Abstract
Aim
The aim of this study was to determine long-term results of renal artery denervation for treatment of treatment-resistant hypertension in the SYMPLICITY HTN-2 study.
Methods
SYMPLICITY HTN-2 randomized 106 subjects with treatment-resistant hypertension to renal denervation or medical therapy alone. At 6 months, 37 control subjects crossed over to renal denervation. Office blood pressure measurements, antihypertensive medication use, and safety events were followed every 6 months through 3 years.
Results
Follow-up was available at 36 months in 40 of 52 subjects in the initial renal denervation group and at 30 months in 30 of 37 subjects who crossed over and received renal denervation at 6 months. Baseline blood pressure was 184 ± 19/99 ± 16 mmHg in all treated subjects. At 30-month post-procedure, systolic blood pressure decreased 34 mmHg (95% CI: −40, −27, P < 0.01) and diastolic blood pressure decreased 13 mmHg (95% CI: −16, −10, P < 0.01). The systolic and diastolic blood pressure reduction at 36 months for the initial renal denervation group was −33 mmHg (95% CI: −40, −25, P < 0.01) and −14 mmHg (95% CI: −17, −10, P < 0.01), respectively. Procedural complications included one haematoma, and one renal artery dissection before energy delivery that was treated successfully. Later complications included two cases of acute renal failure, which fully resolved, 15 hypertensive events requiring hospitalization, and three deaths.
Conclusion
Renal denervation resulted in sustained lowering of blood pressure at 3 years in a selected population of subjects with severe, treatment-resistant hypertension without serious safety concerns.
Clinical trial registration
Keywords: Resistant hypertension, Renal denervation, Symplicity
Introduction
The prevalence of hypertension is increasing worldwide at alarming rates.1–3 Furthermore, the prevalence of uncontrolled hypertension is also increasing, in spite of advances in pharmacotherapy.3,4 Patients with treatment-resistant hypertension (blood pressure ≥140/90 mmHg despite ≥3 antihypertensive medications, including a diuretic)5,6 often have other risk factors for cardiovascular disease and consequently are at even greater risk for end-organ damage and cardiovascular morbidities.5 When confounding causes, such as white-coat hypertension, non-adherence to drug therapy, and inappropriate drug selection or dosing, are eliminated, the estimated proportion of patients with treatment-resistant hypertension ranges from 5 to 16%.7–11
Renal sympathetic outflow is commonly overactive in patients with essential hypertension; this has been demonstrated by measurements of norepinephrine spillover from renal sympathetic nerves.12,13 Pre-clinical work revealed that surgical sectioning of the renal nerves in experimental animal models of hypertension lowers blood pressure or can prevent development of hypertension.14,15
Percutaneous catheter-based renal denervation (RDN) using the Symplicity™ Renal Denervation System (Medtronic, Inc., Santa Rosa, CA, USA) involves the application of low-power (∼8 W) radiofrequency energy to the main renal arteries in a helical pattern of ablations (four to six per artery). The renal artery nerves passing to and from the kidneys transit in the adventitia of the renal artery or just beyond16,17 and are therefore within reach of energy delivery. The Symplicity generator uses a proprietary algorithm to monitor and control temperature, impedance, and power output to assure safe delivery of energy to each site. The SYMPLICITY HTN-1 proof-of-principle trial demonstrated the feasibility of this procedure and showed that subjects with severe, treatment-resistant hypertension experienced a significant reduction in blood pressure that was sustained over at least 3 years (−32/−14 mmHg at 36 months, P < 0.01).18–20
The SYMPLICITY HTN-2 randomized clinical trial compared the safety and effectiveness of RDN plus medical management to medical management alone (control group) in subjects with severe treatment-resistant hypertension.21,22 At 6-month post-randomization, there was a significant decrease from baseline blood pressure for the initial RDN group (n = 49; −32/−12 mmHg, P < 0.0001) and no change in blood pressure from baseline in the control group (n = 51).21 No serious device or procedure-related adverse events were reported at the 6-month primary endpoint.
The SYMPLICITY HTN-2 trial allowed control subjects to crossover to receive RDN after the initial 6-month primary endpoint evaluation. We now report 36-month follow-up of the initially denervated subjects and 30-month follow-up of the control subjects who crossed over to RDN at 6 months.
