Abstract
Background
Although marital status has been reported as a prognostic factor in different cancer types, its prognostic effect on hormone receptor (HR) positive male breast cancer (MBC) is unclear. The objective of the present analysis was to assess the effects of marital status on survival in patients with HR positive MBC.
Material/Methods
Patients diagnosed with HR positive MBC from 1990 to 2014 in the Surveillance, Epidemiology, and End Results (SEER) database were included. Kaplan-Meier survival analysis and Cox proportional hazard regression were used to identify the effects of marital status on cancer-specific survival (CSS) and overall survival (OS).
Results
A total of 3612 cases were identified in this study. Married patients had better 5-year CSS and 5-year OS than unmarried men. In multivariate Cox regression models, unmarried patients also showed higher mortality risk for both CSS and OS, independent of age, race, grade, stage, PR status, HER2 status, and surgery. Subgroup survival analysis according to different ER/PR status showed that married patients had beneficial CSS results only in ER+/PR+ subtype, and CSS in the married and unmarried groups did not significantly differ by TNM stage. The results were further confirmed in the 1: 1 matched group.
Conclusions
Marital status was an important prognostic factor for survival in patients with HR positive MBC. Unmarried patients are at greater risk of death compared with married groups. The survival benefit for married patients remained even after adjustment, which indicates the importance of spousal support in MBC.
MeSH Keywords: Breast Neoplasms, Male; Marital Status; Receptors, Estrogen; Survival Analysis
Background
Male breast cancer (MBC) is a rare disease, accounting for around 1% of all breast cancers [1]. Although rare, its incidence has steadily increased [2]. In 1991, an estimated 900 men in the United States were diagnosed with breast cancer; the number increased to 2550 men by 2018 [3,4]. Although the mortality and survival rates of both male and female breast cancer patients have significantly improved, progress in men has been slower [5]. Due to lack of prospective data and limited retrospective series, MBC usually has been treated according to recommendations for female breast cancer (FBC) [6]. Although MBC shares some features with FBC, it significantly differs in prognostic factors, epidemiological factors, and biological behavior [7,8]. For example, MBC tends to have higher rates of hormone receptor (HR) positivity compared to FBC [5,7]. MBC is frequently positive for ERα (91–95%) and/or PR (80–81%) [5,9,10]. Therefore, identifying prognostic factors in HR positive MBC can help to manage the majority of MBC cases.
Most cancer research focuses on biological aspects; the effect of social or psychological factors, such as marital status, on survival in cancer patients is much less studied. However, marriage has been shown to function as a positive social support with a survival benefit for cancer patients [11]. The relationship between marital status and survival has been studied for some cancers, including hepatocellular cancer [12], gastric cancer [13], biliary tract cancer [14], colorectal cancer [15], prostate cancer [16], pancreatic cancer [17] and breast cancer [18]. Marital status is an independent prognostic factor for survival, and married patients gain a significant survival benefit versus the unmarried, who are single, widowed, or separated/divorced patients [19,20]. As for MBC, only 1 previous study reported that unmarried men were more likely to present with advanced disease at diagnosis and were at greater risk for poorer outcomes compared with married men [21]. However, in that study, researchers did not control for confounding variables and the outcomes may have been subject to a selection bias. Additionally, they only took stage into consideration and could not discuss the effect of marriage on survival from other aspects, such as different ER/PR subtypes.
To our knowledge, no study has analyzed the influence of marital status on prognosis in HR positive MBC. Therefore, data from Surveillance, Epidemiology, and End Results (SEER) database was used to investigate the influence of marital status on survival and on potential subtypes in HR positive MBC.
Material and Methods
Patient population and study design
We obtained permission to access SEER research-data files using the reference number 15983-Nov2016. Because no information from the SEER database requires informed patient consent, it is considered exempt from the ethical approval requirements of the institutional review board. The case listing in this retrospective cohort study was generated by SEER *Stat version 8.3.5, which contained data from 18 population-based cancer registries (1973–2014) and covered approximately 28% of the United States population (http://seer.cancer.gov/). Male patients with first primary stages I–III and HR positive breast cancer diagnosed between 1990 and 2014 were selected from the SEER database. We selected the period starting from 1990 because HR status was introduced to SEER in 1990. We choose 3612 patients according to the following criteria: (a) at least 18 years old at diagnosis; (b) male; (c) diagnosed between 1990 and 2014; (d) known marital status; (e) known race; (f) known residence type; (g) pathologically confirmed breast cancer; (h) breast cancer as the first and only malignant cancer diagnosis; (i) known histology; (j) known grade; (k) American Joint Committee on Cancer stages I–III at diagnosis; (l) known tumor size; (m) known lymph node status; (n) HR positive (ER+ or PR+); (o) known HER2 status; (p) known surgical condition; (q) known radiotherapy condition; (r) active follow-up; (s) known survival months after diagnosis; and (t) known cause of death. We excluded patients for whom the aforementioned data was missing. Eligible patients were categorized by marital status, age at diagnosis, race, residence type, histology, tumor grade, pathologic T stage, pathologic N stage, ER status, PR status, HER2 status, surgery and radiotherapy. Marital status at diagnosis was the primary variable of interest, and classified as married or unmarried, the latter of which included patients who were single, divorced, separated, and widowed. The methods were performed in accordance with the approved guidelines.
Statistical analyses
Clinicopathological features were compared between different marital groups using the t-test and the χ2 test as appropriate. Cancer-specific survival (CSS) and overall survival (OS) were estimated with the Kaplan-Meier method; differences were calculated by the log rank test. Multivariate Cox proportional hazards regression models were built for analyzing hazard ratios of different prognostic variables. OS was defined as the interval from breast cancer diagnosis until death due to all causes (including breast cancer) or last follow-up. CSS was measured from the date of diagnosis to either the date of breast cancer death or the date of last contact. All variables for which P<0.05 in univariate analyses were initially included in multivariate analyses; for the Cox proportional hazards regression, age, race, PR, and radiotherapy were included although P>0.05 for their respective univariate analyses, because they are common confounders of MBC. We performed a 1: 1 case-matched analysis based on marital status and matching for age, race, residence, histology, grade, T-stage, N-stage, ER status, PR status, HER2 status, surgery and radiotherapy, using the propensity score matching method to control for confounding variables. These analyses were performed with SPSS software version 23.0 (IBM Corporation, Armonk, NY, USA). P<0.05 (2-sided) was considered significant.
Results
Patient baseline characteristics
From 1990 to 2014, 7959 men were diagnosed with invasive breast cancer in the SEER database. From these records, we excluded patients with missing records or exact data on any of the abovementioned variables. The flow diagram of the study selection process is shown in Figure 1. Finally, we identified 3612 eligible patients with MBC.
Figure 1.
