Abstract
The present study aimed to evaluate the anti-cancer effect of ellagic acid in human non-small cell lung cancer (NSCLC) A549 cells and to reveal the potential underlying mechanism. The effects of ellagic acid on the cell proliferation of A549 cells were determined by MTT assay. Cell cycle and apoptosis were measured with flow cytometry and Annexin V-propidium iodide staining. Western blotting was used to measure the expression levels of the phosphatidylinositol 3-kinase (PI3K)/protein kinas B (Akt) signaling pathway and apoptosis-associated proteins. It was demonstrated that ellagic acid exerted an inhibitory effect in the proliferation of human NSCLC A549 cells. Flow cytometry demonstrated that G1 phase retention and apoptosis rates were significantly increased after treatment with ellagic acid. Further investigation revealed that ellagic acid treatment diminished the phosphorylation of PI3K and Akt and regulated the expression of apoptosis-associated proteins in A549 cells. In conclusion, the present results indicated that ellagic acid suppresses cell proliferation, arrests cell cycle and induces apoptosis in human NSCLC A549 cells by inhibiting the PI3K/Akt signaling pathway.
Keywords: non-small cell lung cancer, A549 cells, apoptosis, phosphoinositide 3-kinase/protein kinase B
Introduction
According to GLOBOCAN statistics in 2012, lung cancer is the most diagnosed cancer worldwide (1). As the leading cause of cancer death, lung cancer accounted for 1.6 million deaths in 2012. Lung cancer includes small cell lung cancer and non-small cell lung cancer (NSCLC), and the latter accounts for 80–85% of total lung cancer (2). Although improvements have made in the diagnosis and treatment of NSCLC in previous decades, the 5-year survival rate of patients remains <15% (3). In addition, many current treatments of NSCLC have various adverse effects including and easily induce drug-resistance (4,5). For example, coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab were exhibited in a patient with non-small-cell lung cancer (6). Consequently, exploring new therapeutic agents from Traditional Chinese Medicine has gained increasing attention.
Dietary polyphenols constitute a large amount of secondary metabolites and have been widely identified as chemopreventive or anti-cancer agents (7). It has also been documented that dietary polyphenols exert various biological activities, such as anti-inflammatory, immunomodulatory and anti-tumor properties (8–10). Ellagic acid [2,3,7,8-tetrahydroxy-chromeno (5,4,3-cde) hromene-5,10-dione], a natural polyphenolic compound, is widely found in grapes, pomegranates, nuts, strawberries and green tea (11). Accumulating evidence has suggested an anti-tumor activity of ellagic acid due to its ability to prevent tumor growth (12,13), inhibit proliferation (14), arrest cell cycle (15), induce apoptosis (16) and suppress cell metastasis and angiogenesis (13). Furthermore, previous studies have elucidated that ellagic acid treatment significantly ameliorated obstructive jaundice-induced lung damage (17) and acute lung injury (18), which suggests the pulmonary protective effect of ellagic acid. Despite these biological activities of ellagic acid, the effects of ellagic acid on human NSCLC A549 cells remain unclear.
Therefore, the objective of the present study was to investigate whether ellagic acid could inhibit human NSCLC A549 cells, and to reveal the potential underlying mechanism. It was demonstrated that ellagic acid may suppress cell proliferation, arrest cell cycle and induce apoptosis in human NSCLC A549 cells via the suppression of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. The present findings provide evidence that ellagic acid may be developed as a potential therapy for the treatment of lung cancer.
Materials and methods
Chemicals and reagents
Ellagic acid, dimethylsulfoxide (DMSO), propidium iodide (PI) and MTT were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640 medium, fetal bovine serum (FBS), penicillin and streptomycin were purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The Annexin V-FITC Apoptosis Detection kit and Cell cycle detection kit were obtained from BD Biosciences (Franklin Lakes, NJ, USA). Antibodies against phosphorylated (p)-PI3K, PI3K, p-Akt, Akt, cyclin D1, p21, B cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax) and cleaved-Caspase-3 were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
The NSCLC line A549 was obtained from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China). A549 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified incubator under 5% CO2 at 37°C.
