Abstract
Low socioeconomic status (SES) has been repeatedly linked with decreased academic achievement, including lower reading outcomes. Some lower SES children do show skills and scores commensurate with those of their higher SES peers, but whether their abilities stem from the same systems as high SES children or are based on divergent strategies is unknown. We here investigated a potential interactive relationship between SES and real-word reading skill in the white matter in 42 typically developing children. SES was determined based on parental education; reading skill and age were not significantly related to SES. There was a significant neural interaction: Clusters in the bilateral inferior longitudinal fasciculus (ILF), left superior longitudinal fasciculus, and left corticospinal tract demonstrated interactive skill–SES relationships in fractional anisotropy. Follow-up analyses demonstrated that higher SES children showed a positive relationship between fractional anisotropy, reflecting tract coherence, and reading skill in left hemisphere tract clusters, whereas lower SES children showed a positive relationship in the right hemisphere homologues. Broadly, the ILF has been demonstrated to support orthographic skill on the left and more general visuospatial processing on the right, so high reading achievement in lower SES children may rely on supplementary visuospatial processing more than for higher SES readers. This pattern is consistent with previous work reporting low SES children’s environments to include less rich verbal experience, which may lead them to disproportionately draw on visuospatial skills for success. Further, these results indicate that group SES differences may be best described by an adaptive, not a deficit, model.
Introduction
Familial socioeconomic status is an important environmental variable with significant influence on many skills and behaviors, including cognitive control, language development, and academic achievement (Bradley & Corwyn, 2002). Specifically, children from lower SES households have repeatedly been demonstrated to show decreased educational skills and attainment from school entrance, with the achievement gap between higher and lower SES groups widening across years of school (Brooks-Gunn & Duncan, 1997; Panel, 2008; Statistics, 2011). Lower SES children’s reading performance may be three grade levels below that of their higher SES peers by the end of fifth grade (Cooper, Borman & Fairchild, 2010). This between-group discrepancy represents a large barrier in lower SES children’s ability to improve their societal situation.
A limited number of studies have explored whether there are neural differences between higher and lower SES children commensurate with these noted behavioral achievement differences. Electrophysiological and functional MRI studies have consistently revealed SES-related differences in neural activation patterns during verbal processing, executive function, and reading (for reviews, see Hackman & Farah, 2009; Nelson & Sheridan, 2011; Tomalski & Johnson, 2010). In each case, higher SES children exhibited increased functional specialization in task-relevant regions (Hackman & Farah, 2009; Pakulak, Sanders, Paulsen & Neville, 2005; Raizada, Richards, Meltzoff & Kuhl, 2008; Stevens, Lauinger & Neville, 2009). For example, Raizada et al. (2008) showed that SES (defined by parental education and occupation) was positively correlated with the degree of left-lateralization of 5-year-olds’ inferior frontal gyrus activity during a rhyming task. Lower SES children’s impaired academic performance could thus be related to lessened functional support from the neural systems supporting these skills.
Little work has examined whether there are structural brain differences between SES groups that are related to behavioral performance or are independent of skill. Group differences appear to be most pronounced for regions similar to those reported from functional neuroimaging, i.e. frontal, temporal, and hippocampal areas (for review, see Brito & Noble, 2014; Hanson, Chandra, Wolfe & Pollak, 2011; Hanson, Hair, Shen, Shi, Gilmore et al., 2013; Noble, Houston, Kan & Sowell, 2012), which are central to verbal processing and executive function. SES is positively related to gray matter volume and degree of gyrification in these areas (Hanson et al., 2013; Jednoróg, Altarelli, Monzalvo, Fluss, Dubois et al., 2012; Lawson, Duda, Avants, Wu & Farah, 2013; Noble et al., 2012; Raizada et al., 2008). More recently, Noble, Houston, Brito, Bartsch, Kan et al. (2015) demonstrated that parental education and income were each significantly related to brain surface area, but not cortical thickness, in many bilateral temporal, frontal, limbic, and parietal regions across a large sample of children. Surface area mediated the relationship between parental income and executive function task scores, but not the relationship between income and vocabulary or reading scores. As such, the relationship between brain structure and SES-related differences on language skills cannot be determined from this work alone.
Potential relationships between socioeconomic status and white matter structure during development, though, remain relatively unexplored. Complex, later-developing skills like reading recruit networks of connected regions spread across the brain (Pugh, Mencl, Jenner, Katz, Frost et al., 2001); examination of the white matter supporting these connections may be important for determining potential causes of lower SES children’s difficulties. While significant correlations between adults’ educational attainment and structural connectivity have been noted (Chiang, McMahon, de Zubicaray, Martin, Hickie et al., 2011; Gianaros, Marsland, Sheu, Erickson & Verstynen, 2013; Noble, Korgaonkar, Grieve & Brickman, 2013; Piras, Cherubini, Caltagirone & Spalletta, 2011), Jednoróg et al. (2012) did not find any association between parental SES (based on maternal education and profession) and children’s white matter integrity in a small sample of children ages 9 to 11. In a larger study, Chiang et al. (2011) did not see a direct association between their adult (twin) subjects’ parental socioeconomic status (defined by occupation) and fractional anisotropy (FA), but did find an interaction between SES and white matter integrity genes. Genetics explained more FA variance in higher than in lower SES individuals in the thalamus, left middle temporal gyrus, and splenium, meaning that environmental influences may have generally had more influence on lower SES individuals’ brain structure. To our knowledge, though, no other work has examined the developmental influence of SES on white matter, or its relationship with tracts supporting academic skill.
One reason for this gap in the literature is that the relationship between achievement and brain structure might not be simply magnified in lower SES individuals: it could be different from that seen in higher SES children. Lower SES children may perform tasks using different strategies, potentially building and relying on connections between different neural systems, from their higher SES counterparts. Indeed, presenting lower SES as simply inducing a deficit may be a mischaracterization (see D’Angiulli, Lipina & Olesinska, 2012, for review). In addition, individual variability is an important factor to consider. Some children from low SES homes do demonstrate strong academic skills and positive outcomes commensurate with those of their higher SES peers, indicating resilience to their environment or successful implementation of an alternative strategy. Conversely, not all high SES children show high academic achievement. Thus, contrasts or regressions that collapse across high and low achievers in each group, as in Jednoróg et al. (2012), may mask any significant interactive differences within these samples.
