Abstract
TRAF1 is a member of the TRAF protein family, which regulates the canonical and noncanonical NF-κB signaling cascades. Although aberrant TRAF1 expression in tumors has been reported, the role of TRAF1 remains elusive. Here, we report that TRAF1 is required for solar UV-induced skin carcinogenesis. Immunohistochemical analysis showed that TRAF1 expression is up-regulated in human actinic keratosis and squamous cell carcinoma. In vivo studies indicated that TRAF1 expression levels in mouse skin are induced by short-term solar UV irradiation, and a long-term skin carcinogenesis study showed that deletion of TRAF1 in mice results in a significant inhibition of skin tumor formation. Moreover, we show that TRAF1 is required for solar UV-induced extracellular signal-regulated kinase–5 (ERK5) phosphorylation and the expression of AP-1 family members (c-Fos/c-Jun). Mechanistic studies showed that TRAF1 expression enhances the ubiquitination of ERK5 on lysine 184, which is necessary for its kinase activity and AP-1 activation. Overall, our results suggest that TRAF1 mediates ERK5 activity by regulating the upstream effectors of ERK5 and also by modulating its ubiquitination status. Targeting TRAF1 function might lead to strategies for preventing and treating skin cancer.
INTRODUCTION
Nonmelanoma skin cancer is the most frequently diagnosed cancer in the United States. Nonmelanoma skin cancers comprise basal cell carcinoma and squamous cell carcinoma (SCC) and most develop on skin exposed to sunlight. The International Agency for Research on Cancer concluded in 1992 that sufficient evidence existed in humans supporting the idea that solar radiation causes cancer, and UVR was classified as a group 1 carcinogen (International Agency for Research on Cancer, 1992). The actual number of nonmelanoma skin cancers is very difficult to estimate because reporting these cases to cancer registries is not required. However, the most recent assessment of occurrence for these skin carcinomas was estimated at 5.4 million cases diagnosed among 3.3 million people in 2012 (American Cancer Society, 2016). Previous epidemiologic evidence suggests that solar UVR is the most important risk factor for skin carcinogenesis (Berwick et al., 2008; Fartasch et al., 2012; Milon et al., 2014). Therefore, understanding the mechanisms of solar UV-induced skin carcinogenesis might lead to effective strategies for preventing and treating skin cancer.
The extracellular signal-regulated kinase (ERK)-5 is a member of the conventional MAPKs consisting of ERK1/2; c-Jun N-terminal kinases 1–3; and p38-α, -β, -γ, and -δ and is similar to ERK1/2, which contain the Thr-Glu-Tyr activation motif (Nishimoto and Nishida, 2006). ERK5 activity is up-regulated by ERK kinase 5 (MEK5) phosphorylation on the Thr-Glu-Tyr motif, and the activation of the MEK5/ERK5 pathway results in AP-1 activation through the induction of c-Fos, c-Jun, and Fra1, which can form AP-1 complexes (Amano et al., 2015; Kayahara et al., 2005; Morimoto et al., 2007; Ren et al., 2007; Terasawa et al., 2003). ERK5 has been shown to be important for human cancers including breast, prostate, liver, and colon cancer (Lochhead et al., 2012; Ortiz-Ruiz et al., 2014; Ramsay et al., 2011; Simoes et al., 2015; Zen et al., 2009). The ERK5 pathway was more recently reported to be involved in keratinocyte proliferation and skin tumorigenesis (Finegan et al., 2015; Wu et al., 2015). Although the ERK5 signaling cascade is activated by various stimuli including cytokines, mitogens, and cellular stresses (Kamakura et al., 1999; Kato et al., 1998; Kayahara et al., 2005; Nakaoka et al., 2003; Nicol et al., 2001; Wang and Tournier, 2006), UVR reportedly failed to induce ERK5 activation (Kamakura et al., 1999; Kato et al., 1997).
The TRAF family includes seven TRAF member proteins (TRAF1–7), and these proteins regulate the canonical and noncanonical NF-κB signaling cascades. TRAF1 is unique among the TRAF proteins because it lacks the RING finger domain (Lee and Choi, 2007), which is associated with E3 ubiquitin ligase activity. TRAF1 expression is detected in a limited number of normal tissues such as lung, spleen, thymus, testis, and skin (Mosialos et al., 1995; Rothe et al., 1994; Zapata et al., 2000). Aberrant expression has been observed in blood and solid tumors including stomach, ovary, kidney, and skin cancers (Guo et al., 2009; Kang et al., 2014; Kiaii et al., 2013; Muehleisen et al., 2012; Rajandram et al., 2012; Wang et al., 2014). Although previous reports suggest that TRAF1 might function and be associated with various cancers, the role of TRAF1 in carcinogenesis remains elusive.
In this study, we showed that TRAF1 is a crucial component of 7, 12-dimethylbenz[a]anthracene (DMBA)/solar UV-induced skin carcinogenesis. Solar UV resulted in ERK5 activation followed by the induction of c-Fos and c-Jun expression, which can form an AP-1 complex in mouse skin. The activation of the ERK5/AP-1 signaling cascade by solar UV was inhibited by the deletion of TRAF1. Moreover, we showed that TRAF1 functions as a modulator of ERK5 ubiquitination, which is required for ERK5 kinase activity.