MTT assay
The effect of ellagic acid on the viability of human NSCLC A549 cells was measured by MTT assay. A total of 1×104 cells/well were cultured in 96-well plates and stimulated with various concentrations of (5, 10 and 20 µM) of ellagic acid for 48 h at 37°C. Then, the medium was removed, MTT (100 µl) was added and incubated at 37°C for additional 4 h. Then 150 ml DMSO was added, and the absorbance was read at 570 nm with a micro-plate reader (Thermo Fisher Scientific, Inc.).
Cell cycle assay
To detect the effect of ellagic acid in the cell cycle, a PI cell cycle analysis kit was used. In brief, human NSCLC A549 cells (5×105 cells per well) were cultured in 6-well plates and stimulated with various concentrations of (5, 10 and 20 µM) of ellagic acid for 48 h at 37°C. Cells were then fixed in 70% ethanol at −20°C for 1 h, and 2 mg/ml RNaseA and 50 mg/ml PI were added and incubated for 45 min at 37°C. Stained cells were analyzed using the Guava easyCyte 5HT flow cytometry system (EMD Millipore, Billerica, MA, USA).
Cell apoptosis assay
Human NSCLC A549 cells were treated with various concentrations of ellagic acid (5, 10 and 20 µM) for 48 h at 37°C. Cell apoptosis was assessed using flow cytometry with staining of the cells using an Annexin V/propidium iodide (PI) kit (cat. no. 556547; BD Biosciences, Franklin Lakes, NJ, USA). Cells were incubated with Annexin V-FITC (5 µl) and PI (5 µl) at room temperature for 30 min in the dark. Then a flow cytometer (Cytomics FC 500 MPL; Beckman Coulter, Inc., Brea, CA, USA) was used to measure the apoptosis rates by detecting the relative amount of Annexin V-FITC positive and PI negative cells.
Western blotting
After A549 cells were treated with 5, 10, or 20 µM ellagic acid for 48 h at 37°C, total proteins were extracted from the A549 cells with radio immunoprecipitation assay lysis buffer (Pierce; Thermo Fisher Scientific, Inc.). Protein samples were then quantified with a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein (25 µg/pore) were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes. The membrane was then blocked overnight with skimmed milk at 4°C. and incubated with antibodies against p-PI3K (1:1,000; cat. no. 4228), PI3K (1:1,000; cat. no. 4257), p-Akt (1:1,000; 4060), Akt (1:1,000; cat. no. 4685), cyclin D1 (1:1,000; cat. no. 2978), p21 (1:1,000; cat. no. 2947), Bax (1:1,000; cat. no. 5023), Bcl-2 (1:1,000; cat. no. 15071), cleaved-caspase-3 (1:1,000; cat. no. 9661) and GAPDH (1:2,000, cat. no. 5174). After washing with PBST, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000; cat. no. ab191866 and ab218695; Abcam, Cambridge, UK) and visualized with an enhanced chemiluminescence kit (Thermo). GAPDH acted as an internal control, and quantified using the Bio-Rad GS-700 imaging densitometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the means + standard deviation. The differences between control and treatment groups were analyzed using one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Treatment of ellagic acid inhibits cell viability in A549 cells
Initially, the effects of ellagic acid on viability of A549 cells were measured by MTT assay. As presented in Fig. 1, the viability rates were significantly reduced by ellagic acid treatment in a dose-dependent manner, compared with the control group (5 µM, P<0.05; 10 µM, P<0.01, 20 µM; P<0.001). These results indicate that ellagic acid significantly inhibited the viability of human NSCLC A549 cells.
Figure 1.
