Gadolinium-based contrast agents (GBCAs) are used over 10 million times worldwide each year in magnetic resonance imaging (MRI) scans to diagnose and characterize disease states affecting the central nervous system (CNS). Primary and metastatic brain cancers, multiple sclerosis, and stroke all result in a disrupted blood brain barrier that is readily detected by GBCA enhanced MRI.1 Seven of the eight FDA-approved GBCAs are indicated for imaging abnormal blood-brain barrier and this represents by far the most common clinical application of GBCAs. GBCAs are neutral or anionic, hydrophilic gadolinium complexes comprising an octadentate chelator and a water co-ligand. Upon intravenous injection, GBCAs provide immediate enhancement of the arteries and then rapidly distribute throughout the extracellular space. CNS lesions show prolonged enhancement because of compromised endothelium and/or lymphatic drainage.
In 2006 an association was established between GBCAs and a rare but devastating, and sometimes fatal, fibrosing disorder termed nephrogenic systemic fibrosis (NSF) in patients with impaired kidney function. GBCAs are excreted primarily by the kidneys and when kidney function decreases the residency time of the GBCA in the body increases, leading to increased risk of toxicity. This led to the avoidance of GBCAs in patients with poor kidney function and resulted in no new cases of NSF in the last few years. In a series of seminal reports starting in late 2013 it was demonstrated that in patients who had received multiple GBCA doses over time that there was sometimes MR signal hyperintensity in the globus pallidus and dentate nucleus that persisted long after the last GBCA dose and that this enhancement was due to Gd retention as proven by elemental analysis of autopsy specimens.2 Deposition of Gd in the brain and other organs, e.g. bone, has been observed with all GBCAs regardless of kidney function, although GBCAs based on macrocyclic chelators appear to deposit lower levels of Gd relative to GBCAs based on acyclic chelators. Although no symptoms signaling neurotoxicity have yet been attributed to the irreversible Gd deposition within the CNS, this startling revelation sparked a flurry of 2017 regulatory activity. The FDA assigned a new class warning to all GBCAs advising physicians to carefully consider the retention characteristics of a GBCA before prescribing contrast enhanced examinations. Japan’s Pharmaceuticals and Medical Devices Agency also recently updated their GBCA package inserts, advising the use of less stable GBCAs only when more stable GBCAs are not appropriate. The European Medicines Agency took a more cautious approach and suspended the marketing authorization for the 3 GBCAs considered to have the highest propensity for CNS Gd deposition and restricted the use of two others to liver imaging applications.
All intravenous contrast agents approved for use and that are commercially available contain Gd. These growing safety concerns around Gd deposition have led to active research for non-Gd alternatives. Superparamagnetic iron oxide nanoparticles can generate excellent MR contrast properties. Ferumoxytol (Feraheme®) is a carboxymethyldextran coated iron oxide nanoparticle that is approved as an intravenous iron supplement for treating anemia. Ferumoxytol has been used off-label as a contrast agent, however it has very different pharmacokinetic properties to GBCAs, making it best suited to different indications.5 Unlike GBCAs, ferumoxytol is too large to rapidly extravasate from the blood vessels into tumors and other lesions as a result of a compromised blood-brain barrier. Lesion enhancement is observed at a much later time point, typically the next day after injection, while GBCAs enhance tumors within minutes. Ferumoxytol has a blood elimination half-life of 14h, whereas a typical GBCA elimination half-life ranges between 1–2 hours. The long blood half-life and slow kinetics of tumor accumulation can be exploited to characterize the tumor vasculature. Figure 1a shows an image of a brain tumor taken shortly after ferumoxytol injection where the blood vessels in the tumor appear dark. The long residency time of ferumoxytol makes it easy to calculate the relative cerebral blood volume (Figure 1b) of the tumor which can be be highly predictive of malignancy and post-surgical outcomes. Imaging the patient again the next day after the nanoparticle has had time to accumulate in the tumor shows strong positive contrast with a T1-weighted acquisition (Figure 1c) and negative contrast with a T2-weighted acquisition (Figure 1d).
Figure 1.
