Abstract
Systemic amyloid A (AA) amyloidosis is a major cause of morbidity and mortality among captive cheetahs. The self-aggregating AA protein responsible for this disease is a byproduct of serum amyloid A (SAA) protein degradation. Transcriptional induction of the SAA1 gene is dependent on both C/EBPβ and NF-κB cis-acting elements within the promoter region. In cheetahs, 2 alleles exist for a single guanine nucleotide deletion in the putative NF-κB binding site. In this study, a novel genotyping assay was developed to screen for the alleles. The results show that the SAA1A−97delG allele is associated with decreased SAA protein concentrations in the serum of captive cheetahs (n = 58), suggesting genetic differences at this locus may be affecting AA amyloidosis prevalence. However, there was no significant difference in the frequency of the SAA1A−97delG allele between individuals confirmed AA amyloidosis positive versus AA amyloidosis negative at the time of necropsy (n = 48). Thus, even though there is evidence that having more copies of the SAA1A−97delG allele results in a potentially protective decrease in serum concentrations of SAA protein in captive cheetahs, genotype is not associated with this disease within the North American population. These results suggest that other factors are playing a more significant role in the pathogenesis of AA amyloidosis among captive cheetahs.
Keywords: Acute phase response, amyloidosis, cheetah, genetic typing of insertion/deletion, serum amyloid A
The acute phase response (APR) is a systemic reaction that occurs early during inflammation, initiated by cells of the innate immune system such as macrophages (Baumann and Gauldie 1994; Paltrinieri 2008). The liver is one of the primary targets for systemic inflammatory mediators such as the IL-1 type cytokines, IL-1β, and TNF-α, as it is the primary location of acute phase protein (APP) production (Baumann and Gauldie 1994; Marhaug and Dowton 1994; Jensen and Whitehead 1998; Cerón et al. 2005; Paltrinieri 2008). APPs, by definition, are proteins whose concentration in serum significantly increase or decrease during the APR and there is a high degree of similarity in the response patterns of several APPs to systemic inflammatory mediators during the APR across species (Baumann and Gauldie 1994; Cerón et al. 2005; Paltrinieri 2008).
The major APPs that have been identified to date in the domestic cat are the serum amyloid A (SAA) protein and alpha-1-acid glycoprotein (AGP) (Kajikawa et al. 1999; Cerón et al. 2005; Paltrinieri 2008). In humans, the concentration of SAA found in circulation bound to high-density lipoproteins increases as much as 1000-fold within 24–48h of the initiation of inflammation (Marhaug and Dowton 1994). The increase in SAA observed in domestic cats during the APR, approximately a 10–100-fold increase, is lower in magnitude compared to humans and other species, but still represents a major APP (Kajikawa et al. 1999; Giordano et al. 2004; Cerón et al. 2005). Additionally, multiple studies have shown that SAA concentrations in sick cats are significantly higher than SAA concentrations in healthy cats (Kajikawa et al. 1999; Sasaki et al. 2003; Giordano et al. 2004; Tamamoto et al. 2008; Kann et al 2012). Similarly, Depauw et al. (2014) identified SAA and haptoglobin as major APPs in cheetah, with significant increases in protein concentrations among unhealthy individuals.
The SAA protein is well conserved throughout evolution, indicating an important biological function (Marhaug and Dowton 1994). Though its function is not completely understood, possible roles of SAA during the APR include the recruitment of inflammatory cells to the primary site of inflammation, lipid metabolism and transport, increasing cytokine production in monocytes/macrophages, stimulating nitric oxide production in macrophages, and scavenging for oxidized cholesterol, preventing prolonged tissue damage (Jensen and Whitehead 1998; Cerón et al. 2005; Paltrinieri 2008; Tamamoto et al. 2012). The SAA protein has a short half-life: ~90min in normal serum (Marhaug and Dowton 1994), and the concentration of SAA in serum returns to normal following the termination of inflammation.
The N-terminal region of the SAA protein is the precursor for the amyloid A (AA) protein, the first 10–15 amino acid residues of which are amyloidogenic (Marhaug and Dowton 1994). In abundance, the AA protein polymerizes to form insoluble fibrils, which are deposited into the liver, kidney, spleen, and other tissues (Marhaug and Dowton 1994). AA amyloidosis is a disease induced by the over-accumulation and incomplete degradation of SAA, polymerization of AA protein into fibrils and subsequent tissue deposition, resulting in a disruption in organ functionality (Marhaug and Dowton 1994). Thus, it is unsurprising that elevated SAA concentrations in serum have been found to correlate with AA amyloidosis and other inflammatory diseases in humans (Marhaug and Dowton 1994; Lachmann et al. 2007).
