Abstract
Due to the ∼86,000 chemicals registered under the Toxic Substances Control Act and increasing ethical concerns regarding animal testing, it is not economically or technically feasible to screen every registered chemical for toxicity using animal-based toxicity assays. To address this challenge, regulatory agencies are investigating high-throughput screening in vitro methods to increase speed of toxicity testing, while reducing the overall cost. One approach for rapid toxicity testing currently being investigated is monitoring of volatile emissions produced by cell lines in culture. Such a metabolomics approach would measure gaseous emissions from a cell line and determine if such gaseous metabolites are altered upon exposure to a xenobiotic. Herein, we describe the history and rationale of monitoring endogenously produced volatiles for identification of pathologic conditions, as well as emerging applications in toxicity testing for such an approach.
Keywords: : cellular volatiles, high-throughput screening, in vitro, mass spectrometry, metabolomics
Introduction
Exhaled breath has long been known to be composed primarily of oxygen, carbon dioxide, nitrogen, and water vapor. However, in the 1970s, Linus Pauling discovered that exhaled breath also contains hundreds of volatile organic compounds (VOCs).1 These VOCs, along with a limited number of inorganic volatiles, are primarily the products and by-products of a host of biochemical processes within cells and microbes, many of which are still poorly elucidated.2 In recent decades, alterations in patterns of such volatiles have been used to identify both lung-specific and systemic pathologic conditions in vivo. Volatiles in exhaled breath are ideal biomarkers of pathologic conditions because they can be collected noninvasively and are essentially inexhaustible, allowing for multiple samples to be collected when needed with minimal inconvenience to the subject.3 An example of a volatile biomarker of exposure to a specific gaseous pollutant was described by Sawyeret al., where levels of several carbonyls, including acetaldehyde, were elevated in exhaled breath of individuals exposed to ozone.4 The ability to repeatedly collect endogenously produced volatiles (EPVs) noninvasively is also what makes the collection and analysis of EPVs appealing for in vitro applications.
Significant in vitro work has been conducted on the use of EPVs to detect the presence of specific microbes to differentiate healthy tissue from pathologic tissue, and to monitor enzyme activity.5–7 Due to increasing emphasis on in vitro toxicity testing, particularly high-throughput screening (HTS), there is significant interest in determining whether rapidly monitoring for alterations in EPVs in vitro can identify chemicals of concern.8 However, limited research has been conducted on how EPVs may be altered when mammalian cell cultures are exposed to xenobiotics. Therefore, to determine whether EPVs are viable biomarkers of cellular responses, including cytotoxicity, background EPVs must first be characterized. Upon characterization of background volatiles, xenobiotic-induced alterations of EPVs, if any, can then be identified. In this study, we focus on analyzing the formation of endogenous metabolites as a novel method for toxicity screening of household, pharmaceutical, and environmental agents for toxicity.
Rationale of Screening for Toxicity Using EPVs
Due to the vast number of chemicals registered for use in the United States, it is not feasible to collect toxicity data for every available chemical using traditional animal models. Therefore, over the past decade, there has been a paradigm shift toward using in vitro assays to rapidly screen a large number of chemicals to determine the need for more in-depth in vivo tests.
In vitro toxicity tests are primarily designed to identify biological perturbations at the molecular or cellular level, termed “key events,” which may lead to an adverse outcome.9 Such steps from chemical exposure to an adverse effect are described in an OECD-endorsed (Organization for Economic Co-operation and Development) framework termed an adverse outcome pathway (AOP).10 The overarching goal of an AOP is to clearly articulate the sequential key events necessary for a chemical to cause an adverse effect. Identifying and screening for such chemically induced key events, rather than the adverse outcome, is typically the primary approach of in vitro toxicity screens. In addition, the in vitro screens may provide detailed information regarding a chemical's mechanism of toxicity. In vitro toxicity screens are valuable for the vast majority of chemicals, including environmental pollutants, industrial chemicals, pharmaceuticals, and emerging nanomaterials. However, one significant challenge is that in vitro toxicity tests, particularly HTS, are liquid-based assays that are incapable of screening volatile chemicals for toxicity. Approximately 10% of registered chemicals are considered insoluble or excessively volatile for currently available liquid-based in vitro toxicity tests.11 In addition, current liquid-based in vitro toxicity tests are incompatible with monitoring the levels of endogenously produced gaseous molecules, such as nitric oxide (NO), carbon monoxide (CO), and VOCs, which may be indicators of specific key events within various AOPs. Therefore, the development of novel in vitro toxicity tests capable of screening volatile chemicals and collecting gaseous biomarkers is needed.
Utility of Examining EPVs
Hippocrates was the first to record a link between changes in mammalian EPVs and pathologic conditions when he linked alterations in breath odor to late-stage liver failure. The alterations in breath odor in liver failure patients, termed fetor hepaticus, are largely due to an increase in exhaled volatiles, such as dimethyl sulfide, ketones, and trimethylamine.12,13 Furthermore, a study by Sehnert et al. compared the exhaled breath VOC profile of subjects with chronic liver diseases to subjects with normal healthy livers.14 The authors determined that chronic liver failure is also associated with elevations in carbonyl sulfide, carbon disulfide, and isoprene. In addition, in a recent article published by Fernández del Río et al., alterations in volatiles in the exhaled breath of individuals with liver cirrhosis before and after liver transplantation surgery were studied using proton-transfer-reaction mass spectrometry (PTR-MS).15 The authors identified a statistically significant decrease in limonene, methanol, and 2-pentanone after liver transplantation, indicating that these three VOCs may be viable biomarkers of liver cirrhosis.
Acetone, another EPV with well-elucidated links to specific pathologies in vivo, is found at approximately 300–1000 parts per billion by volume (ppbv) in healthy adults.16 Acetone is significantly elevated in exhaled breath during ketoacidosis.17 Ketoacidosis occurs in diabetics when cells have insufficient levels of glucose absorption, resulting in production of ketones through metabolism of fatty acids for energy.18 The majority of these excess ketones, such as acetone, are released from the blood into the lungs, where they are then released in exhaled breath.
Isoprene is a close second to acetone as a highly studied breath VOC with median levels of ∼100 ppbv; it is produced as part of the human cholesterol synthesis pathway.19,20 It is found in every human breath sample along with acetone, but it is much more variable as isoprene concentration depends upon recent activity with concentrations increasing during sleep and decreasing during strenuous activity.21
In addition, the inorganic EPVs NO and CO have well-established links to specific pathologies. Endogenously produced NO is generated by a family of enzymes termed NO synthase, which comprises endothelial NO synthase, neuronal NO synthase, and inducible NO synthase.22 Through vasodilation by relaxation of smooth muscle, NO plays a vital role in regulating blood pressure, erectile function, and minimizing ischemic injury.23–25 However, NO also plays a vital role in neurotransmission and immune system function.26,27 Alterations in NO levels have been illustrated in disease states, such as cystic fibrosis, asthma pulmonary arterial hypertension, as well as by xenobiotic exposure.7,28–30 There is significant interest in monitoring NO in the air directly above cell cultures, termed headspace, as a marker of cell stress.
