Abstract
Following the accident at the Fukushima Daiichi Nuclear Power Plant in 2011, a number of evacuees were forced to live in temporary housing and suffered mental and physical stress. However, few reports have used objective or quantitative indicators to determine the evacuee's level of stress. The aim of the present study was to serially estimate the mental and physical stress of the evacuees from 2013 to 2015 by using the oxidative stress marker, urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG). A total of 773 evacuees from Namie town in Fukushima prefecture participated in the study. In the first year, 486 evacuees participated (age, 62.8±18.2 years; male/female, 217/269). Of these, 127 continually participated in the study for 3 years (age, 69.5±13.5 years; males/female 52/75) and 18.1% had no chronic disease after the first year. Urine samples were collected once per year. Urinary 8-OHdG was measured using immunochromatography and corrected by the concentration of urinary creatinine. For all the participants examined each year, mean values of urinary 8-OHdG significantly increased over time. For the 127 continual participants, mean values of urinary 8-OHdG were significantly higher in 2014 and 2015 than those in 2013. Age, gender and presence of chronic disease did not significantly influence the 8-OHdG values, suggesting that the stress level of the evacuees was not associated with these factors. The stress level of the individuals increased with the length of time spent living in the temporary housing. The evacuees in radiation disasters have different stressors from other natural disasters, which may accelerate mental and physical stress.
Keywords: oxidative stress, urinary 8-hydroxy-2′-deoxyguanosine, evacuees, Fukushima Daiichi Nuclear Power Plant, disaster
Introduction
On March 11, 2011, a huge earthquake occurred off the Pacific coast of the Tohoku region of Japan, followed by a giant tsunami that caused unprecedented damage. Although it was initially named ‘The 2011 Tohoku earthquake and tsunami,’ it was later renamed ‘The Great East Japan Earthquake’ because the affected area was not limited to the Tohoku region. However, the prefectures of Iwate, Miyagi, and Fukushima, located on the Pacific coast of the Tohoku region, were particularly affected. Numerous lives were lost and many cities, towns, and villages were destroyed. Following the earthquake and tsunami, infrastructure such as the traffic network, electricity supply, water supply, sewerage system, and telephone network suffered widespread damage. To make matters worse, due to the power outage caused by the tsunami, an accident occurred at the Fukushima Daiichi Nuclear Power Plant (FDNPP), forcing many people to evacuate from their homes.
The FDNPP is located astride the neighboring towns Okuma and Futaba on the Pacific coast of Fukushima prefecture. The evacuation zone included 13 neighboring towns and villages, designated according to the level of radioactive contamination. A large proportion of the residents were forced to evacuate to temporary housing or to other areas. The number of evacuees peaked at 164,000 (pref.fukushima.lg.jp/site/portal-english/en03-08.html). At present, 6 years after the disaster, approximately 90,000 evacuees are still forced to live outside their hometowns (reconstruction.go.jp/topics/main-cat2/sub-cat2-1/20170728_hinansha.pdf). Temporary housing was quickly built to provide accommodation for the evacuees in the months following the disaster. Assembly facilities were constructed for each 50 houses (approximately) to enhance the community and improve communication among the residents (fdma.go.jp/concern/publication/higashinihondaishinsai_kirokushu/pdf/honbun/03-06.pdf).
Motoya (1) reported the issues experienced by the evacuees living in the temporary housing 2 years after the FDNPP accident. He indicated that because the temporary housing was located in the highlands to avoid tsunamis, it lacked convenience and isolated the evacuees from public transport. Further, temporary housing was allocated almost independently of preexisting social ties. In addition, prefabricated housing was used for the temporary dwellings, and this is often too hot in summer and too cold in winter. The residents also complained of high levels of noise and a lack of privacy. An increase in mental and physical health problems was reported in evacuees following the earthquake and FDNPP disaster. Recent studies have also demonstrated increases in depression, disturbed sleep, lack of exercise and increases in body weight in the evacuees after the disaster (2–4).
Studies reporting objective or quantitative indicators expressing the severity of stress in the evacuees following the disaster were not found. Therefore, we decided to use urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker to quantitatively and noninvasively assess oxidative stress in the evacuees living in temporary housing to determine appropriate levels of support for the evacuees. 8-OHdG is known as one of the dominant forms of oxidatively generated base modifications produced by the reaction of a hydroxyl radical on the C8 position of 2′-deoxyguanosine (dG) (5) in DNA or on the guanine in the nucleotide pool (6). Because 8-OHdG is relatively stable, it is excreted into urine in the unchanged form following its intracellular production (7,8). The biomarker 8-OHdG is associated with aging, smoking, exercise level, employment status, obesity, diabetes, and depression (9,10). Thus, we inferred that 8-OHdG could be used as an indicator to evaluate the mental and physical stress of the evacuees.
