Highlights
-
•
We present a case of PAAG mediated breast augmentation resulting in breestfeeding difficulty.
-
•
Overall management involved clinical examination, ultrasound, X-ray, MRI, microscopic evaluation, antibiotics and surgical removal of PAAG material.
-
•
PAAG has been associated with worse breast cancer outcomes.
Keywords: Polyacrylamide hydrogel, PAAG, Breast augmentation, Breastfeeding complications, Mammography, Modified radial surgery
Abstract
Introduction
Breast augmentation using polyacrylamide hydrogel (PAAG) has been routinely used in the past as a minimal invasive procedure. However, several patients undergoing this procedure have started to report complications. We report a case of breast augmentation using PAAG leading to a delayed infection and breastfeeding complication.
Clinical case
A 36-year-old Danish female who was treated with PAAG fifteen years earlier, presented with difficulty in breastfeeding and fistulation. Clinical evaluation revealed structural deformity of the right breast and a 5 × 5 mm skin defect. Mammography showed diffuse microcalcification density grade 4. Ultrasound and MRI displayed inhomogeneous gelatinous material in both breasts diffused into the pectoralis major muscle. Initial management involved aspiration of the material. The patient developed infection and was subjected to modified radical debridement removing the PAAG. The patient healed without any further complications.
Discussion
The prevalence of PAAG mediated breast augmentation related complications are increasing. The most prominent complication being late infections, breast hardening and subsequent breastfeeding difficulties. In this case, the difficulty in breastfeeding was induced by the PAAG within the breast tissue. The inhomogeneous gelatinous material was surgically removed leading to complete remission.
Conclusion
Long-term complications, among others breast feeding difficulty, in women treated with PAAG are increasing and need appropriate management strategy. PAAG mediated breast augmentation may cause irreversible damage to the breast in healthy women necessitating complex debridement.
1. Introduction
Polyacrylamide hydrogel (PAAG) has been widely used for breast augmentation among women especially in Iran, China and India [1], [2]. The technique was developed in 1987 and had broad acceptance up until the millennium. The technique of PAAG breast augmention is described as an aseptic injection of PAAG into the subcutaneous fat, meanwhile sparring the breast tissue, in af fan-like manner [3]. The technique was approved by the China Food and Drug Administration (CFDA) for its broader clinical usage in 1997, but the approval has since been withdrawn [4]. The exact number of patients treated with PAAG in breast augmentation are unknown, but it is estimated that approximately 300,000 women have undergone this procedure [2]. The number of women complaining about the late adverse effects associated with PAAG treatment continues to increase and breastfeeding complications have been reported among several women [5]. Besides breastfeeding difficulties, breast autoinflation, displacement of the injected material, formation of lumps and hematoma have been reported [6]. Patients facing the complications are often left with the choice of debridement surgery [1]. Development of appropriate management strategies to address PAAG associated complications subsequent to augmentation mammoplasty are ongoing [7].
We report a 36-years-old Danish woman with breastfeeding complication due to breast augmentation using PAAG 15 years earlier in Iran. This case report is in line with the SCARE criteria [8].
2. Presentation of case
A 36-year-old Danish female who received breast augmentation with PAAG-injection in Iran 15 years earlier, presented with a 5 × 5 mm skin defect in the upper medial quadrant of the right breast 2 months after giving birth (Fig. 1A). She was unable to breastfeed. Thick, yellow gelatinous material with small transparent particles and necrotic fluid emerged from the defect. Initial examination of the breasts revealed resilience and deformation of the right breast. The deformity was observable along both radial lines and vertical segments of the breast. Mammography of the right breast (Fig. 2C) revealed diffuse microcalcification density grade 4. Bilateral ultrasound showed inhomogeneous gelatinous material, thus ruling out any cyst or cancerous lump (Fig. 2A + B). Magnetic Resonance Imaging (MRI) revealed that the injected PAAG material had diffused into the pectoralis major muscle bilaterally (Fig. 2D). Initial management strategy involved extraction of the material from the right breast. A total of 200 ml material was aspirated. Microscopic evaluations showed no findings of any leukocytes or bacteria (Fig. 3). Two weeks after initial treatment, the patient developed infection in the right breast and was treated with oral moxifloxacin and azithromycin. Following successful treatment of the infection, the woman was subjected to debridement surgery removing PAAG from the subcutaneous tissue, breast tissue and pectoralis major muscle bilaterally. The patient had no further complications at 3-months follow-up.