RESULTS
TRAF1 is up-regulated in actinic keratoses (AKs), SCCs, and solar UVR-irradiated skin
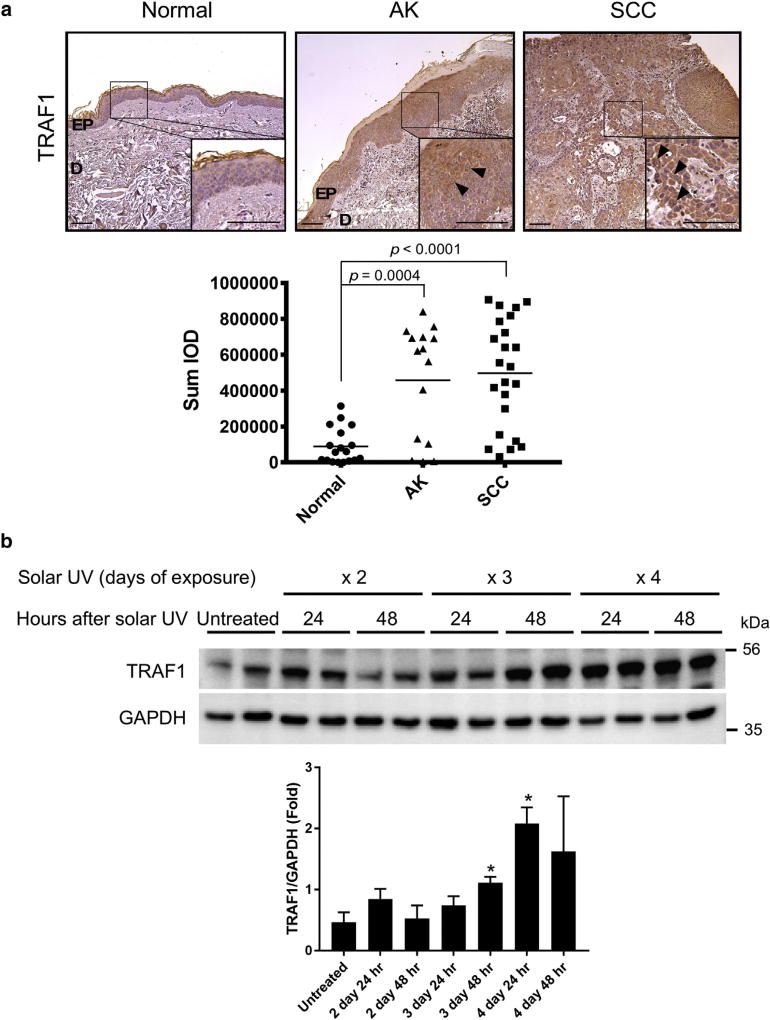
We first examined the protein expression of TRAF1 in human skin tissues, including normal skin, AK, and cutaneous SCC. The mRNA expression of TRAF1 is reportedly increased in human SCC (Muehleisen et al., 2012). Consistent with previous findings, we observed that the protein expression of TRAF1 was up-regulated in both AK and SCC compared with normal skin (Figure 1a), suggesting that the induction of TRAF1 expression might be associated with tumorigenesis. Because the most important risk factor for nonmelanoma skin cancer is UV irradiation (Diepgen et al., 2012; International Agency for Research on Cancer, 2005), we next determined whether solar UV irradiation could induce TRAF1 expression. Mice were subjected to a short-term exposure to solar UV as described in the Materials and Methods. The results showed that TRAF1 expression was up-regulated and sustained after a third irradiation (Figure 1b).
Figure 1. TRAF1 is up-regulated in AKs, SCCs, and solar UV-irradiated skin.
(a) TRAF1 protein expression levels in human skin were analyzed by immunohistochemistry. Representative photos are shown (upper panels), and a density scores obtained from each sample were determined (lower panel). Representative TRAF1 staining is indicated by the arrowheads in the AK and SCC photographs. Statistical significance was determined by one-way analysis of variance. Normal (n = 6), AK (n = 5), SCC (n = 8). Scale bar = 200 µm. (b) TRAF1 expression levels in mouse skin after solar UV irradiation. The shaved backs of BALB/c mice were irradiated with solar UV at a dosage of 149 kJ/m2 UVA and 7.2 kJ/m2 UVB per day for 2–4 days. Mouse skin was collected at the indicated time point. Endogenous TRAF1 expression levels were assessed by immunoblotting (upper) and the quantification of TRAF1 normalized to GAPDH is shown as mean values ± standard deviation (lower, n = 3; *P < 0.05 vs. untreated). Statistical significance was determined by Student t test. AK, actinic keratosis; D, dermis; EP, epidermis; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; IOD, integrated optical density; SCC, squamous cell carcinoma.
TRAF1 is required for DMBA/solar UVR-induced skin carcinogenesis
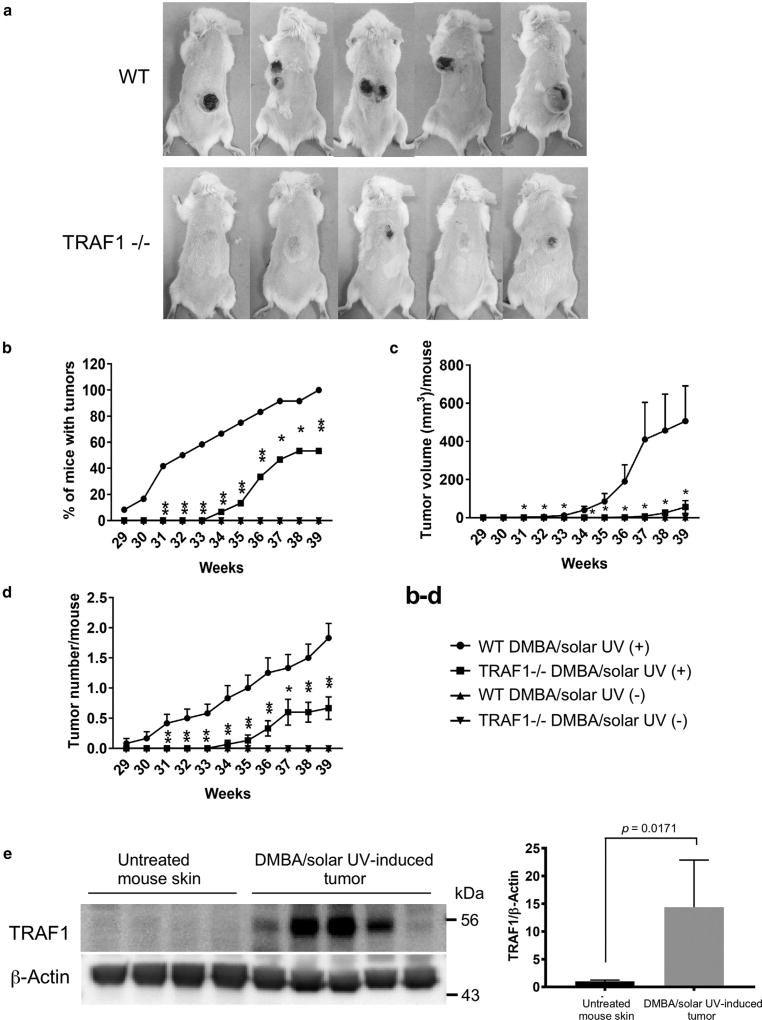
The traditional two-stage skin carcinogenesis model comprises a single administration of a carcinogen, DMBA, followed by promotion with 12-O-tetradecanoylphorbol-13-acetate (TPA) and induces an irreversible H-Ras mutation at the second nucleotide of codon 61 (Fujiki et al., 1989). On the other hand, the UVA and/or UVB-induced skin carcinogenesis model gives rise to mutations of the TP53 gene and has been established to understand the mechanism of skin cancer development (Brash et al., 1991; Liu et al., 2013; Oi et al., 2012). We previously determined whether the BALB/c mouse strain was susceptible to UVR to develop skin tumorigenesis when exposed to chronic solar UVR. The results showed that this mouse model failed to generate any tumors (data not shown). In general, mutations of RAS genes are not frequently observed in actinic keratosis or cutaneous SCCs, and ras genes are rarely mutated in mice skin exposed to UVB radiation (Khan et al., 1996). However, a more recent report showed that 20.5% of patients with aggressive cutaneous SCCs have mutations in the HRAS gene (Pickering et al., 2014). Therefore, mice were subjected to a two-stage carcinogenesis protocol, which consisted of initiation with DMBA and promotion with solar UVR, as described in the Materials and Methods. This protocol resulted in the development of SCC, but not papillomas, on mouse dorsal skin exposed to solar UVR (Figure 2a). However, mice required long-term exposure to solar UVR for tumor development, indicating that this mouse strain is somewhat resistant to UVR. Moreover, we evaluated the requirement for TRAF1 in DMBA/solar UV-induced skin carcinogenesis. Results showed that the incidence, volume, and number of tumors were markedly inhibited in TRAF1-null mice (Figure 2b–d) compared with wild-type mice. Consistent with the results shown in Figure 1a, TRAF1 was strongly expressed in skin tumor samples (Figure 2e). These results suggest that TRAF1 is required for solar UV-induced skin cancer formation with a mutation of the HRAS gene.