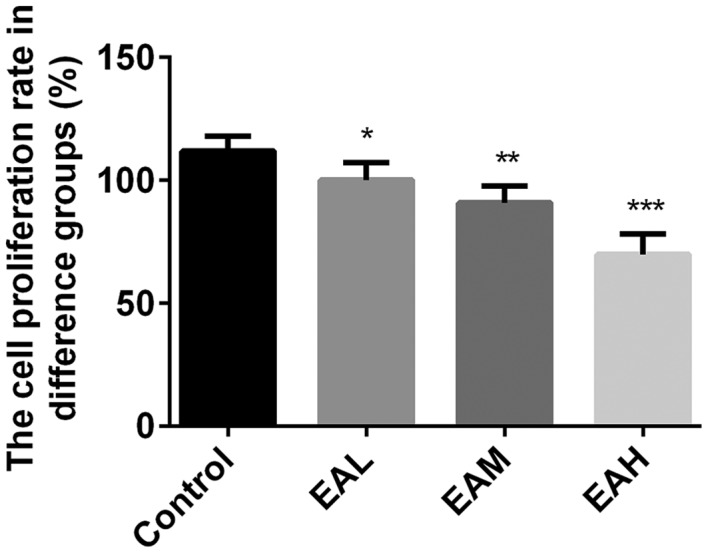
MTT assay following ellagic acid treatment. Cell viability was reduced by ellagic acid treatment in a dose-dependent manner compared with the control group. Data are presented as the mean ± standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control. EAL, low concentration (5 µM) ellagic acid, EAM, middle concentration (10 µM) ellagic acid; EAH, high concentration (20 µM) ellagic acid.
Treatment of ellagic acid induces apoptosis in A549 cells
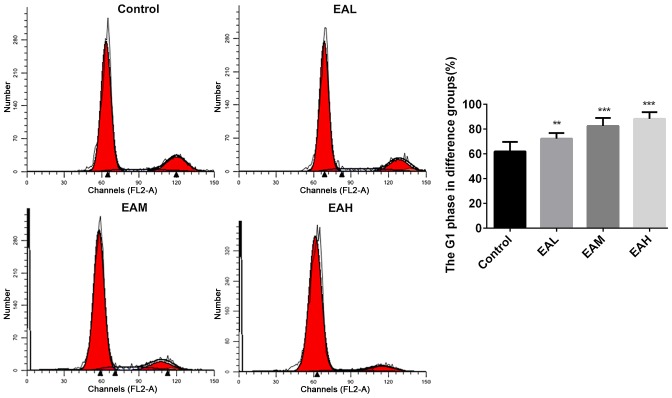
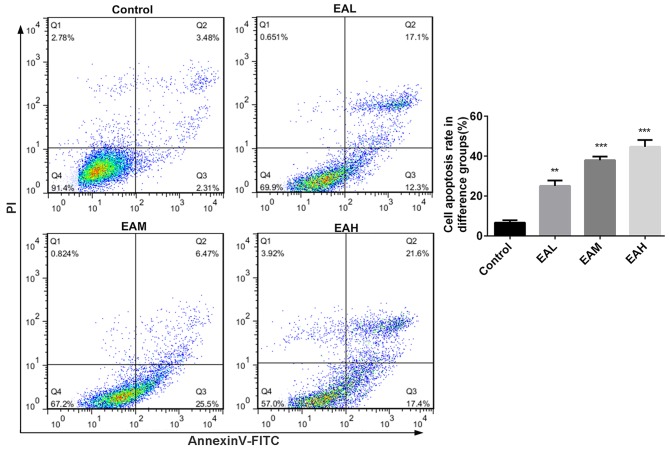
The cell cycle between different groups was measured with PI cell cycle analysis kit, and the relative proportion of A549 cells in the G1 phase was calculated. Fig. 2 demonstrated that in the control group, the number of cells in G1 phase were low, whereas, after treatment with ellagic acid for 48 h, the rates of cells in G1 phase were significantly increased (P<0.05). Annexin V and PI double staining results in Fig. 3 demonstrated that the ellagic acid-treated groups exhibited a significantly increased apoptosis rate in comparison with controls (P<0.01). These observations indicate that the inhibitory effect of ellagic acid on human NSCLC A549 cells may be due to increased apoptosis.
Figure 2.
Cell cycle in A549 cells. In the control group, the proportion of cells in the G1 phase was low. After treatment with ellagic acid for 48 h, the proportion of cells in G1 the phase increased significantly compared to control group. Data are presented as the mean ± standard deviation (n=3). **P<0.01, ***P<0.001 vs. control. EAL, low concentration (5 µM) ellagic acid; EAM, middle concentration (10 µM) ellagic acid; EAH, high concentration (20 µM) ellagic acid.
Figure 3.