The CCAAT-enhancer-binding proteins (C/EBPs) are primarily responsible for the upregulation of SAA transcription during the APR (Ray and Ray 1994). The C/EBPβ binding site in the promoter of the human SAA gene is located within the 265bp region upstream of the transcription start site (Marhaug and Dowton 1994). Also located within this region is a binding site for the transcription factor NF-κB (nuclear factor kappa-light chain enhancer of activated B cells) (Marhaug and Dowton 1994). In the presence of stimulation from proinflammatory cytokines, such as TNF-α (Beg et al. 1993; Scheinman et al. 1995) and IL-1β (Barnes and Karin 1997), the activated forms of C/EBPβ and NF-κB enter the nucleus and bind to the promoter region of target genes associated with inflammatory and immune responses, such as SAA (Beg et al. 1993; Ray and Ray 1994; Barnes and Karin 1997). IL-1β, TNF-α, and IL-6 proinflammatory cytokines have all been shown to stimulate hepatic SAA production during the APR (Baumann and Gauldie 1994; Marhaug and Dowton 1994; Jensen and Whitehead 1998; Cerón et al. 2005; Paltrinieri 2008).
Four SAA protein genes (SAA1A, SAA1B, SAA3A, SAA3B) have been identified in the cheetah genome, linked on a single chromosome, and are monomorphic such that all polymorphisms are observed in noncoding regions of the genes (Chen et al. 2012). Chen et al. (2012) found that gene expression of both SAA1 and SAA3 is higher in the liver tissue of cheetahs with amyloidosis, suggesting that an increase in SAA protein production by the liver is important in the etiology of systemic amyloidosis. The SAA1 protein encoded by the SAA1 gene is the precursor to the AA protein identified as the major component of amyloid fibrils deposited into tissues during systemic amyloidosis in cheetahs (Johnson et al. 1997; Ofri et al. 1997; Bergstrom et al. 2006). The structure of the cheetah SAA1 genes show high similarity to the human SAA1 and mouse Saa1 genes (Zhang et al. 2008).
Transcriptional induction of the SAA1 gene in cheetahs by IL-1β and IL-6 is dependent on the presence of both C/EBPβ and NF-κB elements (Zhang et al. 2008). Nucleotide sequences of NF-κB and C/EBPβ target sites are highly conserved between cheetah and human (Zhang et al. 2008). However, in contrast to SAA1A, the SAA1B gene is not expressed in the liver, even in the presence of inflammation, suggesting a negative regulatory element exists upstream of −667bp (Chen et al. 2012). Zhang et al. (2008) identified 2 alleles for a polymorphism consisting of a single guanine nucleotide deletion (SNP) in the putative NF-κB binding site of the SAA1A promoter in cheetah. More recently, Chen et al. (2012) identified 3 haplotypes carrying the single guanine nucleotide deletion. These SAA1A−97delG alleles are associated with reduced transcriptional activity of the SAA1A gene in vitro (Zhang et al. 2008), though the effect in vivo has yet to be evaluated.
Systemic amyloid A amyloidosis is an increasingly important cause of morbidity and mortality in captive cheetahs, affecting up to 70% of individuals at necropsy (Ofri et al. 1997; Papendick et al. 1997; Munson et al. 2005) and despite the high prevalence of AA amyloidosis observed in captive cheetahs in both North America and South Africa, wild cheetahs are virtually unaffected (Munson et al. 2005). The aim of this study was to determine if the SAA1A−97delG SNP in the putative NF-κB binding site of the SAA1A promoter is associated with a decreased production of SAA protein in vivo and if so, if the difference in AA amyloidosis prevalence between the wild and captive cheetah populations could be attributable to genetic differences at this locus.
Methods
Sample Collection
Banked cheetah fecal (n = 38) and serum (n = 20) samples from captive North American cheetahs were obtained from a repository at the Smithsonian Conservation Biology Institute (SCBI) in Front Royal, VA, and National Zoological Park (NZP) in Washington, DC for genotypic and SAA protein analysis, respectively. Serum samples came from cheetahs only housed at either the NZP or SCBI. Cheetahs ranged in age from 2 to 12 years old (median age 5.5 years) and were of variable health status (13 healthy, 4 sick, 3 with unknown health status). Animals were housed individually (females, n = 8) or in sibling groups (males, n = 12) in enclosures ranging from ~25×25 m at NZP and ~40×50 m at SCBI and fed a beef-based diet (Natural Balance Pet Foods Inc., Pacoima, CA) 5 day/week, whole prey (rabbit) 1 day/week and beef bones (femur, ox tail) 2 day/week. Cheetahs had ad libitum access to water, a permanent barn structure (with heat when ambient temperature was ≤30°F) and an unheated yard shelter.
