| Target BP (mmHg) | 160/90 | 160/95 | 140 mmHg systolic | 140 mmHg systolic | 150/80 |
| Drop on placebo (mmHg) | 10/7 | 9/6 | 15/6 | 13/2 | 15/7 |
| Difference between active + placebo (mmHg) | 23/9 | 19/9 | 11/3 | 10/5 | 15/6 |
EWPHE, European Working Party on Hypertension in the Elderly; SHEP, The Systolic Hypertension in the Elderly Program; STOP, Swedish Trial in Old Patients with Hypertension; Syst-EuR, the Systolic Hypertension in Europe trial (Syst-Eur); HYVET, Hypertension in the Very Elderly Trial.
‘The only other trial to report a reduction in the point estimate for total mortality was STOP.5 It has been suggested that one of the reasons for the reduction in mortality seen in HYVET was the result of utilizing low doses of the medication employed in the trial and that there was little biochemical disturbance.10 This could be of relevance to potassium changes given the increased risk associated with potassium disturbance and cardiac arrhythmia and particularly on an aged myocardium. Swedish Trial in Old Patients with Hypertension used a combination thiazide and potassium sparing diuretic as first line treatment and reported no differences in potassium between active and placebo treatment.5 Systolic Hypertension in the Elderly Program has reported that those with hypokalaemia did not experience benefit at least in terms of the reduction in cardiovascular benefits.13 Hypertension in the Very Elderly Trial used a low dose regimen of indapamide sustained release 1.5 mg with optional addition of 2–4 mg of perindopril, with most participants taking both; after 2 years of follow-up 73% of those on active medication, 85% of those taking placebo.10 The use of a low dose diuretic in combination with an Angiotensin Converting Enzyme Inhibitor (ACE-I) would be expected to have a neutral impact on potassium and no significant changes were noted at 24 months.10 Adherence in HYVET was high (estimated at 99%) and BP separation and changes were congruent with this’.
The goal BP of <150/80 mmHg in HYVET was a pragmatic choice given concerns related to excessive BP lowering in this group and was higher than those recommended in treatment guidelines at the time. The controversy regarding the J-shaped relationship between BP lowering and cardiac or cardiovascular events continues. That is, the idea that there is a point at which BP lowering becomes associated with an increase in risk. The J-shape has been demonstrated in several studies in younger age groups although populations differ as do the BP measures and nadirs associated with the lowest risk.14,15 Despite this, there must be a point at which lowering BP becomes less beneficial and this may vary with age. Results from the Japanese Trial to Assess Optimal Systolic blood Pressure in Elderly hypertensive patients (JATOS) and the Valsartan in elderly Isolated Systolic Hypertension (VALISH) study suggest no additional benefit from more intensive BP lowering in older populations.16,17 A meta-analysis from 2010, specifically focused on the very elderly, combined the same trials as the Cochrane review and reported similar results with regard to mortality but presented a further meta-regression analysis suggesting that a reduction in mortality was achieved in those trials with the least BP reductions and the lowest intensity of therapy.18
A separate factor that may have influenced the HYVET results is the population. Hypertension in the Very Elderly Trial recruited a population in which the overall was healthier than the average very elderly population. Those with dementia were excluded as were those requiring residential nursing care and participants were largely a primary prevention group without prior stroke or myocardial infarction. Despite this, participants were not the super fit. A mean number of 1.8 co-morbidities/participants were reported by trial investigators (more than 25% reported three or more), in addition to hypertension. Patient self-reported symptoms were also high suggesting a large undiagnosed or unreported co-morbidity.
Overall, the choice of drug, dose, and pragmatic goal BP, a largely primary prevention population and a focus solely on recruiting from the very elderly, may go some way to explain why the HYVET results differ from those seen in the very elderly subgroups of earlier double-blind and open-label trials with regards to mortality, although for cardiovascular benefit the results from all trials appear compellingly consistent.
Dementia
Hypertension is also a recognized risk factor for later cognitive decline and dementia, both vascular and Alzheimer's dementia. Several hypertension trials have included dementia as a secondary outcome allowing some insight into the benefit or not of BP lowering on incident dementia.