Apoptosis rate in A549 cells. Annexin V and PI double staining results revealed that ellagic acid-treated groups exhibited a significant increase in apoptosis rate in comparison with controls. Data are presented as the mean ± standard deviation (n=3). **P<0.01, ***P<0.001 vs. control. PI, propidium iodide; EAL, low concentration (5 µM) ellagic acid; EAM, middle concentration (10 µM) ellagic acid; EAH, high concentration (20 µM) ellagic acid.
Treatment of ellagic acid suppresses the PI3K/Akt signaling pathway in A549 cells
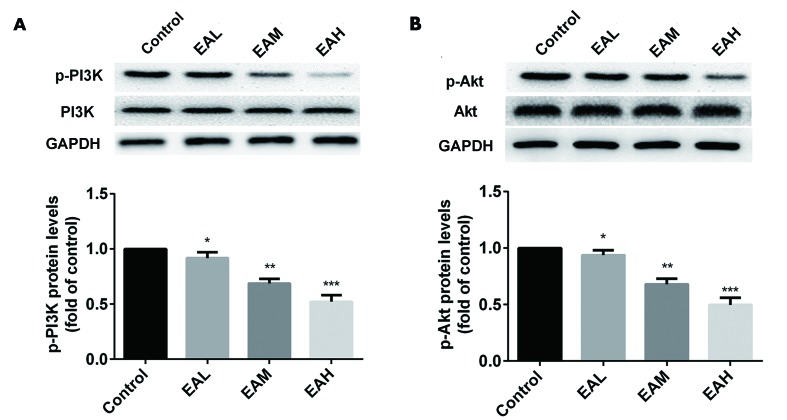
The effects of ellagic acid treatment on the PI3K/Akt signaling pathway in A549 cells were detected by western blotting. Fig. 4 indicates that, compared with the control group, the phosphorylation of PI3K and Akt were significantly downregulated by the treatment of ellagic acid in a dose-dependent manner (5 µM, P<0.05; 10 µM, P<0.01, 20 µM; P<0.001).
Figure 4.
PI3K/Akt signaling pathway was inhibited in A549. Compared with the control group, the phosphorylation of both (A) PI3K and (B) Akt were downregulated by the treatment of ellagic acid in a dose-dependent manner. Data are presented as the mean ± standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control. PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; p, phosphorylated; EAL, low concentration (5 µM) ellagic acid; EAM, middle concentration (10 µM) ellagic acid; EAH, high concentration (20 µM) ellagic acid.
Treatment of ellagic acid regulates apoptosis regulator protein expression in A549 cells
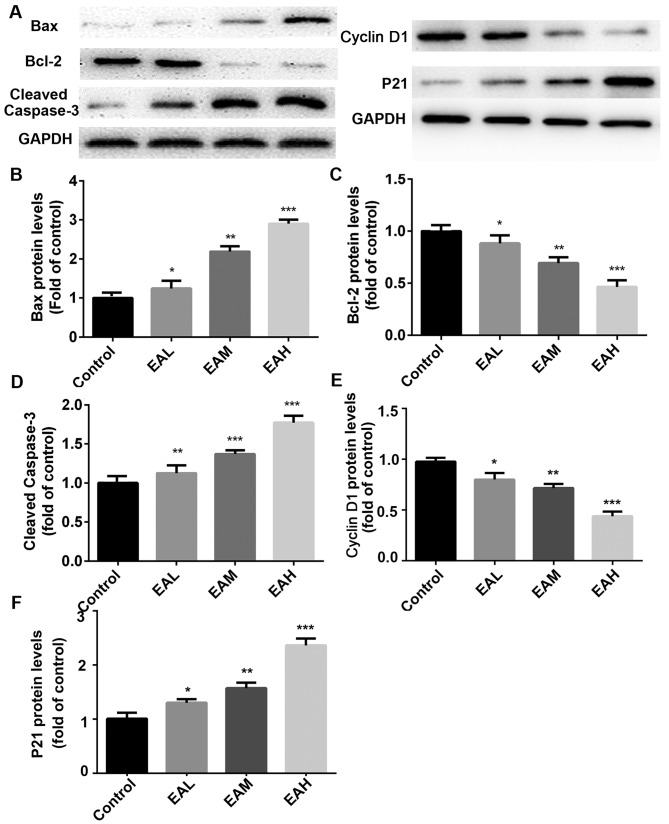
Finally, the effect of ellagic acid on expression of apoptosis-related protein in A549 cells was detected. As presented in Fig. 5, compared with the control group, ellagic acid stimulation (5, 10 and 20 µM) for 48 h significantly increased p21, Bax and cleaved caspase-3 protein expression in a dose-dependent manner in A549 cells (5 µM, P<0.05; 10 µM, P<0.01, 20 µM; P<0.001). Conversely, cyclin D1 and Bcl-2 protein levels were significantly downregulated after the treatment of ellagic acid (5 µM, P<0.05; 10 µM, P<0.01; 20 µM, P<0.001).
