Abstract
Motivation: Mathematical modeling and optimization have been used for detecting enzyme targets in human metabolic disorders. Such optimal drug design methods are generally differentiated as two stages, identification and decision-making, to find optimal targets. We developed a unified method named fuzzy equal metabolic adjustment to formulate an optimal enzyme target design problem for drug discovery. The optimization framework combines the identification of enzyme targets and a decision-making strategy simultaneously. The objectives of this algorithm include evaluations of the therapeutic effect of target enzymes, the adverse effects of drugs and the minimum effective dose (MED).
Results: An existing generalized mass action system model of human uric acid (UA) metabolism was used to formulate the fuzzy optimization method for detecting two types of enzymopathies: hyperuricemia caused by phosphoribosylpyrophosphate synthetase (PRPPS) overactivity and Lesch–Nyhan syndrome. The fuzzy objectives were set so that the concentrations of the metabolites were as close as possible to the healthy levels. The target design included a diet control of ribose-5-phospahate (R5P). The diet control of R5P served as an extra remedy to reduce phosphate uptake entering the purine metabolic pathway, so that we could obtain a more satisfactory treatment than obtained for those without a diet control. Moreover, enhancing UA excretion resulted in an effective treatment of hyperuricemia caused by PRPPS overactivity. This result correlates with using probenecid and benbromazone, which are uricosuric agents present in current clinical medications. By contrast, the Lesch–Nyhan syndrome required at least three enzyme targets to cure hyperuricemia.
Contact: chmfsw@ccu.edu.tw
Supplementary information: Supplementary data are available at Bioinformatics online.
1 INTRODUCTION
One of the most potentially valuable approaches used to accelerate the development of new drugs is through the simulation of human metabolic disorders. Simulations involve the construction of mathematical models that integrate genomic, proteomic and metabolic information (Cascante et al., 2002; Kell, 2006; Leung et al., 2013; Materi and Wishart, 2007; Voit, 2002). In mathematical models, parameters are manipulated to identify biomedical systems that have defective enzymes, and mathematical optimization methods are then applied to correct those systems (Banga, 2008; Sams-Dodd, 2006; Vera et al., 2010).
One of the genetic causes of hyperuricemia is a functional defect in phosphoribosylpyrophosphate synthetase (PRPPS), which provokes an increase in the activity of this enzyme and promotes the metabolic flux yielding uric acid (UA; Scriver et al., 1989). Another instance of hyperuricemia is caused by a deficiency of the activity of hypoxanthine-guanine phosphoribosy1-transferase (HGPRT), leading to Lesch–Nyhan disease (Lesch and Nyhan, 1964). Patients with hyperuricemia are at risk of nephropathy, urinary tract stone disease, gouty arthritis and tophaceous deposits. In addition, those with the Lesch–Nyhan disease also exhibit neurological symptoms and self-mutilating behavior. The oxypurines hypoxanthine and xanthine (XA) accumulate in HGPRT deficiency. These oxypurines are thought to contribute to the pathogenesis of the neurological symptoms of Lesch–Nyhan disease because of their cytopathic effects (Palmour et al., 1989). The current treatment of hyperuricemia usually includes a symptomatic treatment for joint pain, a restricted diet in which foods with high purine concentrations are limited, and the prescription of medications, such as uricosuric agents and XA oxidase inhibitors, that increase the excretion of UA in the urine and block UA production, respectively (Schlesinger, 2010).
The development of a new drug is a high-risk and costly activity involving multi-criteria decision making and trade-offs. In a model-based approach, systems biological data and optimization technologies are integrated to surmount these drawbacks (Guillen-Gosalbez and Sorribas, 2009; Leung et al., 2013; Li et al., 2011; Materi and Wishart, 2007; Stone et al., 2010). The optimization program for drug design (OPDD) is a two-stage procedure used to identify enzyme targets for remedying hyperuricemia (Vera et al., 2007), which is caused by PRPPS overactivity in the purine metabolic pathway. The first stage of the OPDD is to enumerate each enzyme to identify a set of candidate enzyme targets that minimize the difference between a high concentration of UA and its healthy levels. The purine metabolic pathway was originally expressed using a generalized mass action (GMA) model (Curto et al., 1998). The GMA model was first transformed into an S-system model, so that the OPDD can be applied to a linear programming method in solving the drug target design problem to obtain a set of candidate enzyme targets. The second stage of the OPDD is a posterior decision-making determining a satisfactory target from the candidate enzyme targets. Three objectives, therapeutic effect, adverse effect and low dose, are considered to make the decision. The therapeutic effect objective consists of ensuring that each candidate enzyme target is able to return the concentration of UA to <105% of the healthy levels. The adverse effect objective consists of ensuring that the least-square error between the remedied metabolite concentration and its corresponding healthy levels is small. Furthermore, the drug dose objective is based on the fact that higher enzyme activities imply using a lower dose of drugs, and low drug doses minimize adverse effects.
In this study, we introduce a fuzzy multi-objective optimization approach to formulate the enzyme target design problem for drug discovery. The design problem is a unified optimization framework, in which the identification of enzyme targets is combined with multi-criteria decision-making to identify enzyme targets for curing hyperuricemia caused by PRPPS overactivity and HGPRT deficiency. In this work, we not only consider the therapeutic effect and adverse effect objectives, but also the minimum effective dose (MED) to represent the low dose objective. The MED is defined as the lowest dose level of a pharmaceutical product that provides a clinically significant response with average efficacy, and that is also significantly superior to the response provided by the placebo (Fillon, 1995). Although fuzzy multi-objective optimization approaches have been applied to the enzyme intervention problem metabolic networks of microbe (Wu et al., 2011), almost no literature has so far discussed the approach for evaluating therapeutic effect, adverse effect and MED, simultaneously, to combine the detection of candidate enzyme targets and decision-making to select satisfactory targets.
