Abstract
Introduction
We sought to evaluate the pathological results of renal masses in comparison with Bosniak III renal cystic lesions to determine the actual malignancy risk.
Methods
A retrospective review of Bosniak III renal lesions identified by computed tomography (CT) or magnetic resonance imaging (MRI) were collected from our patients between August 1, 2013 and December 31, 2015 who underwent surgical excision. TNM stage, histology, Fuhrman grade, and maximum lesion size data was collected. Lesion size relationship with prevalence of malignancy was completed by two-tailed t-test, using the homogeneity hypothesis between malignant and benign groups.
Results
Fifteen of 25 (60%) of Bosniak III lesions were determined to be malignant. All malignant lesions were classified as either Fuhrman grade 1 or 2 with no evidence of progression to Bosniak IV. Average size of malignant lesions was smaller than those of benign pathology (3.52±1.99 cm vs. 5.66±2.53 cm; p=0.041). Smaller lesions (size <4 cm) were more likely to be malignant than lesions of a larger size (p=0.047).
Conclusions
The malignancy risk of Bosniak III renal lesions was 60% in our study. All Bosniak III lesions were of low Fuhrman grade with no evidence of progression. No patient in this study developed metastatic disease within the three-year followup period. Smaller (<4 cm) Bosniak III cysts were more likely to be malignant and lesion size should be taken into consideration when considering management of complex cysts. Active surveillance may be a reasonable option for Bosniak III cystic lesions, regardless of overall size, based upon their universal low grade and no patient developing metastatic disease.
Introduction
The Bosniak renal cyst classification system is a well-established method that uses contrast-enhanced computed tomographic (CT) findings to categorize cystic renal masses into groups with associated risk of malignancy.1,2 Briefly, this system separates simple and mildly complex cystic renal lesions as Bosniak categories I and II, respectively, which do not require further followup. Since its original description, an additional category of IIF has been added to identify increased abnormal findings with recommendation for regular followup. 3,4 Bosniak category IV is considered to have malignancy risk greater than 80% and surgical excision is recommended in able-bodied patients. Bosniak category III cystic masses are of indeterminate origin, thought to have a malignant risk of 40–60%, and surgical excision is recommended.1,5,6
While the malignant potential of Bosniak III cysts are stated to be approximately 40–60%, several studies have demonstrated different rates ranging from 41%7–84%8, correlating with final pathology. Most studies did not require a single-observer interpretation of radiological findings, as it has been shown that a good degree of interobserver agreement exists in assessment of complex renal cysts.9
Although the original Bosniak classification was based on CT findings, many institutions use non-radiation modalities, such as magnetic resonance imaging (MRI), for categorizing complex renal cysts. Studies have shown that MRI are equally as useful as CT for characterization of cystic masses.10,11
Here, we performed a retrospective review of Bosniak III renal cystic lesions classified radiographically by CT and MRI at one academic care centre, with evaluation of pathological diagnosis and lesions to determine the actual malignancy risk.
Methods
Institutional board review and informed consent was obtained for this study. We performed a retrospective review of Bosniak III lesions at our academic institution to evaluate pathological diagnosis and radiographical findings. The hospital information system (Horizon [WP] Provider Portal, McKesson, San Francisco, CA, U.S.) and radiology information system (PACS Centricity Enterprise, GE Healthcare, Chicago, IL, U.S.) was searched from August 1, 2013 to December 31, 2015 for adult patients (>18 years of age) in our clinic with Bosniak III lesions. Inclusion criteria consisted of patients who had one or more Bosniak III lesions characterized by CT or MRI and underwent surgical excision. This was correlated to final pathology. Fuhrman grade 1 and 2 were considered a less aggressive malignancy, while Fuhrman grade 3 and 4 were classified as aggressive subtypes.
Data from 25 patients were reviewed for pathological malignancy in the final analysis. These cases were not controlled for single radiologist interpretation over the time period. These cases’ most recent radiographical findings on CT or MRI prior to surgical excision were all classified as Bosniak III cystic renal lesions. Nephron-sparing surgeries were performed in 11 cases and radical nephrectomies were performed in the remaining 14 cases.
