Abstract
It has been widely reported that the major histocompatibility complex (MHC) is under balancing selection due to its immune function across terrestrial and aquatic mammals. The comprehensive studies at MHC and other neutral loci could give us a synthetic evaluation about the major force determining genetic diversity of species. Previously, a low level of genetic diversity has been reported among the Indo-Pacific humpback dolphin (Sousa chinensis) in the Pearl River Estuary (PRE) using both mitochondrial marker and microsatellite loci. Here, the expression and sequence polymorphism of 2 MHC class II genes (DQB and DRB) in 32 S. chinensis from PRE collected between 2003 and 2011 were investigated. High ratios of non-synonymous to synonymous substitution rates, codon-based selection analysis, and trans-species polymorphism (TSP) support the hypothesis that balancing selection acted on S. chinensis MHC sequences. However, only 2 haplotypes were detected at either DQB or DRB loci. Moreover, the lack of deviation from the Hardy–Weinberg expectation at DRB locus combined with the relatively low heterozygosity at both DQB locus and microsatellite loci suggested that balancing selection might not be sufficient, which further suggested that genetic drift associated with historical bottlenecks was not mitigated by balancing selection in terms of the loss of MHC and neutral variation in S. chinensis. The combined results highlighted the importance of maintaining the genetic diversity of the endangered S. chinensis.
Keywords: balancing selection, DQB, DRB, MHC expression, trans-species polymorphism
Understanding the levels of genetic variation and the mechanism by which genetic variation is maintained in threatened species is of fundamental interest to evolutionary and conservation biologists. Loss of genetic variation in small populations may increase the extinction risk due to decreased reproductive fitness and increased disease susceptibility (Miller et al. 2010). To date, studies of genetic diversity in threatened populations have mainly focused on neutral genetic markers, such as mitochondrial DNA (mtDNA) or microsatellites. However, patterns of neutral genetic variation do not necessarily correlate with quantitative variation for ecologically important traits or variation of adaptively important genes (Knopp et al. 2007). Thus, the variation at neutral loci cannot provide direct information on selective processes, such as the interaction of individuals with their environment or adaptive potential for future environmental change (Meyers and Bull 2002), which are issues of particular relevance for conservation.
The MHC genes are well-known examples of adaptive significance that have been used as indicators of populations and species in various studies. The MHC genes are the most polymorphic genes in vertebrates, which encode a series of cell-surface glycoproteins that bind and present antigens to T cells, and thereby triggering a cascade of adaptive immune response (Piertney and Oliver 2006). The variability of the amino acids found in the groove of an MHC molecule, especially those comprising the peptide-binding region (PBR), specifies the range of pathogen-derived peptides that can be presented to T lymphocytes. The high levels of polymorphisms usually observed in the MHC genes presumably occur due to balancing selection acting over functional regions of the MHC proteins such as the PBR (Gu and Nei 1999). Selection in this region of the MHC genes is driven by the presence of pathogens in a frequency-dependent manner or by heterozygote advantage (Piertney and Oliver 2006). Other processes that are believed to maintain MHC polymorphisms include linkage disequilibrium and epistatic gene–gene interactions, gene conversion and recombination, sexual selection, and maternal–fetal interactions (Sutton et al. 2011).
The Indo-Pacific humpback dolphin (Sousa chinensis), locally known in China as the Chinese white dolphin, was once widely found in the coastal and inshore waters of the Indian to western Pacific oceans (Jefferson and Karczmarski 2001). This species is listed in the First Order of the National Key Protected Wild Aquatic Animals List in China and has been included in the International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species since 2010 (Reeves et al. 2010). The largest population comprises approximately 1500 individuals (Jefferson and Hung 2004) and inhabits the Pearl River Estuary (PRE) of China, which is one of the world’s busiest and most polluted ports. All of the other reported populations (including Leizhou Bay, Xiamen harbor, Beibu Gulf, and western Taiwan) occur in estuarine areas and include no more than 300 individuals (Wang et al. 2004; Zhou et al. 2007; Chen et al. 2008). Many studies noticed the low level of genetic diversity for humpback dolphins in China. Lin et al. (2010) proposed the repeated bottleneck during the glaciation cycle lead to the loss of gene load. However, this hypothesis was based primarily on the coastline evolutionary history and remained to be tested. Another possible cause for the lack of genetic diversity is the recent population contraction due to extremely strong anthropogenic pressures, such as chemical pollution, coastal development, heavy vessel traffic, depletion of prey resources, and irresponsible ecotourism. For example, it is believed that the largest population size still declines at a continuous rate of 2.46% per annum and the decline rate may further accelerate in the future, which greatly increases the risk of extinction (Huang et al. 2012). There are few academic descriptions of S. chinensis in China prior to 2000, but it is generally believed that the current populations were isolated at some point (Wang et al. 2010), and might tend to lose their genetic diversity as a consequence of genetic drift. Previous genetic studies based exclusively on mtDNA or microsatellite data have revealed an extremely low level of molecular diversity in S. chinensis in Chinese waters (Lin et al. 2010; Lin et al. 2012). However, relatively little is known about the MHC diversity of this species and even less about MHC gene expression. The MHC study on other cetacean species supported the presence of balancing selection on marine mammals (Munguia et al. 2007; Vassilakos et al. 2009; Xu et al. 2012). Given the infection of bacteria (Streptococcus faecium, Vibrio cholera, andVibrio spp.) and nematode (Halocercus pingi) reported from the S. chinensis (Parsons and Jefferson 2000; Jefferson et al. 2006), the species might withstand a high degree of pathogen stress.
In this study, we investigated the expression and diversity of 2 different MHC genes (DQB and DRB) in S. chinensis in the PRE of China. Specifically, we performed a careful evaluation and determined the primary genetic factors responsible for the present MHC diversity in this species. We sought to provide evidence of the mechanism responsible for the presence of identical alleles in S. chinensis and other cetaceans by investigating trans-species polymorphisms (TSP). We hope our findings in this study will clarify and deepen our understanding of the genetic diversity in S. chinensis, and therefore enable a more comprehensive conservation measure for this species in China.