One study explored whether the relationship between behavioral skill and task-related brain activity was different in higher versus lower SES children in the domain of reading. Specifically, Noble, Wolmetz, Ochs, Farah and McCandliss (2006b) examined whether the relationship between phonological awareness and reading activity was modulated by SES (based on parental education, income, and profession) in a group of elementary school children. Importantly, the two SES groups were matched on phonological awareness, though the distribution of scores in both groups was relatively low compared to the population average. Higher SES children showed greater overall activity in brain regions typically thought to support reading, specifically the left fusiform gyrus (particularly involved in orthographic recognition and processing, e.g. Cohen, Lehericy, Chochon, Lemer, Rivaud et al., 2002; Dehaene, Le Clec’H, Poline, Le Bihan & Cohen, 2002; McCandliss, Cohen & Dehaene, 2003) and perisylvian cortex (active in phonological and crossmodal processing, e.g. Calvert, 2001; Hickok & Poeppel, 2007). Moreover, children at the lower end of the SES gradient showed a strong positive relationship between phonological awareness skill and activation in these areas, while children at the higher end did not show a significant relationship. The authors suggested that for higher SES children, rich environmental resources and verbal input might serve to buffer low phonological skill, which could result in their weaker correlations between skill and activity. Without this additional buffer, though, lower SES children’s reading ability may be more tightly tied to such subskills (see also Noble, Farah & McCandliss, 2006a), even in nonphonological areas. This evidence of different relationships between behavioral skill and brain function between SES groups indicates that academic achievement may arise through different neural networks in different groups. However, because both groups showed relatively low scores, this study could not examine whether lower SES children use an alternative network to attain reading success.
The mechanisms by which some lower SES children develop strong reading skills thus remain unknown. High achieving low SES children could use the same neural systems in the same manner as their high achieving high SES compatriots, indicating reliance on the same underlying mechanisms. Alternatively, they could employ divergent strategies, either by drawing on typical regions in an atypical manner, as found by Noble et al. (2013), or by relying on a different set of regions, avoiding use of relatively weak skills or under-stimulated areas (see Brito & Noble, 2014). We investigated a potential interactive relationship between SES and real-word reading skill in white matter across the brain in a sample of typically developing children using diffusion tensor imaging (DTI). DTI allows for examination of the strength or coherence of the connections between individual regions. As reading builds on extended networks across the brain, use of this method can allow for examination of the multi-area systems supporting successful reading in children across different levels of SES. Directly testing for such interactive relationships can help better characterize both SES’s overall influence on the brain, and also better describe its relationship with academic skill outcomes.
Methods
Participants
Participants were 42 (19 F) children, ages 7;9–13; 8 (mean = 10;5 years) recruited from the Chicago metropolitan area. Parents of children were interviewed to ensure that children met the inclusionary criteria of the study. Children were all native English speakers with normal hearing and normal or corrected-to-normal vision. All were right-handed, with no history of attention deficit hyperactivity disorder (ADHD), psychiatric illness, or neurological disorder or damage, and were not taking medication affecting central nervous system function. Informed consent was obtained from participants and their parents, and the Institutional Review Board at Northwestern University approved all procedures.
Parents were asked to complete several initial questionnaires, including reporting the occupation and level of education completed by each parent or guardian. The average education level of both parents was used as the measure of socioeconomic status, given that parental education is more stable than income or occupation, is closely related to parent–child interactions and home learning environment, and is considered to be a stronger predictor of academic achievement than income and occupation (Duncan & Magnuson, 2012; Lewis & Mayes, 2012). SES was used as a continuous variable in all initial analyses; follow-up analyses divided children into lower and higher SES subgroups based on both parents’ education. For these analyses, lower SES was defined as 10–14 years of education for both parents (N = 21, mean = 12.5), and higher SES as 16–18 years (N = 21, mean = 16.5) (see Table S1 for frequency distribution of years of education). No sets of parents with 15 years of education were chosen for this sample to allow for flexibility in continuous or categorical analyses. The lower SES subgroup thus included children whose parents may have finished high school and some post-secondary education, but had not completed four-year college programs; higher SES children’s parents had both at least completed four-year college programs.
Standardized testing
Children first participated in a comprehensive standardized testing session to ensure that all participants were of at least average IQ and reading ability. Tests included the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), using two verbal (vocabulary, similarities) and two performance (block design, matrix reasoning) subtests; the Woodcock-Johnson III Tests of Achievement (Woodcock, McGrew & Mather, 2001), including the word identification subtest; and the Tests of Word Reading Efficiency (TOWRE; Torgesen, Wagner & Raschotte, 1999), including the sight word efficiency subtest. A real-word reading score was calculated from the average of the word identification and sight word efficiency standardized scores. This composite thus captures untimed word identification and fluency, which are both critical for successful reading. All children demonstrated full-scale IQ standardized scores between 81 and 129, and real-word reading standardized scores between 81 and 110.5 (see Table 1 for demographic and standardized test score information). As no participants were statistical outliers, and these scores do not exceed 1.5 standard deviations below the mean, scores between 80 and 85 are taken to indicate low-normal participants.
Table 1.
Demographic characteristics and standardized reading scores, as shown for lower and higher SES subgroup children
| Lower SES | Higher SES | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Mean (SD) |
Range | Mean (SD) |
Range | t-value | |
| Parent education (mean) | 12.5 (1.3) | 10–14 | 16.5 (.68) | 16–18 | 12.73 |
| Age | 10.4 (1.6) | 7.8–13.7 | 10.6 (1.4) | 7.8–12.6 | 0.17 |
| Real-Word Reading score | 95 (7.3) | 81.5–110.5 | 93.2 (6.3) | 84–107 | 0.80 |
| Full-Scale IQ | 104.7 (14.3) | 81–128 | 109.4 (10.5) | 89–129 | 1.21 |
Importantly, the lower and higher SES children selected here did not differ on standardized test performance (real-word reading p = .43; full-scale IQ p = .24), nor on age (p = .86) or gender distribution (chi-squared p = .75). Higher and lower skilled readers were thus distributed across the sampled SES levels, ages, and IQs.