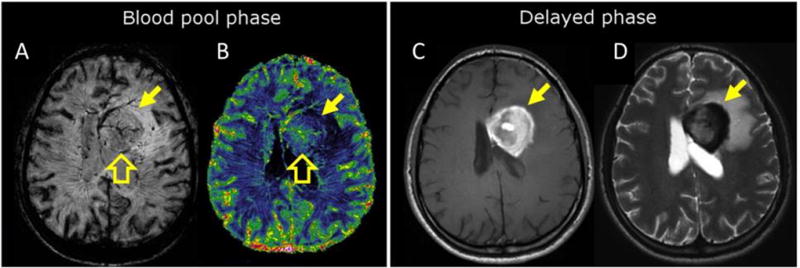
Characterization of a newly diagnosed primary central nervous system lymphoma (yellow arrow) during the (A, B) blood pool, and (C, D) delayed phases after ferumoxytol injection. (A) T2*-weighted image shows curvilinear branching hypointensities compatible with abnormal tumor vasculature (open block arrow). (B) Steady state CBV map with a high resolution confirms increased blood volume (open block arrow). Steady state CBV maps such as that depicted in B cannot be acquired using most GBCAs. (C) Axial T1-weighted scan demonstrates a typical enhancement 24 hours after ferumoxytol administration due to iron accumulation; this information would typically be acquired minutes after GBCA injection. (D) Axial T2-weighted MRI shows marked hypointensity in the tumor 24 hours after ferumoxytol administration due to iron accumulation. Reprinted with permission from reference 5.
While iron oxide nanoparticles can provide complementary information to GBCAs, they lack the ability to rapidly identify areas of blood brain barrier disruption. To match the pharmacokinetics of GBCAs, a small molecule complex is required. Complexes of Mn2+ may represent the most viable alternative to GBCAs. Like Gd3+ (S=7/2), the high spin Mn2+ ion is also strongly paramagnetic (S = 5/2) and has a long electronic T1. Water exchange of coordinated water ligands at Mn2+ is very fast (>106 s−1) and the metal-H(water) distance is shorter than for Gd3+ complexes which leads to increased relaxation and offsets the lower spin quantum number of Mn2+ compared to Gd3+. Unlike Gd3+, Mn is an essential trace nutritional element that the human body can process and clear via endogenous mechanisms.
There is a high barrier to developing effective Mn2+-based contrast agents that are stable to dissociation in the body. The d5 Mn2+ ion has no ligand field stabilization energy and lies to the left of the Irving-Williams series resulting in complexes that are kinetically labile and lacking in thermodynamic stability. Contrast agents require a site for water to coordinate and for Mn2+ this means that hexa- or pentadentate chelators must be used, whereas for GBCAs the chelators are all octadentate. Many Mn2+ complexes are unstable to dissociation of the Mn2+ ion in vivo once confronted with competing metal ions like Zn2+ and/or with coordinating anions. Alternately, there are stable, inert complexes that are coordinatively saturated and provide no site for water coordination resulting in much lower relaxivity (contrast efficiency).
We recently reported the Mn2+ complex Mn-PyC3A (Figure 2a) as a stable contrast agent.. The trans-1,2-cyclohexylenediamine backbone imparts a rigidifying effect that strongly disfavors molecular distortions that occur en route to Mn release, while the picolyl moiety further rigidifies the molecule and renders it more inert to transmetallation.3. Under conditions of extreme challenge with a high excess of Zn2+ competitor ion, transmetallation occurs 20 times more slowly with Mn-PyC3A than with Gd-DTPA, the widely used GBCA Magnevist®. The relaxivity of Mn-PyC3A in blood plasma is similar to other GBCAs like Magnevist® and Dotarem®. After intravenous administration to mice, Mn-PyC3A enhances the blood vessels in MRI and then is rapidly cleared from the body via the kidneys into the urine and via the liver into the bile and feces. Twenty-four hours after dosing, less than 0.1% of the administered Mn remains in the body of the mice.
Figure 2.
