Abstract
Aims
Angiotensin receptor blockers (ARBs) are available in different dosages and it is common clinical practice to uptitrate if blood pressure goal is not achieved with the initial dose. Data on the incremental antihypertensive efficacy with uptitration are scarce. It is also unclear if antihypertensive efficacy of losartan is comparable with other ARBs.
Methods and results
We systematically reviewed PubMed/EMBASE/Cochrane databases for all randomized clinical trials until December 2012 reporting 24 h ambulatory blood pressure (ABP) for most commonly available ARBs in patients with hypertension. Reduction in ABP with ARBs was evaluated at 25% of the maximum (max) dose, 50% of the max dose, and at the max dose. Comparison was made between 24 h BP-lowering effect of losartan 50 and 100 mg and other ARBs at 50% max dose and the max dose, respectively. Sixty-two studies enrolling 15 289 patients (mean age 56 years; 60% men) with a mean duration of 10 weeks were included in the analysis. Overall, the dose–response curve with ARBs was shallow with decrease of 10.3/6.7 (systolic/diastolic), 11.7/7.6, and 13.0/8.3 mmHg with 25% max dose, 50% max dose, and with the max dose of ARBs, respectively. Losartan in the dose of 50 mg lowered ABP less well than other ARBs at 50% max dose by 2.5 mmHg systolic (P < 0.0001) and 1.8 mmHg diastolic (P = 0.0003). Losartan 100 mg lowered ABP less well than other ARBs at max dose by 3.9 mm Hg systolic (P = 0.0002) and 2.2 mmHg diastolic (P = 0.002).
Conclusion
In this comprehensive analysis of the antihypertensive efficacy of ARBs by 24 h ABP, we observed a shallow dose–response curve, and uptitration marginally enhanced the antihypertensive efficacy. Blood pressure reduction with losartan at starting dose and at max dose was consistently inferior to the other ARBs.
Keywords: Angiotensin receptor blockers, Ambulatory blood pressure monitoring, Hypertension, Meta-analysis
Introduction
Hypertension is an asymptomatic condition and should remain so when treated. Angiotensin receptor blockers (ARBs) are known to provide a good blood pressure reduction with little, if any, adverse effects.1 The magnitude and duration of antihypertensive response of various ARBs is thought to vary due to differences in pharmacokinetic and pharmacodynamic properties.2 Conflicting results have been reported in several reviews and meta-analyses regarding the antihypertensive efficacy of various ARBs; some suggesting no difference within the class,1,3 whereas others suggesting losartan being inferior.4,5 Twenty-four hour ambulatory blood pressure (ABP) monitoring is considered as the most objective and accurate tool to assess antihypertensive efficacy and is shown to predict cardiovascular events even after adjusting for office blood pressure measurement.6 Our objective was two-fold: (i) to evaluate the antihypertensive efficacy of ARBs as assessed by 24 h ABP at 25% maximum (max), 50% max, and max dose, and (ii) to evaluate ABP reduction with losartan compared with other ARBs.
Methods
Search strategy
A systematic search was performed in PubMed, EMBASE, and Cochrane Central Register of Clinical Trials (Cochrane Library Issue 6, June 2012) using the key terms ‘Angiotensin Receptor Blockers’, ‘ARBs’, and names of all individual ARBs. We limited our search to randomized controlled trials in human subjects and in peer-reviewed journals until December 2012. No language restriction was applied. The reference lists of identified articles and bibliographies of original articles were also reviewed. Trials in the abstract form without a manuscript published were excluded for this analysis. Authors of the individual trials were contacted in case of inadequate data.
Selection criteria
To be included in the analysis, a trial had to fulfil the following criteria: (i) randomized clinical trials that assessed the antihypertensive efficacy by 24 h ABP comparing ARB with other antihypertensive drug classes (including other ARBs) or with placebo, (ii) patient population with hypertension, (iii) ARB used as monotherapy, (iv) no uptitration of ARB dose throughout the trial, and (v) trial duration of at least 4 weeks. Studies were excluded if ARB doses were uptitrated or if additional antihypertensive drugs were added to control the blood pressure. None of the included studies had patients with severe hypertension. Studies with tasosartan were excluded, since it was never marketed.
Data extraction
Two authors (H.M. and J.R.) searched the data independently and in duplicate. Disagreements were resolved by consensus. We extracted characteristics of each trial, duration of intervention and methods, baseline demographics, type of ARB used with the dose, 24 h ABP at baseline and after the intervention, for our analysis.
Quality assessment
The criteria used for quality assessment were sequence generation of allocation, allocation concealment, masking of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias, as recommended by the Cochrane Collaboration.7
Statistical analysis
The statistical analysis was done in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,8 using Review Manager (RevMan), version 5.1.7, the Cochrane Collaboration, 2012. Heterogeneity was assessed using the I2 statistics. I2 is the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error (chance) and we considered I2 < 25% as low and I2 > 75% as high. Random-effects model of DerSimonian and Laird9 was used to calculate the effect sizes if I2 > 25%. Analysis was performed on intention-to-treat basis. Data from changes in baseline blood pressure were combined using weighted mean difference method. For trials that did not provide complete information about variance for net change in BP, the information was obtained from confidence intervals (CIs), P-value, or from t-statistics. Variance was estimated from pre-test–post-test (parallel group and factorial design) and crossover designs as suggested by Follmann et al.10 All the studies were stratified based on 25% max dose, 50% max dose, and the max dose of ARB as defined in hypertension guidelines of the Joint National Committee11 (Table 1). Separate head-to head comparison was performed between losartan and other ARBs when data were available. Publication bias was estimated visually by funnel plots, and/or using Begg's test and the weighted regression test of Egger et al.12 Sensitivity analyses was performed for BP reduction at 50% max and max dose of ARBs based on the quality of study, mean baseline blood pressure (above vs. below mean BP), number of patients in the study (≤100 vs. >100), and study duration (≤8 vs. >8 weeks). We estimated difference between subgroups according to the tests of interaction.13
Table 1.
Type of angiotensin receptor blockers with doses at 25% maximum, 50% maximum, and at maximum dose
| Type of ARB | 25% max dose (mg) | 50% max dose (mg) | Max dose (mg) |
|---|---|---|---|
| Azilsartan | – | 40 | 80 |
| Candesartan | 8 | 16 | 32 |
| Irbesartan | 75 | 150 | 300 |
| Losartan | 25 | 50 | 100 |
| Olmesartan | 5–10 | 20 | 40 |
| Telmisartan | 20 | 40 | 80 |
| Valsartan | 80 | 160 | 320 |
ARB, angiotensin receptor blocker; max, maximum.
Results
Study characteristics
We identified 2684 articles, out of which 146 abstracts were retrieved and reviewed for possible inclusion. Sixty-two studies14–75 enrolling 15 289 patients (mean age 56 ± 7 years; 60% men) and the mean duration of 10 weeks fulfilled the inclusion criteria and were included in the analysis (Table 2). These 62 trials were with azilsartan (n = 1), candesartan (n = 8), eprosartan (n = 1), irbesartan (n = 6), losartan (n = 25), olmesartan (n = 12), telmisartan (n = 14), and valsartan (n = 12) (Table 1). Forty-six trials were excluded: uptitrated dose of ARBs (n = 13), inadequate data (n = 15), ARBs combined with other drugs (n = 12), baseline study population without hypertension (n = 3), and studies with tasosartan (n = 3) (Figure 1).
Table 2.