Various longitudinal open-label or active comparator trials have reported cognitive function results and shorter trials have looked at neuropsychological outcomes and antihypertensive use over periods of several weeks. Four double-blind placebo-controlled trials have used dementia as an outcome.6,19–21 We will focus here on studies with dementia outcomes and with follow-up of >1 year.
Of the double-blind placebo-controlled trials carried out in older adults, the results of Syst-Eur stand out. They reported a significant 50% reduction in incident dementia over a mean follow-up of 2 years in those aged 60 or over (from 7.7/1000 patient years in the placebo group to 3.8 in the actively treated group).19 Systolic Hypertension in the Elderly Program (mean age 72) found a non-significant effect of treatment, with 36 (1.5%) incident cases in the active group and 44 (1.9%) in the placebo group.6 The Perindopril Protection against Recurrent Stroke Study (PROGRESS) trial, a secondary prevention population post stroke or transient ischaemic attack (mean age 64 years) found fewer cases in the actively treated groups but no significant differences overall. When incident dementia was combined with recurrent stroke, the outcome became significant in favour of the actively treated group.20
Hypertension in the Very Elderly Trial reported higher rates of incident dementia, as would be expected in an older population and fewer cases in the active group although this was not significant (HR 0.86, 95% CI 0.67–1.09).21 Open-label trials have reported similar results and there have been several meta-analyses. Each has combined a slightly different selection of trials but all report a point estimate <1.0, with just one reaching significance (HR 0.87, 95% CI 0.76–1.00, P = 0.045).21 The results of Syst-Eur may be a chance finding or may reflect CCB use conferring some additional benefit either in terms of lowering the risk of ischaemic events or in relation to Alzheimer'-type pathology.22 Currently, therefore, there is no overwhelming evidence that treating hypertension in the very elderly prevents dementia but equally there is no evidence that treatment increases risk.
A further factor to take into consideration in the very elderly is the possibility of a J-shaped relationship between BP and cognitive decline or dementia, i.e. an increased risk associated with low as well as high BP. There has been the suggestion that there may be a J-shape at least for diastolic pressure and cognitive function given that the very elderly with stiffer arteries are potentially less capable of regulating cerebral perfusion.23 As BP tends to fall prior to a diagnosis of dementia it is hard to fully disentangle such associations. It may be that BP lowering decreases risk in some and increases risk in others. Recent results from HYVET have reported a J-shape for diastolic BP, active treatment, and dementia although not for systolic BP and a wider pulse pressure had the strongest relationship with incident dementia.24 The concept of a J-shape and an the appropriate BP goal when lowering pressure using antihypertensives in the very elderly remains controversial and is an area that requires further studies designed specifically to address this issue. The results from HYVET would suggest a target of 150/80 mmHg in such a group. This does not rule out additional benefit from lower levels but such benefit cannot be assumed and adverse effects may occur.
Renal function and biochemistry
An area of concern when treating the very elderly is renal function. Hypertension and older age are both associated with an increased risk of impaired renal function. Research in hypertensive very elderly groups is limited and the equations used to calculate estimated glomerular filtration rate (eGFR) remain largely unvalidated in this group. In those aged 75 and over with an eGFR calculated using the CKD-EPI (Chronic Kidney disease epidemiology collaboration) equation, low eGFR has been associated with an increased risk of hospitalization and mortality independent of known cardiovascular co-morbidity at baseline.25 In HYVET, there was a suggestion of a U-shape with low (<45 mL/min/1.73 m2) and high (≥75 mL/min/1.73 m2) eGFR being associated with a potential increase in risk of later cardiovascular events and mortality albeit not significantly.26 It should be noted that those with significantly impaired renal function were excluded from HYVET. The pattern of results was similar when the analyses were repeated using different formulae for calculating eGFR, although each equation performed slightly differently.26 Active treatment in HYVET had no impact on eGFR or on creatinine, potassium, glucose or urea. There was a statistically but not clinically significant rise in uric acid over 12 but not 24 months with no increase in gout reported.
