Abstract
Patient: Male, 24
Final Diagnosis: Delayed onset serotonin syndrome
Symptoms: Agitation • autonomic instability • fever • hyperreflexia • hypertonia • inducable clonus
Medication: Fluoxetine
Clinical Procedure: —
Specialty: Toxicology
Objective:
Unexpected drug reaction
Background:
Serotonin syndrome is a condition characterized predominantly by neuromuscular symptoms and altered thermoregulation in response to serotonergic overtone. Treatment is focused on withdrawal of serotonergic agents, which leads to resolution in the majority of cases. In the setting of serotonergic overdose, the onset of serotonin syndrome is usually within 4 to 13 h. Here, we report a case of delayed-onset serotonin syndrome in a patient who ingested a mixture of longer-acting serotonin agonists with serotonin antagonists.
Case Report:
A 24-year-old male was transferred to our medical intensive care unit with hypotension and altered mental status after an overdose of fluoxetine, cyproheptadine, trazodone, olanzapine, risperidone, and bupropion. After approximately 72 h, the patient developed symptoms of fever, lower leg clonus, hyperreflexia, and agitation. He was diagnosed with delayed-onset serotonin syndrome, which responded well to re-administration of cyproheptadine, leading to resolution of symptoms by day 5 of his stay.
Conclusions:
In this present case, our patient presented with the longest reported delay in the onset of serotonin syndrome after intentional ingestion. This was likely secondary to co-ingestion of long-acting serotonin agonists with protective shorter-acting serotonin antagonists (cyproheptadine and olanzapine). Clinicians should consider delayed-onset serotonin syndrome when patients ingest longer-acting serotonergic agents with serotonin antagonists.
MeSH Keywords: Cyproheptadine, Delayed Diagnosis, Drug Overdose, Fluoxetine, Serotonin Syndrome
Background
Serotonin syndrome is a condition characterized predominantly by neuromuscular symptoms and altered thermoregulation in response to serotonergic overtone. Treatment is focused on withdrawal of serotonergic agents, which leads to resolution in the majority of cases. However, if not recognized and treated, this syndrome can progress to a life-threatening state. Here, we describe a patient who overdosed on long-acting serotonergic agents in combination with several anti-serotonergic agents, including the 5-HTP2A antagonist cyproheptadine, resulting in a delayed-onset serotonin syndrome.
Case Report
A 24-year-old man with past medical history significant for depression and treatment-refractory schizoaffective disorder was brought in by ambulance after being found unresponsive. He was last seen normal approximately 7 h prior; at the scene, he was surrounded by multiple empty prescription bottles and intentional drug overdose was suspected. In the field, he was administered naloxone without a change in his status.
Upon arrival to the emergency department, the patient was afebrile (temperature 37.2°C) but was hypotensive (mean arterial pressure 59 mm Hg) and tachycardic (heart rate 116). Physical examination was notable for fixed pupils bilaterally and a Glasgow Coma Score (GCS) of 3. Laboratory studies were significant for a lactate 14.8 mmol/L, bicarbonate 16 mEq/L, a white blood cell count of 14.2×109/L, creatinine 1.5 mg/dl, AST 121 U/L, ALT 55 U/L, and creatinine kinase 12 643 U/L.
QTc was prolonged at 549 ms, but QRS interval was normal. Urine toxicology screen was positive for amphetamines. All other laboratory parameters (including electrolytes, blood urea nitrogen, and blood gases) were normal at this time. The patient was subsequently intubated and admitted to the intensive care unit. Treatment initially consisted of supportive care, including vasopressor support for hemodynamic instability in the form of persistent hypotension, volume resuscitation for his acute kidney injury/rhabdomyolysis, and empiric antibiotic coverage with vancomycin and piperacillin-tazobactam for suspected aspiration pneumonia. He was also maintained on infusions of fentanyl, midazolam, and propofol for sedation.
On the second hospital day, the patient’s hemodynamic instability resolved, leading to weaning of sedation and vasopressors. In addition, initial electrolyte abnormalities, elevated white count, and creatinine had normalized, with the exception of creatinine kinase and liver function tests, which were down-trending. However, physical examination was notable for fixed and pinpoint pupils, but with mildly improved neurological function. At this time, the patient’s family provided the medications presumed to have caused his overdose, which included fluoxetine, bupropion, olanzapine, cyproheptadine, and risperidone (Table 1).
Table 1.
