Abstract
Breast cancer (BC) is the second most common cause of cancer-related deaths in women worldwide. The availability of reliable biomarkers of response/resistance to cancer treatments would benefit patients and clinicians allowing for a better selection of BC patients most likely to respond to a specific treatment. Phosphatidylinositol 3-kinase (PI3K) enzymes are involved in numerous cellular- functions and processes. The gene encoding for PI3K catalytic subunit p110α is mutated in 20-40% of BC. We performed a meta-analysis of the current literature on randomized clinical trials, investigating the role of PIK3CA mutational status as prognostic factor and predictor of response to anti-cancer treatments. Overall 1929 cases were included. The pooled analysis confirmed that the presence of a PIK3CA mutation represents an independent negative prognostic factor (HR = 1.67, 95% CI: 1.15-2.43; p = 0.007) in BC, as previously reported. Since PI3K signalling is also a result of other pathways’ hyperactivation, further investigation of potential biomarkers able to predict likelihood of response to anti-PI3K/mTOR, anti-HER2 and other TKRs is warranted in future randomized clinical trials. This article is protected by copyright. All rights reserved
Keywords: Breast Cancer, Meta-analysis, PIK3CA, Prognostic Factor, Anti-cancer treatment response
1. Introduction
In the new era of personalised medicine, breast cancer (BC) patients are routinely offered a “molecular diagnosis” in order to allow for tailored treatments that can potentially improve their survival outcomes. These patients have benefited from major scientific and medical advances, and the fact that new targeted drugs labels now include pharmacogenomics information constitutes evidence of it. In this scenario, predictors of response to targeted therapies are needed to select the patients that are more likely to respond and monitor the therapeutic benefit in real-time.
Phosphatidylinositol 3-kinase (PI3K) proteins are a family of highly conserved enzymes involved in regulating important cellular processes, such as protein synthesis, metabolism, cell survival, proliferation, motility, intracellular trafficking, angiogenesis and apoptosis. There are three different classes of PI3K1. The PI3K class 1 is a heterodimer composed of a regulatory and a catalytic subunit. This class is divided into Subclasses 1A and 1B on the basis of functional and structural biochemical differences2. Class 1A heterodimer contains a p110 catalytic subunit isoform and a p85 regulatory subunit isoform. Class 1B has a similar structure and function, but lacks the p85-binding domain 3. Under normal physiological conditions, activation of PI3K Class IA requires coupling to growth factor receptor tyrosine kinases (RTKs), including members of the human epidermal growth factor receptor (HER) family and insulin-like growth factor 1 (IGF-1) receptor 4. On the other hand, activation of Class IB depends on the interaction with G protein-coupled receptors (GPCRs) 5,6.
PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5- triphosphate (PIP3). Accumulation of PIP3 at the plasma membrane results in the recruitment of both AKT, a downstream serine/threonine kinase, and phosphoinositide-dependent kinase 1 (PDK1), an essential enzyme for the phosphorylation of AKT at Ser 308. Once phosphorylated, AKT interacts with many different effectors, including the TSC complex, constitutive inhibitor of mTORC1 7,8, thereby regulating RNA translation, cell growth, autophagy and protein synthesis 9. A suppressor of this pathway is the tumour suppressor protein phosphatase and tensin homolog (PTEN), which catalyses the de-phosphorylation of PIP3 to PIP2. Cellular PIP3 levels depend therefore on the competition between PI3K and PTEN 10,11.
The PIK3CA gene encodes the PI3K catalytic subunit p110α, which is often mutated or amplified in human cancers, including BC 12,13. Since PIK3CA is mutated in 20-40% of BC 14,15, we performed a meta-analysis of the current literature, investigating the role of PIK3CA mutational status as a prognostic factor and a predictor of response to anti-cancer treatments.
2. Material and Methods
The studies were identified according to the following inclusion criteria: 1) participants with BC; 2) outcome results expressed in relation to the presence of a PIK3CA mutation; 3) a primary outcome (disease free survival, overall survival or progression free survival) expressed as hazard ratio (HR). The following exclusion criteria were used: 1) insufficient data available to estimate outcomes; 2) animal studies; 3) size of each study arm less than 10 participants.
The summary estimates were generated using a fixed-effect model (Mantel–Haenszel method) 16 or a random-effect model (DerSimonian–Laird-method) 17 depending on the absence or presence of heterogeneity (I2). A subgroup analysis was performed to highlight any differences between studies in terms of Overall Survival (OS), Disease Free Survival (DFS), Progression Free Survival (PFS), as summarized in table 1.