Figure 2. TRAF1 is required for solar UV-induced skin carcinogenesis.
(a) Mouse skin tumors were induced by chronic solar UV irradiation. One group each of WT and TRAF1−/− mice received no solar UVR (n = 10 each) but received topical treatment with a single dose of 7, 12-dimethyl-benzanthracene (DMBA). One group each of WT and TRAF1−/− mice were irradiated with solar UVR (n = 15 each; maximum dose = 60 kJ/m2 UVA and 2.9 kJ/m2 UVB) after treatment with a single dose of DMBA. The solar UV irradiation was administered 3 times a week for 35 weeks. Representative photos of mice are shown. (b) Tumor incidence was determined, and data are shown as mean values ± standard error. *P < 0.05, **P < 0.01 versus WT mice treated with solar UV (+). Statistical significance was determined by one-way analysis of variance. (c) Average tumor volume per mouse was calculated according to the following formula: tumor volume (mm3) = length × width × 0.52. Data are shown as mean values ± standard error, and statistical significance was determined by one-way analysis of variance. The asterisk indicates a significant decrease in tumor volume of the solar UVR-treated TRAF−/− (+) group compared to the WT solar UVR (+)-treated group (P < 0.05). (d) Tumor number was determined, and data are shown as mean values ± standard error. Statistical significance was determined by one-way analysis of variance. The asterisk indicates a significant decrease in tumor number of the solar UV-treated TRAF−/− (+) group compared with the WT solar UV (+)-treated group. *P < 0.05, **P < 0.01. (e) TRAF1 expression is increased in solar UVR-induced skin tumors. At the end of this study, mouse skin and tumor samples were collected from the WT no-solar UV group and the WT solar UV (+) group. TRAF1 expression levels of skin and tumor samples were assessed by immunoblotting (left), and the quantification of TRAF1 normalized to β-actin is shown as mean values ± standard deviation (lower; n = 4–5, P-value versus normal). Statistical significance was determined by Student t test. WT, wild type.
TRAF1 regulates the ERK5/AP-1 pathway
Reportedly, ERK5 is not activated by UV irradiation in COS7 and CHO-K1 cells (Kamakura et al., 1999; Kato et al., 1997). We determined whether solar UVR could induce the phosphorylation of ERK5, and we found that treatment of adult human epidermal keratinocyte (HEKa) cells with solar UVR or EGF resulted in strong phosphorylation of ERK5 and phosphorylation of ERK1/2 (Figure 3a, left), followed by the induction of c-Fos (Figure 3a, right, and see Supplementary Figure S1a online). Total ERK5 and ERK1/2 were not changed (Figure 3a, middle, and see Supplementary Figure S1a). We also determined whether TNF-α treatment affected the ERK5 pathway, because two transcription factor complexes, AP-1 and NF-κB, are strongly implicated in mediating the UVR response. The results showed that TNFα could induce a weak ERK5 phosphorylation resulting in no c-Fos accumulation (see Supplementary Figure S2 online). Because solar UVR could induce the phosphorylation of both ERK5 and ERK1/2, we determined whether the induction of c-Fos by solar UV is affected by inhibition of the MEK5/ERK5 pathway. The results showed that the induction of c-Fos in HEKa cells was suppressed by treatment with PD98059, a specific inhibitor of MEK1, or BIX02188, a specific inhibitor of MEK5 (Figure 3b, and see Supplementary Figure S1b), suggesting that the ERK5, as well as the ERK1/2, signaling pathway independently affected the induction of c-Fos by solar UVR. We next examined whether the genetic loss of TRAF1 influences the activation of the solar UVR-induced ERK1/2 or ERK5/AP-1 pathway. Mice were subjected to a short-term exposure to solar UVR, and then epidermal lysates were analyzed by immunoblotting. The deletion of TRAF1 reduced ERK5, but not ERK1/2, phosphorylation and inhibited c-Fos and c-Jun induction by solar UV irradiation (Figure 3c and d, and see Supplementary Figure S1c and d). These results show that TRAF1 is involved in the solar UVR-induced ERK5/AP-1 pathway.
Figure 3. TRAF1 regulates the ERK5/AP-1 pathway.
(a) Solar UV irradiation induces ERKS phosphorylation. HEKa cells were cultured in EpiLife medium (Thermo Fisher Scientific, Waltham, MA) with human keratinocyte growth supplement and then incubated in defined keratinocyte–serum-free medium with supplement for 24 hours before being exposed to solar UVR at a dose of 60 kJ/m2 UVA and 2.9 kJ/m2 UVB. EGF (5 ng/ml) was used as a positive control. Cells were harvested at the indicated time point after solar UVR or EGF exposure, and samples were prepared for Western blot analysis. (b) Inhibition of the MEKS/ERKS pathway impairs solar UVR-induced c-Fos expression. HEKa cells were incubated in defined keratinocyte–serum-free medium with supplement for 24 hours and then treated with 25 µmol/L PD980S9 and/or 25 µmol/L BIX02188 for 1 hour before being exposed to solar UVR at a dose of 60 kJ/m2 UVA and 2.9 kJ/m2 UVB. Cells were harvested at the indicated time point, and samples were prepared for Western blot analysis. (c, d) TRAF1 is required for solar UVR-induced activation of the ERKS/AP-1 pathway in mouse skin. The shaved back of mice was irradiated with solar UVR at a dosage of 149 kJ/m2 UVA and 7.2 kJ/m2 UVB per day for 2 days. Mice were killed at the required time point (c, at 1 hour; d, at 1, 3, or 6 hours) and then skin was collected. The epidermis from each piece of mouse skin was scraped and homogenized with RIPA buffer and then analyzed by Western blot (left). See Supplementary Figure S2 for quantitation. ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HEKa, adult human epidermal keratinocyte; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; min, minutes; p-, phosphorylated; WT, wild type.
