Abstract
Recent studies have shown contradictory associations between calcium levels and health outcomes. We suspected these conflicting results were the consequence of more general issues with how biomarkers are analyzed in epidemiological studies, particularly in the context of aging. To demonstrate the risks of typical analyses, we used three longitudinal aging cohort studies and their demographic subsets to analyze how calcium levels change with age and predict risk of mortality and frailty. We show that calcium levels either increase or decrease with age depending on the population, and positively or negatively predict frailty depending on the population and analysis; both age and frailty results showed substantial heterogeneity. Mortality analyses revealed few significant associations but were likely underpowered. Variation in population composition (demographics, diseases, diet, etc.) leads to contradictory findings in the literature for calcium and likely for other biomarkers. Epidemiological studies of biomarkers are particularly sensitive to population composition both because biomarkers generally have non-linear and often non-monotonic relationships with other key variables, notably age and health outcomes, and because there is strong interdependence among biomarkers, which are integrated into complex regulatory networks. Consequently, most biomarkers have multiple physiological roles and are implicated in multiple pathologies. We argue that epidemiological studies of aging using biomarkers must account for these factors, and suggest methods to do this.
Keywords: Aging biomarkers, Population composition, Physiological complexity, Calcium, Frailty
1. Introduction
Clinical and epidemiological studies of biomarkers during aging often characterize associations between a single marker and either age or relevant health outcomes (Glei et al., 2011; Liu et al., 2015; Loeffen et al., 2015; Schottker et al., 2015; Schram et al., 2007). However, we and others have previously observed that many standard clinical biomarkers (cholesterol, glucose, blood panel markers, circulating proteins, inflammatory markers, etc.) have surprisingly variable associations with age in different populations (Cohen et al., 2015b; Martin-Ruiz and von Zglinicki, 2014; Sebastiani et al., 2016), and we were worried that many biomarker findings in the literature might be poorly general-izable. We note that our focus here is largely on clinical biomarkers used in epidemiological research of aging, not on single biomarkers of the aging process per se. Such single biomarkers have not really been found, though there is some controversy about telomeres (Johnson, 2006).
Individual biomarkers do not necessarily change linearly with, or independently of, other aspects of physiology, but rather are integrated into physiological regulatory networks that can exhibit properties of formally complex systems (Cohen, 2016; Cohen et al., 2012). Accordingly, many biomarkers have multiple physiological determinants and roles and can fluctuate for diverse physiological and pathophysiological reasons (see below for details, taking calcium as an example). Biomarkers are thus rarely a direct window into the underlying physiological processes of interest, but are rather proxies, the efficacy of which can depend heavily on other biomarkers, disease state, and demographics.
Another consequence of complex physiological integration is that changes with age are generally non-linear and usually non-monotonic as well (i.e., showing periods of both increase and decrease). For example, a long-term study of seven biomarkers in the Framingham Heart Study found that six showed non-monotonic changes across the adult life course (Yashin et al., 2010). Thus, since disease prevalence and physiological traits can vary greatly across populations, population composition should have a strong role in determining biomarker-age associations in any given study. For example, if a biomarker happens to be elevated in diabetics, observed changes in the biomarker with age may depend strongly on the prevalence and severity of diabetes in the population, the age structure of diabetes incidence, and impacts of diabetes on other outcomes except aging and mortality, all of which can be quite variable but are unrelated to the fundamental question of how the biomarker may or may not predict aging. Such effects are likely to be present even in longitudinal studies, where biomarkers may evolve differently with age and have different impacts in specific subpopulations.
This article attempts to address this challenge by combining aspects of a review and aspects of an empirical study. We first conduct analyses of how calcium associates with age, mortality, and clinical frailty in three different cohort studies of aging and their demographic subsets. We demonstrate that results are highly heterogeneous across populations. Obviously, calcium is but a single example, so our discussion then goes well beyond our particular findings to consider the theoretical basis of why biomarker studies should generate such unstable results, and how to address the problem. We propose a number of concrete methods to help mitigate the problem.
2. The example of calcium
Within the aging context, calcium has not particularly been proposed as a single biomarker of aging, and is generally analyzed rather as one among many markers in a panel (Martin-Ruiz et al., 2011; Szewieczek et al., 2015). Nevertheless, calcium levels are increasingly believed to be associated with risk of mortality and morbidity. Normal calcium reference ranges are 8.9–10.1 mg/dl in adults. While many studies with middle-aged adults report associations between high calcium levels and increased risk of mortality (Larsson et al., 2010; Reid et al., 2016), findings in oldest adults tend to show reduced adverse outcomes for higher calcium levels, perhaps reflecting the concomitant higher albumin levels within this group (Szewieczek et al., 2015). Nonlinear associations have also been reported (Larsson et al., 2010), notably U- or J-shaped curves for risk (Durup et al., 2012; Lu et al., 2016).
