Abstract
Sepsis with severe systemic inflammation remains a great challenge for the intensive care unit in clinics. Although biomarkers have been identified to diagnose, monitor and predict these syndromes, novel therapeutic approaches are required for the amelioration of symptoms of sepsis and septic shock. The present study demonstrated that interleukin (IL)-31 was able reduce the mortality rate of lipopolysaccharide (LPS)-induced sepsis with the reduction of inflammatory cytokines in the sera. IL-31 also inhibited IL-1β production in the peritoneal lavage fluid in LPS-induced or cecal ligation and puncture-induced sepsis. The in vitro mechanism responsible for IL-31 regulation on peritoneal IL-1β activation following LPS challenge was explored. It was demonstrated that IL-1β secretion was suppressed by IL-31 treatment from LPS-challenged peritoneal macrophages following adenosine triphosphate stimulation, which is an activator of NLR family, pyrin domain-containing 3 (NLRP3). Furthermore, IL-31 inhibited the expression of NLRP3 at the transcriptional level. In human THP-1 cells, anti-IL-31/anti-IL-31 receptor (R) neutralizing antibody enhanced NLRP3 expression as well as IL-1β activation, suggesting a role of the IL-31-IL-31R-NLRP3-IL-1β signaling axis in the physiological status of sepsis. On the other hand, IL-31 displayed a negative effect on the NLRP1 inflammasome, but not on NLRP3 on the LPS-primed human peripheral blood monocytes, resulting in reduction of the inflammatory cytokine, tumor necrosis factor (TNF)-α, in the supernatant. Taken together, the present data implied that T helper 2-type cytokine, IL-31, may be a promising therapeutic option for treatment of sepsis and septic shock in clinics.
Keywords: sepsis, interleukin-31, inflammasomes, NLRP3, NLR family, pyrin domain-containing 3, interleukin-1β
Introduction
Sepsis is a clinical syndrome defined by physiological changes with a systemic inflammation response, and it remains a major medical challenge in pediatric and critical care medicine (1,2). Sepsis is often caused by documented or suspected infection with bacteria that produce lipopolysaccharide (LPS), and septic shock is the progression of those physiological changes to the extent that delivery of oxygen and metabolic substrate to tissues is compromised (3). Sepsis is usually diagnosed when the symptoms of the systemic inflammatory response syndrome (SIRS) develop. SIRS is defined clinically by the activation of the immune/inflammatory response, resulting in abnormal temperature or leukocyte count (4). Biomarkers have been identified that have the potential to diagnose, monitor, stratify and predict the outcome of these syndromes, for example, C-reactive protein and interleukin (IL)-18 elevation have been used as biomarkers indicating sepsis (5,6). However, many clinicians in the intensive care unit still face the challenge of diagnosing and accurately assessing the risk of outcome. More importantly, initiating appropriate therapy for sepsis may help improve patient management and decrease sepsis-related morbidity and mortality.
IL-31 is a cytokine derived from T cells, namely T helper 2 (Th2) cells, that shares several structural and functional characteristics with IL-6, oncostatin M, leukemia inhibitory factor and cardiotrophin-1 (7). IL-31 signals through a receptor complex comprised of GPL (also known as gp130-like receptor or IL-31RA) and oncostatin M receptor (OSMR) (8–10). GPL/OSMR signaling is a strong activator of signal transducer and activator of transcription STAT3 and STAT5, and also activates STAT1, Janus kinase (JAK)1 and JAK2 signaling pathways (11). IL-31-regulated immune responses have been implicated in skin physiology and inflammatory skin diseases (12). Research has reported that IL-31 may negatively regulate lung type 2 inflammation by IL-31/IL-31 receptor (R) interaction (13). In addition, IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection (14). However, the effect of IL-31 on sepsis and its underlying mechanisms remain uncertain. NLR family, pyrin domain-containing 3 (NLRP3, CIAS1, PYPAF or cryopyrin, is a cytosolic member of the NLRP family of proteins expressed in leukocytes, particularly neutrophils (15). As a component of the inflammasome, NLRP3 activates caspase 1 and causes the maturation cleavage of pro-IL-1β and pro-IL-18 (16). Defects in NLRP3 may cause autoinflammatory syndromes. A subgroup of the nucleotide-binding domain, leucine-rich repeat containing (NLR) proteins are key mediators of the inflammasome. IL-1β functions in the systemic responses to infection and chronic and acute inflammation (17). IL-1β (p17) is the mature active form of pro-IL-1β (p35) that has to be cleaved by caspase 1 at Asp116 (15,18). The present study examined the role IL-31 on experimental sepsis and investigated the mechanisms of IL-31 efficacy responsible for the regulation of NLRP3 as well as IL-1β.