2 METHODS
2.1 Kinetic model
The dynamics of a metabolic network can be generically governed by a set of nonlinear differential equations with the following structure:
| (1) |
where x ∈ Rn is a vector of the concentrations of metabolites or pools, α ∈ Rr is a vector of the enzyme levels corresponding to the enzyme activities, v ∈ Rr is a vector of reaction rates and N ∈ Rn×r is the stoichiometric matrix describing the interconnecting fluxes. The stoichiometric property of a network is time invariant. Each reaction rate in this study was expressed based on the power-law function in Equation (2).
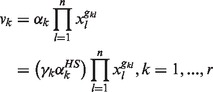 |
(2) |
where αk is the rate constant or enzyme activity for the kth reaction rate and gkl are the kinetic orders. The rate constant αk in Equation (2) was also expressed as a ratio γk of its healthy state 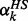 to easily modulate the enzyme activity in the following drug discovery problem.
to easily modulate the enzyme activity in the following drug discovery problem.
2.2 Fuzzy equal metabolic adjustment problem
As mentioned in the introduction, the OPDD is a two-stage procedure used to identify enzyme targets for remedying hyperuricemia (Vera et al., 2007). We introduce a fuzzy equal metabolic adjustment (FEMA) problem, which is a unified optimization framework that combines the detection of candidate enzyme targets and decision-making to select satisfactory targets in one framework. The FEMA framework is described as the following formulation.
 |
(3) |
 |
(4) |
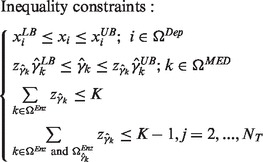 |
(5) |
The three fuzzy equals in Equation (3) are applied to evaluate therapeutic effect, adverse effect and MED, simultaneously, combining the detection of candidate enzyme targets and decision-making to select satisfactory targets. Fuzzy equal operation, which is different from traditional optimization problems, is applied as an index for evaluating therapeutic effect, as well as adverse effect and MED, that is used in identifying enzyme targets to restore the desired metabolite concentrations to their corresponding healthy levels as close as possible. The second and third fuzzy equal objectives in Equation (3) serve as the measures for decision making. In the second objective, the metabolite concentrations are used to evaluate adverse effects. However, the therapeutic effect set ΩTE is different from the adverse effect set ΩAE. In the MED objective of fuzzy equal objectives, each enzyme activity (i.e. the rate constant of the GMA model) is assumed to be as close to its healthy level as possible. The logarithmic expression 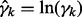 of the rate constant ratio γk is used in the FEMA formulation to modulate the enzyme activity.
of the rate constant ratio γk is used in the FEMA formulation to modulate the enzyme activity.
Each rate equation shown in Equation (2) was formulated as the power-law expression. This expression may result in a computational failure when a concentration approaches zero. We used the exponential relationship in Equation (4) to prevent this numerical problem. Here, the logarithmic expression of the healthy enzyme activity is defined as 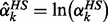 . The first inequality constraint in Equation (5) is used to restrict each metabolite concentration within its corresponding lower and upper bounds. A binary variable zk is embedded in the second inequality constraint to determine whether the enzyme target is modulated or not. The last inequality is used as a control indicator for enumerating alternative enzyme targets from the FEMA problem. Here, K is the allowable number of enzymes that can be selected in the modulated set ΩEnz to be modulated, e.g. K = 2 means that we can select two enzymes from the modulated set. The last inequality in Equation (5) is ≤K − 1, which is used to enumerate alternate enzyme targets. Therefore, the selected candidate targets at the current iteration should be at least one enzyme different from the candidate targets obtained in the previous iterations. The set
. The first inequality constraint in Equation (5) is used to restrict each metabolite concentration within its corresponding lower and upper bounds. A binary variable zk is embedded in the second inequality constraint to determine whether the enzyme target is modulated or not. The last inequality is used as a control indicator for enumerating alternative enzyme targets from the FEMA problem. Here, K is the allowable number of enzymes that can be selected in the modulated set ΩEnz to be modulated, e.g. K = 2 means that we can select two enzymes from the modulated set. The last inequality in Equation (5) is ≤K − 1, which is used to enumerate alternate enzyme targets. Therefore, the selected candidate targets at the current iteration should be at least one enzyme different from the candidate targets obtained in the previous iterations. The set 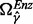 corresponds to the set of candidate enzyme targets selected in the previous kth iterations. Here, NT is the number of iterations used to solve the FEMA problem.
corresponds to the set of candidate enzyme targets selected in the previous kth iterations. Here, NT is the number of iterations used to solve the FEMA problem.
2.3 Solving strategy
To solve the FEMA problem, we first define a membership function for each fuzzy equal objective to quantify each corresponding satisfactory grade. The membership function is described in Equation (6) as a two-side function:
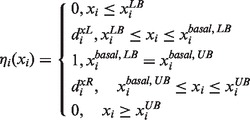 |
(6) |
The membership function on the left-hand side is a strictly monotonically increasing function 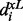 . In contrast, the right-hand side is a strictly monotonically decreasing function
. In contrast, the right-hand side is a strictly monotonically decreasing function 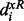 . Sakawa (1993) proposed five types of membership functions: linear, exponential, hyperbolic inverse and piecewise linear functions, to quantify the behavior of fuzzy objectives or constraints. For concise illustration of the FEMA problem, the linear membership function of each fuzzy equal objective is shown in the Supplementary Additional File 1. Here,
. Sakawa (1993) proposed five types of membership functions: linear, exponential, hyperbolic inverse and piecewise linear functions, to quantify the behavior of fuzzy objectives or constraints. For concise illustration of the FEMA problem, the linear membership function of each fuzzy equal objective is shown in the Supplementary Additional File 1. Here, 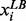 and
and 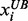 are the lower and upper bounds of the ith metabolite concentration or enzyme activity provided by the user. The satisfactory grade or membership function value is equal to 1 if the corresponding metabolite concentration or enzyme activity is between
are the lower and upper bounds of the ith metabolite concentration or enzyme activity provided by the user. The satisfactory grade or membership function value is equal to 1 if the corresponding metabolite concentration or enzyme activity is between 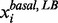 and
and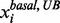 , and the small lower and upper bounds are near the basal value
, and the small lower and upper bounds are near the basal value 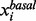 . The satisfactory grade is 0 if the metabolite concentration or enzyme activity is beyond its lower or upper bounds. The satisfactory grade is between 0 and 1 if the metabolite concentration or enzyme activity is within its range.