Calcium is involved in many crucial physiological functions, notably neuronal transmission, immune cell stimulation, apoptosis, bone health maintenance, muscle contraction –including the heart – and blood coagulation (Shaker and Deftos, 2000). In the blood, its ionized form is believed to be the more closely regulated one, and accounts for approximately 50% of total serum calcium, the rest being bound to serum proteins, mainly albumin (Brown, 2001). Abnormalities in albumin and globulins may affect total serum calcium levels, but the ionized level should remain unchanged for a given pH (Payne et al., 1973). Hypercalcaemia has various causes, notably cancer and hyperparathyroidism, whereas hypocalcaemia is generally due to hypoparathyroidism or chronic renal failure (Parfitt, 1979). Many undetected pathological conditions related to age may influence calcium homeostasis and as such remain undiagnosed for long-time.
The relationship between calcium and generalized risks of adverse outcomes during aging thus appears to be a complex one. First, both high and low levels might have detrimental effects on physiological homeostasis. Thus, population composition, most notably age, might strongly affect possible associations between calcium and risk of health outcomes. For instance, while high calcium levels at mid-life may be linked to adverse outcomes, the inverse relationship might take place at older ages. To demonstrate this idea, we looked at age-related changes in calcium, and its potential association with frailty and mortality, across three longitudinal cohort studies of aging. Analyses were repeated in different cohort subsets (sex, race, and age) to assess the effect of population composition on potential associations. Our objective was to demonstrate the limits of such single-biomarker epidemiology in the context of the complex process of aging.
Detailed Methods are provided in the online Appendix; here we note principally that we have taken care to reproduce the methods most common in the literature. For example, it is common to use measures of calcium adjusted for albumin levels, and we thus used both a raw and albumin-adjusted version. There are two principal exceptions where we opted for methods more sophisticated than are standard. First, many analyses use tertiles or other quantiles, and we disfavour this approach because it causes a loss of statistical power and can cause important biases and inflation of Type-I error without any important justification (Barnwell-Ménard et al., 2015). We thus always consider calcium as a continuous variable. Second, age was sometimes controlled using a cubic spline, a more sophisticated approach than age categories or age as a linear variable.
3. Findings on calcium
3.1. Association of calcium with age
First, we looked at the association between calcium and age through correlation analyses in three longitudinal cohort studies of community dwelling older adults (Table 1, see online Appendix for details), as might be obtained in many cross-sectional studies. As shown in Table 2, calcium is consistently negatively correlated with age, except for WHAS I (nearly significant) and WHAS II; however albumin-adjusted calcium (alb-adj ca) shows unstable results (see Table A.1 for other versions). Alb-adj ca is clearly positively correlated with age in the InCHIANTI cohort, but not in the other two data sets. Calcium is correlated with albumin in all three cohorts (0.33 ≤ r ≤ 0.44, all p < 0.001). Calcium and alb-adj ca also correlate strongly (0.78 ≤ r ≤ 0 .87, p < all 0.001). Surprisingly, alb-adj ca is not much more correlated with ionized calcium than the unadjusted measure (r = 0.45 compared to r = 0.43) and thus might not serve as a good proxy for ionized calcium (Fig. A.1).
Table 1.
Characteristics of study populations (at first visit).
| Characteristic | InCHIANTI | WHAS I | WHAS II | BLSA |
|---|---|---|---|---|
|
|
|
|
|
|
| Characteristic | n = 1310 | n = 796 | n = 430 | n = 1290 |
| Age | ||||
| Mean ± SD | 68.3 ± 15.4 | 78.5 ± 7.9 | 74.7 ± 2.9 | 64.9 ± |
| Range (min–max) | 21.3–98.4 | 65.8–100.3 | 70.2–82.5 | 26.4–99.7 |
| Female (%) | 727 (55.5) | 796 | 430 | 636 (49.3) |
| (100.0) | (100.0) | |||
| Race (white, %) | 1310 | 570 (71.6) | 348 (80.9) | 830 (64.3) |
| (100.0) | ||||
| Deaths, number (%) | 366 (27.9) | 238 (29.9) | 173 (40.2) | 109 (8.4) |
| Frailty criteria, number | ||||
| 0 (%) | 501 (38.2) | 81 (10.2) | 262(60.9) | – |
| 1 (%) | 255 (19.5) | 170 (21.4) | 126 (29.3) | – |
| 2 (%) | 128 (9.8) | 217 (27.3) | 30 (7.0) | – |
| 3 (%) | 59 (4.5) | 121 (15.2) | 10 (2.3) | – |
| 4 (%) | 33 (2.5) | 65 (8.2) | 1 (0.2) | – |
| 5 (%) | 10 (0.8) | 31 (3.9) | 1 (0.2) | – |
| Ca (mmol/L), mean ± SD | 2.36 ± 0.11 | 2.32 ±0.11 | 2.38 ±0.10 | 2.30 ±0.11 |
| Alb-adj ca (mmol/L), mean ± SD | 2.31 ± 0.11 | 2.32 ± 0.11 | 2.32 ± 0.11 | 2.30 ± 0.10 |
Table 2.