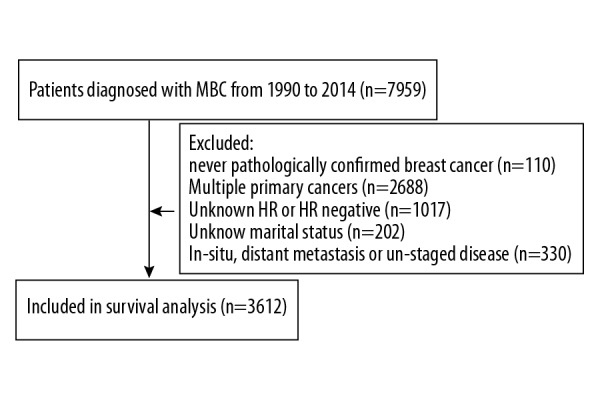
Diagram of analytic cohort for survival analysis. HR – hormone receptor; MBC – male breast cancer.
When we stratified HR positive MBC patient by marital status, significant differences emerged (Table 1). Of these patients, 2548 (70.5%) were married and 1064 (29.5%) were unmarried. The 2 groups significantly differed in age, race, pathologic T stage, pathologic N stage, and surgical history. The mean age of the entire cohort was 65 years (range: 23–103 years). Unmarried patients were younger (64.8±14.3 vs. 65.3±12.3 years old, P=0.003), and had a lower proportion (77.0% vs. 89.2%, P<0.0001) of white patients and a higher proportion (19.7% vs. 9.9%, P<0.0001) of black patients than the married group. The married group was also more likely to have tumors that were smaller in size (35.0% vs. 26.6%, P<0.0001), less likely to have lymph node metastases (50.3% vs. 43.6%, P<0.0001) and had a higher rate of surgery (87.5% vs. 85.2%, P=0.013).
Table 1.
Baseline characteristic of male patients with HR positive breast cancer in SEER database, by marital status.
| Characteristic (%) | Total (%) | Married (%) | Unmarried (%) | P value |
|---|---|---|---|---|
| 3612 (100.0) | 2548 (70.5) | 1064 (29.5) | ||
| Age | 0.003 | |||
| <50 | 445 (12.3) | 283 (11.1) | 162 (15.2) | |
| 50–64 | 1259 (34.9) | 895 (35.1) | 364 (34.2) | |
| ≥65 | 1908 (52.8) | 1370 (53.8) | 538 (50.6) | |
| Race | <0.0001 | |||
| White | 2932 (81.2) | 2113 (82.9) | 819 (77.0) | |
| Black | 462 (12.8) | 252 (9.9) | 210 (19.7) | |
| Other | 202 (5.6) | 171 (6.7) | 31 (2.9) | |
| Unknown | 16 (0.4) | 12 (0.5) | 4 (0.4) | |
| Residence type | 0.935 | |||
| Metropolitan | 3238 (89.6) | 2287 (89.8) | 951 (89.4) | |
| Non-metropolitan | 360 (10.0) | 251 (9.9) | 109 (10.2) | |
| Unknown | 14 (0.4) | 10 (0.4) | 4 (0.4) | |
| Histology | 0.103 | |||
| Ductal | 3153 (87.3) | 2230 (87.5) | 923 (86.7) | |
| Lobular | 33 (0.9) | 28 (1.1) | 5 (0.5) | |
| Others | 426 (11.8) | 290 (11.4) | 136 (12.8) | |
| Grade | 0.369 | |||
| Well/moderately differentiated | 2208 (61.1) | 1574 (61.8) | 634 (59.6) | |
| Poorly/undifferentiated | 1183 (32.8) | 825 (32.4) | 358 (33.6) | |
| Unknown | 221 (6.1) | 149 (5.8) | 72 (6.8) | |
| Pathologic T stage | <0.0001 | |||
| T0–T1 | 1174 (32.5) | 891 (35.0) | 283 (26.6) | |
| T2 | 1166 (32.3) | 778 (30.5) | 388 (36.5) | |
| T3 | 139 (3.8) | 86 (3.4) | 53 (5.0) | |
| Unknown | 1133 (31.4) | 793 (31.1) | 340 (32.0) | |
| Pathologic N stage | <0.0001 | |||
| N0 | 1746 (48.3) | 1282 (50.3) | 464 (43.6) | |
| N1 | 1008 (27.9) | 729 (28.6) | 279 (26.2) | |
| N2 | 335 (9.3) | 227 (8.9) | 108 (10.2) | |
| N3 | 172 (4.8) | 109 (4.3) | 63 (5.9) | |
| Unknown | 351 (9.7) | 201 (7.9) | 150 (14.1) | |
| ER status | 0.192 | |||
| Negative | 31 (0.9) | 19 (0.7) | 12 (1.1) | |
| Positive | 3578 (99.1) | 2528 (99.2) | 1050 (98.7) | |
| Unknown | 3 (0.1) | 1 (0.0) | 2 (0.2) | |
| PR status | 0.549 | |||
| Negative | 374 (10.4) | 265 (10.4) | 109 (10.2) | |
| Positive | 3161 (87.5) | 2233 (87.6) | 928 (87.2) | |
| Unknown | 77 (2.1) | 50 (2.0) | 27 (2.5) | |
| HER2 status | 0.866 | |||
| Negative | 1130 (31.3) | 792 (31.1) | 338 (31.8) | |
| Positive | 144 (4.0) | 100 (3.9) | 44 (4.1) | |
| Unknown | 2338 (64.7) | 1656 (65.0) | 682 (64.1) | |
| Surgery | 0.013 | |||
| No | 101 (2.8) | 58 (2.3) | 43 (4.0) | |
| Yes | 3143 (87.0) | 2230 (87.5) | 913 (85.8) | |
| Unknown | 368 (10.2) | 260 (10.2) | 108 (10.2) | |
| Radiation | 0.605 | |||
| No | 2696 (74.6) | 1908 (74.9) | 788 (74.1) | |
| Yes | 916 (25.4) | 640 (25.1) | 276 (25.9) |
ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; PR – progesterone receptor. SEER – The Surveillance Epidemiology and End Results.