CO is another well-studied inorganic EPV. While CO is primarily known for its asphyxiant properties, it is also produced endogenously and has a vital cellular function. The role of endogenously produced CO is primarily as a cell signaling molecule, resulting in antiproliferative, antiapoptotic, anticoagulative, and anti-inflammatory effects.31 The mechanism of such physiological effects is predominantly due to modulation of activity of soluble guanylyl cyclase, resulting in the induction of cGMP activity. In addition, exposure to a wide host of xenobiotics has been illustrated to alter the heme oxygenase 1 (HO-1) activity, resulting in altered endogenously produced CO levels. However, while CO levels are altered in numerous disease states and exposure to certain xenobiotics, the underlying response to alterations of HO activity is elevated cellular stress.32 Therefore, alterations in CO levels in cell culture headspace may be a viable tool for in vitro toxicity screenings.
In general, there are many presumed endogenously produced compounds, although in most cases, it is difficult to determine their actual source. Consider that the human body includes not only its own cells but also ∼10 times as many bacterial cells, which contribute their metabolites to the systemic circulation.33,34 The skin microbiome also affects EPV emanations from the body's surface.35 Common skin EPVs include aldehydes, hydrocarbons, ketones, esters, and alcohols.2 Compounds emanating from skin may originate from blood or result from metabolism by epidermal microbiota, sweat from apocrine and eccrine glands, or lipid peroxidation of sebum from sebaceous glands.36,37
There is an overlap between compounds produced by human cells and those available from the environment, for example, aldehydes, ketones, and alcohols could come from food and consumer products or as contaminants in ambient air. However, the majority of true EPVs are formed through complex biological processes.14 A recent compendium of volatiles in healthy individuals identified 1849 compounds.38 Of these, 874 compounds were identified in breath, 504 in skin, 381 in feces, 279 in urine, and 154 in blood. Some volatiles are specific to a bodily fluid or tissue, while others are common to all emanation sources, as illustrated in the tables of de Lacy Costello et al..38 However, the exact mechanisms of production and tissue of origin are poorly understood for a significant portion of these volatiles.
To help identify which EPVs are created by specific cell types in the body, in vitro studies must be conducted. A 2016 compendium of VOCs released by a limited number of cell lines reliably identified ∼125 volatiles.39 The VOCs identified in the headspace were primarily alcohols, aldehydes, ketones, hydrocarbons, and esters. In addition, inorganic EPVs, such as NO, CO, and hydrogen sulfide (H2S), have been identified from cell culture emissions.22
Screening for Alterations in Volatiles In Vitro
Historically, studies involving the collection and analysis of volatiles from in vitro cell cultures were primarily designed to identify differences in patterns of volatiles, termed fingerprints, between pathogenic bacteria and healthy human cells or between diseased and healthy human cells, with the overarching goal of noninvasively identifying or monitoring the specific microbe or pathology in vivo. In a study conducted by Allardyce et al., metabolic VOCs were collected from the headspace of blood cultures infected with one of five bacterial strains that commonly cause bacteremia.40 The VOC profiles from blood bottles affected by specific bacteria strains were sufficiently unique to identify the particular strain. In a similar study by Dryahina et al., the unique volatile metabolites from common cystic fibrosis pathogens allowed for pathogen identification.41 Furthermore, Bean et al. identified 28 unique volatiles in the headspace above cultured Pseudomonas aeruginosa, a common cystic fibrosis-associated pathogen.42 Rapidly identifying the bacterial strain associated with infection may be particularly valuable because it can allow physicians to treat the infection with a narrow-spectrum antibiotic, resulting in more efficacious treatment and reduced risk of development of antibiotic resistance. In addition, monitoring of VOCs has been illustrated to be a useful tool for identifying the particular growth phase of various strains of bacteria.43
More recently, in vitro research has focused on identifying alterations in the volatile profile of cultured human diseased cells from that of normal healthy cells because EPV analysis of human subjects can be affected by age, gender, and comorbidities. Initial profiling of EPV biomarkers of disease using cultured cells can help focus the EPV profile for a disease/condition by eliminating inherent variabilities associated with human samples. Examples of disease-associated EPV changes using in vitro methods include induction of gaseous NO production by IFNγ in primary epithelial cell cultures from healthy individuals, but not in primary cell cultures from cystic fibrosis patients.7 Filipiak et al. observed that numerous gas-phase VOC concentrations were altered in the headspace of cultured cancer cells relative to controls, mirroring the ability to identify gas-phase biomarkers of lung cancer in vivo.6,44,45 Furthermore, Kwak et al. reported altered metabolism of melanoma cells compared to normal skin melanocytes.46 In headspace analyses of the cultures, the melanoma EPV profile exhibited higher isoamyl alcohol and lower isovaleric acid concentrations. Given that these compounds have leucine as a common precursor, the results suggest that the cancer cell metabolism produces less oxidized VOCs, which could aid in melanoma diagnoses.