Correlation coefficients between age and calcium level at first visit.
| Dataset | n | Calcium | Alb-adj ca | ||
|---|---|---|---|---|---|
|
|
|
||||
| r | p | r | p | ||
| InCHIANTI | 1305 | −0.073 | 0.008 | 0.129 | <0.001 |
| WHAS | 1189 | −0.115 | <0.001 | −0.029 | 0.32 |
| WHAS I | 766 | −0.063 | 0.08 | −0.017 | 0.64 |
| WHAS II | 423 | −0.029 | 0.55 | −0.030 | 0.54 |
| BLSA | 1242 | −0.126 | <0.001 | 0.036 | 0.21 |
| All | 3736 | −0.092 | <0.001 | 0.024 | 0.14 |
Unadjusted calcium and albumin-adjusted calcium (alb-adj ca) variables were centered at zero and divided by their standard deviation such that mean was 0 and standard deviation 1. Significant correlation coefficients are indicated in bold.
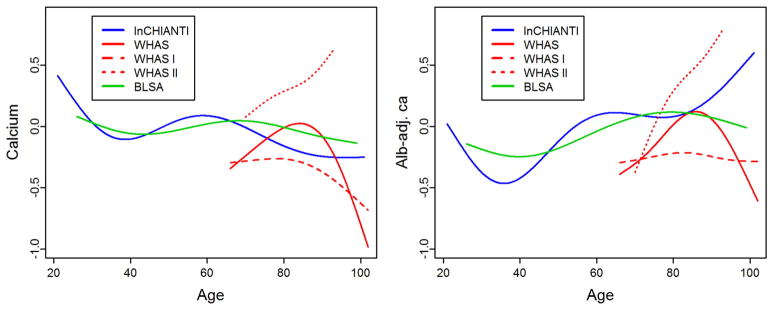
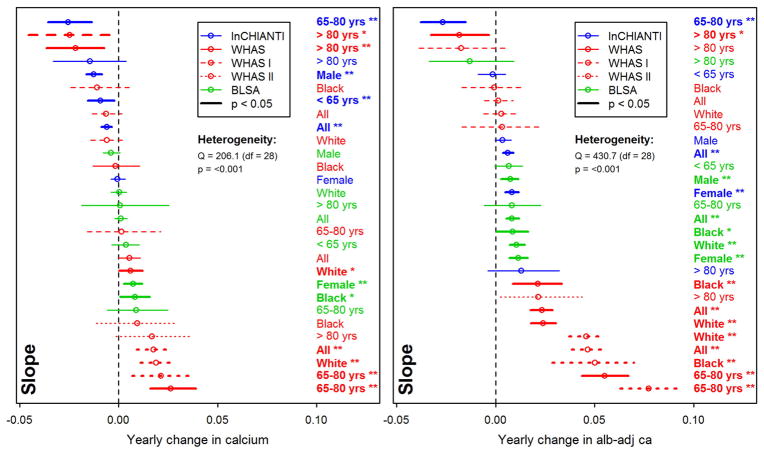
Longitudinal changes in calcium levels with age varied markedly across populations and sub-populations. Across full datasets, alb-adj ca always increases up to 80 years or so, but diverges across datasets after this age (Fig. 1). In contrast, unadjusted calcium shows much more variation across populations. These findings are sometimes but not always in agreement with the correlations based on a cross-sectional approach (Table 2). Using finer-scale sub-populations (Fig. 2), both unadjusted calcium and alb-adj ca can increase or decrease significantly with age depending on the population, and heterogeneity across analyses is substantial and highly significant (Table A.2). Calcium slopes (i.e. mean yearly changes in SDs) varied greatly across these analyses, ranging from −0.02 to +0.08 SDs/year (Fig. 2). Calcium trajectories also varied substantially according to sex and age group within each cohort (Figs. A.2–A.5). Analysis of calcium trajectories with age in a merged dataset revealed significant interactions between dataset and slope (Table A.3). Sex also influenced calcium changes with age for unadjusted calcium, but not the alb-adj ca, with women showing steeper increases in calcium with age than men when considering all three datasets (β = 0.008, p < 0.001).
Fig. 1.
Calcium trajectory with age. Changes with age are shown for unadjusted calcium (left panel) and albumin-adj ca (right panel) variables. Trajectories were estimated using Bayesian mixed models with age as a cubic spline (with 5 knots for InCHIANTI and BLSA, and 4 knots for WHAS, see Appendix for details) for three longitudinal cohorts, as indicated with different colors. Analyses for WHAS were performed either combining WHAS I and WHAS II (solid lines), or with either one (dashed lines for WHAS I and dotted lines for WHAS II). Age started at 66 for WHAS, while InCHIANTI and BLSA had a small fraction of younger subjects.