Suspected ingested medications (including elimination half-lives) with quantities available to patient based on prescription fill data.
| Drug and dose | Quantity available | Half-life [1,4] | Receptors involved [1,3,4] |
|---|---|---|---|
| Serotonin antagonists | |||
| Olanzapine (5 mg and 10 mg) | 60 | 33 hours | 5HT2A, 5HT2C, dopamine and HT3 antagonist |
| Risperidone (3 mg) | 120 | 3 to 20 hours | 5HT2A, 5HT7, D2 antagonist |
| Cyproheptadine (4 mg) | 60 | 16 hours | 5HT2 Antagonist |
| Serotonergic agents/serotonergic agonists | |||
| Fluoxetine (20 mg) | 30 | Fluoxetine: 4 to 6 days Norfluoxetine: 7 to 15 days |
5HT reuptake inhibition |
| Trazodone (50 mg) | 30 | 6 h | 5HT2A, 5HT2C agonist |
| Other Adrenergic Agents Ingested | |||
| Bupropion SR (100 mg) and XL (150 mg) | 60 | 14 to 21 h | Norepinephrine, dopamine reuptake inhibition |
On the third hospital day, the patient’s temperature rose to 39.1°C and he was noted to have a marked change on examination, with spontaneous myoclonic jerks, rigors, asymmetric patellar reflexes, and nystagmus. This was accompanied by features of autonomic instability (systolic blood pressure of 189 mm Hg). Despite the delay in presentation, serotonin syndrome was suspected as the patient clearly had documented exposure to a serotonergic agent and per Hunter’s Decision Rules for the diagnosis of serotonin toxicity, demonstrated spontaneous clonus (bilateral lower extremities), agitation, ocular clonus, fever, and hyperreflexia [1]. It was thought that upon clearance of olanzapine and cyproheptadine (anti-serotonergic agents), serotonergic overtone from fluoxetine overdose was unmasked due to the longer half-life of this medication (Table 1). To decrease additional serotonergic agent exposure, fentanyl was switched to hydromorphone and a lorazepam drip was initiated to manage his clonus and agitation. However, the patient’s temperature continued to increase (Figure 1); therefore, a single dose of cyproheptadine 12 mg was administered. This resulted in a substantial decrease in temperature (39.9°C to 36.2°C), resolution of clonus and hyperreflexia, and improvement in GCS. Thus, cyproheptadine was continued at 2 mg every 2 h as needed, with an additional 12 mg over the next 72 h. By hospital day 5, no further signs of serotonin syndrome were exhibited and the patient demonstrated a normalization in vital signs (mean arterial pressure 86, heart rate 88) and laboratory parameters (creatinine 0.64 mg/dL, alkaline phosphatase 145 U/L, alanine aminotransferase 79 U/L, creatine kinase 728 U/L, lactate 1.0 mmol/L, bicarbonate 25 mEq/L) He was subsequently extubated and was discharged to inpatient psychiatry on hospital day 8 for further psychiatric management.
Figure 1.
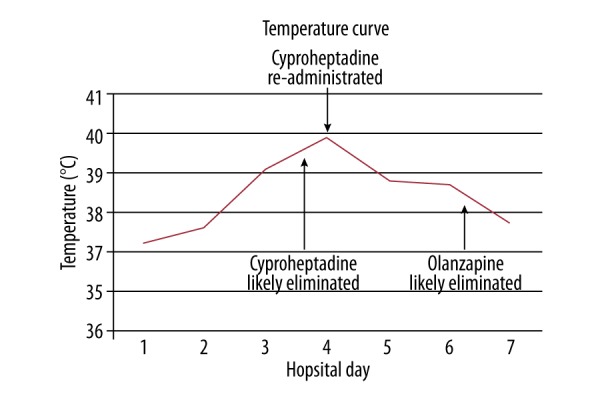
Temperature curve of patient over the patient’s 8-day hospital course (overlaid with time points of elimination of ingested olanzapine and cyproheptadine).
Discussion
This patient presented with a unique manifestation of delayed serotonin syndrome due to co-ingestion of shorter-half-life serotonin antagonists (olanzapine, cyproheptadine) with longer-acting serotonergic agents (fluoxetine). The patient’s poly-pharmacy for depression (fluoxetine), schizoaffective disorder (olanzapine, risperidone), and insomnia (cyproheptadine) contributed to the complexity of this case.
Cyproheptadine and olanzapine are reported to have 16-h [2] and 30-h half-lives, respectively. [3] Doses as low as 18 mg of cyproheptadine provide near-complete antagonism of serotonin receptors [2]. At 80 h post-ingestion, over 95% of cyproheptadine should be eliminated from a given patient. This time course corresponds to the delayed-onset of our patient’s symptoms, thus leading to the high suspicion of delayed serotonin syndrome. In addition, the patient had a marked improvement in his fever curve, muscle clonus, rigidity, and mental status when cyproheptadine was re-administered with lorazepam. At this time, his laboratory abnormalities had grossly normalized, minimizing the likelihood of alternative etiologies. The prompt response to re-administration of cyproheptadine is consistent with prior reports of this agent’s use in serotonin syndrome [4]. Although olanzapine has not been utilized for the treatment of serotonin syndrome, withdrawal of this agent has been implicated in revealing serotonin syndrome in a patient receiving concurrent serotonergic agents [5]. Risperidone may have also conferred a protective effect [6], although serotonin syndrome has been reported with this agent in cases of co-administration with SSRIs [7].