While recent in vitro studies have focused on identifying gas-phase biomarkers of disease, minimal research has been conducted on screening for alterations of EPVs upon xenobiotic exposure. While the concept of monitoring for alterations of EPVs in vitro as biomarkers of toxicity is relatively novel, recent advances in analytical techniques have made it technically feasible for rapid toxicity screening. Until recently, the primary challenge in monitoring for potential alterations in both organic and inorganic EPVs in vitro is their low concentrations in cell culture headspace. However, significant advances in real-time gas-phase detection systems have been made in recent years. Real-time analytical devices, such as PTR-MS, selected ion flow-tube mass spectrometry (SIFT-MS), and ion mobility spectrometry (IMS) have shown potential to detect EPVs in the low ppb to parts per trillion range in or near real time.47–51 Real-time sampling of volatiles in headspace is advantageous for toxicity screenings because of the potential for longitudinal sampling and rapid analysis. Unlike many liquid-based toxicity screens, analysis of headspace does not disturb the cells or the media surrounding the cells. In addition, many volatiles, such as aldehydes, may potentially form adducts with proteins, or may be metabolized by enzymes, in culture media, limiting detection to some extent.52 However, this challenge may be minimized through the culture of cells under air-liquid interface (ALI) conditions, optimizing the release of some reactive volatiles directly into the headspace, and using cell lines that do not require serum to avoid metabolism or binding. Furthermore, instead of analyzing multiple plates at single time points, volatiles in headspace may be collected over multiple time points using the same cell culture without disturbing the cells, or if paired with a real-time gas-phase detector, may be continuously analyzed.8 However, the novel cell culture system should be ideally capable of exposing cells to xenobiotics in solid phase (particles), liquid phase (media), or gaseous phase (inlet air), which is not currently feasible with existing rapid in vitro toxicity screening assays. Furthermore, such recently developed quantitative mass analyzers minimize sample handling and may not require chromatographic separation, further reducing analysis time and potential for preparation error, which may allow for higher throughput applications.53
Novel Cell Culture Systems for Monitoring EPVs
To address the shortcomings of existing cell culture systems, novel cell culture systems are being designed and optimized to improve the collection and analysis of EPVs in cell culture headspace. A system currently in development relies on an isolated cell culture chamber with an inlet port that introduces 5% CO2 filtered air and an outlet port directly connected to a quantitative mass analyzer. The introduced 5% CO2 filtered air mixes with the cell culture headspace and creates a pressure gradient within the enclosed vesicle, which moves EPVs from the headspace through the outlet port, resulting in a rapid mechanism for headspace sampling. There is no requirement for collection by, for example, solid-phase microextraction (SPME) fibers, thermal desorption tubes, chemically inert Tedlar® bags, or summa canisters, potentially reducing analysis time and risk of human error. By designing a system with a constant pressure gradient that moves EPVs to the quantitative mass analyzer, the VOCs in cell culture headspace may be analyzed in real time when paired with a real-time gas-phase detector, such as a PTR-MS or a SIFT-MS.54
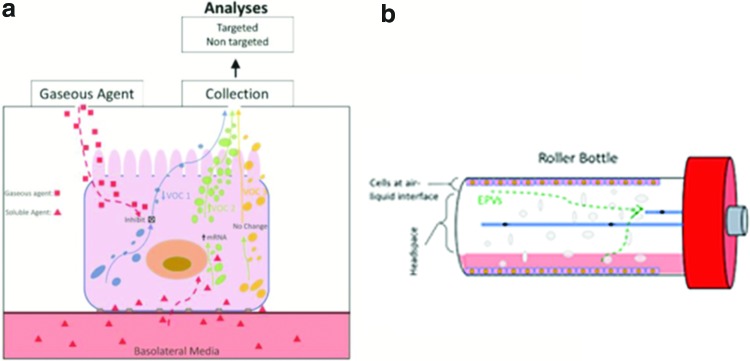
To allow for direct exposure of cell culture to insoluble chemicals of interest and to facilitate the release of EPVs to cell culture headspace, cells can be cultured under ALI conditions (Fig. 1a). Under ALI conditions, specific cell types, such as bronchial epithelial cells and skin keratinocytes, can differentiate, resulting in a more tissue-like morphology, which may produce an in vitro VOC response that reflects that of the in vivo response. In addition, ALI conditions allow for exposure to gaseous xenobiotics for toxicity screening, while also facilitating the release of endogenously produced gaseous metabolites into headspace without interference from the medium on the apical surface. The most readily available method for culturing cells under ALI conditions is by using Transwell® permeable membrane supports, whose gaseous environment can be controlled and sampled using a gas in vitro exposure system.55 However, if a large number of cells under ALI conditions is necessary for detection of low concentration EPVs, a roller bottle approach may be ideal (Fig. 1b). In this approach, cells are cultured in a cylindrical bottle that is constantly rotating (0.5–50 rpm) to bathe cells attached to the inner surface in medium. Approximately 15% of cells attached to the inner surface of the bottle are bathed in medium at any one time. The other 85% of cells remain under ALI conditions. Use of glass avoids any potential release of VOCs from Transwell materials.
FIG. 1.
Schematic diagrams illustrating the two cell culture systems designed for screening gaseous chemicals for toxicity and to facilitate the collection of released EPVs. (a) Illustration of a bronchial epithelial cell cultured under ALI conditions on Transwell®. ALI conditions facilitate the release of EPVs into cell culture headspace for straightforward collection and analysis. In addition, ALI conditions allow for exposure to gaseous agents for toxicity. Exposure to nonvolatile chemicals may occur through dissolution in basolateral medium. Xenobiotic-induced alterations in EPVs may occur through a variety of pathways, as illustrated in (a). (b) The culture system relies on a roller bottle approach, where cells are cultured on the interior of a borosilicate glass bottle, which is constantly rotated to hydrate cells. Approximately 15% of the cells within the bottle are under media, while the remaining 85% of cells in culture are under ALI conditions at any time. Filtered air is pumped through inlet port to create pressure gradient to move EPVs from cells to outlet port for collection to an adsorbent or for analysis using a real-time MS. ALI, air-liquid interface; EPVs, endogenously produced volatiles; MS, mass spectrometry. Color images available online at www.liebertpub.com/aivt
Any primary cell or cell line that can endure and perhaps differentiate under ALI culture conditions can be used with this novel roller bottle cell culture system. This is particularly valuable for inhalation toxicology models. Bronchial epithelial cell lines, such as the SV-40 transformed BEAS-2B, are ideal inhalation toxicology models for testing, optimizing, and implementing toxicity screening using the novel roller bottle system. The BEAS-2B cell line thrives under ALI conditions, is a well-validated cell line for inhalation toxicology, and can be readily transduced to express proteins of interest, which would be useful if protoxicants are being tested because these cells have limited basal CYP450 expression.56–58 Alternatively, an alveolar cancer cell line, such as the A549 cell line, and primary bronchial epithelial or nasal epithelial cells could be cultured in the novel cell culture system to identify EPVs. We believe that alterations in EPVs seen in the bronchial-alveolar cell lines upon exposure to a xenobiotic will be most relevant to the respiratory system. There is precedent in that the responses of BEAS cells exposed to ambient airborne particulate matter and ozone mimic some responses observed in rodents and humans exposed in vivo.59,60 It is currently unclear whether similar perturbations in EPVs will exist in cell lines originating from other organ systems. In addition, one must be cautious with the choice of culture conditions as the absence or presence of some components, for example, fetal bovine serum (FBS) in growth medium of BEAS-2B cells, has been shown to affect genome-wide gene expression and, in turn, affect several biological pathways, including glycolysis, basal and maximal respiration, and response to toxicants like arsenite.61 Bolton et al. first used a roller bottle design to study the effects of ozone on RNA synthesis in bovine kidney cultures. They and others expressed concern about the influence of complex medium components (e.g., FBS) on cellular response to gaseous toxicants as components may alter the function of cells, resulting in altered production of EPVs. Alternatively, certain medium components may introduce an exogenous source of volatiles to the system.62–64 Others have also shown that VOCs from medium components and polystyrene vessels can overlap with and/or affect VOCs emitted from the cultures.64–66 For instance, the lung cancer lines A549 and Lu7466 were noted by Schallschmidt et al. to be nonoptimal for breath biomarker detection of cancer because their VOC profiles were indistinguishable from that of the culture medium.66 These effects can be potentially minimized by using glass culture vessels and extensively characterizing defined culture media. In addition, although normal primary cells are more difficult to obtain and maintain, they may produce VOC profiles more representative of cells in vivo.