Fig. 2.
Yearly changes in calcium with age. Trajectories for unadjusted calcium (left panel) and albumin-adj ca (right panel) variables were estimated using Bayesian linear mixed models (see Appendix for details). Each line represents a single analysis in a single subpopulation, as indicated by the colors, lines, and labels at right; the relative positions of the lines thus permits us to assess the heterogeneity of relationship between calcium levels and age. Note that the x-axes are for change in the variables after a standard normal transformation, such that a change of 1 represents a change of 1 standard deviation from the population mean of 0. Slopes greater than zero thus indicate that calcium is increasing with age, and slopes smaller than zero that it is declining with age. Slopes (points) obtained are plotted with their 95% confidence intervals (CIs; segments). Analyses for WHAS were performed either combining WHAS I and WHAS II (solid lines), or with either one (dashed lines for WHAS I and dotted lines for WHAS II). Results for heterogeneity tests are also indicated. Significant results are plotted in bold (and CIs do not overlap the vertical line), with asterisks indicating the significance level (***, p < 0.001; **, p < 0.01; *, p < 0.05).
3.2. Association with mortality and frailty
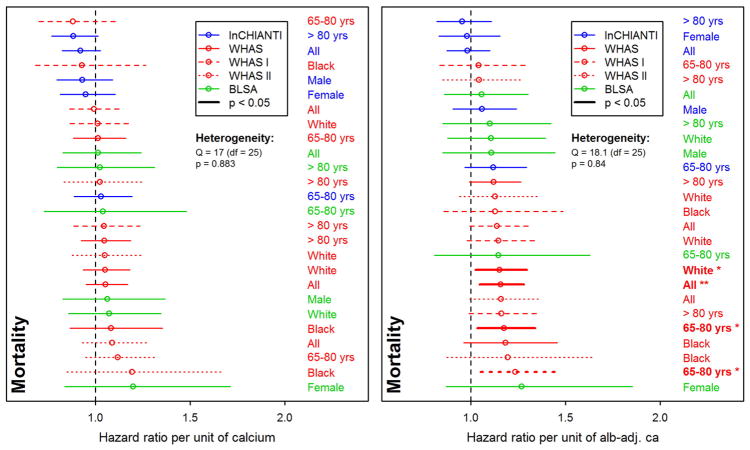
Our mortality analyses appear to be underpowered, failing to reach normal significance thresholds even when effect sizes were large (ranging from 0.88 to 1.27, see Fig. 3). For the oldest subjects (>80 yrs), we note a lower mortality risk due to unadjusted calcium in the InCHIANTI dataset, standing in contrast to previous findings in older populations (Martin-Ruiz et al., 2011; Szewieczek et al., 2015). Nonetheless, meta-regression of effect sizes shows that the associations between mortality and calcium do differ across datasets, even when these associations are not themselves significant: using BLSA led to stronger effects compared to WHAS I (β = 0.14, p = 0.04) and to InCHIANTI (β = 0.13, p = 0.04), an effect that failed to reach significance for WHAS II (β = 0.10, p = 0.13). Using alb-adj ca compared to unadjusted calcium led to higher effect sizes (β = 0.13, p = 0.02).
Fig. 3.
Relationships between calcium and mortality. Each line represents a single analysis in a single subpopulation, as indicated by the colors, lines, and labels at right; the relative positions of the lines thus permits us to assess the heterogeneity of relationship between calcium levels and mortality. Estimated hazard ratios (points) together with 95% confidence intervals (CIs; segments) are plotted for unadjusted calcium (left panel) and albumin-adj ca (right panel) variables, based on counting process Cox proportional hazards models. See Fig. 2 for further explanations.
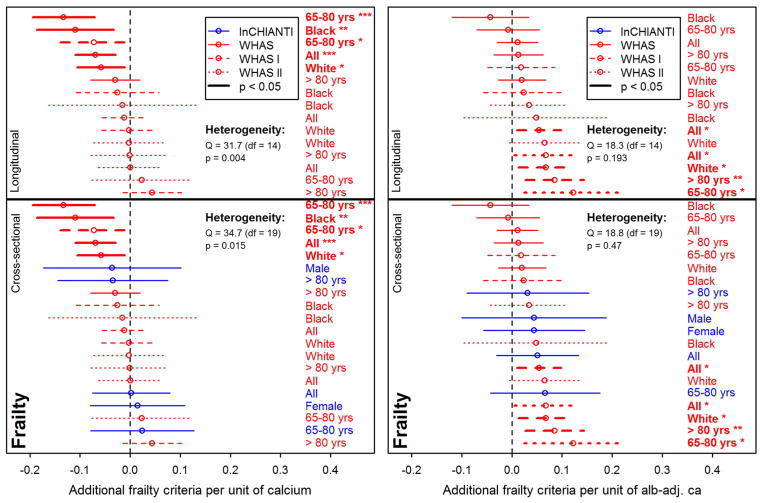
Frailty was sometimes associated with calcium levels, but in opposite directions for unadjusted calcium and alb-adj ca: more frailty criteria were significantly associated with lower levels of unadjusted calcium, but with higher levels of alb-adj ca in WHAS I and WHAS II (Fig. 4). However, there was also substantial heterogeneity of these results for unadjusted calcium, and most results were not significant. Negative associations between calcium and frailty were more present in WHAS (meta-regression β = −0.05, p = 0.06 and β = −0.07, p = 0.005, respectively for WHAS I and WHAS II) than in InCHIANTI. Type of calcium measurement also significantly affected the effect size (β = 0.07, p < 0.001, for considering alb-adj ca compared to unadjusted calcium).