Materials and methods
Mouse strains and reagents
C57BL/6 mice (50 female mice, 20–30 g and 6–10 weeks old) were obtained from Vital River Laboratories Co., Ltd., (Beijing, China) and were housed in plastic cages (3–4 mice per cage) with free access to drinking filtered tap water and food under controlled conditions of humidity (50±10%), light (12-h light/dark cycle) and temperature (23±2°C). Mice were maintained in a specific pathogen free facility in accordance with institutional guidelines. The protocol was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University (Beijing, China).
Septic shock model
To induce in vivo cytokine secretion, 7-week old female mice were injected intraperitoneally with LPS (10 mg/kg body weight) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and their health status was monitored at regular intervals. A total of 6 h after the injection, the peritoneal cavities were washed with 0.8 ml phosphate-buffered saline (PBS) containing 1% fetal bovine serum (FBS, Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cytokines in the peritoneal lavage fluids and in the sera were then measured by ELISA. The cecal ligation and puncture (CLP) technique was used to induce intraabdominal sepsis in mice (19). All the mice received LPS injection or CLP operation, and 6–8 mice were checked in each group. Control mice were injected with PBS compared with IL-31 injection after LPS or CLP treatment. The mice were divided into the following groups: Vehicle control group (IL-31-), which received PBS injection + LPS or CLP operation; IL-31 treatment group (IL-31+), which received IL-31 injection + LPS or CLP operation. In total, four groups (PBS+LPS, IL-31+LPS, PBS+CLP and IL-31+CLP) were used for the study (6–8 mice per group).
Cells
In preparation for the isolation of peritoneal macrophages as described previously (20), mice were intraperitoneally injected with 1 ml 4% thioglycollate (Sigma-Aldrich; Merck KGaA, B2551), and peritoneal exudate cells were isolated from the peritoneal cavity 4 days post-injection. The cells were then incubated at 37°C for 6 h and washed three times with Hank's Balanced Salt Solution (HBSS; Thermo Fisher Scientific, Inc.). The remaining adherent cells were used as the peritoneal macrophages described in previous experiments (20). Unless otherwise indicated, the macrophages were primed with 200 ng/ml LPS from Escherichia coli 0111:B4 (Sigma-Aldrich; Merck KGaA) for 4 h at 37°C before stimulation with 5 mM adenosine triphosphate (ATP; Sigma-Aldrich; Merck KGaA, A6419) for 30 min at 37°C.
Human peripheral blood monocytes (PBMC) were obtained from healthy donors who provided written informed consent. The cells were adjusted to 5×106 cells/ml and resuspended in RPMI-1640 culture medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 50 mg/ml gentamicin, 2 mM L-glutamine and 1 mM pyruvate. Human THP-1 cells were purchased from the China Center for Type Culture Collection (Wuhan, China). THP-1 cells were cultured in RPMI-1640 supplemented with 10% FBS, 1% HEPES, 1% L-glutamine, and 50 µg of cefotaxime. The cells were treated with 5 nM phorbol myristate acetate (Sigma-Aldrich; Merck KGaA) overnight and then washed three times. Cells were rested 3 days following chemical differentiation to ensure that they reverted to a resting phenotype.
Proteins and antibodies
All reagents used in the present study were from Sigma-Aldrich (Merck KGaA), unless stated otherwise. Recombinant murine IL-31 was obtained from PeproTech Company (Suzhou, China). A total of 6–8 mice for each IL-31 treatment group were used in the three groups of experiments: i) Survival study of IL-31 treatment and control treatment (8 mice in PBS+LPS vs. 8 mice in IL-31+LPS for survival rate check); ii) cytokine analysis of LPS-induced sepsis for the IL-31 treatment and control treatment (8 mice in PBS+LPS vs. 8 mice in IL-31+LPS for cytokine analysis); and iii) cytokine analysis of CLP operation for the IL-31 treatment and control treatment (8 mice in PBS+CLP vs. 8 mice in IL-31+CLP for cytokine analysis). A total of 100 µg of IL-31 cytokine in PBS for one time i.p. injection was given per mouse in vivo. Control mice were injected with PBS vehicle compared with IL-31 injection after LPS or CLP treatment. Functional anti-human IL-31 antibody (cat. no. AF2824, 1 µg/ml; R&D Systems China Co., Ltd., Shanghai, China) and anti-human IL-31RA antibody (Nemolizumab; cat. no. TAB-439CQ, 1 µg/ml; Creative Biolabs; New York, NY, USA).