Incorporation of Three-Dimensional Cell Culture Models into EPV Analyses
The second greatest source of EPVs is skin, with ∼530 individual volatiles secreted.38 Skin-derived EPVs could potentially mix with other extradermal-derived EPVs by mixing in the blood and excretion through breath, or potentially diffuse into room air with subsequent rebreathing. Therefore, the identification of skin-derived EPVs using isolated skin cells is particularly important to identify the true origin and magnitude of EPVs in exhaled breath. Identification of the specificity of dermal-derived EPVs would be useful in determining the contribution of skin volatiles to samples collected in vivo.
To better elucidate the origins of these individual volatiles and determine if alterations in skin cell EPVs occur upon exposure to a xenobiotic, such as a pollutant or components of skincare products, immortalized HaCaT cells or primary keratinocyte cells may be cultured under ALI conditions in the novel roller bottle system. However, in skin, keratinocytes differentiate from the basal layer at the basement membrane to the cornified layer at the skin's surface. Each layer exhibits different expression patterns and unique histology. It would be useful to know if monolayer keratinocyte cultures have the same EPV profile as the in vivo tissue. Comparison of monolayer cultures with three-dimensional (3D) skin reconstructs have shown that the 3D skin reconstructs exhibit more robust cytokine expression, express xenobiotic-metabolizing enzymes better, and are more resistant to toxicity induced by surfactants.67–71
3D-reconstructed tissues, unlike monolayer cultures, usually contain several cell types that recapitulate the tissue of origin and exhibit gene expression profiles of differentiated cells.68,69,72–74 Cell-cell interactions are important for normal tissue responses. 3D reconstructs for skin and lung have been used in toxicological screens.75–78 Skin and lung 3D reconstructs are grown on transmembranes at an ALI that, along with changes in growth factor concentrations, stimulates differentiation and recapitulates the in vivo tissue's exposure to air.79 Several organ systems are available commercially (e.g., MatTek Corp, Ashland, MA and Epithelix) or can be made in-house.80–84 3D reconstructs have been treated with toxic gases and vapors in vitro using cell viability as the endpoint.55,85 The in vitro system could potentially be adapted to measure EPVs and would require testing and validation for each 3D system. It is interesting that 3D lung reconstructs are more resistant to the toxic effects of ozone compared with the two-dimensional cancer cell line A549.55 The cell-cell communication of the 3D tissues may make them more resilient, as well as the presence of protective surface factors such as mucous made by goblet cells in lung reconstructs and the cornified layer of skin reconstructs. Although the 3D reconstructs may be the best in vitro models of tissues, they have been limited by the size of the cultures and their high cost. Therefore, if a large number of cells are needed, it may be difficult to adapt 3D transmembrane systems to roller bottle ALI. The only 3D reconstructed tissue that has been grown in a roller bottle is liver on pleated bottles coated with collagen.86
Analytical Methods for EPVs
There are primarily two methods for sampling and analysis of cellular produced VOCs: on-line and off-line. Both have distinct advantages; the choice is based on the purpose of the experiment, the logistical (timing) constraints, and availability of laboratory instrumentation.
On-line analysis avoids the conventional sample collection step and draws a small gas flow directly from the cell exposure system into an analytical system. The analytical instrument could be a simple nonspecific detector, such as a thermal conductivity detector, electrochemical cell, chemical array detector (e.g., electronic nose), or a more sophisticated real-time mass-based detector, such as IMS, PTR-MS, or SIFT-MS. Ions are separated based on speed while moving through a drift tube in IMS, which utilizes radioactive or alkali cation sources for ionization.87 IMS instruments have the advantage of being much smaller than PTR-MS and SIFT-MS, making them more portable.88 Standard PTR-MS instruments contain quadrupole mass spectrometers, which have unit mass resolution. To achieve higher resolution, time-of-flight (ToF) mass analyzers have been coupled to PTR-MS instruments.48 PTR-ToF-mass spectrometry (MS) instruments have sub-ppbv detection limits and reported mass resolution between 4000 and 5000.48,89 SIFT-MS instruments also utilize quadrupole mass filters separated by a flow tube. These instruments are fast, allowing for individual breaths to be analyzed in real time.87
Typically, these on-line strategies focus on targeted analysis; one monitors for compounds deemed probative, ignoring the rest of the matrix. Furthermore, results are essentially instantaneous; changes in analyte production are monitored longitudinally to track time scales and the effect of intervention scenarios. On-line techniques have been utilized to analyze the headspace of cellular and bacterial cultures as well as biological media, including urine and feces. Cell culture off-gas has been monitored using PTR-MS to identify volatiles unique to certain growth conditions.90 SIFT-MS has been utilized to monitor volatiles emitted by bacteria to identify biomarkers of infection in cystic fibrosis as well as fecal headspace for colorectal cancer screening.91–93 Urinary headspace has been sampled using IMS to identify differences between Celiac disease and irritable bowel syndrome.93
Off-line analysis is also referred to as “central laboratory analysis,” wherein samples are collected over fixed time periods (e.g., 10 or 20 minutes) and periodically transferred to a laboratory for bulk analysis. The sample format is typically an adsorbent tube (e.g., Tenax, carbosieve, or activated charcoal), although canisters or various vial/headspace (i.e., SPME fiber) collection methods are also available. The instrumentation relies on three steps: sample concentration, analyte separation, and analyte identification/quantitation. For VOCs, the original sampling tube (or other format) is thermally desorbed onto a secondary trap invoking cryogenic or adsorbent focusing to achieve a higher density of analyte to boost sensitivity. Separation is achieved with capillary column gas chromatography (GC), and detection is performed with conventional single mass/charge unit (Da) MS, or high-resolution ToF-MS.