. The satisfactory grade is 0 if the metabolite concentration or enzyme activity is beyond its lower or upper bounds. The satisfactory grade is between 0 and 1 if the metabolite concentration or enzyme activity is within its range.
The FEMA problem is used to find the metabolite concentrations, enzyme activities and selection variables that ensure that the fuzzy equal objectives are as close to their healthy levels as possible. Having elicited the membership functions for each fuzzy equal objective, the FEMA problem is then transferred to the maximizing decision problem,
where the crisp feasible domain Ψ consists of the kinetic model in Equation (4) and inequality constraints in Equation (5). The maximizing decision problem is used to find the maximum intersection of the fuzzy equal objectives. The intersection of the fuzzy equal objectives is conducted according to the minimization operation, which is a discontinuous function. Thus, the maximizing decision problem can be rewritten as the equivalent problem to avoid a discontinuous computation.
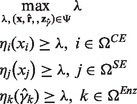 |
(7) |
The advantage of this approach is that the optimal membership grade corresponds to the satisfactory level for each objective, and the optimal decision λ represents the overall satisfactory grade (equivalent to the lower bound) of the problem.
3 RESULTS
3.1 Design conditions
The kinetic model of the purine metabolic pathway described in Curto et al. (1998) was expressed based on a GMA formulation consisting of 16 dependent variables, 1 diet control variable (ribose-5-phospahate, R5P), 1 constant variable (phosphate, Pi) and 28 independent variables for modulating enzyme activities. Detailed information regarding this model is included in Supplementary Additional File 2. The model equations were accessed from the BioModels Database (Li et al., 2010a, b; Le Novère et al., 2006). Generally, human hyperuricemia is caused by either PRPPS overactivity or HGPRT deficiency (which is related to Lesch–Nyhan disease). These two pathologic cases were considered in this study. In the first case, we assumed that the activity of PRPPS doubled its healthy level (Vera et al., 2007). In the second case (HGPRT deficiency), we assumed that both rate constants of guanine phosphoribosyltransferase (GPRT) and hypoxantine phosphoribosyltransferase (HPRT) were 1% of their healthy values (Curto et al., 1998). Using these pathological states, the abnormal UA concentrations caused by PRPPS-related hyperuricemiain and Lesch–Nyhan disease were 131.0 and 145.5 µM, respectively. By contrast, the UA concentration in the healthy state was 100.3 µM.
Considering the therapeutic effect of target enzyme selection, the upper limit of UA was set as 105% of the healthy-state value. To prevent neuronal toxicity, the upper limits of hypoxanthine and XA were set as 120% of the healthy state value. Other metabolites were allowed to vary between 0.5 and 10 times their normal values. The combination of diet control was also considered, and we set a constraint for the concentration of R5P to be between 0.5 and 1.5 times the normal intake concentrations. The enzyme activities were restricted to be 0.1–2 times their normal state values. We used various allowable numbers of enzymes for the FEMA problem to determine optimal enzyme targets with or without the prescribed diet control. The FEMA problem can be used to enumerate alternative optimal enzyme targets; therefore, we performed 10 iterations for each run to obtain a set of optimal enzyme targets. All optimization problems were solved using the BONMIN and KNITRO solvers accessed from GAMS (version 23.8) on a 3.07 GHz Intel Core i7 CPU with 12 GB of RAM.
3.2 Pathological state caused by PRPPS overactivity
We first used the therapeutic effect objective only for UA [i.e. the first fuzzy objective in Equation (3)] to identify candidate enzyme targets, and make a posterior decision to choose treatment targets. This computation was similar to the two-stage design of the OPDD. In the first stage, the satisfactory grade of the therapeutic effect objective for each run was ∼100%. The next stage involved computing the membership grade for each metabolite and enzyme, and choosing the lowest grade (equivalent to minimum satisfaction) from the set of corresponding grades as the adverse effect objective and MED objective to choose treatment targets. The computational satisfaction values between adverse effects and MED considered without or with prescription of diet control are shown in Figures 1 and 2, respectively. The color figures are shown in Supplementary Additional File 3. In practice, it was difficult to simultaneously apply both objectives to make a decision because some targets had a higher membership grade of the adverse effect objective but accompanying with a lower grade of the MED objective, or vice-versa.