Impact of marital status on cancer-specific survival of HR positive MBC patients
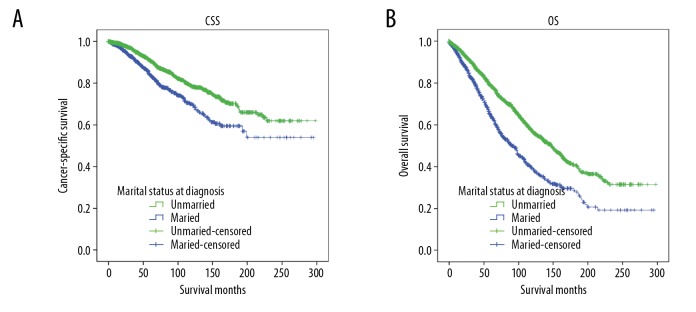
We used Kaplan-Meier analysis and log-rank test to evaluate the impact of marital status on CSS of HR positive MBC patients (Figure 2A). The married group had a better 5-year CSS rate than the unmarried group (90.8% vs. 83.8%, χ2=28.501, P<0.0001). In univariate analyses, race (P<0.0001), histology (P<0.0001), grade (P<0.0001), pathologic T stage (P<0.0001), pathologic N stage (P<0.0001), PR status (P<0.0001), HER2 status (P=0.039), surgery (P<0.0001), and radiotherapy (P<0.0001) were also significantly associated with CSS in HR positive MBC patients (Table 2). In multivariate Cox regression analysis of these factors, the unmarried group were found to have a significantly greater risk for cancer-specific mortality (hazards ratio: 1.394, 95% CI: 1.153–1.687, P=0.001). Race, histology, grade, pathologic T stage, pathologic N stage, PR status, and surgery were validated as independent prognostic factors as well.
Figure 2.
Kaplan-Meier survival curves for cancer-specific survival (CSS) and overall survival (OS) in married vs. unmarried male patients with hormone receptor (HR) positive breast cancer. (A) CSS: χ2=28.501, P<0.0001; (B) OS: χ2=79.335, P<0.0001.
Table 2.
Univariate and multivariate analyses for of CSS predictors in men with hormone receptor-positive breast cancer.
| Variables | 5-year CSS (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log Rank χ2 test | P value | HR | 95% CI | P value | ||
| Marital status | 28.501 | <0.0001 | ||||
| Married | 90.8 | Reference | ||||
| Unmarried | 83.8 | 1.394 | 1.153–1.687 | 0.001 | ||
| Age | 1.214 | 0.545 | ||||
| <50 | 89.8 | Reference | ||||
| 50–64 | 91.0 | 0.950 | 0.728–1.238 | 0.702 | ||
| ≥65 | 87.0 | 1.203 | 0.925–1.566 | 0.169 | ||
| Race | 37.467 | <0.0001 | ||||
| White | 89.9 | Reference | ||||
| Black | 79.9 | 1.731 | 1.369–2.189 | <0.0001 | ||
| Other | 91.5 | 0.935 | 0.617–1.417 | 0.753 | ||
| Residence type | 0.734 | 0.693 | ||||
| Metropolitan | 89.1 | |||||
| Non-metropolitan | 86.4 | |||||
| Histology | 16.697 | <0.0001 | ||||
| Ductal | 88.1 | Reference | ||||
| Lobular | 92.4 | 0.761 | 0.240–2.412 | 0.642 | ||
| Others | 93.9 | 0.600 | 0.416–0.867 | 0.007 | ||
| Grade | 55.794 | <0.0001 | ||||
| Well/moderately differentiated | 92.1 | Reference | ||||
| Poorly/undifferentiated | 82.8 | 1.611 | 1.336–1.942 | <0.0001 | ||
| Pathologic T stage | 69.301 | <0.0001 | ||||
| T0–T1 | 96.5 | Reference | ||||
| T2 | 84.9 | 2.199 | 1.577–3.067 | <0.0001 | ||
| T3 | 77.2 | 2.838 | 1.649–4.883 | <0.0001 | ||
| Pathologic N stage | 313.683 | <0.0001 | ||||
| N0 | 95.2 | Reference | ||||
| N1 | 88.7 | 2.366 | <0.0001 | |||
| N2 | 79.4 | 4.235 | <0.0001 | |||
| N3 | 67.7 | 6.261 | <0.0001 | |||
| ER status | 0.156 | 0.925 | ||||
| Negative | 89.2 | |||||
| Positive | 88.9 | |||||
| PR status | 26.386 | <0.0001 | ||||
| Negative | 84.2 | Reference | ||||
| Positive | 89.3 | 0.669 | 0.531–0.844 | 0.001 | ||
| HER2 status | 6.467 | 0.039 | ||||
| Negative | 93.1 | Reference | ||||
| Positive | 83.8 | 1.316 | 0.575–3.012 | 0.516 | ||
| Surgery | 57.175 | <0.0001 | ||||
| No | 74.2 | Reference | ||||
| Yes | 90.3 | 0.505 | 0.290–0.880 | 0.016 | ||
| Radiation | 17.788 | <0.0001 | ||||
| No | 90.1 | Reference | ||||
| Yes | 85.3 | 0.982 | 0.802–1.203 | 0.860 | ||
CI – confidence interval; CSS – cause-specific survival; ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; R – hazard ratio; PR – progesterone receptor.
Interestingly, we observed a better 5-year CSS in the no-radiotherapy group (90.1%) than among those who received radiotherapy (85.3%). Complicated influence of unadjusted confounders was a possible reason, but the 2 groups showed no significant difference in the multivariate analysis (Table 2).
Impact of marital status on overall survival (OS) of HR positive MBC patients
Univariate analysis (Kaplan-Meier analysis) and multivariate analysis (multivariate Cox regression analysis) were also used to evaluate the effect of marital status on the overall survival (OS) of HR positive MBC patients (Table 3). Unmarried men had worse 5-year OS than did married men (64.2% vs. 78.6%; χ2=79.335, P<0.0001; Figure 2B and Table 3). In univariate analysis, age (P<0.0001), race (P<0.0001), histology (P=0.002), grade (P<0.0001), pathologic T stage (P<0.0001), pathologic N stage (P<0.0001), PR status (P=0.017), HER2 status (P=0.008), and surgery (P<0.0001) were also associated with OS and they were further included in multivariate Cox regression analyses (Table 3). Marital status was also an independent prognostic factor in the multivariate analysis after adding the other prognostic factors. Unmarried status significantly increased overall mortality risk (hazard ratio: 1.548, 95% CI: 1.373–1.746, P<0.0001). We also included radiotherapy in the multivariate analysis because it is an important confounder of MBC, although the P value of radiotherapy in univariate analysis was >0.05; radiotherapy still demonstrated a protective effect on OS (hazard ratio: 0.824, 95% CI: 0.717–0.947, P=0.006) after multivariate Cox regression. Age, race, grade, pathologic T stage, pathologic N stage, HER2 status, and surgery were also associated with OS in multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analyses of OS predictors in men with hormone receptor-positive breast cancer.