Off-line analysis focuses on nontargeted strategies where as many compounds in the matrix as possible are documented. Extended sample collection time (10 or 20 minutes) in nontargeted analysis allows many (often hundreds) of low-level organic compounds to be detected due to the extra concentration steps. Once compounds of interest are defined from nontargeted analysis, these techniques can be further augmented for sensitivity by reverting to a targeted approach. ToF-MS has fast scanning speed and high mass accuracy (around 10 ppm), making it an advantageous technique for nontargeted analysis when high resolution is required.94 GC-ToF-MS has been applied to study the VOC profiles of lung cancer cellular headspace as well as VOCs emitted in the headspace of eosinophils and neutrophils.95,96 Recently, GC has also been coupled with Orbitrap mass analyzers capable of achieving mass resolution from 7500 to 140,000 and sub-ppm mass accuracy, allowing for more compounds to be detected in a single analysis.94,97–99 A GC-quadrupole linear ion trap (QCL) Orbitrap has been utilized to detect semivolatile compounds in environmental samples and metabolites from plant extracts.97 Exactive GC-Orbitrap mass spectrometers have also been used to analyze Candida albicans and Staphylococcus aureus metabolites.99 In the future, the use of Orbitrap mass analyzers for volatile analysis has the potential to significantly increase the number of biomarkers that can serve as useful probes of endogenous response to exposure.
Analytical techniques for inorganic EPVs in cell culture headspace such as NO and CO vary considerably from analytical techniques for VOCs. To minimize degradation of NO and CO before analysis, cell culture headspace can be collected using chemically inert polyvinyl fluoride bags, such as Tedlar bags. Alternatively, headspace can be pumped directly to the analytical device for measurement.100 Analysis of gaseous NO from cell culture headspace is primarily conducted using ultrasensitive chemiluminescent instruments, such as the Sievers NOA 280 (General Electric, Fairfield, CT), which are capable of NO detection down to <1 ppb.101 Gaseous CO analysis is conducted using electrochemical sensors, such as the Bedfont Smokerlyzer®, a device traditionally used to monitor smoking cessation. CO electrochemical sensors have detection ranges in the ppbv range.102
Screening for Alterations in Activity of Select Enzymatic Pathways
Today, ∼50% of Americans take two or more prescription drugs, while nearly 20% take five or more medications.103 The use of multiple prescription drugs, termed polypharmacy, introduces the risk of a drug-drug interaction, where the presence of one drug alters the activity of the xenobiotic-metabolizing enzyme responsible for the metabolism of another drug, resulting in reduced efficacy or increased risk of deleterious side effects. Thus, in vitro methods of detecting and quantifying changes in enzyme activities by measuring the formation of volatile metabolites without perturbing the cell culture would be useful and have numerous applications.
Approaches that involve introducing isotopically labeled substrates to an enzyme of interest have previously been used to monitor the activity of specific enzymes. Upon metabolism by the enzyme of interest, isotopically labeled volatiles, such as 13CO2, are formed and released directly into the headspace, where they can be collected and quantified without ever disturbing the cell culture. This approach, which is used to measure the activity of certain xenobiotic-metabolizing enzymes as well as to monitor for the presence of pathogenic bacteria such as Helicobacter pylori, allows for noninvasive monitoring of activity of enzymes of interest.104 Such noninvasive approaches may allow for multiple time-point samples of enzyme activity.
Angrish et al. detailed a similar approach for measuring cytochrome p450 enzymes.105 However, rather than using radiolabeled substrates, a substrate with a known volatile metabolite is introduced to the cell culture. The activity of the enzyme of interest is determined by collecting the cell culture headspace and conducting a quantitative analysis for the volatile metabolite using GC-MS. Furthermore, the approach is not limited to monitoring activity of enzymes of the cytochrome P450 superfamily; it is amenable to a host of other enzymes, provided the substrate is primarily metabolized by the enzyme of interest and has a single known volatile substrate.
In the event an enzyme of interest is not expressed at significant levels in a cell line or primary cell type is not compatible with ALI culture conditions, bronchial epithelial cell lines such as the BEAS-2B may be stably transduced to express the enzyme of interest.57
Handling of Cellular VOC Data for Analyses
One major historical challenge in applying GC-MS-based EPV metabolomics to toxicity screening is the relatively limited metabolome databases and software available for interpreting results from metabolomic studies. Some of the most complete metabolomics databases that contain volatiles, such as U.S. Department of Agriculture's Duke Phytochemical and Ethnobotanical Database and the UC Davis MassBank of North America (MONA), are primarily focused on the metabolome of plants.106 Currently, metabolomics databases for mammalian cells, specifically gaseous metabolites produced by mammalian cells, are still in their infancy. However, in 2007, The Human Metabolome Database (www.hmdb.ca) was constructed as a human-specific metabolome database containing spectroscopic, quantitative, and physiological analytic data on small molecule metabolites and their relationships to specific diseases.107 The database is designed to contain or link three kinds of data: (1) chemical data, (2) clinical data, and (3) molecular biology/biochemistry data. The database contains 74,507 metabolite entries, including both water- and lipid-soluble metabolites as well as metabolites that would be regarded as either abundant (>1 μM) or relatively rare (<1 nM; www.hmdb.ca). Such databases are crucial to the development of metabolome sample collection methods, analytical chemistry methods for analysis and quantitation, and statistical evaluations of results. The new collection and analytic methods described above for cellular VOCs will identify more compounds to add to the Human Metabolome Database.
Summary
The potential applications of monitoring EPVs for toxicity testing continue to develop. While their use in toxicity testing has historically been limited to in vivo applications, increasingly sensitive analytical instrumentation in conjunction with novel cell culture methods has allowed monitoring of EPVs to be applied to in vitro scenarios, which tend to limit the confounding complexities of in vivo sampling. Recent studies have illustrated the feasibility of monitoring EPVs to distinguish pathologic cell cultures from healthy cell cultures, although culture conditions have to be controlled. Future applications of monitoring EPVs released by cell cultures may include identifying xenobiotic-induced perturbations for toxicity screening. The overarching benefits of metabolomics-based toxicity screening, specifically the analysis of EPVs, are the ability for real-time analysis, longitudinal sampling, and the ability to noninvasively identify xenobiotic-induced perturbations in cell cultures.