Figure 5.
(A) Apoptosis-associated protein expression. Compared with the control group, p21, Bax and cleaved caspase-3 protein expression was increased in a dose-dependent manner following treatment. Conversely, cyclin D1 and Bcl-2 protein levels were downregulated after the treatment with ellagic acid. (B-F) Quantitation of Western blot signal intensities. Data are presented as the mean ± standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control. Bcl-2, B cell lymphoma 2; Bax, Bcl-2 associated X protein; EAL, low concentration (5 µM) ellagic acid; EAM, middle concentration (10µM) ellagic acid; EAH, high concentration (20 µM) ellagic acid.
Discussion
In the present study, the inhibitory influence of ellagic acid on the viability of NSCLC A549 cells was investigated. The data demonstrated that stimulation with ellagic acid for 48 h significantly inhibited cell viability in a dose-dependent manner. Additionally, ellagic acid significantly induced cell apoptosis in human NSCLC A549 cells in a dose-dependent manner. Ho et al (19) have previously reported that ellagic acid may inhibit viability and promote apoptosis in human bladder cancer cells.
Increasing evidence has demonstrated that the PI3K/Akt signaling pathway is associated with a large number of pathophysiological processes including cell viability, cell cycle progression, survival, apoptosis and metastasis (20,21). The aberrant activation of the PI3K/Akt signaling pathway has also been observed in NSCLC cell lines and NSCLCs, and has been demonstrated to serve a key role in the initiation and development of this cancer (22,23). In addition, it has previously been reported that ellagic acid may inhibit viability and induce apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells (24). These findings suggest that the PI3K/Akt signaling pathway is a novel potential target of NSCLC therapy. In accordance with these studies, the present study demonstrated that the phosphorylation of PI3K and Akt were significant lower in ellagic acid-treated groups than the control group, identifying the inhibitory effect of ellagic acid on the PI3K/Akt signaling pathway in A549 cells. These findings suggest that ellagic acid inhibits the cell viability and promotes cell apoptosis in A549 cells via the suppression of PI3K/Akt signaling pathway. As the present findings indicated, after the treatment of ellagic acid for 48 h, the proportion of cells in G1 phase increased significantly in ellagic acid-treated groups, and ellagic acid-treated groups exhibited a significant increase in apoptosis rate in comparison with controls, suggesting that ellagic acid may accelerate cell apoptosis in a dose-dependent manner. Western blot analysis revealed that the phosphorylation of PI3K and Akt were downregulated by the treatment of ellagic acid, indicating that PI3K and Akt signaling pathway was inhibited. p21, Bax and cleaved Caspase-3 protein expression were increased with ellagic acid treatment in a dose-dependent manner, consistent with the results of apoptosis rate increase. Conversely, cyclin D1 and Bcl-2 protein levels were downregulated after treatment with ellagic acid, further indicating that ellagic acid promotes cell apoptosis at the protein level.