Experimental procedure
Participants were given a standardized test battery and completed a practice MRI session, then completed the experimental MRI sessions.
MRI images were acquired at the Northwestern University Center for Translational Neuroimaging using a 3.0 T Siemens Trio MRI scanner, with a standard 16-channel headcoil. Head position was secured using foam pads. Participants wore sound-attenuating headphones to minimize the effects of the ambient scanner noise. A diffusion-weighted image (echo-planar spin echo imaging) was acquired for each subject (TR = 9512 ms, TE = 89 ms, matrix size = 128 × 128 mm2, field of view = 256 × 256 mm2, slice thickness = 2 mm, b = 1000 s/mm2, 64 non-collinear diffusion-encoding directions, one image b = 0 s/mm2).
Analysis
DTI analysis
All DTI data analysis was performed using FSL software (http://www.fmrib.ox.ac.uk/fsl version 5.0.6). All images were first examined for artifact by creating mean, standard deviation, and signal-to-noise maps using the fslmaths command. Between-volume motion was also inspected; all participants demonstrated run motion < 0.5 mm across the scan, indicating minimal movement less than the size of a voxel. Preprocessing steps for all subjects included eddy current correction, brain extraction (fractional intensity threshold 0.25), and diffusion tensor fitting, using standard FSL parameters. Fractional anisotropy (FA) maps were then calculated for each subject. As participants were at least 8 years of age, their brain sizes were expected to be at least 95% of adult size (Burgund, Kang, Kelly, Buckner, Snyder et al., 2002; Kang, Burgund, Lugar, Petersen & Schlaggar, 2003), allowing for use of an adult-based template for alignment and warping. As such, the adult FMRIB58 1-mm template was used for map normalization, and the FA skeleton generated from this standard template for individual FA map projection and skeletonization.
Tract-based spatial statistics were implemented across the whole brain to determine voxels where FA values were predictive of demographic or behavioral measures. Only voxels with FA greater than 0.25 were included in the analysis (Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols et al., 2006). One regression was run using the randomize tool, which included average parental education for SES, real-word reading score, and their interaction, as well as participant age, as modeled factors. This method allows comparative demonstration of the potential unique effects of each factor while partialling out effects due to simple maturation. Randomize implements Monte Carlo permutation testing to determine significance; all results are reported at n = 5000 iterations, p < .05 corrected for multiple comparisons, k > 5, using the threshold-free cluster enhancement option (Smith et al., 2006). P-values were corrected using the FDR tool available in the FSL package (http://fsl.fmri-b.ox.ac.uk/fsl/fslwiki/FDR).
Significant clusters were defined by the tract and section of which they were a part, e.g. the temporal section of the right inferior longitudinal fasciculus, using the JHU white-matter tractography atlas (Mori, Kaufmann, Davatzikos, Stieltjes, Amodei et al., 2002; Wakana, Caprihan, Panzenboeck, Fallon, Perry et al., 2007). As the posterior (y < −25) sections of the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF) cannot be reliably distinguished at this level of analysis, this area was referred to as the ‘ILFOF’. Participants’ average FAs in significant clusters in a tract section (including multiple significant clusters within a region) were extracted and used in post-hoc correlations with regressor values to describe significant relationships.
Results
The relationships between FA across the whole brain and SES (continuous), real-word reading ability (continuous), and their interaction, controlled for participant age, were examined (see Table 2 for full cluster information). Overlapping and unique clusters between these contrasts were also examined.
Table 2.
Relationships between SES, reading skill, and FA across participants
| Test | Tract | x | y | z | k | |
|---|---|---|---|---|---|---|
| 2A. | SES: | L corticospinal tract | −21 | 7 | 61 | 46 |
| Positive corr. | R anterior IFOF | 31 | 3 | −17 | 17 | |
| 34 | 14 | −14 | 14 | |||
| L SLF | −42 | −30 | 29 | 12 | ||
| L temporal ILF | −14 | −7 | −32 | 11 | ||
| SES: Negative corr. | None | |||||
| 2B. | Reading skill: | L anterior SLF | −35 | −7 | 42 | 123 |
| Positive corr. | −36 | −1 | 35 | 24 | ||
| L corticospinal tract | −18 | −3 | 62 | 117 | ||
| −19 | −22 | 65 | 31 | |||
| −11 | −40 | 52 | 23 | |||
| L anterior IFOF | −24 | 19 | −16 | 52 | ||
| −34 | 24 | −13 | 42 | |||
| −8 | 43 | −18 | 26 | |||
| L ILFOF | −23 | −82 | 8 | 43 | ||
| −8 | −85 | −7 | 35 | |||
| −26 | −77 | 8 | 27 | |||
| R ILFOF | 37 | −41 | −15 | 40 | ||
| R corticospinal tract | 15 | −51 | 63 | 25 | ||
| R posterior SLF | 32 | −30 | 29 | 23 | ||
| 27 | −58 | 42 | 22 | |||
| R anterior IFOF | 34 | 25 | −13 | 20 | ||
| 28 | 20 | −19 | 20 | |||
| R temporal ILF | 48 | −16 | −28 | 20 | ||
| Reading skill: | None | |||||
| Negative corr. | ||||||
| 2C. | SES × Reading: | L medial corticospinal tract | −5 | −15 | 52 | 42 |
| Positive Int. | −17 | 14 | 57 | 8 | ||
| R anterior IFOF | 9 | 39 | 7 | 31 | ||
| 35 | 14 | −14 | 10 | |||
| L anterior SLF | −28 | −4 | 37 | 8 | ||
| L temporal ILF | −26 | −21 | −35 | 8 | ||
| SES × Reading: | R temporal ILF | 19 | −24 | −33 | 99 | |
| Negative Int. | R ILFOF | 41 | −65 | 11 | 10 |
First, there was a main effect of SES on FA. SES was positively predictive of FA in clusters in the left corticospinal tract, right anterior inferior fronto-occipital fasciculus (IFOF), left superior longitudinal fasciculus (SLF), and left temporal inferior longitudinal fasciculus (ILF) (see Table 2A). There were no regions where SES was negatively related to FA. Real-word reading skill was also positively predictive of FA in clusters in several tracts across the brain. Significant areas included clusters in the bilateral SLF, corticospinal tract, anterior IFOF, and posterior ILFOF, and the right temporal ILF (see Table 2B). There were no regions where reading skill was negatively related to FA. Two clusters (in the left corticospinal tract and right anterior IFOF) were significant in both main effect analyses; no other voxels were significant in both.