Fracture and falls
Those treating the very elderly with hypertension often express concern that BP lowering increases the risk of falling and thus fracture with the subsequent adverse outcomes that ensue. However, thiazide and thiazide-like diuretics have been associated with reduced risk of fracture, assumed to be due to inhibition in the distal nephron of the Na–Cl co-transporter leading to a hyperpolarization, increasing the electrical driving force for calcium reabsorption, subsequent decrease in urinary calcium loss and the suggestion that this could preserve bone mineral density.27,28 It has also been suggested that such drugs exert there effect on bone independent of their effect on the kidney via inhibition of Na–Cl transporter expressed on osteoblasts resulting indirectly in bone mineral formation.29
Accurate data on falls are hard to capture and there is no clear threshold for BP lowering below which risk increases. A meta-analysis of nine drug classes and falls in older adults did show an association between falling and both diuretics and beta-blockers. However, when this was adjusted for co-variates the association was no longer significant.30 Orthostatic hypotension (OH) too has been associated with BP lowering medication and OH itself is associated with an increased risk of falling and with adverse outcomes in terms of mortality and worsening cognition.31
Incident fractures were also recorded as a secondary endpoint in HYVET. There were in fact fewer fractures in the actively treated group (38 vs. 52) and following adjustment for baseline predictors of fracture, active treatment was associated with a just significant reduction in incident fractures (HR 0.58, 95% CI 0.33–1.00, P = 0.0498).32 When all reported fractures (both those validated independently and those classified as unconfirmed) were included in the analysis the results were even more strong in favour of active treatment (HR 0.54, 95% CI 0.32–0.94, P = 0.028).32 Preliminary analyses with regard to OH at baseline from HYVET have suggested that OH was associated with increased mortality but when the results were adjusted for baseline cardiovascular risk this association was no longer significant.
Depression, quality of life, and adverse events
Prevalence of depression is reported to rise at older age.33 Alongside this there is a substantial literature linking cardiovascular risk and depression and linking depression to an increased risk of dementia.34,35 Currently, limited data exist on the impact of treatment for depression on cardiovascular outcomes or of cardiovascular treatment on affect related outcomes. In HYVET data were collected annually on depression as part of the quality-of-life (QoL) side project and using the Geriatric Depression Scale (GDS). There was a strong association between worse depression scores at baseline and later events, both for cardiovascular (HR 2.10, 95% CI 1.50–2.96) and total mortality (HR 1.78, 95% CI 1.40–2.27) and cardiovascular events (HR1.59, 95% CI 1.21–2.09).36 Furthermore, neither depression scores nor scores from the Short Form 36 (SF-36) (a standard QoL questionnaire also collected annually) showed any difference between the two groups over time with regard to trial treatment.37 Overall, active treatment had neither a negative nor a positive impact on QoL as assessed by the SF-36 and GDS in the HYVET trial.
Older adults are more likely to experience side effects from medication and to be taking multiple medications which influences adherence. In advanced age with an inevitably limited life expectancy the balance of treatment to reduce future cardiovascular events against increased symptoms and potentially lower QoL is important. In HYVET, a validated symptom questionnaire for use in hypertension was administered annually. From 23 symptoms, 3 differed significantly between the two groups. Active treatment was associated with cough (P = 0.005), nocturia (P = 0.001), and pain in joints (P = 0.015).32 Over 90% of those completing the questionnaire (n = 2656) reported three or more as having bothered them within the last week. Examples include wheezing, severe joint pain, and muscle cramps reported in between 30 and 50% of patients with nocturia reported by over 80%.32 This is in line with previous work in older adults with hypertension with higher levels of symptomology reported with increasing age and greater reporting in females.38
The number of Serious Adverse Events (SAE) reported in HYVET was 806 (448 with placebo, 358 with active treatment), this approximates to 10/100 patient years of follow-up. The changes in European legislation in this area mean that it is not possible to compare these rates with those from earlier trials where a portion of the participants were aged 80 and over. Given the definition of an SAE which includes events that are fatal, life threatening or requiring hospitalization, these rates are not unexpected in older populations. As the number of the trial endpoints, e.g. mortality, is also classified as SAEs higher number of SAEs would also be expected in the placebo arm of the trial. Nonetheless, only five of the SAEs were felt to be related to the trial medication; three in the placebo and two in the actively treated group.