Previously extracted DNA or buffy coat samples (n = 47) and banked serum samples (n = 38; 24 females, 14 males) were obtained from the Cheetah Conservation Fund (CCF) in Namibia from wild-born cheetahs for genotypic and SAA protein analysis, respectively. Serum samples were collected from cheetahs housed in captivity, either temporarily or permanently, at CCF. Cheetahs ranged in age from 0 to 15 years old (median age 6 years) and were of variable health status (3 sick, 35 with unknown health status). Animals were housed in groups, varying in size from 1 to 8 individuals. Only one group had mixed sexes (a sibling group with both males and females). Enclosure sizes ranged from 0.05 to 48 hectares. The diet consisted of 1.5–2.5kg (meat on the bone) fresh horse and donkey meat per day, 6 day/week, with a vitamin and mineral supplement (Predator exotict, Healthtech Laboratories, Midrand, Gauteng, 1685, Republic of South Africa; 25–30g/animal). Cheetahs had ad libitum access to water, playtrees, and shade.
The Association of Zoos and Aquariums (AZA) Cheetah Species Survival Plan® (SSP) pathology archives were reviewed for cases of amyloidosis by a board certified veterinary pathologist (KAT). Sections of liver and kidney were routinely processed for histopathology and stained with hematoxylin and eosin as well as Congo Red stains and evaluated for the presence or absence of amyloid per previously published methods (Papendick et al. 1997). Sections of liver, kidney, spleen, or muscle tissue from a subset of cases (n = 48) with known amyloidosis status and available either frozen (−80 °C) or formalin-fixed were obtained for DNA extraction and genotyping.
DNA Isolation
DNA was extracted from fecal samples, buffy coat, and fresh or formalin fixed tissues using the QIAamp® DNA Stool Mini Kit (QIAGEN®: following the protocol for the isolation of DNA from stool for human DNA analysis), the QIAamp® DNA Blood Mini Kit (QIAGEN®: following the protocol for DNA purification from blood or body fluids, spin protocol), and the PUREGENE® Genomic DNA Purification Kit (Gentra Systems: following the solid tissue protocol with an extended incubation time in the Cell Lysis Buffer), respectively.
Amplification and Genotyping of the NF-κB Binding Site in the SAA1A Promoter Region
The putative NF-κB binding site within the SAA1A promoter region −90 to −99bp upstream of the transcription start site was amplified using a semi-nested polymerase chain reaction (PCR). Semi-nested PCR (using initial PCR product as template for second reaction) was used to get adequate concentrations of the product from degraded DNA samples (feces). The initial external primer pair (F3A, SAA1R; Table 1) amplified a 716bp segment of the SAA1A promoter specifically; the seminested reaction with an alternative forward primer (SAA1F; Table 1) produced a smaller PCR product (186bp) containing the putative NF-κB binding site. The first PCR product was either purified using the QIAquick® Gel Extraction Kit (QIAGEN®) or was treated with ExoSAP-IT® (Affymetrix) before being used as template for the second reaction. Both polymerase chain reactions were carried out using GoTaq® Green Master Mix (Promega). The touchdown PCR protocol entailed an initial denaturing step of 94 °C for 10min, followed by 10 cycles of 94 °C for 15s (denaturation), 60–50 °C for 30s (annealing; temperature was dropped by 1 °C per cycle) and 72 °C for 45s (extension), then 30 cycles of 94 °C for 15s (denaturation), 50 °C for 30s (annealing) and 72 °C for 45s (extension), followed by a final extension step of 72 °C for 30min. A subset of the PCR products (n = 86) were genotyped by standard Sanger sequencing via GENEWIZ® Inc. To prepare samples for shipment, the PCR products were purified using the QIAquick® Gel Extraction Kit (QIAGEN®) and premixed with the SAA1F primer. Chromatograms were interpreted for determination of genotype. SAA1A+/+ and SAA1A−97delG/−97delG genotypes contained clear distinguishable peaks on the chromatogram and were distinguished from each other by the number of guanine residues at position −97. SAA1A+/−97delG heterozygotes generated a chromatogram with overlapping peaks beginning at the −97 guanine deletion site in the SAA1A promoter (Supplementary Figure 1).
Table 1.
Primer sequences used for amplification of the SAA1A promoter
| Primer | Locationa | Sequence | Use |
|---|---|---|---|
| F3Ab | −745 to −724 bp | 5′-CAGATGGCCTCTGGTAGGTTTC-3′ | SAA1A specific forward primer |
| SAA1Rc | −30 to −49 bp | 5′-ACTGTGCCCTCCCCGTTGGG-3′ | SAA1 nonspecific reverse primer |
| SAA1Fc | −215 to −196 bp | 5′-GACCGGCCAAGCTGGCTTCC-3′ | SAA1 nonspecific forward primer |
| SAA1F-FAMc | −215 to −196 bp | 5′-/6-FAM/GACCGGCCAAGCTGGCTTCC-3′ | Fluorescently labeled SAA1 nonspecific forward primer |
aPosition upstream of the SAA1A transcription start site.
bChen et al. (2012).
cThis study, modified from Zhang et al. (2008).
