Highlights
-
•
Double cancers of the bile duct are often associated with pancreaticobiliary maljunction.
-
•
Double cancers of the bile duct without pancreaticobiliary maljunction are very rare.
-
•
Double cancers of the bile duct may have a terribly poor prognosis.
Abbreviations: PBM, pancreaticobiliary maljunction; CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography
Keywords: Double cancers, Common bile duct cancer, Extrahepatic bile duct cancer, Pancreaticobiliary maljunction
Abstract
Background
Double cancers of the biliary tract system are rare. Most of these cancers are synchronous double cancers of the gall bladder and bile duct, associated with pancreaticobiliary maljunction (PBM). Synchronous double cancers of the extrahepatic bile duct without PBM are especially rare, and only 4 cases have been reported.
Case presentation
A 78-year-old woman was admitted to our hospital for examination of hyperbilirubinemia and liver dysfunction. Contrast-enhanced abdominal computed tomography, Magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography revealed 2 stenotic regions in the common bile duct: at its junction with the cystic duct and in the distal bile duct. No findings suggested PBM, such as a markedly long common channel. The diagnosis based on endoscopic brush cytology from both stricture portions was adenocarcinoma. The patient had a pylorus-preserving pancreaticoduodenectomy with regional lymph node resection. Macroscopically, there were 2 stenotic regions at the cystic duct junction and in the distal bile duct. Microscopically, the tumor at the junction of the cystic duct was a well-to-moderately differentiated adenocarcinoma. On the other hand, the tumor of the distal bile duct was a poorly differentiated adenocarcinoma. There was no evidence of communication between these 2 cancers.
Conclusion
Double cancers of the extrahepatic bile duct without PBM are very rare. Therefore, an accurate diagnosis prior to surgery is necessary. Furthermore, this rare condition seems to be associated with a poor prognosis.
1. Background
Double cancers of the biliary tract system are rare. Most reports are of synchronous gall bladder and bile duct cancers, and cases are often associated with pancreaticobiliary maljunction (PBM) [1]. Synchronous double cancers of the extrahepatic bile duct without PBM are especially rare, and to date only 4 cases have been reported in the English literature [2], [3], [4], [5]. We herein report a case of synchronous double primary cancers of the extrahepatic bile duct and review the relevant literature. This study has been reported in line with the SCARE criteria [6].
2. Case presentation
A 78-year-old woman visited her local doctor with appetite loss, abdominal fullness, and dark urine. She had suffered from a drug-induced hepatitis due to an agricultural chemical in youth and she was receiving treatment for hypertension, hyperlipidemia, and vertigo. On admission, the patient had jaundice, and there was no palpable mass in the abdomen. Laboratory test results revealed hyperbilirubinemia (total bilirubin 7.6 mg/dl; normal range 0.3–1.3 mg/dl, direct bilirubin 6.1 mg/dl; normal range 0.0–0.4 mg/dl) and high serum levels of several liver enzymes (aspartate aminotransferase 240 U/L; normal range 11–34 U/L, alanine aminotransferase 347 U/L; normal range 7–34 U/L, alkaline phosphatase 1426 U/L; normal range 110–340 U/L, r-glutamyltranspeptidase 1097 U/L; normal range 5–55 U/L). Carbohydrate antigen 19-9 levels were high (328 U/ml; normal range 0–37 U/ml), and carcinoembryonic antigen levels were normal. An abdominal ultrasonography showed a dilation of the intrahepatic bile duct in both liver lobes and the common bile duct measuring 11 mm in diameter. No tumors were detected in the common bile duct or in the head of the pancreas. A contrast-enhanced abdominal computed tomography (CT) scan revealed thickening of the common bile duct wall at its junction with the cystic duct and dilation of the peripheral bile duct from this point. On CT, another obstruction was found in the distal portion of bile duct. There was no lymph node swelling around the extrahepatic bile duct (Fig. 1). Magnetic resonance cholangiopancreatography (MRCP) revealed 2 stenotic regions in the common bile duct. One portion was at its junction with the cystic duct and the other was in the distal bile duct (Fig. 2a). Endoscopic retrograde cholangiopancreatography (ERCP) showed similar findings to the MRCP (Fig. 2b). There were no findings indicative of PBM, such as a long common channel, in either the MRCP or ERCP examinations. The diagnosis based on endoscopic brush cytology from the upper stricture of the common bile duct was adenocarcinoma and the diagnosis from the distal stricture was suspicious of adenocarcinoma. The patient had a pylorus-preserving pancreaticoduodenectomy with regional lymph node resection. The postoperative course was uneventful, with the exception of “biochemical leak” postoperative pancreatic fistula [7] and chylous ascites (Grade I according to the Clavien-Dindo classification [8]). The patient was discharged on postoperative day 26.