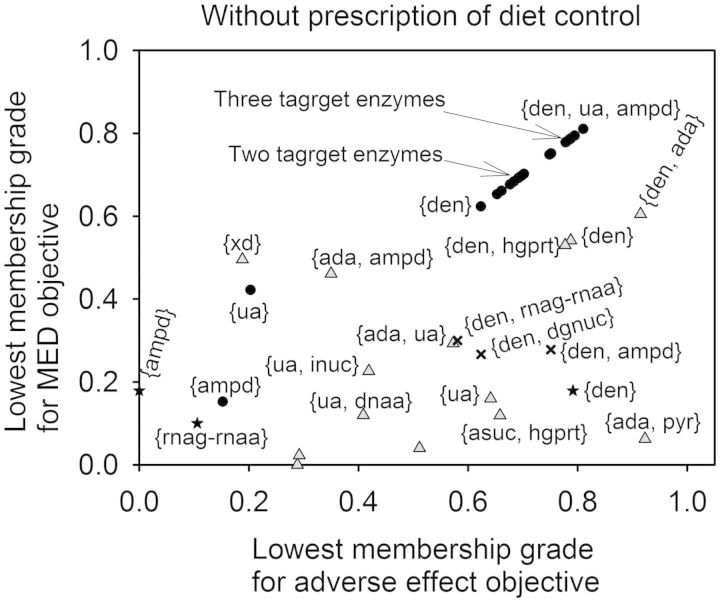
Fig. 1.
Relationship between the lowest membership grades for adverse effect objectives and MED objectives. The upward-facing triangles indicate the results obtained from the therapeutic effect objective FEMA problem considered one and two enzyme targets without a diet control. Circles indicate the results obtained from the triple-objective FEMA problem considered one, two and three enzyme targets without a diet control. Stars (single enzyme target) and crosses (two enzyme targets) indicate the corresponding lowest membership grades for adverse effect objectives and MED objectives computed by using the optimal solutions obtained from Vera et al. (2007)
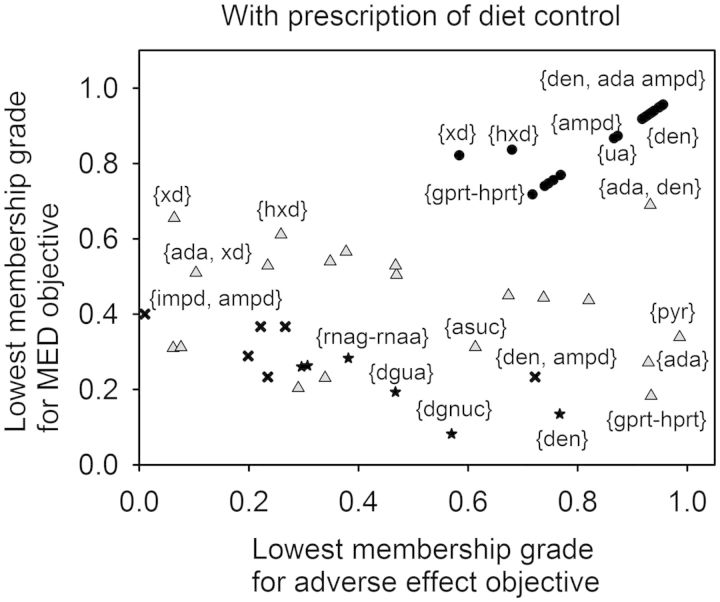
Fig. 2.
Relationship between the lowest membership grades for adverse effect objectives and MED objectives. The upward-facing triangles indicate the results obtained from the therapeutic effect objective FEMA problem considered one and two enzyme targets with the diet control of R5P. Circles indicate the results obtained from the triple-objective FEMA problem considered one, two and three enzyme targets with the diet control of R5P. Stars (single enzyme target) and crosses (two enzyme targets) indicate the corresponding lowest membership grades for adverse effect objectives and MED objectives computed by using the optimal solutions obtained from Vera et al. (2007)
For one enzyme target without prescription of diet control, amidophosphoribosyltransferase {den} and XA oxidase {xd} were downregulated from 5.27 and 0.949 to 1.83 and 0.297 µmol min−1 kg−1, respectively, to progressively reduce UA formation. As a result, the therapeutic effect objective concerning UA was nearly 100% satisfactory. However, the adverse effect objectives for the enzyme targets {den} and {xd} had satisfactory grades of 78.85 and 18.8%, respectively, and the MED objectives were 54.1 and 49.6%, respectively (Fig. 1). In contrast, UA excretion {ua} was upregulated from 8.74 × 10−5 to 1.57 × 10−4 µmol min−1 kg−1 to enhance UA secretion. The membership grades for adverse effect objective and MED objective were 64.1 and 16%, respectively. We also solved the therapeutic effect objective FEMA problem considered one and two enzyme targets with prescription diet of R5P. The optimal results for 10 iterations could achieve 100% the therapeutic effect objective. However, adverse effect and MED objectives as shown in Figure 2 are similar to the results obtained by one enzyme target. For one enzyme target with prescription of diet, 10 different enzyme targets could be obtained to achieve 100% therapeutic effect. However, only three enzyme targets were found for the case of one enzyme target without prescription of diet. The membership grades for adverse effect objective and MED objective had similar situations as observed from Figures 1 and 2.
The optimal enzyme targets obtained by the OPDD (Vera et al., 2007) were also applied to evaluate the membership grades for adverse effect objective and MED objective, which were listed as stars (single enzyme target) and crosses (two enzyme targets) in Figures 1 and 2 for comparison. The therapeutic effect for UA could be 100% achievable if we considered the first objective only, but the adverse effect and MED were incapable of fulfillment simultaneously. Note that the OPDD is to transform GMA model to the S-system model so that a linear programming method can be applied to detect enzyme targets. As a result, the OPDD can determine an aggregated enzyme target {rnag-rnaa} due to this linearization transformation.