ELISA
Mouse (m)TNFα (cat. no. MTA00B), mIL-18 (cat. no. 7625), mIL-1β (cat. no. MLB00C), human (h)IL-1β (cat. no. DLB50), hIL-6 (cat. no. D6050) and hTNF-α (cat. no. DTA00C) ELISA kits for cytokine detection in the peritoneal lavage fluids, sera or cell culture supernatant were obtained from R&D Systems China Co., Ltd. To detect low levels of cytokines in the samples, a standard curve was obtained by diluted standard reagents.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from cell culture was extracted using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and subjected to RT-qPCR in triplicates, using SYBR Green chemical dye (Toyobo Life Science, Osaka, Japan).
Relative expression levels of IL-31, IL-31RA, NLRP1/3/6 and tumor necrosis factor (TNF) were calculated using the 2−ΔΔCq method (21) normalized to the internal control, β-actin. To quantify cytokine mRNA, the assays were performed in 2 µg total RNA for reverse transcription using cDNA synthesis kits (Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 11756050). Reverse transcription was performed at 50°C for 30 min. qPCR was performed using a RT-qPCR kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling conditions used for the assays were as follows: 1 cycle at 94°C for 15 min, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The following primer sequences were used: IL-31, forward 5′-TCGGTCATCATAGCACATCTGGAG-3′ and reverse 5′-GCACAGTCCCTTTGGAGTTAAGTC-3′; IL-31RA, forward 5′-AGAATGTTCCAGATACAATGG-3′ and reverse 5′-CGAAGCATGCATACTAAAGGAA-3′; NLRP1, forward 5′-GGACCTCATGGTGGTTACTTTC-3′ and reverse 5′-TCCCAGGGGCCGTAAACTT-3′; NLRP3, forward 5′-ATTACCCGCCCGAGAAAGG-3′ and reverse 5′-CATGAGTGTGGCTAGATCCAAG-3′; NLRP6, forward 5′-TCTCTCCGTGTCAGCGTTCA-3′ and reverse 5′-CGGAAGAGCCGATTAAAAGTGT-3′; TNF, forward 5′-TCCCCAAAGGGATGAGAAGTTC-3′ and reverse 5′-TCATACCAGGGTTTGAGCTCAG-3′; and β-actin, forward 5′-ATGGGTCAGAAGGACTCCTACG-3′ and reverse 5′-AGTGGTACGACCAGAGGCATAC-3′.
Statistical analysis
Data were presented as the mean ± standard deviation of three independent experiments. The statistical comparisons between the different treatments were performed using an unpaired Student's t-test. Analysis was performed using SPSS software, Version 22.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
IL-31 protects against experimental sepsis in vivo
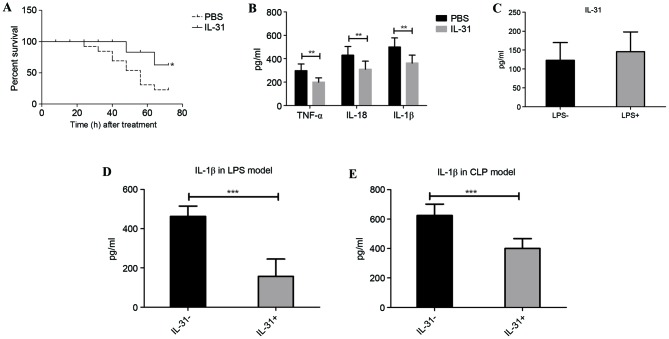
In LPS-induced sepsis, the susceptibility of mice treated with PBS or IL-31 cytokine was compared. Mice that received the high dose of LPS and that were treated with recombinant murine IL-31 injection demonstrated a significantly lower mortality rate than the vehicle control-treated (PBS) mice from 24 to 72 h (P=0.0473; Fig. 1A). Furthermore, the septic shock marker and inflammatory cytokine levels in the serum, including TNF-α, IL-18 and IL-1β, were significantly suppressed by IL-31 injection compared with treatment with PBS (P<0.01; Fig. 1B). There was no significant difference in IL-31 production following LPS challenge (Fig. 1C), whereas the IL-1β level in the peritoneal lavage fluid was significantly downregulated by IL-31 treatment compared with no IL-31 treatment (P<0.001; Fig. 1D), suggesting that IL-31 may regulate IL-1β activation-related signaling in vivo. Furthermore, to confirm whether IL-31 was protective against infection and septic shock, IL-31 production in the CLP model of sepsis was measured. As expected, mice that received IL-31 treatment demonstrated a significant reduction of IL-1β in the CLP model compared with the vehicle control group (P<0.001; Fig. 1E).