In this study, we aimed to evaluate the changing level of mental and physical stress in the evacuees by serially measuring urinary 8-OHdG after the FDNPP disaster and to relate this to health problems of the evacuees living in difficult circumstances in temporary housing.
Materials and methods
Subjects and methods
Evacuees who originally lived in Namie town in Fukushima prefecture and moved to temporary housing following the FDNPP disaster were recruited to this study (Table I). The number of participants was 486, 346 and 195 in 2013, 2014, and 2015, respectively. Average ages [± standard deviation, (SD)] in 2013, 2014, and 2015 were 62.8±18.2, 66.5±14.6, and 68.3±14.8 years, respectively. The male/female ratios were 217/269, 160/186, and 81/114, respectively. Among the participants chronic diseases including cardiovascular disease and diabetes were present, which were diagnosed and categorized by a physician (Yasushi Mariya, MD, PhD), based on the detailed self-reports from them concerning health condition and disease. The participants were inspected once a year for internal radiation exposure using the whole-body counter FASTSCAN (FASTSCAN™, Canberra Inc., Meriden, CT, USA) owned by Namie town and none showed a committed effective dose over 1 mSv. Five adult healthy volunteers also participated in this study as control. Written informed consent was obtained from all the participants. The study was performed in accordance with ethical standards and was approved by the Ethics Committee of the Hirosaki University Graduate School of Medicine (approval no. 2013-115).
Table I.
Background of the study participants.
| Year | |||
|---|---|---|---|
| Characteristic | 2013 | 2014 | 2015 |
| Participants (n) | 486 | 346 | 195 |
| Male/female (n) | 217/269 | 160/186 | 81/114 |
| Age (years) mean ± SD | 62.8±18.2 | 66.5±14.6 | 68.3±14.8 |
In 2014 and 2015, the age composition of the participants was higher than expected based on the 2013 results. SD, standard deviation.
We collected urine samples in November or December at the first year. Thus, the urine sampling of the second year and the third year was also in November or December to prevent effects of seasonal fluctuation of oxidative stress. Analysis of urinary 8-OHdG was performed using Oxidative Stress Analyzer ICR-001 (Techno Medica, Japan). Measurements of 8-OHdG and creatinine were made by immunochromatography and Jaffe's method, respectively (www.technomedica.co.jp/t01/products/labo_tests/5/icr-001.html). In a vial tube, 100 µl of urine sample and 100 µl of pure water were mixed according to the analyzer protocol, and then measured. Urinary 8-OHdG was corrected by the concentration of urinary creatinine (CRE) and the value was expressed as the corrected value (ng/mg CRE). The detection limits of 8-OHdG and CRE were estimated to be 1.0 ng/ml and 10 mg/dl, respectively (technomedica.co.jp/t01/EnglishPage/products/labo_tests/2/icr-001.html). The median value of the coefficient of variance was 0.130 (range: 0.093–0.197) obtained from urine samples of the five healthy volunteers.
Each year, data from all the participants were analyzed. At the same time, to exclude the influence of the age of the participants, data were analyzed from 127 participants who provided urine samples continually over the 3 years. Of these participants, the average age in 2013 was 69.5±13.5 years, and the male/female ratio was 52/75. In 2014 and 2015, the age composition of the participants was significantly older than expected from the 2013 results (Table I).
The 127 participants who provided samples for 3 years continually were separated into two groups based on their age. Those aged 65 or older in 2013 were defined as the ‘elderly’ group, and those younger than 65 as the ‘non-elderly’ group. The elderly group comprised 90 participants with an average age of 76.2±7.6 years. The non-elderly group comprised 37 participants with an average age of 53.0±10.0 years. In addition, of the 127 continual participants, 23 (18.1%) had no chronic disease, 83 (65.4%) had cardiovascular disease, 26 (20.5%) suffered from diabetes, and 76 (59.8%) had been diagnosed with other chronic diseases. There were a proportion of the 127 participants with several diseases.