The triple-objective FEMA problem was then applied to determine the optimal enzyme targets using various requirements to fulfill these objectives simultaneously. The lowest membership grades between adverse effects and MED for various pairs of enzyme targets are shown as circles in Figures 1 and 2. Both grades were nearly identical to the overall satisfaction, except for the enzyme targets, {ua}, {xd} and {hxd}, because a trade-off procedure occurred in the triple-objective FEMA problem that resulted in a compromise. Figure 3 shows the overall satisfactory grade attained with or without prescribed diet control. The FEMA problem can be used to enumerate alternative optimal enzyme targets, so that the top three satisfactory grades among the solutions are depicted. The enzyme targets with a prescription diet control of R5P had higher than 86% satisfactory grade, and are more satisfactory than those without the prescription diet control. When there was no prescription diet control, pyrimidine synthesis {pyr} was upregulated from 1.295 to 1.592 µmol min−1 kg−1 for two enzyme targets, and to 1.5 µmol min−1 kg−1 for three enzyme targets, to detour PRPP synthesis in purine metabolism. This effect is similar to the prescription diet control of R5P, in which half of uptake entering the purine metabolic pathway is reduced. As a result, one enzyme target with the prescription diet control could achieve a higher satisfaction than those without the prescription diet control.
Fig. 3.
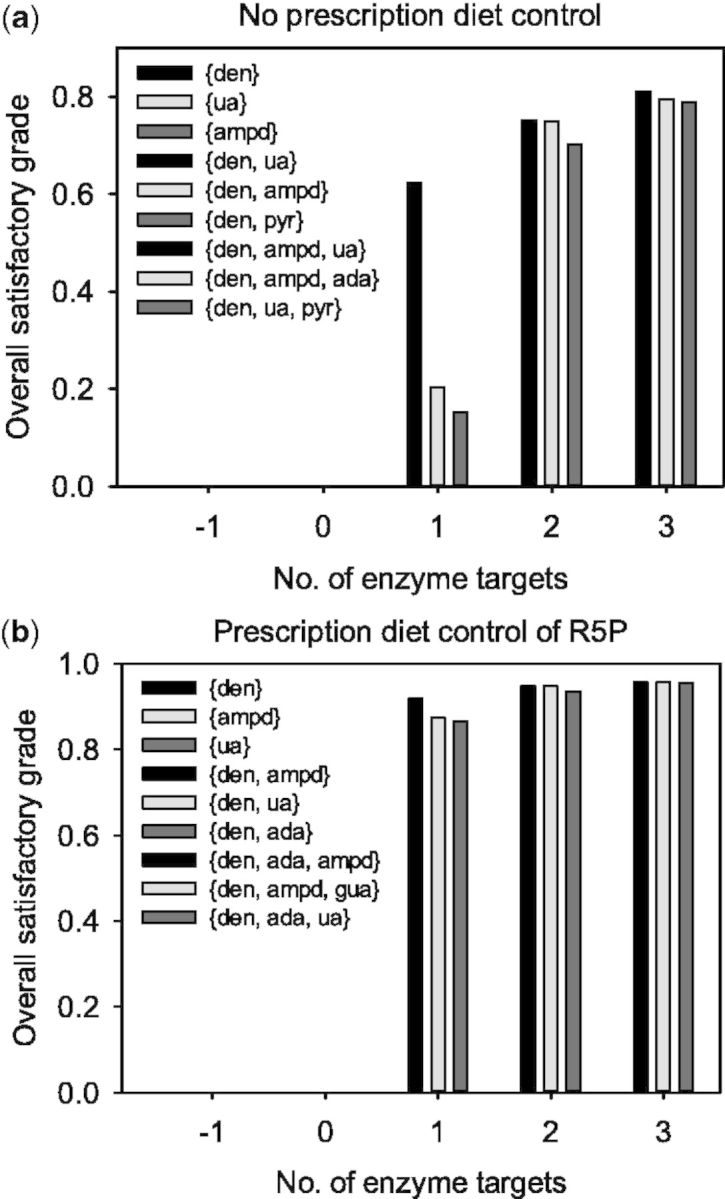
The overall satisfactory grades for the treatment of hyperuricemia caused by PRPPS hyperactivity, which were obtained by applying the triple-objective FEMA problem with or without diet control
There are two kinds of drugs, namely allopurinol and uricosuric agents, used for the present treatment of hyperuricemia (Schlesinger, 2010). Allopurinol, which acts as an XA oxidase {xd} inhibitor, inhibits the production of UA (Shoji et al., 2004). Uricosuric agents, including Benzbromazone, Probenecid and Sulfinpyrazone, increase the excretion of UA {ua}. According to this case study of one enzyme target, we used the therapeutic effect objective to obtain the enzyme targets {xd} and {ua}, which the objective concerning UA were ∼100% satisfactory. Without diet control, {xd} was downregulated to 0.297 and {ua} was upregulated to 1.57 × 10−4 mol min−1 kg−1. With diet control, {xd} was downregulated to 0.429 and {ua} was upregulated to 1.52 × 10−4 mol min−1 kg−1. This result is compatible with the clinical treatment guideline that a diet control is recommended to enhance the effect of drugs for hyperuricemia (Choi et al., 2004).
3.3 Pathological state caused by HGPRT deficiency
The pathological state considered in the second case study is Lesch–Nyhan syndrome, which is a rare inherited disorder caused by HGPRT deficiency, and is produced by mutations in the HPRT gene located on X chromosome. In this case study, HGPRT deficiency was assumed by
reducing the activity of the enzymes GPRT and HPRT to 1% so that the abnormal UA concentrations increased to 145.5 µM, representing a 45% increase from the normal levels.
