Abstract
Aims
We studied the unclear question whether blood pressure (BP) lowering reduces cardiovascular disease (CVD) in elderly individuals with systolic BP <160 mm Hg.
Methods and results
We initiated a randomized placebo-controlled stratified 2 × 2 factorial clinical trial evaluating the effects of BP lowering in 11 000 elderly individuals with systolic blood pressure (SBP) between 130 and 159 mm Hg, for 5 years. Following 5-week active run-in, participants were randomized to aliskiren (300 mg) or placebo, and to an additional antihypertensive [hydrochlorothiazide (25 mg) or amlodipine (5 mg)], or their respective placeboes. Study was terminated by sponsor after 1759 subjects (age 72.1 ± 5.2 years, 88% receiving at least one antihypertensive) were randomized and followed for 0.6 year. Study drugs were well tolerated with few serious adverse events during run-in and after randomization, with no significant differences between treatment groups. By design, three levels of BP reductions were achieved, adjusted mean BP reductions of 3.5/1.7 mm Hg (P < 0.001) by aliskiren, 6.8/3.3 mm Hg (P < 0.001) by hydrochlorothiazide or amlodipine, and 10.3/5.0 mm Hg (P < 0.001) by double therapy compared with placebo. Twenty-five major CVD events occurred. Non-significant trends towards fewer CVD events with greater BP reductions are evident: hazard ratios (HR) 0.82 [95% confidence interval (CI): 0.37–1.81] for 3.5 mm Hg SBP reduction; HR 0.45 (95% CI: 0.19–1.04) for 6.8 mm Hg; and HR 0.25 (0.05–1.18) for 10.3 mm Hg reduction for primary composite of CV death, MI, stroke, or significant heart failure.
Conclusions
Sizeable reductions in BP, with potential for substantial CVD reduction, can be safely achieved using combinations of BP drugs in the elderly with normal high and Stage 1 hypertension.
Clinical trial registration
Keywords: Lowering stage 1 hypertension in elderly, Clinical trial, Aliskiren, Direct renin inhibitor, Combination therapy
See page 1703 for the editorial comment on this article (doi:10.1093/eurheartj/ehu129)
Introduction
Hypertension is a major risk factor for cardiovascular disease (CVD), especially in the elderly, and accounts for ∼6% of adult deaths worldwide.1,2 Several guidelines recommend initiating antihypertensive drug treatment in those with systolic blood pressure (SBP) > 140 mm Hg or diastolic blood pressure (DBP) > 90 mm Hg, and some suggest that BP should be lowered in individuals with diabetes or other high-risk conditions who have an SBP over 130 mm Hg.3–5 While blood pressure (BP) reduction has been clearly shown to reduce CVD in elderly people with SBP ≥ 160 mm Hg, there is at present conflicting evidence on whether this is the case also when SBP values are 159 mm Hg or less, especially if individuals are already receiving antihypertensive medications.6,7 This gap in knowledge is highlighted by the recent ESH/ESC guidelines which indicate that there is limited evidence from randomized clinical trials that drug treatment for grade 1 hypertension reduces clinical events in the elderly.8
Achieving target levels of BP recommended by guidelines frequently requires combinations of various drugs with different mechanisms of action. However, use of multiple BP lowering medications may often be associated with drug interactions, intolerance, poor adherence and increased costs, particularly in the elderly. Therefore, outcome data showing that use of multiple drugs reduces CVD and is safe, are needed to clarify BP lowering and to justify treating or augmenting therapy in the elderly with SBP between 130 and 159 mm Hg, without offsetting adverse experiences from the treatment and is of major clinical, public health and economic importance.
The direct renin inhibitor, aliskiren, was approved as an effective agent to treat hypertension, alone and in combination with other agents.9–14 The Aliskiren Prevention of Later Life Outcomes (APOLLO) trial was designed to provide information on the impact of aliskiren on clinical events when used alone or in combination with other common drugs [amlodipine or hydrochlorothiazide (HCTZ)], relative to risks, in elderly hypertensive subjects who are already receiving other commonly used drugs, using a stratified factorial design. Recruitment started on January 2011 but was prematurely stopped for non-scientific reasons on 3 May 2012 at the request of the sponsor without any knowledge of blinded trial data and despite the objections of the Steering Committee and the independent Data and Safety Monitoring Board (DSMB) (see Supplementary material online). Thus, the original study objectives on the effects of treatment on clinical outcomes could not be achieved. Instead, we report the tolerability of the various regimens and the effects on BP lowering associated with aliskiren alone and the intensification of the BP lowering by adding either HCTZ or amlodipine in high-risk patients already receiving other BP lowering drugs. In addition, we report the impact on major clinical events (as safety outcomes) relative to BP lowering, although the power to detect plausible differences is substantially compromised.