TRAF1 mediates ERK5 ubiquitination
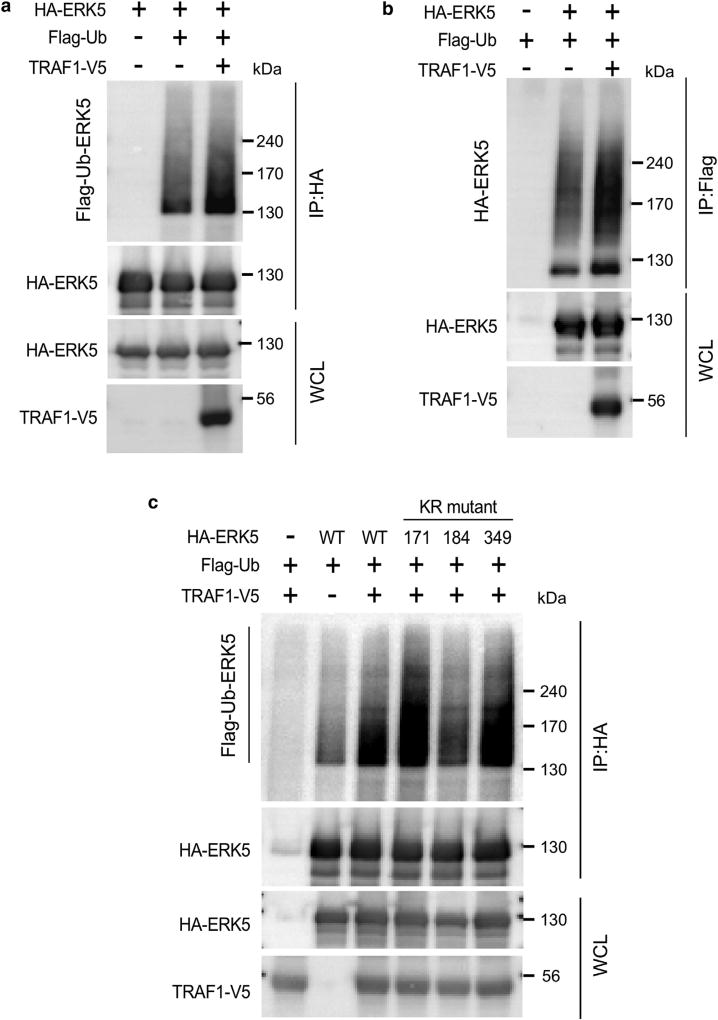
We found that when TRAF1-V5 and hemagglutinin (HA)-ERK5 were co-transfected into human embryonic kidney 293 (HEK293)T cells, TRAF1 expression increased ubiquitination of ERK5 (Figure 4a and b). To identify the sites of ERK5 that are responsible for ubiquitination, we generated a series of ERK5 plasmids containing a lysine residue mutation on lysine (K) 171, K184, or K349 to arginine based on mass spectrometry analysis (Table 1) and then examined the susceptibility of these ERK5 mutants to ubiquitination. The results showed that TRAF1-mediated ERK5 ubiquitination was significantly decreased in the K184R mutant but not the K171R or K349R mutant (Figure 4c). These results indicate that TRAF1 expression induces ubiquitination of ERK5 on K184.
Figure 4. TRAF1 mediates ERK5 ubiquitination.
(a, b) TRAF1 expression induces the ubiquitination of ERKS. HA-ERKS was co-expressed with TRAF1-VS along with Flag-ubiquitin in HEK293T cells. Cells were harvested, and then samples were prepared for Western blot analysis. HA-ERKS or Flag-ubiquitin was immunoprecipitated with anti-HA or anti-Flag and then ubiquitinated-ERKS was detected by immunoblot analysis. (c) TRAF1 expression induces the ubiquitination of ERKS on K184. HA-ERKS WT or the K171 R, K184R, or K349R mutant protein was co-expressed with TRAF1-VS, along with Flag-ubiquitin in HEK293T cells. HA-ERKS was immunoprecipitated with anti-HA, and then ubiquitinated-ERKS was detected by immunoblot analysis. ERK, extracellular signal-regulated kinase; HA, hemagglutinin; HEK, human embryonic kidney; IP, immunoprecipitation; K, keratin; KR, lysine to arginine; Ub, ubiquitin; WCL, whole cell lysates; WT, wild type.
Table 1.
Mass spectrometry analysis was used to identify the ubiquitination sites of ERK5
| Conf | Sc | Prec m/z | z | Sequence | Theor MW | ΔMass | Site |
|---|---|---|---|---|---|---|---|
| 99 | 16 | 562.2949 | 3 | GLK-ubY1 M 2 HASQVIHR | 1,683.8628 | −0.0293 | K171 |
| 41.4 | 12 | 1008.999 | 2 | DLK-ubP2 SNLLVNENC 3 ELK | 2,015.9834 | −0.0197 | K184 |
| 44.1 | 14 | 816.8583 | 4 | HPFLAK-ubY1 H 2 DPDDEPD C3 APPFDFAFDR | 3,263.4043 | −0.0269 | K349 |
Abbreviations: ΔMass, the difference between theoretical MW and experimental MW of the matching peptide sequence; Conf, the confidence for the peptide identification; ERK5, extracellular signal-regulated kinase 5; K, lysine; Prec m/z, precursor m/z; Sc, the score for the peptide; Theor MW, theoretical precursor molecular weight for peptide sequence; z, the charge for the fragmented ion.
Tyrosine oxidation to 2-aminotyrosine.
Oxidation.
Carbamidomethyl.