Materials and Methods
Sample Origin, DNA and RNA Isolation, and cDNA Synthesis
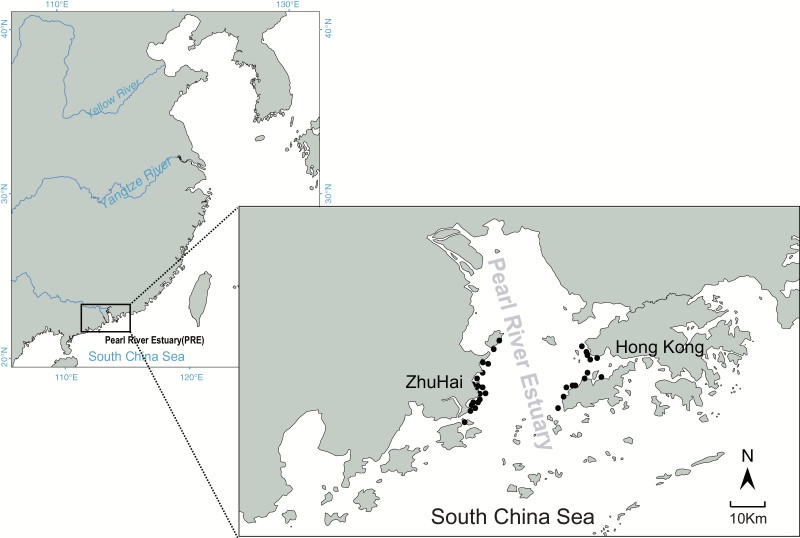
Skin or muscle samples were collected from 32 stranding S. chinensis along the coast of the PRE (Figure 1) between 2003 and 2011. Although these samples were collected from different locations around the PRE, no variation evidence for population genetic structure was found in this region based on mitochondrial markers (Lin et al. 2012). Samples from dolphins stranded in Hong Kong (n = 13) were provided by the Ocean Park Conservation Foundation, Hong Kong (OPCFHK), with authorization from the Agriculture, Fisheries, and Conservation Department (AFCD). Samples from dolphins stranded Zhuhai (n = 19) were preserved at the School of Marine Sciences, Sun Yat-sen University. Genomic DNA (gDNA) from these dead stranded dolphins was extracted by standard proteinase K digestion and phenol–chloroform procedures. Additionally, we took advantage of a unique opportunity to obtain blood samples from a freshly dead individual (ZH-SC-37). Total RNA was obtained using RNAiso Plus (TaKaRa Bio Inc., Shiga, Japan) according to the manufacture’s protocol. Extracted RNA samples were stored at −80 °C until use. cDNA was constructed using oligo (dT) primers and the RNase H Minus reverse transcriptase enzyme following the instructions provided by the PrimeScriptTM RT-PCR Kit (TaKaRa Bio Inc., Shiga, Japan).
Figure 1.
Map of the approximate sampling locations of S. chinensis in the Pearl River Estuary (PRE).
PCR Amplification, Cloning, and Sequencing
In this study, the exon 2 of DQB and DRB loci was amplified from 32 S. chinensis DNA samples, and sequences comprised from exon 1 to exon 4 of DQB and DRB loci were also amplified from 1 S. chinensis cDNA sample. Specifically, a 172bp fragment from exon 2 of the DQB gene was amplified with the universal primers DQF (5′-CATGTGTTACTTCACCAACGGC-3′) and DQR (5′-CACAACT ACAGGRTTGATGAGA-3′) as reported by Murray and White (1998). Similarly, a 215bp fragment was amplified from exon 2 of the DRB gene with the forward primer DRR (5′-CCGCTGCA CCGTTGAAGCT-3′) and reverse primer DRF (5′-CAGTTTAAGKSCGAGTGTC-3′) (Baker and Vant 2006). Meanwhile, 2 cDNA segments of DQB and DRB (705 and 758bp, respectively) including the region from exons 1 to 4 were amplified using the newly designed locus-specific primers based on bottlenose dolphin (T. truncatus) sequences (GenBank: EF507863.1~EF507878.1). These primers are: forward SoDQ1 (5′-TGATGCTGACGGTGCTGAG-3′), reverse SoDQ2 (5′-TCCTGTGACGGATGA TGAGAC-3′) and forward SoDR1 (5′-GGTGTCCCTGTATTTCTCC-3′), reverse SoDR2 (5′-GAGT GTCCTTTCTGATTCCT-3′). Amplifications were performed by PCR in a total volume of 50 μl containing 20–50ng of template DNA, 25 μl 2 × GC buffer I (TaKaRa Bio Inc.), 4mM MgCl2, 400 μM dNTPs, 0.2 μM of each primer, 80ng μl−1 BSA, and 0.3U of LA Taq DNA polymerase (TaKaRa Bio Inc). Standard PCR protocols were used as follows: an initial denaturation at 94 °C for 5min, followed by 35 cycles of denaturation at 94 °C for 1min, annealing at 55 °C for 1min, and extension at 72 °C for 1min. A final extension step was performed at 72 °C for 10min. PCR products were visualized on 1.5% agarose gels with an ethidium-bromide gel stain. Fragments of the expected size were excised from the gel, extracted, and purified using the TaKaRa PCR purification kit. Then, the purified products were ligated into pMD-18 Simple T vectors (TaKaRa Bio Inc) prior to transformation into DH5α cells. At least 10 recombinant plasmids for each individual were selected and sequenced using the ABI PRISMTM Big Dye Terminator cycle sequencing ready reaction kit (PE Biosystems) on an ABI 310 DNA analyzer. To further guarantee that all alleles were detected and artificial mutants were excluded, the purified PCR products were sequenced bidirectionally so that the sites of mutation could be directly detected based on doublets.
Allele Identification and Nomenclature
A new allele was identified only when it met the criteria summarized by Kennedy et al. (2002). The criteria were that at least 3 identical clones had to be present in either 2 separate PCRs from the same individual or from PCRs from at least 2 different individuals during DNA cloning and sequencing. A majority of the sequences considered to be PCR artifacts only differed from the accepted alleles identified by the same PCR by 1 or 2 base pairs. In accordance with the proposed nomenclature for MHC in nonhuman species (Klein et al. 1993), we designated the exon 2 alleles Soch-DQB and Soch-DRB for S. chinensis with the serial numbers attached. The putative PBR of the DQB/DRB gene was estimated in comparison with the β chain of the HLA-DR 1, of which molecular structure is well-known (Brown et al. 1993).
Data Analysis
All of the sequences were aligned using the program Clustal X (Thompson et al. 1997). The observed and expected heterozygosities (Ho and He) were calculated using Arlequin 3.5 (Excoffier and Lischer 2010). Deviation from the Hardy–Weinberg equilibrium was tested using GENEPOP 4.0 (Rousset 2008). DnaSP 5.0 (Librado and Rozas 2009) was used to calculate haplotype diversity (Hd) and nucleotide diversity (π).
Because the presence of recombinant sequences in the data set can significantly influence selection and phylogenetic analysis (Anisimova et al. 2003), we tested the DQB and DRB sequences for the presence of intralocus recombination events using the RDP3 software (Martin et al. 2010). RDP3 simultaneously applies various statistical methods to generate a weighted consensus concerning the presence and characteristics of recombination events. Three approaches were used to determine whether positive selection had acted on the evolution of the DQB and DRB genes in S. chinensis. First, non-synonymous (dN) and synonymous (dS) substitution rates were calculated for the PBR and non-PBR regions according to the method of Nei and Gojobori (1986) using the Jukes–Cantor correction for multiple substitutions; the significance levels were determined by a Z-test using MEGA 6.0 (Tamura et al. 2013). Under neutral evolution, the synonymous mutation rate should be equal to the non-synonymous mutation rate; thus, the dN/dS ratio will be close to one. Positive selection tends to maintain non-synonymous mutations to increase the ratio, whereas purifying or negative selection acts in an opposite way by removing the mutants from the gene pool. Second, evidence for positive selection at the codon level within the gene was assessed using the CODEML subroutine contained in the PAML 4 package (Yang 2007). We used site models implemented in the CODEML program for this purpose. Models M1a and M7 restricted sites with ω (dN/dS) ≤ 1, whereas models M2a and M8 included a class of sites with ω > 1. M0 assumes the same ω ratio for all codons, whereas M3 allows for 3 site classes with the proportions (p0, p1, p2) and ω ratios (ω0, ω1, ω2). Values of ω < 1, = 1, and >1 indicate negative purifying selection, neutral evolution and positive selection, respectively. We compared M0 and M3 to test for the significance of heterogeneity in ω across sites, whereas M1a was compared with M2a and M7 with M8 to test for the existence of positive selection. The sites with a Bayes empirical Bayes (BEB) posterior probability > 0.9 were considered to be candidates for selection. Finally, we analyzed the DQB and DRB sequences using a statistically more robust fast unconstrained Bayesian approximation (FUBAR) (Murrell et al. 2013) to test for selection, which served as a comparison to the CODEML program. Deviations from neutral expectations of molecular evolution were also tested with Tajima’s D-test using DnaSP 5.0 (Librado and Rozas 2009). In a population of constant size, Tajima’s D is expected to be zero in the absence of selection, positive in the presence of balancing selection, and negative in the presence of purifying selection against deleterious mutations.