Future directions
Although the number of individuals reaching 80 who are considered to be healthy is increasing, the very elderly are likely to have long-term conditions, to report symptoms and/or be taking at least one regular medication. The impact of antihypertensive treatment has to be taken into account in this context. The treatment regimen in HYVET with a goal BP of <150/80 mmHg has been shown to provide benefits in terms of a reduction in risk of total mortality, stroke, and cardiovascular events with potential benefits and no evidence of increased risk for fracture, dementia, depression, and QOL outcomes. Subgroup analyses suggest that these benefits are consistent for those aged 80–85 or 85 and over male and female, primary or secondary prevention regardless of baseline systolic BP in HYVET.39
Questions remain however as to the J-shape, whether antihypertensive treatment would still be beneficial in the extreme elderly, those aged 90 or more, in a frailer cohort, particularly if residential nursing care and in those with cognitive impairment. With increasing age of those aged over 90 and 100 years, the balancing of benefit and risk becomes more sophisticated. Should we continue to treat or is there a limit to benefit and thus a point when treatment should be stopped? It is also unclear whether consistent control of BP, achieving different levels of BP lowering and lowering BP below the targets used in HYVET, or using a different treatment regimen would give similar positive results. With increasing age, there is greater BP variability. There is recent evidence to suggest that BP variability has a greater impact on outcome than mean arterial BP and that different classes of antihypertensive have differing impact.40 Whether this has a greater impact in the very elderly is unknown. Future trials are needed to let us know whether we should treat the oldest old, 90 and over, the frailer elderly, whether we should be aiming for a lower BP goal, differences between monotherapy and combination therapy choice of a particular treatment combination or a more consistent control of BP. With the potential exception of trials recruiting the oldest old and frail future trials would need to include active comparators and as such would be well placed to examine questions such as appropriate level of BP lowering and drug combination.
Perspectives
The beneficial effects associated with the antihypertensive treatment regimen used in the HYVET trial outweighed the risks from treatment and should be considered for the general ambulant very elderly population. However, further evidence is needed in the frailer elderly, with regard to clarifying benefits, defining ideal BP targets, the potential for treatment combinations that may best protect cognitive function, as well as treatment in other areas where cardiovascular risk is high simply from age alone irrespective of the presence of an established cardiovascular risk factor; for example the true benefits of statin use in this population.
Funding
C.B., N.B., and R.P. received financial support from grants made to Imperial College London by the British Heart Foundation and Servier International supporting the delivery of the HYVET trial. The HYVET was funded by the British Heart Foundation and Servier International. No funding was received to support the generation of this manuscript.
Conflict of interest: All of the authors have several years of experience working on or with cardiovascular drug trials and other studies. C.B., N.B., and R.P. have received speaker fees from Servier International. N.B. and T.M. are currently practising medicine but have no further conflict of interest to declare relating to this manuscript.