Method
Participants
We included individuals ≥65 years of age, with stable SBP between 130 and 159 mm Hg on two consecutive visits one or more months apart, in whom background BP therapy was unchanged for at least 1 month. Patients were required to have either CVD (coronary heart disease, stroke or TIA, peripheral artery disease) or one additional CV risk factor (dyslipidaemia, smoking, abdominal obesity, hyperglycaemia, renal dysfunction, or evidence of left ventricular hypertrophy) or be >70 years (advanced age increases risk). Individuals with indications, contraindications, or intolerance to the drugs tested were excluded. The following were also excluded: those with SBP > 160 mm Hg or DBP > 100 mm Hg, symptomatic heart failure, complex cardiac or valvular heart disease, stroke within the previous 3 months or TIA within the previous 7 days, acute coronary syndrome within the previous 1 month, cardiac surgery or percutaneous coronary intervention within the previous 3 months or planned in next 3 months, eGFR < 30 mL/min/1.73 m2 (Modification of diet and renal disease formula), known renal artery stenosis, serum potassium >5.3 mmol/L, chronic liver disease, malignancy (except localized skin basal cell carcinoma) within the past 5 years, concurrent treatment with cyclosporine, quinidine or systemic conazoles, chronic use of non-steroidal anti-inflammatory drugs or cyclooxygenase-2 inhibitors in subjects with eGFR < 60 mL/min/1.73 m2.
Design
This was a randomized placebo-controlled 2 × 2 stratified factorial design clinical trial on 11 000 subjects to be followed for 5 years. Participants were randomized to aliskiren 300 mg daily or placebo and also to an additional BP lowering drug (amlodipine 5 mg daily or HCTZ 25 mg daily) or placebo (Table 1). Substudies on Ambulatory Blood Pressure Monitoring and Brain MRI were to be conducted to examine in greater details the effects of the therapies.
Table 1.
Study design (as planned)
| Aliskiren 300 mg + additional BP lowering drug (amlodipine 5 mg or HCTZ 25 mg) N = 2750 (actual randomized: 433) |
Placebo for aliskiren 300 mg + additional BP lowering drug (amlodipine 5 mg or HCTZ 25 mg) N = 2750 (actual randomized: 447) |
Additional BP lowering drug (amlodipine or HCTZ) N = 5500 (actual randomized: 880) vs. |
| Aliskiren 300 mg + placebo for additional BP lowering drug N = 2750 (actual randomized: 427) |
Placebo for aliskiren 300 mg + placebo for additional BP lowering drug N = 2750 (actual randomized: 452) |
Placebo for additional BP lowering drug (amlodipine or HCTZ) N = 5500 (actual randomized: 879) |
|
Aliskiren N = 5500 (actual randomized: 860) vs. |
Placebo for aliskiren N = 5500 (actual randomized: 899) |
Randomized placebo-controlled 2 × 2 factorial design, with planned sample size (N = 11 000) and actual number randomized (N = 1759). For primary objective 1, treatment effect with an aliskiren-based regimen (left column) was to be compared with that of non-aliskiren-based regimen (right column), i.e. the aliskiren arm vs. placebo for aliskiren arm. For primary objective 2, treatment effect of aliskiren + amlodipine or HCTZ (combination therapy, left upper cell) was to be compared with double placebos (right lower cell).
National Leaders, clinical monitors, and site investigators supervised the recruitment of patients. The trial was coordinated and the data were gathered and analysed by the Population Health Research Institute (PHRI) at McMaster University and Hamilton Health Sciences. The Steering Committee designed and oversaw the trial. An Operations Committee consisting of academic leaders, representatives from PHRI, and the sponsor met frequently and regularly to ensure smooth running of the trial. All primary study outcomes and all deaths were adjudicated by a central committee whose members were unaware of study-group allocations, with the use of standard criteria. All serious adverse events were reviewed by the DSMB.
Study objectives and outcomes
Originally, our primary objectives were to determine whether: (i) treatment with an aliskiren-based regimen compared with a non-aliskiren-based regimen, both on top of existing BP lowering agents, reduced the risks of the composite of CV death, non-fatal MI, non-fatal stroke, and clinically significant heart failure and (ii) intensified therapy with aliskiren plus an additional BP lowering drug (amlodipine or HCTZ) compared with their placebos, reduced the risk of the same composite clinical outcome (also see Table 1). Secondary objectives were to determine whether treatment with the two regimens reduced the risk of fatal and non-fatal stroke, prevented decline in ability to perform everyday activities independently using a health-related successful aging instrument, prevented decline in renal function or reduced total mortality. Tertiary objectives were to determine the effect of treatment including measures of cognition, independence and function of daily living activities, individual CV outcomes and new diagnosis of diabetes, components of health-related successful living instrument and depression.
Run-in, randomization and follow-up
The study protocol was reviewed and approved by the institutional review board/ethics committee of each participating centre. Participants entered a pre-run-in phase (after written informed consent was obtained), which was designed to ensure that study participants' BP remained stable and in range while receiving background antihypertensive therapies for 1 month before entering run-in. Eligible participants then entered a 5-week run-in phase when, based on their pre-existing BP lowering therapy, participants were stratified into those receiving a calcium channel blocker such as amlodipine or a thiazide diuretic. At run-in, participants who had received a calcium channel blocker were assigned to active HCTZ, those on a thiazide to amlodipine. Those receiving neither were randomly assigned to HCTZ or amlodipine. Starting low dose active study medications were titrated upwards to the full study doses to ensure tolerance to the study medications without serious adverse effects (Table 2). Renal function and potassium levels were checked midway and at the end of run-in.
Table 2.
Run-in treatments
| Period | Treatments |
|
|---|---|---|
| For patients on thiazide backgrounda (N = 1001) | For patients on CCB backgrounda (N = 1335) | |
| First 3 days | Amlodipine 5 mg | HCTZ 12.5 mg |
| Next 7 days: Days 4–10 (±2 days) | Amlodipine 5 mg | HCTZ 25 mg |
| Next 10 days: Days 11–21 (±2 days) | Amlodipine 5 mg + aliskiren 150 mg | HCTZ 25 mg + aliskiren 150 mg |
| Next 14 days: Days 22–36 (±3 days) | Amlodipine 5 mg + aliskiren 300 mg | HCTZ 25 mg + aliskiren 300 mg |
aPatients who were on neither thiazide nor CCB background were randomly assigned to one of the two treatments.