To establish the phylogenetic relationship of the S. chinensis DQB and DRB alleles with the alleles from other cetaceans, phylogenetic trees were reconstructed using MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003). The best-fit evolution model for MHC was selected on the basis of the Akaike Information Criterion (AIC) using MODELTEST 3.7 (Posada and Crandall 1998). Two independent runs of Markov chain Monte Carlo (MCMC) with 4 chains were each performed for 1.0×106 generations and sampled every 1000 generations. The first 10% of the trees were discarded as burn-in. To calculate the posterior probability of each bipartition, the majority-rule consensus tree was computed from these 900 sampled trees. Sequences of the human (H. sapiens), American bison (B. bison), and wild goat (C. aegagrus) alleles were used as outgroups.
Data Archiving
In fulfillment of data archiving guidelines (Baker 2013), we have deposited the primary data underlying these analyses with Dryad.
Results
Expression of MHC Class II Genes
Total RNA was successfully extracted from the freshly dead individual (ZH-SC-37). The expression of MHC class II genes including the region from exons 1 to 4 was confirmed by amplification from cDNA using the primer pairs SoDQ1/SoDQ2 and SoDR1/SoDR2. Both loci were found to be heterozygous and did not contain a stop codon or insertions/deletions. The segments covering exon 2 were further amplified from the gDNA of the same individual with the primer pairs DQF/DQR and DRF/DRR. The cDNA and gDNA sequences were fully matched.
MHC Variability
Segments covering part of exon 2 of DQB and DRB (172 and 215bp, respectively) were successfully amplified from 32 individuals. None of the detected sequences contained any insertions/deletions or stop codons, suggesting that all of the sequences might represent functional molecules in the genome. No more than 2 DQB/DRB sequences were detected in each individual, indicating that only 1 DQB locus and 1 DRB locus were amplified using these primer sets. Analysis of the 172bp fragment revealed 2 alleles from the second exon of the DQB locus (Soch-DQB*01, KR559266 and Soch-DQB*02, KR559267). Pairwise comparisons of DQB locus revealed that 7.56% (13 of 172) of the nucleotides and 17.54% (10 of 57) of the amino acids were variable. When the position of the variations was considered, 6 out of the 14 amino acids within the PBR regions were variable (42.86%), whereas only 4 out of the remaining 43 amino acids in the non-PBR regions were polymorphic (9.3%), thereby suggesting that the genetic variations predominately occurred in the PBR region (Table 1). The DRB locus of S. chinensis was identified for the first time in this study (Soch-DRB*01, KR559268 and Soch-DRB*02, KR559269). Twenty-eight of the 215 nucleotides (13.02%) and 17 of the 71 amino acids (23.94%) were variable. Similarly, the variations in the PBR region of DRB (52.63%) were much higher than those in the non-PBR region (13.46%). Table 2 shows the estimates of genetic diversity parameters for S. chinensis. For DQB, the values of Hd, π, and AR were 0.373, 2.82% and 2, respectively. For DRB, the values of Hd, π, and AR were 0.441, 5.46% and 2, respectively. Both DQB and DRB had a large number of homozygous to heterozygous genotypes (5:1 and 3:2, respectively). He (0.3728) was significantly higher than Ho (0.1613) under the Hardy–Weinberg equilibrium for DQB (P = 0.02), whereas for DRB the He (0.4193) was close to the Ho (0.4063) and there was no significant departure from the expected frequencies of alleles (P = 0.65).
Table 1.
Alignment of predicted amino acid sequences of S. chinensis MHC class II genes (DQB and DRB)
| * * * | ** | * * | ** * | * ** * | * ** | |||
|---|---|---|---|---|---|---|---|---|
| 1112222222 | 2223333333 | 3334444444 | 4445555555 | 5556666666 | 6667777777 | 7778888888 | 8 | |
| 7890123456 | 7890123456 | 7890123456 | 7890123456 | 7890123456 | 7890123456 | 7890123456 | 7 | |
| Soch-DRB*01 | FSNGTERVRL | LVRDIYNQEE | TLRYDSDVGE | YRAVTELSRQ | EAEKRNSQKD | LLERRRAEVD | TVCRHNYRLG | E |
| Soch-DRB*02 | .........F | VM.RT..R.. | F..F...... | .......G.R | T...W..... | ...QN..... | .Y......VV | . |
| Soch-DQB*01 | ----.....F | VN.N...R.. | YV.F...... | F......G.R | T..YW..... | ....K...L. | .--------- | - |
| Soch-DQB*02 | ----.....H | VS.Y...R.. | FV.F...... | F......G.P | D..YW...E. | ....K..D.. | .--------- | - |
Dots indicate residues identical to the reference sequence Soch-DRB*01. Putative peptide binding sites according to the 3-dimensional structure of human HLA DR1 molecules are marked with asterisks.
Table 2.
Diversity and selection parameters of DQB and DRB loci for S. chinensis in PRE waters
| Locus | N | A R | π | Hd | He | Ho | P value | Tajima’s D |
|---|---|---|---|---|---|---|---|---|
| DQB | 38 | 2 | 2.82% | 0.373 | 0.3728 | 0.1613 | 0.02 | 2.17* |
| DRB | 31 | 2 | 5.46% | 0.441 | 0.4193 | 0.4063 | 0.65 | 3.14 ** |
AR, allelic richness, π: nucleotide diversity, Hd, haplotype diversity, He, expected heterozygosity; Ho, observed heterozygosity; N, number of individuals.
*P < 0.05, **P < 0.01.
Selection Analysis
No evidence of recombination events was found according to the RDP3 software. Large positive values of Tajima’s D were found for both DQB (2.17, P < 0.05) and DRB (3.14, P < 0.01) with significant statistical support (Table 2). Both the DQB and DRB sequences showed significantly higher non-synonymous substitution rates than synonymous substitution rates (Table 3). When compared with the non-PBR region, the Z-test gave an overall higher ω (dN/dS) ratio for the PBR region, indicating positive selection occurred at these loci. Moreover, results from the codon-based analysis in CODEML confirmed that positive selection had acted on the DQB and DRB loci (i.e., M2a, M8, and M3 fit the data significantly better than the uniform ω and the nearly neutral models M1a, M0, and M7) (Table 4). The specific codons identified by the BEB approach with posterior probabilities of at least 95% constituted 8 and 18 codons in DQB and DRB, respectively (Table 5). With the use of FUBAR, we also detected 4 and 6 codons that evolved under positive selection, with posterior probabilities of > 95% in DQB and DRB, respectively (Table 5). The codons identified under positive selection by CODEML and FUBAR overlapped considerably; most of the detected codons were involved in antigen binding or located adjacent to the PBR region.