Importantly, some of these main effects may be qualified by the interaction of SES and real-word reading. There were significant positive interactions between SES and reading skill in clusters in the medial part of the left corticospinal tract, anterior SLF, and temporal ILF, and right anterior IFOF. In addition, there were significant negative interactions in clusters in the right temporal ILF and posterior ILFOF (see Table 2C for cluster information). Cohen’s f2 was then calculated to determine the size of the relationship between the interaction of SES and reading skill, and FA in each of the tract clusters with significant interaction results (left medial corticospinal tract: 0.437; left anterior SLF: 0.03; left temporal ILF: 0.167; right anterior IFOF: 0.245; right temporal ILF: 0.205; right posterior ILFOF: 0.142). These interaction results overlapped with the SES main effect clusters in the left corticospinal tract, right anterior IFOF, and left temporal ILF (see Figure 1). Thus, the only result unique to the main effect of SES was a left posterior SLF cluster. Further, the clusters in the left corticospinal tract, anterior SLF, and right temporal ILF and ILFOF overlapped with the reading skill main effect results, though the rest of the skill main effects were unique.
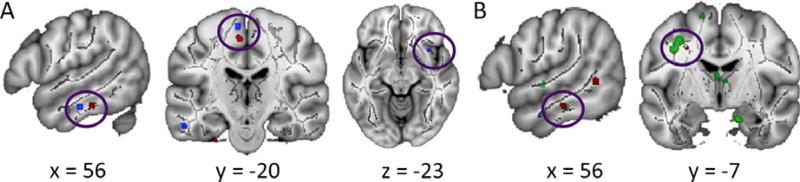
Figure 1.
Socioeconomic status and reading skill main effects with socioeconomic status by reading skill interaction effects. (a) Main effect of SES shown in blue, interaction effect in red, clusters significant for both analyses shown in purple. Areas, left to right, include the left temporal inferior longitudinal fasciculus (ILF), left corticospinal tract, and right anterior inferior fronto-occipital fasciculus (IFOF). (b) Main effect of reading skill shown in green, interaction effect in red, clusters significant for both analyses shown in brown. Areas, left to right, include the right temporal inferior longitudinal fasciculus (ILF) and left anterior superior longitudinal fasciculus (SLF).
Within-SES subgroup analyses
To determine whether these interactive effects were reflective of significant within-group relationships, follow-up analyses examined the relationship between reading skill and FA within each lower and higher SES subgroup. Whole-brain analyses using real-word reading skill as a variable of interest were performed within each subgroup. These results were then compared with the results of the whole-group interaction to determine overlapping and unique clusters between SES subgroups across analyses (see Figure 2 for Venn diagram of unique and overlapping clusters).
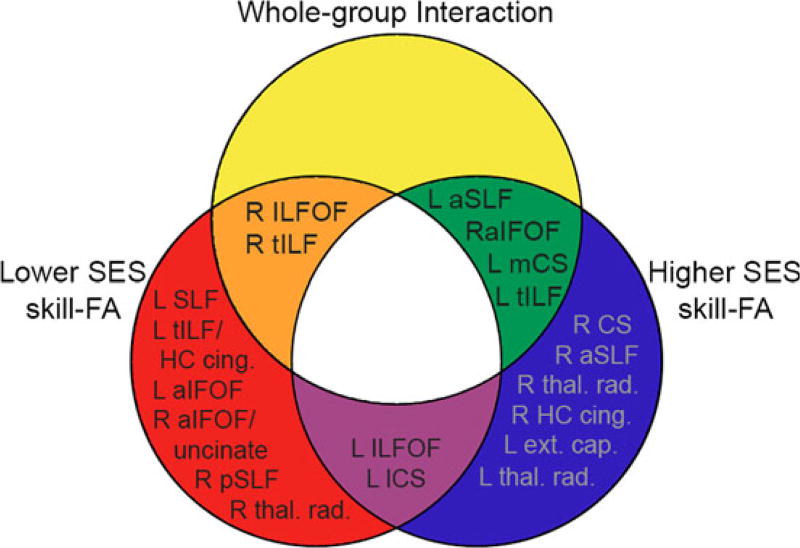
Figure 2.
Relationships between clusters significant for whole-group interaction, lower SES skill–FA correlation, and higher SES skill–FA correlation. There were overlapping and unique regions between the whole-group interaction and within-subgroup skill–FA analyses. Clusters significant in the interaction and lower SES subgroup listed in orange section (right ILFOF, right temporal ILF); clusters significant in the interaction and higher SES subgroup listed in green section (left anterior SLF, left medial corticospinal tract (mCS), left temporal ILF, right anterior IFOF). Clusters significant in only the lower SES subgroup listed in red section (left SLF, left temporal ILF/hippocampal cingulum, left anterior IFOF, right anterior IFOF/uncinate fasciculus, right parietal SLF, right thalamic radiation); clusters significant in only higher SES subgroup listed in blue section (right corticospinal tract (CS), right anterior SLF, right thalamic radiation, right hippocampal cingulum, left external capsule, left thalamic radiation); clusters significant in both SES subgroup analyses but not in whole-group interaction listed in purple section (left ILFOF, left lateral corticospinal (lCS) tract).
In the lower SES subgroup, there was a significant positive reading skill–FA relationship in clusters in the right temporal ILF, left temporal ILF and hippocampal cingulum, bilateral anterior IFOF and right uncinate fasciculus, bilateral ILFOF, left SLF, bilateral parietal SLF, and right thalamic radiations. No significant negative relationships were found (see Table 3A for full cluster information). The right temporal ILF and ILFOF clusters overlapped with the results of the whole-group interaction.
Table 3.