In selective serotonin reuptake inhibitor (SSRI) overdose, the reported onset of serotonin syndrome is normally within 13 h, with the majority of overdoses leading to symptom onset within 4 h [8]. Isolated case reports of delayed-onset serotonin syndrome have been described, although not exceeding 24 h [9]. Our patient overdosed on fluoxetine (time to peak concentration – Tmax 6 h), but the drug’s active metabolite, norfluoxetine, possesses a markedly prolonged Tmax of 48 h [10]. The delay to serotonergic symptoms in this patient (80 h) is the longest reported delay in an intentional SSRI overdose, likely secondary to the co-ingestions described above. Notably, other toxidromes were considered given his mixed ingestion, including anti-cholinergic toxicity and neuroleptic malignant syndrome. The patient did not receive any medications that could have induced malignant hyperthermia. Neuroleptic malignant syndrome is typically hyporeflexic in nature with rigid rigidity in all muscle groups; this patient had hyper-reflexia localized to the lower extremities. Although this patient had agitated delirium consistent with anticholinergic toxidromes, the initial presentation of miosis, with altered muscle tone made this syndrome less likely. The profound response to cyproheptadine this patient exhibited would not be expected with either of these toxidromes but has been documented in serotonin syndrome. Alcohol withdrawal was a possible cofounder in this patient given the delayed hyperadrenergic state that this syndrome generates; however, if it was the sole toxidrome present, the continuous lorazepam infusion should have adequately treated his symptoms without the marked response to cyproheptadine administration. Other conditions such as meningoencephalitis and metabolic encephalopathy were felt to be less likely given the timing of the case with an intentional overdose and profound response to cyproheptadine. Potential infectious and metabolic etiologies were considered less likely given how rapidly the patient’s laboratory parameters normalized, while the patient remained symptomatic. A major limitation of this case report was the inability to obtain serum or urine levels of the ingested agents. This would have allowed a clear depiction of the toxicokinetics of these drugs in this patient and would have allowed trending of serum levels to symptom manifestations.
Conclusions
Combined ingestion of cyproheptadine and olanzapine in a multi-drug overdose resulted in an early “protective” effect, leading to delayed serotonin syndrome in the setting of concurrent fluoxetine and trazodone overdose. Evidence from this report demonstrates that the onset of serotonin syndrome can be delayed and that a high index of suspicion is required for diagnosis, especially in patients with co-ingestion of serotonin antagonists. Serum level monitoring may be useful if multiple agents such as these are co-ingested. Lastly, further study is needed to investigate the potential role of olanzapine and other serotonin antagonists in the early treatment of this condition.
Footnotes
Conflicts of interest
None.
References:
- 1.Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: Simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635–42. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- 2.Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: A potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884. doi: 10.1176/ajp.154.6.884a. [DOI] [PubMed] [Google Scholar]
- 3.Mauri MC, Volonteri LS, Colasanti A, et al. Clinical pharmacokinetics of atypical antipsychotics: A critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46(5):359–88. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- 4.Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615–19. doi: 10.1016/s0736-4679(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 5.Himmighoffen H, Seifritz E, Boeker H. Serotonin syndrome after discontinuation of olanzapine in a combined treatment with duloxetine – case report. Pharmacopsychiatry. 2011;44(2):75–76. doi: 10.1055/s-0030-1268420. [DOI] [PubMed] [Google Scholar]
- 6.Nisijima K, Yoshino T, Ishiguro T. Risperidone counteracts lethality in an animal model of the serotonin syndrome. Psychopharmacology (Berl) 2000;150(1):9–14. doi: 10.1007/s002130000397. [DOI] [PubMed] [Google Scholar]
- 7.Isbister GK. Comment: combination risperidone and SSRI-induced serotonin syndrome. Ann Pharmacother. 2003;37(10):1531–32. doi: 10.1345/aph.1C228a. author reply 1532–33. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LS, Erdman AR, Booze LL, et al. Selective serotonin reuptake inhibitor poisoning: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2007;45(4):315–32. doi: 10.1080/15563650701285289. [DOI] [PubMed] [Google Scholar]
- 9.Pearce S, Ahned N, Varas GM. A case study of delayed serotonin syndrome: Lessons learned. Consult Pharm. 2009;24(1):64–68. doi: 10.4140/tcp.n.2009.64. [DOI] [PubMed] [Google Scholar]
- 10.Moraes MO, Lerner FE, Corso G, et al. Fluoxetine bioequivalence study: Quantification of fluoxetine and norfluoxetine by liquid chromatography coupled to mass spectrometry. J Clin Pharmacol. 1999;39(10):1053–61. doi: 10.1177/00912709922011827. [DOI] [PubMed] [Google Scholar]