Disclaimer
The U.S. EPA through its Office of Research and Development has subjected this article to Agency administrative review and approved it for publication. Mention of trade names or commercial products does not constitute endorsement for use. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Acknowledgments
B.R.W. was supported as a predoctoral candidate, in part, by NIEHS Toxicology Training Grant T32 ES007126 and UNCEPA Training Agreement CR-83591401-0. This work was partly supported by the National Institute for Occupational Safety and Health (R21-OH010550 and T42-OH008673). Also, we are grateful for the expert advice and guidance from Dr. Michael Davis of Virginia Commonwealth University (Richmond, VA).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Corradi M, Mutti A. News from the breath analysis summit 2011. J Breath Res 2012:6;020201. [DOI] [PubMed] [Google Scholar]
- 2.Amann A, de Lacy Costello B, Miekisch W, et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res 2014:8;034001. [DOI] [PubMed] [Google Scholar]
- 3.Amann A, Smith D. Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring. Singapore: World Scientific Publishing Co.; 2005 [Google Scholar]
- 4.Sawyer K, Samet JM, Ghio AJ, et al. Responses measured in the exhaled breath of human volunteers acutely exposed to ozone and diesel exhaust. J Breath Res 2008:3;037019. [DOI] [PubMed] [Google Scholar]
- 5.Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut 1999:45;18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipiak W, Sponring A, Mikoviny T, et al. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro. Cancer Cell Int 2008:24;8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowski LE, Stewart D, Hazucha M. Interferon γ stimulates accumulation of gas phase nitric oxide in differentiated cultures of normal and cystic fibrosis airway epithelial cells. Lung 2012:190;563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleil JD. Cellular respiration: replicating in vivo systems biology for in vitro exploration of human exposome, microbiome, and disease pathogenesis biomarkers. J Breath Res 2016:10;010201. [DOI] [PubMed] [Google Scholar]
- 9.Vinken M. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 2013:312;158–165 [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve DL, Crump D, Garcia-Reyero N, et al. Adverse outcome pathway development II: best practices. Toxicol Sci 2014:142;321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EPA: U.S. Environmental Protection Agency, Office of Research and Development. Toxcast chemical inventory: data management and data quality considerations, 2014. Research Triangle Park, NC [Google Scholar]
- 12.Mitchell S, Ayesh R, Barrett T, et al. Trimethylamine and foetor hepaticus. Scand J Gastroenterol 1999:34;524–528 [DOI] [PubMed] [Google Scholar]
- 13.Van den Velde S, Nevens F, Van Hee P, et al. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci 2008:875;344–348 [DOI] [PubMed] [Google Scholar]
- 14.Sehnert SS, Jiang L, Burdick JF, et al. Breath biomarkers for detection of human liver diseases: preliminary study. Biomarkers 2002:7;174–187 [DOI] [PubMed] [Google Scholar]
- 15.Fernández del Río R, O'Hara ME, Holt A, et al. Volatile biomarkers in breath associated with liver cirrhosis—comparisons of pre- and post-liver transplant breath samples. EBioMedicine 2015:2;1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wang C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J Breath Res 2013:7;037109. [DOI] [PubMed] [Google Scholar]
- 17.Crofford OB, Mallard RE, Winton RE, et al. Acetone in breath and blood. Trans Am Clin Climatol Assoc 1977:88;128–129 [PMC free article] [PubMed] [Google Scholar]
- 18.Blaikie TP, Edge JA, Hancock G, et al. Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J Breath Res 2014:8;046010. [DOI] [PubMed] [Google Scholar]
- 19.Kushch I, Arendacká B, Štolc S, et al. Breath isoprene—aspects of normal physiology related to age, gender and cholesterol profile as determined in a proton transfer reaction mass spectrometry study. Clin Chem Lab Med 2008:46;1011–1018 [DOI] [PubMed] [Google Scholar]
- 20.Karl T, Prazeller P, Mayr D, et al. Human breath isoprene and its relation to blood cholesterol levels: new measurements and modeling. J Appl Phys 2001:91;762–770 [DOI] [PubMed] [Google Scholar]
- 21.King J, Kupferthaler A, Frauscher B, et al. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol Meas 2012:33;413–428 [DOI] [PubMed] [Google Scholar]
- 22.Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—a tale of three gases. Pharmacol Therap 2009:123;386–400 [DOI] [PubMed] [Google Scholar]
- 23.Burnett AL, Lowenstein CJ, Bredt DS, et al. Nitric oxide: a physiologic mediator of penile erection. Science 1992:257;401–403 [DOI] [PubMed] [Google Scholar]
- 24.Murohara T, Asahara T, Silver M, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 1998:101;2567–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995:377;239–242 [DOI] [PubMed] [Google Scholar]
- 26.Taqatqeh F, Mergia E, Neitz A, et al. More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci 2009:29;9344–9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowenstein CJ, Snyder SH. Nitric oxide, a novel biologic messenger. Cell 1992:70;705–707 [DOI] [PubMed] [Google Scholar]
- 28.Kharitonov SA, Yates D, Robbins RA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994:343;133–135 [DOI] [PubMed] [Google Scholar]
- 29.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995:333;214–221 [DOI] [PubMed] [Google Scholar]
- 30.Steerenberg PA, Snelder JB, Fischer PH, et al. Increased exhaled nitric oxide on days with high outdoor air pollution is of endogenous origin. Eur Respir J 1999:13;334–337 [DOI] [PubMed] [Google Scholar]
- 31.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006:86;583–650 [DOI] [PubMed] [Google Scholar]
- 32.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res 1991:51;974–978 [PubMed] [Google Scholar]
- 33.Pleil JD, Stiegel MA, Risby TH. Clinical breath analysis: discriminating between human endogenous compounds and exogenous (environmental) chemical confounders. J Breath Res 2013:7;017107. [DOI] [PubMed] [Google Scholar]
- 34.Pleil JD, Miekisch W, Stiegel MA, et al. Extending breath analysis to the cellular level: current thoughts on the human microbiome and the expression of organic compounds in the human exposome. J Breath Res 2014:8;029001. [DOI] [PubMed] [Google Scholar]
- 35.Dormont L, Bessière JM, Cohuet A. Human skin volatiles: a review. J Chem Ecol 2013:39;569–578 [DOI] [PubMed] [Google Scholar]
- 36.Broza YY, Mochalski P, Ruzsanyi V, et al. Hybrid volatolomics and disease detection. Angew Chem Int Ed Engl 2015:54;11036–11048 [DOI] [PubMed] [Google Scholar]