Relationships between reading skill and FA within each SES subgroup
| Test | Tract | x | y | z | k | |
|---|---|---|---|---|---|---|
| 3A. | Lower SES: | L corticospinal tract | −19 | −4 | 62 | 137 |
| Positive corr. | R temporal ILF | 21 | −20 | −33 | 60 | |
| L anterior IFOF, uncinate | −24 | 18 | −18 | 58 | ||
| −35 | 29 | −7 | 51 | |||
| −12 | 21 | −8 | 17 | |||
| −34 | 25 | −12 | 15 | |||
| L ILFOF | −13 | −89 | −3 | 33 | ||
| R ILFOF | 40 | −48 | −16 | 32 | ||
| R anterior IFOF, uncinate | 12 | 23 | −9 | 29 | ||
| 35 | 25 | −12 | 27 | |||
| 42 | 26 | −5 | 19 | |||
| 24 | 19 | −23 | 19 | |||
| 42 | 34 | 1 | 18 | |||
| R uncinate fasciculus | 15 | 18 | −9 | 17 | ||
| 9 | 23 | −13 | 16 | |||
| R parietal SLF | 43 | −53 | 39 | |||
| L temporal ILF | −26 | −9 | −33 | 12 | ||
| Hippocampal cingulum | −42 | −26 | −29 | 21 | ||
| −34 | −28 | −33 | 19 | |||
| −51 | −36 | 10 | 17 | |||
| L parietal SLF | −28 | −60 | 41 | 24 | ||
| R thalamic radiations | 3 | −3 | 8 | 19 | ||
| L anterior SLF | −36 | 9 | 45 | 15 | ||
| Lower SES: | None | |||||
| Negative corr. | ||||||
| 3B. | Higher SES: | L corticospinal tract | −29 | −24 | 46 | 67 |
| Positive corr. | −5 | −16 | 52 | 12 | ||
| −30 | 0 | 39 | 11 | |||
| L anterior SLF | −36 | −2 | 34 | 47 | ||
| −32 | 1 | 20 | 17 | |||
| R anterior IFOF, uncinate | 17 | 28 | −20 | 32 | ||
| 23 | 20 | −16 | 14 | |||
| R SLF | 35 | −17 | 35 | 30 | ||
| 51 | −24 | 25 | 19 | |||
| L ILFOF | −24 | −82 | 6 | 29 | ||
| −17 | −71 | 13 | 13 | |||
| R hippocampal cingulum | 15 | −6 | −23 | 18 | ||
| R corticospinal tract | 11 | 5 | 59 | 16 | ||
| L temporal ILF | −56 | −12 | −19 | 16 | ||
| −25 | −19 | −33 | 12 | |||
| L external capsule | −32 | 4 | 6 | 13 | ||
| R thalamic radiations | 11 | −29 | 14 | 11 | ||
| L thalamic radiations | −2 | −8 | 13 | 10 | ||
| Higher SES: | None | |||||
| Negative corr. |
In the higher SES subgroup, there were significant positive relationships between reading skill and FA in clusters in the bilateral corticospinal tract, bilateral anterior SLF, bilateral thalamic radiation, right anterior IFOF, right hippocampal cingulum, left temporal ILF, left ILFOF, and left external capsule (see Table 3B for full cluster information). The left medial corticospinal, anterior SLF, temporal ILF, and right anterior IFOF clusters overlapped with the whole-group interaction results. The left ILFOF cluster was common to the two SES groups, but not the interaction results: higher and lower SES children both demonstrated positive skill–FA relationships in this cluster, and so there was no difference in skill–FA relationship between the groups in this area.
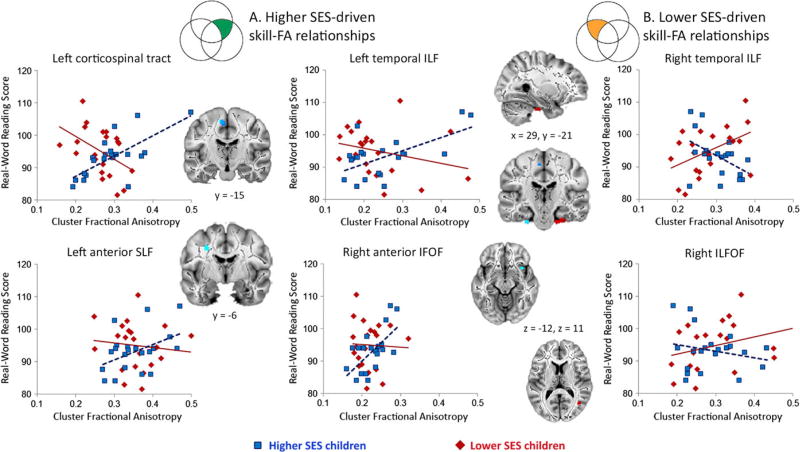
Participants’ mean FAs were then extracted from tract clusters which were significant in at least one of the SES subgroup analyses and overlapped with the whole-group interaction (i.e. clusters in the bilateral temporal ILF, right ILFOF, right anterior IFOF, left medial corticospinal tract, and left anterior SLF), and scatterplots between FA and reading skill were visualized for descriptive purposes (see Figure 3). In the left hemisphere regions and the right anterior IFOF, scatterplots demonstrated positive skill–FA relationships for higher SES children, but no relationships for lower SES children. In the right temporal ILF and ILFOF, the relationship was reversed, i.e. scatterplots demonstrated positive skill–FA relationships for lower SES children. As such, the interactions found to be significant across the whole group were driven by opposite skill–FA relationships between the two groups.
Figure 3.
Interaction between socioeconomic status group and reading skill for FA. FA was extracted from clusters significant in the whole-group interaction analysis and in one SES subgroup analysis (e.g. in green and orange sections of Figure 2) and plotted against reading skill for visualization purposes. (A) In the left medial corticospinal tract, left anterior SLF, left temporal ILF, and right anterior IFOF, higher reading skill was related to higher FA in higher SES children (blue squares), but was related to lower FA, or was not significantly related to FA, in lower SES children (red diamonds). (B) In the right temporal ILF and right ILFOF, higher reading skill was instead related to higher FA in lower SES children, but lower FA in higher SES subjects.
Discussion
The goal of the present study was to examine the neural systems supporting better reading skill at different SES levels. While previous work has examined individual differences in skill and SES separately, especially in the gray matter of the brain, to our knowledge no research examining white matter has directly tested for such an interactive effect. We here investigated an interactive relationship between SES and reading skill in white matter fractional anisotropy across the brain.