Statistical analyses
Continuous variables were expressed as mean average ± SD. One-way analysis of variance (ANOVA) was used to evaluate the differences between groups for all participants, followed by post-hoc analysis using the Games-Howell test. For the 127 continual participants, repeated ANOVA was used to evaluate the differences between groups, followed by post-hoc analysis using Tukey's HSD test or the Friedman test as appropriate. Two sample t-test was used to compare the urinary 8-OHdG values of all the participants with those for the 127 continual participants. Two-way repeated-measures ANOVA was used to compare the mean urinary 8-OHdG values between the elderly and non-elderly groups. The Mann-Whitney test was used to evaluate the difference between the values by gender.
Statistical analysis was performed using IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
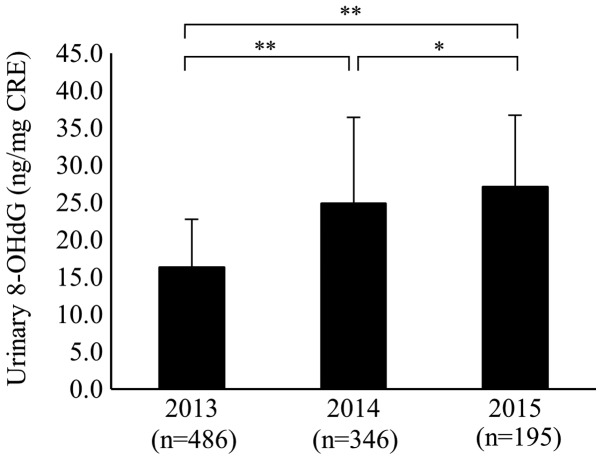
The mean values of urinary 8-OHdG for all subjects were 16.4±6.4 (ng/mg CRE), 24.9±11.6, and 27.1±9.5 in 2013, 2014, and 2015, respectively. The mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than that in 2013 (P<0.01). Furthermore, the mean value of urinary 8-OHdG in 2015 was significantly higher than that in 2014 (P<0.05; Fig. 1).
Figure 1.
Serial changes in the level of urinary 8-OHdG for all subjects. Error bars indicate standard deviation. The mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than in 2013. The urinary 8-OHdG in 2015 was significantly higher than that in 2014. (Coefficient of variance for measuring instrument: 9.3–19.7%). *P<0.05 and **P<0.01. 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
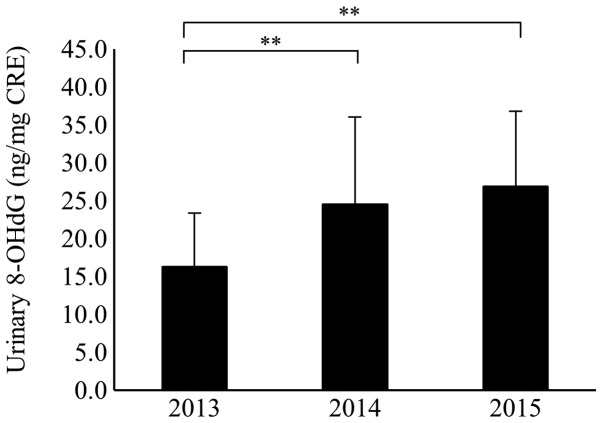
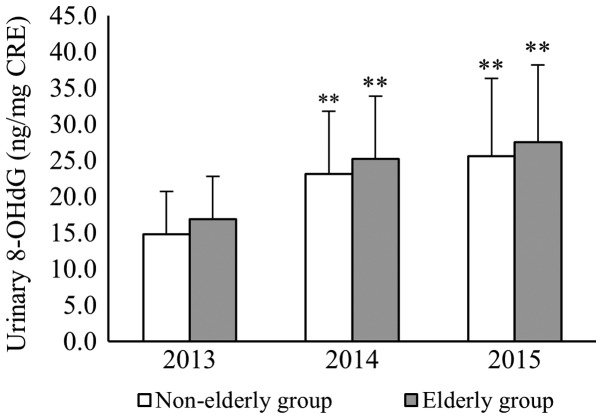
The 127 participants who continually provided urine samples for 3 years were analyzed. The mean values of urinary 8-OHdG were 16.3±7.1, 24.6±11.5, and 26.9±9.8 in 2013, 2014, and 2015, respectively. Among them the mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than that in 2013 (P<0.01; Fig. 2). When comparing the level of urinary 8-OHdG of the 127 continual participants with that of all the participants, there was no significant difference in the mean values between the two groups each year (Table II). Furthermore, in the elderly group of the continual participants, the mean values of urinary 8-OHdG were 16.9±7.5, 25.2±12.5, and 27.5±9.5 in 2013, 2014, and 2015, respectively. In the non-elderly group, values were 14.8±5.9, 23.1±8.7, and 25.6±10.7 in 2013, 2014, and 2015, respectively. In both of the age groups, the mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than that in 2013 (P<0.01). For each year, there was no significant difference in the mean values of urinary 8-OHdG between the elderly and non-elderly groups within the 127 continual participants (Fig. 3).