Table 3.
The estimated rates of nonsynonymous (dN) and synonymous (dS) substitutions for the PBR and other regions (non-PBR) and their ratios in the S. chinensis DQB and DRB loci
| Locus | Positions | Codons | d N | d S | ω(dN/dS) | P value |
|---|---|---|---|---|---|---|
| DQB | All | 57 | 0.037 (0.012) | 0.009 (0.009) | 4.13 | 0.037 |
| PBR | 14 | 0.080 (0.028) | 0 (0) | ∞ | 0.003 | |
| Non-PBR | 43 | 0.024 (0.013) | 0.012 (0.013) | 2 | 0.26 | |
| DRB | All | 71 | 0.073 (0.019) | 0.016 (0.008) | 4.54 | 0.0003 |
| PBR | 19 | 0.195 (0.073) | 0.031 (0.023) | 6.29 | 0.009 | |
| Non-PBR | 52 | 0.036 (0.015) | 0.011 (0.008) | 3.27 | 0.014 |
d N and dS were computed according to the Nei–Gojobori method, with standard errors obtained through 1000 bootstrap replicates in parentheses; P is the probability that dN and dS are different using a Z-test.
Table 4.
Likelihood ratio test analyses to compare different models of codon evolution implemented in CODEML for the exon 2 sequences at the DQB and DRB loci in S. chinensis
| Models compared | df | DQB | DRB | ||
|---|---|---|---|---|---|
| 2Δl | P value | 2Δl | P value | ||
| M3 versus M0 | 4 | 348.6 | <0.0001 | 888.4 | <0.0001 |
| M2a versus M1a | 2 | 91.4 | <0.0001 | 268.2 | <0.0001 |
| M8 versus M7 | 2 | 91.6 | <0.0001 | 258.2 | <0.0001 |
Differences in log-likelihood values (2(lb – la)) were compared with degrees of freedom using the χ2 distribution. df indicates the number of free parameters in the ω distribution, ω the selection parameter, and 2Δl the likelihood ratio statistic computed as 2(lb – la), where la and lb are the log-likelihood values for each of the models being compared.
Table 5.
Codon sites under positive selection identified using CODEML and FUBAR
| Locus | Codons identified |
|---|---|
| DQB | 26 n, 28*b, 30b, 37b, 56b, 57*n, 70*b,71*b |
| DRB | 26 n, 27n, 28b, 29n, 30b, 34n, 37*b, 40n, 56b, 57*n, 60b, 67*n, 70*b, 71*b, 78b, 84*n, 85b, 86b |
All sites indicated here were identified by models M2 and M8 with at least > 95% posterior probability, whereas sites in bold were identified by model M8 with posterior probabilities > 99%. Sites marked with asterisks were identified by the FUBAR method with posterior probabilities > 95%. Codon numbering corresponds to exon 2 amino acid positions.
bPredicted antigen-binding sites, nSites neighboring predicted antigen-binding sites.
Phylogenetic Analysis
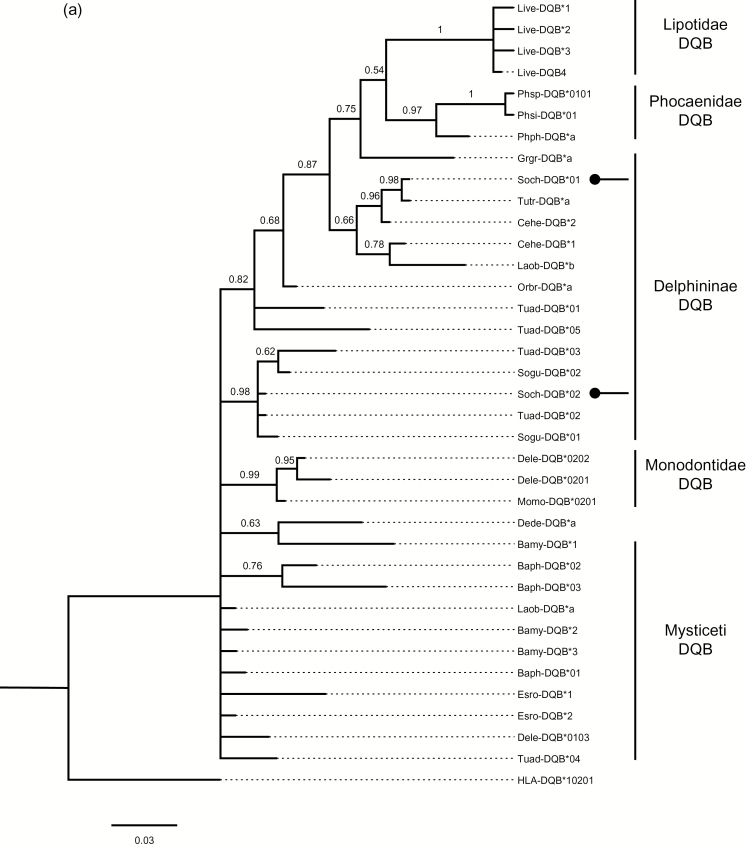
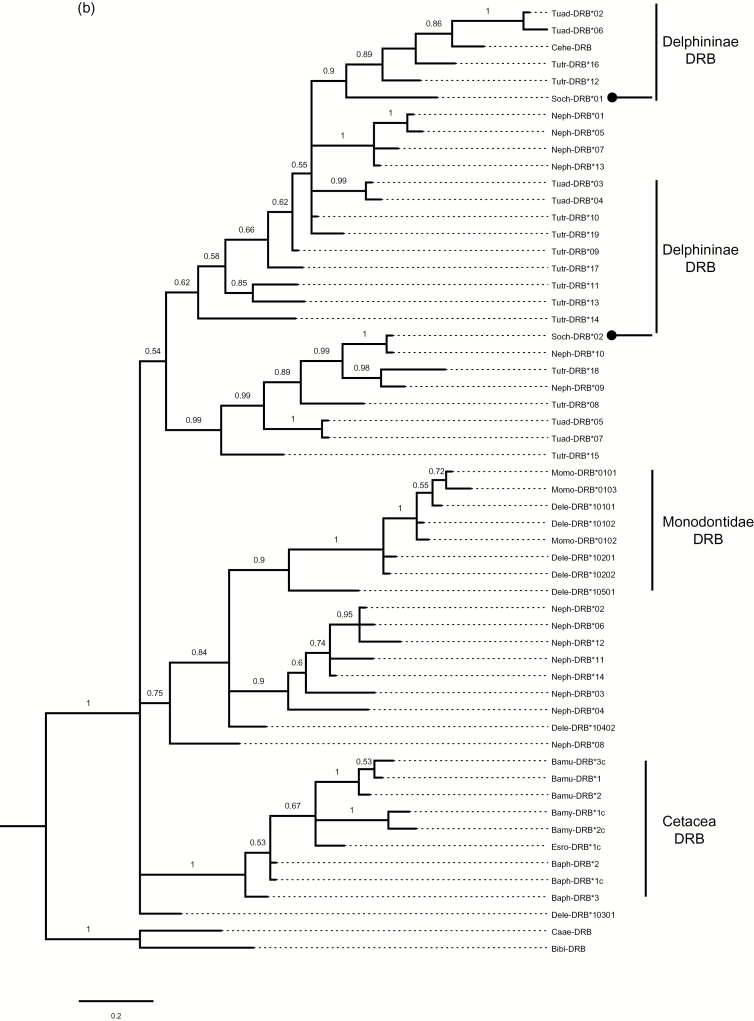
Using the AIC criterion, HKY + G was identified as the most appropriate model for the present DQB data and K2 + G for the DRB data. Phylogenetic reconstructions of the DQB and DRB data sets revealed well-supported branches for cetacean-specific clades that were distinct from the outgroup HLA-DQB, Bibi-DRB, and Caae-DRB sequences (Figure 2). Alleles of S. chinensis did not form a monophyletic clade, but intermixed with other closely related species. Phylogenetic analysis of the DQB alleles revealed the TSP for the identical allele found in S. chinensis and T. truncatus (Soch-DQB*01 and Tutr-DQB*a). This TSP was closely related with a high Bayesian posterior probability to allele Ceha-DQB*2 from the Hector’s dolphin (C. hectori). We also found that Soch-DQB*02 clustered with closely related species, such as the Indo-Pacific bottlenose dolphin (T. aduncus) and the Guyana river dolphin (S. guianensis). In the Bayes tree for DRB, Soch-DRB*02 was identical to Neph-DRB*10 from the finless porpoise (N. phocaenoides). Soch-DRB*01 clustered with the genera Tursiops and Cephalorhynchus. These clades strongly suggest the presence of trans-species and trans-genus allelic polymorphisms.