On completion of run-in, eligible participants who tolerated the run-in medications were randomized to the study drugs or matching placebo. At randomization, all participants were assigned to aliskiren or placebo and simultaneously those receiving active HCTZ to HCTZ or placebo and those receiving amlodipine to amlodipine or placebo, in a stratified 2 × 2 factorial design (Table 1). Study subjects and personnel from study sites and the central coordinating centre were blinded to study medication allocations. Follow-up visits occurred 6 weeks, 3 and 6 months after randomization and then every 6 months until the end of the trial, projected to last an average of 5 years. At each visit, resting sitting brachial BP was measured on the same arm following a standardized technique using an Omron Automatic BP Monitor (Model HEM-711DLXCAN). Two measurements were made on the same arm, 2 min apart, after the participant has sat quietly for at least 5 min and the average of the two readings were taken as the BP for the visit.
Statistical analysis
Given that the Sponsor stopped recruitment on 23 December 2011, the original study objectives could not be achieved and so the original statistical analysis planned (to examine the impact on clinical outcomes) in the protocol was no longer appropriate. Therefore, an alternative statistical analysis plan was formulated prior to study close-out and database lock for a supplementary pre-specified key outcome to assess the effect of study treatments on mean sitting systolic and diastolic BP, within the randomized follow-up period (up to 6 months after randomization) when compared with values at the run-in visit. BP measurements recorded in participants from the time they had undergone administrative censoring, that is they had stopped the study medication because they had diabetes and on background ACE inhibitor or ARB, were not included within the primary analysis. Analyses for safety and tolerability were performed, with descriptive analysis of all clinical efficacy outcomes.
Means and standard deviations were used for continuous variables, and numbers and percentages for dichotomous variables. Comparisons between groups were made by t-tests or χ2-tests. Comparisons on the effects of treatments on BP were made using mixed model regression analysis with the following covariates: run-in BP value, treatment factor, treatment time by time interaction term, and two stratification factors. The first stratification factor has four levels representing: baseline with thiazide, baseline with calcium channel blocker, baseline with neither thiazide nor calcium channel blocker but assigned to HCTZ at run-in, and baseline with neither thiazide nor calcium channel blocker and assigned to amlodipine at run-in. The second stratification has two factors representing being randomized to other BP lowering therapy or placebo. Since randomization was stratified by site, we did not model the random effect of site for changes in BP. Hazard ratios (HR) were calculated using Cox regression model stratified by the two stratification factors described above. Two-sided tests were used and P < 0.05 was accepted as significant.
Results
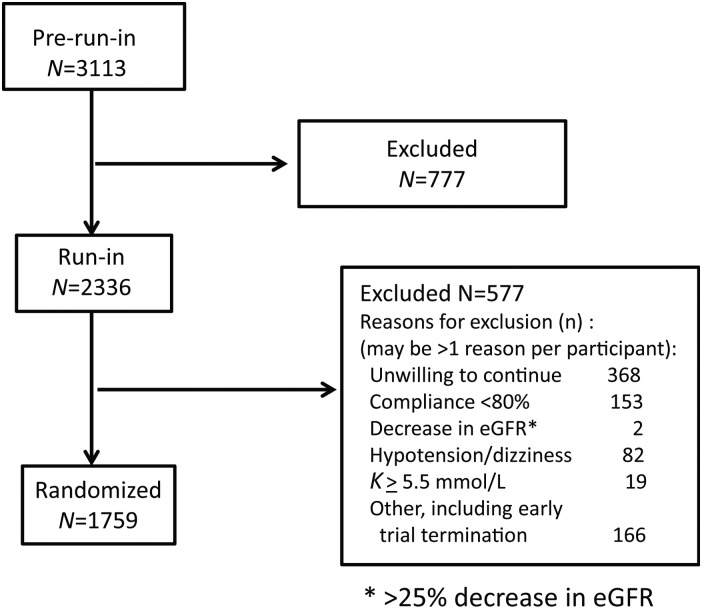
The trial started enrolment in January 2011 in 17 countries involving 145 centres. When the Sponsor stopped recruitment on 23 December 2011, instructions were given to the study centres to discontinue aliskiren or placebo in participants with diabetes and in whom an ACE inhibitor or ARB was to be continued. Those in whom the site investigators decided that the ACE inhibitor or ARB could be stopped were continued in the study but were administratively censored for the purpose of BP analysis in this study. The sponsor made the decision to terminate and close out the APOLLO trial on 3 May 2012, with 1759 randomized participants (Figure 1). The median follow-up time was 0.6 (interquartile range: 0.5–0.8) years, representing <2% of the expected 55 000 person-years of follow-up to be obtained within APOLLO. Average age was 72.1 ± 5.2 years, 46.2% were women, 29.7% had a history of vascular disease and 40.6% diabetes. Table 3 shows the baseline demographics, medical history and open label background BP medications.
Figure 1.
Recruitment flow chart.
Table 3.