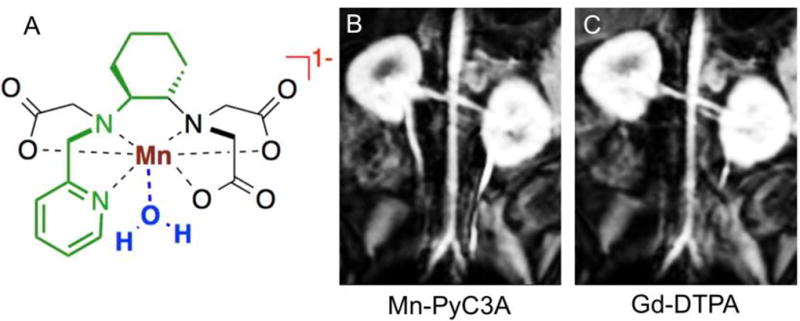
(A) Mn-PyC3A was a rationally designed as a direct GBCA replacement. The PyC3A ligand is constructed from a rigidifying N-picolyl-trans-1,2-diaminocyclohexane backbone (highlighted in green), which engenders a high degree of kinetic stability and the complex is thus highly resistant to degredation by Mn release. The Mn-PyC3A chelate also accommodates a rapidly exchanging water co-ligand (blue) that provides strong positive contrast to throughout the local tissue environment. The anionic charge (red) of Mn-PyC3A ensures extracellular distribution. (B and C) Multiplanar reformatted coronal images in baboons from 3D T1-weighted images acquired at 3.0 T showing the abdominal aorta and renal arteries (B) 9 seconds after injection of 0.1 mmol/kg Mn-PyC3A, and (C) 9 seconds after injection of 0.1 mmol/kg Gd-DTPA.
Encouraged by these favorable results in rodents, we sought to evaluate Mn-PyC3A in a more clinical scenario by imaging the arteries of baboons at 3T using a clinical scanner and clinical imaging protocols while comparing directly to Gd-DTPA. In this study the dose (0.1 mmol/kg), the formulation of the contrast agent, the injection rate, and scanning protocol were identical for both contrast agents. Figures 2b and 2c show multiplanar reformatted coronal T1-weighted images that delineate the abdominal aorta and renal arteries taken immediately after contrast administration. The images are virtually identical. We quantified artery-to-muscle contrast-to-noise ratio (CNR) for the abdominal aorta and for the brachiocephalic arteries and found no statistical difference in CNR between Mn-PyC3A enhanced images compared to Gd-DTPA. Dynamic imaging of the abdomen following the angiographic scan revealed rapid renal excretion as well as clearance via the hepatobiliary system. This mixed renal/ hepatobiliary excretion implies that Mn-PyC3A can also be efficiently excreted by renally impaired patients. Like GBCAs, Mn-PyC3A exhibits pharmacokinetics consistent with extracellular distribution and is eliminated from the blood plasma of baboons with an elimination half-life of 40.1 ± 3.1 min, which is comparable to GBCAs. HPLC-ICP-MS analysis of blood plasma and urine revealed no significant evidence of any free Mn or of Mn containing species other than Mn-PyC3A. Given the similar contrast properties and pharmacokinetics of Mn-PyC3A and GBCAs, we hypothesize that Mn-PyC3A will offer a suitable alternative to GBCAs for visualization of CNS lesions. Such studies are ongoing.
Attitudes toward the safety of GBCAs are growing increasingly cautious, and we expect that concerns among clinicians and regulators will continue to drive innovation in the coming years to provide alternatives to GBCAs. Direct replacements like Mn-PyC3A could provide the same diagnostic and prognostic information of GBCAs while innovative contrast agents with different mechanisms of action, like ferumoxytol, can provide further and novel characterization of disease.
References
- 1.Essig M, Dinkel J, Gutierrez JE. Use of Contrast Media in Neuroimaging. Magn. Reson. Imaging Clin. N. Am. 2012;20:633–648. doi: 10.1016/j.mric.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology. 2015;275(3):630–634. doi: 10.1148/radiol.2015150805. [DOI] [PubMed] [Google Scholar]
- 3.Gale EM, Atanasova I, Blasi F, Ay I, Caravan P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015;137(49):15548–15557. doi: 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale EM, Wey HY, Ramsay I, Yen YF, Sosnovik D, Caravan P. A Manganese-Based Alternative to Gadolinium: Contrast Enhanced MR Angiography, Pharmacokinetics, and Metabolism. Radiology. 2018 doi: 10.1148/radiol.2017170977. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP, Gahramanov S, Harisinghani M, Macdougall I, Neuwelt A, Vasanawala SS, Ambady P, Barajas R, Cetas JS, Ciporen J, DeLoughery TJ, Doolittle ND, Fu RW, Grinstead J, Guimaraes AR, Hamilton BE, Li X, McConnell HL, Muldoon LL, Nesbit G, Netto JP, Petterson D, Rooney WD, Schwartz D, Szidonya L, Neuwelt EA. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney International. 2017;92(1):47–66. doi: 10.1016/j.kint.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