Table 1.
Characteristics of the analysed trials.
| Study | Subtype | Reference |
|---|---|---|
| 240 HER2+ early stage BC patients receiving adjuvant treatment were assessed before taking anti-HER2 trastuzumab therapy. | HER2+ | Jensen JD et al., 2011 |
| 292 invasive BC patients treated with adjuvant therapy or chemotherapy were assessed. | Of the overall patients 68% were ER+; 57% were PR+ ; and 18% were HER2 +. | Lopez-Knowles E. et al., 2009 |
| 452 unilateral invasive primary BC patients were assessed. Adjuvant therapy was given to 366 patients, which consisted of chemotherapy alone in n = 94, hormone therapy alone in n = 177, and both treatments in n = 95. None of the HER2+ patients received anti-HER2 trastuzumab therapy. |
Of the overall patients 12% were HR+ (ER+ or PR+ or both) ERBB2+; 63% were HR + (ER+ or PR + or both) ERBB2 -; 11% were HR - (ER- and PR-) ERBB2 +; and 14% were HR- (ER- and PR-) ERBB2 - . | Cizkova M. et al., 2012 |
| 188 primary BC were assessed. One hundred patients received adjuvant hormonal therapy. Forty-seven patients received the combination of chemotherapy and hormonal therapy. Fifty-six patients developed metastases. | All subtypes | Maruyama N. et al., 2007 |
| 446 BC samples collected before radio- or chemotherapy were analyzed. Three hundred sixty-one patients received adjuvant therapy, 20% consisting of chemotherapy alone, 39% consisting of hormone therapy alone, and 22% both treatments. One hundred and sixty- four patients developed distant metastases. | Of the overall patients 15% were HR- ERBB2-; 9% were HR-ERBB2+; 64% were HR+ ERBB2-; and 12% were HR+ ERBB2+. | Tury S. et al., 2016 |
| 55 BC patients HER2+ with metastasis were analyzed. The following treatments were given to patients: anti-ERBB2 therapy monotherapy (5%) or anti-HER2 trastuzumab therapy plus taxane (15%), or vinorelbine (10%), or other chemotherapy (7%). | Of the overall patients 62% were HER2+. From the overall patients 25% were ER+ and 34% were ER-. | Berns K. et al., 2007 |
| 256 metastatic breast cancer patients HER2+ treated with anti-HER2 trastuzumab or lapatinib treated were analyzed. | HER2+ | Razis E. et al., 2011 |
When we used the keywords “PIK3CA mutations in early breast cancer”, “PIK3CA mutations in metastatic breast cancer”, “PIK3CA impact in breast cancer”, the PubMed search yielded 133 potentially relevant articles; 75 studies were excluded, as duplicates. After viewing the titles and abstracts of the 58 remaining studies, the full texts of 30 studies were retrieved and 7 studies 13,18–23 were included in the analysis (table 1).
3. Results and discussion
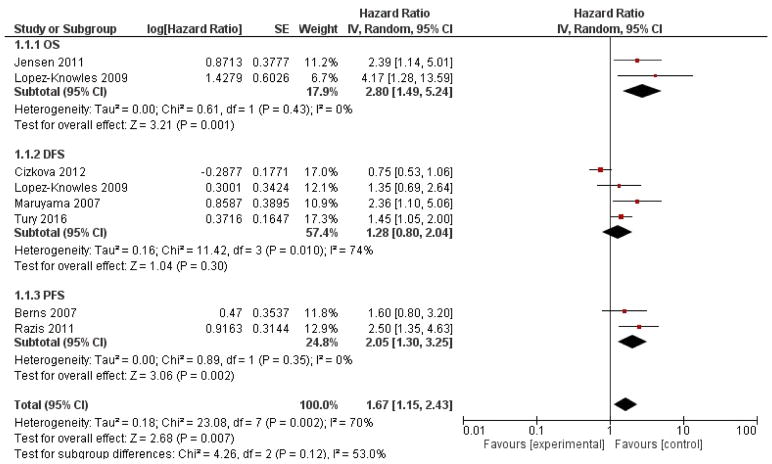
A total of 1929 cases were included. BC patients were treated with adjuvant chemotherapy (such as docetaxel, cyclophosphamide, methotrexate, fluorouracil, epirubicin, vinorelbine), anti-HER2 (trastuzumab or lapatinib), endocrine therapy (such as goserelin, tamoxifen), or a combination of these treatments, including a surgical component in some cases (table 1). The pooled analysis revealed that the presence of a PIK3CA mutation is a negative prognostic factor (HR = 1.67, 95% CI: 1.15-2.43; p = 0.007, figure 1) in BC. The analysis was performed using a random-effects model due to the high heterogeneity (I2=70%).