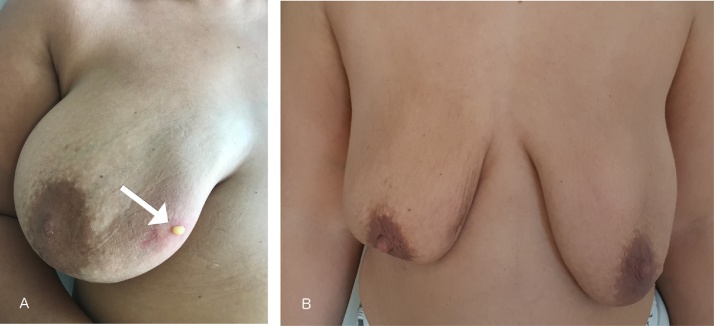
Fig. 1.
A: Clinical examination showing a 5 × 5 mm skin defect in the right breast with PAAG fistulation Fig. 1B: Follow up after 3 months.
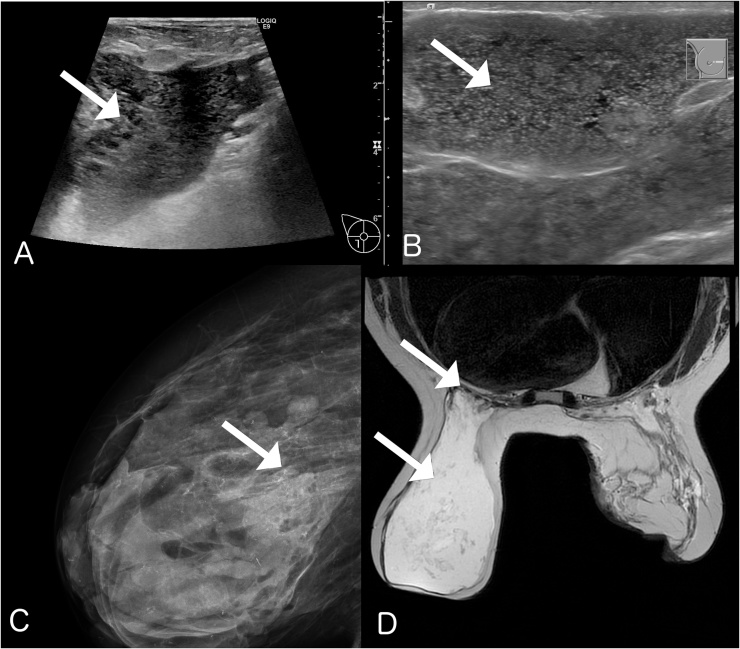
Fig. 2.
A: Ultrasound of right breast showing diffuse PAAG in the subcutaneous tissue and breast tissue (Arrow). B: Ultrasound of the left breast showing homogeneous distribution and intact skin (Arrow). C: X-ray of the right breast showing microcalcification in the remaining mammary tissue (Arrow). D: T2-weighted MRI-scan showing distribution of PAAG in both breasts and diffusion through m. pectoralis bilaterally (Arrow).
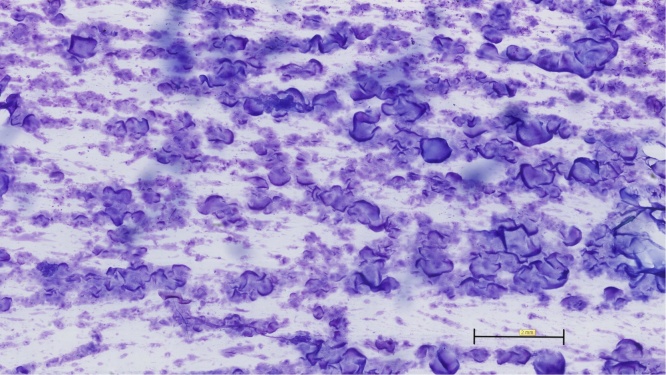
Fig. 3.
HE-stain showing diffuse PAAG in the breast tissue with no bacteria.
3. Discussion
This case report describes the management of complications in a Danish female patient who had PAAG mediated breast augmentation in adultescence. The overall management involved clinical examination, ultrasound, X-ray, MRI, extraction of material for microscopic evaluation, followed by treatment with antibiotics and finally surgical removal of PAAG material from both breasts.
This case highlights an unfortunate retrospect of a treatment method labelled as safe and “minimally invasive” for breast augmentation [9]. The use of PAAG for breast augmentation was practiced without appropriate empirical knowledge of consequences or human clinical trials [2]. Injected PAAG is not supposed to migrate or alter location, unfortunately, reports of displacement of injected material into e.g. extra pleural spaces have started to emerge [10], [11], [12]. Migration of PAAG was also seen in our patient with the PAAG being in the pectoralis muscle bilaterally.
The patient received PAAG mediated breast augmentation 15-years earlier and never had any complications prior to childbirth. Presumably, the majority of women receiving PAAG for breast augmentation were young, which is why long-term complications start to emerge among others in the form breastfeeding complications as described in this case [1], [13]. Other reported complications to PAAG breast augmentation are pain, breast hardening, breast deformity, lumps, gel migration and leakage problems [14].