To this end, we demonstrated an interaction between SES and reading skill in clusters within several tracts, including the bilateral temporal ILF, right ILFOF, right anterior IFOF, left corticospinal tract, and left anterior SLF. Importantly, several of these clusters overlap with those showing significant main effects for SES, suggesting that the SES effects are qualified by the interaction between SES and skill. Independent analyses conducted within each SES subgroup confirmed significant skill–FA relationships in these areas. Lower SES children demonstrated positive relationships between skill and FA in the right hemisphere clusters, meaning that higher FA (i.e. white matter coherence, Pierpaoli & Basser, 1996) in these areas was related to better reading. In contrast, higher SES children showed positive relationships between skill and FA particularly in left-sided clusters and the right anterior IFOF.
Only one previous study has directly compared white matter coherence in higher and lower SES children, and reported no group differences (Jednoróg et al., 2012). However, this study’s design differed from ours in two important ways. First, the sample used demonstrated a significant correlation between SES and literacy score, while we specifically determined that SES and reading skill were independent in our sample. Second, we examined interactive effects between SES and skill, while Jednoróg et al. (2012) focused on main effects. Thus, the group differences we find are attributable to the interactive SES subgroup–reading skill relationships.
We found additional significant relationships between reading skill and fractional anisotropy. Clusters in the left corticospinal tract and left SLF overlapped with areas showing a significant skill–FA interaction across the whole group, but the other clusters were unique, suggesting that skill effects cannot entirely be captured by their interaction with SES. Our findings are consistent with past research reporting that white matter structure was significantly related to reading skill in multiple tracts, including the SLF, ILF, IFOF, and corticospinal tract (see Vandermosten, Boets, Wouters & Ghesquiere, 2012b, for review); coherence in these tracts has been previously shown to increase with both age and reading skill (Wandell & Yeatman, 2013; Yeatman, Dougherty, Ben-Shachar & Wandell, 2012). Our results show that these relationships between reading skill and white matter structure are impactful beyond socioeconomic status.
Connectivity along the ILF and IFOF has previously been linked to visual perception and recognition (Ffytche, 2008; Ross, 2008), and in the left hemisphere specifically to orthographic processing skill (Epelbaum, Pinel, Gaillard, Delmaire, Perrin et al., 2008; Vandermosten, Boets, Poelmans, Sunaert et al., 2012a; Vandermosten et al., 2012b; Wandell & Yeatman, 2013). Each of these tracts begins in the occipital lobe and progresses forward, with the ILF including endpoints in the temporal lobe and the IFOF in the inferior frontal lobe, thus connecting regions involved in simple visuospatial responsivities and grapheme and bigram processing. As such, higher reading achievement in lower SES children may be disproportionately reliant on the occipital-based right-sided visuospatial processing supported by these tracts. Indeed, it may be adopted as a supplementary mechanism, in addition to use of the typical reading-related tracts shown in the whole-group reading skill main effect. In contrast, higher SES children may not require such an alternative strategy supported by these tracts, and instead simply use the canonical posterior left hemisphere tracts and left and right anterior tracts.
One reason for such SES differences in relating skill to white matter may be the relative emphases of the instructional strategies. For example, lower SES parents are more likely than their higher SES counterparts to endorse instruction of specific literacy skills which emphasize visual letter features and to value decoding activities to a greater extent than higher SES parents (Lynch, Anderson, Anderson & Shapiro, 2006; Stipek, Milburn, Clements & Daniels, 1992). In contrast, higher SES parents may take a more holistic approach to literacy, where children are exposed to written language in a variety of ways (Lynch et al., 2006). In addition, higher versus lower SES schools could differ in instructional emphases, influencing reading strategies: for example, Duke (2000) noted that first-grade classrooms in high SES school districts had significantly greater levels of print exposure (e.g. larger classroom libraries, more classroom print, more activities involving print), which has been clearly tied to reading skill (Cipielewski & Stanovich, 1992), than classrooms in low SES districts. These environmental differences could lead lower SES children to continue to rely on visuospatial letters features for reading, and higher SES children to transition to verbal skills.
Differential environmental exposure to visuospatial versus verbal information may also influence reading behavior. There are larger SES differences in children’s verbal than visuospatial processing (Noble, McCandliss & Farah, 2007). Higher SES children are more likely to have richer verbal experience and input than their lower SES peers (Hart & Risley, 1995; Levine, Ratliff, Huttenlocher & Cannon, 2012). In contrast, the few studies examining group differences in exposure to visuospatial stimulation, such as puzzle play, did not report significant differences according to parental education (Levine et al., 2012). Some did find differences in parental visuospatial language input (Dearing, Casey, Ganley, Tillinger, Laski et al., 2012), but this result may be more reflective of the established group verbal input differences. Thus, lower SES children may disproportionately draw on their visuospatial skills to achieve strong reading outcomes, an interpretation consistent with the findings of Noble et al. (2006a).
SES differences in the nature of verbal input may also underlie the degree of neural lateralization for linguistic processing. Behavioral work using dichotic listening paradigms has demonstrated that lower SES children may not develop strong left hemispheric asymmetries for linguistic information, but may instead show bilateral processing (Boles, 2011; Cai, Lavidor, Brysbaert, Paulignan & Nazir, 2008; Raizada et al., 2008). In the context of our study, lessened verbal input may be related to the association of white matter with skill in the right hemisphere tracts for the lower SES children.
Our findings for the lower SES children are also consistent with studies examining skill differences in reading disabilities. Individuals with dyslexia who develop increased reading proficiency often show a compensatory pattern of increased right hemisphere activity in both anterior (inferior frontal) and posterior (perisylvian, fusiform) regions (Eden, Jones, Cappell, Gareau, Wood et al., 2004; Shaywitz, Shaywitz, Pugh, Mencl, Fulbright et al., 2002). In the present study, the positive relationship found between reading skill and FA in right posterior visuospatial/orthographic fasciculi for higher achieving lower SES children may thus reflect a common strategy adopted in the face of lessened verbal stimulation or decreased verbal processing. However, we note that lower SES should not be understood as a reading impairment itself, but instead a socio-environmental situation which may encourage the development of alternative strategies for success.