Methods
Study population and protocol
SYMPLICITY HTN-2 enrolled subjects from June 2009 to January 2010 at 24 clinical sites in Europe, Australia, and New Zealand. The trial was approved by the local Ethics Committees in accordance with the Declaration of Helsinki and all subjects provided written informed consent.
Details of the trial methods have been previously reported.21 In summary, hypertensive adult subjects with a systolic blood pressure (SBP) ≥160 mmHg (≥150 mmHg if they had type-2 diabetes mellitus) while receiving ≥3 antihypertensive medications were eligible for randomization in the trial. Exclusion criteria included a history of a prior renal artery intervention, main renal arteries <4 mm in diameter, or <20 mm in length and haemodynamically or anatomically significant renal artery abnormalities. The baseline estimated glomerular filtration rate (eGFR) was required to be >45 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula.23 Subjects with type 1 diabetes mellitus and stenotic valvular heart disease, or myocardial infarction, unstable angina, or cerebrovascular accident within 6 months prior to enrolment were also excluded.
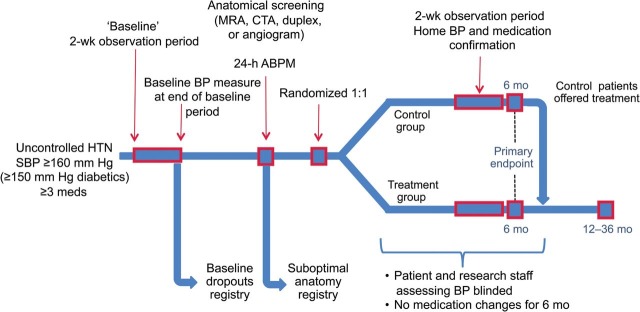
All the subjects underwent a 2-week screening period both before randomization and before their 6-month follow-up to establish adherence to a stable pharmacological treatment regimen and to confirm that SBP was ≥160 mmHg. Subjects were asked to record medications in a diary for 2 weeks at baseline and again at 6-month post-randomization. Control subjects, however, were not required to keep a diary again before the 6-month measurement post-RDN treatment. The study did not include any dietary recommendations. Physicians were encouraged to maintain subjects on a stable drug regimen throughout the study period, although clinically necessary modifications of drug choice or dose were allowed. All the subjects underwent renal artery imaging (renal duplex, computed tomography, MRI, or angiogram) before randomization to establish anatomical eligibility. Among the 190 subjects assessed for eligibility, 84 subjects were excluded from eligibility, and 106 were randomly allocated 1 : 1 to the RDN group (n = 52) or control group (n = 54)21 (Appendix Figure 1). The RDN procedure has been previously described.21,24 Neither study personnel nor subjects were blinded to the study group allocation. At the 6-month follow-up visit, control group subjects were eligible to crossover to receive RDN treatment. The treated crossover group reported here had a SBP ≥160 mmHg (≥150 mmHg in subjects with type II diabetes mellitus) at the 6-month visit. Control group subjects (n = 9) with a SBP <160 mmHg (<150 mmHg in subjects with type II diabetes mellitus) at the 6-month visit who chose to proceed to RDN on compassionate grounds are excluded from the present blood pressure analysis but are included in the safety analysis. Participating study sites, where subjects were treated, were designated European hypertension centres of excellence in 16 of 24 instances.
Statistical analysis
Descriptive statistics were used to present results for the initial RDN and crossover groups. Comparisons of blood pressure and heart rate measurements at trial milestones were compared with pre-procedure measurements using the paired t-test. A change was considered significant if the two-side alpha level was ≤0.05. Variability of office blood pressure in all follow-up visits was calculated as the standard deviation (SD) or coefficient of variation (%, 100 * SD/mean). Between group differences were compared using confidence intervals and tested using unpaired t-tests. Statistical analyses were performed using SAS version 9.2.