Fig. 4.
Relationships between calcium and frailty. Each line represents a single analysis in a single subpopulation, as indicated by the colors, lines, and labels at right; the relative positions of the lines thus permits us to assess the heterogeneity of relationship between calcium levels and frailty. Poisson regression models were performed on the number of Fried's frailty criteria (Fried et al., 2001) for unadjusted calcium (left panel) and albumin-adj ca (right panel) variables, adjusting for age with a cubic spline. Estimations (points) together with 95% confidence intervals (CIs; segments) are plotted. Longitudinal analyses are shown on the top panels (for WHAS only) and cross-sectional analyses (using the number of frailty criteria at first visit only) are presented on the lower panels. See Fig. 2 for further explanations.
4. Discussion
Our analyses demonstrate the challenges in using a single biomarker in an epidemiological study based on a single population. Using the example of calcium, a biomarker appearing frequently in recent epidemiological studies (Larsson et al., 2010; Lu et al., 2016; Reid et al., 2016), we show that we can detect effects differing not just in magnitude but direction depending on the population studied and variable definition. For example, using unadjusted calcium in the whole WHAS data set, we could have published an article showing that calcium levels increase with age, are negatively associated with frailty, and unassociated with mortality. However, this conclusion would not have generalized or yielded any important insight: not only is it not replicated across datasets, it is not even replicated within WHAS subsets by age and race.
Of course, for the reasons outlined in the Introduction, it would be naïve to hypothesize a general relationship between calcium and age, mortality, and/or frailty. We should expect high levels and low levels to arise through different types of underlying pathology (Parfitt, 1979), and to have different consequences for individual health in different physiological contexts (Szewieczek et al., 2015). Because these pathologies and contexts vary across age groups and populations, population composition can easily become the primary driver of the results. Our principal objective here is thus not to illuminate calcium epidemiology during aging, and accordingly our study has a number of clear limitations in that context. First, we do not consider (nor generally have access to) sufficient data on dietary consumption or supplements. Second, kidney disease can be a major factor (Parfitt, 1979), but we had insufficient data to perform such analyses. Third, we did not target specific subpopulations. For example, calcium might conceivably be a useful biomarker for the efficacy of certain medications to treat osteoporosis, and its use as a single biomarker in that context might be appropriate.
While our principal conclusion concerns biomarkers in aging research generally, calcium is not representative of all such biomarkers. Some, such as telomere length, might be markers of the aging process itself, though evidence is mixed (Sanders and Newman, 2013) and hope for a single silver-bullet biomarker of aging is dimming with increasing realization that aging is a multi-factorial, complex phenomenon (Cohen, 2016; Kirkwood, 2005), and that many biomarkers of aging correlate only weakly with each other (Sanders et al., 2014). Others, such as hepcidin, may be indicators of very specific aspects of physiological functioning or disease states (Nemeth et al., 2003). Calcium is a problematic biomarker because it plays multiple roles and thus can be implicated in multiple pathologies; its levels need to be considered in light of multiple other markers (minimally vitamin D, parathyroid hormone, and albumin, Bushinsky and Monk, 1998). Nonetheless, most common clinical biomarkers play multiple roles or present similar levels of complexity for interpretation, among them cholesterol, albumin, C-reactive protein (CRP), insulin-like growth factor (IGF-1), haemoglobin, uric acid, and glucose. These common but challenging markers are increasingly the focus in aging studies (Beekman et al., 2016; Martin-Ruiz et al., 2011; Mitnitski et al., 2015). A final class of aging biomarkers increasingly thought to be important (but not always available) are dynamic biomarkers that measure a change in response to a challenge or stimulus (Kalyani et al., 2012; Varadhan et al., 2008).