Figure 1.
Effect of IL-31 on experimental sepsis. (A) Survival rate of IL-31-treated or PBS-treated (vehicle control) mice with LPS-induced sepsis. (B) Proinflammatory cytokine production in the sera from each group. (C) IL-31 level in the peritoneal lavage fluid of mice with LPS-induced sepsis and normal mice without LPS treatment. (D) IL-1β level in the peritoneal lavage fluid of mice with LPS-induced sepsis in the presence or absence of IL-31 injection. (E) IL-1β level in the peritoneal lavage fluid of mice with CLP-induced sepsis in the presence or absence of IL-31 injection. *P=0.0473 vs. PBS from 24 to 72 h; **P<0.01 and ***P<0.001, as indicated. Experiments were performed in triplicate. IL, interleukin; PBS, phosphate-buffered saline; LPS, lipopolysaccharide; TNF, tumor necrosis factor; CLP, cecal ligation and puncture.
IL-31 regulates IL-1β production by targeting NLRP3 inflammasome transcription in vitro
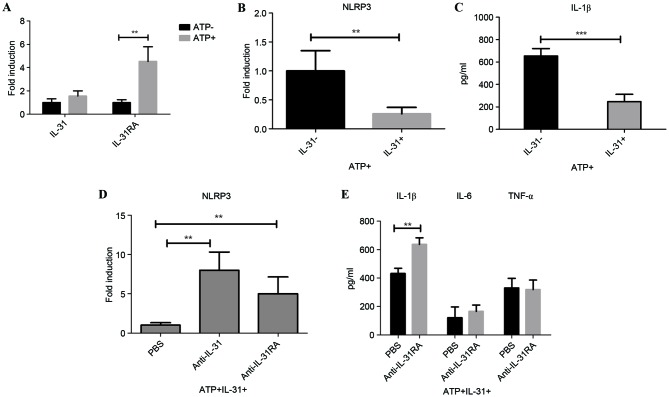
As IL-31 may be involved in IL-1β production in vivo, the role of IL-31 on IL-1β signaling was examined in vitro. RT-qPCR results demonstrated that IL-31RA mRNA was significantly induced following ATP stimulation on the peritoneal macrophages (P<0.01); however, there was no significant change of IL-31 expression on the cell following ATP induction (Fig. 2A). ATP is an activator of the NLRP3 inflammasome. To analyze the IL-31-IL-1β signaling axis, the in vitro mRNA expression of NLRP3 following IL-31 treatment was measured. Results demonstrated that IL-31 was able to significantly reduce the expression of NLRP3 (P<0.01; Fig. 2B). Furthermore, IL-31 also significantly decreased LPS-induced IL-1β secretion in ATP-treated cells (P<0.001; Fig. 2C).
Figure 2.
In vitro role of IL-31 on IL-1β activation. (A) Expression of IL-31 and IL-31RA on the peritoneal macrophage with or without ATP stimulation. (B) Expression of NLRP3 on peritoneal macrophages with or without IL-31 treatment under ATP stimulation. (C) Secretion of IL-1β on peritoneal macrophage supernatant with or without IL-31 treatment under ATP stimulation. (D) Expression of NLRP3 on the THP-1 cell with anti-IL-31 antibody or anti-IL-31RA antibody treatment under ATP stimulation plus IL-31. (E) Secretion of cytokines in THP-1 cell supernatant with anti-IL-31RA antibody treatment under ATP stimulation plus IL-31. **P<0.01 and ***P<0.001, as indicated. Experiments were performed in triplicate. IL, interleukin; IL-31RA, interleukin-31 receptor A; ATP, adenosine triphosphate; PBS, phosphate-buffered saline.