| Variables | 5-year OS (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log Rank χ2 test | P value | HR | 95% CI | P value | ||
| Marital status | 79.335 | <0.0001 | ||||
| Married | 78.6 | Reference | ||||
| Unmarried | 64.2 | 1.548 | 1.373–1.746 | <0.0001 | ||
| Age | 280.203 | <0.0001 | ||||
| <50 | 86.9 | Reference | ||||
| 50–64 | 85.4 | 1.167 | 0.930–1.464 | 0.182 | ||
| ≥65 | 64.0 | 3.126 | 2.534–3.857 | <0.0001 | ||
| Race | 18.314 | <0.0001 | ||||
| White | 74.9 | Reference | ||||
| Black | 67.4 | 1.378 | 1.166–1.629 | <0.0001 | ||
| Other | 82.5 | 0.791 | 0.601–1.043 | 0.097 | ||
| Residence type | 1.771 | 0.412 | ||||
| Metropolitan | 74.6 | |||||
| Non-metropolitan | 72.7 | |||||
| Histology | 12.566 | 0.002 | ||||
| Ductal | 73.5 | Reference | ||||
| Lobular | 92.4 | 0.435 | 0.179–1.056 | 0.066 | ||
| Others | 80.2 | 0.825 | 0.679–1.001 | 0.052 | ||
| Grade | 35.760 | <0.0001 | ||||
| Well/moderately differentiated | 78.8 | Reference | ||||
| Poorly/undifferentiated | 66.5 | 1.327 | 1.175–1.498 | <0.0001 | ||
| Pathologic T stage | 113.607 | <0.0001 | ||||
| T0–T1 | 87.4 | Reference | ||||
| T2 | 68.0 | 1.858 | 1.531–2.255 | <0.0001 | ||
| T3 | 58.9 | 2.363 | 1.680–3.324 | <0.0001 | ||
| Pathologic N stage | 470.864 | <0.0001 | ||||
| N0 | 85.2 | Reference | ||||
| N1 | 74.4 | 1.669 | 1.444–1.930 | <0.0001 | ||
| N2 | 66.0 | 2.479 | 2.035–3.019 | <0.0001 | ||
| N3 | 58.6 | 2.805 | 2.226–3.534 | <0.0001 | ||
| ER status | 0.265 | 0.876 | ||||
| Negative | 76.1 | |||||
| Positive | 74.5 | |||||
| PR status | 8.173 | 0.017 | ||||
| Negative | 71.6 | Reference | ||||
| Positive | 74.5 | 0.870 | 0.738–1.026 | 0.098 | ||
| HER2 status | 9.636 | 0.008 | ||||
| Negative | 76.7 | Reference | ||||
| Positive | 66.1 | 1.625 | 1.019–2.591 | 0.041 | ||
| Surgery | 109.767 | <0.0001 | ||||
| No | 39.7 | Reference | ||||
| Yes | 77.1 | 0.694 | 0.494–0.976 | 0.036 | ||
| Radiation | 0.113 | 0.737 | ||||
| No | 74.0 | Reference | ||||
| Yes | 75.8 | 0.824 | 0.717–0.947 | 0.006 | ||
CI – confidence interval; ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; HR – hazard ratio; OS – overall survival; PR – progesterone receptor.
Survival analysis in matched groups
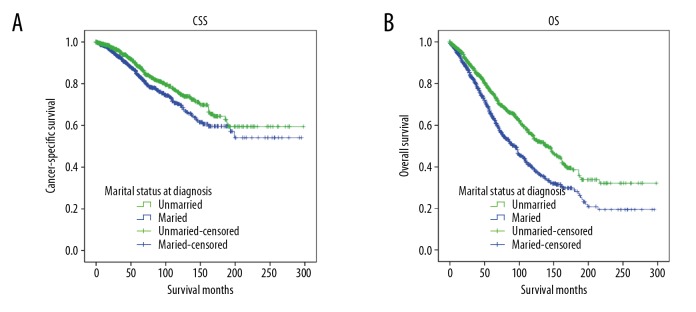
To control for confounding variables, we used case matching to determine if these factors were responsible for the benefit seen with marital status. A total of 1049 cases in the married group were successfully matched with 1049 cases from the unmarried group (Table 4). We also analyzed CSS and OS by marital status with the case-matched cohorts. As with the total group, the married group showed significant CSS and OS benefits in stratified log-rank tests with matched pairs (Figure 3), which was confirmed through multivariate analysis with the Cox proportional hazards model performed on the propensity-matched cohort. Univariate analysis of CSS and OS in matched groups also showed results similar to Tables 2 and 3. However, when compared with an unmatched cohort, race and histology were not significantly associated with OS in the matched cohort. In addition to marital status, multivariate Cox analyses further confirmed the independent prognostic significance of tumor grade, pathologic T stage, and pathologic N stage in CSS and OS. We also found that PR status and surgery were significantly associated with CSS (hazard ratio: 0.473,95% CI: 0.555–0.995, P=0.046), but not OS. Although race did not reach significance in univariate analysis, white race was associated with improved OS in multivariate analysis when compared to black race (hazard ratio: 1.285,95% CI: 1.063–1.553, P=0.009). The results are summarized in Tables 5 and 6.
Table 4.
Characteristics of male patients with breast cancer by marital status, in 1: 1 matched groups.