PAAG mediated tissue expansion and particularly breast augmentation came into practice without any reliable scientific evidence [2]. The issue of safety was never evaluated in humans, and no clinical trials were conducted to assess the usage of this material for breast augmentation. The technique got its popularity mainly in China, Iran and India. However, women receiving PAAG mediated treatment is seen across the world [5]. It has been estimated that almost 300,000 women have undergone PAAG injection as augmentation mammoplasty [2]. One can expect an increase in reported complications such as the described case, due to the unknown long-term complications and consequences of PAAG [15], [16].
PAAG is mainly composed of 2.5% polymerized (cross-linked) polyacrylamide in non-pyrogenic water [17]. The high water content of PAAG allows the material to assimilate into the connective tissues of the mammary glands. Due to high amounts of water, T2-weighted MRI provides the best resolution to assess the location of the PAAG-material. Uncomplicated PAAG in the breast is observed by MRI in the form of a single blob, whereas migration is in the form of a diffused pattern as seen in our patient (Fig. 2D) [18]. It is presumed that PAAG is mainly resistant to migration. However, the current case and several other studies suggest that polymerized material can transmigrate subcutaneously into different layers of the mammary glands as observed in this case [13].
The migration of PAAG into the breast tissue can make breast imaging difficult and thus missing a breast malignancy. There is no information on whether breast cancer diagnosis has been delayed in patients with PAAG injections [14]. However, if gel remnants are still present, this may challenge future breast imaging. Recently published case reports describes cases of breast cancer in women after PAAG injection and possibly an association [19], [20].
4. Conclusions
Difficulty in breastfeeding among women previously treated with PAAG for breast augmentation is an increasing issue. In the past, thousands of women across the world underwent breast augmentation using PAAG. Due to lack of human studies and knowledge about consequences and long-term outcomes, complications have started to emerge. This case report highlights the issue of breast complications among women who have been treated with PAAG in the past. Management of PAAG associated breastfeeding complications through surgical procedure as described in this case, provides empirical information to the potential treatment of such complications in the future.
Conflicts of interest
No conflict of interest.
Funding
No funding.
Consent
The authors solemnly that we have the patient consent for the publication of this case report.
Author contributions
Rami Mossad Ibrahim: Drafted the first version of the manuscript and critical revision of the manuscript for important intellectual content.
Elisabeth Lauritzen and Caspar Weel Krammer: Critical revision of the manuscript for important intellectual content.
Guarantor
Rami Mossad Ibrahim.
Acknowledgement
The authors thank Dr. Mai-Liss from Department of Pathology Rigshospitalet for assisting in preparing pathology photos.
References
- 1.Jin R., Luo X., Wang X., Ma J., Liu F., Yang Q., Yang J., Wang X. Complications and treatment strategy after Breast augmentation by polyacrylamide hydrogel injection: summary of 10-year clinical experience. Aesthet. Plast. Surg. 2018:402–409. doi: 10.1007/s00266-017-1006-9. https://www.ncbi.nlm.nih.gov/pubmed/29124374 [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Li S., Wang L., Zhang S., Jiang Y., Chen J., Luo D. Polyacrylamide hydrogel injection for breast augmentation: another injectable failure. Med. Sci. Monit. 2012:CR399–CR408. doi: 10.12659/MSM.882910. https://www.ncbi.nlm.nih.gov/pubmed/22648256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Jean Carruthers and Alastair Carruthers; 2018. Soft Tissue Augmentation, Book. (page 73) [Google Scholar]
- 4.Cheng N.X., Xu S.L., Deng H., Ding X.B., Zhang X.M., Wu D.H., Zhong H., Sun Z.H. Migration of implants: a problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthet. Plast. Surg. 2006:215–225. doi: 10.1007/s00266-005-0081-5. https://www.ncbi.nlm.nih.gov/pubmed/16547628 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z.X., Luo D.L., Dai X., Yu P., Tao L., Li S.R. Polyacrylamide hydrogel injection for augmentation mammaplasty: loss of ability for breastfeeding. Ann. Plast. Surg. 2012:123–128. doi: 10.1097/SAP.0b013e318225931c. https://www.ncbi.nlm.nih.gov/pubmed/21785335 [DOI] [PubMed] [Google Scholar]
