Abstract
The diagnostic value of detection of serum β-human chorionic gonadotropin (β-HCG) and Chlamydia trachomatis immunoglobulin G (CT-IgG) combined with transvaginal ultrasonography in early tubal pregnancy was investigated. A total of 55 patients with early tubal pregnancy were selected as the tubal pregnancy group, while 55 subjects of normal intrauterine pregnancy were enrolled as the intrauterine pregnancy group. Transvaginal ultrasonography and quantitative detection of serum β-HCG and CT-IgG were performed for all patients, and the clinical examination results were analyzed and compared. The endometrial thickness and serum β-HCG level of patients with early tubal pregnancy were significantly lower than those of women with intrauterine pregnancy (6.7±1.5 vs. 11.6±1.2 mm; 776±109 vs. 5,598±187 U/l), and the differences were statistically significant (p<0.01); the serum CT-IgG antibody positive rate of patients in tubal pregnancy group (49.1%) was significantly higher than that in intrauterine pregnancy group (12.7%) (p<0.01); the serum CT-IgG antibody positive rates of patients with degree I, II and III of pelvic adhesion intubal pregnancy group were 28.6, 75.0 and 81.8%, respectively; the more severe the pelvic adhesion was, the higher the CT-IgG positive rate would be. The diagnostic coincidence rate of combined detection was significantly higher than that of single detection of serum β-HCG, progesterone and endometrial thickness. The detection of serum β-HCG and CT-IgG combined with transvaginal ultrasonography can diagnose the early tubal pregnancy soonest possible, and help choose the appropriate therapeutic methods depending on the situation to reduce the tubal damage of patients, so as to provide a reliable basis for the diagnosis, treatment and prognosis, and it has important clinical application value.
Keywords: early tubal pregnancy, transvaginal ultrasonography, serum β-human chorionic gonadotropin, Chlamydia trachomatis immunoglobulin G
Introduction
The implantation site of the fertilized egg in the fallopian tube, is known as tubal pregnancy and is the most common ectopic pregnancy (1). The early diagnostic methods of tubal pregnancy include transvaginal ultrasonography, laparoscopy, detection of progesterone, serum β-human chorionic gonadotropin (β-HCG) and other biochemical indexes. The study of the correlation between Chlamydia trachomatis immunoglobulin G (CT-IgG) antibody and salpingitis, tubal pregnancy and tubal infertility has been reported (2,3). The clinical manifestations of early tubal pregnancy are not specific and similar to the symptoms of early intrauterine pregnancy and abortion, and in addition to the individual differences of pregnant women, sometimes the single detection method is not enough to diagnose tubal pregnancy. Life will be threatened once the rupture or abortion occurs (4,5).
In this study, tubal pregnancy was diagnosed via the detection of serum β-HCG and CT-IgG combined with transvaginal ultrasonography, to explore a safe, effective and highly accurate diagnostic scheme to guide the clinical practice.
Patients and methods
Patients
A total of 55 patients with early tubal pregnancy treated in Linyi People's Hospital (Linyi, China) from September 2015 to September 2016 were collected as the tubal pregnancy group, while 55 subjects of early intrauterine pregnancy were collected as the intrauterine pregnancy group. Before the study, patients signed the informed consent. The general data, such as age, pregnancy times and past medical history, had no statistically significant differences between the two groups and were comparable. The Ethics Committee of Linyi People's Hospital approved this study [201502-007]. Signed informed consents were obtained from all the patients or guardians.
Inclusion criteria and exclusion criteria
Inclusion criteria were: i) A slightly larger uterus and an appendage or thickening of the uterus with obvious pain in gynecologic examination; ⅱ) a history of menopause; ⅲ) β-HCG test results were positive; and ⅳ) there was no obvious echo in the womb of the patient's uterus, and the echo of abnormal mass was found in the accessory area of the para uteri.
Exclusion criteria were: Ultrasound examination shows the echo of the intrauterine gestation sac, which shows the yolk sac, the germ, the intrauterine pregnancy of the primitive heart tube pulsation.