- 37.Martin HJ, Turner MA, Bandelow S, et al. Volatile organic compound markers of psychological stress in skin: a pilot study. J Breath Res 2016:10;046012. [DOI] [PubMed] [Google Scholar]
- 38.de Lacy Costello B, Amann A, Al-Kateb H, et al. A review of the volatiles from the healthy human body. J Breath Res 2014:8;014001. [DOI] [PubMed] [Google Scholar]
- 39.Filipiak W, Mochalski P, Filipiak A, et al. A compendium of volatile organic compounds (VOCs) released by human cell lines. Curr Med Chem 2016:23;2112–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allardyce RA, Langford VS, Hill AL, et al. Detection of volatile metabolites produced by bacterial growth in blood culture media by selected ion flow tube mass spectrometry (SIFT-MS). J Microbiol Methods 2006:65;361–365 [DOI] [PubMed] [Google Scholar]
- 41.Dryahina K, Sovová K, Nemec A, et al. Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex. J Breath Res 2016:10;037102. [DOI] [PubMed] [Google Scholar]
- 42.Bean HD, Dimandja JM, Hill JE. Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2012:901;41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunge M, Araghipour N, Mikoviny T, et al. On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry. Appl Environ Microbiol 2008:74;2179–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filipiak W, Filipiak A, Sponring A, et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J Breath Res 2014:8;027111. [DOI] [PubMed] [Google Scholar]
- 45.Phillips M, Cataneo RN, Cummin AC, et al. Detection of lung cancer with volatile markers in the breath. Chest 2003:123;2115–2123 [DOI] [PubMed] [Google Scholar]
- 46.Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromatogr B Analyt Technol Biomed Life Sci 2013:931;90–96 [DOI] [PubMed] [Google Scholar]
- 47.Jordan A, Haidacher S, Hanel G, et al. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int J Mass Spectrom 2009:286;122–128 [Google Scholar]
- 48.Herbig J, Müller M, Schallhart S, et al. On-line breath analysis with PTR-TOF. J Breath Res 2009:3;027004. [DOI] [PubMed] [Google Scholar]
- 49.Smith D, Španěl P, Herbig J, et al. Mass spectrometry for real-time quantitative breath analysis. J Breath Res 2014:8;027101. [DOI] [PubMed] [Google Scholar]
- 50.Fink T, Baumbach J, Kreuer S. Ion mobility spectrometry in breath research. J Breath Res 2014:8;027104. [DOI] [PubMed] [Google Scholar]
- 51.Cumeras R, Figueras E, Davis CE, et al. Review on ion mobility spectrometry. Part 1. Analyst 2015:140;1376–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richard LM, Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol 2014:27;1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbig J, Amann A. Proton transfer reaction-mass spectrometry applications in medical research. J Breath Res 2009:3;020201. [DOI] [PubMed] [Google Scholar]
- 54.Pleil JD. Breath biomarkers in toxicology. Arch Toxicol 2016a:90;2669–2682 [DOI] [PubMed] [Google Scholar]
- 55.Zavala J, O'Brien B, Lichtveld K, et al. Assessment of biological responses of EpiAirway 3-D cell constructs versus A549 cells for determining toxicity of ambient air pollution. Inhal Toxicol 2016:28;251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diabaté S, Mülhopt S, Paur HR, et al. The response of a co-culture lung model to fine and ultrafine particles of incinerator fly ash at the air-liquid interface. Altern Lab Anim 2008:36;285–298 [DOI] [PubMed] [Google Scholar]
- 57.Tal TL, Simmons SO, Silbajoris R, et al. Differential transcriptional regulation of IL-8 expression by human airway epithelial cells exposed to diesel exhaust particles. Toxicol Appl Pharmacol 2010:243;46–54 [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Canton C, Minet E, Anadon A, et al. Metabolic characterization of cell systems used in in vitro toxicology testing: lung cell system BEAS-2B as a working example. Toxicol In Vitro 2013:27;1719–1727 [DOI] [PubMed] [Google Scholar]
- 59.Dye JA, Lehmann JR, McGee JK, et al. Acute pulmonary toxicity of particulate matter filter extracts in rats: coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect 2001:109 (Suppl 3);395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dye JA, Madden MC, Richards JH, et al. Ozone effects on airway responsiveness, lung injury, and inflammation. Comparative rat strain and in vivo/in vitro investigations. Inhal Toxicol 1999:11;1015–1040 [DOI] [PubMed] [Google Scholar]
- 61.Zhao F, Klimecki WT. Culture conditions profoundly impact phenotype in BEAS-2B, a human pulmonary epithelial model. J Appl Toxicol 2015:35;945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolton DC, Tarkington BK, Zee YC, et al. An in vitro system for studying the effects of ozone on mammalian cell cultures and viruses. Environ Res 1982:27;466–475 [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen RE. In vitro systems for exposure of lung cells to NO2 and O3. J Toxicol Environ Health 1984:13;397–411 [DOI] [PubMed] [Google Scholar]
- 64.Schallschmidt K, Becker R, Jung C, et al. Investigation of cell culture volatilomes using solid phase micro extraction: options and pitfalls exemplified with adenocarcinoma cell lines. J Chromatogr B Analyt Technol Biomed Life Sci 2015:1006;158–166 [DOI] [PubMed] [Google Scholar]
- 65.Acevedo CA, Sánchez EY, Reyes JG, et al. Volatile organic compounds produced by human skin cells. Biol Res 2007:40;347–355 [PubMed] [Google Scholar]
- 66.Schallschmidt K, Becker R, Zwaka H, et al. In vitro cultured lung cancer cells are not suitable for animal-based breath biomarker detection. J Breath Res 2015:9;027103. [DOI] [PubMed] [Google Scholar]
- 67.Nygaard U, van den Bogaard EH, Niehues H, et al. The “Alarmins” HMBG1 and IL-33 downregulate structural skin barrier proteins and impair epidermal growth. Acta Derm Venereol 2017:97;305–312 [DOI] [PubMed] [Google Scholar]
- 68.Götz C, Pfeiffer R, Tigges J, et al. Xenobiotic metabolism capacities of human skin in comparison with a 3D epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: activating enzymes (phase I). Exp Dermatol 2012a:21;358–363 [DOI] [PubMed] [Google Scholar]
- 69.Götz C, Pfeiffer R, Tigges J, et al. Xenobiotic metabolism capacities of human skin in comparison with a 3D-epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: phase II enzymes. Exp Dermatol 2012b:21;364–369 [DOI] [PubMed] [Google Scholar]
- 70.Wiegand C, Hewitt NJ, Merk HF, et al. Dermal xenobiotic metabolism: a comparison between native human skin, four in vitro skin test systems and a liver system. Skin Pharmacol Physiol 2014:27;263–275 [DOI] [PubMed] [Google Scholar]
- 71.Lu B, Miao Y, Vigneron P, et al. Measurement of cytotoxicity and irritancy potential of sugar-based surfactants on skin-related 3D models. Toxicol In Vitro 2017:40;305–312 [DOI] [PubMed] [Google Scholar]
- 72.Gibbs S, van de Sandt JJ, Merk HF, et al. Xenobiotic metabolism in human skin and 3D human skin reconstructs: a review. Curr Drug Metab 2007:8;758–772 [DOI] [PubMed] [Google Scholar]