To investigate the role of IL-31 signaling in human cells, a specific antibody that directly blocks IL-31 (anti-IL-31) or IL-31R (IL-31RA antibody) was utilized. NLRP3 mRNA expression increased significantly following IL-31 signal blocking with neutralizing antibody targeting IL-31/IL-31RA compared with cells treated with PBS (P<0.01; Fig. 2D), suggesting that IL-31 inhibited IL-1β activation through decreasing the NLRP3 inflammasome. In concordance with this, IL-31RA antibody significantly enhanced the level of IL-1β compared with the level in cells treated with PBS (P<0.01; Fig. 2E), without significantly affecting the levels of Toll-like receptor signaling-related IL-6 and TNF-α in the supernatants of the cell culture.
IL-31 modulates NLRP1 in human PBMC
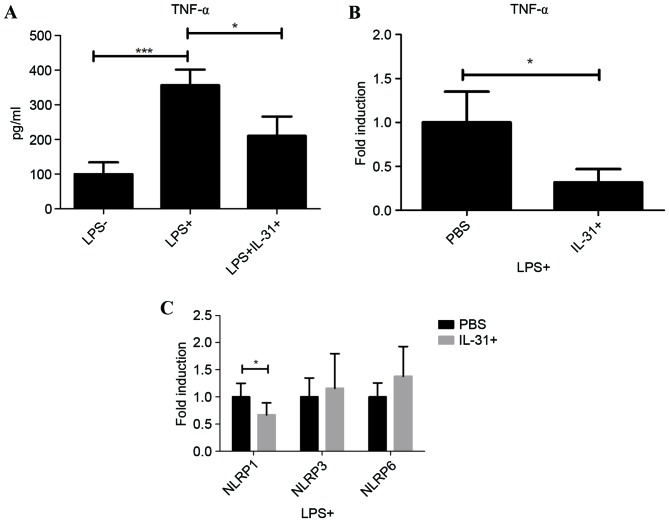
To systematically investigate the effect of IL-31 on septic shock, LPS-primed human PBMC from healthy donors were cultured and treated with IL-31 in vitro. As a typical inflammatory cytokine in sepsis, TNF-α was significantly induced in the supernatants by LPS treatment compared with when LPS was not used (P<0.001), suggesting the activation of the LPS pathway in human PBMC (Fig. 3A). Following IL-31 treatment, it was demonstrated that IL-31 was able to significantly inhibit the protein expression and mRNA expression of TNF-α following LPS stimulation compared with PBS treatment (P<0.05; Fig. 3B), suggesting a regulatory role of IL-31 on PBMC. However, results indicated that IL-31 negatively regulated the transcription of inflammasome subtype NLRP1 significantly compared with PBS treatment (P<0.05), without significantly affecting NLRP3 or NLRP6 in the macrophages in human PBMC (Fig. 3C). These results suggested a dual role of IL-31 on the septic shock in mice and human cells; IL-31 may regulate the NLRP3-IL-1β in the macrophage and also mediate NLRP1 on the LPS-primed human PBMC, exhibiting therapeutic effects on septic shock.
Figure 3.
Effect of IL-31 on human PBMC inflammasome. (A) Secretion of TNF-α in PBMC supernatant with LPS and IL-31 treatment. (B) mRNA expression level of TNF-α on PBMC with or without IL-31 treatment under LPS stimulation. (C) mRNA expression level of NLRP1, NLRP3 and NLRP6 on PBMC with or without IL-31 treatment under LPS stimulation. *P<0.05 and ***P<0.001, as indicated. Experiments were performed in triplicate. PBMC, peripheral blood monocytes; TNF, tumor necrosis factor; LPS, lipopolysaccharide; IL, interleukin; PBS, phosphate-buffered saline.