| Characteristic (%) | Total (%) | Married (%) | Unmarried (%) | P value |
|---|---|---|---|---|
| 2098 (100.0) | 1049 (100.0) | 1049 (100.0) | ||
| Age | 0.088 | |||
| <50 | 349 (16.6) | 189 (18.0) | 160 (15.3) | |
| 50–64 | 686 (32.7) | 323 (30.8) | 363 (34.6) | |
| ≥65 | 1063 (50.7) | 537 (51.2) | 526 (50.1) | |
| Race | 0.633 | |||
| White | 1649 (78.6) | 830 (79.1) | 819 (78.1) | |
| Black | 372 (17.7) | 177 (16.9) | 195 (18.6) | |
| Other | 67 (3.2) | 36 (3.4) | 31 (3.0) | |
| Unknown | 10 (0.5) | 6 (0.6) | 4 (0.4) | |
| Residence type | 0.599 | |||
| Metropolitan | 1861 (88.7) | 924 (88.1) | 937 (89.3) | |
| Non-metropolitan | 227 (10.8) | 119 (11.3) | 108 (10.3) | |
| Unknown | 10 (0.5) | 6 (0.6) | 4 (0.4) | |
| Histology | 0.929 | |||
| Ductal | 1818 (86.7) | 908 (86.6) | 910 (86.7) | |
| Lobular | 9 (0.4) | 4 (0.4) | 5 (0.5) | |
| Others | 271 (12.9) | 137 (13.1) | 134 (12.8) | |
| Grade | 0.649 | |||
| Well/moderately differentiated | 1246 (59.4) | 619 (59.0) | 627 (59.8) | |
| Poorly/undifferentiated | 701 (33.4) | 349 (33.3) | 352 (33.6) | |
| Unknown | 151 (7.2) | 81 (7.7) | 70 (6.7) | |
| Pathologic T stage | 0.706 | |||
| T0–T1 | 563 (26.8) | 280 (26.7) | 283 (27.0) | |
| T2 | 747 (35.6) | 365 (34.8) | 382 (36.4) | |
| T3 | 100 (4.8) | 48 (4.6) | 52 (5.0) | |
| Unknown | 688 (32.8) | 356 (33.9) | 332 (31.6) | |
| Pathologic N stage | 0.756 | |||
| N0 | 958 (45.7) | 494 (47.1) | 464 (44.2) | |
| N1 | 539 (25.7) | 260 (24.8) | 279 (26.6) | |
| N2 | 207 (9.9) | 100 (9.5) | 107 (10.2) | |
| N3 | 125 (6.0) | 62 (5.9) | 63 (6.0) | |
| Unknown | 269 (12.8) | 133 (12.7) | 136 (13.0) | |
| ER status | 0.732 | |||
| Negative | 26 (1.2) | 15 (1.4) | 11 (1.0) | |
| Positive | 2070 (98.7) | 1033 (98.5) | 1037 (98.9) | |
| Unknown | 2 (0.1) | 1 (0.1) | 1 (0.1) | |
| PR status | 0.397 | |||
| Negative | 237 (11.3) | 128 (12.2) | 109 (10.4) | |
| Positive | 1812 (86.4) | 898 (85.6) | 914 (87.1) | |
| Unknown | 49 (2.3) | 23 (2.2) | 26 (2.5) | |
| HER2 status | 0.418 | |||
| Negative | 688 (32.8) | 356 (33.9) | 332 (31.6) | |
| Positive | 93 (4.4) | 49 (4.7) | 44 (4.2) | |
| Unknown | 1317 (62.8) | 664 (61.4) | 673 (64.2) | |
| Surgery | 0.792 | |||
| No | 68 (3.2) | 32 (3.1) | 36 (3.4) | |
| Yes | 1813 (86.4) | 905 (86.3) | 908 (86.6) | |
| Unknown | 217 (10.3) | 112 (10.7) | 105 (10.0) | |
| Radiation | 1.000 | |||
| No | 1550 (73.9) | 775 (73.9) | 775 (73.9) | |
| Yes | 548 (26.1) | 274 (26.1) | 274 (26.1) |
ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; PR – progesterone receptor.
Figure 3.
Kaplan-Meier survival curves of 1: 1 matched group for cancer-specific survival (CSS) and overall survival (OS) in married vs. unmarried male patients with hormone receptor (HR) positive breast cancer: (A) CSS: χ2=4.730, P=0.030. (B) OS: χ2=30.037, P<0.0001.
Table 5.
Univariate and multivariate analyses of CSS predictors in 1: 1 matched groups of men with breast cancer.
| Variables | 5-year CSS (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log Rank χ2 test | P value | HR | 95% CI | P value | ||
| Marital status | 4.730 | 0.030 | ||||
| Married | 87.4 | Reference | ||||
| Unmarried | 84.3 | 1.273 | 1.021–1.586 | 0.032 | ||
| Age | 1.737 | 0.420 | ||||
| <50 | 88.8 | Reference | ||||
| 50–64 | 86.7 | 1.028 | 0.754–1.401 | 0.863 | ||
| ≥65 | 84.2 | 1.203 | 0.882–1.641 | 0.242 | ||
| Race | 12.183 | 0.007 | ||||
| White | 87.0 | Reference | ||||
| Black | 80.6 | 1.475 | 1.130–1.926 | 0.004 | ||
| Other | 84.9 | 0.889 | 0.454–1.744 | 0.733 | ||
| Residence type | 1.899 | 0.387 | ||||
| Metropolitan | 86.4 | |||||
| Non-metropolitan | 81.5 | |||||
| Histology | 7.669 | 0.022 | ||||
| Ductal | 85.0 | Reference | ||||
| Lobular | 85.7 | 1.358 | 0.187–9.867 | 0.762 | ||
| Others | 90.9 | 0.749 | 0.505–1.109 | 0.149 | ||
| Grade | 28.095 | <0.0001 | ||||
| Well/moderately differentiated | 89.0 | Reference | ||||
| Poorly/undifferentiated | 79.4 | 1.438 | 1.142–1.811 | 0.002 | ||
| Pathologic T stage | 27.715 | <0.0001 | ||||
| T0–T1 | 94.0 | Reference | ||||
| T2 | 81.2 | 1.879 | 1.248–2.828 | 0.003 | ||
| T3 | 76.6 | 2.370 | 1.287–4.365 | 0.006 | ||
| Pathologic N stage | 169.063 | <0.0001 | ||||
| N0 | 92.9 | Reference | ||||
| N1 | 85.3 | 2.354 | 1.728–3.207 | <0.0001 | ||
| N2 | 77.6 | 3.979 | 2.764–5.727 | <0.0001 | ||
| N3 | 67.1 | 5.452 | 3.745–7.939 | <0.0001 | ||
| ER status | 0.519 | 0.772 | ||||
| Negative | 90.8 | |||||
| Positive | 85.8 | |||||
| PR status | 8.441 | 0.015 | ||||
| Negative | 85.0 | Reference | ||||
| Positive | 85.6 | 0.743 | 0.555–0.995 | 0.046 | ||
| HER2 status | 5.322 | 0.070 | ||||
| Negative | 90.9 | Reference | ||||
| Positive | 77.3 | 1.448 | 0.581–3.608 | 0.427 | ||
| Surgery | 30.247 | <0.0001 | ||||
| No | 71.5 | Reference | ||||
| Yes | 87.0 | 0.438 | 0.227–0.848 | 0.014 | ||
| Radiation | 11.689 | 0.001 | ||||
| No | 87.1 | Reference | ||||
| Yes | 82.7 | 1.054 | 0.821–1.352 | 0.681 | ||
CI – confidence interval; CSS – cause-specific survival; ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; HR – hazard ratio; PR – progesterone receptor.
Table 6.