Figure 1.
Forest plots of hazard ratios (HRs) according PIK3CA mutation in breast cancer.
The PI3K/AKT/mTOR pathway is one of the most commonly dysregulated pathways in patients with BC. Our meta-analysis evaluates the impact that mutations of PIK3CA have over prognosis of patients in different clinical settings. The most common point mutations in this gene occur at the p110α cluster around 2 hotspots: E542/5 (exon 9) in the helical domain, and H1047 (exon 20), close to the catalytic domain. Such mutations result in amino acid substitutions (E545K, E542K, and H1047R) 12, ultimately increasing the PI3K holoenzyme activity 24 and resulting in constitutive AKT activity 24,25.
Due to the complexity of this signalling pathway, targeting PI3K is challenging. While pan-PI3K inhibition is often plagued by high toxicity 26, targeting only one of the multiple PI3K isoforms could eventuate in parallel activation of other signalling pathways and ultimately lead to drug resistance 27–30. Both pan-PI3K (e.g. NVP-BKM-120/Buparlisib, GDC-0941/Pictilisib and BAY 806946/Copanlisib) and PI3K isoform-specific inhibitors (BYL719/Alpelisib and GDC-0032/Taselisib) were developed. Pan-PI3K inhibitors Pictilisib and Buparlisib were discontinued due to the high toxicity, while the isoform-specific inhibitors Alpelisib and Taselisib have shown promising results in terms of anti-tumour activity (in monotherapy and in combination with anti-hormone therapies), with “expected” and more manageable side effects 31,32.
PI3K/AKT is the major pathway downstream of HER2. Mutations of PIK3CA occur in nearly 25% of HER2 overexpressing BC and are associated with poorer outcome and response to tyrosine-kinase inhibitors, such as lapatinib and trastuzumab 33. Moreover, PIK3CA mutations or PTEN loss are associated with resistance to trastuzumab, via hyperactivation of PIK3-mTOR pathway 34–38,34 both at a preclinical and clinical level. The combination of anti-HER2 targeted therapies trastuzumab and lapatinib blocks PI3K signalling reverting trastuzumab resistance 35,39. A recent pooled analysis of data on neoadjuvant clinical trials showed that patients with PI3KCA mutations had lower rates of pCR in comparison to WT patients 40. Interestingly, further results from the EMILIA trial showed that PFS was not affected by the PIK3CA mutational status in patients treated with TDM-1, while patients harbouring PIK3CA mutations, treated with standard HER2 therapy, had shorter PFS compared to PIK3CA wild-type patients (4.3 vs. 6.4 months respectively) 41.
More recently, Toomey et al. were the first to report that PI3KCA/ERBB mutations in patients receiving neoadjuvant docetaxel, carboplatin, trastuzumab and lapatinib may be more likely to experience pCR in comparison to wild type patients42.
In breast cancer models, the introduction of a pan-PIK3 inhibitor reverted the anti-HER2 resistance38. Based on this observation, clinical trials testing the dual pathway blockade are ongoing (NCT02038010; NCT02705859).
AKT inhibitors, such as MK-2206, and mTOR inhibitors, such as everolimus 33,43 are also used in BC. Notwithstanding the promising results obtained in clinical trials using PI3K/AKT/mTOR inhibitors, pharmacological resistance has been shown to occur with this type of treatment. HER2+ PIK3CA mutated breast cancer cell line KPL-4 expressing PIK3CA mutant allele H1047R showed constitutive activation of the PI3K signalling pathway and pharmacological resistance to GDC-0941. PIK3CA knock-down by siRNA restored sensitivity to PI3K inhibition as for the parental cells.44. Dual blockade of mTOR 1/2 and HER2 resulted in anti-tumour activity in in vitro pre-clinical models of breast cancer resistant to anti-HER2 therapies 45. Garay et al. used the SK-BR3 cell line to demonstrate that only kinase domain (H1047R) mutations and not helical domain (E545K) mutations 46 confer resistance to lapatinib. Le et al. investigated molecular mechanisms of resistance to PI3K inhibitors demonstrating that expression of proviral insertion site in murine leukaemia virus (PIM) is able to bypass AKT inhibition, thus conferring resistance to selective PI3Kα inhibitor BYL719/Alpelisib in BC cell lines. Concomitant PIM1 and PI3K blockade restored sensitivity to inhibition 47. It would be interesting to investigate the same pharmacological approach in future clinical trials.