Baseline characteristics of the included trials
| Trial, year | Number of patients | Men (%) | Age (years) | Follow-up (weeks) | Comparison group |
|---|---|---|---|---|---|
| Andersen et al.,14 2000 | 16 | 63 | 42 | 8 | Losartan 50 mg vs. losartan 100 mg vs. placebo |
| Baguet et al.,15 2006 | 256 | 60 | 54 | 6 | Candesartan 8 mg vs. losartan 50 mg vs. placebo |
| Bakris et al.,16 2001 | 406 | 56 | 53 | 8 | Losartan 100 mg vs. verapamil 360 mg vs. enalapril 20 mg vs. placebo |
| Brunner et al.,17 2003 | 635 | 57 | 52 | 8 | Candesartan 8 mg vs. olmesartan 20 mg |
| Byyny,18 1996 | 122 | 68 | 53 | 4 | Losartan 50 mg vs. losartan 50 mg b.i.d. vs. losartan 100 mg vs. placebo |
| Chanudet and De Champvallins,19 2001 | 277 | 51 | 59 | 12 | Losartan 50 mg vs. perindopril 2 mg/indapamide 0.625 mg |
| Chrysant et al.,20 2003 | 440 | 63 | 52 | 8 | Olmesartan 20 mg vs. amlodipine 5 mg vs. placebo |
| Chung et al.,21 2000 | 263 | 53 | 57 | 12 | Losartan 50 mg vs. mibefradil 50 mg Losartan 100 mg vs. mibefradil 100 mg |
| Crowe et al.,22 2003 | 17 | NR | NR | 8 | Losartan 50 mg vs. losartan 100 mg |
| de Champlain et al.,23 2007 | 47 | 81 | 57 | 8 | Valsartan 160 mg vs. amlodipine 10 mg |
| Destro et al.,24 2005 | 107 | 56 | NR | 8 | Olmesartan 20 mg vs. valsartan 160 mg |
| Ding et al.,25 2004 | 61 | 77 | 61 | 6 | Losartan 50 mg vs. telmisartan 40 mg |
| Duprez et al.,26 2011 | 108 | 54 | 78 | 4 | Valsartan 160 mg vs. HCTZ 12.5 m vs. valsartan/HCTZ 160/12.5 |
| Düsing et al.,27 2012 | 822 | 53 | 56 | 12 | Telmisartan 80 mg vs. aliskiren 300 mg |
| Fagard et al.,28 2001 | 9 | NR | 46 | 6 | Losartan 50 mg vs. enalapril 20 mg vs. placebo |
| Fogari et al.,29 2006 | 130 | 55 | 60 | 4 | Olmesartan 20 mg vs. valsartan 160 mg |
| Fogari et al.,30 2008 | 126 | 55 | 60 | 8 | Olmesartan 20 mg vs. telmisartan 80 mg |
| Galzerano et al.,31 2004 | 69 | 55 | 54 | 52 | Telmisartan 80 mg vs. HCTZ 25 mg |
| Galzerano et al.,32 2005 | 82 | 57 | 60 | 44 | Telmisartan 80 mg vs. carvedilol 25 mg |
| Guasti et al.,33 2002 | 22 | NR | NR | 8 | Losartan 50 mg vs. enalapril 20 mg |
| Hermida et al.,34 2005 | 100 | 34 | 68 | 12 | Valsartan 160 mg a.m. vs. valsartan 160 mg p.m. |
| Hermida et al.,35 2007 | 215 | 53 | 46.4 | 12 | Valsartan 80 mg a.m. vs. valsartan 80 mg p.m. |
| Hermida et al.,36 2009 | 144 | 33 | 46.6 | 12 | Olmesartan 20 mg a.m. vs. olmesartan 20 mg p.m. |
| Kawano et al.,37 2008 | 79 | 67 | 58.9 | 6 | Irbesartan 100 mg vs. placebo |
| Kraiczi et al.,38 2000 | 40 | 100 | 57 | 6 | Losartan 50 mg vs. atenolol 50 mg vs. HCTZ 25 mg vs. amlodipine 5 mg vs. enalapril 20 mg |
| Kuschnir et al.,39 2004 | 299 | 45 | 56 | 8 | Losartan 50 mg vs. nifedipine 20 mg |
| Lacourciere and Asmar,40 1999 | 268 | 62 | 55 | 4 | Losartan 50–100 mg vs. candesartan 8–16 mg vs. placebo |
| Lacourciere et al.,41 2006 | 812 | 67 | 53 | 14 | Telmisartan 40–80 mg vs. ramipril 2.5–10 mg |
| Littlejohn et al.,42 2000 | 426 | 68 | 53 | 8 | Telmisartan 80 mg vs. valsartan 80 mg |
| London et al.,43 2006 | 576 | 49 | 59 | 12 | Candesartan 8 mg vs. amlodipine 5 mg vs. indapamide 1.5 mg |
| Mallion et al.,44 1999 | 223 | 67 | 56 | 6 | Losartan 50 mg vs. telmisartan 40–80 mg vs. placebo |
| Matsumoto et al.,45 2009 | 35 | 54 | 61 | 4 | Olmesartan 10 mg vs. amlodipine 2.5 mg |
| Meier et al.,46 2011 | 20 | 50 | 53 | 20 | Losartan 100 mg vs. losartan 200 mg vs. losartan/lisinopril 100/20 |
| Morgan and Anderson,47 2002 | 31 | 90 | 77 | 4 | Candesartan 16 mg vs. felodipine 5 mg vs. placebo |
| Morgan et al.,48 2004 | 23 | 96 | 75 | 4 | Candesartan 16–32 mg vs. lisinopril 20–40 mg vs. placebo |
| Munakata et al.,49 2004 | 41 | 49 | 54 | 12 | Valsartan 80 mg vs. nifedipine 20 mg |
| Neutel et al.,50 1997 | 216 | 83 | 55 | 8 | Valsartan 20 mg vs. valsartan 80 mg vs. valsartan 160 mg vs. valsartan 320 mg vs. placebo |
| Neutel et al.,51 2002 | 334 | 69 | 54 | 8 | Olmesartan 5 mg vs. 2.5 mg b.i.d. vs. 20 mg vs. 10 mg b.i.d. vs. 40 mg b.i.d. vs. 80 mg vs. placebo |
| Neutel et al.,52 2003 | 714 | 57 | 55 | 6 | Telmisartan 80 mg vs. losartan 50 mg/HCTZ 12.5 mg |
| Ogihara et al.,53 2009 | 862 | 68 | 57 | 12 | Olmesartan 20 mg vs. azelnidipine 16 mg vs. olmesartan + azelnidipine 10–20/8–16 |
| Palatini et al.,54 2010 | 654 | 61 | 54 | 9 | Irbesartan 300 mg vs. aliskiren 300 mg vs. ramipril 10 mg |
| Parati et al.,55 2010 | 68 | 60 | 54 | 12 | Losartan 100 mg vs. barnidipine 10 mg/losartan 50 mg |
| Pechere-Bertschi et al.,56 1998 | 20 | 65 | 54 | 12 | Irbesartan 100 mg vs. enalapril 20 mg |
| Podzolkov et al.,57 2003 | 40 | 68 | 51 | 8 | Losartan 50 mg vs. losartan 50 mg/HCTZ 12.5 mg |
| Poirier et al.,58 2004 | 57 | 70 | 59 | 8 | Telmisartan 80 mg vs. amlodipine 10 mg vs. ramipril 10 mg |
| Povedano and Garcia De La Villa,59 2009 | 38 | 42 | 54 | 16 | Olmesartan 40 mg a.m. vs. olmesartan 40 mg p.m. |
| Ragot et al.,60 2000 | 229 | 56 | 56 | 6 | Losartan 50 mg vs. trandolapril 2 mg |
| Rajagopalan et al.,61 2007 | 404 | 53 | 64 | 12 | Valsartan 160 mg vs. valsartan 160 mg/simvastatin 20 mg vs. valsartan 160 mg/simvastatin 80 mg |
| Sasso et al.,62 2002 | 64 | NR | 49 | 8 | Irbesartan 150 mg b.i.d. vs. placebo |
| Smith et al.,63 2005 | 588 | 61 | 52 | 8 | Irbesartan 150 mg vs. olmesartan 20 mg vs. losartan 50 mg vs. valsartan 80 mg |
| Stergiou et al.,64 2002 | 33 | 49 | 47 | 10 | Losartan 50 mg vs. lisinopril 20 mg |
| Stergiou et al.,65 2003 | 36 | 78 | 50 | 10 | Telmisartan 80 mg vs. lisinopril 20 mg |
| Suonsyrja et al.,66 2008 | 208 | 100 | 51 | 4 | Losartan 50 mg vs. bisoprolol 5 mg vs. amlodipine 5 mg vs. HCTZ 25 mg |
| Tedesco et al.,67 1998 | 77 | 53 | 55 | 95 | Losartan 50 mg vs. HCTZ 25 mg |
| Ubaid-Girioli et al.,68 2007 | 63 | 46 | 49.3 | 12 | Irbesartan 150 mg vs. quinapril 20 mg vs. HCTZ 25 mg |
| Weber et al.,69 1995 | 122 | 68 | 53 | 4 | Losartan 50 mg vs. 100 mg vs. 50 mg b.i.d. vs. placebo |
| Weir et al.,70 2011 | 246 | 50 | 52 | 8 | Olmesartan 40 mg vs. losartan 100 mg |
| White et al.,71 2001 | 200 | 69 | 54 | 8 | Eprosartan 600 mg vs. eprosartan 1200 mg vs. placebo |
| White et al.,72 2004 | 490 | 76 | 55 | 8 | Telmisartan 80 mg vs. valsartan 160 mg |
| White et al.,73 2011 | 1291 | 54 | 56 | 6 | Azilsartan 40 mg vs. olmesartan 40 mg vs. azilsartan 80 mg vs. valsartan 320 mg vs. placebo |
| Williams et al.,74 2006 | 801 | 60 | 54 | 14 | Telmisartan 80 mg vs. ramipril 10 mg |
| Yasuda et al.,75 2005 | 87 | 41 | 62 | 12 | Losartan 100 mg vs. amlodipine 10 mg |
All studies had patient population with hypertension.