An alternative genotyping method was developed for this study, using a modified forward primer with a fluorescent label (SAA1F-FAM; Table 1) for the seminested PCR. Fluorescently labeled PCR products (n = 47) were run on the ABI PRISM® 310 Genetic Analyzer and analyzed with fragment size polymorphism using GeneMapper® version 4; homozygotes produced a single peak, and heterozygotes produced 2 peaks. Homozygous genotypes could be distinguished based on PCR product length (Supplementary Figure 2).
It was also tested whether the fluorescently labeled second round PCR primers (SAA1F-FAM/R; nonspecific: amplifies both SAA1A and SAA1B promoters because they have perfect homology up through the −666th position) could be used as an independent genotyping assay, without the initial PCR round with the specific F3A forward primer. For this, the SAA1 promoter region was amplified for 6 cheetahs (2 with each genotype) and results compared to those of the nested PCR. The SAA1B gene lacks the SAA1A−97delG SNP (Chen et al. 2012), thus amplification of both SAA1A and SAA1B promoters simultaneously produced 4 amplified products: 2 (corresponding to the SAA1B gene) always appearing as SAA1A+ and the other 2 being dictated by the SAA1A genotype. Peak number and peak height were used to assign SAA1A genotypes.
Quantification of SAA Protein Concentration in Serum
SAA concentration was measured in serum samples using the PhaseTM Range Multispecies SAA ELISA kit (Tridelta Development Ltd: Cat No. TP 802). Serum samples were diluted 1:100 in the provided sample diluent before use. Anti-SAA/horseradish peroxidase (HRP) conjugate (50 µl) and sample/control/standard (50 µl, in duplicate) were added to each pre-coated well and incubated for 1.5h on a shaker at room temperature. After washing, 100µl of tetramethylbenzidine (TMB) substrate solution was added to each well, and the plate was incubated for 15min at room temperature. Stop solution was added (100 µl) and absorbance for each well was read at 450nm using 620nm as a reference. Limit of detection for the assay was 3.125ng/ml. The interassay coefficients of variation (CV) for 2 internal controls (n = 5 assays) were 8.5% and 27.2%.
Statistical Analysis
Allele frequencies for the captive North American and wild Namibian cheetah populations were estimated using the software MENDEL (Lange et al. 2013) utilizing known genotypes and complete pedigree information available for all individuals. Genotype and allele frequencies were compared between populations using a Chi-square test of homogeneity. Significant associations were declared when P < 0.05.
The additive (a) and dominance (d) genetic effects of the SAA1A−97delG allele on SAA concentration in serum were determined using an animal model:
where the ’s (fixed effects) included population (CCF vs. NZP-SCBI), sex (male vs. female), and health status (healthy, sick, or unknown), age is included as a covariate, and additional polygenic additive variance () is estimated based on pedigree information. Additive and dominance genetic variances were calculated using formulas 8.3b and 8.4, respectively, from Falconer and Mackay (1996) utilizing the allele frequencies under the null hypothesis from the MENDEL analysis described above. Analysis was performed using the MIXED procedure in SAS® Studio 3.1 (SAS Institute Inc, 2014) with the Satterthwaite (DDFM = SAT) option for adjustment of denominator degrees of freedom and incorporation of a covariance structure determined by the numerator relationship matrix generated using PROC INBREED as described in Tempelman and Rosa (2004). Significant associations were declared when P < 0.05. Tukey’s multiple mean comparison test was used to compare SAA concentrations across levels of health status.
Allele frequencies for the deceased representatives of the captive North American cheetah population with known AA amyloidosis diagnosis (based on histology) were estimated using the software MENDEL (Lange et al. 2013) utilizing known genotypes and complete pedigree information available for all individuals. Genotype and allele frequencies were compared between individuals confirmed with and without AA amyloidosis using a Chi-square test of homogeneity.
Data Archiving
In fulfillment of data archiving guidelines (Baker 2013), genotypic and SAA protein concentration data have been deposited with Dryad.
Results
SAA1A Amplification and Genotyping
DNA was isolated and the SAA1A promoter successfully amplified from all fecal, buffy coat, and tissue samples. It was determined that both Sanger sequencing (n = 86) and the alternative genotyping method (using the fluorescent primer—with or without the seminested PCR; n = 47) were effective in determining an individual’s genotype at this locus (−97 position). No additional indels (insertions/deletions) were identified among the 86 sequences. Sequences were not examined for additional SNPs in the 186bp amplified PCR products of the promoter because previous research has not found any additional polymorphisms within this region and the fidelity of the Taq polymerase used was unknown.