Baseline demographics, medical history and background blood pressure lowering therapy
| Overall | Aliskiren | Placebo | HCTZ or amlodipine | Placebo | Double therapy | Double placebo | |
|---|---|---|---|---|---|---|---|
| Randomized (n) | 1759 | 860 | 899 | 880 | 879 | 433 | 452 |
| Age in years mean (SD) | 72.1(5.2) | 72.4 (5.2) | 71.9 (5.2) | 72.4 (5.3) | 72.0 (5.1) | 72.5 (5.3) | 71.8 (5.1) |
| Women n (%) | 813 (46.2) | 408 (47.4) | 405 (45.1) | 419 (47.6) | 394 (44.8) | 210 (48.5) | 196 (43.4) |
| Ethnicity n (%) | |||||||
| European | 729 (41.4) | 359 (41.7) | 370 (41.2) | 367 (41.7) | 362 (41.2) | 177 (40.9) | 180 (39.8) |
| Native Latin | 703 (40.0) | 349 (40.6) | 354 (39.4) | 355 (40.3) | 348 (39.6) | 178 (41.1) | 177 (39.2) |
| Other | 327 (18.6) | 152 (17.7) | 175 (19.5) | 158 (18.0) | 169 (19.2) | 78 (18.0) | 95 (21.0) |
| Medical history/risk factors (%) | |||||||
| CHD | 386 (22.0) | 189 (22.0) | 197 (21.9) | 185 (21.0) | 201 (22.9) | 94 (21.7) | 106 (23.5) |
| Stroke/TIA | 143 (8.1) | 71 (8.3) | 72 (8.0) | 79 (9.0) | 64 (7.3) | 41 (9.5) | 34 (7.5) |
| PAD | 58 (3.3) | 32 (3.7) | 26 (2.9) | 30 (3.4) | 28 (3.2) | 18 (4.2) | 14 (3.1) |
| Heart failure | 25 (1.4) | 14 (1.6) | 11 (1.2) | 8 (0.9) | 17 (1.9) | 3 (0.7) | 6 (1.3) |
| Cancer | 70 (4.0) | 32 (3.7) | 38 (4.2) | 30 (3.4) | 40 (4.6) | 11 (2.5) | 19 (4.2) |
| Hypertension | 1433 (81.5) | 704 (81.9) | 729 (81.2) | 728 (82.7) | 705 (80.3) | 357 (82.4) | 358 (79.4) |
| Diabetes | 713 (40.6) | 352 (40.9) | 361 (40.2) | 347 (39.4) | 366 (41.7) | 179 (41.3) | 193 (42.8) |
| At run-in (%) | |||||||
| Beta-blockers | 793 (46.1) | 397 (46.2) | 396 (44.0) | 396 (45.0) | 397 (46.2) | 201 (46.4) | 201 (44.5) |
| ACE inhibitors | 410 (23.3) | 219 (25.5) | 191 (21.2) | 204 (23.2) | 206 (23.4) | 112 (25.9) | 99 (21.9) |
| ARB | 355 (20.2) | 169 (19.7) | 186 (20.7) | 178 (20.2) | 177 (20.1) | 88 (20.3) | 96 (21.2) |
| Amlodipine | 476 (27.1) | 232 (27.0) | 244 (27.1) | 241 (27.4) | 235 (26.7) | 111 (25.6) | 114 (25.2) |
| Other CCB | 101 (5.7) | 49 (5.7) | 52 (5.8) | 53 (6.0) | 48 (5.5) | 26 (6.0) | 25 (5.5) |
| Alpha blockers | 98 (5.6) | 42 (4.9) | 56 (6.2) | 46 (5.2) | 52 (5.9) | 17 (3.9) | 27 (6.0) |
| Diuretics | 387 (22.0) | 189 (22.0) | 198 (22.0) | 177 (20.1) | 210 (23.9) | 84 (19.4) | 105 (23.2) |
| At randomization (%) | |||||||
| Beta-blockers | 778 (44.2) | 386 (44.9) | 392 (43.6) | 388 (44.1) | 390 (44.4) | 195 (45.0) | 199 (44.0) |
| ACE inhibitors | 392 (22.3) | 212 (24.7) | 180 (20.0) | 197 (22.4) | 195 (22.2) | 110 (25.4) | 93 (20.6) |
| ARB | 334 (19.0) | 155 (18.0) | 179 (19.9) | 167 (19.0) | 167 (19.0) | 82 (18.9) | 94 (20.8) |
| Amlodipine | 458 (26.0) | 223 (25.9) | 235 (26.1) | 233 (26.5) | 225 (25.6) | 109 (25.2) | 111 (24.6) |
| Other CCB | 138 (7.8) | 64 (7.4) | 74 (8.2) | 63 (7.2) | 75 (8.5) | 30 (6.9) | 41 (9.1) |
| Alpha blockers | 64 (3.8) | 30 (3.5) | 34 (3.8) | 32 (3.6) | 32 (3.6) | 13 (3.0) | 15 (3.3) |
| Diuretics | 382 (21.7) | 189 (22.0) | 193 (21.5) | 175 (19.9) | 207 (23.6) | 84 (19.4) | 102 (22.6) |
| At final visit (%) | |||||||
| Beta-blockers | 616 (45.1) | 301 (45.5) | 315 (44.7) | 302 (44.0) | 314 (46.2) | 149 (44.5) | 162 (45.9) |
| ACE inhibitors | 148 (10.8) | 80 (12.1) | 68 (9.6) | 69 (10.0) | 79 (11.6) | 37 (11.0) | 36 (10.2) |
| ARB | 173 (12.7) | 72 (10.9) | 101 (14.3) | 92 (13.4) | 81 (11.9) | 40 (11.9) | 49 (13.9) |
| Amlodipine | 375 (27.5) | 180 (27.2) | 195 (27.7) | 193 (28.1) | 182 (26.8) | 85 (25.4) | 87 (24.7) |
| Other CCB | 121 (8.9) | 55 (8.3) | 66 (9.4) | 51 (7.4) | 70 (10.3) | 26 (7.8) | 41 (11.6) |
| Alpha blockers | 80 (5.9) | 35 (5.3) | 45 (6.4) | 36 (5.2) | 44 (6.5) | 14 (4.2) | 23 (6.5) |
| Diuretics | 303 (22.2) | 138 (20.9) | 165 (23.4) | 132 (19.2) | 171 (25.2) | 59 (17.6) | 92 (26.1) |
Blood pressure
At randomization, participants took an average of 1.5 non-study antihypertensive drugs, 87.5% were taking at least one and 46.8% were on two or more medications. At run-in, average sitting BP was 146.1 ± 11.1/82.6 ± 9.2 mm Hg, and at the end of run-in but prior to randomization, it was 133.7 ± 14.2/76.6 ± 9.3 mm Hg.