Ellagic acid antitumor activity was initially suggested after the observation that aromatase, a key enzyme in breast cancer development which converts androgens to estrogens, is inhibited by polyphenols derived from fresh pomegranate juice (25). Akt, the serine/threonine kinase downstream effector of PI3K, causes tumor cell survival and inhibition of apoptosis, induces viability and cell growth, and stimulates angiogenesis by phosphorylating numerous downstream targets in the presence of different apoptotic stimuli (26). These previous findings suggest that increased constitutive phosphorylation of Akt is associated with decreased apoptosis, whereas Akt inhibition increased apoptosis. Therefore, the Akt signaling pathway is becoming a promising target for cancer chemoprevention and therapy (27). The aim of the present study was to address the cytotoxic effects of ellagic acid on A549 cells. PI3K activates the downstream target Akt to mediate several biological effects. Upon activation, Akt inactivates several downstream targets including Bcl-2 family members and caspase-3, thereby blocking apoptosis (28). Alternatively, the inhibition of phosphorylated Akt increases the expression of proapoptotic Bax, a fact that favors progress of the apoptotic process. Bcl-2 and its helpers compete with Bax and other proapoptotic proteins to regulate the release of cytochrome c from mitochondria, which in turn activates initiator caspases including caspase-3 (29). The present findings suggest that in A549 cells, ellagic acid blocks PI3K phosphorylation, thus decreasing Akt phosphorylation, therefore the PI3K/Akt signaling pathway was blocked. This trigged cell apoptosis as evidenced by increased p21, Bax and cleaved caspase-3 protein levels, and decreased Bcl-2 and cyclin D1 levels.
In conclusion, the results of the present study demonstrated the inhibition of human NSCLC cell viability and induction of apoptosis by ellagic acid treatment. The findings demonstrated that ellagic acid decreased PI3K and AKT phosphorylation, and promoted A549 cell apoptosis. The apoptotic induction capacity of ellagic acid may be attributed to its effect to regulate the apoptosis-related proteins Bax, Bcl-2, and caspase-3 through downregulating the PI3K/Akt pathway. The present study revealed that the mechanism by which ellagic acid exhibits its growth inhibitory effect on human NSCLC in vitro is by inhibiting cell viability and inducing apoptosis. Further studies are required to understand the different molecular mechanisms of action of ellagic acid, which will help provide useful information for its possible application in cancer prevention, and perhaps novel therapies for cancer and other diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangdong Provincial Key Platform and Major Scientific Research Projects (grant no. 2016GXJK213) and Xinhua Institute Teachers Research Fund Project of Sun Yat-sen University (grant no. 2016YB002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QL wrote the manuscript and interpreted the data. XL analyzed the data and revised the manuscript, CN searched the literature and collected the data. XW designed the study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Tieulent Lortet J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Subramaniam S, Thakur RK, Yadav VK, Nanda R, Chowdhury S, Agrawal A. Lung cancer biomarkers: State of the art. J Carcinog. 2013;12:3. doi: 10.4103/1477-3163.107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Huang Y, Zhuo W, Zhu Y, Zhu B, Chen Z. Fisetin, a dietary phytochemical, overcomes Erlotinib-resistance of lung adenocarcinoma cells through inhibition of MAPK and AKT pathways. Am J Transl Res. 2016;8:4857–4868. [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10. doi: 10.1016/j.ctrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Dalvi MP, Wang L, Zhong R, Kollipara RK, Park H, Bayo J, Yenerall P, Zhou Y, Timmons BC, Rodriguez-Canales J, et al. Taxane-platin-resistant lung cancers Co-develop hypersensitivity to jumonjic demethylase inhibitors. Cell Rep. 2017;19:1669–1684. doi: 10.1016/j.celrep.2017.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JH, Lee KY, Hu CJ, Chung CC. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: A case report and literature review. Medicine (Baltimore) 2017;96:e9262. doi: 10.1097/MD.0000000000009262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocanu MM, Nagy P, Szöllősi J. Chemoprevention of breast cancer by dietary polyphenols. Molecules. 2015;20:22578–22620. doi: 10.3390/molecules201219864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar S, Siddiqui AA, Mazumder S, De R, Saha SJ, Banerjee C, Iqbal MS, Adhikari S, Alam A, Roy S, Bandyopadhyay U. Ellagic acid, a dietary polyphenol inhibits tautomerase activity of human macrophage migration inhibitory factor and its pro-inflammatory responses in human peripheral blood mononuclear cells. J Agr Food Chem. 2015;63:4988–4998. doi: 10.1021/acs.jafc.5b00921. [DOI] [PubMed] [Google Scholar]