SAA Concentration in Captive Cheetahs is Associated with Genotype
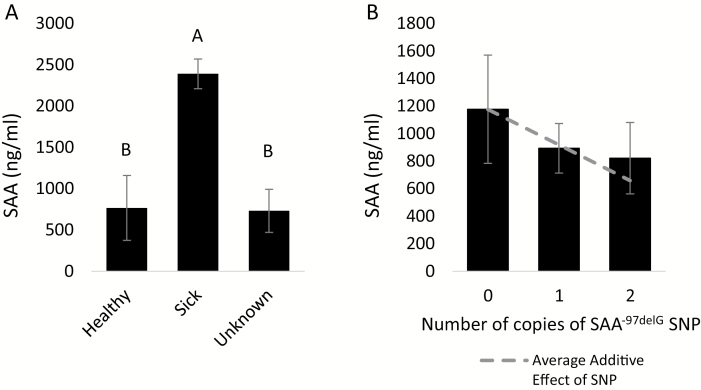
Serum concentrations of SAA ranged from <3.125ng/ml (below the limit of detection)—4707ng/ml across all cheetah serum samples. There were no variations in SAA concentrations attributable to population or sex, and no linear association with age. Health status was significantly associated with SAA, such that animals with known illness’ had significantly higher SAA concentrations in serum than either individuals known to be healthy or with unknown health status (P = 0.0021; Figure 1A). Based on our analysis, the effect of the SAA1A−97delG allele is additive only, and explains ~2.7% of the total phenotypic variance in serum concentrations of SAA protein. Additional copies of the SAA1A−97delG allele are associated with a decrease in serum SAA protein concentrations (P = 0.1911 for the regression coefficient of a; Figure 1B). Both dominance (d) and additional polygenic additive variance () were not significantly different from zero (P = 0.9450 and P = 0.8247, respectively). 90% of the total phenotypic variance in SAA protein concentrations remained unexplained by our model ().
Figure 1.
Effects of health status and genotype on serum concentrations of serum amyloid A (SAA) protein in captive cheetahs at NZP-SCBI and CCF. Means of the SAA concentrations ± SE by health status (A) and genotype (B) are presented.
Differences in SAA1A−97delG Allele Frequencies Between Populations
When examining the genotype frequencies of the SNP in the NF-κB binding site in the SAA1A promoter region of the captive North American and wild Namibian cheetah populations, there was no evidence to reject Hardy–Weinberg equilibrium in either population, thus allele frequencies between populations were compared. The allele frequency of the single base pair deletion in the promoter region of the SAA1A gene, SAA1A−97delG, was significantly different between populations (P = 0.0414, Wright’s FST = 0.0750): the SAA1A−97delG allele is more common in the captive North American cheetah population when compared to wild Namibian cheetahs (Table 2).
Table 2.
Estimated allele frequencies of the SAA1A−97delG SNP for wild or wild-born cheetahs in Namibia and captive cheetahs in North America
| Population | Allele | Estimateda frequency ± standard error | Hardy–Weinberg P valueb |
|---|---|---|---|
| Namibia | SAA1A −97delG | 0.5032c ± 0.0600 | 0.291 |
| (Wild or wild-born, n = 47) | SAA1A + | 0.4968±0.0600 | |
| North America | SAA1A −97delG | 0.7158d ± 0.0796 | 0.422 |
| (Captive, n = 38) | SAA1A + | 0.2842±0.0796 | |
| North America | SAA1A −97delG | 0.6450±0.0700 | 0.438 |
| (Deceased, n = 48) | SAA1A + | 0.3550±0.0700 | |
| AA amyloidosis | SAA1A −97delG | 0.8750±0.1170 | |
| Positive (n = 27) | SAA1A + | 0.1250±0.1170 | |
| AA amyloidosis | SAA1A −97delG | 0.5980±0.0781 | |
| Negative (n = 21) | SAA1A + | 0.4020±0.0781 |
aEstimated using the software MENDEL (Lange et al. 2013) utilizing complete pedigree information available for all individuals.
bGenotypes tested for Hardy–Weinberg equilibrium for each population in MENDEL (Lange et al. 2005).
c,dIndicates a significant difference in allele frequency based on the Chi-square test of homogeneity, P = 0.0414.
Allele frequencies were also compared among deceased cheetahs from the captive North American population between individuals who were confirmed to be AA amyloidosis positive (n = 27) and those confirmed AA amyloidosis negative (n = 21) at the time of necropsy. There was no significant difference in the frequency of the SAA1A−97delG allele between these 2 groups of captive cheetahs, therefore the SAA1A−97delG allele does not appear to be associated with the disease (P = 0.1100, Wright’s FST = 0.2362, Table 2).