Figure 2.
Phylogenetic trees of the S. chinensis DQB (a) and DRB (b) alleles. Alleles of S. chinensis are indicated with arrows. Other homologous cetacean sequences available from GenBank were included. DQB sequences: Yangtze River dolphin (L. vexillifer, AY177150-53), Burmeister’s porpoise (P. spinipinnis, DQ914412), Vaquita porpoise (P sinus, DQ914412), Harbor porpoise (P. phocoena, AB164211), Risso’s dolphin (G. griseus, AB164222), Bottlenose dolphin (T. truncatus, AB164221), Hector’s dolphin (C. hectori, DQ354628-29), Pacific white-sided dolphin (L. obliquidens, AB164224-25), Irrawaddy dolphin (O. brevirostris, AB164223), Indo-Pacific Bottlenose dolphin (T. aduncus, EU698973-77), Guyana river dolphin (S. guianensis, FJ848543-44), Beluga whale (D. leucas, DLU16988-90), Narwhal (M. monoceros, U16991), Saddleback dolphin (D. delphis, AB164220), Bowhead whale (B. mysticetus, DQ354623-25), Fin whale (B. physalus, DQ300261-63), Grey whale (E. robustus, DQ354635-36) and Human (H. sapiens, NM001243962). DRB sequences: Indo-Pacific Bottlenose dolphin (T. aduncus, EF507868-74), Hector’s dolphin (C. hectori, DQ354675), Bottlenosed dolphin (T. truncatus, KJ722698-709), Indo-Pacific Finless porpoise (N. phocaenoides, FJ416157-70), Narwhal (M. monoceros, AF012939-41), Beluga whale (D. leucas, AF012930-37), Blue whale (B. musculus, DQ354666-68), Bowhead whale (B. mysticetus, DQ354669-71), Grey whale (E. robustus, DQ354678), Fin whale (B. physalus, DQ354672-74), Wild goat (C. aegagrus, Z92716), and American bison (B. bison, DQ353805). The Bayesian posterior probabilities of phylogenetic trees larger than 0.5 are indicated above the branches.
Discussion
MHC Expression
Many genetic studies have reported low to moderate levels of MHC gene diversity in marine mammals compared to their terrestrial relatives (Murray and White 1998; Yang et al. 2005; Xu et al. 2009). Moreover, an interrupted reading frame was found in the MHC genes of humpback whales (Baker et al. 2006). These results were previously proposed to be a signal of deficient MHC function or the consequence of weak pathogenic pressure in the marine environment. At present, relatively little is known about the expression of MHC genes in cetaceans, with the exception of the franciscana dolphin (P. blainvillei), southern right whale (E. australis) and Hector’s dolphin (C. hectori) in New Zealand (Heimeier et al. 2009; Heinzelmann et al. 2009). Here, we described the expression of MHC class II genes (DQB and DRB) by amplification of exons 1, 2, 3, and 4 from the cDNA from a freshly dead individual (ZH-SC-37). The agreement of the cDNA and gDNA sequences excluded the possibility of amplifying pseudogenes and confirmed the expression results. Subsequent cloning of amplified fragments from both cDNA and gDNA revealed no more than 2 sequences per individual at each locus, suggesting the presence of 1 locus for each of these genes in the genome of S. chinensis.
Low MHC Variation in S. chinensis
In this study, we described the variation in the MHC class II genes of the endangered S. chinensis from the PRE, China. To the best of our knowledge, this is the first report of MHC class II variation in S. chinensis. Only 2 DQB and 2 DRB alleles were found following the criteria for the acceptance of new MHC alleles, revealing a relatively low level of MHC class II genes variation in S. chinensis. This low level of variation was similar to the 1 DQB allele and 2 DRB alleles found in California harbor porpoises (P. sinus), whose abundance has decreased to only 567 individuals (Munguia et al. 2007). Furthermore, the π and Hd measures that are related to heterozygosity at the nucleotide level are usually remarkably high in MHC loci. However, S. chinensis had relatively low genetic diversity in all MHC class II genes (DQB: π = 2.82%, Hd = 0.373 and DRB: π = 5.46%, Hd = 0.441) compared to other studied cetaceans (π = 0–6.30%, Hd = 0–0.94) (Murray and White 1998; Baker et al. 2006; Munguia et al. 2007; Xu et al. 2009). Previous studies revealed that S. chinensis in PRE was also characterized by low genetic diversity at both maternal and bi-parental markers; only 5 mtDNA haplotypes were characterized in S. chinensis with Soch-A accounting for 85% of all the haplotypes (Lin et al. 2010). Likewise, for 10 microsatellite loci, the average number and allele richness per locus were only 2.5 and 1.7, respectively, and significant heterozygosity deficiency was also detected (Lin et al. 2012).
Even though S. chinensis in PRE have been reported to be decreasing since the end of last century, it is unlikely that the resulting bottleneck has been severe enough to cause the current genome-wide loss of genetic diversity. Generally, demographic collapse will not lead to the severe loss of molecular diversity. For example, the endangered giant panda (A. melanoleuca) with its abundance recently reduced to 1000 individuals revealed 6 DQB alleles and 7 DRB alleles (Chen et al. 2010). In the past decades, the critically endangered Yangtze River dolphin (L. vexillifer) dramatically declined to less than 100 individuals and is now functionally extinct. However, this species still shows considerable MHC diversity, with 43 DQB alleles (Yang et al. 2005) and 9 DRB alleles (Xu et al. 2012). Instead, historical bottlenecks with drift serving as the major long-term force that determined the genetic diversity of small populations were reported in many species (Miller and Lambert 2004; Zeisset and Beebee 2014; Sutton et al. 2015). In accordance with this, our previous studies proposed that the long-term historical bottleneck associated with coastline development might have caused the genome-wide loss of genetic diversity in S. chinensis (Lin et al. 2010; Lin et al. 2012). Therefore, it seems likely that the recent population decline of S. chinensis may not be able to explain the reduced levels of variation observed at the MHC class II loci. Instead, such a dramatic genome-wide loss of genetic diversity may have resulted from long-term historical bottlenecks.