Results
Subject characteristics
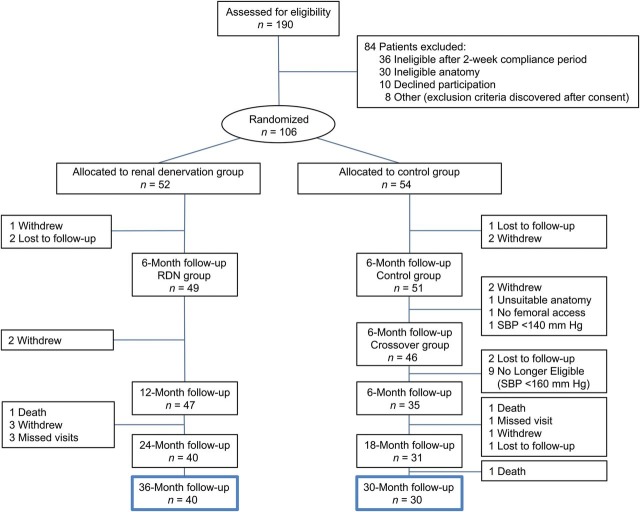
A total of 70 subjects were available for long-term follow-up; 40 subjects in the original RDN treatment group had follow-up to 36-month post-randomization and 30 control subjects who crossed over to RDN treatment at 6-month post-randomization had follow-up to 30 months following denervation (Figure 1). Baseline characteristics are shown in Table 1. Baseline SBP was 178 ± 18 mmHg in the RDN group and 191 ± 20 mmHg in the crossover group prior to the procedure. The baseline BP for all treated subjects combined was 184 ± 19/99 ± 16 mmHg. The crossover group had more women, a higher baseline eGFR, and a higher baseline office SBP. Most subjects received a diuretic, beta-adrenergic blocker, calcium channel blocker, and an angiotensin receptor blocker or angiotensin-converting enzyme (ACE) inhibitor (Table 2).
Figure 1.
Study flow.
Table 1.
Baseline demographics stratified by original renal denervation and crossover groups
| All treated subjects pre-procedure |
All treated subjects followed to 3 yearsa |
|||||
|---|---|---|---|---|---|---|
| Original RDN (n = 52) | Crossover (n = 37b) | P-value | Original RDN (n = 40) | Crossover (n = 30) | P-value | |
| Systolic pressure, means ± SD, mmHg systolic diastolic | 178 ± 18 | 191 ± 20 | 0.002 | 178 ± 17 | 191 ± 21 | 0.008 |
| 97 ± 16 | 101 ± 16 | 0.21 | 96 ± 14 | 100 ± 15 | 0.26 | |
| Age, means ± SD, years | 58 ± 12 | 57 ± 13 | 0.76 | 59 ± 11 | 58 ± 13 | 0.69 |
| Women, n (%) | 18 (35) | 22 (60) | 0.03 | 12 (30) | 19 (63) | 0.008 |
| Caucasian race, n (%) | 51 (98) | 36 (97) | 39 (98) | 29 (97) | ||
| Body mass indexc, mean (SD) | 31 ± 5 | 31 ± 5 | 0.84 | 31 ± 5 | 32 ± 5 | 0.50 |
| Type II diabetes mellitus, n (%) | 21 (40) | 11 (30) | 0.37 | 19 (48) | 9 (30) | 0.22 |
| Coronary artery disease, n (%) | 10 (19) | 2 (5) | 0.11 | 7 (18) | 1 (3) | 0.13 |
| eGFR, means ± SD, mL/min/1.73 m2 | 77 ± 19 | 89 ± 20 | 0.005 | 77 ± 18 | 88 ± 20 | 0.02 |
| Heart rate, means ± SD, b.p.m. | 75 ± 15 | 73 ± 15 | 0.65 | 73 ± 15 | 72 ± 14 | 0.63 |
eGFR, estimated glomerular filtration rate.
aInitial RDN subjects followed to 3-year post-RDN therapy; crossover subjects followed to 30-month post-RDN therapy.
bAmong the 51 control subjects at 6 months, 46 chose to crossover, of whom 37 met the inclusion/exclusion criteria and are included in the efficacy analysis.
cBody mass index is calculated as weight in kilograms and divided by height in meters squared.
Table 2.