Univariate and multivariate analysis of OS predictors in 1: 1 matched groups of men with breast cancer.
| Variables | 5-year OS (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Log Rank χ2 test | P value | HR | 95% CI | P value | ||
| Marital status | 30.037 | <0.0001 | ||||
| Married | 74.5 | Reference | ||||
| Unmarried | 64.8 | 1.519 | 1.315–1.754 | <0.0001 | ||
| Age | 176.879 | <0.0001 | ||||
| <50 | 85.8 | Reference | ||||
| 50–64 | 79.9 | 1.207 | 0.929–1.569 | 0.159 | ||
| ≥65 | 58.0 | 2.965 | 2.332–3.769 | <0.0001 | ||
| Race | 5.568 | 0.135 | ||||
| White | 69.6 | Reference | ||||
| Black | 68.6 | 1.285 | 1.063–1.553 | 0.009 | ||
| Other | 69.5 | 0.835 | 0.547–1.275 | 0.403 | ||
| Residence type | 3.073 | 0.215 | ||||
| Metropolitan | 70.1 | |||||
| Non-metropolitan | 65.0 | |||||
| Histology | 6.614 | 0.037 | ||||
| Ductal | 68.5 | Reference | ||||
| Lobular | 85.7 | 0.747 | 0.183–3.054 | 0.685 | ||
| Others | 76.4 | 0.815 | 0.646–1.028 | 0.084 | ||
| Grade | 28.177 | <0.0001 | ||||
| Well/moderately differentiated | 75.0 | Reference | ||||
| Poorly/undifferentiated | 59.6 | 1.379 | 1.184–1.607 | <0.0001 | ||
| Pathologic T stage | 63.425 | <0.0001 | ||||
| T0–T1 | 84.4 | Reference | ||||
| T2 | 63.2 | 1.971 | 1.516–2.561 | <0.0001 | ||
| T3 | 57.1 | 2.420 | 1.621–3.613 | <0.0001 | ||
| Pathologic N stage | 279.309 | <0.0001 | ||||
| N0 | 81.6 | Reference | ||||
| N1 | 69.9 | 1.588 | 1.310–1.926 | <0.0001 | ||
| N2 | 64.0 | 2.332 | 1.815–2.996 | <0.0001 | ||
| N3 | 59.6 | 2.517 | 1.908–3.319 | <0.0001 | ||
| ER status | 0.656 | 0.720 | ||||
| Negative | 75.1 | |||||
| Positive | 69.5 | |||||
| HER2 status | 9.335 | 0.009 | ||||
| Negative | 72.8 | Reference | ||||
| Positive | 61.3 | 1.557 | 0.882–2.748 | 0.127 | ||
| Surgery | 64.162 | <0.0001 | ||||
| No | 36.9 | Reference | ||||
| Yes | 72.2 | 0.694 | 0.464–1.040 | 0.077 | ||
| Radiation | 0.324 | 0.569 | ||||
| No | 68.1 | Reference | ||||
| Yes | 73.7 | 0.865 | 0.728–1.029 | 0.101 | ||
CI – confidence interval; ER – estrogen receptor; HER2 – human epidermal growth factor receptor 2; HR – hazard ratio; OS – overall survival; PR – progesterone receptor.
Stratification analysis according to ER/PR status and tumor stage
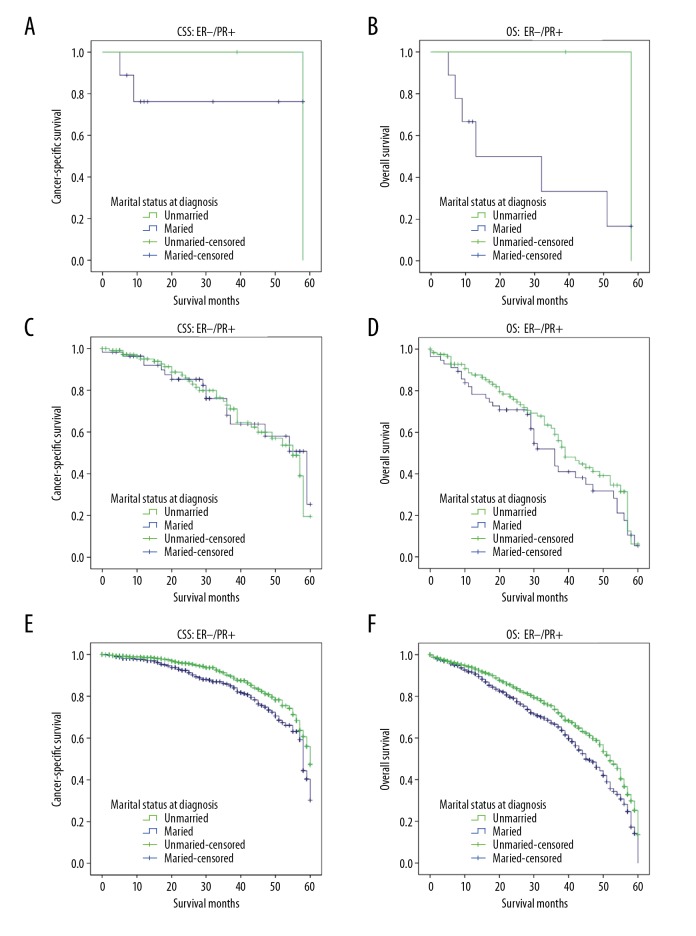
Based on ER and PR expression, HR positive MBC can be further classified as ER−/PR+, ER+/PR− and ER+/PR+ subtypes. To further investigate the prognostic effect of marital status on CSS and OS in different subtypes, we stratified all the cases by ER and PR expression and performed univariate analyses. Of the 3532 cases, 31 were ER−/PR+, 374 were ER+/PR− and 3127 were ER+/PR+. Distribution of these subgroups did not significantly differ among the married and unmarried groups (P=0.513; Supplementary Table 1). Kaplan-Meier curves for the 3 subgroups showed that only married patients with ER+/PR+ subtypes had better 5-year CSS and OS, but not the other 2 subtypes (Figure 4). Consequently, marriage clearly benefited HR positive MBC prognosis among patients with ER+/PR+ subtype. Relevance between marital status and stage at diagnosis was also shown by univariate logistic regression models (see Supplementary Table 2), which found no significant difference in CSS between the married and unmarried groups with respect to TNM stage, which was further confirmed in matched groups.
Figure 4.
Kaplan-Meier survival analysis of the effect of marital status on cancer-specific survival (CSS) and overall survival (OS) in 3612 male patients with breast cancer by estrogen receptor (ER) and progesterone receptor (PR) status. (A) CSS ER−/PR+: χ2=0.016, P=0.899; (B) OS ER−/PR+: χ2=0.968, P=0.325; (C) CSS ER+/PR−: χ2=0.030, P=0.862; (D) OS ER+/PR−: χ2=1.578, P=0.209; (E) CSS ER+/PR+: χ2=9.557, P=0.002; (F) OS ER+/PR+: χ2=16.475, P<0.001.
Discussion
Because MBC is a relatively rare disease, prognostic evaluation in MBC is often modeled after FBC. However, it is known that FBC and MBC differ biologically. Incidence of hormone receptor expression is strikingly different, and it is reportedly higher in MBC than in FBC [22]. Among MBC cases, receptor phenotypes were: ER+/PR+ (86%), ER+/PR− (6%), ER−/PR+ (3%) and ER−/PR− (5%) [23]. Moreover, the presence of HR positive tumors in men does not increase with age, which is common observed in FBC [24]. As most MBC are HR positive, we carried out this population-based study to better characterize prognostic factors.