Discussion
AA amyloidosis has been identified in many species. In some cases, susceptibility appears to be genetic (familial amyloidosis), as observed in Shar-Pei dogs (Rivas et al. 1993), Abyssinian (Boyce et al. 1984) and Siamese (van der Linde-Sipman et al. 1997) breeds of domestic cats, and black-footed cats (Terio et al. 2008). Among other species, AA amyloidosis is considered “reactive” and does not have a genetic basis, but rather appears secondary to other inflammatory conditions, as observed in bighorn sheep (Hadlow and Jellison 1962), bat (Gruber and Linke 1996), Dorcas gazelle (Rideout et al. 1989) and humans (De Beer et al. 1982). Domestic cats are an interesting species to examine because the traditional domestic short hair (DSH) cat is not often affected by amyloidosis, while the Siamese and Abyssinian breeds have inherited forms of the disease, though the genetic variants implicated in the heritable disease are different between these 2 breeds (Niewold et al. 1999). The cheetah AA protein amino acid sequence is more similar to that of Abyssinian cats than DSH cats, having concordance at 5 of 8 positions of variability with Abyssinian cats but only 2 with DSH cats (Johnson et al. 1997), which suggests the possibility that cheetah AA protein may be structured in a way that makes it more amyloidogenic compared to the domestic cat AA protein. However, AA amyloidosis is uncommon among wild cheetahs (Munson et al. 2005), suggesting that the amyloidosis observed in cheetahs is not of a true “familial” type and rather secondary to chronic inflammation.
The identification of the SNP in the promoter region of the SAA1A gene in cheetahs by Zhang et al. (2008) suggested that AA amyloidosis in captive cheetahs may still be linked to genetic differences, as the SAA1A−97delG allele is associated with a decrease in transcription of the SAA1A gene in vitro (Zhang et al. 2008). This study shows that the SAA1A−97delG allele is similarly associated with a decrease in the SAA concentration in serum of captive cheetahs. However, genetic variation only accounted for 2.7% of the total phenotypic variation in SAA concentration. Much greater significant differences in SAA concentrations were observed between sick and healthy cheetahs. Sick cheetahs had significantly higher SAA concentrations compared to healthy cheetahs. This result is unsurprising given that SAA is an APP which is upregulated in response to inflammation. Among the deceased cheetahs with known AA amyloidosis diagnoses, 88.5% of cheetahs confirmed positive for AA amyloidosis also had moderate to chronic inflammation of any kind, compared to only 28.6% of cheetahs confirmed AA amyloidosis negative at necropsy.
In human populations, there is evidence that a SNP could explain population differences in secondary AA amyloidosis prevalence in rheumatoid arthritis patients: a thymine (T) nucleotide at the −13 position in the promoter region of the SAA1 gene is primarily associated with amyloidosis risk in these human populations (Moriguchi et al. 2001). Interestingly, cheetahs have a T at the −13 position in the SAA1 promoter (Zhang et al. 2008) and this nucleotide position is not polymorphic (Chen et al. 2012), therefore all cheetahs may be predisposed to developing secondary AA amyloidosis in the presence of chronic inflammation.
It has been shown that persistently elevated SAA concentration leads to increased amyloid load and organ deterioration in humans (Gillmore et al. 2001): mortality, amyloid burden, and renal prognosis are all significantly correlated with serum SAA concentration (De Beer et al. 1982; Lachmann et al. 2007). Similar to cheetahs, the main manifestation of secondary amyloidosis in human patients is renal dysfunction and failure (Lachmann et al. 2007).
Papendick et al. (1997) found that all cheetahs diagnosed with amyloidosis also suffered from other significant chronic inflammatory diseases in organs other than kidney, most commonly chronic lymphoplasmacytic gastritis. The prevalence of severe gastritis in captive cheetahs increased from 16% of individuals in pre-1990 necropsies to 43% of individuals in 1995, an increase almost parallel to the increase in AA amyloidosis prevalence observed in the same study (Papendick et al. 1997). While close to 100% of captive cheetahs have some form of gastritis (Munson 1993; Munson et al. 1999; Munson et al. 2005), 76% of cheetahs with amyloidosis have moderate to severe gastritis (Papendick et al. 1997). Therefore, the high prevalence of AA amyloidosis only in captive cheetahs is more likely a result of chronic inflammatory conditions, such as gastritis, inducing the overproduction of SAA.
This study has also shown that the SAA1A−97delG allele is significantly more common among the captive North American population compared to the wild Namibian cheetah population. While it could be argued that captivity-induced chronic inflammation has imposed natural selection pressure within the captive population to favor the SAA1A−97delG allele (associated with less SAA production), the lack of a relationship between the SNP and AA amyloidosis diagnosis found in this study and the Chen et al. (2012) study suggests that the population differences observed in allele frequencies are most likely due to an unrepresentative founder population or genetic drift within the captive North American population, as opposed to selection.
Conclusion
This study was the first to demonstrate that the concentration of SAA protein in serum in captive cheetahs is associated with the SAA1A−97delG SNP in the promoter region of the SAA1A gene. However, based on the significant difference observed between sick and healthy cheetahs and the lack of association between the indel and AA amyloidosis diagnosis in captive North American cheetahs, the high prevalence of AA amyloidosis observed among captive cheetahs is not primarily attributable to genetic differences at this locus, but rather appears to be related to chronic inflammation and/or other environmental factors.
Supplementary Material
Supplementary material can be found at http://www.jhered.oxfordjournals.org/.