Antihypertensive medication usage at baseline and at 30 and 36 months
| All subjects followed to 30-month post-RDN |
All subjects followed to 36-month post-RDN (original RDN) |
|||||
|---|---|---|---|---|---|---|
| Subjects at baseline (n = 69) | Subjects at 30 months (n = 69) | P-value | Subjects at baseline (n = 40) | Subjects at 36 months (n = 40) | P-value | |
| Number of antihypertensive medications, means ± SD | 5.1 ± 1.4 | 4.8 ± 1.5 | 0.06 | 5.1 ± 1.5 | 4.6 ± 1.6 | 0.02 |
| Antihypertensive medications, n (%) | (n = 69) | (n = 69) | (n = 40) | (n = 40) | ||
| ACE inhibitor | 33 (48) | 27 (39) | 0.03 | 19 (48) | 14 (35) | 0.03 |
| Angiotensin receptor blocker | 53 (77) | 50 (73) | 0.18 | 28 (70) | 28 (70) | >0.99 |
| Calcium channel blocker | 52 (75) | 49 (71) | 0.44 | 30 (75) | 27 (68) | 0.26 |
| Diuretic | 60 (87) | 52 (75) | 0.05 | 35 (88) | 32 (80) | 0.37 |
| Aldosterone antagonists | 13 (19) | 22 (32) | 0.007 | 8 (20) | 14 (35) | 0.08 |
| Centrally acting sympatholytics | 35 (51) | 24 (35) | 0.005 | 23 (58) | 13 (33) | 0.002 |
| Direct renin inhibitors | 14 (20) | 6 (9) | 0.02 | 8 (20) | 3 (8) | 0.06 |
| Beta-blockers | 52 (75) | 49 (71) | 0.26 | 33 (83) | 30 (75) | 0.08 |
| Alpha-adrenergic blocker | 3 (4) | 2 (3) | 0.32 | 2 (5) | 2 (5) | >0.99 |
| Direct-acting vasodilators | 4 (6) | 5 (7) | 0.66 | 3 (8) | 4 (10) | 0.56 |
ACE inhibitor, angiotensin-converting enzyme inhibitor.
Blood pressure and heart rate measurements
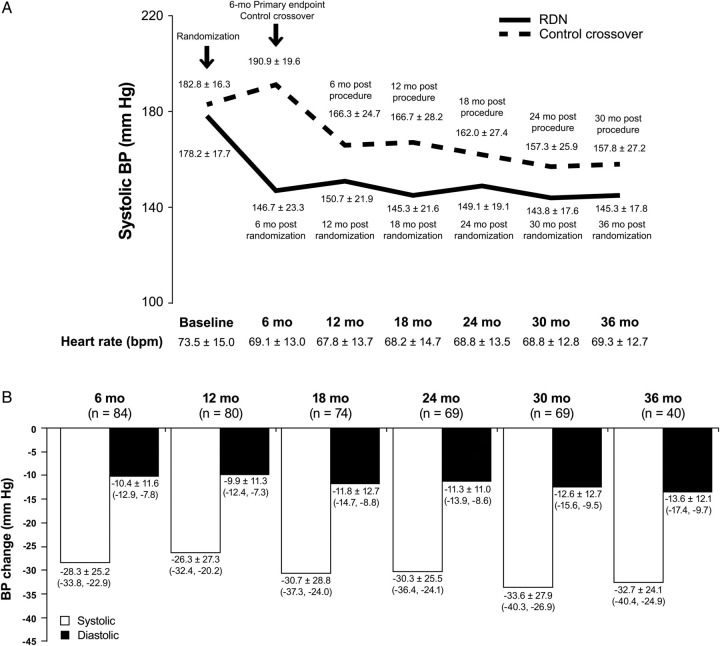
Systolic blood pressure and diastolic blood pressure (DBP) measurements were significantly lower than the pre-procedure measurements at all-time points (6, 12, 24, 30, and 36 months, Figure 2A and B); blood pressure reduction did not diminish during extended follow-up. For the 69 subjects (1 initial RDN subject missed the 30-month visit) with 30-month post-procedure follow-up, the change in SBP was −34 mmHg (95% CI: −40, −27, P < 0.01) and the change in DBP was −13 mmHg (95% CI: −16, −10, P < 0.01). The SBP and DBP reduction at 36 months in the initial RDN group was 33 mmHg (95% CI: −40, −25, P < 0.01) and 14 mmHg (95% CI: −17, −10, P < 0.01), respectively. Both the original RDN group and the crossover subjects had a similar reduction in SBP at 30-month post-RDN (−34 ± 23 vs. −33 ± 33, respectively). The coefficient of variation of systolic office blood pressure measurements (based on all subjects with at least two measurements) was 9.5 ± 4.7 (n = 80).