Figure 2.
Serial changes in the level of urinary 8-OHdG for 127 subjects who continually participated for 3 years. The mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than that in 2013. **P<0.01. 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Table II.
Mean values of urinary 8-OHdG.
| Mean 8-OHdG (ng/mg creatine) | |||
|---|---|---|---|
| Group | 2013 | 2014 | 2015 |
| All subjects | 16.4±6.4 | 24.9±11.6 | 27.1±9.5 |
| 127 subjects | 16.3±7.1 | 24.6±11.5 | 26.9±9.8 |
Data are presented as the mean ± standard deviation. There was no significant difference in the mean values of 8-OHdG between the two groups each year. 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Figure 3.
Serial changes in the mean value of urinary 8-OHdG for different age groups. In each group the mean values of urinary 8-OHdG in 2014 and 2015 were significantly higher than in 2013. **P<0.01. 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
For gender, the mean values of urinary 8-OHdG of all the male participants were 13.9±4.6, 22.7±9.2, and 26.4±9.1 in 2013, 2014, and 2015, respectively. For all females, the mean values of urinary 8-OHdG were 18.0±7.9, 25.9±12.8, and 27.3±10.4 in 2013, 2014, and 2015, respectively. Only in 2013, the mean value of urinary 8-OHdG of the females was significantly higher than that of the males (P<0.01), and there was no significant difference in the values by gender in 2014 and 2015.
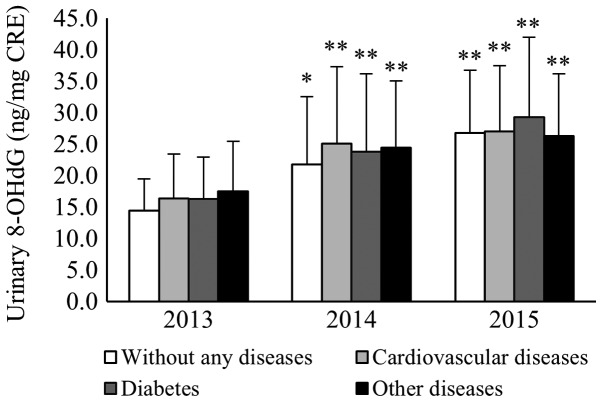
The mean values of urinary 8-OHdG for the 127 continual participants with no chronic diseases were 14.4±5.0, 21.8±10.8, and 26.7±10.0 in 2013, 2014, and 2015, respectively. The mean values of urinary 8-OHdG in 2014 and 2015 were higher than that in 2013 (P<0.05 and P<0.01, respectively). In contrast, the mean values of urinary 8-OHdG for participants with cardiovascular disease were 16.3±7.1, 25.1±12.2, and 27.0±10.4 in 2013, 2014, and 2015, respectively. Those with diabetes had values of 16.3±6.7, 23.8±12.4, and 29.3±12.7 in 2013, 2014, and 2015, respectively. Mean values for those with other chronic diseases were 17.5±8.0, 24.5±10.6, and 26.3±9.9 in 2013, 2014, and 2015, respectively. The mean values of urinary 8-OHdG for participants with cardiovascular disease, diabetes, and other chronic diseases in 2014 and 2015 were significantly higher than those in 2013 (P<0.01; Fig. 4).
Figure 4.
The 127 subjects who provided samples continually for 3 years were divided into four categories according to their chronic diseases. This fig. Shows the mean values of urinary 8-OHdG in each category. The mean values of 8-OHdG with no chronic diseases in 2014 and 2015 were significantly higher than in 2013. The mean values of 8-OHdG with cardiovascular disease, diabetes, and other chronic diseases in 2014 and 2015 were significantly higher than those in 2013. *P<0.05 and **P<0.01. 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Discussion
There are several phases of evacuation. In the initial phase, evacuees go into emergency shelters, if possible, or stay in their cars. They face medical issues such as emotional stress and/or sleep disturbance, and may not have access to regular medication. In addition, limited living space increases the risk of thrombosis. After several months, evacuees move into temporary housing. To evaluate the mental and physical stress of evacuees, we analyzed the serial change of urinary 8-OHdG in the evacuees living in temporary housing following the 2011 FDNPP disaster, starting from 2013, once a year for 3 years. The level of urinary 8-OHdG significantly increased in the second and third years compared with that in the first year.