Multidetector CT scans were performed according to standard protocol covering the abdomen while the patient was placed in the supine position. Pathology specimen reports were reviewed and categorized as benign or malignant and used the 2010 American Joint Committee on Cancer (AJCC) TNM staging classification for kidney cancer.12
Statistical analysis
Prediction of lesion size in relation to prevalence of malignancy was done by means of a two-tailed t-test using the homogeneity hypothesis between pathology results between malignant and benign groups. A statistically significant difference by which the two groups would be considered different was taken to be indicated by a p value of less than 0.05. A hypothesis test for two population proportions was done with 1 cm intervals to compare proportions of benign vs. malignant lesions at different lesion sizes using Fischer’s exact test.
Results
Twenty-five patients with Bosniak III lesions were included in this study, with an average age of 58.6±12.2 years; nine were male and 16 were female. There were 15 lesions (60%) on the right side and 10 (40%) on the left side. Mean lesion size of all cases was 4.41±2.42 cm (range 1.0–12.0 cm).
Of the 25 cases, 15 were malignant (60%) and 10 were benign complex cysts (40%). Surgical pathology of the malignant lesions revealed 2/15 (13%) were papillary renal cell carcinoma (RCC), while the remaining 13/15 (87%) were clear-cell RCC. Of the malignant lesions, 5/15 (33%) were Fuhrman grade 2, 9/15 (60%) Fuhrman grade 1, while Fuhrman grade was not available for one lesion.
According to TNM staging, 9/15 (60%) malignant lesions were pT1a (<4 cm in diameter, limited to the kidney), 4/15 (27%) were pT1b (4–7 cm in diameter, limited to the kidney), and 1/15 (7%) was pT2a (7–10 cm in diameter, limited to the kidney). One lesion was suspicious for RCC and was sent for consultation, which confirmed unilocular RCC, although the tumour cells were well under 1% of the mass; staging the lesion as pT2 based on its 12 cm size would not be appropriate and it was therefore staged as pTX. For this reason, this specimen was not included in the size comparisons of lesions. Radiological and pathological findings are summarized in Table 1.
Table 1.
Summary of radiological and pathological findings of 25 cases of renal masses
| Patient # | Gender | Age (at surgery) | Classification (on CT/MRI) | Pathology/Fuhrman grade | Maximum mass dimension (cm) |
|---|---|---|---|---|---|
| 1 | F | 66 | Bosniak III (CT) | pT2a, pNX Clear-cell RCC G2 |
7.50 |
| 2 | F | 75 | Bosniak III (CT) | pT1b, pNX Clear-cell RCC G1 |
4.50 |
| 3 | M | 66 | Bosniak III (CT) | pT1b, pNX Clear-cell RCC G1 |
3.00 |
| 4 | F | 41 | Bosniak III (MRI) | pT1b, pNX Clear-cell RCC G1 |
7.00 |
| 5 | F | 80 | Bosniak III (MRI) | pT1b, N0 Papillary RCC |
5.00 |
| 6 | F | 37 | Bosniak III (MRI/CT) | pT1a, pNX Clear-cell RCC G2 |
3.50 |
| 7 | M | 66 | Bosniak III (CT) | pT1a, pNX Clear-cell RCC G1 |
3.50 |
| 8 | F | 60 | Bosniak III (MRI) | pT1a, pNX Clear-cell RCC G1 |
3.00 |
| 9 | M | 47 | Bosniak III (MRI) | pT1a, pNX Clear-cell RCC G1 |
1.50 |
| 10 | M | 51 | Bosniak III (CT) | pT1a, pN0 Papillary RCC G1 |
1.50 |
| 11 | F | 66 | Bosniak III (CT) | pT1a, pN0 Clear-cell RCC G1 |
3.00 |
| 12 | M | 57 | Bosniak III (CT) | pT1a, pNX Clear-cell RCC G2 |
4.00 |
| 13 | F | 68 | Bosniak III (CT) | pT1a, pN0 Clear-cell RCC G2 |
1.30 |
| 14 | F | 31 | Bosniak III (MRI) | pT1a, pN0 Clear-cell RCC G2 |
1.00 |
| 15* | M | 55 | Bosniak III (CT) | pTX, pNX Clear-cell RCC G1 |
12.00 |
| 16 | M | 52 | Bosniak III (CT) | No obvious tumour mass Cystic structure Negative for RCC |
6.00 |
| 17 | F | 47 | Bosniak III (CT) | Negative RCC (cystic area) Multiloculated cystic area with fibrotic wall <0.1 cm (4 cm total cystic area) |
4.00 |
| 18 | F | 53 | Bosniak III (CT) | Multicystic mass | 7.00 |
| 19 | F | 70 | Bosniak III (CT) | Simple cortical cysts with calcifications | 4.50 |
| 20 | M | 75 | Bosniak III (MRI) | Simple benign cortical cyst | 4.00 |
| 21 | M | 59 | Bosniak III (CT) | Hemorrhagic cyst | 2.50 |
| 22 | F | 54 | Bosniak III (CT) | Cystic structure | 3.00 |
| 23 | F | 56 | Bosniak III (MRI) | Cystic nephroma | 6.50 |
| 24 | F | 69 | Bosnaik III (MRI) Bosniak III (CT) |
Cystic nephroma | 10.10 |
| 25 | F | 64 | Bosniak III (CT) | Complex renal cyst | 9.00 |
CT: computed tomography; F: female; M: male; MRI: magnetic resonance imaging; RCC: renal cell carcinoma.