This demonstration of visuospatial versus verbal brain system use in lower versus higher SES individuals is also consistent with work from functional neuroimaging of arithmetic processing. Demir, Prado and Booth (2015) demonstrated that lower SES children (based on parental education) showed a positive relationship between math skill and right superior parietal activity during subtraction problem solving, reflecting visuospatial processing; in contrast, higher SES children showed the opposite pattern. The greater reliance on visuospatial rather than verbal brain regions in the lower SES children was interpreted as being due to differences in parental verbal input. Greater engagement of visuospatial mechanisms may therefore be a consistent strategy for higher academic achievement in low SES children across domains.
The present sample of children includes a broad range of ages. Prior work has demonstrated that FA tends to increase with age in most of the brain. SES was not related to participant age in our sample, which should ensure that results from the between-subjects comparisons and the interaction analyses are independent from developmental changes. Further, our inclusion of age as a covariate also allowed us to directly partial out potential variance in FA associated with maturation. As such, the interactive effects found are not likely to be developmentally based or biased.
Parental education was used as a proxy for socioeconomic status in our sample. Previous works have varied in their use of maternal (Stevens et al., 2009), primary caregiver (Kishiyama, Boyce, Jimenez, Perry & Knight, 2009), and average parental (Noble et al., 2006a) educational attainment for measurement of this aspect of SES. We defined SES based on both parents’ educational attainment; as such, participant subgroup membership would not change if reports from only one parent were used instead. By our definitions, lower SES parents had at most two years of post-secondary education, while higher SES parents had completed at least four years of college. While there may be further differences between parents who were able to attain some post-secondary schooling and those who had at most only a high school education, these differences may be more qualitative than quantitative (Duncan & Magnuson, 2003) and we did not have enough lower SES children in this sample to further separate these potential subgroups. As such, the ‘lower’ SES participants may not completely reflect ‘low’ SES children, but are still significantly lower than the higher SES children. Further, using a relatively restricted range enabled us to examine the effect of normal SES variation without the confounding influences associated with extremely low SES, such as high stress and poor nutrition (Bradley & Corwyn, 2002). The differences found in our sample indicate that even normative variance in SES is important for academic skills and brain development.
While parental education is only one dimension of SES, it is strongly related to the richness of children’s verbal input and thus may be particularly impactful on children’s literacy development; in contrast, parental income or environmental stress may have a greater impact on social, emotional, or executive function skill development (Brito & Noble, 2014; Duncan & Magnuson, 2012; Noble et al., 2015). Furthermore, parental education is temporally a more stable measure and has stronger effects on academic development than parental occupation and income (Duncan & Magnuson, 2012; Gottfried, Gottfried, Bathurst, Guerin & Parramore, 2003; Noble et al., 2007). The use of parental education in the current study is thus appropriate as we investigated the neural systems supporting reading, a linguistic academic skill.
Parental IQ may also impact children’s reading scores. IQ is in part heritable (Bouchard, Lykken, McGue, Segal & Tellegen, 1990), though potentially less so in lower SES environments (Turkheimer, Haley, Waldron, D’Onofrio & Gottesman, 2003), and may influence both parents’ and children’s educational achievement (Walberg, 1984). However, our lower and higher SES groups were matched on IQ but differed in level of parental education achieved. As such, our results are independent of participant IQ; parental IQ may have some indirect effects, but these cannot be parsed apart through our study design.
Our results contribute to a newly emerging literature suggesting that SES effects on brain structure and function might not simply be main effects (e.g. D’Angiulli et al., 2012). Instead, depending on their environmental experiences, children might rely on different neural systems to succeed on academic tasks. Thus, our results provide information about the potential mechanisms by which some lower SES children develop strong reading skills. Given their environmental conditions, lower SES children may use both typical left hemisphere language and orthographic tracts for reading achievement, but especially draw on the right homologues of these visuospatial connections. In higher SES children, such a strategy may not be necessary (and indeed, reliance on these posterior right hemisphere tract areas is more indicative of poorer than better reading), and so they use the canonical left verbal tracts. The current paper focused on single word reading skill; the long-term implications of these early differences on later reading skill and for more complex reading tasks remains an open question for additional correlational work and future experimental manipulations.
In summary, our study is the first to demonstrate unique relationships between academic skill and white matter fractional anisotropy in higher versus lower socioeconomic status children. We showed that higher reading ability is supported by some common tracts across SES levels, but may also involve unique regions for each subgroup, with lower SES children relying more on a visuospatial network for reading. Given the effects of socioeconomic status on academic outcomes, the identification of neural systems supporting individual and group differences in reading achievement is important for the development effective instructional strategies. The identification of brain structure and function underlying skill in lower versus higher SES children could potentially lead to different educational techniques that emphasize visuospatial versus verbal strategies.
Supplementary Material
Research highlights.
Socioeconomic status has a significant impact on academic achievement, including reading.
We investigated whether the relation of reading skill to white matter depends on socioeconomic status.
For lower SES children, higher reading skill was correlated with white matter in right hemisphere visuospatial tracts.
Lower SES children may rely more on visuospatial orthographic processing strategies for reading success.
Acknowledgments
This research was supported by National Institute of Child Health and Human Development Grant (grant number HD042049 to JRB). The first author was supported by a Ruth L. Kirschstein NRSA Institutional Research T32 Training Grant from the National Institute on Deafness and Other Communication Disorders (grant number T32 DC009399-01A10).
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article:
Table S1. Frequencies of years of parental education, averaged between parents, of children included in sample.