4.1. Risks of one-at-a-time biomarker analyses
These caveats on the use of biomarkers may seem obvious, but they are rarely incorporated into study design and present a number of specific risks, namely:
Identification of a biomarker that is conditionally associated with an outcome despite lack of a clear causal role. For example, CRP is thought to be a marker of inflamm-aging and heart disease risk; however, it is very high in the Tsimane hunter-gatherers despite an absence of heart disease in this population (Gurven et al., 2009). This paradox is due to the multiple roles and associations CRP has with different aspects of the inflammatory response triggered under different conditions (Bandeen-Roche et al., 2009). Indeed, despite its popularity, CRP is far from the best marker of inflamm-aging, and multivariate indices perform better than univariate ones (Cohen et al., in press). This problem cannot be avoided by simple control for other biomarkers in regression models; the complex systems dynamics and feedback effects among the markers break fundamental assumptions of such approaches.
Identification of associations that are not generalizable, as would have happened had we attempted to publish our calcium findings based only on one of our datasets. While this risk is present for any study (“external validity”), it is particularly acute for biomarker studies given the physiological complexity and the history of conflicting findings.
Misidentification of “aging biomarkers”. Many biomarkers are thought to change with age and are accordingly used in analyses to understand the aging process. Often, a cross-sectional correlation with age is used to determine a set of aging biomarkers for a more complex analysis (Levine, 2013). However, in many cases these age-related changes are either not reproduced in longitudinal analyses (as is the case here) or are not reproduced across datasets (also true here and in our previous analyses (Cohen et al., 2015b)). For example, both the Pace of Aging (Belsky et al., 2015) and the Klemera-Doubal biological age algorithm (Klemera and Doubal, 2006; Levine, 2013) assume linear change in biomarkers with age, and identify sets of biomarkers based on detected age associations. While both work well in young cohorts where the changes are closer to linear, non-monotonicity presents major challenges for implementation in older cohorts (D. Belsky, pers. comm.).
Perpetuation of a simplified model of physiology and epidemiology. There is no controversy surrounding either the complex physiological interdependence of many biomarkers (Cohen, 2016; Cohen et al., 2012), nor the challenges of considering population composition in epidemiological studies. Nonetheless, much of the literature continues to ignore one or both of these factors, generating not just ungeneralizable results but perpetuating a simplified worldview across generations of researchers.
Variation in population composition (demographics, diseases, diet, etc.) leads to contradictory findings in the literature for calcium and likely for other biomarkers. Epidemiological studies of biomarkers are particularly sensitive to population composition both because biomarkers generally have non-linear and often non-monotonic relationships with other key variables, notably age and outcomes, and because there is strong interdependence among biomarkers, which are integrated into complex regulatory networks. Consequently, most biomarkers have multiple physiological roles and are implicated in multiple pathologies.
4.2. Potential solutions
We suggest that epidemiological studies using biomarkers, particularly in the context of aging, must consider three factors: (1) population composition; (2) non-linear and often non-monotonic relationships between biomarkers and other key variables; and (3) interdependence among biomarkers due to their integration into complex regulatory networks. The first factor is often important because of the second and/or the third, but in practical terms each can be considered as a separate methodological challenge. Each factor alone is a major challenge, and proper incorporation of all three simultaneously may seem daunting. Nonetheless, we are convinced it is both possible and essential to make progress in the field despite these challenges. Here are several suggestions.
First, problems related to population composition can be addressed by replication, both across datasets and within data subsets as shown here. Emphasis should be put on results that are robust across populations, or where there is a clear explanation for heterogeneous results. The increasing availability of data from diverse cohorts (Erten-Lyons et al., 2012) implies that it should rarely be acceptable to publish analyses based on a single cohort without independent replication. This is particularly true for biomarker analyses where there is a high risk of non-replicability. Beyond replication, it is important to consider what physiological processes or diseases might be linked to the biomarker in question and how these might vary across populations, particularly when these processes/diseases are not the focus of the study.
Second, multiple methods exist to incorporate non-linearity into analyses, ranging from a simple quadratic term to generalized additive models to cubic splines; indeed, in the case of calcium, several studies have used such methods, demonstrating their importance (Durup et al., 2012; Lu et al., 2016). Often, optimal biomarker values are intermediate rather than very high or very low, a reflection of the need to maintain homeostasis in a complex system (Cohen, 2016), and may also be related to the principle of hormesis (Rattan, 2008). This generally produces a non-monotonic association with the outcome (e.g. U- or J-shaped). In a multivariate context, statistical distance approaches account for this (Cohen et al., 2015a); in a univariate context, extreme high and low quantiles or absolute values of standard-normal-transformed variables may be used (Martin-Ruiz et al., 2011; Seplaki et al., 2005).