During the study, in participants randomized to aliskiren, 35.0% had average SBP <130 mm Hg and 11.0% had SBP <120 mm Hg compared with 22.8 and 6.8%, respectively, in those receiving placebo. Corresponding proportions were 37.5 and 12.6% for those assigned to HCTZ or amlodipine and 20.0 and 5.1% for placebo, and 43.0 and 14.8% for those receiving double therapy and 13.5 and 3.1% for double placebo.
Postrandomization, aliskiren reduced adjusted mean SBP by 3.5 (SE 0.5) mmHg, (P < 0.001), and DBP by 1.7 (SE 0.3) mmHg (P < 0.001) compared with placebo (first co-primary outcome), HCTZ or amlodipine by 6.8 (SE 0.5) mmHg, (P < 0.001) for SBP and 3.3 (SE 0.3) mmHg (P < 0.001) for DBP. The reduction in SBP in the double therapy compared with double placebo (second co-primary outcome) was 10.3 (SE 0.8) mmHg (P < 0.001) for SBP, and 5.0 (SE 0.5) mmHg, P < 0.001 in mean DBP (Figure 2).
Figure 2.
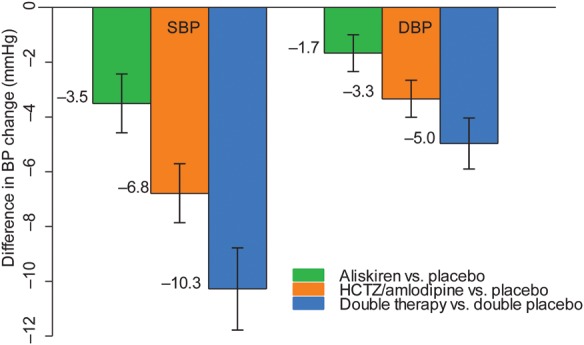
Mean (95% confidence interval) differences (mm Hg) in SBP and DBP between active and placebo treatments (alikiren vs. placebo, hydrochlorothiazide, or amlodipine vs. placebo and double therapy vs. double placebo. Mean blood pressure between blood pressure at run-in and average blood pressure after randomization (Week 6 and Month 6 measurements) were compared.
Potassium and renal function
Mean potassium was 4.32 ± 0.42 mmol/L at run-in; after randomization, 15 participants randomized to aliskiren had K > 5.5 mmol/L vs. 4 in the placebo group, while 12 randomized to amlodipine or HCTZ and 7 to placebo had K levels of >5.5 mmol/L. In the group that received double active drugs, there were 10 with potassium >5.5 mmol/L and 2 in the double placebo group (Table 4). None had levels of >6.0 mmol/L.
Table 4.
Prevalence of hyperkalaemia and renal impairment from randomization to the one-month wash-out at study end
| Alisk + Amlod or HCTZ K> 5.5 mmol/L (N = 10) eGFR < 30 mL/min/1.73 m2 (N = 2) |
Placebo for alisk + Amlod or HCTZ K > 5.5 mmol/L (N = 2) eGFR < 30 mL/min/1.73 m2 (N = 4) |
Amlod or HCTZ K > 5.5 mmol/L (N = 12) eGFR < 30 mL/min/1.73 m2 (N = 6) vs. |
| Alisk + placebo for Amlod or HCTZ K > 5.5 mmol/L (N = 5) eGFR < 30 mL/min/1.73 m2 (N = 1) |
Placebo for alisk + placebo for Amlod or HCTZ K > 5.5 mmol/L (N = 2) eGFR < 30 mL/min/1.73 m2 (N = 0) |
Placebo for Amlod or HCTZ K > 5.5 mmol/L (N = 7) eGFR < 30 mL/min/1.73 m2 (N = 1) |
|
Alisk K > 5.5 mmol/L (N = 15) eGFR < 30 mL/min/1.73 m2 (N = 3) vs. |
Placebo for alisk K > 5.5 mmol/L (N = 4) eGFR < 30 mL/min/1.73 m2 (N = 4) |
At run-in, average creatinine levels were 84.4 ± 21.9 μmol/L and 25% of participants had eGFR between 30 and 60 mL/min/1.73 m2. This proportion increased to over 30% in those who received aliskiren or double active drugs after randomization. An eGFR of <30 mL/min/1.73 m2 was seen during the study in seven participants (Table 4).