- 73.Taylor JM, Street TL, Hao L, et al. Dynamic and physical clustering of gene expression during epidermal barrier formation in differentiating keratinocytes. PLoS One 2009:4;e7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brinkmann J, Stolpmann K, Trappe S, et al. Metabolically competent human skin models: activation and genotoxicity of benzo[a]pyrene. Toxicol Sci 2013:131;351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ponec M. Skin constructs for replacement of skin tissues for in vitro testing. Adv Drug Deliv Rev 2002:54 (Suppl 1);S19–S30 [DOI] [PubMed] [Google Scholar]
- 76.Bernstam L, Lan CH, Lee J, et al. Effects of arsenic on human keratinocytes: morphological, physiological, and precursor incorporation studies. Environ Res 2002:89;220–235 [DOI] [PubMed] [Google Scholar]
- 77.Black AT, Hayden PJ, Casillas RP, et al. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol 2010:249;178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sexton K, Balharry D, Brennan P, et al. Proteomic profiling of human respiratory epithelia by iTRAQ reveals biomarkers of exposure and harm by tobacco smoke components. Biomarkers 2011:16;567–576 [DOI] [PubMed] [Google Scholar]
- 79.Groeber F, Holeiter M, Hampel M, et al. Skin tissue engineering-in vivo and in vitro applications. Adv Drug Deliv Rev 2011:63;352–366 [DOI] [PubMed] [Google Scholar]
- 80.Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp 2011:54;pii:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalabis J, Wong GS, Vega ME, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc 2012:7;235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh JW, Hsi TC, Guerrero-Juarez CF, et al. Organotypic skin culture. J Invest Dermatol 2013:133;1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gildea J, McGrath HE, Van Sciver RE, et al. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. In: Epithelial Cell Culture Protocols 2nd ed. Randall SH. Fulcher ML. (eds); pp. 329–345. New York: Humana Press; 2012 [DOI] [PubMed] [Google Scholar]
- 84.Balogh K, Sivars U, Hornberg E, et al. A 3D human airway model enables prediction of respiratory toxicity of inhaled drugs in vitro. Toxicol Sci 2017:162;1–8 [DOI] [PubMed] [Google Scholar]
- 85.Holt K. Validation of a xenobiotic vapor generation and exposure chamber system for quantification of exposure effect in 3dimensional human tissue reconstructs. MSPH, Department of Environmental Sciences and Engineering; 2014 [Google Scholar]
- 86.Michalopoulos GK, Bowen WC, Mulè K, et al. Histological organization in hepatocyte organoid cultures. Am J Pathol 2001:159;1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujii T. Ion/Molecule Attachment Reactions: Mass Spectrometry. New York, NY: Springer; 2015 [Google Scholar]
- 88.Baumbach JI. Ion mobility spectrometry coupled with multi-capillary columns for metabolic profiling of human breath. J Breath Res 2009:3;034001. [DOI] [PubMed] [Google Scholar]
- 89.Kohl I, Herbig J, Dunkl J, et al. Smokers breadth as seen by proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF-MS). In: Volatile Biomarkers. Amann A. Smith D. (eds); pp. 89–116. Amsterdam: Elsevier; 2013 [Google Scholar]
- 90.Schmidberger T, Gutmann R, Bayer K, et al. Advanced online monitoring of cell culture off-gas using proton transfer reaction mass spectrometry. Biotechnol Prog 2014:30;496–504 [DOI] [PubMed] [Google Scholar]
- 91.Shestivska V, Dryahina K, Nunvář J, et al. Quantitative analysis of volatile metabolites released in vitro by bacteria of the genus Stenotrophomonas for identification of breath biomarkers of respiratory infection in cystic fibrosis. J Breath Res 2015:9;027104. [DOI] [PubMed] [Google Scholar]
- 92.Batty CA, Cauchi M, Lourenco C, et al. Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PloS One 2015:10;e0130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arasaradnam RP, Westenbrink E, McFarlane MJ, et al. Differentiating coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis—a pilot study. PloS One 2014:9;107312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee YJ, Smith EA, Jun JH. Gas chromatography-high resolution tandem mass spectrometry using a GC-APPI-LIT orbitrap for complex volatile compounds analysis. Mass Spec Lett 2012:3;29–38 [Google Scholar]
- 95.Hanai Y, Shimono K, Oka H, et al. Analysis of volatile organic compounds released from human lung cancer cells and from the urine of tumor-bearing mice. Cancer Cell Int 2012:12;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Florence NS, Jan WD, Monique H, et al. Volatile organic compounds discriminate between eosinophilic and neutrophilic inflammation in vitro. J Breath Res 2016:10;016006. [DOI] [PubMed] [Google Scholar]
- 97.Peterson AC, McAlister GC, Quarmby ST, et al. Development and characterization of a GC-enabled QLT-Orbitrap for high-resolution and high-mass accuracy GC/MS. Anal Chem 2010:82;8618–8628 [DOI] [PubMed] [Google Scholar]
- 98.Peterson AC, Hauschild JP, Quarmby ST, et al. Development of a GC/quadrupole-orbitrap mass spectrometer, part I: design and characterization. Anal Chem 2014:86;10036–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weidt S, Haggarty J, Kean R, et al. A novel targeted/untargeted GC-Orbitrap metabolomics methodology applied to Candida albicans and Staphylococcus aureus biofilms. Metabolomics 2016:12;189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suresh V, Mih JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2007:37;97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barreto M, Villa MP, Martella S, et al. Off-line exhaled nitric oxide measurements in children. Pediatr Pulmonol 2001:32;159–167 [DOI] [PubMed] [Google Scholar]
- 102.Obermeier J, Trefz P, Wex K, et al. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J Breath Res 2015:9;016008. [DOI] [PubMed] [Google Scholar]
- 103.Zhong W, Maradit-Kremers H, St. Sauver JL, et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clin Proc 2013:88;697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klein PD, Malaty HM, Martin RF, et al. Noninvasive detection of Helicobacter pylori infection in clinical practice: the 13C urea breath test. Am J Gastroenterol 1996:91;690–694 [PubMed] [Google Scholar]
- 105.Angrish MM, Madden MC, Pleil JD. Probe molecule (PrM) approach in adverse outcome pathway (AOP) based high-throughput screening (HTS): in vivo discovery for developing in vitro target methods. Chem Res Toxicol 2015:28;551–559 [DOI] [PubMed] [Google Scholar]
- 106.Misra BB. Plant volatilome resources. Curr Metabol 2016:4;148–150 [Google Scholar]
- 107.Wishart DS, Tzur D, Knox C, et al. HMDB: the human metabolome database. Nucleic Acids Res 2007:35;D521–D526 [DOI] [PMC free article] [PubMed] [Google Scholar]