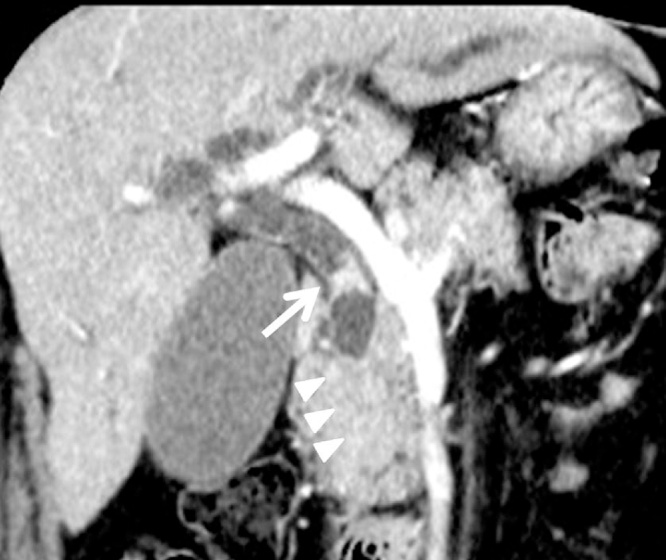
Fig. 1.
Coronal image of contrast-enhanced computed tomography of the abdomen. The common bile duct wall in the junction of cystic duct is thickened (arrows). The common bile duct does not trace until the papilla of Vater, which suggests that there is stenosis in the distal bile duct (arrowheads).
Fig. 2.
(a) Magnetic resonance cholangiopancreatography; There are 2 stenotic portions in the common bile duct. One portion is in the junction of the cystic duct (arrows), and the other is in the distal bile duct.The common channel is of normal length. (b) Endoscopic retrograde cholangiopancreatography; There are 2 stenotic portions in the common bile duct. One portion is in the middle bile duct (arrows), and the other is in the distal bile duct (arrowheads).The common channel is of normal length.
Macroscopically, there were 2 stenotic regions with rough mucous membrane: in the common bile duct and cystic duct junction and in the distal bile duct (Fig. 3). Microscopically, the tumor at the junction of the cystic duct was a well-to-moderately differentiated adenocarcinoma. The tumor cells had spread to the middle bile duct and invaded the under layer of the serosa (Fig. 4a). On the other hand, the tumor of the distal bile duct was a poorly differentiated adenocarcinoma (Fig. 4b). The tumor cells had invaded the pancreas and the fatty tissue outside the bile duct. Sections of the tumor had neuroendocrine carcinoma-like features, but immunohistochemical staining for neuroendocrine tumor markers, such as CD56, chromogranine A, and synaptophysin, was negative. There was no communication between the 2 cancers (Fig. 4c) and there was no lymph node metastasis. According to the TNM classification [9], the tumor of the cystic duct junction was classified as pT1N0M0, Stage IA, and the tumor of the distal bile duct was classified as pT3N0M0, Stage IIA.
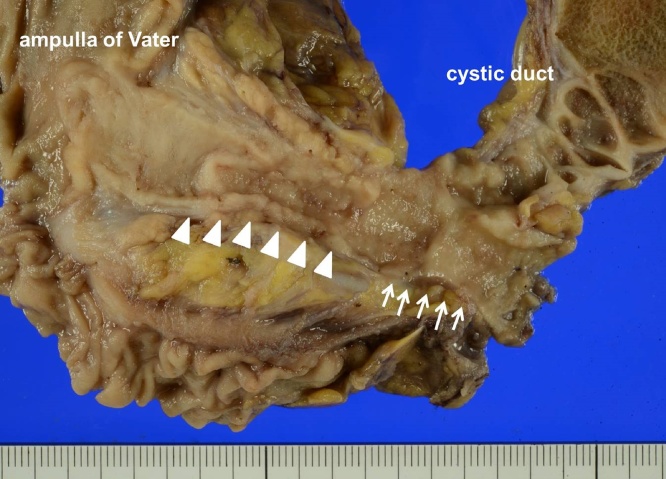
Fig. 3.
Macroscopic findings of the extrahepatic duct. There are 2 stenotic portions with rough mucous membrane in the junction of the cystic duct (arrows) and in the distal bile duct (arrowheads).