It has been confirmed that marital status is considered as a protective survival factor in different cancer types [25–27]. However, effects of marital status on HR positive MBC survival have not been fully examined. In this study, we first explored the influence of marital status on CSS and OS in patients with HR positive MBC; we found that both CSS and OS were better in married patients than in their single, divorced, separated, or widowed counterparts. In multivariable analyses, the beneficial effect for married patients remained, even after adjusting for age, race, residence, histology, grade, pathologic T stage, pathologic N stage, ER status, PR status, HER2 status, surgery, and radiotherapy. As HR status is an important biologic prognostic indicator in breast cancer, subgroup analysis later evaluated the impact of marital status on survival by different HR phenotypes.
To our knowledge, this is the first study to find that marriage is only associated with improved CSS among patients with the ER+/PR+ subtype. An earlier hypothesis for worse survival among unmarried patients was that they tended to present with delayed diagnoses at advanced tumor stages [18,20]. However, we found no significant difference in CSS between the married and unmarried groups by TNM stage, which was confirmed in matched groups. Obviously, delayed diagnosis alone cannot explain the poorer survival outcomes in unmarried patients.
Our result show that marital status is associated with survival in patients with HR positive MBC and have emphasized the relationship between marital status and survival rather than causal relationships. Why marital status of married patients serves as a protective factor warrants further study. However, accumulating evidence suggested that physiological changes that accompany stress and depression may affect cancer outcomes through different mechanisms. Decreased psychosocial support and psychological stress has been reportedly associated with immune dysfunction, which may contribute to tumor progression and mortality [28,29]; and lack of social support can depress natural killer cell activity [30], which could result in disorders of various endocrine hormones [31,32]. Sex hormone disorder is closely related to occurrence and development of breast cancer. A cohort study has associated depression and anxiety with breast cancer recurrence [33]. Breast cancer patients, and male patients in particular, suffer from significant psychological and socioeconomic stress [34]. With no spouses to share their emotional burdens, unmarried cancer patients may experience more distress, depression, and anxiety than married patients [35,36]. Although unmarried patients may have support from friends and family, this support did not lead to lower psychological distress, whereas any beneficial social support received by male cancer patients from friends and family may be mediated by spousal support [36]. Psychosocial support from a spouse may ultimately translate to less distress and greater fighting spirit to improve adherence to cancer treatment [37,38]. Married patients are also more likely than unmarried patients to have better family financial circumstances, to seek treatment at more prestigious medical centers, to accept curative therapies, and to comply with treatment, all of which may contribute to better outcomes [39–41].
This study had some limitations. First, as important information regarding chemotherapy or systemic therapy was not provided in SEER database, and could not be adjusted by our analyses, whether they contributed to survival differences by marital status is unclear. Second, the SEER database only provides the marital status at diagnosis, but details about the duration or quality of the marriage, or any changes in marital status, were not tracked, which might influence the prognosis of MBC patients. Third, some important demographic factors were not recorded in the SEER databases, such as education, insurance, income status, and family status, all of which may influence the effect of marital status on cancer survival [42,43]. Fourth, data on ER, PR, and HER2 status were collected from different local pathology laboratories and could not be further verified, which might increase the possibilities of bias.
Conclusions
Despite these potential limitations, this study demonstrated that marital status is an independent prognostic factor for survival in HR positive MBC patients. Unmarried patients are at greater risk for overall and tumor cause-specific mortality independent of age, race, grade, stage, surgery, and radiotherapy. Particularly, subgroup analysis showed that the beneficial survival results of married patients in HR positive MBC is associated with ER+/PR+ subtype. The main reasons for poor survival in unmarried patients can be explained hypothetically by social support and psychological factors. Therefore, more social and psychological supports should be provided for unmarried patients. Further understanding of the potential associations among the marital status, psychosocial factors and survival outcomes may help to identify sound strategies of treatment in HR positive MBC patients.
Supplementary Tables
Supplementary Table 1.
Men with breast cancer by ER/PR status.
| Subtype | Total (%) | Married (%) | Unmarried (%) | P value |
|---|---|---|---|---|
| 3532 (100.0) | 2497 (100.0) | 1035 (100.0) | ||
| ER–PR+ | 31 (0.9) | 19 (0.8) | 12 (1.2) | 0.513 |
| ER+PR− | 374 (10.6) | 265 (10.6) | 109 (10.5) | |
| ER+PR+ | 3127 (88.5) | 2213 (88.6) | 914 (88.3) |
ER – estrogen receptor; PR – progesterone receptor.
Supplementary Table 2.
Characteristics and subgroup analysis of the effect of marital status on CSS by tumor stage in men with hormone receptor-positive breast cancer.
| Stage | Married (%) | Unmarried (%) | Log rank χ2 test (c) | P value | Log rank χ2 test (c) | P value |
|---|---|---|---|---|---|---|
| I | 13.8% | 11.3% | 0.117 | 0.732 | 2.462 | 0.117 |
| II | 16.4% | 18.5% | 3.677 | 0.055 | 0.678 | 0.410 |
| III | 6.0% | 7.7% | 1.120 | 0.290 | 1.181 | 0.277 |
CSS – cause-specific survival; Log Rank χ2 test (a), adjusted Log Rank χ2 test (adjusted for age, race, residence, histology, grade, pathologic T stage, pathologic N stage, ER status, PR status, HER2 status, surgery and radiotherapy); Log Rank χ2 test (c), crude Log Rank χ2 test.
Acknowledgment
The authors would like to thank the SEER program for providing open access to the database.
Footnotes
Disclosure
The authors declare that they have no competing interests.