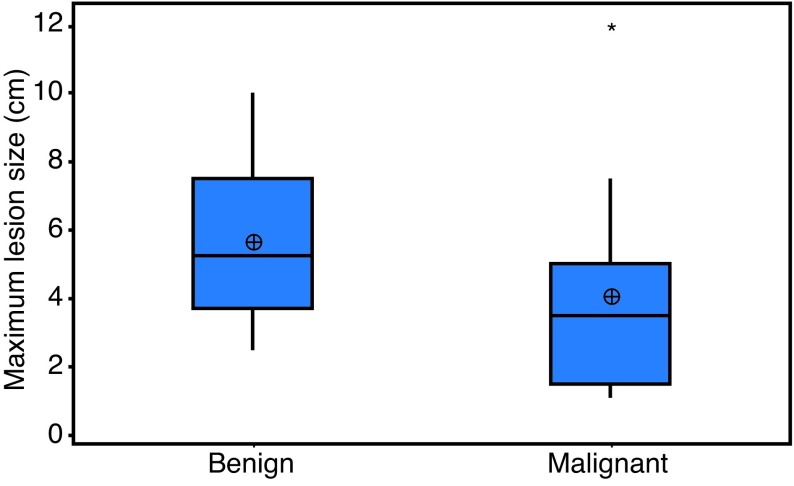
Comparison of lesion size as a predictor of malignancy on pathological analysis displayed statistical difference of average lesion size and prevalence of malignancy (p=0.041) (Table 2, Fig. 1).
Table 2.
Average lesion size
| Sample size | Mean size (cm) | |
|---|---|---|
| Benign | 10 | 5.66±2.53 |
| Malignant | 14 | 3.52±1.99 |
Fig. 1.
Boxplot of lesion size vs. malignancy in Bosniak III renal masses.
*Statistical outliers. Outliers were not included in lesion size calculations due to inability to classify histologically by TNM stage.
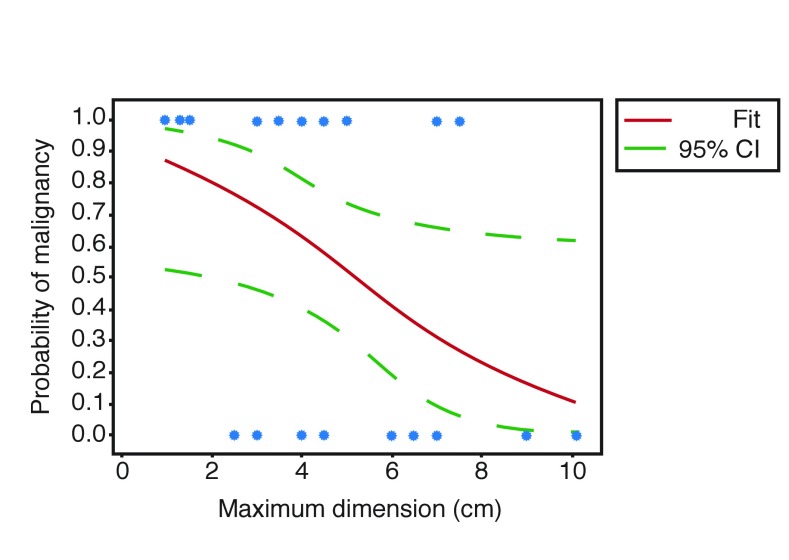
Smaller lesions (size <4 cm) were shown to be more likely to be malignant than lesions of a larger size (Fischer’s exact test p=0.047) based on a proportional comparison of maximum lesion dimension shown in Table 3. A binary regression of the data revealed a trend identifying a decreasing probability of malignancy with larger lesion size (decreased likelihood of 1.554 for every 1.0 cm increase in size) on average, although further investigation with increasing sample sizes may provide clearer data (pseudo R2=15.44%) (Fig. 2).