Discussion
Currently, a patient is diagnosed with sepsis when symptoms of SIRS develop, and SIRS results from documented or suspected infection (22). Although various clinical studies have identified monitoring markers in sepsis and respiratory infection (23,24), effective therapeutic approaches are lacking. One of the earliest discovered biomarkers used to diagnose infection was C-reactive protein and procalcitonin, which are often elevated in the serum during sepsis (3). However, research has demonstrated that inflammatory cytokines, including IL-6, TNF-α and IL-1, have important roles in the disease (3). Recently, IL-8 and IL-18 have also been used as newer candidate biomarkers. It was reported that IL-8 was identified in genome-wide expression profiling in pediatric septic shock as an effective predictor of outcome in children with septic shock who were receiving standard care (25). On the other hand, IL-18 is a cytokine produced by activated macrophages and is involved in infection-induced cell immunity (26). However, further study is required to clarify the utility of this biomarker in the diagnosis of sepsis. Meanwhile, the role of cytokines in experimental sepsis need to be elucidated for drug discovery for septic shock
In the present study, novel cytokine IL-31 functions were identified regarding the immune response. IL-31 was identified as an inflammatory cytokine induced by activated cluster of differentiation (CD)4+ T-helper cells and has an important role in the pathogenesis of atopic dermatitis and allergic diseases in human eosinophils (8–10). The glycoprotein 130 (gp130) family constitutes the signaling receptors for the IL-6/IL-12 family of cytokines, such as IL-6, IL-12, IL-23, leukemia inhibitory factor and oncostatin M, many of which have important pro- and anti-inflammatory functions in immune cells (27). The most recent addition to this family was the gp130-like monocyte receptor or IL-31RA, which heterodimerizes with OSMR to form the IL-31R signaling complex (28). Signaling through IL-31R primarily results in the phosphorylation of STAT in the JAK-STAT signaling pathway (29). The ligand for IL-31R, IL-31, is predominantly expressed by activated the CD4+ Th2 subset. Therefore, the interactions of IL-31-IL-31R may regulate various allergic and infectious diseases (30). For example, anti-IL-31R antibody has been demonstrated to be a potential therapeutic option for treating itch and dermatitis in mice; scratching behavior was inhibited by treatment with anti-IL-31Rα-neutralizing antibody, BM095 (9). In the lung, macrophages derived from IL-31RA-knockout mice promoted enhanced ovalbumin-specific CD4+ T cell proliferation and purified naive CD4+ T cells from IL-31RA knockout mice exhibited enhanced proliferation and expression of Th2 cytokines (13). Furthermore, IL-31RA-knockout mice also exhibited increased Th2 cytokine responses in the mesenteric lymph nodes and elevated serum immunoglobulin (Ig)E and IgG1 levels compared with wild type mice with intestinal helminth infection (14). IL-31RA-knockout mice also displayed enhanced goblet cell hyperplasia and a notable increase in secretion of goblet cell-derived resistin-like molecule β into the intestinal lumen (14).
In the present study, it was demonstrated that IL-31 treatment was able to rescue the symptoms of septic shock by reducing inflammatory cytokines, particularly IL-1β in serum and peritoneal lavage fluid. In vitro data indicated that IL-31 inhibited the expression of NLRP3 in macrophages, thus reducing IL-1β secretion following LPS treatment. In the human T cell line, it was demonstrated that anti-human IL-31 neutralizing antibody or anti-human IL-31RA neutralizing antibody consistently enhanced the expression of NLRP3 as well as IL-1β, indicating the involvement of IL-31-IL-31R-NLRP3-IL-1 signaling in ATP-stimulated LPS-mediated inflammation in vitro. In contrast, IL-31 suppressed TNF activation through another inflammasome subtype, NLRP1, in human PBMC, which was a different signaling pathway in this specific cell type. In conclusion, the present data demonstrated that IL-31 may be a potential therapeutic target for the treatment of sepsis and septic shock.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the project of the Natural Science Foundation of Beijing (grant no. 7153169).
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XYG and CW designed and performed the experiments, analyzed the data, and wrote the manuscript. XSZ performed the evaluation and analysis. FPL, BS and XFZ assisted with performing the experiments.