References
- 1.Lanzieri G. The greying of the baby boomers, a century-long view of ageing in European populations. Eurostat Statistics in focus 23/2011 http://ec.europa.eu/digital-agenda/futurium/sites/futurium/files/futurium/library/The%20Greying%20of%20the%20Baby%20Boomers.%20A%20Century-long%20View%20of%20Ageing%20in%20European%20Populations.pdf . (01 November 2013) [Google Scholar]
- 2.Gueyffier F, Bulpitt C, Boissel J-P, Schron E, Ekbom T, Fagard R, Casiglia E, Kerlikowske K, Coope J for the INDANA Group. Antihypertensive drugs in very old people: a sub-group meta-analysis of randomised controlled trials. Lancet. 1999;353:793–796. doi: 10.1016/S0140-6736(98)08127-6. [DOI] [PubMed] [Google Scholar]
- 3. United Nations. World Population Ageing 1950–2050 http://www.un.org/esa/population/publications/worldageing19502050/ (26 May 2012)
- 4.Amery A, Birkenhäger WH, Brixko P, Bulpitt C, Clement D, Deruyttere M, De Schaepdryver A, Dollery C, Fagard R, Forette F. Mortality and morbidity results from the European Working Party on High blood pressure in the Elderly trial. Lancet. 1985;I:1349–1354. doi: 10.1016/S0140-6736(85)91783-0. [DOI] [PubMed] [Google Scholar]
- 5.Dahlöf B, Hansson L, Lindholm L, Scherstén B, Ekbom T, Wester P-O. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338:1281–1285. doi: 10.1016/0140-6736(91)92589-T. [DOI] [PubMed] [Google Scholar]
- 6.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP) J Am Med Assoc. 1991;272:1932–1938. [PubMed] [Google Scholar]
- 7.Staessen J, Fagard R, Thijs L, Celis H, Arabidze G, Birkenhäger W, Bulpitt C, Leeuw P, Dollery C, Fletcher A, Forette F, Leonetti G, Nachev C, O'Brian E, Rosenfeld J, Rodicio J, Tuomilehto J, Zanchetti A for the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators . Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. doi: 10.1016/S0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 8.Bulpitt C, Beckett N, Cooke J, Dumitrascu D, Gil-Extremera B, Nachev C, Nunes M, Peters R, Staessen J, Thijis L on behalf of the HYVET-pilot investigators. Results of the pilot study for the Hypertension in the Very Elderly Trial (HYVET Pilot) J Hypertens. 2003;21:2409–2417. doi: 10.1097/00004872-200312000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu LS, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ on behalf of the HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. New Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 11.Beckett N, Peters R, Tuomilehto J, Swift C, Sever P, Pooer J, McCormack T, Forette F, Gil-Extramera B, Dumitrascu D, Staessen J, Thijs L, Fletcher A, Bulpitt C. Immediate and late benefits of treating very elderly people with hypertension: results from the active treatment extension to hypertension in the very elderly randomised controlled trial. Br Med J. 2012;344:d7541. doi: 10.1136/bmj.d7541. [DOI] [PubMed] [Google Scholar]
- 12.Musini V, Tejani A, Bassett K, Wright J. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000028.pub2. Issue 4. Art. No.: CD000028. DOI: 10.1002/14651858.CD000028.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Franse LV, Pahor M, Di Bar M, Somes GW, Cushman WC, Applegate WB. Hypokaleamia associated with diuretic use and cardiovascular events in the systolic hypertension in the elderly programme. Hypertension. 2000;35:1025–1030. doi: 10.1161/01.HYP.35.5.1025. [DOI] [PubMed] [Google Scholar]
- 14.Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27:1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- 15.Wang JG, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45:907–913. doi: 10.1161/01.HYP.0000165020.14745.79. [DOI] [PubMed] [Google Scholar]
- 16.Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, Imai Y, Kikuchi K, Ita S, Eto T, Kimura G, Imaizumi T, Takishita S, Ueshima H for the Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly. Hypertension. 2010;56:196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 17.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31:2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 18.Bejan-Angoulvant T, Saadatian-Elahi M, Wright J, Schron E, Lindholm L, Fagard R, Staessen J, Gueyffier F. Treatment of hypertension in patients 80 years and older: the lower the better? A meta-analysis of randomized controlled trials. J Hypertens. 2010;28:1366–1372. doi: 10.1097/HJH.0b013e328339f9c5. [DOI] [PubMed] [Google Scholar]
