Abstract
The risk of rupture, the most feared clinical consequence of abdominal aortic aneurysm, increases with the enlargement of aorta. MicroRNA-29b (miR-29b) has emerged as a key modulator of extracellular matrix (ECM) homeostasis and thereby is proposed to play a crucial role in vascular remodeling. However, agents that alter miR-29b expression are relatively inefficient in the aorta, likely due to inferior uptake. Herein we found that miR-29b was upregulated in aortic smooth muscle cells upon prostaglandin E2 (PGE2) stimulation whereas indomethacin treatment downregulated miR-29b expression. In order to obtain insight into the pathological processes associated with the vascular remodeling that accompanies aortic dilatation, we compared expression profiles of several representative ECM components in aortic walls. Notably, PGE2 induced a dramatic decline in these ECM components, which was rescued by introduction of indomethacin. In addition, COL1A1 was validated as a direct target gene of miR-29b by dual-luciferase reporter assay. In aggregate, our study suggests that PGE2 may accelerate ECM degradation through decreasing miR-29b expression. Thus those anti-inflammatory drugs that inhibit PGE2 synthesis represent an effective means of inducing an augmented profibrotic response in the aortic walls and thereby inhibiting aneurysmal expansion.
Keywords: fibrosis, abdominal aortic aneurysm, prostaglandin, microRNA-29b, extracellular matrix
Introduction
An abdominal aortic aneurysm (AAA) occurs if the abdominal aorta becomes focally enlarged, resulting from a weakened abdominal aortic wall. The prevalence of AAA has continued to increase worldwide over the last four decades. Data from clinical investigations involving different populations demonstrate that the prevalence is 2.4–16.9% in male and 0.5–2.2% in female patients older than 65 years of age (1). AAA usually develops asymptomatically until the outbreak of acute aneurysm rupture, which can cause severe internal bleeding and accounts for a mortality of 85–90% (2). The pathogenesis of AAA initiation, progression, and ultimate rupture has been rarely elucidated by previous studies. Dilatation and weakening of the aorta is accompanied by other phenotypic changes including local inflammation, smooth muscle cell apoptosis, oxidation stress increase and, in particularly, dramatic extracellular matrix (ECM) degradation (3).
MicroRNAs (miRNAs) are a class of endogenous non-coding RNAs encompassing 18–23 nucleotides that regulate the expression of mRNAs by targeting their 3′-untranslated regions (3′-UTRs) and inhibiting translation (4). Recently, miRNAs have been deemed as vital modulators of diverse cell events and have been implicated in a wide array of pathologies, such as cardiovascular disorders (5). Expression alterations in miRNAs have been revealed to impact the vascular angiogenesis, inflammation, and remodeling (6,7). As a member of the miR-29 family, microRNA-29b (miR-29b) has been previously proven to be remarkably upregulated in patients with AAA in comparison to the normal population (8). The miR-29 family can accelerate fibrosis in various tissues via regulating downstream ECM genes (9–11), including multiple collagens isoforms (COL1A1, COL5A1, COL3A1) and components of aortic wall, such as and elastin (ELN) and fibrillin-1 (FBN1). Dysregulation of ECM homeostasis is responsible for several pathological conditions, such as fibrosis and cancerous invasion. In addition, the aforementioned collagens that are widely expressed in the aortic wall play a vital role in aneurysm formation (12–14). Also, matrix metalloproteinases (MMPs, such as MMP2 and MMP9) have been identified as direct target genes of miR-29b and relate to AAA onset and progression (15,16). These data in aggregate suggest that miR-29b may participate in AAA development via altering ECM microenvironment and suppressing ECM protein expression.
Prostaglandins (PGs) are essentially a category of fatty acids that can result in inflammation, pain, redness and swelling. There are four major bioactive PGs that are ubiquitously generated in vivo and function as lipid mediators in autocrine and paracrine manner. Among them, prostaglandin E2 (PGE2) is one of the most abundant PGs synthesized in the human body and possesses versatile physiological and/or pathological functions. While the pro-inflammatory property of PGE2 during acute inflammatory response is profoundly established, increasing studies have been launched with regard to its role in multiple vascular pathological conditions. For example, PGE2 induces augmentation of arterial dilatation and enhances microvascular permeability, thereby increasing blood flow into the inflamed tissues (17). On the other hand, PGE2 restrains the aortic smooth muscle cell (ASMC) proliferation and decreases cytokine secretion in vitro (18).