- 6.Kang G.C., Ong Y.S. Large unilateral breast autoinflation after breastfeeding linked to polyacrylamide hydrogel injection augmentation mammaplasty. Aesthet. Plast. Surg. 2011:122–124. doi: 10.1007/s00266-010-9550-6. https://www.ncbi.nlm.nih.gov/pubmed/20652566 [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Sha L., Huang S.P., Li S.R., Wang Z.X. Treatment for displacement of PAAG mixture after injection augmentation mammoplasty. Int. J. Clin. Exp. Med. 2015:3360–3370. https://www.ncbi.nlm.nih.gov/pubmed/26064226 [PMC free article] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S. Orgill DP, for the SCARE group. the SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. https://www.sciencedirect.com/science/article/pii/S0717201X16300288 [DOI] [PubMed] [Google Scholar]
- 9.Swami V., Jones J., Einon D., Furnham A. Men’s preferences for women’s profile waist-to-hip ratio, breast size, and ethnic group in Britain and South Africa. Br. J. Psychol. 2009:313–325. doi: 10.1348/000712608X329525. https://www.ncbi.nlm.nih.gov/pubmed/18625082 [DOI] [PubMed] [Google Scholar]
- 10.Cheng N.X., Wang Y.L., Wang J.H., Zhang X.M., Zhong H. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthet. Plast. Surg. 2002:375–382. doi: 10.1007/s00266-002-2052-4. https://www.ncbi.nlm.nih.gov/pubmed/12432479 [DOI] [PubMed] [Google Scholar]
- 11.Margolis N.E., Bassiri-Tehrani B., Chhor C., Singer C., Hernandez O., Moy L. Polyacrylamide gel breast augmentation: report of two cases and review of the literature. Clin. Imaging. 2015:339–343. doi: 10.1016/j.clinimag.2014.12.008. https://www.ncbi.nlm.nih.gov/pubmed/25670236 [DOI] [PubMed] [Google Scholar]
- 12.Xu L.Y., Kong X.Q., Tian Z.X., Qiu D.S. Magnetic resonance imaging on complications of breast augmentation with injected hydrophilic polyacrylamide gel. Chin. Med. J. (Engl.) 2006:1311–1314. https://www.ncbi.nlm.nih.gov/pubmed/16919193 [PubMed] [Google Scholar]
- 13.Ghasemi H.M., Damsgaard T.E., Stolle L.B., Christensen B.O. Complications 15 years after breast augmentation with polyacrylamide. JPRAS Open. 2015:30–34. https://www.sciencedirect.com/science/article/pii/S2352587815000194 [Google Scholar]
- 14.Unukovych D., Khrapach V., Wickman M., Liljegren A., Mishalov V., Patlazhan G., Sandelin K. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J. Surg. 2012:695–701. doi: 10.1007/s00268-011-1273-6. https://www.ncbi.nlm.nih.gov/pubmed/21932147 [DOI] [PubMed] [Google Scholar]
- 15.Chen B., Song H. Management of Breast deformity after removal of injectable polyacrylamide hydrogel: retrospective study of 200 cases for 7 years. Aesthet. Plast. Surg. 2016:482–491. doi: 10.1007/s00266-016-0646-5. https://www.ncbi.nlm.nih.gov/pubmed/27251750 [DOI] [PubMed] [Google Scholar]
- 16.Patlazhan G., Unukovych D., Pshenisnov K. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthet. Plast. Surg. 2013:312–320. doi: 10.1007/s00266-012-0045-5. https://www.ncbi.nlm.nih.gov/pubmed/23381651 [DOI] [PubMed] [Google Scholar]
- 17.Brahm J., Lessel R., Ditlev S., Schmidt R. Flux of selected body fluid constituents and beylpencillin in polyacrylamide hydrogel (PAAG) J. Tissue Eng. Regen. Med. 2012:793–8202. doi: 10.1002/term.485. https://www.ncbi.nlm.nih.gov/pubmed/22052857 [DOI] [PubMed] [Google Scholar]
- 18.Lui C.Y., Ho C.M., Iu P.P., Cheung W.Y., Lam H.S., Cheng M.S., Liu H.L. Evaluation of MRI findings after polyacrylamide gel injection for breast augmentation. AJR Am. J. Roentgenol. 2008:677–688. doi: 10.2214/AJR.07.2733. https://www.ncbi.nlm.nih.gov/pubmed/18716094 [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z.B., Liu Y. The relationship between breast cancer and breast augmentation with injected polyacrylamide gel: two case reports. J. Plast Reconstr. Aesthet. Surg. 2008;61(8):981–982. doi: 10.1016/j.bjps.2008.01.003. https://www.ncbi.nlm.nih.gov/pubmed/18395503 [DOI] [PubMed] [Google Scholar]
- 20.Cheng N.X., Liu L.G., Hui L. Breast cancer following augmnetation mammaplasty with polyacrylamide hydrogel(PAAG) injection. Aesthet. Plast. Surg. 2009;33:563–569. doi: 10.1007/s00266-008-9298-4. https://www.ncbi.nlm.nih.gov/pubmed/18716094 [DOI] [PubMed] [Google Scholar]