b.i.d., twice daily; HCTZ, hydrochlorothiazide; mg, milligrams; NR, not reported.
Figure 1.
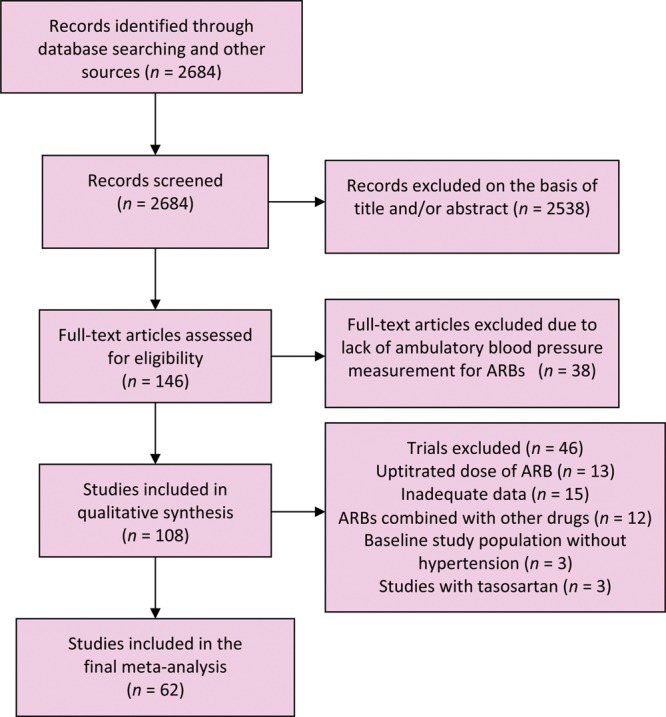
Selection of studies. ARBs, angiotensin receptor blockers.
All the included studies were done in patients with mild to moderate hypertension. Of the 62 trials, 18 trials reported adequate generation of allocation sequence and adequate allocation concealment, and 39 reported adequate masking of participants, personnel, and outcome assessors. On the basis of quality assessment, 18 were deemed as low-bias risk trials and the rest as high-bias risk trials.
Antihypertensive efficacy of angiotensin receptor blockers
Reduction in blood pressure was measured at three separate doses—25% max dose, 50% max dose, and at the max dose for all the ARBs (Figure 2).
Figure 2.
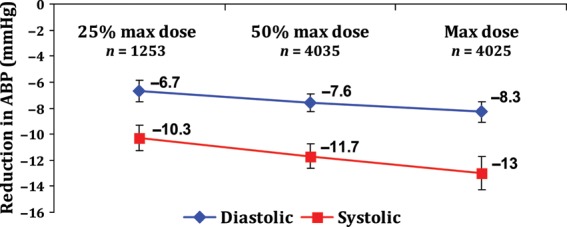
Antihypertensive efficacy of angiotensin receptor blockers at 25% maximum, 50% maximum, and the maximum dose. Error bars represent 95% confidence intervals. ABP, ambulatory blood pressure; n, number of patients; max, maximum.
Twenty-five per cent maximum dose of angiotensin receptor blockers
Data were available from 12 studies with the total of 1253 patients. Reduction in BP was 10.3 mmHg (95% CI: 9.3–11.3) systolic and 6.7 mmHg (95% CI: 5.8–7.5) diastolic with 25% max dose of ARBs.
Fifty per cent maximum dose of angiotensin receptor blockers
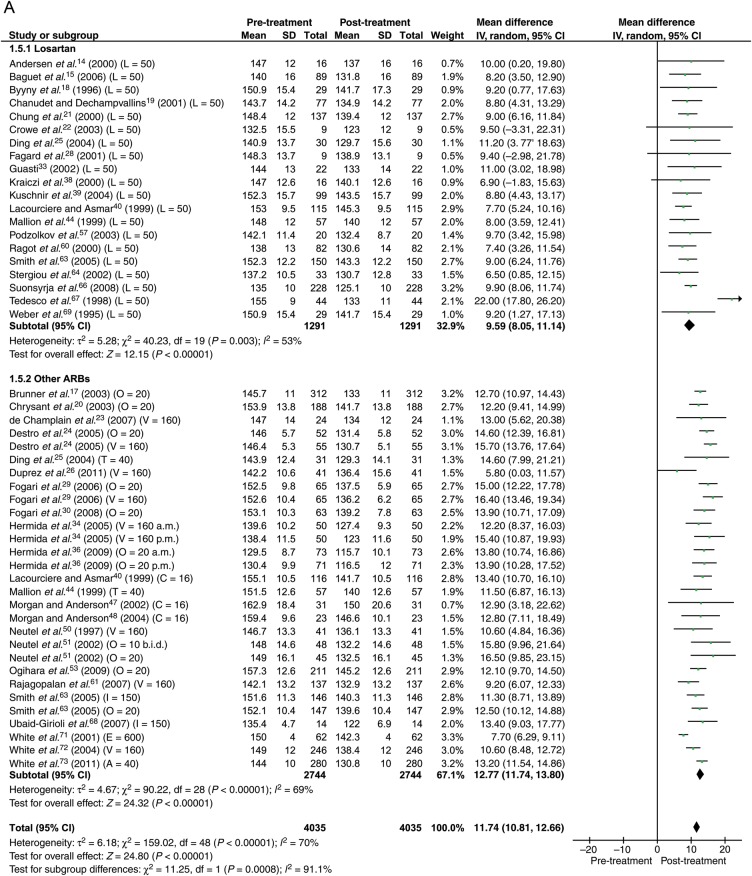
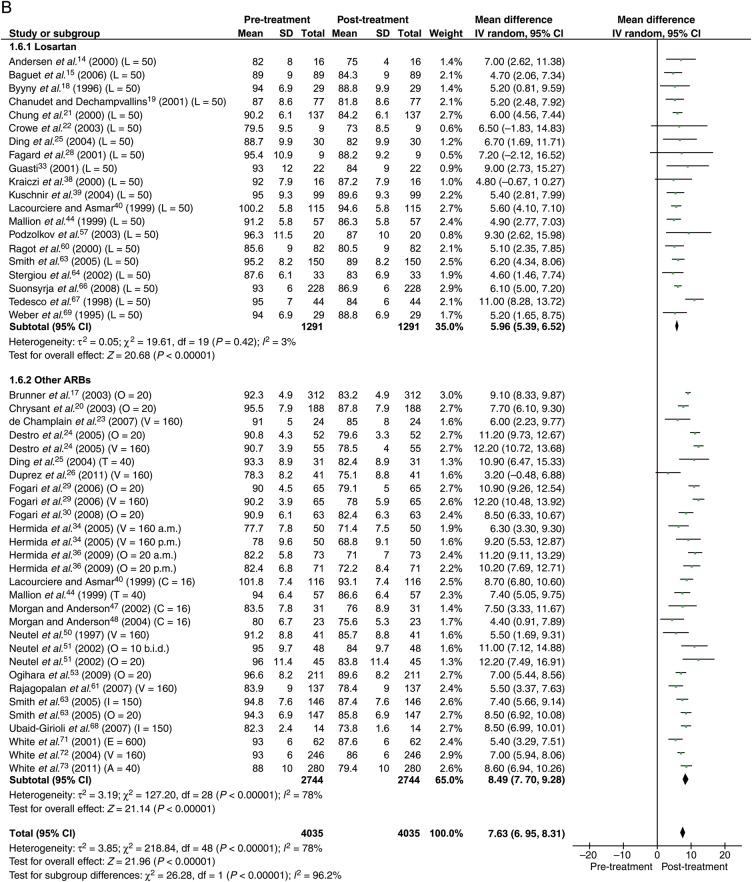
Data were available from 40 studies with the total of 4035 patients. With 50% max dose, the reduction in BP was 11.8 mmHg (95% CI: 10.8–12.7) systolic and 7.6 mmHg (95% CI: 7.0–8.3) diastolic (Figure 3).