Prior studies have also shown that PGE2 is abundantly produced in the aneurysm wall, which may exert inhibitory effects on collagen synthesis (19,20). In addition, PGE2 is significantly implicated in vascular wall remodeling via the regulation of MMP activities in human AAA (21). It has been demonstrated that the miR-29 family members were obviously upregulated in trabecular meshwork cells by exogenous PGE2-evoked stimuli (22). Fortunately we found that the expression of miR-29b in the ASMCs was elevated on PGE2 treatment in our tentative trial, justifying the assumption that PGE2 improves miR-29b-mediated ECM remodeling in AAA development.
Materials and methods
Cell culture
The Ethics Committee of the Provincial Hospital Affiliated to Shandong University approved the study (Jinan, China). Human ASMCs (passage no. 3) propagated in growth media SmGM-2 were both purchased from Lonza (Walkersville, MD, USA) supplemented with 5% fetal bovine serum (FBS) following the manufacturer's instructions. PGE2 and indomethacin were purchased from Cayman Chemical (Ann Arbor, MI, USA). Cells were treated with 500 ng/ml PGE2 or 10 mmol/l indomethacin, with DMSO employed as a control. Cell containing plates were harvested for RNA or protein analysis at ~90% confluence.
In particular, indomethacin solution was first prepared by dropwise addition of 1 mol/l Na2CO3 to the drug powder until dissolved, and afterwards DMSO was added to make the solution concentration of 10.0 mmol/l, followed by sterile filtering.
Transfection of cultured cells
The ASMCs were transfected with miRNA-29b mimic, inhibitor or Scr-miR (Dharmacon, Chicago, IL, USA) using Lipofectamine 2000 (Invitrogen, Burlington, ON, Canada). miRNA transfection efficiency was confirmed by RT-qPCR. Two hours after transfection, cells were treated with PGE2 or indomethacin for 24 h before they were harvested.
miRNA extraction and quantification
miRNAs were extracted from cells using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA). Briefly, the cell samples were collected and washed two times using PBS, prior to the addition of miRNA additive (1:10) on ice for 15 min. The cell lysate was added with equal volumes of acid-phenol:chloroform, before centrifugation and removal of the aqueous phase, and then the mixture was added 1.25-fold to 100% ethanol. The mixture was passed through the filter cartridge and eluted. RT-qPCR was carried out with a final reaction volume of 20 ml containing 10 ml TaqMan Universal PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific Inc., Waltham, MA, USA), 8 ml DEPC-treated water, 1 ml TaqMan microRNA assay (Applied Biosystems; Thermo Fisher Scientific Inc.), and 1 ml RT product. The data were normalized to RNU6B small nuclear RNA to calculate fold-changes using the method of ∆∆Cq.
Dual-luciferase reporter assay
Two online databases, miRBase and TargetScan, were used to predict the potential binding sites for miR-29b. For dual-luciferase reporter assays, the full-length 3′-UTR of COL1A1 containing three miR-29b binding sites was cloned into the downstream of a pMIR-Report (Ambion) to generate pMir-COL1A1 3′-UTR, which was co-transfected with miR-29b mimics or Scr-miR into ASMCs. The pRL-SV40 vector (Promega, Madison, WI, USA) carrying the Renilla luciferase gene was used as an internal control to normalize the transfection efficiency. Luciferase activities were determined by using the Dual-Luciferase Reporter assay system (Promega) following the manufacturer's instructions. All the reactions were performed in triplicate.
mRNA quantification by RT-qPCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The total RNA 40 µg was used as template and then reverse transcribed with M-MLV Reverse Transcriptase kit (Promega Biotech Co., Ltd, Beijing, China) to synthesize the cDNA. Expression levels of target genes were normalized to β-actin, the internal positive control. RT-qPCR was carried out in a LightCycler (Roche Diagnostics, Laval, QC, Canada) machine with the SYBR-Green probe (SYBR Premix Ex Taq™ II; Takara, Dalian, China). Melting-curve analysis was used to determine the melting temperature (Tm) of specific amplification products and primer dimers, which were used for the signal acquisition step (2–3°C below Tm) for each gene. The comparative Cq (2-ΔΔCq) method was introduced to account for the relative fold-changes of the amount of template differences.