Figure 2.
(A) Office systolic blood pressure (all P < 0.01 by 6 months after renal denervation and after crossover) and heart rate (P < 0.01 at 6, 12, 18 months, P = 0.06 at 24 months, P = 0.16 at 30 months, and P = 0.04 at 36 months and for difference in the mean heart rate in the pooled population from baseline to follow-up). RDN, renal denervation. Solid bar: original renal denervation treatment group. Hashed bar: crossover to renal denervation treatment from control group. (B) Post-procedure change in office blood pressure. Systolic blood pressure, SBP; diastolic blood pressure, DBP. P < 0.01 at all-time periods for blood pressure difference from baseline. White bar: SBP. Black bar: DBP.
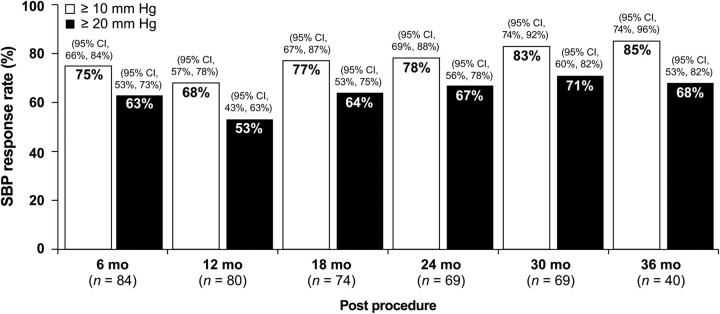
At 30 months after RDN, 83% of the subjects had achieved a reduction in SBP of ≥10 mmHg, and among the 40 RDN subjects with 36-month follow-up, 85% of the subjects had achieved a reduction in SBP of ≥10 mmHg (Figure 3). This response rate was similar in both the original RDN subjects and in crossover subjects: at 30 months after RDN 72% of original RDN subjects and 70% of crossover subjects had ≥20 mm reduction in SBP.
Figure 3.
Systolic blood pressure response rate. Systolic blood pressure, SBP, white bar: ≥10 mm reduction in SBP, black bar: ≥20 mm reduction in SBP, CI, confidence interval.
Heart rate was slowed following RDN and this decrease persisted to 36 months, with a mean decrease of 4 b.p.m. (n = 36, 95% CI: −8, −0.1, P = 0.04) (Figure 2A).
Medications and body weight
Changes in medication class and/or prescribed dose were permitted beyond 6 months. Comparison of the mean number of antihypertensive medications at 36-month follow-up showed a significant reduction vs. baseline (4.6 ± 1.6 vs. 5.1 ± 1.5, P = 0.02) due in part to significantly lower usage of ACE inhibitors and centrally acting sympatholytics at 3 years (Table 2). At 30 months in all 69 subjects, the mean change in antihypertensive medications was 5.1 ± 1.4 at baseline vs. 4.8 ± 1.5 at 6 months, P = 0.06, due in part to a significant reduction in ACE inhibitors, diuretics, centrally acting sympatholytics, and direct renin inhibitors, and a significant increase in aldosterone antagonists (Table 2).
Weight was measured at all-time periods. Among subjects with 30-month follow-up, mean body weight was 91.9 ± 19.4 kg at baseline (n = 68) vs. 93.0 ± 19.9 kg at 30-month post-RDN (n = 65, P = 0.17).
Safety
Previous safety results to 12 months on the RDN and crossover subjects have been published.22 Peri-procedural complications included one haematoma at the femoral access site in the original RDN therapy group and one renal artery dissection before energy delivery that required renal artery stenting in the crossover group. Clinical events to 1 year included nine hypertensive events requiring hospitalization and two hypotensive events requiring hospitalization.