Serious adverse events and adherence
There were few serious adverse events, both during run-in (Table 5) and after randomization (Table 6), with no excess associated with any treatment group. Of the 24 participants who experienced serious adverse events that resulted in permanent study drug discontinuation after randomization, 13 were in the aliskiren group vs. 11 in the placebo group; 13 in the amlodipine or HCTZ vs. 11 in the placebo group and 8 in the double therapy vs. 7 in the double placebo group (Table 5). At the final visit, study drug discontinuation was ∼10% and this was similar in the 4 randomized groups (Table 6). Participants who stopped study medications due to premature study termination were administratively censored, and were not counted as drug discontinuations. Their data were included up to the time they stopped their study medications.
Table 5.
Prevalence of SAEs during run-in by study drug allocation
| Overall (n = 2336) | Aliskiren + HCTZ (n = 1335) | Aliskiren + amlodipine (n = 1001) | |
|---|---|---|---|
| Gastrointestinal disorders | 2 | 1 | 1 |
| Drug interaction/intolerance | 2 | 2 | 0 |
| Bronchitis/pneumonia | 2 | 1 | 1 |
| Creatinine/urea increased | 2 | 2 | 0 |
| Hyperkalaemia | 3 | 2 | 1 |
| Hypokalaemia | 2 | 2 | 0 |
| Hyponatraemia | 1 | 1 | 0 |
| Syncope | 2 | 0 | 2 |
| Hypotension | 2 | 0 | 2 |
| Angioedema | 2 | 0 | 2 |
| Other | 4 | 0 | 4 |
| Total | 20 | 10 | 10 |
A total of 2336 participants underwent the run-in phase.
Table 6.
Incidence of clinical outcomes, serious adverse events and proportions of participants who permanently discontinued their study medications during the study
| Aliskiren | Placebo | HCTZ or amlodipine | Placebo | Double therapy | Double placebo | |
|---|---|---|---|---|---|---|
| Clinical outcomes: incidence (%; annualized % event rates) | ||||||
| Primary composite outcome | 11(1.3%; 2.1%) | 14(1.6%; 2.6%) | 8 (0.9%; 1.5%) | 17(1.9%; 3.2%) | 2 (0.5%; 0.8%) | 8 (1.8%; 3.0%) |
| Composite of total mortality, non-fatal stoke, non-fatal MI or significant heart failure | 11(1.3%; 2.1%) | 16(1.8%; 3.0%) | 9 (1.0%; 1.7% | 18(2.0%; 3.4%) | 2 (0.5%; 0.8%) | 9 (2.0%; 3.4%) |
| SAE | ||||||
| Cardiac arrest | 0 | 2 | 1 | 1 | 0 | 1 |
| Stroke | 1 | 1 | 1 | 1 | 1 | 1 |
| Gastrointestinal | 1 | 5 | 2 | 4 | 0 | 4 |
| New diabetes | 0 | 1 | 1 | 0 | 0 | 0 |
| Hyperkalaemia | 3 | 1 | 3 | 1 | 2 | 0 |
| Renal failure | 3 | 0 | 1 | 2 | 1 | 0 |
| Impairment | 1 | 1 | 0 | 2 | 0 | 1 |
| Angioedema | 4 | 0 | 4 | 0 | 4 | 0 |
| Syncope | ||||||
| Study drug discontinuations (no. stopped/no. followed up, %) | ||||||
| 6 weeks | 32/826 (3.9) | 27/873 (3.1) | 30/850 (3.5) | 30/849 (3.3) | 16/417 (3.8) | 15/440 (3.4) |
| 6 months | 46/409 (11.3) | 34/411 (8.4) | 44/400 (11.0) | 38/430 (9.1) | 23/199 (11.6) | 17/210 (8.1) |
| 1 year | 4/35 (11.4) | 3/29 (10.3) | 6/33 (18.2) | 1/31 (3.2) | 3/16 (18.8) | 0/12 (0) |
| Final visit | 67/669 (10.0) | 70/712 (9.8) | 75/694 (10.8) | 68/687 (9.9) | 34/339 (10.0) | 34/357 (9.5) |
In patients with diabetes who received ACE inhibitor or ARB as background therapy, of 251 diabetic patients who were randomized to aliskiren, 5 (2.0%) SAEs of hyperkalaemia, syncope or renal failure occurred compared with none in 247 patients who received placebo for aliskiren.