Detection protocols
Transvaginal ultrasonography was performed using the LOGIQ-3 digital color Doppler ultrasound diagnostic apparatus (General Electric, Schenectady, NY, USA). Fasting blood (2 ml) was drawn from the two groups of subjects in the morning for the detection of IgG. Serum CT-IgG was detected using the enzyme-linked immunosorbent assay (ELISA) kit (Biovision, Beijing, China). Serum β-HCG was detected using the ELECSYS 2010 electrochemiluminescence full automated immunoassay analyzer (Roche Diagnostics, Basel, Switzerland).
Observation indexes
The detection results of serum β-HCG and CT-IgG in subjects were observed, and the endometrial thickness was detected via ultrasound. Levels of serum β-HCG in subjects were detected again after 48 h, and whether the chronic pelvic adhesion existed and its degree in patients with tubal pregnancy were detected during operation. Degree I: No adhesion or mild membranous adhesion; degree II: Adhesion easy to be separated; degree III: Severe adhesion that cannot be separated. The diagnostic coincidence rate was based on the mean values of the three kinds of detection methods of normal pregnancy. Endometrial thickness <11.2 mm and β-HCG level <1,500 U/l may indicate early tubal pregnancy.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 19.0 software (IBM Corp., Armonk, NY, USA) was used for the statistical treatment and analysis of research data. Measurement data are presented as mean ± standard deviation. Comparison between groups was done using one-way ANOVA test followed by post hoc test (Least Significant Difference). Chi-square test was used for the comparison of enumeration data. P<0.05 indicates that the difference is statistically significant.
Results
Comparison of clinical data
Basic data for the patients in tubal pregnancy and intrauterine pregnancy groups are shown in Table I. There was no significant difference in age, pregnancy times, history of pelvic inflammation, history of spontaneous abortion and history of implanting intrauterine device (IUD) between the two groups (p>0.05).
Table I.
Comparisons of general clinical data between tubal and intrauterine pregnancy group.
| Pregnancy Group | No. | Age | Pregnant times | History of pelvic inflammation | History of spontaneous abortion | History of implanting IUD |
|---|---|---|---|---|---|---|
| Tubal | 55 | 25.2±3.6 | 1.4±0.1 | 14 (25.5%) | 4 (7.2%) | 8 (14.5%) |
| Intrauterine | 55 | 25.9±4.1 | 1.5±0.5 | 11 (20.0%) | 3 (5.5%) | 7 (12.7%) |
| P-value | 0.563 | 0.472 | 0.338 | 0.415 | 0.511 |
In the comparisons between the two groups, p>0.05. IUD, intrauterine device.
Comparison of serum β-HCG and CT-IgG between tubal and intrauterine pregnancy group
The levels of serum β-HCG and CT-IgG of patients with early tubal pregnancy on the admission day and after 48 h were significantly lower than those in women with intrauterine pregnancy (p<0.01) (Table II). The level of serum β-HCG in women with intrauterine pregnancy after 48 h was significantly higher than that on the admission day (p<0.01). There was no statistically significant difference in the comparison of serum β-HCG level in patients with early tubal pregnancy on the admission day and after 48 h (p>0.05). The serum CT-IgG antibody-positive rate of patients in tubal pregnancy group (49.1%) was significantly higher than that in intrauterine pregnancy group (12.7%) (p<0.01) (Fig. 1). that in intrauterine pregnancy group (12.7%) (p<0.01) (Fig. 1). Human monoclonal CT-IgG antibody (dilution: 1:2,000; cat. no. ab108720) was purchased from Abcam (Cambridge, MA, USA).
Table II.
Comparison of serum β-HCG level between tubal and intrauterine pregnancy group.
| β-HCG (U/l) | |||||
|---|---|---|---|---|---|
| Group | No. | On admission | 48 h | t | P-value |
| Tubal pregnancy group | 55 | 776±109 | 758±111 | 0.707 | 0.415 |
| Intrauterine pregnancy group | 55 | 5,598±187 | 10,997±1,798 | 19.898 | 0.008 |
| t | 150.166 | 45.733 | |||
| P-value | <0.001 | <0.001 | |||
β-HCG, β-human chorionic gonadotropin.
Figure 1.