Table 3.
Proportional comparison of lesion size and malignancy
| Number of malignancies/total cases (%) | |
|---|---|
| <4 cm | ≥4 cm |
| 81.82% | 38.46% |
Fig. 2.
Fitted line plot for probability of malignancy based on maximum lesion dimension. CI: confidence interval.
Discussion
This study demonstrated probability of malignancy of 60% in renal lesions classified as Bosniak III by CT or MRI. Several studies have demonstrated various different malignancy rates of Bosniak III that are in agreement with the findings described in this series.7,13–15 Bata et al have recently shown much higher probability of malignancy (84%) in Bosniak III lesions in their series,8 although the sample size was 19 patients.
While both papillary RCC and clear-cell RCC were present in this series, the Bosniak III lesions were predominantly clear-cell RCC, the most common type of RCC.16 This reinforces the principle that cystic lesions that are malignant do not necessarily equate to clear-cell RCC. It remains an important finding, as various malignant neoplasms exist that can potentially alter management options.17,18
The short-term results of this series showed that 100% of all malignancies were Fuhrman grade 1 or 2 and no development of metastatic disease occurred during the three-year followup period in this study. Therefore, active surveillance may be an option for Bosniak III cystic masses regardless of size, as all pathologies were low-grade with low metastatic potential.
Lesion size does appear to play a statistically significant (p=0.041) role in the determination of malignancy of Bosniak III classified renal masses of this series. The results of this study identified that a smaller lesion size tended to be associated with malignancy, although further investigation with larger sample sizes may be required to further reinforce this finding. Han et al15 also reported that lesion size should be taken into account for formulation of treatment, although they identified that lesions smaller than 2 cm are likely benign. The previous study only reported on lesions less than or greater than 2 cm and included Bosniak II–IV lesions, but did not comment on the spectrum of lesion size. Proportional comparison of the data in our study used several cutoff values for lesion size, at 1.0 cm intervals, which could identify benign from malignant tumours. Significance was identified at the 4 cm boundary.
These results showing that smaller Bosniak III lesions had higher risk of being malignant were somewhat unexpected. Although a direct relationship between size and malignancy exists for solid renal masses, this is less well-understood in cystic lesions. This relationship between the larger cystic lesion sizes may be, in part, due to relatively increased functional fluid secretion tissue vs. that of malignant lesions that secrete less fluid. Although we attempted to compare the proportion of preoperative images that contained solid components to lesion size, no significance was identified within our sample; this may be an area for further study.
Goenka et al14 developed a clinical prediction model for malignancy risk of Bosniak III renal lesions, concluding that smaller lesion size confers higher risk of malignancy (with a large sample size of 107 in agreement with this data).
There were some limitations in our study, including the retrospective design. Radiologists who were involved in interpretation of CT or MRI were not standardized and although there is evidence that interobserver effect is minimal in complex renal cysts,9 application of the Bosniak classification system remains subjective. Finally, the overall sample size of this series was relatively small; a study involving a larger number of patients may be helpful in validating the overall malignancy risk and prediction power of lesion size in Bosniak III cysts.
Conclusion
The malignancy risk of Bosniak III renal cysts is 60% in our study. The data collected from this series suggests that the smaller Bosniak III cysts carry higher risk of malignant final pathology, although larger studies may be required in the future to clarify the data. Lesions <4 cm showed a significant increase in the likelihood of malignancy. The decision for surveillance vs. surgical excision of complex renal cysts that may represent RCC should take into account lesion size as a primary factor. There was no evidence of progression from Bosniak III to Bosniak IV of patients in this study or development of locally advanced or metastatic disease within the three-year followup period. Finally, 100% of all malignancies were classified as a less aggressive grade (Fuhrman grade <2). This data can be used to counsel patients with regards to the option of active surveillance for Bosniak III masses regardless of size, as all pathologies were low-grade and no patient developed metastatic disease.