Third, multiple solutions exist to at least partially tackle physiological complexity. Biomarker dynamics can be evaluated using a stimulus-response paradigm (Varadhan et al., 2008). Multivariate biomarker analyses are generating progress on the problem of physiological interdependence (Arbeev et al., 2016). Both the Pace of Aging (Belsky et al., 2015) and Klemera-Doubal biological age (Levine, 2013) have produced impressive results despite the above-mentioned caveat with the linearity assumption, likely because errors related to individual markers wash out when enough markers are included. Homeostatic dysregulation, as measured via statistical distance, is also a promising method that does not rely on assumptions of linearity or associations with age (Cohen et al., 2015a). Use of such integrative approaches does not necessarily solve all questions of population composition (Cohen et al., 2016), but can at least avoid some of the problems of interdependence among markers.
We emphasize that there is no single recipe for how to deal with these challenges, and not every study will be able to deal with them perfectly. Rather, the point is to consider them both in study design (e.g. longitudinal cohort studies designed to permit analysis of multisystem dynamics) and in interpretation. There may be cases where a single bio-marker is linearly related with health outcomes in a stable fashion across populations, but there is every reason to expect such cases to be few and far between, particularly when the outcomes are the generalized health conditions common in aging studies (mortality, frailty, disability, quality of life, physical functioning, cognitive decline, etc.). The burden should be shifted to authors to prove generalizability and validity, rather than to suppose them in the absence of evidence, by favouring the design of studies integrating the above outlined criteria. Given the recognized importance of gene-by-environment interactions, and more problematically yet gene-by-environment-by-age interactions, we should be relatively modest about what biomarker studies of aging may achieve; nonetheless, some progress should be possible if appropriate care is exercised to avoid the main pitfalls.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR, grant numbers 119485 and 145585). AAC is also supported by a CIHR New Investigator Salary Award and is a member of the Fonds de recherche du Québec – Santé funded Centre de recherche du CHUS and Centre de recherche sur le vieillissement. GF is supported by the European Union's Horizon 2020 research and innovation program (grant number 633589).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.exger.2017.07.011.
References
- Arbeev KG, Ukraintseva SV, Yashin AI. Dynamics of biomarkers in relation to aging and mortality. Mech Ageing Dev. 2016;156:42–54. doi: 10.1016/j.mad.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell-Ménard JL, Li Q, Cohen AA. Effects of categorization method, regression type, and variable distribution on the inflation of type-I error rate when categorizing a confounding variable. Stat Med. 2015;34:936–949. doi: 10.1002/sim.6387. [DOI] [PubMed] [Google Scholar]
- Beekman M, Uh H-W, Van Heemst D, Wuhrer M, Ruhaak LR, Gonzalez-Covarrubias V, Hankemeier T, Houwing-Duistermaat JJ, Slagboom PE. Classification for longevity potential: the use of novel biomarkers. Frontiers in Public Health. 2016:4. doi: 10.3389/fpubh.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE. Quantification of biological aging in young adults. Proc Natl Acad Sci. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Levine A. Physiology of calcium homeostasis. In: Bilezikian JPM, editor. The Parathyroids. 2001. [Google Scholar]
- Bushinsky DA, Monk RD. Electrolyte quintet: calcium. Lancet. 1998;352:306–311. doi: 10.1016/s0140-6736(97)12331-5. [DOI] [PubMed] [Google Scholar]
- Cohen AA. Complex systems dynamics in aging: new evidence, continuing questions. Biogerontology. 2016;17:205–220. doi: 10.1007/s10522-015-9584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Martin LB, Wingfield JC, McWilliams SR, Dunne JA. Physiological regulatory networks: ecological roles and evolutionary constraints. Trends Ecol Evol. 2012;27:428–435. doi: 10.1016/j.tree.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Li Q, Milot E, Leroux M, Faucher S, Morissette-Thomas V, Legault V, Fried LP, Ferrucci L. Statistical distance as a measure of physiological dysregulation is largely robust to variation in its biomarker composition. PLoS One. 2015a;10:e0122541. doi: 10.1371/journal.pone.0122541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Li Q, Bergeron P, Poirier R, Dusseault-Belanger F, Fulop T, Leroux M, Legault V, Metter EJ, Fried LP, Ferrucci L. Detection of a novel, integrative aging process suggests complex physiological integration. PLoS One. 2015b;10:e0116489. doi: 10.1371/journal.pone.0116489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Morissette-Thomas V, Ferrucci L, Fried LP. Deep biomarkers of aging are population-dependent. Aging (Albany NY) 2016;8:2253. doi: 10.18632/aging.101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Bandeen-Roche K, Morissette-Thomas V, Fulop T. A robust characterization of inflammaging and other immune processes through multivariate analysis of cytokines from longitudinal studies. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on Immunosenescence: Basic Understanding and Clinical Applications. Doderecht: Springer Science + Business Media BV; (in press) [Google Scholar]
- Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97:2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- Erten-Lyons D, Sherbakov LO, Piccinin AM, Hofer SM, Dodge HH, Quinn JF, Woltjer RL, Kramer PL, Kaye JA. Review of selected databases of longitudinal aging studies. Alzheimers Dement. 2012;8:584–589. doi: 10.1016/j.jalz.2011.09.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Lin YH, Weinstein M. Age-related changes in biomarkers: longitudinal data from a population-based sample. Res Aging. 2011;33:312–326. doi: 10.1177/0164027511399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Rodriguez DE, Vasunilashorn S, Kim JK, Finch CE, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol Ser A Biol Med Sci. 2012;67:1300–1306. doi: 10.1093/gerona/glr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, Sundstrom J. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–339. doi: 10.1161/ATVBAHA.109.196675. [DOI] [PubMed] [Google Scholar]
- Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol Ser A Biol Med Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Huang J, Chu X, Qian D, Wang Z, Sun X, Chen F, Xu J, Li S, Jin L, Wang X. Blood biomarkers and functional disability among extremely longevous individuals: a population-based study. J Gerontol A Biol Sci Med Sci. 2015;70:623–627. doi: 10.1093/gerona/glu229. [DOI] [PubMed] [Google Scholar]
- Loeffen R, Winckers K, Ford I, Jukema JW, Robertson M, Stott DJ, Spronk HM, ten Cate H, Lowe GD, Group PS. Associations between thrombin generation and the risk of cardiovascular disease in elderly patients: results from the PROSPER study. J Gerontol A Biol Sci Med Sci. 2015;70:982–988. doi: 10.1093/gerona/glu228. [DOI] [PubMed] [Google Scholar]
- Lu JL, Molnar MZ, Ma JZ, George LK, Sumida K, Kalantar-Zadeh K, Kovesdy CP. Racial differences in association of serum calcium with mortality and incident cardio- and cerebrovascular events. J Clin Endocrinol Metab. 2016;101:4851–4859. doi: 10.1210/jc.2016-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, von Zglinicki T. Biomarkers of healthy ageing: expectations and validation. Proc Nutr Soc. 2014;73:422–429. doi: 10.1017/S0029665114000147. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, Dunn M, Hilkens C, Keavney B, Pearce SH, den Elzen WP, Talbot D, Wiley L, Bond J, Mathers JC, Eccles MP, Robinson L, James O, Kirkwood TB, von Zglinicki T. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev. 2011;132:496–502. doi: 10.1016/j.mad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:1. doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Equilibrium and disequilibrium hypercalcemia. New light on an old concept. Metab Bone Dis Relat Res. 1979;13:279–293. [Google Scholar]
- Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4:643–646. doi: 10.1136/bmj.4.5893.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Reid IR, Gamble GD, Bolland MJ. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J Intern Med. 2016;279(6):524–540. doi: 10.1111/joim.12464. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs008. mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Ding V, Arnold AM, Kaplan RC, Cappola AR, Kizer JR, Boudreau RM, Cushman M, Newman AB. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol Ser A Biol Med Sci. 2014;69:174–181. doi: 10.1093/gerona/glt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottker B, Saum KU, Jansen EH, Boffetta P, Trichopoulou A, Holleczek B, Dieffenbach AK, Brenner H. Oxidative stress markers and all-cause mortality at older age: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2015;70:518–524. doi: 10.1093/gerona/glu111. [DOI] [PubMed] [Google Scholar]
- Schram MT, Trompet S, Kamper AM, de Craen AJ, Hofman A, Euser SM, Breteler MM, Westendorp RG. Serum calcium and cognitive function in old age. J Am Geriatr Soc. 2007;55:1786–1792. doi: 10.1111/j.1532-5415.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, Thyagarajan B, Sun F, Honig LS, Schupf N, Cosentino S, Feitosa MF, Wojczynski M, Newman AB, Montano M, Perls TT. Age and sex distributions of age-related biomarker values in healthy older adults from the long life family study. J Am Geriatr Soc. 2016;64:e189–e194. doi: 10.1111/jgs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, Weinstein M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp Gerontol. 2005;40:438–449. doi: 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Shaker JL, Deftos L. Calcium and phosphate homeostasis. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA): 2000. [Google Scholar]
- Szewieczek J, Dulawa J, Francuz T, Legierska K, Hornik B, Wlodarczyk-Sporek I, Janusz-Jenczen M, Batko-Szwaczka A. Mildly elevated blood pressure is a marker for better health status in Polish centenarians. Age. 2015;37:9738. doi: 10.1007/s11357-014-9738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129:666–670. doi: 10.1016/j.mad.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Akushevich I, Arbeeva L, Kravchenko J, Il'yasova D, Kulminski A, Akushevich L, Culminskaya I, Wu D, Ukraintseva SV. Dynamic determinants of longevity and exceptional health. Curr Gerontol Geriatr Res. 2010 doi: 10.1155/2010/381637. http://dx.doi.org/10.1155/2010/381637 (Article ID 381637, 13 pages) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.