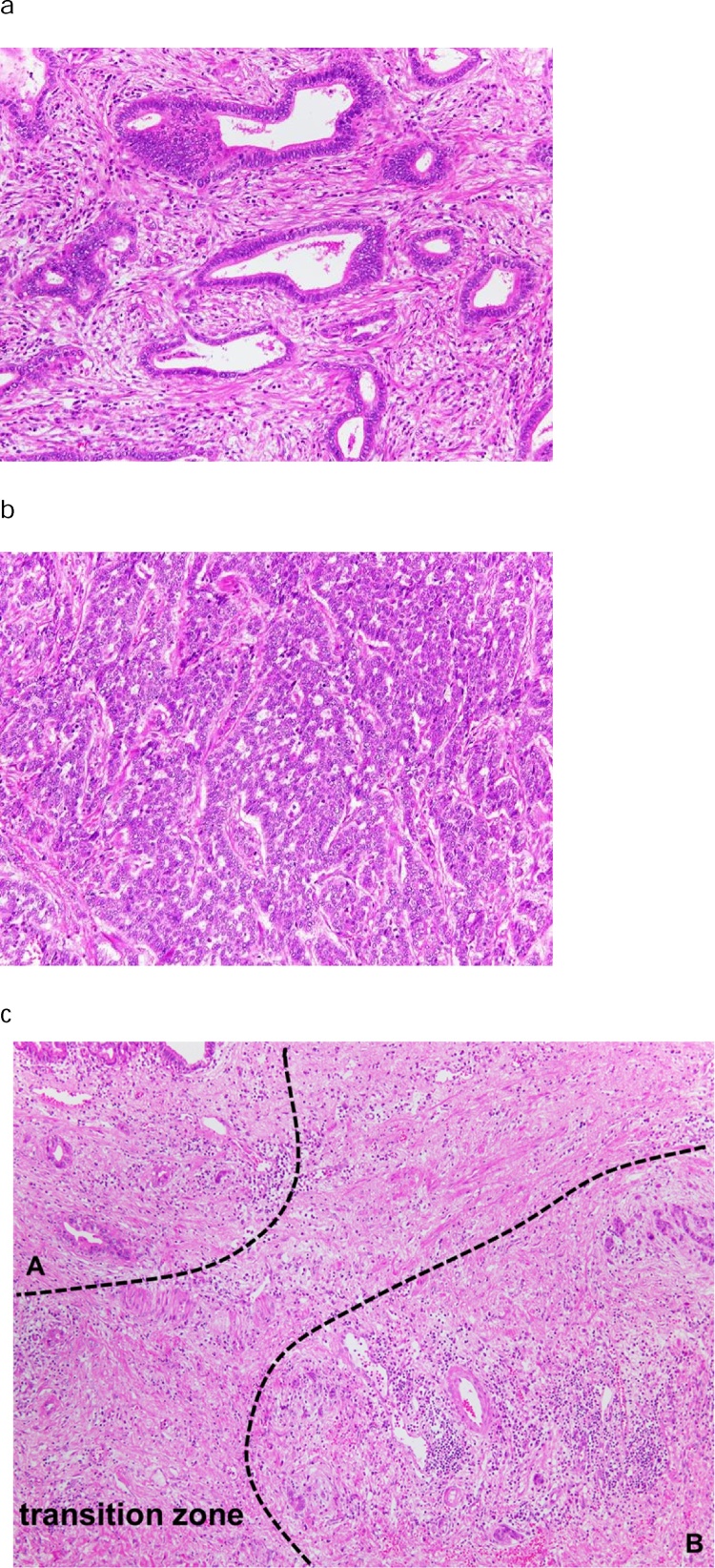
Fig. 4.
(a) Microscopic findings of the tumor in the junction of the cystic duct. The atypical cells form irregular duct structures, and the tumor was diagnosed as a well-to-moderately differentiated adenocarcinoma (hematoxylin and eosin, magnification 20×). (b) Microscopic findings of the tumor in the distal bile duct. The tumor cells spread invasively with solid and trabecular formation. The tumor was diagnosed as a poorly differentiated adenocarcinoma (hematoxylin and eosin, magnification 20×). (c) Microscopic findings of transition zone between the tumor in the junction of the cystic duct and the tumor in the distal bile duct. There was no communication between the 2 cancers (A; the tumor in the junction of the cystic duct, B; the tumor in the distal bile duct) (hematoxylin and eosin, magnification 10×).