Figure 3.
Forest plot showing reduction in ambulatory blood pressure for losartan and other angiotensin receptor blockers at 50% maximum dose. (A) Systolic. (B) Diastolic. The number in brackets represent angiotensin receptor blocker dose in milligrams. ABP, ambulatory blood pressure; ARB, angiotensin receptor blocker; b.i.d., twice daily; a.m., morning; p.m., evening; A, azilsartan; C, candesartan; E, eprosartan; I, irbesartan; L, losartan; O, olmesartan; T, telmisartan; V, valsartan.
Maximum dose of angiotensin receptor blockers
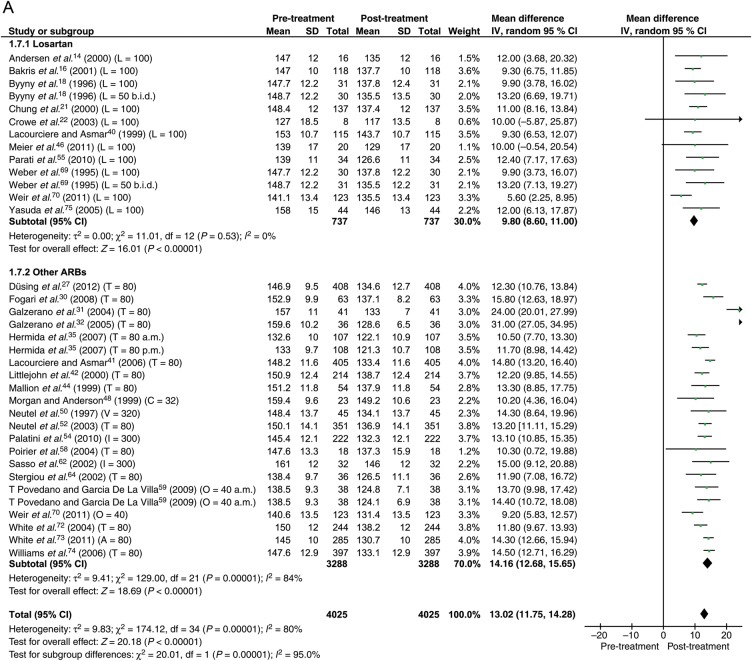
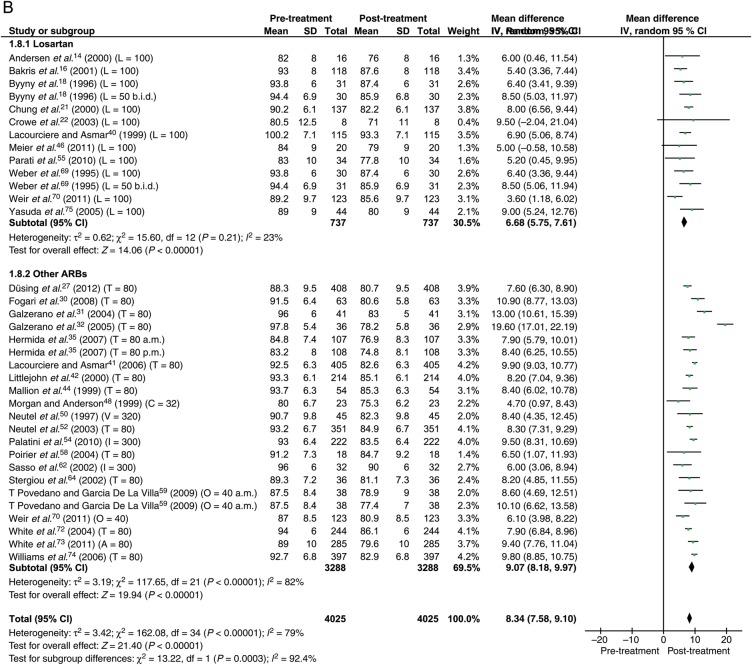
Data were available from 30 studies with the total of 4025 patients. With the maximum dose of ARBs, the reduction in BP was 13.0 mmHg (95% CI: 11.8–14.3) systolic and 8.3 mmHg (95% CI: 7.6–9.1) diastolic (Figure 4).
Figure 4.
Forest plot showing reduction in ambulatory blood pressure for losartan and other angiotensin receptor blockers at maximum dose. (A) Systolic. (B) Diastolic. Abbreviations as in Figure 3.
On comparing ARBs at 25% max dose with 50% max dose, there was a significant reduction of systolic ABP (P = 0.04), but not diastolic ABP (P = 0.08). On comparing ARBs at 50% max dose with the max dose, there was no significant difference in both systolic (P = 0.11) and diastolic (P = 0.18) ABP reduction. There was a significant reduction in both systolic (P = 0.0008) and diastolic ABP (P = 0.004) when ARBs at 25% max dose were compared with the ARBs at the max dose, but the four-fold increase in dose resulted in a meagre 2.7 mmHg (mean) decrease in systolic pressure. Since this is an indirect comparison, the data should be interpreted with caution.
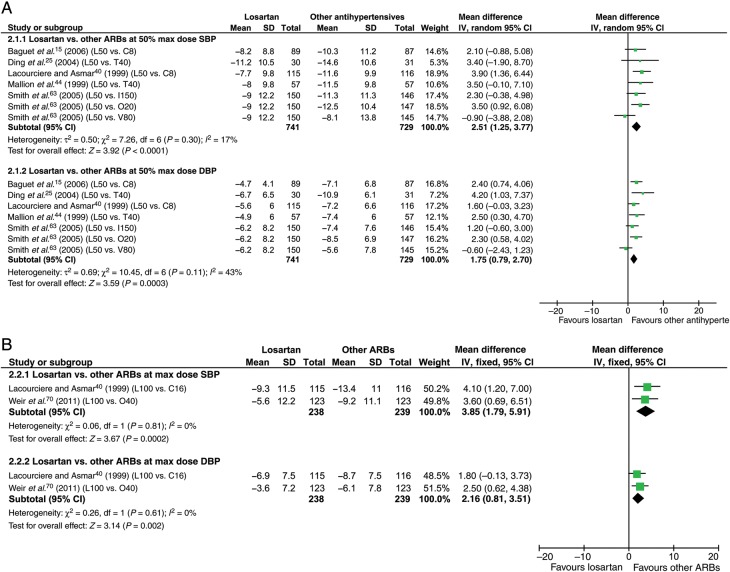
Comparison of losartan 50 and 100 mg with other angiotensin receptor blockers at 50% maximum dose and at maximum dose
Head-to-head comparison between losartan and other ARBs was available in six studies (Figure 5). Losartan in the dose of 50 mg lowered ABP less well than other ARBs at 50% max dose by 2.5 mmHg systolic (P < 0.0001) and 1.8 mmHg diastolic (P = 0.0003). Losartan in the dose of 100 mg lowered ABP less well than other ARBs at max dose by 3.9 mmHg systolic (P = 0.0002) and 2.2 mmHg diastolic (P = 0.002) (Figure 5).