This work has limitations. In particular, the retrospective nature of the study is intrinsically prone to bias. Furthermore, the patients included in the study had different treatment regimens (e.g. trastuzumab, chemotherapy or celecoxib) and different stages of disease that could both affect the analysis’ results.
PI3K mutational status is currently used as a biomarker to identify patients likely to benefit from pan-PI3K48 and PI3K-α49 targeted inhibition. In our analysis, we looked at breast cancer patients who underwent different types of treatment, not necessarily targeted therapies only. Our result is in agreement with the currently accepted view that the presence of PI3KCA mutations constitutes an independent negative prognostic factor in breast cancer patients, providing a relative indication of disease “aggressiveness”.
The importance of PI3K signalling and high prevalence of mutations activating PI3K in breast cancer warrants further investigations to assess other potential biomarkers able to predict the likelihood of response to anti-PI3K/mTOR, anti-HER2 and other TKRs.
Highlights.
Breast cancer is the second most common cause of cancer-related deaths in women.
More accurate biomarkers of response to treatment and predictors of prognosis are needed
Phosphatidylinositol 3-kinase gene is mutated in 20-40% of BC
In our meta-analysis PI3K is an independent negative prognostic factor and correlates with a worse prognosis (p = 0.007)
Footnotes
4. COMPLIANCE WITH ETHICAL STANDARDS
CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent for publication was obtained from all authors of this short communication.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci U S A. 1997;94:4330–5. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–3. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 4.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Immunity, Homeostasis, and Cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 5.Krugmann S, Hawkins PT, Pryer N, Braselmann S. Characterizing the interactions between the two subunits of the p101/p110gamma phosphoinositide 3-kinase and their role in the activation of this enzyme by G beta gamma subunits. J Biol Chem. 1999;274:17152–8. doi: 10.1074/jbc.274.24.17152. [DOI] [PubMed] [Google Scholar]
- 6.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, et al. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–14. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 7.Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science (80- ) 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J. Targeting the Phosphoinositide-3 (PI3) Kinase Pathway in Breast Cancer. Oncologist. 2011;16:12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 9.Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Avan A, Narayan R, Giovannetti E, Peters GJ. Role of Akt signaling in resistance to DNA-targeted therapy. World J Clin Oncol. 2016;7:352–369. doi: 10.5306/wjco.v7.i5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN Tumor Suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science (80- ) 2004;304:554–554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JD, Knoop A, Laenkholm AV, Grauslund M, Jensen MB, Santoni-Rugiu E, et al. PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol. 2012;23:2034–2042. doi: 10.1093/annonc/mdr546. [DOI] [PubMed] [Google Scholar]
- 14.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo W-L, Davies M, et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA Mutations Correlate with Hormone Receptors, Node Metastasis, and ERBB2, and Are Mutually Exclusive with PTEN Loss in Human Breast Carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 19.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Tury S, Becette V, Assayag F, Vacher S, Benoist C, Kamal M, et al. Combination of COX-2 expression and PIK3CA mutation as prognostic and predictive markers for celecoxib treatment in breast cancer. Oncotarget. 2016;7:85124–85141. doi: 10.18632/oncotarget.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cizkova M, Susini A, Vacher S, Cizeron-Clairac G, Andrieu C, Driouch K, et al. PIK3CAmutation impact on survival in breast cancer patients and in ERα, PR and ERBB2- based subgroups. Breast Cancer Res. 2012;14:R28. doi: 10.1186/bcr3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Knowles E, O’Toole SA, McNeil CM, Millar EKA, Qiu MR, Crea P, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S. Clinicopathologic Analysis of Breast Cancers with PIK3CA Mutations in Japanese Women. Clin Cancer Res. 2007;13:408–414. doi: 10.1158/1078-0432.CCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 24.