Kimura et al (11) measured urinary 8-OHdG in 248 healthy Japanese volunteers using enzyme-linked immunosorbent assay (ELISA), and the mean urinary 8-OHdG concentration was 15.2±5.71 ng/mg CRE. In the present study, measurement of urinary 8-OHdG was initiated 2 years after the FDNPP disaster. The level of urinary 8-OHdG in the first year in our participants was similar to those in healthy Japanese people reported by Kimura et al (11). In 2004, the Chuetsu earthquake occurred in Niigata prefecture in Japan, and Saito et al (12) reported the level of oxidative stress of 73 elderly residents living in temporary housing. The survey was initiated 10 months after the earthquake and continued for ~1 year. They also measured urinary 8-OHdG as an oxidative stress marker using ELISA and reported that the urinary 8-OHdG level remained within normal range during the observation (12). However, our results revealed that the level of urinary 8-OHdG rose in the second and third years compared with that in the first year. We consider that the different period until the measurement was initiated, 10 months and 2 years after the disaster, played an important role in the difference in results between the two surveys.
There was a shift in age composition of participants in this study to significantly older participants in the second and third years. The elderly generally have a higher prevalence of chronic diseases (9). Therefore, at first, we speculated that aging and chronic diseases might influence the level of urinary 8-OHdG. We separated 127 participants who continually provided urine samples for 3 years, into elderly and non-elderly groups. There was no significant difference in the level of urinary 8-OHdG between the two groups. Further, we categorized the 127 continual participants into four groups: those with no chronic diseases, those with cardiovascular disease, those with diabetes, and those with other chronic diseases. The urinary 8-OHdG levels increased in the second and third years regardless of the presence of each chronic disease. A similar result was obtained regarding gender. These results suggest that the stress level of the evacuees was not associated with age, gender, or presence of chronic disease.
None of the evacuees showed the committed effective dose over 1 mSv, and the physical effect of radiation was negligible. However, there are specific properties of a radiation disaster, which are derived from the psychosocial effects. Participants' concerns included radioactive fallout, radiation exposure, food safety, loss of employment, and loss of their own homes. Furthermore, they lost social connection due to the evacuation distance (13,14). Consequently, the evacuees in radiation disasters have different stressors from other natural disasters, which could accelerate mental and physical stress.
It has been reported that there are several effective interventional programs providing exercise or a combined program of exercise and craftwork for the evacuees (15,16). Itaki et al (16) suggested that these attempts were intended to improve physical conditions and quality of life and that they reduced the risk of progression of cognitive dysfunction. We consider that it is important to provide opportunities for stress relief for the evacuees in the chronic phase. In this case, 6 years have passed since the FDNPP disaster, and public housing has been recently built in safe areas. The temporary housing that we visited to conduct the survey was closed; therefore, our survey ended at 3 years.
There are several limitations to the present study. We measured only urinary 8-OHdG as an oxidative stress marker. Also, detailed clinical information was not obtained according to the wishes of the evacuees. Nevertheless, we believe that the results are important because the data were obtained from the rare situation of a nuclear-associated disaster.
In conclusion, evaluation of the stress level of evacuees living in temporary housing following the FDNPP disaster, employing the oxidative stress marker urinary 8-OHdG, demonstrated a significant increase in stress level as the length of time living in temporary housing increased.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- CRE
creatinine
- FDNPP
Fukushima Daiichi Nuclear Power Plant
Funding
This study was in part supported by JSPS KAKENHI (grant no. 25461900).
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
CI and YM designed the research. YF, AN, CI and YM conducted the research. YM provided essential reagents or materials. YF and AN analyzed the data and ST and MY interpreted the data. YF, CI and YM performed the statistical analyses. YF, CI, ST, MY and YM wrote the paper. YM had primary responsibility for the final content. All authors contributed to and approved the final version of the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from all the participants. The study was performed in accordance with recognized ethical standards and was approved by The Ethics Committee of the Hirosaki University Graduate School of Medicine (approval number 2013-115).