Ubiquitination of ERK5 on K184 is required for kinase activity and AP-1 activation
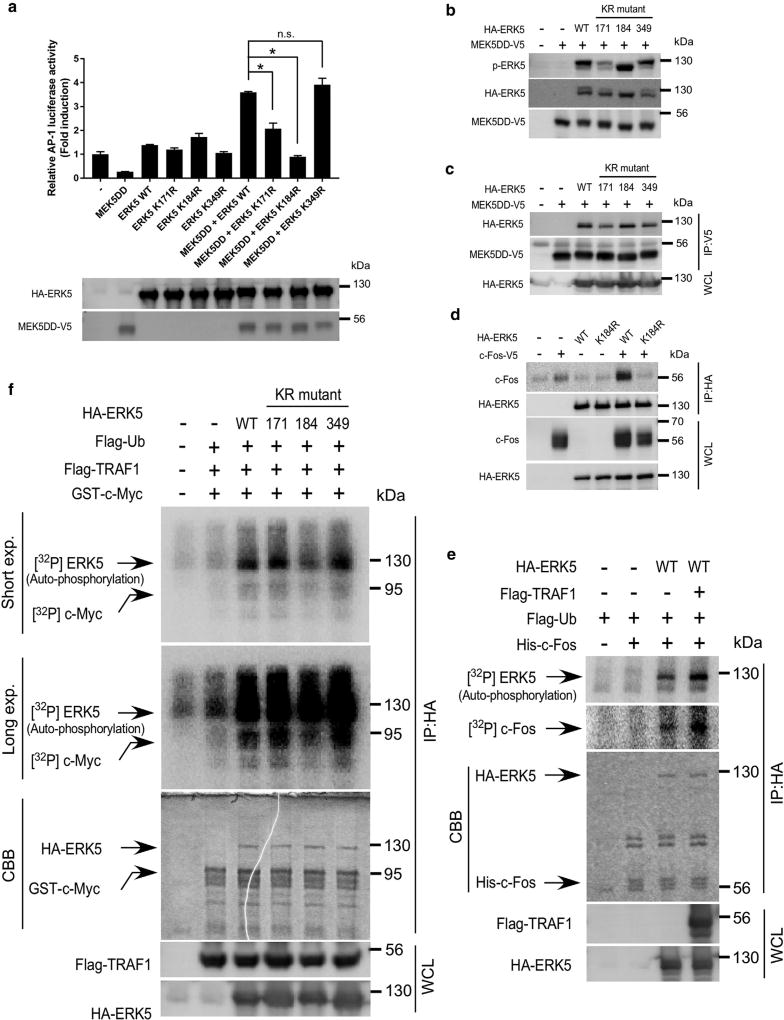
To examine the role of ERK5 ubiquitination, we next determined how the mutant ERK5 K171R, K184R, or K349R affects AP-1 activity, which is induced by a constitutively active mutant of MEK5 (MEK5 DD). The results clearly showed that K184R failed to induce AP-1 activation when K184R was expressed with the MEK5 DD protein under conditions in which the respective proteins were expressed at similar levels in HEK293T cells (Figure 5a, upper and lower panels). These results indicate that ubiquitination of ERK5 on K184 is necessary for AP-1 activation induced by MEK5 DD. We then determined how ERK5 ubiquitination on K184 affects AP-1 activity. Wild-type (WT) HA-ERK5, K171R, K184R, or K349R and MEK5 DD-V5 were co-expressed in HEK293T cells, and then the phosphorylation of HA-ERK5 or co-immunoprecipitation of HA-ERK5 and MEK5DD-V5 was detected by immunoblotting. We observed that both the phosphorylation of ERK5 and its interaction with MEK5 DD were not impaired in the K184R mutant compared with WT (Figure 5b and c), indicating that K184 ubiquitination is not required for its phosphorylation by or interaction with MEK5. Moreover, to understand how the K184R mutant affects the interaction of ERK5 with c-Fos, a substrate of ERK5, anti-HA immunoprecipitates of HEK293T cell lysates, expressing HA-ERK5 WT or K184R along with c-Fos-V5, were subjected to anti-V5 immunoblotting. The results showed that WT, but not K184R, interacted with c-Fos, indicating that ERK5 ubiquitination on K184 is required for its interaction with c-Fos (Figure 5d). Furthermore, we determined whether TRAF1-induced ubiquitination of ERK5 contributes to its kinase activity. Anti-HA immunoprecipitates of HEK293T cell lysates, expressing HA-ERK5 and/or FLAG-TRAF1 along with FLAG-ubiquitin, were subjected to an in vitro kinase assay with or without His-tagged c-Fos proteins, which were purified from Escherichia coli. We found that both the autophosphorylation of ERK5 and the phosphorylation of c-Fos were increased when ERK5 was expressed with TRAF1 (Figure 5e), suggesting that the ubiquitination of ERK5 enhances its kinase activity. Moreover, we evaluated the effects of a series of mutants with another known substrate, c-Myc. Results showed that K184R exhibited decreased kinase activity (Figure 5f). Overall, these results suggest that ERK5 kinase activity is not only regulated by MEK5 but also depends on its ubiquitination status.
Figure 5. Ubiquitination of ERK5 on K184 is required for its kinase activity and AP-1 activation.
(a) Ubiquitination of ERKS on K184 is required for MEK5-induced AP-1 activation. HA-ERK5 WT or the K171R, K184R, or K349R mutant protein was co-expressed with a MEKS DD-VS mutant along with pGL3-AP-1-luc and pCMV-β-gal in HEK293T cells. At 24 hours after transfection, cells were disrupted with lysis buffer, and then the lysates were used for a reporter assay and immunoblotting. Firefly luciferase activities were normalized against β-galactosidase activity. Data are represented as mean values ± standard deviation from three independent experiments performed with triplicate samples. The level of statistical significance was determined by the Student t test. The asterisk indicates a significant decrease in two of the MEK5 DD mutants (ERK5 K171R, ERK5 K184R) compared with the MEK5 DD ERK5 WT group (P < 0.01). (b) ERK5 phosphorylation is impaired in the K171R and K349R mutant proteins. MEK5 DD-V5 was co-expressed with HA-ERK5 WT or the mutant K171R, K184R, or K349R protein in HEK293T cells. Cells were harvested and samples were prepared for Western blot analysis. (c) The ERK5 interaction with MEK5 DD is impaired in the K171R and K349R mutant proteins. MEK5 DD-V5 was co-expressed with HA-ERK5 WT or the K171R, K184R, or K349R mutant protein in HEK293T cells. Cells were disrupted with lysis buffer, and MEK5 DD-V5 was immunoprecipitated with anti-V5, and then HA-ERK5 or MEK5 DD-V5 was detected by immunoblot analysis. (d) ERK5 ubiquitination on K184 is required for the interaction with c-Fos. HA-ERK5 WTor K184R was co-expressed with c-Fos-V5 in HEK293T cells. Cells were solubilized with lysis buffer, and HA-ERK5 WTor HA-ERK5 K184R was immunoprecipitated with anti-HA, and then HA-ERK5 or c-Fos was detected by immunoblotting analysis. (e) TRAF1 expression induces the kinase activation of ERK5. Anti-HA immunoprecipitates of HEK293T cell lysates, expressing HA-ERK5 and/or FLAG-TRAF1 along with FLAG-ubiquitin, were subjected to an in vitro kinase assay with or without His-tagged c-Fos proteins. Auto-phosphorylation of ERK5 or ERK5 phosphorylation of c-Fos was detected by autoradiography. Protein levels of HA-ERK5 and His-c-Fos used for an in vitro kinase assay were assessed by Coomassie brilliant blue (CBB) staining. (f) ERK5 ubiquitination on K184 is required for its kinase activity. Anti-HA immunoprecipitates of HEK293T cell lysates, expressing HA-ERK5 WT or the mutant K171R, K184R, or K349R protein and/or FLAG-TRAF1 along with FLAG-ubiquitin, were subjected to an in vitro kinase assay with or without GST-tagged c-Myc proteins. Auto-phosphorylation of ERK5 or ERK5 phosphorylation of c-Myc was detected by autoradiography. Protein levels of HA-ERK5 and GST-c-Myc used for in vitro kinase assay were assessed by Coomassie brilliant blue (CBB) staining. CBB, Coomassie brilliant blue; ERK, extracellular signal-regulated kinase; exp., exposure; GST, glutathione S-transferase; IP, immunoprecipitation; KR, lysine to arginine; HA, hemagglutinin; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase; n.s., not significant; p-, phosphorylated; Ub, ubiquitin; WT, wild type.