Between 12 and 36 months, there were five hypertensive events requiring hospitalization as well as one case of mild transient acute renal failure due to dehydration that responded to volume expansion and temporary discontinuation of antihypertensive medications. There was one report of acute renal failure due to acute interstitial nephritis that resolved with conservative treatment and was deemed unrelated to the RDN treatment. Two subjects were hospitalized with atrial fibrillation, one of whom underwent cardioversion. There were three deaths (two occurred at home of unknown causes and one occurred due to cardiogenic shock following aortic valve replacement) that were adjudicated to be unrelated to the device or therapy by the independent safety and monitoring board. There was no change in the mean eGFR, and no renal vascular events were reported.
Discussion
SYMPLICITY HTN-2, the first randomized controlled RDN trial, now with data out to 3 years, adds additional long-term data to the body of evidence concerning the safety and efficacy of RDN first reported in SYMPLICITY HTN-1.18–20 Our similar results confirm the substantial magnitude and long-term durability and safety of the RDN antihypertensive effects. Observations are available at 36 months in the group initially randomized to RDN, and at 30 months following denervation in the crossover group, the group initially randomized to control who subsequently received RDN after the 6-month primary endpoint assessment. At 36 months SBP was reduced by 33 mmHg (95% CI: −40, −25, P < 0.01). This consistent long-term effect was achieved without increasing medication or serious long-term safety concerns.
While results in SYMPLICITY HTN-2 were consistent with results in SYMPLICITY HTN-1 and other reports with the Symplicity catheter, results in SYMPLICITY HTN-3 have been inconsistent to previous studies. SYMPLICITY HTN-3 was a rigorously designed study that required screening with 24-h ambulatory blood pressure monitoring to exclude subjects with white-coat hypertension and randomized 535 subjects in a 2 : 1 fashion to active denervation or a sham procedure.25 Both subjects and the involved staff (outside the catheterization laboratory) were blinded to treatment. But there is an Achilles heel with most clinical trials of RDN for hypertension, including this one. Typically no validated test is applied during the procedure, or subsequently to document that adequate renal denervation is performed. The 6-month reduction in office SBP was −14 ± 24 mmHg in the denervation group compared with −12 ± 26 mmHg in the sham-procedure group (P = 0.26 for pre-specified superiority with a margin of 5 mmHg).26 The lack of significant improvement in SBP over a control arm may be related to study design, patient selection criteria and procedural details that may have caused failure to consistently achieve renal denervation. The level of procedural training for the 100+ operators in SYMPLICITY HTN-3 at 88 centres, all new to renal denervation, was far less than that in SYMPLICIYT HTN-2, which was conducted at only 24 centres with hands-on renal denervation experience for all operators prior to their performing their first denervation in the trial. This flaw in the design of SYMPLICITY HTN-3, the use of renal denervation novices, with minimal training, was likely based on the notion that renal denervation is easily achieved, which it is not.21 Additionally, participating SYMPLICITY HTN-2 study sites were designated European hypertension centres of excellence in 16 of 24 instances, whereas subjects in SYMPLICITY HTN-3 were often referred to the study from outside institutions and may not have exhausted all treatment options.
Despite rational multidrug antihypertensive prescribing in severe essential hypertension and patient adherence to both lifestyle measures and to medication dosing, blood pressure targets are not met in a significant proportion of patients.5,7 In the early phases of SYMPLICITY HTN-1 and SYMPLICITY HTN-2 trials, antihypertensive medications were not changed unless clinically required prior to assessing the primary BP endpoint at 6 months following RDN to allow the evaluation of the antihypertensive effect of RDN free from any confounding by dosing or medication changes. Subsequently, dosing or medication changes could be initiated by treating physicians when clinically required. On average, at the current limits of follow-up, there was a significant decrease in the number of medication classes. Thus, the sustained decrease in blood pressure beyond 6 months and out to at least 3 years cannot be readily explained by increased drug regimens. Indeed, the intent of the trial was to lower blood pressure and thereby reduces cardiovascular risk. The aim was not to achieve medication-free control of hypertension; therefore down-titration of antihypertensive drug treatment after the procedure was not part of the study protocol.