Protein extraction and western blotting
The harvested cells were pelleted and resuspended in RIPA lysis buffer, followed by incubating on ice for further lysing and centrifuged at 15,000 × g for 5 min at 4°C. After centrifugation, protein supernatant was kept at −80°C for future analysis.
For immunoblotting, 30 µg of total protein was separated on 10% SDS-PAGE and transferred to PVDF membrane at 250 mA for 1 h. The membrane was blocked with 5% silk milk and then incubated overnight at 4°C with mouse monoclonal primary antibody mouse monoclonal anti-COL1A1 (sc-293182; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by subsequent incubation with HRP-conjugated secondary goat anti-mouse polyclonal IgG (SA132; 1:2,000; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) at room temperature for 1 h. Then, the membrane was washed with PBST three times (5 min/time) before photographed using ECL reagents (Millipore, Billerica, MA, USA).
Soluble collagen assay
The total soluble collagen secreted from ASMCs was evaluated following the manufacturer's instructions (QuickZyme Biosciences, Leiden, Netherlands). In brief, after treatment with PGE2 or indomethacin for 24 h, companied with transfection with miR-29b mimics, inhibitor, or scr-miR, conditioned culture medium was collected and centrifuged to remove cell debris. Samples were incubated with Sirius Red color dye for 10 min at room temperature. After precipitation in a 96-well plate, data analysis was performed on a microplate reader (Multiskan MK3; Thermo Labsystems, Franklin, MA, USA) based on the absorbance at 540 nm. The experiment was performed in triplicate.
Statistical analysis
Data are presented as means ± SD. All in vitro experiments included at least 3 replicates per group. Groups were compared using the two-tailed Student's t-test for parametric data. When comparing multiple groups, data were analyzed by ANOVA with Bonferroni's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-29b expression was significantly increased on PGE2 treatment
In our earlier study, we found that upon PGE2 incitement, miR-29b was significantly upregulated in ASMCs, unusual undifferentiated muscle cell possessing the capacity to produce ECM. In order to further confirm this, ASMCs were treated with 500 ng/ml PGE2 or 10 mmol/l indomethacin, a non-steroidal anti-inflammatory drug (NSAID), in prior to the evaluation of miR-29b expression in vitro (Fig. 1). Strictly consistent with previous results, miR-29b was dramatically increased in PGE2-treated ASMCs compared to untreated cells (2.162±0.117 vs. 1.004±0.010, P<0.001), whereas miR-29b was significantly downregulated in the presence of indomethacin (0.665±0.015 vs. 1.004±0.010, when compared with untreated cells; P<0.01).
Figure 1.
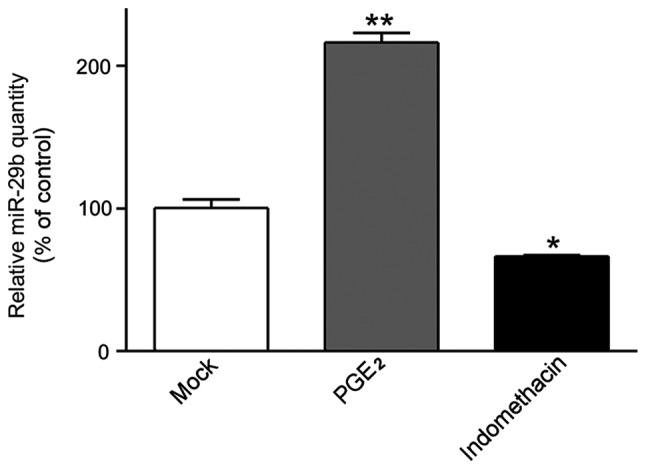
miR-29b expression was significantly increased on PGE2 treatment. AMSCs were treated with PGE2 or indomethacin. After 24 h, miR-29b were isolated from cells and subjected to RT-qPCR assay for quantification. miR-29b expression in untreated cells were defined as 100%. All data were presented as mean ± standard deviation. *P<0.01 and **P<0.001. PGE2, prostaglandin E2; AMSCs, aortic smooth muscle cells.