Funding
Animal Sciences Graduate Student Association (ASGSA) at the University of Maryland College Park; the Cosmos Club Foundation (Washington, DC); Emanuel J. Friedman Philanthropies; and a Smithsonian Institution Fellowship to A.D.F.
Supplementary Material
Acknowledgments
We thank the following institutions for allowing the use of samples collected from their cheetahs for this study: Binder Park Zoo, Cheetah Conservation Fund, Fort Worth Zoo, San Antonio Zoological Gardens and Aquarium, Smithsonian National Zoo, Smithsonian Conservation Biology Institute, White Oak Conservation Center, and Wildlife Safari. The research on the Namibian cheetahs was conducted under research permit #1967/2014 granted by the Namibian Ministry of Environment and Tourism to the Cheetah Conservation Fund. The authors also acknowledge the late Dr. Linda Munson who started the AZA Cheetah SSP pathology survey and archive.
References
- Baker CS. 2013. Journal of heredity adopts joint data archiving policy. J Hered. 104:1. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. 1997. Nuclear factor-κB - a pivotal transcription factor in chronic inflammatory diseases. New Engl J Med. 336:1066–1071. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. 1994. The acute phase response. Immunol Today. 15:74–80. [DOI] [PubMed] [Google Scholar]
- Beg AA, Finco TS, Nantermet PV, Baldwin AS. 1993. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol Cell Biol. 13:3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J, Ueda M, Une Y, Sun X, Misumi S, Shoji S, Ando Y. 2006. Analysis of amyloid fibrils in the cheetah (Acinonyx jubatus). Amyloid. 13:93–98. [DOI] [PubMed] [Google Scholar]
- Boyce JT, DiBartola SP, Chew DJ, Gasper PW. 1984. Familial renal amyloidosis in Abyssinian cats. Vet Pathol. 21:33–38. [DOI] [PubMed] [Google Scholar]
- Cerón JJ, Eckersall PD, Martinez-Subiela S. 2005. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Path. 34:84–99. [DOI] [PubMed] [Google Scholar]
- Chen L, Une Y, Higuchi K, Mori M. 2012. Cheetahs have 4 serum amyloid A genes evolved through repeated duplication events. J Hered. 103:115–129. [DOI] [PubMed] [Google Scholar]
- De Beer FC, Fagan EA, Hughes GRV, Mallya RK, Lanham JG, Pepys MB. 1982. Serum amyloid-A protein concentration in inflammatory disease and its relationship to the incidence of reactive systemic amyloidosis. The Lancet. 320:231–234. [DOI] [PubMed] [Google Scholar]
- Depauw S, Delanghe J, Whitehouse-Tedd K, Kjelgaard-Hansen M, Christensen M, Hesta M, Tugirimana P, Budd J, Dermauw V, Janssens GPJ. 2014. Serum protein capillary electrophoresis and measurement of acute phase proteins in a captive cheetah (Acinonyx jubatus) population. J Zoo Wildlife Med. 45:497–506. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics (4th edn). England: Longman Group Ltd. [Google Scholar]
- Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. 2001. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. The Lancet. 358:24–29. [DOI] [PubMed] [Google Scholar]
- Giordano A, Spagnolo V, Colombo A, Paltrinieri S. 2004. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet J. 167:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AD, Linke RP. 1996. Generalized AA-amyloidosis in a bat (Pipistrellus pipistrellus). Vet Pathol. 33:428–430. [DOI] [PubMed] [Google Scholar]
- Hadlow WJ, Jellison WL. 1962. Amyloidosis in Rocky Mountain bighorn sheep. J Am Vet Med Assoc. 141:243–247. [PubMed] [Google Scholar]
- Jensen LE, Whitehead AS. 1998. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 334(Pt 3):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KH, Sletten K, Munson L, O’Brien TD, Papendick R, Westermark P. 1997. Amino acid sequence analysis of amyloid protein A (AA) from cats (captive cheetahs: Acinonyx jubatus) with a high prevalence of AA amyloidosis. Amyloid. 4:171–177. [Google Scholar]
- Kajikawa T, Furuta A, Onishi T, Tajima T, Sugii S. 1999. Changes in concentrations of serum amyloid A protein, α-1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet Immunol Immunop. 68:91–98. [DOI] [PubMed] [Google Scholar]
- Kann RKC, Seddon JM, Henning J, Meers J. 2012. Acute phase proteins in healthy and sick cats. Res Vet Sci. 93:649–654. [DOI] [PubMed] [Google Scholar]
- Lachmann HJ, Goodman HJB, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN. 2007. Natural history and outcome in systemic AA amyloidosis. New Engl J Med. 356:2361–2371. [DOI] [PubMed] [Google Scholar]