The antihypertensive response rate in this trial was 85% for reduction in office systolic BP of ≥10 mmHg, and 68% for ≥20 mmHg fall at 3 years. The introduction of RDN into clinical patient care may be aided by tests to identify good responders. A higher baseline SBP has been consistently associated with greater blood pressure reduction but other consistent predictors of response have not been identified to date.19,27–29 Lower-response to RDN could be due to multiple possible causes including technical failure of the RDN procedure, variable patient drug adherence, or non-neurally driven pathogenesis of the hypertension in an individual subject.30
Renal denervation as a treatment option for severe uncontrolled hypertension should only be considered after careful clinical evaluation to eliminate both white-coat hypertension (using 24-h ambulatory blood pressure monitoring) and secondary causes of hypertension (primary aldosteronism being most likely to remain unidentified), and after pharmacological and other non-pharmacological treatment options have been optimally applied.24,31
The results of our trial should be interpreted in the context of some limitations. First, the number of subjects enrolled was relatively small, although significant changes in BP were observed. Secondly, our findings should not be extrapolated to patients who do not fulfil the inclusion and exclusion criteria of the SYMPLICITY HTN-2 study. Thirdly, there were losses to clinical follow-up. Fourthly, after the 6-month primary endpoint, control subjects had the option to crossover and therefore form a registry. There may have been a selection bias in terms of control subjects who chose to crossover and still met the inclusion and exclusion criteria of the study. Fifthly, neither study personnel nor subjects were blinded to the study group allocation. Sixthly, ambulatory blood pressure measurement (ABPM) was commonly measured at baseline in order to avoid white-coat hypertension, but this was not part of the inclusion and exclusion criteria. The study previously reported 6-month change in ABPM in initially enrolled subjects.21 Owing to subject non-adherence and incomplete records, an overall change in ABPM is unavailable. After the 6-month end point, 24-h blood pressure records were not collected. Seventhly, crossover subjects began with a higher BP at the time of RDN treatment when compared with the initial RDN group. The crossover subjects represent a subset of the control group; there may have been a selection bias of subjects who continued to have a SBP ≥160 mmHg and who chose to crossover, and this increase in blood pressure could represent a natural tendency for blood pressure to increase in subjects with treatment-resistant hypertension. However, at 30-month post-RDN, the original RDN group and the crossover group had a similar reduction in SBP and similar response rates regardless of a different baseline measurement at time of treatment.
In conclusion, we have demonstrated that renal artery nerve ablation results in sustained lowering of blood pressure out to at least 3 years (30 months for crossover subjects) in a selected population of subjects with severe, treatment-resistant hypertension. This long-term benefit occurred with minimal procedural or late safety concerns and was not associated with increased drug therapy.
Funding
This work was supported by Medtronic, Inc.
Conflict of interest: Nicole Brilakis, MS, MBA, Colleen Gilbert, PharmD, and Sidney A. Cohen, MD, PhD from Medtronic, Inc. provided editorial support. Martin Fahy, MS and Minglei Liu, PhD from Medtronic, Inc. provided statistical support.
Acknowledgements
The sponsor designed the study in collaboration with the study investigators and was responsible for data collection and data analysis. The authors are responsible for data interpretation and writing of the report. The corresponding author, M.D.E., had full access to all the study data and had final responsibility for the decision to submit.