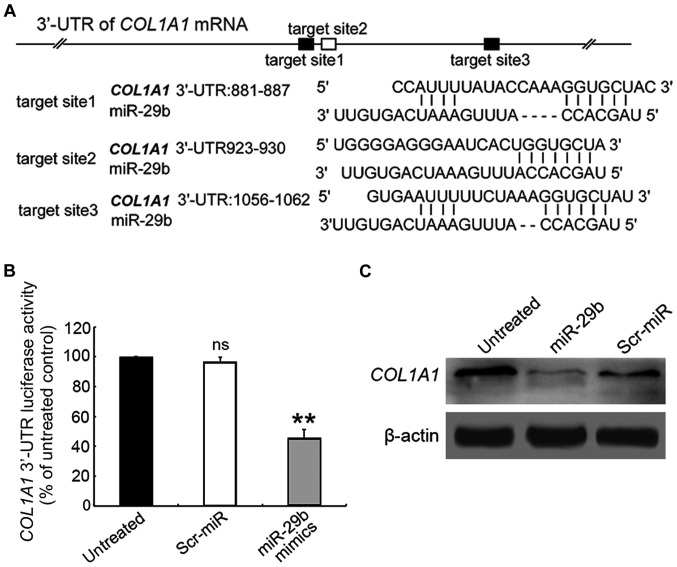
miR-29b directly targets the 3′-UTR of COL1A1 gene in cultured SVMCs
Although various ECM components have been identified as targets of miR-29b in other tissues, the downstream target genes in ASMCs remain unknown. As shown in Fig. 2A, the putative miR-29 binding sites are enriched in the 3′-UTR region of COL1A1 gene. To confirm whether miR-29b could directly target COL1A1, the 3′-UTR region of COL1A1 gene was cloned into a luciferase reporter vector, which was co-transfected into ASMCs with the miR-29b mimics or Scr-miR, prior to the performance of the luciferase reporter assay. In the presence of miR-29b mimics, the relative luciferase activity of COL1A1-3′-UTR-transfected ASMCs was significantly decreased compared with those untransfected cell control (Fig. 2B), whereas Scr exposure had no significant effect on the fluorescence intensity of the COL1A1-3′-UTR-transfected ASMCs.
Figure 2.
COL1A1 is a direct target of miR-29b. (A) The full-length 3′-UTR of COL1A1 mRNA contains three miR-29b-binding sites. (B) ASMCs were co-transfected with either miR-29b mimics or Scr-miR control, together with COL1A1-3′-UTR sequence. The relative luciferase activities were measured and normalized by Renilla luciferase value after 72 h. All data are presented as mean ± standard deviation. Similar results were obtained from 3 independent experiments. **P<0.01. (C) COL1A1 protein level in ASMCs cells co-transfected with either miR-29b mimics or Scr-miR control. 3′-UTR, 3′-untranslated region; ASMCs, aortic smooth muscle cells.
We next assessed the effect of miR-29b on the COL1A1 expression level in ASMCs by using RT-qPCR assay. The results demonstrated that the expression of COL1A1 in miR-29b mimics-transfected ASMCs was reduced at mRNA level compared to untransfected cells (Fig. 3A; 0.587±0.178-fold, P<0.05), which was corroborated by immunoblotting assay (Fig. 2C). These results suggest that miR-29b exerts an inhibitory effect on COL1A1 expression through directly targeting the 3′-UTR.
Figure 3.