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115:429–30. doi: 10.1007/s10549-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 3.Boring CC, Squires TS, Tong T. Cancer statistics, 1991. Cancer J Clin. 1991;41:19–36. doi: 10.3322/canjclin.41.1.19. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2018. Atlanta, Ga: American Cancer Society; 2018. [Google Scholar]
- 5.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: A population-based comparison with female breast cancer. J Clin Oncol. 2010;28:232–39. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severson TM, Zwart W. A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr Relat Cancer. 2017;24:R27–34. doi: 10.1530/ERC-16-0225. [DOI] [PubMed] [Google Scholar]
- 7.Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24:1434–43. doi: 10.1093/annonc/mdt025. [DOI] [PubMed] [Google Scholar]
- 8.Rizzolo P, Silvestri V, Tommasi S, et al. Male breast cancer: Genetics, epigenetics, and ethical aspects. Ann Oncol. 2013;24(Suppl 8):viii75–82. doi: 10.1093/annonc/mdt316. [DOI] [PubMed] [Google Scholar]
- 9.Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: A population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson C, Koliadi A, Johansson I, et al. High proliferation is associated with inferior outcome in male breast cancer patients. Mod Pathol. 2013;26:87–94. doi: 10.1038/modpathol.2012.145. [DOI] [PubMed] [Google Scholar]
- 11.Nipp RD, El-Jawahri A, Fishbein JN, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122:2110–16. doi: 10.1002/cncr.30025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Wang X, Huang R, et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep. 2017;7:41695. doi: 10.1038/srep41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Gan L, Wu Z, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of adult patients with gastric cancer: A population-based study. Oncotarget. 2017;8:22385–405. doi: 10.18632/oncotarget.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song W, Miao DL, Chen L. Survival rates are higher in married patients with biliary tract cancer: A population-based study. Oncotarget. 2018;9:9531–39. doi: 10.18632/oncotarget.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Dai W, Li Y, et al. The effect of marital status by age on patients with colorectal cancer over the past decades: A SEER-based analysis. Int J Colorectal Dis. :2018. doi: 10.1007/s00384-018-3017-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Huang TB, Zhou GC, Dong CP, et al. Marital status independently predicts prostate cancer survival in men who underwent radical prostatectomy: An analysis of 95,846 individuals. Oncol Lett. 2018;15:4737–44. doi: 10.3892/ol.2018.7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XD, Qian JJ, Bai DS, et al. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: An analysis of the SEER database. Oncotarget. 2016;7:24880–87. doi: 10.18632/oncotarget.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne C, Ostir GV, Du X, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Chen P, Qian JJ, et al. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: An analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget. 2016;7:79442–52. doi: 10.18632/oncotarget.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–76. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adekolujo OS, Tadisina S, Koduru U, et al. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am J Mens Health. 2017;11:1190–99. doi: 10.1177/1557988316669044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shandiz F, Tavassoli A, Sharifi N, et al. Hormone receptor expression and clinicopathologic features in male and female breast cancer. Asian Pac J Cancer Prev. 2015;16:471–74. doi: 10.7314/apjcp.2015.16.2.471. [DOI] [PubMed] [Google Scholar]
- 23.Fentiman IS. The biology of male breast cancer. Breast. 2018;38:132–35. doi: 10.1016/j.breast.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Hotko YS. Male breast cancer: Clinical presentation, diagnosis, treatment. Exp Oncol. 2013;35:303–10. [PubMed] [Google Scholar]
- 25.Song W, Tian C. The effect of marital status on survival of patients with gastrointestinal stromal tumors: A SEER database analysis. Gastroenterol Res Pract. 2018;2018:5740823. doi: 10.1155/2018/5740823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa LJ, Brill IK, Brown EE. Impact of marital status, insurance status, income, and race/ethnicity on the survival of younger patients diagnosed with multiple myeloma in the United States. Cancer. 2016;122:3183–90. doi: 10.1002/cncr.30183. [DOI] [PubMed] [Google Scholar]
- 27.Shi RL, Qu N, Lu ZW, et al. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 2016;5:2145–54. doi: 10.1002/cam4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. 1999;85:51–61. doi: 10.1016/s0165-1781(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 29.Sklar LS, Anisman H. Stress and coping factors influence tumor growth. Science. 1979;205:513–15. doi: 10.1126/science.109924. [DOI] [PubMed] [Google Scholar]
- 30.Levy SM, Herberman RB, Whiteside T, et al. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73–85. doi: 10.1097/00006842-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS, Biron CA, Brunson KW, et al. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 33.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser NC, Hartoonian N, Owen JE. Toward a cancer-specific model of psychological distress: Population data from the 2003–2005 National Health Interview Surveys. J Cancer Surviv. 2010;4:291–302. doi: 10.1007/s11764-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 35.Baine M, Sahak F, Lin C, et al. Marital status and survival in pancreatic cancer patients: a SEER based analysis. PLoS One. 2011;6:e21052. doi: 10.1371/journal.pone.0021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: What do oncologists need to know? Ann Oncol. 2010;21:877–83. doi: 10.1093/annonc/mdp398. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi K, Akechi T, Suzuki S, et al. Lack of marital support and poor psychological responses in male cancer patients. Support Care Cancer. 2003;11:604–10. doi: 10.1007/s00520-003-0495-z. [DOI] [PubMed] [Google Scholar]
- 38.Saito-Nakaya K, Nakaya N, Fujimori M, et al. Marital status, social support and survival after curative resection in non-small-cell lung cancer. Cancer Sci. 2006;97:206–13. doi: 10.1111/j.1349-7006.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med. 2003;57:2137–47. doi: 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 40.Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol. 2010;39:710–17. doi: 10.1093/ije/dyq048. [DOI] [PubMed] [Google Scholar]
- 41.Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804. doi: 10.1186/1471-2458-11-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyson MD, Andrews PE, Etzioni DA, et al. Marital status and prostate cancer outcomes. Can J Urol. 2013;20:6702–6. [PubMed] [Google Scholar]
- 43.Fossati N, Nguyen DP, Trinh QD, et al. The impact of insurance status on tumor characteristics and treatment selection in contemporary patients with prostate cancer. J Natl Compr Canc Netw. 2015;13:1351–58. doi: 10.6004/jnccn.2015.0164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Men with breast cancer by ER/PR status.
| Subtype | Total (%) | Married (%) | Unmarried (%) | P value |
|---|---|---|---|---|
| 3532 (100.0) | 2497 (100.0) | 1035 (100.0) | ||
| ER–PR+ | 31 (0.9) | 19 (0.8) | 12 (1.2) | 0.513 |
| ER+PR− | 374 (10.6) | 265 (10.6) | 109 (10.5) | |
| ER+PR+ | 3127 (88.5) | 2213 (88.6) | 914 (88.3) |
ER – estrogen receptor; PR – progesterone receptor.
Supplementary Table 2.
Characteristics and subgroup analysis of the effect of marital status on CSS by tumor stage in men with hormone receptor-positive breast cancer.
| Stage | Married (%) | Unmarried (%) | Log rank χ2 test (c) | P value | Log rank χ2 test (c) | P value |
|---|---|---|---|---|---|---|
| I | 13.8% | 11.3% | 0.117 | 0.732 | 2.462 | 0.117 |
| II | 16.4% | 18.5% | 3.677 | 0.055 | 0.678 | 0.410 |
| III | 6.0% | 7.7% | 1.120 | 0.290 | 1.181 | 0.277 |
CSS – cause-specific survival; Log Rank χ2 test (a), adjusted Log Rank χ2 test (adjusted for age, race, residence, histology, grade, pathologic T stage, pathologic N stage, ER status, PR status, HER2 status, surgery and radiotherapy); Log Rank χ2 test (c), crude Log Rank χ2 test.