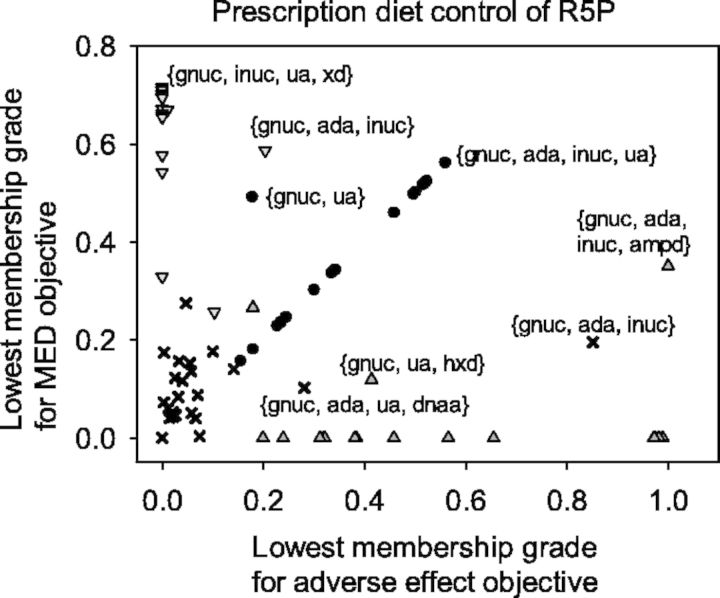
Figure 4 shows the overall satisfactory grades and optimal enzyme targets obtained from various FEMA problems considering different objectives. Although we used a prescription diet control of R5P, only one enzyme target was unable to achieve the goal of curing hyperuricemia caused by the HGPRT deficiency. For the triple-objective FEMA problem with a diet control of R5P, the overall satisfactory grade was >50% for three or four enzyme targets. The triple-objective FEMA problem is a unified optimization framework in which enzyme target identification is combined with decision-making to choose satisfactory results. The unified optimization framework consists of the simultaneous evaluation of therapeutic effect, adverse effect and MED, so that a compromise among these effects can be obtained. Figure 5 shows the lowest membership grades for adverse effects and MED corresponding to various pairs of enzyme targets. For various pairs of enzyme targets, both membership grades (circles in Fig. 5) for adverse effects and MED were nearly identical to the overall satisfaction (Fig. 4a), excluding the enzyme targets {gnuc, ua}.
Fig. 4.
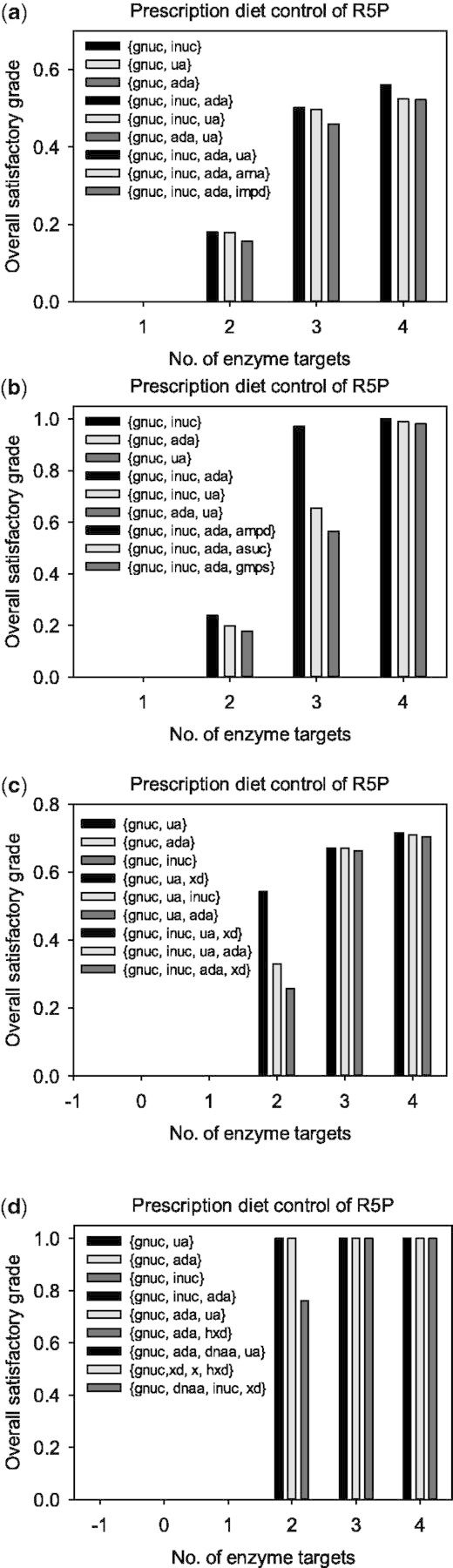
Overall satisfactory grades for the treatment of Lesch–Nyhan disease, which were obtained by applying the single-objective, bi-objective and triple-objective FEMA problem with a diet control; (a) is the triple-objective FEMA problem; (b) and (c) are the bi-objective FEMA problems in which the first and second objectives, or the first and third objectives in Equation (3), respectively, were considered; and (d) is the single-objective FEMA problem
Fig. 5.
Relationship between the lowest membership grades for adverse effect objectives and MED objectives. The circles indicate the results obtained by using the triple-objective FEMA problem. The upward-facing triangles indicate the results of the first- and second-objective FEMA problems. The downward-facing triangles indicate the results of the first- and third-objective FEMA problems. Crosses indicate the results of the first objective FEMA problem
We also omitted the adverse effect or MED objective and both objectives of the FEMA problem to investigate the effectiveness of the study. Figure 4b–d show the overall satisfactory grades and optimal enzyme targets obtained by applying the bi-objective and single FEMA problems. The results shown in Figure 4b correspond to the therapeutic and adverse effect objectives, which are the first and second objectives in Equation (3). For the four enzyme targets, the overall satisfactory grade was 98%, representing a substantial increase compared with the triple-objective problem (Fig. 4a). However, the lowest membership grades (upward-facing triangles in Fig. 5) for MED were lower than those obtained by applying the triple-objective problem, indicating that the enzyme targets required higher doses. By contrast, the other bi-objective problem was used to omit the adverse effect objective. For the three or four enzyme targets, the overall satisfactory grades were >66%, which were greater than those obtained by applying the triple-objective problem. The lowest membership grades (downward-facing triangles in Fig. 5) for the adverse effects were lower than those obtained by applying the triple-objective problem. Both satisfactory grades for adverse effects and MED were considerably low (crosses in Fig. 5), even though we could obtain therapeutic results with a grade of ∼100% (Fig. 4d) by solving the single objective problem.