The Effect of Balancing Selection on MHC Variation
Several lines of evidence support the hypothesis that balancing selection acted on the S. chinensis MHC sequences: high dN/dS ratios and a positive Tajima’s D value were revealed across the DQB and DRB sequences; various approaches involving different statistical constraints identified specific codons affected by selection; and TSP served as evidence of long-term persistence of balancing selection. Generally, balancing selection is expected to increase the level of heterozygosity and play a central role in preserving high levels of MHC polymorphism (Hedrick 1998; Muirhead 2001). However, the unexpectedly low level of MHC allelic diversity in S. chinensis seems to contrast with this expectation. Initial studies suggested the presence of decreased pathogenic pressure in marine compared with terrestrial environments, leading to the hypothesis that balancing selection was weaker in marine mammals than in terrestrial mammals; this hypothesis might explain the limited MHC variation in marine mammals. However, other studies in marine mammals found strong evidence of balancing selection that maintained considerable MHC diversity (Osborne et al. 2013; Arbanasić et al. 2014). The strength of balancing selection seems to vary among cetacean species. Moreover, the coastal living S. chinensis actually face much greater pathogenic pressure in the PRE than other pelagian cetaceans (Yan et al. 2013).
Another proposed explanation was that the ability for selection to retain adaptive genetic variation in small populations is expected to decrease; therefore, balancing selection may become ineffective in the face of other microevolutionary forces such as genetic drift. PRE S. chinensis had significantly less heterozygosity than expected at both DQB locus (P = 0.02) and microsatellite loci (Lin et al. 2012), and no deviation from the Hardy–Weinberg expectation was observed at the DRB locus (p = 0.81). These combined results indicated that balancing selection might not be sufficiently strong. Based on the detected evidence of weak balancing selection in combination with a genome-wide loss of genetic diversity at both adaptive markers (MHC) and neutral markers (mtDNA and microsatellite) in our previous study (Lin et al. 2010; 2012), we suggest that the effect of genetic drift on MHC variation is dominant during long-term bottlenecks. Similar genetic diversity patterns were also observed in the Australian bush rat (R. fuscipes), the Eurasian beaver (C. fiber), and the rhesus macaque (M. mulatta), which exhibited patterns of MHC variation that were congruent with those observed for mtDNA and/or microsatellites (Hinten et al. 2003; Babik et al. 2005; Yao et al. 2014). Such congruence implies the dominant influence of genetic drift.
Trans-Species Polymorphism
Identical and homologous sequences found at the DQB and DRB loci between S. chinensis and other toothed whales revealed the existence of TSP. In fact, TSP has also been reported for many other cetaceans. However, shared alleles in the entire nucleotide sequences are an extreme case of TSP. Generally, 3 rationales could be proposed. Firstly, the similarity of alleles among related species has been explained by their common ancestry—the persistence of allelic lineages through speciation and their passage from species to species. If such polymorphism is present, sequences should not be clustered according to species. We confirmed these results by showing clear phylogenetic evidence of the sharing of certain alleles among species and on the presence of well-supported clusters containing alleles intermingled from different species (Figure 2a,b). Moreover, the genus Tursiops first appeared approximately 16 Mya. The speciation of N. phocaenoides can be inferred in comparison to Dall’s porpoise (P. dalli) and the harbor porpoise (P. phocoena) to have occurred approximately 12.8–13.1 Mya (Nikaido et al. 2001; Arnason et al. 2004), and the speciation of S. chinensis was determined to have taken place approximately 10.4 Mya based on mitochondrial evidence (Lin et al. 2010). The time since speciation combined with the current distribution regions of these 3 species (S. chinensis, N. phocaenoides, and T. truncatus) suggest that they may have diverged from a common ancestor in the late Pleistocene. Secondly, instead of invoking TSP, convergent evolution was used to explain the TSP between 2 river dolphins inhabiting the Yangtze River [the Yangtze River dolphin and finless porpoise; (Xu et al. 2008)] as a result of sharing the same environmental pressure. Our study revealed identical alleles shared between S. chinensis and other Delphinidae species (T. truncatus and N. phocaenoides). These species inhabit tropical, subtropical or temporal zones around the Pacific Ocean. However, it should be noted that these species do not generally share the same habitats. S. chinensis tends to live in estuarine areas, whereas N. phocaenoides inhabits higher saline waters and T. truncatus lives in pelagic areas (Gao and Zhou 1995; Gao et al. 1995; Wells and Scott 2009). Therefore, we propose that convergent evolution alone may not be adequate to maintain the TSP between these species. Another possible explanation for TSP is introgressive hybridization. Recent study shows that cetaceans may have the potential to produce viable hybrid offspring more easily than other mammals (Amaral et al. 2007), and these hybridization events have been recorded between bottlenose dolphin and common dolphin (Delphinus capensis) in captivity (Zornetzer and Duffield 2003). However, over several years of monitoring S. chinensis in the PRE, we failed to identify any potential hybrids.
Conservation Implications
Low levels of genetic variation at MHC genes (or even the lack thereof) may render a low adaptive potential and high susceptibility to novel infectious diseases for the entire population (Miller et al. 2010). Diseases (such as those caused by mycobacterial species) that were seldom referenced in the earlier marine mammal literature have emerged as significant causes of morbidity and mortality in both wild and captive populations of marine mammals (Dierauf and Gulland 2001). S. chinensis is most widely distributed in estuarine and inshore waters of the PRE. Many species of known opportunistic pathogens have been detected in the PRE (Wu et al. 2004; Yan et al. 2013). Debilitating bacterial disease increases the likelihood of predation of affected individuals, and thus may result in an increase of such mortalities. However, to date we have not found any evidence of large-scale infectious diseases in S. chinensis. This may be explained by the “divergent allele advantage” hypothesis. This hypothesis suggests that if the 2 alleles in a MHC genotype are highly divergent in their sequences, their respective molecules are expected to differ proportionally in the repertoire of antigens they can bind and thus confer a more comprehensive immune surveillance than genotypes with less divergent alleles (Lenz et al. 2013). In our study, a high level of divergence was detected in both DQB and DRB (7.56 and 13.02%, respectively) which was confirmed in the phylogenetic trees. In fact, the evidence that the loss of MHC variation negatively affects population survival is equivocal at present (Radwan et al. 2010). Some species with depleted MHC variation seem to be particularly susceptible to infection, but other species thrive and expand following severe bottlenecks that drastically limit their MHC variation (Weber et al. 2004; Babik et al. 2005; Jamieson et al. 2006). Comparatively speaking, their viability seems to depend mainly on outside factors rather than their genetic makeup, such as human-induced mortality.
Supplementary Material
Supplementary material can be found at http://www.jhered.oxfordjournals.org/.
Funding
National Natural Science Foundation of China (41276147 and 41576128); Ocean Park Conservation Foundation; Science and Research Project of Marine Commonwealth (201105011-5).