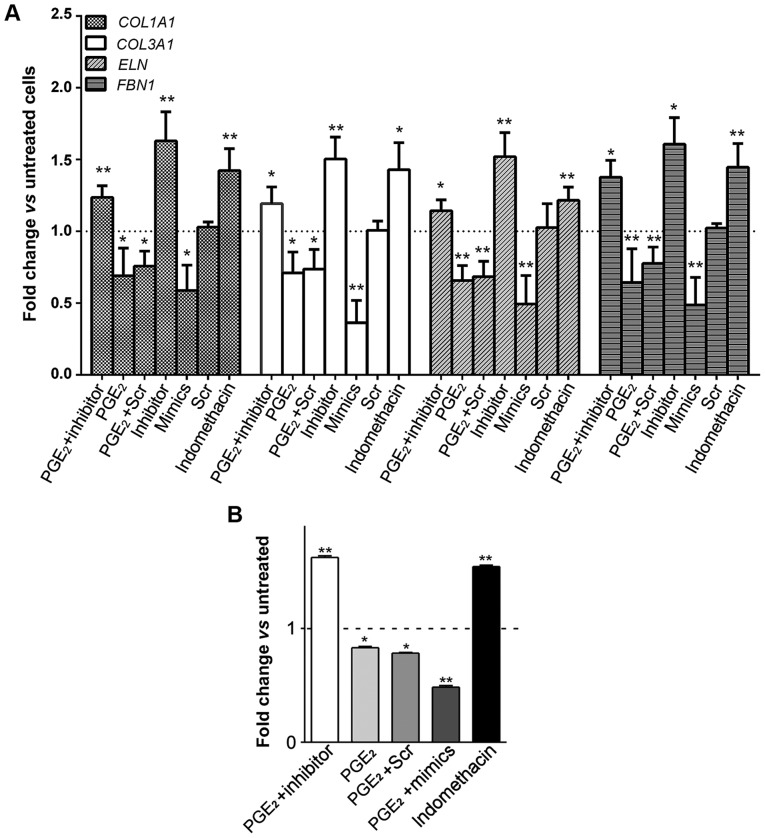
Alteration in the expression profiles of ECM components under different conditions. (A) Expression levels of ECM genes in ASMCs were assessed by RT-qPCR. (B) Soluble collagens produced in ASMCs. Data are presented as mean ± standard deviation. *P<0.05, **P<0.01 vs. untreated cells. ECM, extracellular matrix; ASMCs, aortic smooth muscle cells.
PGE2 promotes miR-29b-mediated ECM degradation in ASMCs
It has been reported that PGE2 participates in vascular wall remodeling via modulating MMP activities (21), thus we speculated whether PGE2 is able to regulate other profibrosis-associated ECM components. Collagen gene expression alterations (represented by COL1A1 and COL3A1) in AMSCs were assessed in the presence of PGE2. As shown in Fig. 3A, upon PGE2 treatment, the mRNA expression of COL1A1 and COL3A1 was significantly repressed (0.690±0.193- and 0.710±0.145-fold, P<0.05, respectively). Likewise, PGE2 also inhibited the expression of ELN and FBN1 in ASMCs (0.657±0.105-fold and 0.643±0.235-fold, P<0.01, respectively). Of note, ECM levels were augmented in response to indomethacin.
To further investigate whether PGE2 exerts inhibitory effects on ECM expression via upregulating miR-29b, miR-29b in ASMCs was then suppressed by transfecting with its inhibitor, followed by assessing the ECM expression profiles. Successful inhibition of miR-29b (<30% of expression), regardless of PGE2 treatment, was confirmed by RT-qPCR analysis (data not shown). Interestingly, expression levels of ECM genes in PGE2-stimulated and miR-29b inhibitor-transfected ASMCs were not inhibited but even increased compared to the untreated control (Fig. 3A). These collectively indicated that PGE2 regulates ECM production by altering miR-29 expression.
The promoted effect of PGE2 on miR-29b-mediated ECM downregulation in ASMCs was re-confirmed by monitoring soluble collagen synthesis after incitement. As shown in Fig. 3B, soluble collagen production was decreased in PGE2-treated ASMCs in comparison to that in untreated control. Notably, transfection of miR-29b inhibitor significantly compromised the inhibitory effect of PGE2 upon soluble collagen synthesis, whereas miR-29b mimics exacerbated ECM degradation caused by PGE2 treatment.
Discussion
The pathogenesis of an aneurysm as well as its progression and ultimate rupture involve series of complicated pathological processes. The determination of underlying mechanisms and identification of effective medical therapy remains a major challenge in recent aneurysmal medicine. In particular, novel molecular therapies in conquering AAA seem to be of vital importance. As far, patients with larger aneurysms depend on elective surgery. Understanding the miR-mediated regulation of ECM perturbations in pathological conditions will potentially provide insightful prospective in seeking innovative medical strategies to fight AAA.
In a separate case-control study, the expansion of AAAs in patients taking NSAIDs was significantly repressed compared with the control subjects (18), suggesting that inhibition of PGs synthesis in AAA development. PGs are ubiquitously generated in all cell types and function as autocrine and paracrine regulators to maintain local homeostasis or inflammatory response. Among them, PGE2 is the most prevalent and bioactive of the mammalian PG, which was found previously to be abundantly generated in the aneurysm wall and involved in the regulation of collagen synthesis (19). It has been demonstrated that during viral infection, there was a dramatic increase in the expression level of miR-29, which inhibited DNA methyl transferase (DNMT) activity and thereby contribute to the activation of COX2 and consequent enhancement of PGE2 production (23). Collectively, we conclude that miR-29 promotes PGE2 accumulation in response to inflammation through epigenetic modification-induced COX2 activation. However the effects of PGE2 on miR-29 and the sequential ECM-mediated inflammatory signaling pathways have rarely been studied.