- 19.Forette F, Seux M, Staessen J, Lutgarde T, Birkenhäger W, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in a randomised double blind placebo controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/S0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 20.The PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 21.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C for the HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 22.Birkenhager W, Forette F, Seux M, Wang J, Staessen J. Blood pressure, cognitive functions, and prevention of dementias in older patients with hypertension. Arch Intern Med. 2001;161:152–156. doi: 10.1001/archinte.161.2.152. [DOI] [PubMed] [Google Scholar]
- 23.Yam A, Lang E, Lagopoulos J, Yip K, Griffith J, Mudaliar Y, Dorsch N. Cerebral autoregulation and ageing. J Clin Neurosci. 2005;12:643–645. doi: 10.1016/j.jocn.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Peters R, Beckett NS, Fagard R, Thijs L, Wang J, Forette F, Pereira L, Fletcher A, Bulpitt C. Increased pulse pressure linked to dementia: further results from the Hypertension in the Very Elderly Trial-HYVET. J Hypertens. 2013;31:1868–1875. doi: 10.1097/HJH.0b013e3283622cc6. [DOI] [PubMed] [Google Scholar]
- 25.Nitsch D, Nonyane B, Smeeth L, Bulpitt C, Roderick P, Fletcher A. CKD and hospitalisation in the elderly: a community-based cohort study in the United Kingdom. Am J Kidney Dis. 2011;57:664–672. doi: 10.1053/j.ajkd.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters R, Beckett N, Poulter R, Pereira L, Narkiewicz K, Fagard R, Liu L, Tzekova M, Nitsch D, Wang N, Li M, Fletcher A, Bultpitt C. Kidney function in the every elderly with hypertension: data from the hypertension in the very elderly [HYVET] trial. Age Ageing. 2013;42:253–258. doi: 10.1093/ageing/afs109. [DOI] [PubMed] [Google Scholar]
- 27.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels R. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:516–526. doi: 10.7326/0003-4819-133-7-200010030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak MM, De Joussinseau C, Carter DH, Pisitkun T, Knepper MA, Gamba G, Kemp PJ, Riccardi D. Thiazide diuretics directly induce osteoblast differentiation and mineralizes nodule formation by interacting with a sodium chloride co-transporter in bone. J Am Soc Nephrol. 2007;18:2509–2516. doi: 10.1681/ASN.2007030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 31.Bevnenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24:135–144. doi: 10.1038/ajh.2010.146. [DOI] [PubMed] [Google Scholar]
- 32.Ruth Peters, Nigel Beckett, Lisa Burch, Marie-Christine de Vernejoul, Lisheng Liu, Joe Duggan, Cameron Swift, Blas Gil-Extremera, Astrid Fletcher, Christopher Bulpitt. The effect of treatment based on a diuretic (indapamide) {+/−} ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39:609–616. doi: 10.1093/ageing/afq071. [DOI] [PubMed] [Google Scholar]
- 33.McDougall F, Kvaal K, Matthews F, Paykel E, Jones PB, Dewey ME, Brayne C. Prevalence of depression in older people in England and Wales: the MRC CFA study. Psychol Med. 2007;37:1787–1795. doi: 10.1017/S0033291707000372. [DOI] [PubMed] [Google Scholar]
- 34.Wulsin L. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.PSY.0000058371.50240.E3. [DOI] [PubMed] [Google Scholar]
- 35.Beekman A, Copeland J, Prince M. Review of community of depression in later life. Br J Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- 36.Peters R, Pinto E, Beckett N, Swift C, Potter J, McCormack T, Nunes M, Grimley-Evans J, Fletcher A, Bulpitt J. Association of depression with subsequent mortality, cardiovascular morbidity and incident dementia in people aged 80 and over and suffering from hypertension. Data from the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39:439–445. doi: 10.1093/ageing/afq042. [DOI] [PubMed] [Google Scholar]
- 37.Peters R. Additional benefits beyond cardiovascular events. XIXth World Congress of Gerontology and Geriatrics; 2009. Paris, pp. 142. [Google Scholar]
- 38.Fletcher A, Bulpitt C, Thijs L, Tuomilehto J, Antikainen R, Bossini A, Browne J, Duggan J, Kawacka-Jaszcz K, Kivinen P, Sarti C, Terzoli L, Staessen J on behalf of the Syst-Eur Trial Investigators. Quality of life on randomised treatment for isolated-systolic hypertension: results from the Syst-Eur trial. J Hypertens. 2002;20:2069–2079. doi: 10.1097/00004872-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Beckett N, Peters R, Leonetti G, Duggan J, Fagard R, Thijs L, Narkiewiez K, McCormack T, Banya W, Fletcher A, Bulpitt C. Benefits in total mortality and cardiovascular events in The Hypertension In The Very Elderly Trial (HYVET) by major subgroups. Hypertension. 2008;52:748. doi: 10.1161/01.HYP.0000335248.34837.A2. [DOI] [Google Scholar]
- 40.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]