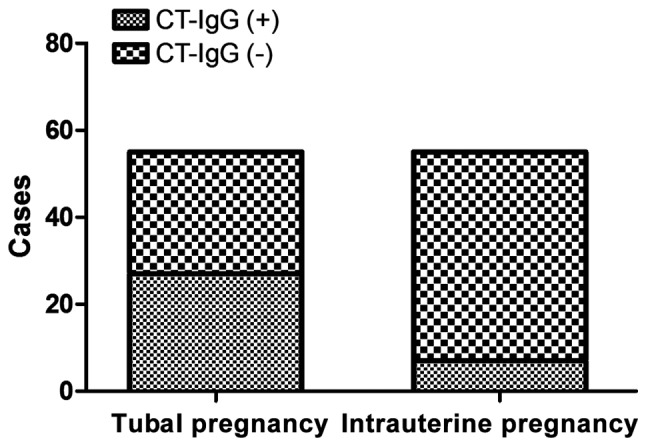
CT-IgG positive cases in tubal and intrauterine pregnancy groups: CT-IgG-positive rate in tubal pregnancy was significantly higher than that of the intrauterine pregnancy group (p<0.01). CT-IgG, Chlamydia trachomatis immunoglobulin G.
Comparison of endometrial thickness and mass size in adnexa area between tubal and intrauterine pregnancy groups
Endometrial thickness in patients with early tubal pregnancy on the admission day and after 48 h was significantly lower than those in women with intrauterine pregnancy (p<0.01). Mass size in adnexa area of patients with early tubal pregnancy on the admission day and after 48 h was significantly bigger than those in women with intrauterine pregnancy (p<0.01). There was no statistically significant difference in the comparison of pelvic effusion between the two groups on the admission day or after 48 h (p>0.05) (Table III).
Table III.
Comparison of transvaginal ultrasound examination results between tubal and intrauterine pregnancy group.
| Transvaginal ultrasonography | ||||
|---|---|---|---|---|
| Group | No. | Endometrial thickness (mm) | Mass size in adnexa area (cm2) | Pelvic effusion (cm) |
| Tubal pregnancy group | 55 | 6.9±1.7 | 60.5±46.2 | 2.4±1.2 |
| Intrauterine pregnancy group | 55 | 11.6±1.2 | 34.2±25.7 | 2.0±1.4 |
| P-value | 0.003 | 0.002 | 0.368 | |
Pelvic adhesion grading of tubal and intrauterine pregnancy groups
The serum CT-IgG antibody-positive rates of patients with grade I, II and III of pelvic adhesion in tubal pregnancy group were 28.6, 75.0 and 81.8%, respectively. These results suggested that the more severe the pelvic adhesion was, the higher the CT-IgG positive rate would be (Fig. 2).
Figure 2.
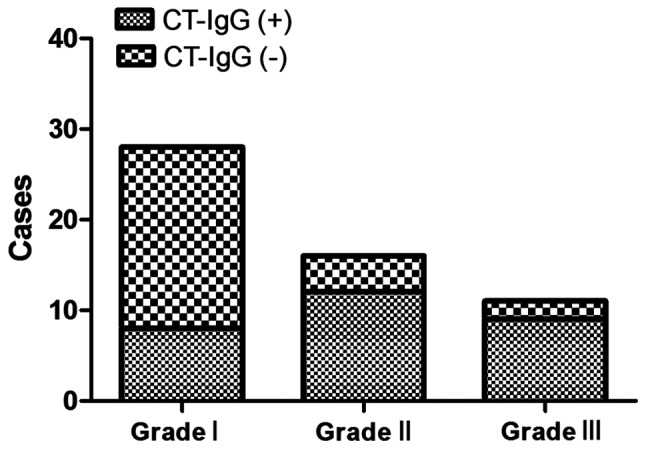
Association between pelvic adhesion grading and CT-IgG positive rates: the more severe the pelvic adhesion is, the higher the CT-IgG positive rate will be (p<0.01). CT-IgG, Chlamydia trachomatis immunoglobulin G.
Comparison of diagnostic results of serum β-HCG, CT-IgG and endometrial thickness
The diagnostic coincidence rate of endometrial thickness was higher than that of the detection of serum β-HCG and CT-IgG, and the difference was statistically significant (p<0.05). The diagnostic coincidence rates of single detection of serum β-HCG, CT-IgG and transvaginal ultrasonography in the tubal pregnancy and intrauterine pregnancy groups were significantly lower than those of the combined detection (p<0.05) (Table IV).