DISCUSSION
The AP-1 transcription factors are key regulators of epidermal keratinocyte survival and differentiation and important drivers of skin cancer development (Briso et al., 2013; Eckert et al., 2013). According to a report, inducible expression of c-Fos in mouse epidermis was shown to promote skin inflammation through chronic CD4 T-cell recruitment, resulting in the acceleration of DMBA-induced papilloma and SCC development (Briso et al., 2013). Although mechanistic studies regarding UVR have suggested that AP-1 activity is increased by UVA, UVB, or UVC through the activation of the MAPK signaling cascades (Bode and Dong, 2003), the role of solar UVR (approximately 95% UVA and 5% UVB) on the MAPK/AP-1 signaling cascade is less known (Liu et al., 2013). In this study, we discovered that solar UV stimulates not only ERK1/2 but also the ERK5 signaling cascade, resulting in the increase of AP-1 components in HEKa cells (primary human keratinocytes) and also in mouse skin. The induction of c-Fos is inhibited by either MEK1 or a MEK5 inhibitor in HEKa cells. These results suggest that not only ERK1/2 but also ERK5 activation are required for AP-1 induction by solar UVR. Remarkably, we found that the deletion or the depletion of TRAF1 results in the inhibition of ERK5 phosphorylation and AP-1 activity. The deletion or the inhibition of ERK5 has been reported to result in the inhibition of DMBA/TPA-induced skin tumor formation (Finegan et al., 2015), suggesting that ERK5 kinase activity is important for a carcinogen-induced skin tumorigenesis. Another report indicated that TRAF4 acts as an adaptor protein for the Act1-mediated MAPK kinase kinase-3–ERK5 pathway, and TRAF4 has been shown to be required for IL-1 7–induced ERK5 phosphorylation and DMBA/TPA-induced skin carcinogenesis (Wu et al., 2015). Our results showed that TRAF1 also regulates ERK5 phosphorylation. These findings lead to the idea that TRAF1 and TRAF4 might form a complex and regulate ERK5 activity. More mechanistic studies are needed for not only TPA- or IL-1 7–induced skin cancer but also solar UVR-induced skin carcinogenesis. Collectively, these results suggest that the TRAF1/ERK5/AP-1 signaling cascade might be important for solar UVR-induced cutaneous SCC formation.
Ubiquitin is a highly evolutionarily-conserved protein consisting of 76 amino acids. The ubiquitination cascade comprises three enzymes referred to as E1, E2, and E3 (Yang et al., 2010). Seven lysine residues in ubiquitin have been identified, and the roles of K48-linked and K63-linked poly-ubiquitination are well-studied. K48-linked ubiquitination triggers protein degradation, whereas K63-linked ubiquitination regulates a variety of nonproteolytic cellular functions, including DNA damage repair, stress responses, and inflammatory pathways (Zhang et al., 2013). Although ERK1/2 are ubiquitinated by MEKK1, resulting in their degradation (Lu et al., 2002), no group has reported ERK5 ubiquitination and its role. We found that TRAF1, which lacks a RING finger domain needed for E3 ligase activity, induces ERK5 ubiquitination on K184, where the kinase domain is located. Mechanistic studies showed that a K184R ERK5 mutant, which prevents ubiquitination at that site, permits MEK5 phosphorylation of ERK5 on the Thr-Glu-Tyr activation motif. Our results have shown that the phosphorylatable ERK5 mutant with K184R failed to induce AP-1 activity. Our findings provide evidence supporting a regulation of MAPKs in skin carcinogenesis.
Overall, our results clearly show a role of TRAF1 in solar UVR-induced skin carcinogenesis mediated through the ERK5/AP-1 signaling cascade. TRAF1 functions as a regulator of ERK5 by modulating its ubiquitination. Clarifying how ERK5 kinase activity is regulated other than by its phosphorylation on the Thr-Glu-Tyr activation motif by upstream kinase MEK5 will be interesting. In conclusion, inhibiting ERK5/AP-1 by targeting TRAF1 might be a strategy for preventing and treating skin cancer.
MATERIALS AND METHODS
Cell culture and transfection
Primary HEKa cells were obtained from Thermo Fisher Scientific (Waltham, MA) and cultured at 37 °C in a humidified incubator with 5% CO2 in EpiLife medium (Thermo Fisher Scientific) supplemented with 60 µM CaCl2 and human keratinocyte growth supplement (Thermo Fisher Scientific). The second passage of HEKa cells was used in these studies. HEK293 cells and HEK293T cells (stably expressing the SV40 large Tantigen in HEK293 cells) from the American Type Culture Collection (Manassas, VA) were cultured at 37 °C in a humidified incubator with 5% CO2 according to American Type Culture Collection protocols in DMEM (Corning, Manassas, VA), supplemented with 10% (volume/volume) fetal bovine serum (Corning) and 1% penicillin/streptomycin (GenDEPOT, Barker, TX). Cells were cytogenetically tested and authenticated before being frozen. Each vial of frozen cells was thawed and maintained for 2 months (10 passages). Cells were transfected using iMFectin poly DNA transfection reagent (GenDEPOT) according to the manufacturer’s instructions.
Animal studies
All animal procedures were performed following guidelines approved by the University of Minnesota Institutional Animal Care and Use Committee. BALB/c WT (+/+) and BALB/c-TRAF1 knockout (−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and TRAF1−/− mice were genotyped by PCR analysis with the primers 5′-GCCAGAGGCCACTTGTGTAG-3′, 5′-CAGAACCCCTTGCCTAATCC-3′, and 5′-TCCTAGAGGCCTGCTGCTAA-3′. Only male mice were included for solar UV irradiation using UVA-340 lamps (Q-Lab Corporation, Westlake, OH). The animals were housed in climate-controlled quarters with a 12-hour light/dark cycle and allowed full access to food and water.
For skin carcinogenesis, the shaved backs of mice (6–7 weeks old) were subjected to a single application of DMBA (50 µg in 0.25 ml of acetone). Two weeks after DMBA application, the dorsal skin was irradiated with solar UVR. The solar UVR irradiation was administered 3 times a week for 35 weeks, as will be described. At week 1, mice were irradiated with solar UVR at a dose of 36 kJ/m2 UVA and 1.8 kJ/m2 UVB 3 times a week. The dose of solar UVR was progressively increased (10% each week). At week 6, the dose of solar UV reached 60 kJ/m2 UVA and 2.9 kJ/m2 UVB, and this dose was maintained from weeks 6 through 35. Mice were weighed and tumors measured by caliper once a week until week 39 or until tumors reached 1 cm3 total volume, at which time mice were killed, and then skin was collected for further analysis.
For short-term exposure to solar UVR irradiation, the shaved backs of the mice (6–8 weeks old) were irradiated with solar UVR at a dosage of 149 kJ/m2 UVA and 7.2 kJ/m2 UVB per day for 2 to 4 days. Mice were killed at the required time point, and then skin was collected for further analysis.
Preparation of epidermal samples
The epidermis from each mouse skin was scraped onto a dish on dry ice. Each epidermis was homogenized with RIPA buffer (50 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, and a protease inhibitor mixture).