Finally, we also considered the extreme pathological state caused by the combination of PRPPS overactivity and HGPRT deficiency, which resulted in a UA concentration ∼71% higher than the normal levels. Following similar procedures, we solved the FEMA problems by considering different objectives to determine the overall satisfactory grades and optimal enzyme targets. The computational results are shown in Supplementary Additional File 3, and are similar to those reported in previous case studies. However, we were unable to identify the optimal solution if two enzyme targets were required because this case study is conducted on the most serious pathology. Our results showed that four enzyme targets had a higher overall satisfactory grade than did three enzyme targets. The triple-objective FEMA problem consists of the simultaneous evaluation of therapeutic effect, adverse effects and MED, so that a compromise among these objectives can be achieved. The satisfactory grade for the therapeutic objective can be improved, if one or both of adverse effect and MED objectives are sacrificed in the FEMA problem.
4 DISCUSSION
Many mathematical formulations are available to express reaction rates. Michaelis–Menten rate law is well known in the field of biological systems. Such a model is derived by making quasi steady-state assumptions for some components. More complex rate law expressions, such as Hill model, can be derived using different versions of the quasi steady-state approximation to describe more complex protein-catalyzed dynamics, such as allosteric processes, competitive inhibition and cooperativity. However, a recasting method (Pozo et al., 2011) can be applied to transform Michaelis–Menten–based models as a power-law expression. As a result, the FEMA formulation becomes a convex optimization problem, which is capable of obtaining a global optimal solution.
In the first case study, the enzyme target {den} was selected using FEMA in treating hyperuricemia caused by PRPPS overactivity if one enzyme target was allowable. This enzyme target was also included among the six candidate enzymes selected by the OPDD (Vera et al., 2007). The advantage of the triple objective FEMA problem is that we could simultaneously obtain trade-off satisfactory grades of therapeutic effect, adverse effect and MED objectives. A set of target candidates with higher therapeutic grades can be obtained in the first stage if a two-stage design approach, such as omitting one or both of adverse effects and MED objectives, is used in the FEMA problem. However, membership grades for adverse effect or MED tend to be considerably low. As a result, it is difficult to make a decision based on the candidates suggested.
With a diet control, the treatment of hyperuricemia caused by PRPPS overactivity was effective, which was reflected in enhanced UA excretion {ua} (Fig. 3). Among the current clinical medications for hyperuricemia, uricosuric agents increase the excretion of UA in the urine (Schlesinger, 2010). According to the simulation, the effect of uricosuric agents may be enhanced with diet control. We found that XA oxidase inhibitor {xd}, which is another clinical medication for hyperuricemia, had an overall satisfactory grade of 58% (not shown) for the treatment of hyperuricemia caused by PRPPS overactivity. Other target enzymes selected by using FEMA were not covered by the current medications for hyperuricemia, but they may be good candidates for new drugs development.
The concentration of UA, the pool of inosine derivatives (HX) and XA in the healthy state were 100.3, 9.5 and 5.1 µM, respectively. The concentrations of UA, HX and XA in the hyperuricemic state caused by PRPPS overactivity were 131.0, 40.3 and 14.8 µM, respectively. The concentrations of UA, HX and XA in Lesch–Nyhan disease were 145.4, 69.8 and 22.5 µM, respectively. A higher concentration of HX and XA has a higher probability of causing a cytopathic effect (Palmour et al., 1989). Lesch–Nyhan disease exhibits a higher concentration of HX and XA, which may explain the neurological effects of this disease (Visser et al., 2000). The adjustment of enzyme targets identified by using the triple-objective FEMA could not only reduce the concentration of UA, but also those of HX and XA.
Allopurinol is currently the main drug for hyperuricemia in patients with Lesch–Nyhan disease (Jinnah et al., 2010). However, even with optimal medical care, individuals with Lesch–Nyhan disease typically live into their third or even forth decade of life and few patients live beyond 40 years (Neychev and Jinnah, 2006). According to the computational results, one enzyme target failed to cure hyperuricemia of Lesch–Nyhan disease. Nevertheless, diet control with four enzyme targets, namely {ada, dnaa, gnuc, hxd}, reduced the concentration of UA, HX and XA from 145.4, 69.8 and 22.5 µM to 102.9, 11.1 and 5.6 µM, respectively. This result indicates that the combination of multi-enzyme targets may be beneficial to treat Lesch–Nyhan disease, and points out a direction to develop new medications.
5 CONCLUSION
We introduce fuzzy programming in which the detection of candidate enzyme targets is combined with decision-making strategies to create a unified optimization framework for determining satisfactory targets. This optimal enzyme target design problem is indeed a multi-criteria decision-making problem. A multi-objective optimization method can be generally applied to find the Pareto front for this decision problem, and such Pareto solutions are provided for the designer using his/her biological knowledge to decide candidate enzyme targets. Almost no literature has so far discussed the fuzzy equal operation in the FEMA problem to simultaneously evaluate therapeutic effect, adverse effect and MED. Furthermore, the membership function defined in fuzzy programming can be referred to as an index for determining how to achieve satisfactory levels in the design.