Table IV.
Comparison of diagnostic coincidence rates of different detection methods between tubal and intrauterine pregnancy group.
| Group | No. | β-HCG | CT-IgG | Endometrial thickness | Combined detection |
|---|---|---|---|---|---|
| Tubal pregnancy group | 55 | 28 (50.9%) | 41 (74.5%) | 45 (81.8%) | 53 (96.4%) |
| P-valuea | 0.0152 | 0.0454 | |||
| P-valueb | 0.0113 | 0.0325 | 0.0374 | ||
| Intrauterine pregnancy group | 55 | 34 (61.8%) | 43 (78.2%) | 49 (89.1%) | 54 (98.2%) |
| P-valuea | 0.0207 | 0.0416 | |||
| P-valueb | 0.0127 | 0.0289 | 0.0473 |
Compared with endometrial thickness
compared with combined detection. β-HCG, β-human chorionic gonadotropin; CT-IgG, Chlamydia trachomatis immunoglobulin G.
Discussion
Tubal pregnancy is a common acute abdominal disease in gynecology and obstetrics, but its pathogenesis is not clear. The salpingitis, fallopian tube abnormalities, contraception, fertilized egg migration, and endocrine abnormalities may lead to the occurrence of tubal pregnancy (1). The incidence of tubal pregnancy is currently on the increase. However, the number of deaths due to rupture has been reduced rather than increased, and the subsequent fertility function can also be retained as much as possible (1). The main reason is due to the rapid and effective development of early diagnosis and treatment technology.
The determination of HCG is the most commonly used diagnostic method of tubal pregnancy. β-HCG is produced by syncytiotrophoblast cells and reflects the activity of villus. Continuous detection of blood β-HCG can identify intrauterine pregnancy and ectopic pregnancy. It is important for early diagnosis and is also an important monitoring method for conservative treatment of tubal pregnancy (6–8). As the variation range of absolute value of serum β-HCG is large, it is used to diagnose ectopic pregnancy clinically via monitoring its doubling time. It is reported that the doubling time of serum β-HCG in patients with ectopic pregnancy is later than that in women with normal intrauterine pregnancy (1.4–2.2 vs. 3–8 days) (9). The serum β-HCG level can directly reflect the viability of trophoblasts, and the high β-HCG level indicates the high proliferation activity of trophoblasts and high invasion into fallopian tube. Dynamic monitoring of serum β-HCG changes can predict the prognosis of tubal pregnancy. If the serum β-HCG level is <2,000 IU/l, the drug therapy and non-surgical conservative treatment can be chosen. If the serum β-HCG level continues to rise >8,000 IU/l, the rupture of tubal pregnancy should be identified and treated by surgery as early as possible (10–12). The results of this study showed that the serum β-HCG level in women with early tubal pregnancy was significantly lower than that in women with normal intrauterine pregnancy. Moreover, the serum β-HCG level in women with normal intrauterine pregnancy was obviously increased on the visiting date, suggesting that the serum β-HCG level in women with normal intrauterine pregnancy can be doubled after 48 h. However, there was no significant change in serum β-HCG in the early stage of tubal pregnancy, which suggested that the early pregnancy of oviduct is not able to implant in the endometrium, and the decidua reaction cannot be completed normally. Insufficient blood supply affects the development of trophoblastic cells, so the synthesis of β-HCG is reduced.
Within 1–2 weeks after the fertilized eggs are implanted outside the uterine cavity, the serum β-HCG levels in women with early tubal pregnancy and normal intrauterine pregnancy are similar, so other clinical detection means are often needed to make up for this shortcoming. Due to the high resolution, transvaginal ultrasonography can clearly identify the location of tubal pregnancy and show the small lesions in organs and tissues in pelvic cavity without being affected by the bladder filling and abdominal wall thickness (13). Kirk et al (14) conducted statistical research on transvaginal ultrasonography results of 5,240 menopausal women. The results showed that the positive predictive value and negative predictive value of diagnosis of tubal pregnancy are 96.7 and 99.4%. Ectopic pregnancy is divided into different types according to the symptoms and outcome. No gestational sac but tubal ring sign with specific diagnostic value found in uterine cavity is the important sign of tubal pregnancy, and trophoblastic blood flow can be seen around the tubal ring. In addition, fake gestational sac can occur in ectopic pregnancy due to the hematocele in uterine cavity, and the blood flow resistance is higher because of the blood flow from the endometrial spiral artery, while the trophoblastic blood flow around the true gestational sac is from the original placenta with low resistance (15,16). The results of this study showed that the endometrial thickness in patients with early tubal pregnancy was smaller than that in women with normal intrauterine pregnancy (p<0.05); the diagnostic coincidence rate of endometrial thickness was higher than that of serum β-HCG and CT-IgG detection, and the difference was statistically significant (p<0.05).