Protein kinase assay
At 36 hours after transfection, cells were disrupted with lysis buffer (50 mmol/L Tris-HCl pH 7.4, 1% (volume/volume) Nonidet P-40, 150 mmol/L NaCl, 5 mmol/L EDTA, and protease inhibitor mixture) with 20 mmol/L N-ethylmaleimide (Alfa Aesar, Ward Hill, MA). Whole cell lysates were immunoprecipitated with anti-HA. The immunoprecipitates were washed 3 times with lysis buffer, then once with kinase buffer (25 mmol/L Tris-HCl pH 7.5, 5 mmol/L β-glycerophosphate, 2 mmol/L dithiothreitol, 0.1 mmol/L Na3VO4, and 10 mmol/L MgCl). The immunoprecipitates were resuspended in 30 µl of kinase buffer containing 10 µmol/L unlabeled ATP and 10 µCi γ[32P]ATP in the presence or absence of 0.5 µg of recombinant His-c-Fos protein and incubated for 20 minutes at 30 °C The reaction was terminated by the addition of Laemmli sample buffer, and samples were separated by SDS-PAGE (9%). Subsequently, the gels were subjected to autoradiography.
Ubiquitination assay
The in vivo ubiquitination assay was performed as described previously (Choo and Zhang, 2009). Briefly, transfected cells were disrupted in lysis buffer (2% SDS, 150 mmol/L NaCl, 10 mmol/L Tris-HCl pH 8.0, 20 mmol/L N-ethylmaleimide, and a protease inhibitor mixture). Lysates were boiled for 10 minutes immediately and then sonicated. Sonicated lysates were diluted with buffer (10 mmol/L Tris-HCl pH 8.0, 150 mmol/L NaCl, 2 mmol/L EDTA, and 1% Triton X-100). The lysates were immunoprecipitated as described, and then the immunoprecipitates were washed 4 times with washing buffer (10 mmol/L Tris-HCl pH 8.0, 1 mol/L NaCl, 1 mmol/L EDTA, and 1% Nonidet P-40). The respective specific antibodies were used for detection of ubiquitinated proteins by Western blotting.
Liquid chromatography-mass spectrometry/mass spectrometry analysis to identify ubiquitination of ERK5
HA-ERK5 was co-expressed with or without Flag-TRAF1, along with Flag-ubiquitin, into HEK293T cells. Cells were solubilized with lysis buffer, and then cell lysates were immunoprecipitated with anti-HA. The immunoprecipitates was denatured by urea buffer (7 mol/L urea, 2 mol/L thiourea, 2% 3-[(3-Cholamidopropyl dimethylammonio] propanesulfonate (CHAPS), reduced with 4 mmol/L dithiothreitol for 1 hour at 37 °C, and then alkylated with 14 mmol/L iodoacetamide for 45 minutes at room temperature under dark conditions. Excess iodoacetamide was quenched with excess dithiothreitol to provide a final concentration of 7 mmol/L. Subsequently, the sample was diluted with 25 mmol/L ammonium bicarbonate to ensure less than 1 mol/L urea content, and digested with trypsin (Promega, Madison, WI) at an enzyme content of 2% (weight/weight) for 16 hours at 37 °C. These tryptic peptides were dried by vacuum evaporation using a speed vacuum and then cleaned up with a Sep-Pak C18 cartridge (Waters, Milford, MA). The ABSciex TripleTOF 5600 system (AB Sciex, Framingham, MA) coupled with Eksigent 1D + nano LC system (AB Sciex) was used to identify ERK5 ubiquitination. The mass spectrometry was calibrated by acquisition of [Glu1] fibrinopeptide (25 fmole/µl). The raw data were processed and searched with ProteinPliot software (version 4.0) (AB Sciex) using the Paragon algorithm. Proteins were identified by searching the UniProtKB mouse database (www.uniprot.org).
Statistical analysis
All quantitative data are expressed as mean value ± standard deviation of at least three independent experiments or samples. Significant differences were determined by Student t test or by one-way analysis of variance. A probability value of P less than 0.05 or P less than 0.01 was used as the criterion for statistical significance.
Additional detailed materials and methods are described in Supplementary Materials.
Supplementary Material
Acknowledgments
We thank Clara Curiel (University of Arizona Cancer Center) for human skin tissues, Akihiko Yoshimura (Keio University, Japan) for the Flag-TRAF1 plasmid, Alyssa Langfald for microscopy analysis, and Nicki Brickman for assistance in submitting this manuscript. This work was supported by The Hormel Foundation and National Institutes of Health grants CA196639, CA187027, CA166011, and CA027502.
Abbreviations
- AK
actinic keratosis
- DMBA
7,12-dimethylbenz[a]anthracene
- ERK
extracellular signal-regulated protein kinase
- HA
hemagglutinin
- HEKa
adult human epidermal keratinocyte
- HEK
human embryonic kidney
- K
keratin
- MAPK
mitogen-activated protein kinase
- MEK5
ERK kinase 5
- SCC
squamous cell carcinoma
- TPA
12-O-tetradecanoylphorbol-13-acetate
- WT
wild type
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2016.12.026.