Clinical outcomes
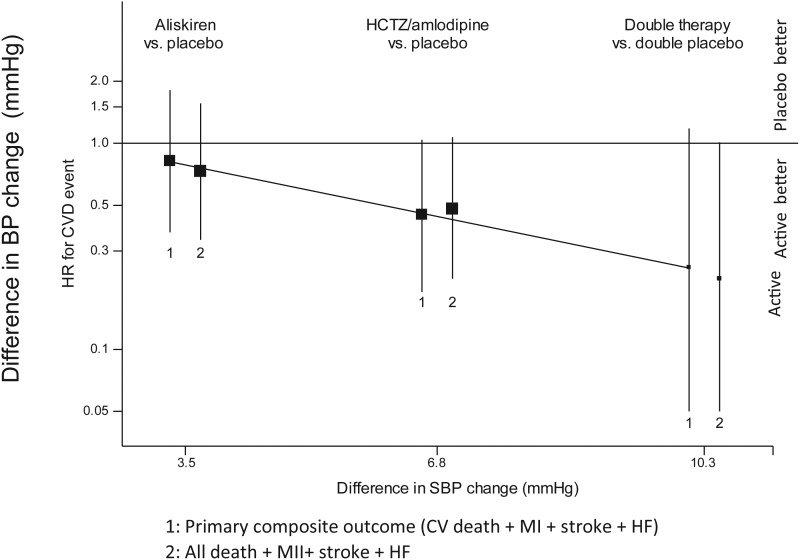
Of 25 primary outcome events (CV death, non-fatal MI, non-fatal stroke, or significant heart failure) (Table 6), non-significant trends toward lower risks of this primary composite outcome, compared with controls, for aliskiren HR was 0.82 (95% CI: 0.37–1.81, P = 0.63), amlodipine or HCTZ (HR 0.45, 95% CI: 0.19–1.04, P = 0.06) and the combination (HR 0.25 (95% CI: 0.05–1.18, P = 0.08). Similar non-significant trends were observed for the composite of all-cause death, non-fatal MI, non-fatal stroke, or significant heart failure, aliskiren: HR 0.73 (95% CI: 0.34–1.56, P = 0.41), amlodipine or HCTZ: HR 0.48 (95% CI 0.22–1.07, P = 0.07) and the combination: HR 0.22 (95% CI: 0.05–1.01, P = 0.05). Non-significant trends towards larger reductions in risk were seen with greater reductions in BP associated with these treatments (Figure 3).
Figure 3.
Clinical composite outcomes (hazard ratios and 95% confidence interval) by treatment (aliskiren vs. placebo, hydrochlorothiazide/amlodipine vs. placebo and double therapy vs. double placebo) for the (1) primary composite outcome of CV death, MI, stroke and significant heart failure and (2) the composite of all-cause mortality, MI, stroke and significant heart failure. Due to small number of events, no significant differences were found between each of the treatments but consistent trends towards reduced event rates with active treatment were observed.
Discussion
This study was designed to clarify whether BP lowering in high-risk elderly individuals, with SBP in a range between 130 and 159 mm Hg, is safe and leads to reductions in major clinical outcomes. Our data show that in addition to other background BP lowering medications (mean of 1.5 drugs), up to two further antihypertensive drugs (for a mean of 3.5 drugs in the double active arm) could be safely administered and were well tolerated. While the effects on major clinical outcomes could not be definitively determined because of early study termination, the present results suggest that such a trial can be conducted safely, with effective BP lowering and high tolerability. After randomization, three levels of BP reductions were achieved, with aliskiren alone (BP reduction of 3.5/1.7 mm Hg), amlodipine or HCTZ alone (6.8/3.3 mm Hg), and the combination of aliskiren plus amlodipine or HCTZ (10.3/5.0 mm Hg) compared with their placebos. Such reductions were not routinely encountered when guidelines are followed in clinical practice. These levels of BP reductions from the single or combination therapies were anticipated in the design of the trial.
There was a trend towards a reduction in clinical events in each of the active arms, and an additive effect with their combination, against a background of 1.5 non-study BP lowering agents. The data suggest the potential for a clinically important benefit on major CVD outcomes from each component of the study medications with possibly an added benefit from the combination. Thus our data suggest that in the elderly with stage 1 and borderline hypertension up to four BP lowering agents at standard doses are often required to reduce BP to acceptable levels and that these can be safely combined. Further, such vigorous attempts at BP lowering with multiple agents have the potential for clinical outcome benefits. These findings are comparable with the results from the ASTRONAUT trial, which while the results did not achieve statistical significance, the trend in reduction of clinical outcomes is encouraging.15
This trial had incorporated a run-in phase during which the active medications were up-titrated in steps to the trial target doses. During run-in, the incidence of serious adverse effects and clinical outcomes were low. None of the randomized treatment groups exhibited any excess adverse outcomes compared with their placeboes. By the final visit, ∼10% of participants had discontinued the study medications permanently, similar to that observed in previous trials such as ONTARGET16 or the HOPE trial.17 This included those individuals who, even though they had tolerated the study drugs, decided to discontinue them because of concerns by participants or physicians about their safety when the decision was made to stop recruitment. The commonest reason was likely related primarily to individuals changing their minds about participating in a long-term trial and not because of adverse effects of the study drugs.
The APOLLO results on safety and tolerability appear to differ from those reported in the ALTITUDE trial which reported an excess of elevated serum potassium levels or renal dysfunction with aliskiren at similar doses.18 However, patients in ALTITUDE all had diabetes and impaired renal function and all also received an ACE inhibitor or ARB as background therapy whereas only ∼40% of participants in our study had diabetes, and 43% were on an ACE inhibitor or an ARB.