In this study, we investigated the underlying mechanism of PGE2 and the pharmacology of its blockade, by introduction of an NSAID indomethacin, in orchestrating the inflammatory response, with particular regard to the AAA. Data from our experiments indicated that treatment of ASMCs with PGE2 induced an increase in miR-29b level, whereas indomethacin resulted in a decrease in miR-29b expression, hence a profibrotic response in AAA.
The miR-29 family is well characterized by its capacity to inhibit the expression of ECM components and thereby block the related fibrosis in a variety of organs. Previously published studies have already manifested the therapeutic implication of miR-29 as a target for fibrosis in heart (11), lung (24), liver (25), and kidney (26), and systemic sclerosis (27). In fibrotic conditions, decreased miR-29b directly results in an aggrandized collagen gene expression. Unfortunately, a profibrotic response in these organs is often pathologic and related to several serious diseases. Whether miR-29b downregulation plays a beneficial role in conquering certain fibrosis-related diseases is not known Although the precise mechanisms remain unclear, repression of miR-29b expression in experimental AAA development has been widely reported in previously published studies (9–11). Fig. 3 shows that modulation of miR-29b levels in ASMCs, especially overexpression with miR-29b mimics or suppression by miR-29b inhibitor, resulted in significant alteration in expression profiles of target collagen genes. Treatment of smooth muscle cells with PGE2 elevated miR-29b expression and thereby suppressed the ECM genes expression compared to untreated cells, while an inhibitor of PG synthesis termed as indomethacin counteracts the promotion by PGE2 of ECM genes expression. Of note, introduction of miR-29b inhibitor compromised the effects of PGE2 on collagen gene expression profiles, suggesting that PGE2 plays an inhibitory role in ECM expression by targeting miR-29b.
Taken together, we proposed that there is a novel bidirectional positive-feedback loop between PGE2 synthesis and miR-29b, functioning as a node in the regulation of ECM homeostasis. When AAA disease occurs, miR-29b expression level is obviously elevated, which inhibits methylation degree of COX2 and enhances its activity, and this will ultimately promote PGE2 accumulation. On the contrary, increased PGE2 can further accelerate miR-29b augmentation and therefore induce the pathological degradation of ECM proteins.
Data from a previous study suggested that a decline in the miR-29b expression triggers a profibrotic process in AAAs (28), which is usually deemed as a pathologic response to aneurismal dilatation. As the aneurysms dilate is companied by an increase in the risk of rupture, the aortic wall may tend, like a balloon, to become thinner and weaker compared to smaller aneurysms. In this case, deposition of new more soluble collagen fibers in aortic wall, leading to the thickening of aneurysmal walls, will occur to compensate the attenuated media and to reduce arterial wall tension (29). Although the new generated soluble collagens are more susceptible to hydrolysis by MMPs, the fibrotic thickening of the adventitia will hinder aneurysm expansion. Our observations highlighted the extent of soluble collagens degradation that took place in ASMCs as exposed to PGE2 treatment, which was rescued by introduction of indomethacin. Furthermore, upon PGE2 treatment, there was a decline in ELN content, which is synthesized constitutively by smooth muscle cells in the media. Since the destruction of ELN fibers has been suggested as a significant pathological process in aortic dilatation and being related to the loss of elastic capacities of the aortic media (30,31), the above suggest that PGE2 as well as miR-29b are crucially involved in the aneurysmal expansion. However, the application of agents that modulate miR-29b expression is relatively less efficient in the aorta compared to that in other organs such as kidney, liver and heart (28), likely due to preferential uptake in these organs. In this study, drugs targeting PGE2 are potentially more effective in control of aneurysmal dilatation.
In conclusion, in this study we propose that more expandable anti-inflammatory drugs that inhibit PGE2 synthesis may emerge as a promising avenue to trigger fibrosis in the aortic wall and thereafter protect the aorta from expansion in human patients with AAA. The in vivo experiments underlying their therapeutic potential to inhibit aneurysm expansion and ultimate rupture are being carried out in our laboratory and further evidence will be published in the future.