Figure 5.
Forest plot showing 24 h ambulatory blood pressure reduction by losartan compared with other angiotensin receptor blockers. (A) Losartan 50 mg vs. other angiotensin receptor blockers at 50% maximum dose. (B) Losartan 100 mg vs. other angiotensin receptor blockers at max dose. DBP, diastolic blood pressure; SBP, systolic blood pressure; other abbreviations as in Figure 3.
Significant heterogeneity was found to be present in most of the analyses and hence random variance model was used. There was no evidence of publication bias for any of the analyses. Sensitivity analysis performed to evaluate the role of baseline blood pressure on BP reduction showed no significant difference between the two subgroups (above vs. below mean BP) (Table 3). Similarly sensitivity analyses for various subgroups based on the risk of bias, number of patients, and study duration did not make any noticeable difference to any of the outcomes (data not shown).
Table 3.
Sensitivity analysis based on baseline blood pressure
| Baseline mean BP (±SD) | Number of trials | Reduction in BP (95% CI) | Interaction P-value | ||
|---|---|---|---|---|---|
| 25% max | |||||
| Systolic | 147.3 ± 4.7 | <147.3 | 6 | 10.03 (8.56–11.50) | 0.63 |
| ≥147.3 | 7 | 10.57 (8.94–12.20) | |||
| Diastolic | 93.2 ± 4.1 | <93.2 | 6 | 7.44 (6.77–8.12) | 0.09 |
| ≥93.2 | 7 | 6.27 (5.18–7.37) | |||
| 50% max | |||||
| Systolic | 146.6 ± 7.3 | <146.6 | 21 | 11.79 (10.55–13.04) | 0.99 |
| ≥146.6 | 28 | 11.78 (10.45–13.10) | |||
| Diastolic | 90.1 ± 5.9 | <90.1 | 19 | 7.22 (6.02–8.42) | 0.56 |
| ≥90.1 | 30 | 7.66 (6.92–8.59) | |||
| Max dose | |||||
| Systolic | 146.7 ± 7.8 | <146.7 | 12 | 12.66 (11.62–13.70) | 0.53 |
| ≥146.7 | 23 | 13.32 (11.53–15.11) | |||
| Diastolic | 90.3 ± 4.9 | <90.3 | 16 | 7.90 (7.29–8.52) | 0.20 |
| ≥90.3 | 19 | 8.71 (7.61–9.80) | |||
Interaction P-value comparing reduction in BP above and below baseline mean BP.
BP, blood pressure; CI, confidence interval; max, maximum; SD, standard deviation
Discussion
In the present analysis of the antihypertensive efficacy of various ARBs with 24 h ABP monitoring, we observed a shallow dose–response curve. Doubling the dose is a common clinical practice when proper blood pressure levels are not reached. In our analysis, doubling the dose merely increased the antihypertensive efficacy by <2 mmHg systolic or diastolic. Losartan had a similarly shallow dose–response curve and, in head-to-head comparisons with other ARBs, was significantly less efficacious at all doses.
The control of blood pressure in the USA remains far from adequate as was observed by the most recent NHANES data.76 Thus, it becomes increasingly important to better control blood pressure with currently available drugs. Monotherapy remains the standard initial treatment for reducing blood pressure in many hypertensive patients. However, if specific blood pressure targets are not reached, most physicians will resort to uptitrating the drug to its max dose before switching to combination therapy. Indeed the American Joint National Committee VII11 advocates uptitration as a primary approach, and combination therapy may be used initially only if a patient's blood pressure is distinctly above the therapeutic goal. British hypertension guidelines77 of 2011 recommend starting monotherapy with either calcium channel blockers or ACE-inhibitors and then adding another antihypertensive agent if blood pressure is not under control. Our data make it clear that uptitration of monotherapy has little benefits for the antihypertensive regimen. Although ARBs may have a particularly shallow dose–response curve, the meta-analysis by Wald et al.78 showed that the response was not much better among other antihypertensive drug classes with the exception of the calcium channel blockers. Wald et al. in this meta-analysis of more than 11 000 patients from 42 trials concluded that combining drugs from two different classes was approximately five times more effective in lowering blood pressure than doubling the dose. In fact, the most recent European Society of Cardiology guidelines on cardiovascular disease prevention79 of 2012 recommend addition of drug from another class rather than uptitration for greater BP control. The guidelines also recommend treatment initiation with combination therapy in patients at high risk in whom early BP control is required.79 In a meta-analysis of 354 trials,80 reduction in blood pressure was only 20% lower with half standard dose compared with standard dose and was consistent among all antihypertensive agents. However, the dose-related adverse events were significantly lower with half standard dose compared with standard dose with thiazides, calcium channel blockers, and beta-blockers, but not with ACE-inhibitors and ARBs.80 In the same meta-analysis, they showed that the reductions in BP were additive with low-dose combination therapy, but the adverse effects were less than additive compared with uptitration.80 Several studies have shown that fixed combinations improve efficacy and adherence without increasing the overall adverse effects.81 In a study comparing combination of valsartan and hydrochlorothiazide (HCTZ) with individual monotherapy, reduction in SBP/DBP was 16.7/8.6 mmHg with combination compared with 14.2/7.9 mmHg with valsartan alone and 9.0/3.9 mmHg with HCTZ alone.26 Similarly, in a study comparing combination of olmesartan and azelnidipine with individual monotherapy, reduction in SBP/DBP was 22.1/13.5 mmHg with combination compared with 12.1/6.9 mmHg with olmesartan and 12.0/6.9 mmHg with azelnidipine.53 Thus, antihypertensive combination therapy may be considered over uptitration of a single agent for better hypertension management. Angiotensin receptor blockers are available in fixed combinations with thiazide diuretics (HCTZ and chlorthalidone) as well as with calcium channel blockers (amlodipine).
Our analysis provides good evidence that antihypertensive efficacy of losartan is weaker compared with other ARBs and increasing the dosage from 50 to 100 mg contributes less to further BP reduction. The antihypertensive efficacy of losartan has been under fire ever since it was marketed.82 Although all ARBs act by blocking angiotensin II receptor blocker, pharmacokinetic differences exist and may be the reason for the difference in antihypertensive efficacy. In a group of normotensive subjects comparing losartan with irbesartan and valsartan, losartan had the weakest angiotensin II antagonist effect; whereas irbesartan showed the slowest decay and longest duration of antagonist effects.83,84 At 4 h, losartan blocked 43% of angiotensin II-induced systolic BP increase, compared with 51% with valsartan and 88% with irbesartan.84 The results were similar when angiotensin II receptor blockade was assessed by the reactive rise in plasma angiotensin II levels and with an in vitro receptor assay.84 In several head-to-head comparisons with other ARBs and meta-analyses, losartan lowered the blood pressure less well than other ARBs; however, for office blood pressure, this may be of questionable significance.1 Its dose–response curve was so shallow that it was initially marketed in one dose only, and instead of uptitration from 50 to 100 mg, add-on therapy with HCTZ was advised.