- Lange K, Papp JC, Sinsheimer JS, Sripracha R, Zhou H, Sobel EM. 2013. Mendel: the Swiss army knife of genetic analysis programs. Bioinformatics. 29:1568–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Sinsheimer JS, Sobel E. 2005. Association testing with Mendel. Genet Epidemiol. 29:36–50. [DOI] [PubMed] [Google Scholar]
- Marhaug G, Dowton SB. 1994. Serum amyloid A: an acute phase apolipoprotein and precursor of AA amyloid. Baillière’s Clin Rheumatol. 8:553–573. [DOI] [PubMed] [Google Scholar]
- Moriguchi M, Terai C, Kaneko H, Koseki Y, Kajiyama H, Uesato M, Inada S, Kamatani N. 2001. A novel single-nucleotide polymorphism at the 5’-flanking region of SAA1 associated with risk of type AA amyloidosis secondary to rheumatoid arthritis. Arthritis Rheum. 44:1266–1272. [DOI] [PubMed] [Google Scholar]
- Munson L. 1993. Diseases of captive cheetahs (Acinonyx jubatus): results of the Cheetah Research Council pathology survey, 1989–1992. Zoo Biol. 12:105–124. [Google Scholar]
- Munson L, Nesbit JW, Meltzer DG, Colly LP, Bolton L, Kriek NP. 1999. Diseases of captive cheetahs (Acinonyx jubatus jubatus) in South Africa: a 20-year retrospective survey. J Zoo Wildl Med. 30:342–347. [PubMed] [Google Scholar]
- Munson L, Terio K, Worley M, Jago M, Bagot-Smith A, Marker L. 2005. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J Wildlife Dis. 41:542–548. [DOI] [PubMed] [Google Scholar]
- Niewold TA, van der Linde-Sipman JS, Murphy C, Tooten PCJ, Gruys E. 1999. Familial amyloidosis in cats: Siamese and Abyssinian AA proteins differ in primary sequence and pattern of deposition. Amyloid. 6:205–209. [DOI] [PubMed] [Google Scholar]
- Ofri R, Nyska A, Linke RP, Shtrasburg S, Livneh A, Gal R. 1997. Systemic amyloidosis in a cheetah (Acinonyx jubatus). Amyloid. 4:98–103. [Google Scholar]
- Paltrinieri S. 2008. The feline acute phase reaction. Vet J. 177:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papendick RE, Munson L, O’Brien TD, Johnson KH. 1997. Systemic AA amyloidosis in captive cheetahs (Acinonyx jubatus). Vet Pathol. 34:549–556. [DOI] [PubMed] [Google Scholar]
- Ray A, Ray BK. 1994. Serum amyloid A gene expression under acute-phase conditions involves participation of inducible C/EBP-β and C/EBP-δ and their activation by phosphorylation. Mol Cell Biol. 14:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout BA, Montali RJ, Wallace RS, Bush M, Phillips LG, Jr, Antonovych TT, Sabnis SG. 1989. Renal medullary amyloidosis in Dorcas gazelles. Vet Pathol. 26:129–135. [DOI] [PubMed] [Google Scholar]
- Rivas AL, Tintle L, Meyers-Wallen V, Scarlett JM, van Tassell CP, Quimby FW. 1993. Inheritance of renal amyloidosis in Chinese Shar-pei dogs. J Hered. 84:438–442. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ma Z, Khatlani TS, Okuda M, Inokuma H, Onishi T. 2003. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J Vet Med Sci. 65:545–548. [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS. 1995. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 270:283–286. [DOI] [PubMed] [Google Scholar]
- Tamamoto T, Ohno K, Goto-Koshino Y, Fujino Y, Tsujimoto H. 2012. Serum amyloid A uptake by feline peripheral macrophages. Vet Immunol Immunop. 150:47–52. [DOI] [PubMed] [Google Scholar]
- Tamamoto T, Ohno K, Ohmi A, Goto-Koshino Y, Tsujimoto H. 2008. Verification of Measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. J Vet Med Sci. 70:1247–1252. [DOI] [PubMed] [Google Scholar]
- Tempelman RJ, Rosa GJM. 2004. Empirical Bayes approaches to mixed model inference in quantitative genetics. In: Saxton A, editor. Genetic analysis of complex traits using SAS. Cary: (NC): SAS Institute Inc; p. 149–176. [Google Scholar]
- Terio KA, O’Brien T, Lamberski N, Famula TR, Munsin L. 2008. Amyloidosis in black-footed cats (Felis nigripes). Vet Pathol. 45:393–400. [DOI] [PubMed] [Google Scholar]
- van der Linde-Sipman JS, Niewold TA, Tooten PCJ, de Neijs-Backer M, Gruys E. 1997. Generalized AA-amyloidosis in Siamese and Oriental cats. Vet Immunol Immunop. 56:1–10. [DOI] [PubMed] [Google Scholar]
- Zhang B, Une Y, Ge F, Fu X, Qian J, Zhang J, Sawashita J, Higuchi K, Mori M. 2008. Characterization of the cheetah serum amyloid A1 gene: critical role and functional polymorphism of a cis-acting element. J Hered. 99:355–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.