Consent for publication
Written informed consent was obtained from all participants for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Motoya R. Health Promotion for the Victims and Evacuees of the Great East Japan Earthquake. Jap J Behavioral Med. 2013;19:68–74. (In Japanese) [Google Scholar]
- 2.Kitajima M, Otsu H, Tomisawa T, Sasatake H, Itaki C, Yonaiyama C, Urushizaka M, Nishizawa Y. The perception of health condition and radiation among elderly evacuees four years after the Fukushima Nuclear Disaster. J Radiolo Nursing Soc Jap. 2017;5:47–55. (In Japanese) [Google Scholar]
- 3.Murakami H, Yoshimura E, Ishikawa-Takata K, Nishi N, Tsuboyama-Kasaoka N, Yokoyama Y, Yaegashi Y, Sakata K, Kobayashi S, Miyachi M. The longitudinal change in physical activity among Great East Japan Earthquake victims living in temporary housing. Nihon Koshu Eisei Zasshi. 2014;61:86–92. doi: 10.11236/jph.61.2_86. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 4.Ohira T, Hosoya M, Yasumura S, Satoh H, Suzuki H, Sakai A, Ohtsuru A, Kawasaki Y, Takahashi A, Ozasa K, et al. Effect of evacuation on body weight after the great East Japan earthquake. Am J Prev Med. 2016;50:553–560. doi: 10.1016/j.amepre.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Kasai H, Nishimura S. Hydroxylation of the C-8 position of deoxyguanosine by reducing agents in the presence of oxygen. Nucleic Acids Symp Ser. 1983:165–167. [PubMed] [Google Scholar]
- 6.Hayakawa H, Taketomi A, Sakumi K, Kuwano M, Sekiguchi M. Generation and elimination of 8-oxo-7,8-dihydro-2′-deoxyguanosine 5′-triphosphate, a mutagenic substrate for DNA synthesis, in human cells. Biochemistry. 1995;34:89–95. doi: 10.1021/bi00001a011. [DOI] [PubMed] [Google Scholar]
- 7.Loft S, Poulsen HE. Estimation of oxidative DNA damage in man from urinary excretion of repair products. Acta Biochim Pol. 1998;45:133–144. [PubMed] [Google Scholar]
- 8.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′- deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 9.Kasai H, Iwamoto-Tanaka N, Miyamoto T, Kawanami K, Kawanami S, Kido R, Ikeda M. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage: Effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res. 2001;92:9–15. doi: 10.1111/j.1349-7006.2001.tb01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–561. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Kimura S, Yamauchi H, Hibino Y, Iwamoto M, Sera K, Ogino K. Evaluation of urinary 8-hydroxydeoxyguanine in healthy Japanese people. Basic Clin Pharmacol Toxicol. 2006;98:496–502. doi: 10.1111/j.1742-7843.2006.pto_217.x. [DOI] [PubMed] [Google Scholar]
- 12.Saito K, Aoki H, Fujiwara N, Goto M, Tomiyama C, Iwasa Y. Association of urinary 8-OHdG with lifestyle and body composition in elderly natural disaster victims living in emergency temporary housing. Environ Health Prev Med. 2013;18:72–77. doi: 10.1007/s12199-012-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada N, Shigemura J, Tanichi M, Kawaida K, Takahashi S, Yasukata F. Mental health and psychological impacts from the 2011 Great East Japan Earthquake Disaster: A systematic literature review. Disaster Mil Med. 2015;1:17. doi: 10.1186/s40696-015-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda M, Oe M. Mental Health Consequences and Social Issues After the Fukushima Disaster. Asia Pac J Public Health. 2017;29(Suppl 2):36S–46S. doi: 10.1177/1010539516689695. [DOI] [PubMed] [Google Scholar]
- 15.Tomata Y, Sato N, Kogure M, Suto S, Imai Y, Aoki H, Sugiyama K, Suzuki R, Sugawara Y, Watanabe T, et al. Health effects of interventions to promote physical activity in survivors of the 2011 Great East Japan Earthquake A longitudinal study. Nihon Koshu Eisei Zasshi. 2015;62:66–72. doi: 10.11236/jph.62.2_66. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 16.Itaki C, Fukushi Y, Kato T, Osanai T, Ohtsu H, Sasatake H, Kitajia M, Tomisawa T, Hosokawa Y, Nishizawa Y. The actual situation of motor functional decline of elderly person who is continuing life as an evacuee by The Fukushima Daiichi nuclear disaster and intervention to physical activity preventive improvement. J Health Sci Res. 2017;7:21–27. (In Japanese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.