The patient did not want to receive adjuvant chemotherapy, and 8 months later, a CT scan revealed multiple liver metastases. The patient then received chemotherapy with gemcitabine and cisplatin, and died 18months after the operation.
3. Discussion
Double cancers of the biliary system sometimes occur in PBM patients [1]. The mechanism of carcinogenesis in PBM appears to be related to the persistent reflux of pancreatic juice into the biliary tract. In PBM, the common channel is so long that sphincter action does not affect the pancreaticobiliary junction, and pancreatic juice frequently refluxes into the biliary tract. Persistent refluxed pancreatic juice injures the epithelium of the biliary tract and promotes cancer development [10]. The most common double cancer combination in PBM patients is gall bladder and bile duct. Synchronous double cancers of the extrahepatic bile duct are very rare, even in PBM patients [1]. We searched a literature review using the PubMed database with the search term “double cancers” and “extrahepatic bile duct” or “common bile duct”. Cases with PBM were excluded. As a result, 4 cases of synchronous double cancers of the extrahepatic bile duct without PBM were identified in the English literature [2], [3], [4], [5]. The findings of these cases are listed in Table 1, along with those of our case. Average patient age was 71.8 years (range 67–78), and there were no remarkable findings regarding gender. The 2-cancer pattern involved the distal bile duct and the middle bile duct or the common hepatic bile duct. All patients displayed some symptoms, including abdominal pain and/or jaundice.
Table 1.
Synchronous double primary cancers of the extrahepatic bile duct without pancreatobiliary maljunction.
| Authors [ref.] | Year | Patient age (years) | Sex | Tumor location | Histology | Clinical symptoms | Prognosis |
|---|---|---|---|---|---|---|---|
| Ogawa [2] | 2001 | 69 | Male | 1.Middle | 1.Poor | Abdominal distension | unkonwn |
| 2.Distal | 2.Moderate | ||||||
| Bedoui [3] | 2011 | 67 | Female | 1.Middle | 1.None | Abdominal pain and jaundice | unkonwn |
| 2.Distal | 2.None | ||||||
| Sumiyoshi [4] | 2012 | 78 | Male | 1.CHD | 1.None | Abdominal pain and jaundice | 31 months |
| 2.Distal | 2.None | ||||||
| Yoo [5] | 2015 | 67 | Male | 1.CHD | 1.Squamous | Jaundice | 8 moths |
| 2.Distal | 2.Moderate | ||||||
| Present study | 2017 | 78 | Female | 1.Middle | 1.Well to moderate | Jaundice | 18 months |
| 2.Distal | 2.poor |
CHD; common hepatic duct.
In 1932, Warren and Gates first proposed the 3 generally accepted criteria for the diagnosis of multiple primary malignant tumors: (1) each tumor must present a definite picture of malignancy, (2) each tumor must be distinct, and (3) the probability that 1 is a metastasis of the other must be ruled out [11]. In double cancers of the extrahepatic bile duct, it is important to distinguish whether the 2 cancers are both primary cancers or a combination of primary cancer and its metastasis. All reviewed cases of double cancers of the extrahepatic bile duct without PBM addressed this problem. Ogawa et al. [2] analyzed loss of heterozygosity (LOH) in double cancers. Both cancers showed common regions of LOH and the authors suggested that 1 cancer in the common bile duct was a metastasis of primary bile duct cancer. Therefore, this case was not a case of synchronous primary double cancers. Bedoui et al. [3] described only pathological findings; in this study, there was no communication between the 2 cancers in either the mucosal layer or the subepithelial layer without periductal lymphatic spread. Sumiyoshi et al. [4] also reported the pathological findings. Here, the 2 cancers were found to be separate entities with different histopathological diagnoses. In the case reported by Yoo et al., it was clear that there were 2 primary cancers because 1 was squamous cell carcinoma and the other was adenocarcinoma [5]. In our case, we diagnosed primary double cancers from histological findings: (1) there was no communication between the 2 cancers, (2) the histological findings of the 2 were different from each other; the tumor at the junction of the cystic duct was a well-to-moderately differentiated adenocarcinoma, and the distal bile duct tumor was a poorly differentiated adenocarcinoma. Furthermore, the latter tumor has neuroendocrine tumor-like features.
One report in the literature described a case that was not diagnosed as double cancers prior to surgery [4]. In this case, another tumor was detected in the distal bile duct on a CT scan performed 11 months after the surgery for the common hepatic bile duct cancer. The author suggested that the presence of the distal bile duct tumor was indicated on CT scans that were performed before the first surgery. This case report demonstrates the importance of minute examinations before surgery, and surgeons should consider the possibility of double cancers of the extrahepatic duct.