- 9.Benvenuto M, Fantini M, Masuelli L, De Smaele E, Zazzeroni F, Tresoldi I, Calabrese G, Galvano F, Modesti A, Bei R. Inhibition of ErbB receptors, Hedgehog and NF-kappaB signaling by polyphenols in cancer. Front Biosci (Landmark Ed) 2013;18:1290–1310. doi: 10.2741/4180. [DOI] [PubMed] [Google Scholar]
- 10.Marzocchella L, Fantini M, Benvenuto M, Masuelli L, Tresoldi I, Modesti A, Bei R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat Inflamm Allergy Drug Discov. 2011;5:200–220. doi: 10.2174/187221311797264937. [DOI] [PubMed] [Google Scholar]
- 11.Talcott ST, Lee JH. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J Agr Food Chem. 2002;50:3186–3192. doi: 10.1021/jf011500u. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Tang SN, Marsh JL, Shankar S, Srivastava RK. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Lett. 2013;337:210–217. doi: 10.1016/j.canlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Ceci C, Tentori L, Atzori MG, Lacal PM, Bonanno E, Scimeca M, Cicconi R, Mattei M, de Martino MG, Vespasiani G, et al. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients. 2016;8 doi: 10.3390/nu8110744. pii: E744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik A, Afaq S, Shahid M, Akhtar K, Assiri A. Influence of ellagic acid on prostate cancer cell proliferation: A caspase-dependent pathway. Asian Pac J Trop Med. 2011;4:550–555. doi: 10.1016/S1995-7645(11)60144-2. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–221. doi: 10.1016/S0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 16.Ho CC, Huang AC, Yu CS, Lien JC, Wu SH, Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al. Ellagic acid induces apoptosis in TSGH8301 human bladder cancer cells through the endoplasmic reticulum stress-and mitochondria-dependent signaling pathways. Environ Toxicol. 2014;29:1262–1274. doi: 10.1002/tox.21857. [DOI] [PubMed] [Google Scholar]
- 17.Gul M, Aliosmanoglu I, Uslukaya O, Firat U, Yüksel H, Gümüs M, Ulger BV. The protective effect of ellagic acid on lung damage caused by experimental obstructive jaundice model. Acta Chir Belg. 2013;113:285–289. doi: 10.1080/00015458.2013.11680929. [DOI] [PubMed] [Google Scholar]
- 18.Favarin Cornelio D, Teixeira Martins M, de Andrade Lemos E, de Freitas Alves C, Chica Lazo JE, Sorgi Artério C, Faccioli LH, Rogerio Paula A. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013;2013:164202. doi: 10.1155/2013/164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho CC, Huang AC, Yu CS, Lien JC, Wu SH, Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al. Ellagic acid induces apoptosis in TSGH8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ Toxicol. 2014;29:1262–1274. doi: 10.1002/tox.21857. [DOI] [PubMed] [Google Scholar]
- 20.Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: Inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–249. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye G, Lu Q, Zhao W, Du D, Jin L, Liu Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biol. 2014;35:11261–11267. doi: 10.1007/s13277-014-2337-7. [DOI] [PubMed] [Google Scholar]
- 22.Scrima M, De Marco C, Fabiani F, Franco R, Pirozzi G, Rocco G, Ravo M, Weisz A, Zoppoli P, Ceccarelli M, et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): New insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7:e30427. doi: 10.1371/journal.pone.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu CX, Jin H, Shin JY, Kim JE, Cho MH. Roles of protein kinase B/Akt in lung cancer. Front Biosci (Elite Ed) 2010;2:1472–1484. doi: 10.2741/e206. [DOI] [PubMed] [Google Scholar]
- 24.Umesalma S, Nagendraprabhu P, Sudhandiran G. Ellagic acid inhibits proliferation and induced apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells. Mol Cell Biochem. 2015;399:303–13. doi: 10.1007/s11010-014-2257-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71:203–217. doi: 10.1023/A:1014405730585. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: Molecular targets for anti-cancer drug development. Current Cancer Drug Targets. 2004;4:235–256. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- 27.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saglam O, Garrett CR, Boulware D, Sayegh Z, Shibata D, Malafa M, Yeatman T, Cheng JQ, Sebti S, Coppola D. Activation of the serine/threonine protein kinase AKT during the progression of colorectal neoplasia. Clinical Colorectal Cancer. 2007;6:652–656. doi: 10.3816/CCC.2007.n.034. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D WR. The hallmarks of cancer. Cell. 2000 doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.