One of the main pathogenesis of female urethritis and salpingitis is CT infection. There are often no obvious subjective symptoms in the early stage. CT damages the uterus and adnexa upwards from the infected cervix, leading to tubal pregnancy, tubal infertility and other sequelae (17,18). It is reported that the serum CT-IgG positive antibody can be detected in women with ectopic pregnancy and normal pregnancy, and the rates are 32–71 and 4–39%, respectively. The relative risk of tubal pregnancy in women with serum CT-IgG positive is 2.4–7.9 (19). Dunne et al (20) studied and suggested that with the decline in CT infection positive rate, the incidence rate of ectopic pregnancy is also rapidly reduced. The results of this study showed that the serum CT-IgG antibody positive rate of patients in tubal pregnancy group was significantly higher than that in intrauterine pregnancy group (p<0.01). At the same time, it was found that the serum CT-IgG antibody-positive rates of patients with degree I, II and III of pelvic adhesion were 28.6, 75.0 and 81.8%, respectively. The more severe the pelvic adhesion was, the higher the CT-IgG positive rate would be. CT infection often shows the subclinical state with no obvious subjective symptoms. If it is not treated and controlled in time, endometrial and endosalpinx injury is caused, further leading to pelvic adhesion, tubal obstruction and other permanent injuries, which is a basic cause of tubal pregnancy.
In conclusion, the results of the present study have shown that the diagnostic coincidence rates of single detection of serum β-HCG, CT-IgG and transvaginal ultrasonography in tubal and intrauterine pregnancy group were significantly lower than those of combined detection. Therefore, the detection of serum β-HCG and CT-IgG combined with transvaginal ultrasonography can improve the diagnostic accuracy of suspected early tubal pregnancy, and provide a reliable basis for clinical treatment and prognosis, which has important application values. However, the sample size of this study is insufficient, and the result is limited. A larger number of samples is needed to confirm the conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HX and PL designed the study, WL performed the ultrasonography, WL and HX collected the data, and WL and PL analyzed the data. HX prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Linyi People's Hospital (Linyi, China). Signed written informed consents were obtained from the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stulberg DB, Cain LR, Dahlquist I, Lauderdale DS. Ectopic pregnancy rates in the Medicaid population. Am J Obstet Gynecol. 2013;208:274.e1–274.e7. doi: 10.1016/j.ajog.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma LK, Cao DY, Yang JX, Liu JT, Shen K, Lang JH. Pregnancy outcome and obstetric management after vaginal radical trachelectomy. Eur Rev Med Pharmacol Sci. 2014;18:3019–3024. [PubMed] [Google Scholar]
- 3.Hoenderboom BM, van Oeffelen AA, van Benthem BH, van Bergen JE, Dukers-Muijrers NH, Götz HM, Hoebe CJ, Hogewoning AA, van der Klis FR, van Baarle D, et al. The Netherlands Chlamydia cohort study (NECCST) protocol to assess the risk of late complications following Chlamydia trachomatis infection in women. BMC Infect Dis. 2017;17:264. doi: 10.1186/s12879-017-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain LR, Stulberg D. Ectopic pregnancy rates in a non-Medicaid population are lower than previously reported. Am J Obstet Gynecol. 2013;209:592. doi: 10.1016/j.ajog.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Akaba GO, Agida TE, Onafowokan O. Ectopic pregnancy in Nigeria's federal capital territory: A six year review. Niger J Med. 2012;21:241–245. [PubMed] [Google Scholar]