Bile duct cancer is known to have a poor prognosis [12]. Regarding the prognosis of double cancers of the extrahepatic bile duct without PBM, 2 reports mentioned it. In 1 case, multiple liver metastases were detected at 10 months after surgery and the patient died 31 months after surgery [4]. In another case, multiple liver metastases were detected at 3 months after surgery and the patient died 8 months after surgery [5]. In our case, multiple liver metastases were also detected at 8 months after surgery and the patient died 18 months after operation. Although a detailed analysis regarding the prognosis of double cancers of the extrahepatic bile duct without PBM is difficult because it is such a rare condition, the prognosis may be terribly poor.
4. Conclusions
Double cancers of the extrahepatic bile duct without PBM are very small in number; therefore, an accurate diagnosis prior to surgery is necessary. Furthermore, this rare condition seems to be associated with a terribly poor prognosis. Further investigation is needed to elucidate the etiology of double cancers of the extrahepatic bile duct without PBM.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This study is exempt from ethnical approval in my institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Author contribution
TN carried out the surgical procedure, designed the report, reviewed the literature, and drafted the manuscript. TH performed the preoperative diagnosis. YS and TK carried out the surgical procedure and participated in designing the report. TT and HM performed the histological analysis of the surgical specimens. TK participated in designing the report and revised the manuscript for submission. All authors have read and approved the final manuscript.
Registration of research studies
Not applicable.
Guarantor
Takeshi Nishi.
Acknowledgement
We would like to thank Editage for providing editorial assistance.
Contributor Information
Takeshi Nishi, Email: nishiken@med.shimane-u.ac.jp.
Yoshitoshi Sato, Email: ys8312002@yahoo.co.jp.
Takuya Hanaoka, Email: manekineko0624@yahoo.co.jp.
Takuya Takahashi, Email: matsue.byouri@gmail.com.
Hiroshi Miura, Email: hmiu3@yahoo.co.jp.
Kenji Takubo, Email: k_tkb50@yahoo.co.jp.
References
- 1.Fujii T., Kaneko T., Sugimoto H., Okochi O., Inoue S., Takeda S. Metachronous double cancer of the gallbladder and common bile duct. J. Hepatobiliary Pancreat. Surg. 2004;11(4):280–285. doi: 10.1007/s00534-003-0880-5. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa A., Sugo H., Takamori S., Kojima K., Fukasawa M., Beppu T. Double cancers in the common bile duct: molecular genetic findings with an analysis of LOH. J. Hepatobiliary Pancreat. Surg. 2001;8(4):374–378. doi: 10.1007/s005340170011. [DOI] [PubMed] [Google Scholar]
- 3.Bedoui R., Ajmi M., Nouira R., Dziri C. Synchronous double cancer of the common bile duct. Am. J. Surg. 2011;201(1):e1–2. doi: 10.1016/j.amjsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Sumiyoshi T., Shima Y., Kozuki A. Synchronous double cancers of the common bile duct. World J. Gastroenterol. 2012;18(41):5982–5985. doi: 10.3748/wjg.v18.i41.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo Y., Mun S. Synchronous double primary squamous cell carcinoma and adenocarcinoma of the extrahepatic bile duct: a case report. J. Med. Case Rep. 2015;9(1):116. doi: 10.1186/s13256-015-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Statement The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Bassi C., Marchegiani G., Dervenis C., Sarr M., Abu Hilal M., Adham M. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobin L.H., Wittekind C., Gospodarowicz M. 7th edn. Wiley-Blackwell; New York: 2009. TNM Classification of Malignant Tumors (UICC) [Google Scholar]
- 10.Kamisawa T., Kuruma S., Tabata T., Chiba K., Iwasaki S., Koizumi S. Pancreaticobiliary maljunction and biliary cancer. J. Gastroenterol. 2015;50(3):273–279. doi: 10.1007/s00535-014-1015-2. [DOI] [PubMed] [Google Scholar]
- 11.Warren S., Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am. J. Cancer. 1932;16:1358–1414. [Google Scholar]
- 12.Kwon H.J., Kim S.G., Chun J.M., Lee W.K., Hwang Y.J. Prognostic factors in patients with middle and distal bile duct cancers. World J. Gastroenterol. 2014;20(21):6658–6665. doi: 10.3748/wjg.v20.i21.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]