Data Availability
Data deposited at Dryad: http://dx.doi.org/doi:10.5061/dryad.18pd8
Supplementary Material
Acknowledgements
We thank OPCFHK and Guangdong Pearl River Estuary Chinese White Dolphin National Nature Reserve for helpful discussions and sample collection. We declare that the research complies with the guidelines or rules for animal care and use for scientific research in China.
References
- Amaral AR, Sequeira M, Martínez-Cedeira J, Coelho MM. 2007. New insights on population genetic structure of Delphinus delphis from the northeast Atlantic and phylogenetic relationships within the genus inferred from two mitochondrial markers. Mar Biol. 151:1967–1976. [Google Scholar]
- Anisimova M, Nielsen R, Yang Z. 2003. Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics. 164:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbanasić H, Đuras M, Podnar M, Gomerčić T, Ćurković S, Galov A. 2014. Major histocompatibility complex class II variation in bottlenose dolphin from Adriatic Sea: inferences about the extent of balancing selection. Mar Biol. 161:2407–2422. [Google Scholar]
- Arnason U, Gullberg A, Janke A. 2004. Mitogenomic analyses provide new insights into cetacean origin and evolution. Gene. 333:27–34. [DOI] [PubMed] [Google Scholar]
- Babik W, Durka W, Radwan J. 2005. Sequence diversity of the MHC DRB gene in the Eurasian beaver (Castor fiber). Mol Ecol. 14:4249–4257. [DOI] [PubMed] [Google Scholar]
- Baker CS, Vant MD, Dalebout ML, Lento GM, O’Brien SJ, Yuhki N. 2006. Diversity and duplication of DQB and DRB-like genes of the MHC in baleen whales (suborder: Mysticeti). Immunogenetics. 58:283–296. [DOI] [PubMed] [Google Scholar]
- Baker CS. 2013. Journal of heredity adopts joint data archiving policy. J Hered. 104:1. [DOI] [PubMed] [Google Scholar]
- Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. 1993. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 364:33–39. [DOI] [PubMed] [Google Scholar]
- Chen BY, Zheng DM, Zhai FM, Xu XR, Sun P, Wang Q, Yang G. 2008. Abundance, distribution and conservation of Chinese white dolphins (Sousa chinensis) in Xiamen, China. Mamm Biol. 73:156–164. [Google Scholar]
- Chen YY, Zhang YY, Zhang HM, Ge YF, Wan QH, Fang SG. 2010. Natural selection coupled with intragenic recombination shapes diversity patterns in the major histocompatibility complex class II genes of the giant panda. J Exp Zool B Mol Dev Evol. 314:208–223. [DOI] [PubMed] [Google Scholar]
- Dierauf L, Gulland F. 2001. Handbook of marine mammal medicine. Boca Raton: (FL: ): CRC Press. [Google Scholar]
- Excoffier L, Lischer HE. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 10:564–567. [DOI] [PubMed] [Google Scholar]
- Gao AL, Zhou KY. 1995. Geographical variation of external measurements and three subspecies of Neophocaena phocaenoides in Chinese waters. Acta Theriol Sinica. 15:81–92. [Google Scholar]
- Gao AL, Zhou KY, Wang YM. 1995. Geographical variation in morphology of bottlenose dolphins (Tursiops sp.) in Chinese waters. Aquat Mammal. 21:121–135. [Google Scholar]
- Gu X, Nei M. 1999. Locus specificity of polymorphic alleles and evolution by a birth-and-death process in mammalian MHC genes. Mol Biol Evol. 16:147–156. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. 1998. Balancing selection and MHC. Genetica. 104:207–214. [DOI] [PubMed] [Google Scholar]
- Heimeier D, Baker CS, Russell K, Duignan PJ, Hutt A, Stone GS. 2009. Confirmed expression of MHC class I and class II genes in the New Zealand endemic Hector’s dolphin (Cephalorhynchus hectori). Mar Mammal Sci. 25:68–90. [Google Scholar]
- Heinzelmann LS, Tavares M, Ott PH, Moreno IMB, Chies JAB. 2009. MHC class II expression in skin biopsies from the franciscana dolphin Pontoporia blainvillei and the southern right whale Eubalaena australis. J Mar Biolog Assoc UK. 89:1009–1013. [Google Scholar]
- Hinten G, Harriss F, Rossetto M, Braverstock PR. 2003. Genetic variation and island biogreography: microsatellite and mitochondrial DNA variation in island populations of the Australian bush rat, Rattus fuscipes greyii. Conserv Genet. 4:759–778. [Google Scholar]
- Huang SL, Karczmarski L, Chen JL, Zhou RL, Lin WZ, Zhang HF, Li HY, Wu YP. 2012. Demography and population trends of the largest population of Indo-Pacific humpback dolphins. Biol Conserv. 147:234–242. [Google Scholar]
- Jamieson IG, Wallis GP, Briskie JV. 2006. Inbreeding and endangered species management: is New Zealand out of step with the rest of the world? Conserv Biol. 20:38–47. [DOI] [PubMed] [Google Scholar]
- Jefferson TA, Hung SK. 2004. A review of the status of the Indo-Pacific humpback dolphin (Sousa chinensis) in Chinese waters. Aquat Mammal. 30:149–158. [Google Scholar]
- Jefferson TA, Hung SK, Lam PKS. 2006. Strandings, mortality and morbidity of Indo-Pacific humpback dolphins in Hong Kong, with emphasis on the role of organochlorine contaminants. J Cetacean Res Manage. 8:181–193. [Google Scholar]
- Jefferson TA, Karczmarski L. 2001. Sousa chinensis. Mamm Spec. 655:1–9. [Google Scholar]
- Kennedy LJ, Ryvar R, Gaskell RM, Addie DD, Willoughby K, Carter SD, Thomson W, Ollier WE, Radford AD. 2002. Sequence analysis of MHC DRB alleles in domestic cats from the United Kingdom. Immunogenetics. 54:348–352. [DOI] [PubMed] [Google Scholar]
- Klein J, Bontrop RE, Dawkins RL, Erlich HA, Gyllensten UB, Heise ER, Jones PP, Parham P, Wakeland EK, Watkins DI. 1993. Nomenclature for the major histocompatibility complexes of different species: a proposal. The HLA system in clinical transplantation. Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Knopp T, Cano JM, Crochet PA, Merilä J. 2007. Contrasting levels of variation in neutral and quantitative genetic loci on island populations of moor frogs (Rana arvalis). Conserv Genet. 8:45–56. [Google Scholar]
- Lenz TL, Mueller B, Trillmich F, Wolf JB. 2013. Divergent allele advantage at MHC-DRB through direct and maternal genotypic effects and its consequences for allele pool composition and mating. Proc Biol Sci. 280:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25:1451–1452. [DOI] [PubMed] [Google Scholar]
- Lin WZ, Chang LH, Frère CH, Zhou RL, Chen JL, Chen X, Wu YP. 2012. Differentiated or not? An assessment of current knowledge of genetic structure of Sousa chinensis in China. J Exp Mar Biol Ecol. 416:17–20. [Google Scholar]
- Lin W, Zhou R, Porter L, Chen J, Wu Y. 2010. Evolution of Sousa chinensis: a scenario based on mitochondrial DNA study. Mol Phylogenet Evol. 57:907–911. [DOI] [PubMed] [Google Scholar]
- Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P. 2010. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics. 26:2462–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LA, Bull JJ. 2002. Fighting change with change: adaptive variation in an uncertain world. Trends Ecol Evol. 17:551–557. [Google Scholar]