Ethics approval and consent to participate
The animal protocol was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University (Beijing, China.).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 2.Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care. 2013;17:R115. doi: 10.1186/cc12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9:71–79. doi: 10.1586/eri.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 5.Alqahtani MF, Smith CM, Weiss SL, Dawson S, Ranaivo Ralay H, Wainwright MS. Evaluation of new diagnostic biomarkers in pediatric sepsis: Matrix Metalloproteinase-9, tissue inhibitor of metalloproteinase-1, mid-regional pro-atrial natriuretic peptide, and adipocyte fatty-acid binding protein. PLoS One. 2016;11:e0153645. doi: 10.1371/journal.pone.0153645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gucyetmez B, Atalan HK. C-reactive protein and hemogram parameters for the non-sepsis systemic inflammatory response syndrome and sepsis: What do they mean? PLoS One. 2016;11:e0148699. doi: 10.1371/journal.pone.0148699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B, et al. The IL-6 signal transducer, gp130: An oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 8.Hänel KH, Pfaff CM, Cornelissen C, Amann PM, Marquardt Y, Czaja K, Kim A, Lüscher B, Baron JM. Control of the physical and antimicrobial skin barrier by an IL-31-IL-1 signaling network. J Immunol. 2016;196:3233–3244. doi: 10.4049/jimmunol.1402943. [DOI] [PubMed] [Google Scholar]
- 9.Kasutani K, Fujii E, Ohyama S, Adachi H, Hasegawa M, Kitamura H, Yamashita N. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br J Pharmacol. 2014;171:5049–5058. doi: 10.1111/bph.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JS, Kim GC, Park E, Kim JE, Chae CS, Hwang W, Lee C, Hwang SM, Wang HS, Jun CD, et al. NFAT1 and JunB cooperatively regulate IL-31 gene expression in CD4+ T cells in health and disease. J Immunol. 2015;194:1963–1974. doi: 10.4049/jimmunol.1401862. [DOI] [PubMed] [Google Scholar]
- 11.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 activates signal transducer and activator of transcription (STAT)-1, STAT-5 and extracellular signal-regulated kinase 1/2 and down-regulates IL-12p40 production in activated human macrophages. Allergy. 2013;68:739–747. doi: 10.1111/all.12152. [DOI] [PubMed] [Google Scholar]
- 12.Kunsleben N, Rüdrich U, Gehring M, Novak N, Kapp A, Raap U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Invest Dermatol. 2015;135:1908–1911. doi: 10.1038/jid.2015.106. [DOI] [PubMed] [Google Scholar]
- 13.Perrigoue JG, Li J, Zaph C, Goldschmidt M, Scott P, de Sauvage FJ, Pearce EJ, Ghilardi N, Artis D. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrigoue JG, Zaph C, Guild K, Du Y, Artis D. IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection. J Immunol. 2009;182:6088–6094. doi: 10.4049/jimmunol.0802459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt RL, Lenz LL. Distinct licensing of IL-18 and IL-1β secretion in response to NLRP3 inflammasome activation. PLoS One. 2012;7:e45186. doi: 10.1371/journal.pone.0045186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Ketelut-Carneiro N, Silva GK, Rocha FA, Milanezi CM, Cavalcanti-Neto FF, Zamboni DS, Silva JS. IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol. 2015;194:4507–4517. doi: 10.4049/jimmunol.1402321. [DOI] [PubMed] [Google Scholar]
- 19.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Caserta S, Kern F, Cohen J, Drage S, Newbury SF, Llewelyn MJ. Circulating plasma microRNAs can differentiate human sepsis and systemic inflammatory response syndrome (SIRS) Sci Rep. 2016;6:28006. doi: 10.1038/srep28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong HR, Cvijanovich N, Wheeler DS, Bigham MT, Monaco M, Odoms K, Macias WL, Williams MD. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008;178:276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei XQ, Leung BP, Niedbala W, Piedrafita D, Feng GJ, Sweet M, Dobbie L, Smith AJ, Liew FY. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–2828. [PubMed] [Google Scholar]
- 27.Klein C, Wüstefeld T, Assmus U, Roskams T, Rose-John S, Müller M, Manns MP, Ernst M, Trautwein C. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115:860–869. doi: 10.1172/JCI23640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin MW, Lee DD, Liu TT, Lin YF, Chen SY, Huang CC, Weng HY, Liu YF, Tanaka A, Arita K, et al. Novel IL31RA gene mutation and ancestral OSMR mutant allele in familial primary cutaneous amyloidosis. Eur J Hum Genet. 2010;18:26–32. doi: 10.1038/ejhg.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edukulla R, Singh B, Jegga AG, Sontake V, Dillon SR, Madala SK. Th2 Cytokines augment IL-31/IL-31RA interactions via STAT6-dependent IL-31RA expression. J Biol Chem. 2015;290:13510–13520. doi: 10.1074/jbc.M114.622126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, Feng ZH, Huang B. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.