References
- Boles DB. Socioeconomic status, a forgotten variable in lateralization development. Brain and Cognition. 2011;76(1):52–57. doi: 10.1016/j.bandc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Lykken DT, McGue M, Segal NL, Tellegen A. Sources of human psychological differences: the Minnesota study of twins reared apart. Science. 1990;250(4978):223–228. doi: 10.1126/science.2218526. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brito NH, Noble KG. Socioeconomic status and structural brain development. Frontiers in Neuroscience. 2014;8:276. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The Future of Children. 1997;7(2):55–71. [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/Nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cai Q, Lavidor M, Brysbaert M, Paulignan Y, Nazir TA. Cerebral lateralization of frontal lobe language processes and lateralization of the posterior visual word processing system. Journal of Cognitive Neuroscience. 2008;20(4):672–681. doi: 10.1162/jocn.2008.20043. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cerebral Cortex. 2001;11(12):1110–1123. doi: 10.1093/Cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, et al. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage. 2011;54(3):2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipielewski J, Stanovich KE. Predicting growth in reading ability from children’s exposure to print. Journal of Experimental Child Psychology. 1992;54(1):74–89. doi: 10.1016/0022-0965(92)90018-2. [DOI] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, et al. Language-specific tuning of visual cortex functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cooper H, Borman G, Fairchild R. School calendars and academic achievement. In: Meece JL, Eccles JS, editors. Handbook of research on schools, schooling and human development. 1. New York: Routledge; 2010. pp. 342–355. [Google Scholar]
- D’Angiulli A, Lipina SJ, Olesinska A. Explicit and implicit issues in the developmental cognitive neuroscience of social inequality. Frontiers in Human Neuroscience. 2012;6:254. doi: 10.3389/fnhum.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing E, Casey BM, Ganley CM, Tillinger M, Laski E, et al. Young girls’ arithmetic and spatial skills: the distal and proximal roles of family socioeconomics and home learning experiences. Early Childhood Research Quarterly. 2012;27:458–470. doi: 10.1016/j.ecresq.2012.01.002. [DOI] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. NeuroReport. 2002;13(3):321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Demir OE, Prado J, Booth JR. Parental socioeconomic status and the neural basis of arithmetic: differential relations to verbal and visuo-spatial representations. Developmental Science. 2015;18(5):799–814. doi: 10.1111/desc.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke NK. For the rich it’s richer: print experiences and environments offered to children in very low- and very high-socioeconomic status first-grade classrooms. American Educational Research Journal. 2000;37(2):441–478. doi: 10.3102/00028312037002441. [DOI] [Google Scholar]
- Duncan GJ, Magnuson K. Off with Hollingshead: socioeconomic resources, parenting, and child development. In: Bornstein M, Bradley R, editors. Socioeconomic status, parenting, and child development. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 83–106. [Google Scholar]
- Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):377–386. doi: 10.1002/Wcs.1176. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, et al. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, et al. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ffytche DH. The hodology of hallucinations. Cortex. 2008;44(8):1067–1083. doi: 10.1016/j.cortex.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cerebral Cortex. 2013;23(9):2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried AW, Gottfried AE, Bathurst K, Guerin DW, Parramore M. Socioeconomic status in children’s development and family environment: infancy through adolescence. In: Bornstein M, Bradley R, editors. Socioeconomic status, parenting, and child development. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 189–207. [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. Plos ONE. 2011;6(5) doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DGG, Shi F, Gilmore JH, et al. Family poverty affects the rate of human infant brain growth. Plos ONE. 2013;8(12):e80954. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley RT. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Paul H. Brookes; 1995. [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/Nrn2113. [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, et al. The influence of socioeconomic status on children’s brain structure. Plos ONE. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19(1):16–28. doi: 10.1016/S1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16(5):641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SC, Ratliff KR, Huttenlocher J, Cannon J. Early puzzle play: a predictor of preschoolers’ spatial transformation skill. Developmental Psychology. 2012;48(2):530–542. doi: 10.1037/a0025913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Mayes LC. The role of environment in development: an introduction. In: Mayes LC, Lewis M, editors. The Cambridge Handbook of Environment in Human Development. Cambridge, UK: Cambridge University Press; 2012. p. 112. [Google Scholar]
- Lynch J, Anderson J, Anderson A, Shapiro J. Parents’ beliefs about young children’s literacy development and parents’ literacy behaviors. Reading Psychology. 2006;27:1–20. doi: 10.1080/02702710500468708. [DOI] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/S1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, et al. Imaging cortical association using diffusion-tensor-based tracts in the human brain axonal tracking. Magnetic Resonance in Medicine. 2002;47(2):215–223. doi: 10.1002/Mrm.10074. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Sheridan MA. Lessons from neuroscience research for understanding causal links between family and neighborhood characteristics and educational outcomes. In: Duncan GJ, Murnane RJ, editors. Whither opportunity. New York: Russell Sage; 2011. pp. 27–46. [Google Scholar]
- Noble KG, Farah MJ, McCandliss BD. Socioeconomic background modulates cognition–achievement relationships in reading. Cognitive Development. 2006a;21(3):349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, et al. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15(4):516–527. doi: 10.1111/j.1467-687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science. 2013;16(5):653–664. doi: 10.1111/Desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science. 2006b;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Pakulak E, Sanders L, Paulsen DJ, Neville H. Semantic and syntactic processing in children from different familial socioeconomic status as indexed by ERPs; Poster presented at the 12th Annual Cognitive Neuroscience Society Meeting; 10–12 April; New York. 2005. [Google Scholar]
- Panel NMA. Foundations for success: The final report of the National Mathematics Advisory Panel. Washington, DC: US Department of Education; 2008. [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Human Brain Mapping. 2011;32(2):282–289. doi: 10.1002/Hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, et al. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Raizada RDS, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage. 2008;40(3):1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED. Sensory-specific amnesia and hypoemotionality in humans and monkeys: gateway for developing a hodology of memory. Cortex. 2008;44(8):1010–1022. doi: 10.1016/j.cortex.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. doi:S0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/J.Neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Statistics, N.C.f.E. The Nation’s Report Card: Mathematics 2011. Washington, DC: 2011. [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipek D, Milburn S, Clements D, Daniels D. Parents’ beliefs about appropriate education for young children. Journal of Applied Developmental Psychology. 1992;13(3):293–310. [Google Scholar]
- Tomalski P, Johnson MH. The effects of early adversity on the adult and developing brain. Current Opinion in Psychiatry. 2010;23(3):233–238. doi: 10.1097/Yco.0-b013e3283387a8c. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Raschotte CA. TOWRE: Test of word reading efficiency. Austin, TX: PROED; 1999. [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, et al. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012a;135:935–948. doi: 10.1093/Brain/Awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012b;36(6):1532–1552. doi: 10.1016/J.Neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/J.Neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg HJ. Improving the productivity of America’s schools. Educational Leadership. 1984;41(8):19–27. [Google Scholar]
- Wandell BA, Yeatman JD. Biological development of reading circuits. Current Opinion in Neurobiology. 2013;23(2):261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. doi:10/1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.