Acknowledgements
Not applicable.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZH and XJ contributed to the conception of the study. TZ contributed significantly to the data analysis and study preparation. YH and GL performed the data analyses and wrote the study. GL helped perform the analysis with constructive discussions. All authors have read and approved the final study.
Ethics approval and consent to participate
The Ethics Committee of the Provincial Hospital Affiliated to Shandong University approved the study (Jinan, China).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no financial or other conflicts of interest in relation to this study and its publication.
References
- 1.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8:623–631. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C. MicroRNomics: A newly emerging approach for disease biology. Physiol Genomics. 2008;33:139–147. doi: 10.1152/physiolgenomics.00034.2008. [DOI] [PubMed] [Google Scholar]
- 5.Seeger T, Boon RA. MicroRNAs in cardiovascular ageing. J Physiol. 2016;594:2085–2094. doi: 10.1113/JP270557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 8.Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T, Sawa Y. Tissue- and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745. doi: 10.1161/JAHA.112.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 10.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Paepe A, Nuytinck L, Hausser I, Anton-Lamprecht I, Naeyaert JM. Mutations in the COL5A1 gene are causal in the Ehlers-Danlos syndromes I and II. Am J Hum Genet. 1997;60:547–554. [PMC free article] [PubMed] [Google Scholar]
- 13.Rahkonen O, Su M, Hakovirta H, Koskivirta I, Hormuzdi SG, Vuorio E, Bornstein P, Penttinen R. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ Res. 2004;94:83–90. doi: 10.1161/01.RES.0000108263.74520.15. [DOI] [PubMed] [Google Scholar]
- 14.Menashi S, Campa JS, Greenhalgh RM, Powell JT. Collagen in abdominal aortic aneurysm: Typing, content, and degradation. J Vasc Surg. 1987;6:578–582. doi: 10.1067/mva.1987.avs0060578. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, Yao JS. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989;10:365–373. doi: 10.1016/0741-5214(89)90409-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: A novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–1728. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- 17.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 18.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: Implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.CIR.100.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Diaz A, Munoz E, Johnston R, Korn JH, Jimenez SA. Regulation of human lung fibroblast alpha 1(I) procollagen gene expression by tumor necrosis factor alpha, interleukin-1 beta, and prostaglandin E2. J Biol Chem. 1993;268:10364–10371. [PubMed] [Google Scholar]
- 20.Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J Vasc Surg. 1997;25:810–815. doi: 10.1016/S0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama U, Ishiwata R, Jin MH, Kato Y, Suzuki O, Jin H, Ichikawa Y, Kumagaya S, Katayama Y, Fujita T, et al. Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS One. 2012;7:e36724. doi: 10.1371/journal.pone.0036724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez P, Luna C, Li G, Qiu J, Epstein DL. miR-29 is induced by prostaglandin E and forms negative feedback loops with the Wnt and TGFbeta pathways in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51:3210. [Google Scholar]
- 23.Fang J, Hao Q, Liu L, Li Y, Wu J, Huo X, Zhu Y. Epigenetic changes mediated by microRNA miR29 activate cyclooxygenase 2 and lambda-1 interferon production during viral infection. J Virol. 2012;86:1010–1020. doi: 10.1128/JVI.06169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther. 2012;20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wu L, Wang Y, Zhang M, Li L, Zhu D, Li X, Gu H, Zhang CY, Zen K. Protective role of estrogen-induced miRNA-29 expression in carbon tetrachloride-induced mouse liver injury. J Biol Chem. 2012;287:14851–14862. doi: 10.1074/jbc.M111.314922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23:252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 28.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakalihasan N, Heyeres A, Nusgens BV, Limet R, Lapière CM. Modifications of the extracellular matrix of aneurysmal abdominal aortas as a function of their size. Eur J Vasc Surg. 1993;7:633–637. doi: 10.1016/S0950-821X(05)80708-X. [DOI] [PubMed] [Google Scholar]
- 30.Martufi G, Satriano A, Moore RD, Vorp DA, Di Martino ES. Local quantification of wall thickness and intraluminal thrombus offer insight into the mechanical properties of the aneurysmal aorta. Ann Biomed Eng. 2015;43:1759–1771. doi: 10.1007/s10439-014-1222-2. [DOI] [PubMed] [Google Scholar]
- 31.Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: Role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.