Limitations
As with other meta-analyses, given the lack of data in each trial, we did not adjust our analysis for adherence to therapy. Also, the results are subject to limitations inherent to any meta-analysis based on pooling of data from different trials with different duration and different patient groups. We tried to minimize the effect of other antihypertensive drugs by excluding the studies that had second- or third-line agents added to control high BP. We also excluded studies that uptitrated the dose of ARB, since this study aimed at measuring 24 h BP at specifically 25% max, 50% max, and at the max dose. Blood pressure response to any drug depends on baseline blood pressure. However, we included only a rather homogeneous patient population with mild to moderate hypertension. Sensitivity analysis comparing studies with above baseline BP with those below baseline did not show a significant difference. Adequate data were not available to perform the head-to-head comparison between different ARBs except losartan.
Conclusion
As evaluated by 24 h ABP, uptitration of ARBs marginally enhances their antihypertensive efficacy. Antihypertensive efficacy of losartan at starting dose and at max dose is consistently inferior to other ARBs.
Authors' contributions
H.M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: H.M., S.B., and F.H.M.; acquisition of data: H.M. and J.R.; analysis and interpretation of data: H.M., S.B., and F.H.M.; drafting of the manuscript: H.M., S.B., and F.H.M.; critical revision of the manuscript for important intellectual content: H.M., S.B., E.A., J.R., A.S., and F.H.M.; statistical analysis: H.M. and A.S.; study supervision: F.H.M. and H.M.
Conflict of interest: F.H.—ad hoc consultant/speaker for the following organizations: Novartis, Daiichi Sankyo, Pfizer, Takeda, Abbott, Medtronic, Servier, and Bayer. S.B.—advisory board: Daiichi Sankyo, Boehringer Ingelheim, Pfizer. H.M., E.A., J.R., A.S.: none.
References
- 1.Heran B, Wong M, Heran I, Wright J. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003822. doi: 10.1002/14651858.CD003822.pub2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JC, White CM. Pharmacologic, pharmacokinetic, and therapeutic differences among angiotensin II receptor antagonists. Pharmacotherapy. 2000;20:130–139. doi: 10.1592/phco.20.3.130.34788. [DOI] [PubMed] [Google Scholar]
- 3.Conlin PR, Spence JD, Williams B, Ribeiro AB, Saito I, Benedict C, Bunt AM. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens. 2000;13:418–426. doi: 10.1016/S0895-7061(99)00237-X. [DOI] [PubMed] [Google Scholar]
- 4.Xi GL, Cheng JW, Lu GC. Meta-analysis of randomized controlled trials comparing telmisartan with losartan in the treatment of patients with hypertension. Am J Hypertens. 2008;21:546–552. doi: 10.1038/ajh.2008.30. [DOI] [PubMed] [Google Scholar]
- 5.Smith DH, Cramer MJ, Neutel JM, Hettiarachchi R, Koval S. Comparison of telmisartan versus losartan: meta-analysis of titration-to-response studies. Blood Press Monit. 2003;8:111–117. doi: 10.1097/00126097-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O'Brien E. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 7.Higgins J, Green S. Assessing Risk of Bias in Included Studies. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2008. p. 672. Version 5.0.0. [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-Q. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57:601–606. doi: 10.1046/j.1523-1755.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 15.Baguet JP, Nisse-Durgeat S, Mouret S, Asmar R, Mallion JM. A placebo-controlled comparison of the efficacy and tolerability of candesartan cilexetil, 8 mg, and losartan, 50 mg, as monotherapy in patients with essential hypertension, using 36-h ambulatory blood pressure monitoring. Int J Clin Pract. 2006;60:391–398. doi: 10.1111/j.1368-5031.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 16.Bakris G, Sica D, Ram V, Fagan T, Vaitkus PT, Anders RJ. A comparative trial of controlled-onset, extended-release verapamil, enalapril, and losartan on blood pressure and heart rate changes. Am J Hypertens. 2002;15:53–57. doi: 10.1016/S0895-7061(01)02254-3. [DOI] [PubMed] [Google Scholar]
- 17.Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Investig. 2003;23:419–430. doi: 10.2165/00044011-200323070-00001. [DOI] [PubMed] [Google Scholar]
- 18.Byyny RL. Antihypertensive efficacy of the angiotensin II AT1-receptor antagonist losartan: results of a randomized, double-blind, placebo-controlled, parallel-group trial using 24-hour blood pressure monitoring. Ambulatory Blood Pressure Monitoring Study Group. Blood Press Suppl. 1996;2:71–77. [PubMed] [Google Scholar]
- 19.Chanudet X, De Champvallins M. Antihypertensive efficacy and tolerability of low-dose perindopril/indapamide combination compared with losartan in the treatment of essential hypertension. Int J Clin Pract. 2001;55:233–239. [PubMed] [Google Scholar]
- 20.Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild-to-moderate hypertension. J Hum Hypertens. 2003;17:425–432. doi: 10.1038/sj.jhh.1001577. [DOI] [PubMed] [Google Scholar]
- 21.Chung O, Hinder M, Sharma AM, Bonner G, Middeke M, Platon J, Unger T. Comparison of the efficacy and safety of losartan (50–100 mg) with the T-type calcium channel blocker mibefradil (50–100 mg) in mild to moderate hypertension. Fundam Clin Pharmacol. 2000;14:31–41. doi: 10.1111/j.1472-8206.2000.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 22.Crowe AV, Howse M, Vinjamuri S, Kemp GJ, Williams PS. The antiproteinuric effect of losartan is systemic blood pressure dependent. Nephrol Dial Transplant. 2003;18:2160–2164. doi: 10.1093/ndt/gfg315. [DOI] [PubMed] [Google Scholar]
- 23.de Champlain J, Karas M, Assouline L, Nadeau R, LeBlanc AR, Dube B, Larochelle P. Effects of valsartan or amlodipine alone or in combination on plasma catecholamine levels at rest and during standing in hypertensive patients. J Clin Hypertens (Greenwich) 2007;9:168–178. doi: 10.1111/j.1524-6175.2007.05938.x. [DOI] [PubMed] [Google Scholar]
- 24.Destro M, Scabrosetti R, Vanasia A, Mugellini A. Comparative efficacy of valsartan and olmesartan in mild-to-moderate hypertension: results of 24-hour ambulatory blood pressure monitoring. Adv Ther. 2005;22:32–43. doi: 10.1007/BF02850182. [DOI] [PubMed] [Google Scholar]
- 25.Ding PY, Chu KM, Chiang HT, Shu KH. A double-blind ambulatory blood pressure monitoring study of the efficacy and tolerability of once-daily telmisartan 40 mg in comparison with losartan 50 mg in the treatment of mild-to-moderate hypertension in Taiwanese patients. Int J Clin Pract Suppl. 2004;125:16–22. doi: 10.1111/j.1742-1241.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 26.Duprez DA, Weintraub HS, Cushman WC, Purkayastha D, Zappe D, Samuel R, Izzo JL., Jr Effect of valsartan, hydrochlorothiazide, and their combination on 24-h ambulatory blood pressure response in elderly patients with systolic hypertension: a ValVET substudy. Blood Press Monit. 2011;16:186–196. doi: 10.1097/MBP.0b013e32834944e9. [DOI] [PubMed] [Google Scholar]
- 27.Düsing R, Brunel P, Baek I, Baschiera F. Sustained decrease in blood pressure following missed doses of aliskiren or telmisartan: the ASSERTIVE double-blind, randomized study. J Hypertens. 2012;30:1029–1040. doi: 10.1097/HJH.0b013e328351c263. [DOI] [PubMed] [Google Scholar]
- 28.Fagard R, Lijnen P, Pardaens K, Thijs L, Vinck W. A randomised, placebo-controlled, double-blind, crossover study of losartan and enalapril in patients with essential hypertension. J Hum Hypertens. 2001;15:161–167. doi: 10.1038/sj.jhh.1001159. [DOI] [PubMed] [Google Scholar]