- Miller HC, Allendorf F, Daugherty CH. 2010. Genetic diversity and differentiation at MHC genes in island populations of tuatara (Sphenodon spp.). Mol Ecol. 19:3894–3908. [DOI] [PubMed] [Google Scholar]
- Miller HC, Lambert DM. 2004. Genetic drift outweighs balancing selection in shaping post-bottleneck major histocompatibility complex variation in New Zealand robins (Petroicidae). Mol Ecol. 13:3709–3721. [DOI] [PubMed] [Google Scholar]
- Muirhead CA. 2001. Consequences of population structure on genes under balancing selection. Evolution. 55:1532–1541. [DOI] [PubMed] [Google Scholar]
- Munguia VA, Esquer GY, Rojas BL, Vazquez JR, Castro PA, Flores RS. 2007. Genetic drift vs. natural selection in a long-term small isolated population: major histocompatibility complex class II variation in the Gulf of California endemic porpoise (Phocoena sinus). Mol Ecol. 16:4051–4065. [DOI] [PubMed] [Google Scholar]
- Murray BW, White BN. 1998. Sequence variation at the major histocompatibility complex DRB loci in beluga (Delphinapterus leucas) and narwhal (Monodon monoceros). Immunogenetics. 48:242–252. [DOI] [PubMed] [Google Scholar]
- Murrell B, Moola S, Mabona A, Weighill T, Sheward D, Pond SLK, Scheffler K. 2013. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol. 30:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gojobori T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 3:418–426. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Matsuno F, Hamilton H, Brownell RL, Jr, Cao Y, Ding W, Zuoyan Z, Shedlock AM, Fordyce RE, Hasegawa M, et al. 2001. Retroposon analysis of major cetacean lineages: the monophyly of toothed whales and the paraphyly of river dolphins. Proc Natl Acad Sci USA. 98:7384–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AJ, Zavodna M, Chilvers BL, Robertson BC, Negro SS, Kennedy MA, Gemmell NJ. 2013. Extensive variation at MHC DRB in the New Zealand sea lion (Phocarctos hookeri) provides evidence for balancing selection. Heredity (Edinb). 111:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons EC, Jefferson TA. 2000. Post-mortem investigations on stranded dolphins and porpoises from Hong Kong waters. J Wildl Dis. 36:342–356. [DOI] [PubMed] [Google Scholar]
- Piertney SB, Oliver MK. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity. 96:7–21. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics. 14:817–818. [DOI] [PubMed] [Google Scholar]
- Radwan J, Biedrzycka A, Babik W. 2010. Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv. 143:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RR, Dalebout ML, Jefferson TA, Karczmarski L, Laidre K, O’Corry-Crowe G, Rojas-Bracho L, Secchi ER, Slooten E, Smith BD. 2010. Sousa chinensis. IUCN Redlist of Threatened Species. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574. [DOI] [PubMed] [Google Scholar]
- Rousset F. 2008. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 8:103–106. [DOI] [PubMed] [Google Scholar]
- Sutton JT, Nakagawa S, Robertson BC, Jamieson IG. 2011. Disentangling the roles of natural selection and genetic drift in shaping variation at MHC immunity genes. Mol Ecol. 20:4408–4420. [DOI] [PubMed] [Google Scholar]
- Sutton JT, Robertson BC, Jamieson IG. 2015. MHC variation reflects the bottleneck histories of New Zealand passerines. Mol Ecol. 24:362–373. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakos D, Natoli A, Dahlheim M, Hoelzel AR. 2009. Balancing and directional selection at exon-2 of the MHC DQB1 locus among populations of odontocete cetaceans. Mol Biol Evol. 26:681–689. [DOI] [PubMed] [Google Scholar]
- Wang JY, Hung SK, Yang SC. 2004. Records of Indo-Pacific Humpback Dolphins, Sousa chinensis(Osbeck, 1765), from the Waters of Western Taiwan. Aquat Mammal. 30:189–196. [Google Scholar]
- Wang X, Yan C, Zhu Q. 2010Distribution and historical decline process of Chinese white dolphin from Xiamen to the Pearl River Estuary. In: International workshop on population connectivity and conservation of Sousa chinensis off Chinese coast, Nanjing, 4–7 June. [Google Scholar]
- Weber DS, Stewart BS, Lehman N. 2004. Genetic consequences of a severe population bottleneck in the Guadalupe fur seal (Arctocephalus townsendi). J Hered. 95:144–153. [DOI] [PubMed] [Google Scholar]
- Wells RS, Scott MD. 2009. Common bottlenose dolphin, Tursiops truncatus. Encyclopedia Mar Mamm. 6:249–255. [Google Scholar]
- Wu M, Song LS, Ren JP, Kan JJ, Qian PY. 2004. Assessment of microbial dynamics in the Pearl River Estuary by 16S rRNA terminal restriction fragment analysis. Cont Shelf Res. 24:1925–1934. [Google Scholar]
- Xu SX, Chen BY, Zhou KY, Yang G. 2008. High similarity at three MHC loci between the baiji and finless porpoise: trans-species or convergent evolution? Mol Phylogenet Evol. 147:36–44. [DOI] [PubMed] [Google Scholar]
- Xu S, Ju J, Zhou X, Wang L, Zhou K, Yang G. 2012. Considerable MHC diversity suggests that the functional extinction of baiji is not related to population genetic collapse. PLoS One. 7:e30423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SX, Ren WH, Li SZ, Wei FW, Zhou KY, Yang G. 2009. Sequence polymorphism and evolution of three cetacean MHC genes. J Mol Evol. 69:260–275. [DOI] [PubMed] [Google Scholar]
- Yan D, Chen Jl, Zheng RQ, Ning X, Duan GQ, Li J, Wu YP. 2013. Bacterial community structure of main habitat for Sousa chinensis in the Zhujiang Estuary. Mar Environ Sci. 32:011. [Google Scholar]
- Yang G, Yan J, Zhou K, Wei F. 2005. Sequence variation and gene duplication at MHC DQB loci of baiji (Lipotes vexillifer), a Chinese river dolphin. J Hered. 96:310–317. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24:1586–1591. [DOI] [PubMed] [Google Scholar]
- Yao YF, Dai QX, Li J, Ni QY, Zhang MW, Xu HL. 2014. Genetic diversity and differentiation of the rhesus macaque (Macaca mulatta) population in western Sichuan, China, based on the second exon of the major histocompatibility complex class II DQB (MhcMamu-DQB1) alleles. BMC Evol Biol. 14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisset I, Beebee TJ. 2014. Drift rather than selection dominates MHC class II allelic diversity patterns at the biogeographical range scale in natterjack toads Bufo calamita. PLoS One. 9:e100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KY, Xu XR, Tian C. 2007. Distribution and abundance of Indo-Pacific humpback dolphins in Leizhou Bay, China. New Zeal J Zool. 34:35–42. [Google Scholar]
- Zornetzer HR, Duffield DA. 2003. Captive-born bottlenose dolphin× common dolphin (Tursiops truncatus×Delphinus capensis) intergeneric hybrids. Can J Zool. 81:1755–1762. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data deposited at Dryad: http://dx.doi.org/doi:10.5061/dryad.18pd8