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuels Y, Diaz LA, Schmidt-Kittler O, Cummins JM, DeLong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Qiu Y, Kong D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm Sin B. 2017;7:27–37. doi: 10.1016/j.apsb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13:2477–88. doi: 10.1158/1535-7163.MCT-14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, et al. Feedback Suppression of PI3Kα Signaling in PTEN-Mutated Tumors Is Relieved by Selective Inhibition of PI3Kβ. Cancer Cell. 2015;27:109–122. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 Levels Reveals an Unexpected Role for p110β in Early Adaptive Responses to p110α-Specific Inhibitors in Luminal Breast Cancer. Cancer Cell. 2015;27:97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518:240–4. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodon J, Tabernero J. Improving the Armamentarium of PI3K Inhibitors with Isoform-Selective Agents: A New Light in the Darkness. Cancer Discov. 2017:7. doi: 10.1158/2159-8290.CD-17-0500. [DOI] [PubMed] [Google Scholar]
- 32.Juric D, Krop I, Ramanathan RK, Wilson TR, Ware JA, Sanabria Bohorquez SM, et al. Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors. Cancer Discov. 2017;7:704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahrami A, Khazaei M, Shahidsales S, Hassanian SM, Hasanzadeh M, Maftouh M, et al. The therapeutic potential of PI3K/Akt/mTOR inhibitors in breast cancer: rational and progress. J Cell Biochem. 2017 doi: 10.1002/jcb.26136. [DOI] [PubMed] [Google Scholar]
- 34.Goel S, Krop IE. Deciphering the role of phosphatidylinositol 3-kinase mutations in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2015;33:1407–9. doi: 10.1200/JCO.2014.60.0742. [DOI] [PubMed] [Google Scholar]
- 35.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33:1334–9. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichhorn PJA, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-Kinase Hyperactivation Results in Lapatinib Resistance that Is Reversed by the mTOR/Phosphatidylinositol 3-Kinase Inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elster N, Collins DM, Toomey S, Crown J, Eustace AJ, Hennessy BT. HER2-family signalling mechanisms, clinical implications and targeting in breast cancer. Breast Cancer Res Treat. 2015;149:5–15. doi: 10.1007/s10549-014-3250-x. [DOI] [PubMed] [Google Scholar]
- 40.Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016;27:1519–1525. doi: 10.1093/annonc/mdw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino AE, et al. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016:22. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toomey S, Eustace AJ, Fay J, Sheehan KM, Carr A, Milewska M, et al. Impact of somatic PI3K pathway and ERBB family mutations on pathological complete response (pCR) in HER2-positive breast cancer patients who received neoadjuvant HER2-targeted therapies. Breast Cancer Res. 2017;19:87. doi: 10.1186/s13058-017-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Leo A, Curigliano G, Diéras V, Malorni L, Sotiriou C, Swanton C, et al. New approaches for improving outcomes in breast cancer in Europe. The Breast. 2015;24:321–330. doi: 10.1016/j.breast.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Huw L-Y, O’Brien C, Pandita A, Mohan S, Spoerke JM, Lu S, et al. Acquired PIK3CA amplification causes resistance to selective phosphoinositide 3-kinase inhibitors in breast cancer. Oncogenesis. 2013;2:e83. doi: 10.1038/oncsis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Garcia C, Ibrahim YH, Serra V, Calvo MT, Guzman M, Grueso J, et al. Dual mTORC1/2 and HER2 Blockade Results in Antitumor Activity in Preclinical Models of Breast Cancer Resistant to Anti-HER2 Therapy. Clin Cancer Res. 2012;18:2603–2612. doi: 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 46.Garay J, Korkola J, Gray J. Abstract P3-03-04: Sensitivity to lapatinib differs between HER2-amplified breast cancer cells harboring kinase and helical domain mutations in PIK3CA and relies on production of PIP3. Cancer Res. 2017:77. [Google Scholar]
- 47.Le X, Antony R, Razavi P, Treacy DJ, Luo F, Ghandi M, et al. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov. 2016;6:1134–147. doi: 10.1158/2159-8290.CD-16-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baselga J, Im S-A, Iwata H, Clemons M, Ito Y, Awada A, et al. PIK3CA Status in Circulating Tumor DNA Predicts Efficacy of Buparlisib Plus Fulvestrant in Postmenopausal Women With Endocrine-resistant HR+/HER2− Advanced Breast Cancer: First Results From the Randomized, Phase III BELLE-2 Trial. Emmanuelle Di Tomaso. :18. [Google Scholar]
- 49.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2 Metastatic Breast Cancer. Clin Cancer Res. 2017:23. doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]