- 29.Fogari R, Zoppi A, Mugellini A, Preti P, Destro M, Rinaldi A, Derosa G. Hydrochlorothiazide added to valsartan is more effective than when added to olmesartan in reducing blood pressure in moderately hypertensive patients inadequately controlled by monotherapy. Adv Ther. 2006;23:680–695. doi: 10.1007/BF02850307. [DOI] [PubMed] [Google Scholar]
- 30.Fogari R, Zoppi A, Mugellini A, Preti P, Destro M, Rinaldi A, Derosa G. Effectiveness of hydrochlorothiazide in combination with telmisartan and olmesartan in adults with moderate hypertension not controlled with monotherapy: a prospective, randomized, open-label, blinded end point (PROBE), parallel-arm study. Curr Ther Res Clin Exp. 2008;69:1–15. doi: 10.1016/j.curtheres.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galzerano D, Tammaro P, Cerciello A, Breglio R, Mallardo M, Lama D, Tuccillo B, Capogrosso P. Freehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre study. J Hum Hypertens. 2004;18:53–59. doi: 10.1038/sj.jhh.1001637. [DOI] [PubMed] [Google Scholar]
- 32.Galzerano D, Tammaro P, del Viscovo L, Lama D, Galzerano A, Breglio R, Tuccillo B, Paolisso G, Capogrosso P. Three-dimensional echocardiographic and magnetic resonance assessment of the effect of telmisartan compared with carvedilol on left ventricular mass a multicenter, randomized, longitudinal study. Am J Hypertens. 2005;18:1563–1569. doi: 10.1016/j.amjhyper.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Guasti L, Zanotta D, Diolisi A, Garganico D, Simoni C, Gaudio G, Grandi AM, Venco A. Changes in pain perception during treatment with angiotensin converting enzyme-inhibitors and angiotensin II type 1 receptor blockade. J Hypertens. 2002;20:485–491. doi: 10.1097/00004872-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Hermida RC, Calvo C, Ayala DE, Mojon A, Rodriguez M, Chayan L, Lopez JE, Fontao MJ, Soler R, Fernandez JR. Administration time-dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int. 2005;22:755–776. doi: 10.1080/07420520500180488. [DOI] [PubMed] [Google Scholar]
- 35.Hermida RC, Ayala DE, Fernandez JR, Calvo C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50:715–722. doi: 10.1161/HYPERTENSIONAHA.107.094235. [DOI] [PubMed] [Google Scholar]
- 36.Hermida RC, Ayala DE, Chayan L, Mojon A, Fernandez JR. Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26:61–79. doi: 10.1080/07420520802548135. [DOI] [PubMed] [Google Scholar]
- 37.Kawano Y, Sato Y, Yoshinaga K. A randomized trial of the effect of an angiotensin II receptor blocker SR47436 (irbesartan) on 24-hour blood pressure in patients with essential hypertension. Hypertens Res. 2008;31:1753–1763. doi: 10.1291/hypres.31.1753. [DOI] [PubMed] [Google Scholar]
- 38.Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1423–1428. doi: 10.1164/ajrccm.161.5.9909024. [DOI] [PubMed] [Google Scholar]
- 39.Kuschnir E, Bendersky M, Resk J, Panart MS, Guzman L, Plotquin Y, Grassi G, Mancia G, Wagener G. Effects of the combination of low-dose nifedipine GITS 20 mg and losartan 50 mg in patients with mild to moderate hypertension. J Cardiovasc Pharmacol. 2004;43:300–305. doi: 10.1097/00005344-200402000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Lacourciere Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study. Candesartan/Losartan study investigators. Am J Hypertens. 1999;12:1181–1187. doi: 10.1016/S0895-7061(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 41.Lacourciere Y, Neutel JM, Davidai G, Koval S. A multicenter, 14-week study of telmisartan and ramipril in patients with mild-to-moderate hypertension using ambulatory blood pressure monitoring. Am J Hypertens. 2006;19:104–112. doi: 10.1016/j.amjhyper.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Littlejohn T, Mroczek W, Marbury T, VanderMaelen CP, Dubiel RF. A prospective, randomized, open-label trial comparing telmisartan 80 mg with valsartan 80 mg in patients with mild to moderate hypertension using ambulatory blood pressure monitoring. Can J Cardiol. 2000;16:1123–1132. [PubMed] [Google Scholar]
- 43.London G, Schmieder R, Calvo C, Asmar R. Indapamide SR versus candesartan and amlodipine in hypertension: the X-CELLENT Study. Am J Hypertens. 2006;19:113–121. doi: 10.1016/j.amjhyper.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Mallion J, Siche J, Lacourciere Y. ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertension. J Hum Hypertens. 1999;13:657–664. doi: 10.1038/sj.jhh.1000925. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto S, Shimodozono M, Miyata R, Kawahira K. Effect of the angiotensin II type 1 receptor antagonist olmesartan on cerebral hemodynamics and rehabilitation outcomes in hypertensive post-stroke patients. Brain Inj. 2009;23:1065–1072. doi: 10.3109/02699050903379404. [DOI] [PubMed] [Google Scholar]
- 46.Meier P, Maillard MP, Meier JR, Tremblay S, Gauthier T, Burnier M. Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. J Hypertens. 2011;29:1228–1235. doi: 10.1097/HJH.0b013e328346d5dc. [DOI] [PubMed] [Google Scholar]
- 47.Morgan T, Anderson A. A comparison of candesartan, felodipine, and their combination in the treatment of elderly patients with systolic hypertension. Am J Hypertens. 2002;15:544–549. doi: 10.1016/S0895-7061(02)02279-3. [DOI] [PubMed] [Google Scholar]
- 48.Morgan T, Anderson A, Bertram D, MacInnis RJ. Effect of candesartan and lisinopril alone and in combination on blood pressure and microalbuminuria. J Renin Angiotensin Aldosterone Syst. 2004;5:64–71. doi: 10.3317/jraas.2004.012. [DOI] [PubMed] [Google Scholar]
- 49.Munakata M, Nagasaki A, Nunokawa T, Sakuma T, Kato H, Yoshinaga K, Toyota T. Effects of valsartan and nifedipine coat-core on systemic arterial stiffness in hypertensive patients. Am J Hypertens. 2004;17:1050–1055. doi: 10.1016/j.amjhyper.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 50.Neutel J, Weber M, Pool J, Smith D, Fitzsimmons S, Chiang YT, Gatlin M. Valsartan, a new angiotensin II antagonist: antihypertensive effects over 24 hours. Clin Ther. 1997;19:447–458. doi: 10.1016/S0149-2918(97)80129-4. Discussion 367–368. [DOI] [PubMed] [Google Scholar]
- 51.Neutel JM, Elliott WJ, Izzo JL, Chen CL, Masonson HN. Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements. J Clin Hypertens (Greenwich) 2002;4:325–331. doi: 10.1111/j.1524-6175.2002.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neutel JM, Kolloch RE, Plouin PF, Meinicke TW, Schumacher H. Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension—a randomised ABPM study. J Hum Hypertens. 2003;17:569–575. doi: 10.1038/sj.jhh.1001592. [DOI] [PubMed] [Google Scholar]
- 53.Ogihara T, Saruta T, Shimada K, Kuramoto K. A randomized, double-blind, four-arm parallel-group study of the efficacy and safety of azelnidipine and olmesartan medoxomil combination therapy compared with each monotherapy in Japanese patients with essential hypertension: the REZALT study. Hypertens Res. 2009;32:1148–1154. doi: 10.1038/hr.2009.163. [DOI] [PubMed] [Google Scholar]
- 54.Palatini P, Jung W, Shlyakhto E, Botha J, Bush C, Keefe DL. Maintenance of blood-pressure-lowering effect following a missed dose of aliskiren, irbesartan or ramipril: results of a randomized, double-blind study. J Hum Hypertens. 2010;24:93–103. doi: 10.1038/jhh.2009.38. [DOI] [PubMed] [Google Scholar]