References
- Amano S, Chang YT, Fukui Y. ERK5 activation is essential for osteoclast differentiation. PLOS One. 2015;10(4):e0125054. doi: 10.1371/journal.pone.0125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- Berwick M, Lachiewicz A, Pestak C, Thomas N. Solar UV exposure and mortality from skin tumors. Adv Exp Med Biol. 2008;624:117–24. doi: 10.1007/978-0-387-77574-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003(167):RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88(22):10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briso EM, Guinea-Viniegra J, Bakiri L, Rogon Z, Petzelbauer P, Eils R, et al. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013;27(18):1959–73. doi: 10.1101/gad.223339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo YS, Zhang Z. Detection of protein ubiquitination. J Vis Exp. 2009;(30):1293. doi: 10.3791/1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepgen TL, Fartasch M, Drexler H, Schmitt J. Occupational skin cancer induced by ultraviolet radiation and its prevention. Br J Dermatol. 2012;167(Suppl. 2):76–84. doi: 10.1111/j.1365-2133.2012.11090.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Adhikary G, Young CA, Jans R, Crish JF, Xu W, et al. AP1 transcription factors in epidermal differentiation and skin cancer. J Skin Cancer. 2013;2013:537028. doi: 10.1155/2013/537028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fartasch M, Diepgen TL, Schmitt J, Drexler H. The relationship between occupational sun exposure and non-melanoma skin cancer: clinical basics, epidemiology, occupational disease evaluation, and prevention. Dtsch Arztebl Int. 2012;109(43):715–20. doi: 10.3238/arztebl.2012.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegan KG, Perez-Madrigal D, Hitchin JR, Davies CC, Jordan AM, Tournier C. ERK5 is a critical mediator of inflammation-driven cancer. Cancer Res. 2015;75:742–53. doi: 10.1158/0008-5472.CAN-13-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Yoshizawa S, Kanazawa H, Sugimura T, Manam S, et al. Codon 61 mutations in the c-Harvey-ras gene in mouse skin tumors induced by 7,12-dimethylbenz[a]anthracene plus okadaic acid class tumor promoters. Mol Carcinog. 1989;2:184–7. doi: 10.1002/mc.2940020403. [DOI] [PubMed] [Google Scholar]
- Guo F, Sun A, Wang W, He J, Hou J, Zhou P, et al. TRAF1 is involved in the classical NF-kappaB activation and CD30-induced alternative activity in Hodgkin’s lymphoma cells. Mol Immunol. 2009;46(13):2441–8. doi: 10.1016/j.molimm.2009.05.178. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Working group reports. Lyon, France: WHO press; 2005. Exposure to artificial UV radiation and skin cancer. [Google Scholar]
- International Agency for Research on Cancer. Solar and ultraviolet radiation. Vol. 55. Lyon, France: WHO press; 1992. IARC monographs on the evaluation of carcinogenic risk of chemicals to man. [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274(37):26563–71. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee MJ, Song JA, Jeong JY, Kim YK, Lee C, et al. Overexpression of goosecoid homeobox is associated with chemoresistance and poor prognosis in ovarian carcinoma. Oncol Rep. 2014;32(1):189–98. doi: 10.3892/or.2014.3203. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16(23):7054–66. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395(6703):713–6. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- Kayahara M, Wang X, Tournier C. Selective regulation of c-jun gene expression by mitogen-activated protein kinases via the 12-o-tetradecanoylphorbol-13-acetate- responsive element and myocyte enhancer factor 2 binding sites. Mol Cell Biol. 2005;25(9):3784–92. doi: 10.1128/MCB.25.9.3784-3792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SG, Mohan RR, Katiyar SK, Wood GS, Bickers DR, Mukhtar H, et al. Mutations in ras oncogenes: rare events in ultraviolet B radiation-induced mouse skin tumorigenesis. Mol Carcinog. 1996;15:96–103. doi: 10.1002/(SICI)1098-2744(199602)15:2<96::AID-MC2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kiaii S, Kokhaei P, Mozaffari F, Rossmann E, Pak F, Moshfegh A, et al. T cells from indolent CLL patients prevent apoptosis of leukemic B cells in vitro and have altered gene expression profile. Cancer Immunol Immunother. 2013;62(1):51–63. doi: 10.1007/s00262-012-1300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Choi Y. TRAF1 and its biological functions. Adv Exp Med Biol. 2007;597:25–31. doi: 10.1007/978-0-387-70630-6_2. [DOI] [PubMed] [Google Scholar]
- Liu K, Yu D, Cho YY, Bode AM, Ma W, Yao K, et al. Sunlight UV-induced skin cancer relies upon activation of the p38alpha signaling pathway. Cancer Res. 2013;73:2181–8. doi: 10.1158/0008-5472.CAN-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead PA, Gilley R, Cook SJ. ERK5 and its role in tumour development. Biochem Soc Trans. 2012;40:251–6. doi: 10.1042/BST20110663. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell. 2002;9:945–56. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- Milon A, Bulliard JL, Vuilleumier L, Danuser B, Vernez D. Estimating the contribution of occupational solar ultraviolet exposure to skin cancer. Br J Dermatol. 2014;170:157–64. doi: 10.1111/bjd.12604. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Kondoh K, Nishimoto S, Terasawa K, Nishida E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J Biol Chem. 2007;282(49):35449–56. doi: 10.1074/jbc.M704079200. [DOI] [PubMed] [Google Scholar]
- Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–99. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- Muehleisen B, Jiang SB, Gladsjo JA, Gerber M, Hata T, Gallo RL. Distinct innate immune gene expression profiles in non-melanoma skin cancer of immunocompetent and immunosuppressed patients. PloS One. 2012;7(7):e40754. doi: 10.1371/journal.pone.0040754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–9. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–67. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–6. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi N, Chen H, Ok Kim M, Lubet RA, Bode AM, Dong Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer Prev Res (Phila) 2012;5:1103–14. doi: 10.1158/1940-6207.CAPR-11-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ruiz MJ, Alvarez-Fernandez S, Parrott T, Zaknoen S, Burrows FJ, Ocana A, et al. Therapeutic potential of ERK5 targeting in triple negative breast cancer. Oncotarget. 2014;5(22):11308–18. doi: 10.18632/oncotarget.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20(24):6582–92. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajandram R, Bennett NC, Wang Z, Perry-Keene J, Vesey DA, Johnson DW, et al. Patient samples of renal cell carcinoma show reduced expression of TRAF1 compared with normal kidney and functional studies in vitro indicate TRAF1 promotes apoptosis: potential for targeted therapy. Pathology. 2012;44:453–9. doi: 10.1097/PAT.0b013e3283557748. [DOI] [PubMed] [Google Scholar]
- Ramsay AK, McCracken SR, Soofi M, Fleming J, Yu AX, Ahmad I, et al. ERK5 signalling in prostate cancer promotes an invasive phenotype. Br J Cancer. 2011;104:664–72. doi: 10.1038/sj.bjc.6606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ma X, Li Y. All-trans retinoic acid regulates c-jun expression via ERK5 in cardiac myoblasts. J Nutr Biochem. 2007;18:832–8. doi: 10.1016/j.jnutbio.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–92. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Simoes AE, Pereira DM, Gomes SE, Brito H, Carvalho T, French A, et al. Aberrant MEK5/ERK5 signalling contributes to human colon cancer progression via NF-kappaB activation. Cell Death Dis. 2015;6:e1718. doi: 10.1038/cddis.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa K, Okazaki K, Nishida E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes to Cells. 2003;8:263–73. doi: 10.1046/j.1365-2443.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Bu G, Feng Q, Liu Z, Xu C, Shen S, et al. The expression level of TRAF1 in human gastric mucosa is related to virulence genotypes of Helicobacter pylori. Scan J Gastroenterol. 2014;49:925–32. doi: 10.3109/00365521.2014.919015. [DOI] [PubMed] [Google Scholar]
- Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–60. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med. 2015;212:1571–87. doi: 10.1084/jem.20150204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Zhang X, Lin HK. Emerging role of Lys-63 ubiquitination in protein kinase and phosphatase activation and cancer development. Oncogene. 2010;29(32):4493–503. doi: 10.1038/onc.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata JM, Krajewska M, Krajewski S, Kitada S, Welsh K, Monks A, et al. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J Immunol. 2000;165:5084–96. doi: 10.4049/jimmunol.165.9.5084. [DOI] [PubMed] [Google Scholar]
- Zen K, Yasui K, Nakajima T, Zen Y, Zen K, Gen Y, et al. ERK5 is a target for gene amplification at 17p11 and promotes cell growth in hepatocellular carcinoma by regulating mitotic entry. Genes Chromosomes Cancer. 2009;48:109–20. doi: 10.1002/gcc.20624. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xu M, Scotti E, Chen ZJ, Tontonoz P. Both K63 and K48 ubiquitin linkages signal lysosomal degradation of the LDL receptor. J Lipid Res. 2013;54:1410–20. doi: 10.1194/jlr.M035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.