While the BP reduction by aliskiren appears less than that from HCTZ or amlodipine, in the background of various background BP therapy, aliskiren is an effective BP lowering agent, and with a favourable tolerability profile, as also shown in the present study.9–14 Aliskiren reduces plasma renin activity9 and this effect had raised hopes that by effectively blocking the renin–angiotensin system it could produce cardiovascular benefits over and above those associated with its BP lowering effect. The hypothesis has also been advanced that with its proximal site of renin–angiotensin system blockade, it could oppose the adverse effects of angiotensin II production more completely than by ACE inhibition or angiotensin receptor antagonism.9 Unfortunately, the report from the ALTITUDE trial that the drug caused hypotension, hyperkalaemia and renal failure when used with another renin angiotensin blocking agent, such as an ACE inhibitor or an ARB, in individuals with diabetes and renal impairment may have precluded its use with these agents in individuals with, or even without, these pre-existing conditions. While the preliminary result of the APOLLO study shows that the drug appeared to be safe and well tolerated, the ALTITUDE trial results were extrapolated (perhaps unwarrantedly) to other populations. This extrapolation has precluded studies to explore whether clinical benefits could be observed in hypertensive individuals when aliskiren used alone or in combination with other drugs to lower BP. The APOLLO trial differed from the ALTITUDE trial in including those who had an elevated BP (and so the potential for benefits from BP lowering could be evaluated), the elderly (among whom BP lowering has a larger absolute benefit), utilizing a run-in phase where tolerability of all drug regimens were evaluated before randomization and where a strategy to achieve an important BP difference (by using combination therapies) was employed. These important differences in trial design and population between APOLLO and ALTITUDE make it possible that the clinical outcomes in the two trials would likely have differed.
In conclusion, the administration of the combination of aliskiren and HCTZ or amlodipine, and used in the background of an additional 1.5 antihypertensive drugs, was associated with an anticipated SBP reduction of ∼10 mmHg and appeared to be safe. There were trends towards fewer clinical outcomes with the active and combination therapies. These findings also suggest that with the sample size and duration of follow-up in the original protocol of APOLLO, significant and substantial reductions in the clinical outcomes would likely to have been observed. However, whether or not these promising results on limited numbers of patients, who were followed for an average of only 0.6 years, would be associated with reductions in CV risk during long-term follow-up could not be determined given the decision to terminate the trial prematurely. Yet the trend towards beneficial reductions in the clinical outcome events supports use of multiple agents to lower BP in elderly individuals with a BP between 130 and 159 mm Hg while definitive trials on this important topic are urgently needed.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The work was supported by Novartis Pharma AG, Basel, Switzerland. The sponsor terminated the study prematurely but had no role in the conduct or analysis of the study or in the decision to submit the manuscript for publication.
Conflict of interest: K.T. and S.Y. received research support from Novartis for the conduct of the APOLLO trial. M.A.P. has the following disclosures: consulting—Aastrom, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Genzyme, Hamilton Health Sciences, Keryx, Medtronic, Merck, Novartis, Roche, Servier, Teva, University of Oxford and Xoma; other activities—Research Grant support—Amgen, Celladon, Novartis, Sanofi-Aventis. Other activities—Government Grant Support—NHLBI—TOPCAT (PI—McKinlay); NHLBI—Rebuilding the Failing Hear (PI—Anversa); other activities—The Brigham and Women's Hospital has patents for the use of inhibitors of the renin–angiotensin system in selected survivors of MI for which Dr Pfeffer is a co-inventor. The licensing agreement with Novartis and Boehringer are irrevocably transferred to charity. G.M. has received honoraria as speaker/chairman at scientific congresses from the following companies: Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Menarini, Novartis, Servier, Takeda.
Supplementary Material
Acknowledgements
S.Y. is supported by the Marion W. Burke endowed chair of the Heart and Stroke Foundation of Ontario, ON, Canada.
References
- 1.Cooper RS. Geographic patterns of hypertension: a global perspective. In: Black HR, Izzo JL, editors. Hypertension Primer. 3rd ed. American Heart Association Dallas: 200. pp. 231–233. [Google Scholar]
- 2.Lakatta EG. Aging, hypertension and the heart. In: Black H, Izzo JL, editors. Hypertension Primer. 3rd ed. Dallas: pp. 166–169. [Google Scholar]
- 3.World Health Organization/International Society of Hypertension. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on the management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ and the National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 5.The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 6.Zanchetti A, Grassi G, Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens. 2009;27:923–934. doi: 10.1097/HJH.0b013e32832aa6b5. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, Cifkova R, Clement D, Coca A, Dominiczak A, Erdine S, Fagard R, Farsang C, Grassi G, Haller H, Heagerty A, Kjeldsen SE, Kiowski W, Mallion JM, Manolis A, Narkiewicz K, Nilsson P, Olsen MH, Rahn KH, Redon J, Rodicio J, Ruilope L, Schmieder RE, Struijker-Boudier HA, van Zwieten PA, Viigimaa M, Zanchetti A. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 8.The Task Force for the management of arterial hypertension on the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) 2013 ESH/ESC guidelines for the management of arterial hypertension . J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 9.Nussberger J, Aubert J-F, Bouzourene K, Pellegrin M, Hayoz D, Mazzolai L. Renin inhibition by aliskiren prevents atherosclerosis progression. Comparison with irbesartan, atenolol, and amlodipine. Hypertension. 2008;51:1306–1311. doi: 10.1161/HYPERTENSIONAHA.108.110932. [DOI] [PubMed] [Google Scholar]
- 10.Brown MJ. Aliskiren. Circulation. 2008;118:773–784. doi: 10.1161/CIRCULATIONAHA.108.787630. [DOI] [PubMed] [Google Scholar]
- 11.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation, 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 12.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 13.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK for the AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 14.Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20:11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz JX, Lesogor A, Maggioni AP for the ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure. The ASTRONAUT Randomized Trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 16.The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 17.HOPE (Heart Outcomes Prevention Evaluation) Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 18.Parving H-H, Brenner BM, McMurray JJV, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA for the ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.