- 6.Senapati S, Barnhart KT. Biomarkers for ectopic pregnancy and pregnancy of unknown location. Fertil Steril. 2013;99:1107–1116. doi: 10.1016/j.fertnstert.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mäkinen J. Current treatment of ectopic pregnancy. Ann Med. 1999;31:197–201. doi: 10.3109/07853899909115978. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AH, Finney M, Lam PM, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9:152. doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gevaert O, De Smet F, Kirk E, Van Calster B, Bourne T, Van Huffel S, Moreau Y, Timmerman D, De Moor B, Condous G. Predicting the outcome of pregnancies of unknown location: Bayesian networks with expert prior information compared to logistic regression. Hum Reprod. 2006;21:1824–1831. doi: 10.1093/humrep/del083. [DOI] [PubMed] [Google Scholar]
- 10.Goksedef BP, Kef S, Akca A, Bayik RN, Cetin A. Risk factors for rupture in tubal ectopic pregnancy: Definition of the clinical findings. Eur J Obstet Gynecol Reprod Biol. 2011;154:96–99. doi: 10.1016/j.ejogrb.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 11.van Mello NM, Mol F, Ankum WM, Mol BW, van der Veen F, Hajenius PJ. Ectopic pregnancy: How the diagnostic and therapeutic management has changed. Fertil Steril. 2012;98:1066–1073. doi: 10.1016/j.fertnstert.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 12.van Mello NM, Mol F, Verhoeve HR, van Wely M, Adriaanse AH, Boss EA, Dijkman AB, Bayram N, Emanuel MH, Friederich J, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60–67. doi: 10.1093/humrep/des373. [DOI] [PubMed] [Google Scholar]
- 13.Reid S, Casikar I, Barnhart K, Condous G. Serum biomarkers for ectopic pregnancy diagnosis. Expert Opin Med Diagn. 2012;6:153–165. doi: 10.1517/17530059.2012.664130. [DOI] [PubMed] [Google Scholar]
- 14.Kirk E, Papageorghiou AT, Condous G, Tan L, Bora S, Bourne T. The diagnostic effectiveness of an initial transvaginal scan in detecting ectopic pregnancy. Hum Reprod. 2007;22:2824–2828. doi: 10.1093/humrep/dem283. [DOI] [PubMed] [Google Scholar]
- 15.Szabó I, Csabay L, Belics Z, Fekete T, Papp Z. Assessment of uterine circulation in ectopic pregnancy by transvaginal color Doppler. Eur J Obstet Gynecol Reprod Biol. 2003;106:203–208. doi: 10.1016/S0301-2115(02)00235-X. [DOI] [PubMed] [Google Scholar]
- 16.Thoma ME. Early detection of ectopic pregnancy visualizing the presence of a tubal ring with ultrasonography performed by emergency physicians. Am J Emerg Med. 2000;18:444–448. doi: 10.1053/ajem.2000.7345. [DOI] [PubMed] [Google Scholar]
- 17.Zele-Starcević L, Plecko V, Budimir A, Kalenić S. Choice of antimicrobial drug for infections caused by Chlamydia trachomatis and Chlamydophila pneumoniae. Acta Med Croatica. 2004;58:329–333. (In Croatian) [PubMed] [Google Scholar]
- 18.Stamatopoulos N, Casikar I, Reid S, Roy B, Branley J, Mongelli M, Condous G. Chlamydia trachomatis in fallopian tubes of women undergoing laparoscopy for ectopic pregnancy. Aust N Z J Obstet Gynaecol. 2012;52:377–379. doi: 10.1111/j.1479-828X.2012.01456.x. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Meng CX, Sun LL, Zhao WH, Zhang M, Zhang J, Cheng L. Reduced prevalence of chronic tubal inflammation in tubal pregnancies after levonorgestrel emergency contraception failure. Pharmacoepidemiol Drug Saf. 2015;24:548–554. doi: 10.1002/pds.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunne EF, Chapin JB, Rietmeijer CA, Kent CK, Ellen JM, Gaydos CA, Willard NJ, Kohn R, Lloyd L, Thomas S, et al. Rate and predictors of repeat Chlamydia trachomatis infection among men. Sex Transm Dis. 2008;35(Suppl):S40–S44. doi: 10.1097/OLQ.0b013e31817247b2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