The FEMA approach can also be applied for drug development for other metabolic diseases. Using multiple objectives enables managing various effects, such as therapeutic effects, adverse effects and MED, and the weighting factor for each objective in a unified optimization framework. The objectives in the FEMA framework can be compromised to obtain a satisfactory result. Furthermore, the concentrations of metabolites in the human body are not fixed, but fluctuate within normal ranges. As a result, boundaries for each objective and constraint are not sharply defined. Fuzzy programming has the advantage of enabling the management of these flexible conditions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the National Science Council, Taiwan, ROC, for funding this study.
Funding: National Science Council, Taiwan, ROC (NSC101-2627-B-194-001, NSC102-2627-B-194-003 and NSC100-2221-E- 194-028-MY3).
Conflicts of Interest: none declared.
REFERENCES
- Banga JR. Optimization in computational systems biology. BMC Syst. Biol. 2008;2:47. doi: 10.1186/1752-0509-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascante M, et al. Metabolic control analysis in drug discovery and disease. Nat. Biotechnol. 2002;20:243–249. doi: 10.1038/nbt0302-243. [DOI] [PubMed] [Google Scholar]
- Choi HK, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- Curto R, et al. Mathematical models of purine metabolism in man. Math. Biosci. 1998;151:1–49. doi: 10.1016/s0025-5564(98)10001-9. [DOI] [PubMed] [Google Scholar]
- Fillon TG. Estimating the minimum therapeutically effective dose of a compound via regression modeling and percentile estimation. Stat. Med. 1995;14:925–932. doi: 10.1002/sim.4780140911. [DOI] [PubMed] [Google Scholar]
- Guillen-Gosalbez G, Sorribas A. Identifying quantitative operation principles inn metabolic pathways: a systematic method for searching feasible enzyme activity patterns leading to cellular adaptive responses. BMC Bioinformatics. 2009;10:386. doi: 10.1186/1471-2105-10-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, et al. Attenuated variants of Lesch-Nyhan disease. Brain. 2010;133:671–689. doi: 10.1093/brain/awq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB. Systems biology, metabolic modeling and metabolomics in drug discovery and development. Drug Discov. Today. 2006;11:1085–1092. doi: 10.1016/j.drudis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Le Novère N, et al. A free, centralized database of curated, published, quantitative kinetic models of biochemical and cellular systems. Nucleic Acids Res. 2006;34:D689–D691. doi: 10.1093/nar/gkj092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am. J. Med. 1964;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- Leung EL, et al. Newtork-based drug discovery by integrating systems biology and computional technologies. Brief. Bioinform. 2013;14:491–505. doi: 10.1093/bib/bbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. BioModels Database: An enhanced, curated and annotated resource for published quantitative kinetic models. BMC Syst. Biol. 2010a;4:92. doi: 10.1186/1752-0509-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. BioModels.net Web Services, a free and integrated toolkit for computational modelling software. Brief. Bioinform. 2010b;11:270–277. doi: 10.1093/bib/bbp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. Two-stage flux balance analysis of metabolic networks for drug target identification. BMC Syst. Biol. 2011;5(Suppl. 1):S11. doi: 10.1186/1752-0509-5-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materi W, Wishart DS. Computational systems biology in drug discovery and development: methods and applications. Drug Discov. Today. 2007;12:295–302. doi: 10.1016/j.drudis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Jinnah HA. Sudden death in Lesch-Nyhan disease. Dev. Med. Child Neurol. 2006;11:923–926. doi: 10.1017/S0012162206002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmour RM, et al. Hypoxanthine accumulation and dopamine depletion in Lesch–Nyhan disease. Adv. Exp. Med. Biol. 1989;253:165–172. doi: 10.1007/978-1-4684-5673-8_27. [DOI] [PubMed] [Google Scholar]
- Pozo C, et al. Steady-state global optimization of metabolic non-linear dynamic models through recasting into power-law canonical models. BMC Syst. Biol. 2011;5:137. doi: 10.1186/1752-0509-5-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakawa M. Fuzzy Sets and Interactive Multiobjective Optimization. New York: Plenum Press; 1993. Fundamental of fuzzy set theory. [Google Scholar]
- Sams-Dodd F. Drug discovery: selecting the optimal approach. Drug Discov. Today. 2006;11:465–472. doi: 10.1016/j.drudis.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Schlesinger N. Diagnosing and treating gout: a review to aid primary care physicians. Postgrad. Med. 2010;122:157–161. doi: 10.3810/pgm.2010.03.2133. [DOI] [PubMed] [Google Scholar]
- Scriver CR, et al. The Metabolic Basis of Inherited Disease. New York: Mc Graw-Hill; 1989. Part I; pp. 965–1094. [Google Scholar]
- Shoji A, et al. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51:321–325. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- Stone JA. Model-based drug development survey finds pharmacometrics impacting decision making in the pharmaceutical industry. J. Clin. Pharmacol. 2010;50:20S–30S. doi: 10.1177/0091270010377628. [DOI] [PubMed] [Google Scholar]
- Vera J, et al. Detection of potential enzyme targets by metabolic modeling and optimization: application to a simple enzymopathy. Bioinformatics. 2007;23:2281–2289. doi: 10.1093/bioinformatics/btm326. [DOI] [PubMed] [Google Scholar]
- Vera J, et al. Optimization of biochemical systems through mathematical programming: methods and applications. Comput. Oper. Res. 2010;37:1427–1438. [Google Scholar]
- Visser JE, et al. Lesh-Nyhan disease and the basal ganglia. Brain Res. Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Voit EO. Metabolic modelling: a tool of drug discovery in the postgenomicera. Drug Discov. Today. 2002;7:621–628. doi: 10.1016/s1359-6446(02)02280-8. [DOI] [PubMed] [Google Scholar]
- Wu WH, et al. Multi-objective optimization of enzyme manipulations in metabolic networks considering resilience effects. BMC Syst. Biol. 2011;5:145. doi: 10.1186/1752-0509-5-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.