Clinical Investigators: Michael Böhm and Felix Mahfoud (Universitätsklinikum des Saarlandes, Homburg, Germany), Horst Sievert and Nina Wunderlich (CardioVascular Center Frankfurt, Frankfurt, Germany), Lars Christian Rump and Oliver Vonend (Universitätsklinikum Düsseldorf, Düsseldorf, Germany), Roland E Schmieder and Michael Uder (Universität Erlangen-Nürnberg, Erlangen, Germany), Mel Lobo and Mark Caulfield (William Harvey Research Institute, Barts and The London NIHR Cardiovascular Biomedical Research unit, Queen Mary University of London and Barts and the London NHS Trust, London, UK), Andrejs Erglis (Pauls Stradins Clinical University Hospital, Riga, Latvia), Michel Azizi and Marc Sapoval (Assistance Publique des Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Paris, France), Suku Thambar (John Hunter Hospital, Newcastle, NSW, Australia), Alexandre Persu and Jean Renkin (Cliniques Universitaires Saint-Luc, Brussels, Belgium), Heribert Schunkert and Joachim Weil (Universitaetsklinikum Schleswig-Holstein, Lübeck, Germany), Hannes Reuter and Uta C Hoppe (Universität zu Köln, Köln, Germany), Henry Krum and Antony Walton (The Alfred Hospital, Melbourne, VIC, Australia), Markus P Schlaich and Murray D Esler (Baker IDI Heart and Diabetes Institute, Melbourne, VIC, Australia), Dierk Scheinert (Universität Leipzig—Herzzentrum, Leipzig, Germany), Thomas Binder (Allgemeines Krankenhaus der Stadt Wien, Vienna, Austria), Andrzej Januszewicz and Adam Witkowski (Samodzielna Pracownia Hemodynamiczna, Warsaw, Poland), Luis M Ruilope (Hospital 12 de Octubre, Madrid, Spain), Robert Whitbourn (St Vincent's Hospital, Melbourne, VIC, Australia), Heike Bruck (Universitätsklinikum Essen, Essen, Germany), Mark Downes and Robert Kaikini (Kent and Canterbury Hospital, Canterbury, UK), Thomas F Lüscher (University Hospital Zurich, Zurich, Switzerland), Alan G Jardine (University of Glasgow, Glasgow, UK), Mark W Webster (Auckland City Hospital, Auckland, New Zealand), Thomas Zeller (Herz-Zentrum Bad Krozingen, Bad Krozingen, Germany), Jerzy Sadowski and Krzysztof Bartus (The John Paul II Hospital, Jagiellonian University, Krakow, Poland).
Appendix
Figure A1.
Study randomization, 24-h ambulatory blood pressure measurement, ABPM; month, mo; week, wk; systolic blood pressure, SBP; classes of antihypertensive medications, meds; magnetic resonance angiography, MRA; computed tomography angiography, CTA; duplex ultrasonography, duplex.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM American Heart Association Professional Education C. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force M. ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 7.Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749–1757. doi: 10.1016/j.jacc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Wojciechowski D, Papademetriou V, Faselis C, Fletcher R. Evaluation and treatment of resistant or difficult-to-control hypertension. J Clin Hypertens (Greenwich) 2008;10:837–843. doi: 10.1111/j.1751-7176.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O'Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 11.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 12.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 14.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 15.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61:806–811. doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25:628–633. doi: 10.1002/ca.21280. [DOI] [PubMed] [Google Scholar]
- 17.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 18.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 19.Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 21.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 22.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA, Symplicity HTNI. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Mahfoud F, Luscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefevre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Bohm M. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–2157. doi: 10.1093/eurheartj/eht154. [DOI] [PubMed] [Google Scholar]
- 25.Kandzari DE, Bhatt DL, Sobotka PA, O'Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, Investigators S-H. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 27.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Bohm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Bohm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140. doi: 10.1161/CIRCULATIONAHA.112.000949. [DOI] [PubMed] [Google Scholar]
- 29.Vogel B, Kirchberger M, Zeier M, Stoll F, Meder B, Saure D, Andrassy M, Mueller OJ, Hardt S, Schwenger V, Strothmeyer A, Katus HA, Blessing E. Renal sympathetic denervation therapy in the real world: results from the Heidelberg registry. Clin Res Cardiol. 2013;103:11–124. doi: 10.1007/s00392-013-0627-5. [DOI] [PubMed] [Google Scholar]
- 30.Ukena C, Cremers B, Ewen S, Bohm M, Mahfoud F. Response and non-response to renal denervation: who is the ideal candidate? EuroIntervention. 2013;9(Suppl. R):R54–R57. doi: 10.4244/EIJV9SRA10. [DOI] [PubMed] [Google Scholar]
- 31.Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Bohm M, Campese VM, Francis DP, Grassi G, Hering D, Katholi R, Kjeldsen S, Krum H, Mahfoud F, Mancia G, Messerli FH, Narkiewicz K, Parati G, Rocha-Singh KJ, Ruilope LM, Rump LC, Sica DA, Sobotka PA, Tsioufis C, Vonend O, Weber MA, Williams B, Zeller T, Esler MD. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–2045. doi: 10.1016/j.jacc.2013.08.1616. [DOI] [PubMed] [Google Scholar]