Footnotes
Competing interests: Dr. Kapoor has been an advisor for and participated in clinical trials supported by Amgen, Astellas, Janssen, GSK, Novartis, Pfizer, and Sanofi. Mr. Lam reports no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. https://doi.org/10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 2.Bosniak MA. The Bosniak renal cyst classification: 25 years later. Radiology. 2012;262:781–5. doi: 10.1148/radiol.11111595. https://doi.org/10.1148/radiol.11111595. [DOI] [PubMed] [Google Scholar]
- 3.Bosniak MA. The use of the Bosniak classification system for renal cysts and cystic tumours. J Urol. 1997;157:1852–3. https://doi.org/10.1016/S0022-5347(01)64883-3. [PubMed] [Google Scholar]
- 4.Bosniak MA. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am J Roentgenol. 1997;169:819–21. doi: 10.2214/ajr.169.3.9275903. https://doi.org/10.2214/ajr.169.3.9275903. [DOI] [PubMed] [Google Scholar]
- 5.Whelan TF. Guidelines on the management of renal cyst disease. Can Urol Assoc J. 2010;4:98–9. doi: 10.5489/cuaj.10023. https://doi.org/10.5489/cuaj.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–8. doi: 10.1016/j.urology.2005.04.003. https://doi.org/10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Cloix PX, Martin X, Pangaud C, et al. Surgical management of complex renal cysts: A series of 32 cases. J Urol. 1996;156:28–30. https://doi.org/10.1016/S0022-5347(01)65928-7. [PubMed] [Google Scholar]
- 8.Bata P, Tarnoki AD, Tarnoki DL, et al. Bosniak category III cysts are more likely to be malignant than we expected in the era of multidetector computed tomography technology. J Res Med Sci. 2014;19:634–8. [PMC free article] [PubMed] [Google Scholar]
- 9.El-Mokadem I, Budak M, Pillai S, et al. Progression, inter-observer agreement, and malignancy rate in complex renal cysts ( ≥ Bosniak category IIF) Urol Oncol. 2014;32:24. doi: 10.1016/j.urolonc.2012.08.018. e21–7. [DOI] [PubMed] [Google Scholar]
- 10.Balci NC, Semelka RC, Patt RH, et al. Complex renal cysts: Findings on MR imaging. AJR Am J Roentgenol. 1999;172:1495–500. doi: 10.2214/ajr.172.6.10350279. https://doi.org/10.2214/ajr.172.6.10350279. [DOI] [PubMed] [Google Scholar]
- 11.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: Comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231:365–71. doi: 10.1148/radiol.2312031025. https://doi.org/10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB. C. American Joint Committee on, editor. In: AJCC cancer staging manual. 7th ed. Edge SB, editor. New York; London: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 13.Smith AD, Remer EM, Cox KL, et al. Bosniak category IIF and III cystic renal lesions: Outcomes and associations. Radiology. 2012;262:152–60. doi: 10.1148/radiol.11110888. https://doi.org/10.1148/radiol.11110888. [DOI] [PubMed] [Google Scholar]
- 14.Goenka AH, Remer EM, Smith AD, et al. Development of a clinical prediction model for assessment of malignancy risk in Bosniak III renal lesions. Urology. 2013;82:630–5. doi: 10.1016/j.urology.2013.05.016. https://doi.org/10.1016/j.urology.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Han HH, Choi KH, Oh YT, et al. Differential diagnosis of complex renal cysts based on lesion size along with the Bosniak renal cyst classification. Yonsei Med J. 2012;53:729–33. doi: 10.3349/ymj.2012.53.4.729. https://doi.org/10.3349/ymj.2012.53.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storkel S, van den Berg E. Morphological classification of renal cancer. World J Urol. 1995;13:153–8. doi: 10.1007/BF00184870. https://doi.org/10.1007/BF00184870. [DOI] [PubMed] [Google Scholar]
- 17.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear-cell renal cell carcinomas: A systematic review and meta-analysis. Eur Urol. 2015;67:740–9. doi: 10.1016/j.eururo.2014.05.010. https://doi.org/10.1016/j.euru-ro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase 2 study (SUPAP) by the French Genitourinary Group (GETUG) Ann Oncol. 2015;26:1123–8. doi: 10.1093/annonc/mdv149. https://doi.org/10.1093/annonc/mdv149. [DOI] [PubMed] [Google Scholar]