- 55.Parati G, Giglio A, Lonati L, Destro M, Ricci AR, Cagnoni F, Pini C, Venco A, Maresca AM, Monza M, Grandi AM, Omboni S. Effectiveness of barnidipine 10 or 20 mg plus losartan 50-mg combination versus losartan 100-mg monotherapy in patients with essential hypertension not controlled by losartan 50-mg monotherapy: a 12-week, multicenter, randomized, open-label, parallel-group study. Clin Ther. 2010;32:1270–1284. doi: 10.1016/j.clinthera.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Pechere-Bertschi A, Nussberger J, Decosterd L, Armagnac C, Sissmann J, Bouroudian M, Brunner HR, Burnier M. Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens. 1998;16:385–393. doi: 10.1097/00004872-199816030-00016. [DOI] [PubMed] [Google Scholar]
- 57.Podzolkov VI, Bulatov VA, Son EA, Os I. Central and peripheral hemodynamic effects of losartan and in combination with hydrochlorothiazide in mild to moderate essential hypertension. Blood Press. 2003;12:239–245. doi: 10.1080/08037050310015467. [DOI] [PubMed] [Google Scholar]
- 58.Poirier L, de Champlain J, Larochelle P, Lamarre-Cliche M, Lacourciere Y. A comparison of the efficacy and duration of action of telmisartan, amlodipine and ramipril in patients with confirmed ambulatory hypertension. Blood Press Monit. 2004;9:231–236. doi: 10.1097/00126097-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Povedano ST, Garcia De La Villa G. 24-hour and nighttime blood pressure monitoring in type 2 diabetic hypertensive patients. J Clin Hypertens (Greenwich) 2009;11:426–431. doi: 10.1111/j.1751-7176.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ragot S, Genes N, Vaur L, Herpin D. Comparison of three blood pressure measurement methods for the evaluation of two antihypertensive drugs: feasibility, agreement, and reproducibility of blood pressure response. Am J Hypertens. 2000;13:632–639. doi: 10.1016/S0895-7061(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 61.Rajagopalan S, Zannad F, Radauceanu A, Glazer R, Jia Y, Prescott MF, Kariisa M, Pitt B. Effects of valsartan alone versus valsartan/simvastatin combination on ambulatory blood pressure, C-reactive protein, lipoproteins, and monocyte chemoattractant protein-1 in patients with hyperlipidemia and hypertension. Am J Cardiol. 2007;100:222–226. doi: 10.1016/j.amjcard.2007.02.085. [DOI] [PubMed] [Google Scholar]
- 62.Sasso FC, Carbonara O, Persico M, Iafusco D, Salvatore T, D'Ambrosio R, Torella R, Cozzolino D. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: a randomized double-blind placebo-controlled crossover study. Diabetes Care. 2002;25:1909–1913. doi: 10.2337/diacare.25.11.1909. [DOI] [PubMed] [Google Scholar]
- 63.Smith DH, Dubiel R, Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5:41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 64.Stergiou GS, Efstathiou SP, Skeva II, Baibas NM, Kalkana CB, Mountokalakis TD. Assessment of drug effects on blood pressure and pulse pressure using clinic, home and ambulatory measurements. J Hum Hypertens. 2002;16:729–735. doi: 10.1038/sj.jhh.1001477. [DOI] [PubMed] [Google Scholar]
- 65.Stergiou GS, Efstathiou SP, Roussias LG, Mountokalakis TD. Blood pressure- and pulse pressure-lowering effects, trough:peak ratio and smoothness index of telmisartan compared with lisinopril. J Cardiovasc Pharmacol. 2003;42:491–496. doi: 10.1097/00005344-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Suonsyrja T, Hannila-Handelberg T, Paavonen KJ, Miettinen HE, Donner K, Strandberg T, Tikkanen I, Tilvis R, Pentikainen PJ, Kontula K, Hiltunen TP. Laboratory tests as predictors of the antihypertensive effects of amlodipine, bisoprolol, hydrochlorothiazide and losartan in men: results from the randomized, double-blind, crossover GENRES Study. J Hypertens. 2008;26:1250–1256. doi: 10.1097/HJH.0b013e3282fcc37f. [DOI] [PubMed] [Google Scholar]
- 67.Tedesco MA, Ratti G, Aquino D, Limongelli G, di Salvo G, Mennella S, Galzerano D, Iarussi D, Iacono A. Effects of losartan on hypertension and left ventricular mass: a long-term study. J Hum Hypertens. 1998;12:505–510. doi: 10.1038/sj.jhh.1000685. [DOI] [PubMed] [Google Scholar]
- 68.Ubaid-Girioli S, Ferreira-Melo SE, Souza LA, Nogueira EA, Yugar-Toledo JC, Coca A, Moreno H., Jr Aldosterone escape with diuretic or angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker combination therapy in patients with mild to moderate hypertension. J Clin Hypertens (Greenwich) 2007;9:770–774. doi: 10.1111/j.1751-7176.2007.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber MA, Byyny RL, Pratt JH, Faison EP, Snavely DB, Goldberg AI, Nelson EB. Blood pressure effects of the angiotensin II receptor blocker, losartan. Arch Intern Med. 1995;155:405–411. doi: 10.1001/archinte.1995.00430040081010. [DOI] [PubMed] [Google Scholar]
- 70.Weir MR, Punzi HA, Flack JM, Stoakes KA, Chavanu KJ, Li W, Dubiel R. A randomized, double-blind, forced-titration study to compare olmesartan medoxomil versus losartan potassium in patients with stage 1 and 2 hypertension. Postgrad Med. 2011;123:80–87. doi: 10.3810/pgm.2011.01.2248. [DOI] [PubMed] [Google Scholar]
- 71.White WB, Anwar YA, Mansoor GA, Sica DA. Evaluation of the 24-hour blood pressure effects of eprosartan in patients with systemic hypertension. Am J Hypertens. 2001;14:1248–1255. doi: 10.1016/S0895-7061(01)02201-4. [DOI] [PubMed] [Google Scholar]
- 72.White WB, Lacourciere Y, Davidai G. Effects of the angiotensin II receptor blockers telmisartan versus valsartan on the circadian variation of blood pressure: impact on the early morning period. Am J Hypertens. 2004;17:347–353. doi: 10.1016/j.amjhyper.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 73.White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–420. doi: 10.1161/HYPERTENSIONAHA.110.163402. [DOI] [PubMed] [Google Scholar]
- 74.Williams B, Gosse P, Lowe L, Harper R. The prospective, randomized investigation of the safety and efficacy of telmisartan versus ramipril using ambulatory blood pressure monitoring (PRISMA I) J Hypertens. 2006;24:193–200. doi: 10.1097/01.hjh.0000194364.11516.ab. [DOI] [PubMed] [Google Scholar]
- 75.Yasuda G, Ando D, Hirawa N, Umemura S, Tochikubo O. Effects of losartan and amlodipine on urinary albumin excretion and ambulatory blood pressure in hypertensive type 2 diabetic patients with overt nephropathy. Diabetes Care. 2005;28:1862–1868. doi: 10.2337/diacare.28.8.1862. [DOI] [PubMed] [Google Scholar]
- 76.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 77.National Institute for Health and Clinical Excellence. Guidance. Hypertension: the clinical management of primary hypertension in adults: update of Clinical Guidelines 18 and 34 August 2011 http://www.nice.org.uk/nicemedia/live/13561/56007/56007.pdf. (12 December 2012)
- 78.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 79.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) . Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 80.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combi-nations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 82.Messerli FH. … and losartan was no better than placebo. J Hum Hypertens. 1999;13:649–650. doi: 10.1038/sj.jhh.1000926. [DOI] [PubMed] [Google Scholar]
- 83.Belz GG, Butzer R, Kober S, Mang C, Mutschler E. Time course and extent of angiotensin II antagonism after irbesartan, losartan, and valsartan in humans assessed by angiotensin II dose response and radioligand receptor assay. Clin Pharmacol Ther. 1999;66:367–373. doi: 10.1053/cp.1999.v66.a101162. [DOI] [PubMed] [Google Scholar]
- 84.Mazzolai L, Maillard M, Rossat J, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade in normotensive subjects: a direct comparison of three AT1 receptor antagonists. Hypertension. 1999;33:850–855. doi: 10.1161/01.HYP.33.3.850. [DOI] [PubMed] [Google Scholar]