Abstract
Background and Purpose
Stroke mortality has been declining since the early twentieth century. The reasons for this are not completely understood, although the decline is welcome. As a result of recent striking and more accelerated decreases in stroke mortality, stroke has fallen from the third to the fourth leading cause of death in the United States. This has prompted a detailed assessment of the factors associated with this decline. This review considers the evidence of various contributors to the decline in stroke risk and mortality and can be used in the design of future interventions regarding this major public health burden.
Methods
Writing group members were nominated by the committee chair and co-chair on the basis of their previous work in relevant topic areas and were approved by the American Heart Association (AHA) Stroke Council’s Scientific Statement Oversight Committee and the AHA’s Manuscript Oversight Committee. The writers used systematic literature reviews, references to published clinical and epidemiology studies, morbidity and mortality reports, clinical and public health guidelines, authoritative statements, personal files, and expert opinion to summarize evidence and indicate gaps in current knowledge. All members of the writing group had the opportunity to comment and approved the final version of this document. The document underwent extensive AHA internal peer review, Stroke Council Leadership review and Scientific Statements Oversight Committee review before consideration and approval by the AHA Science Advisory and Coordinating Committee.
Results
The decline in stroke mortality over the past decades represents a major improvement in population health and is observed for both genders, and all race and age groups. In addition to the overall impact on fewer lives lost to stroke, the major decline in stroke mortality seen among individuals less than 65 years of age represents a reduction on years of potential life lost. The decline in mortality results from reduced stroke incidence and lower case fatality rates. These significant improvements in stroke outcomes are concurrent with cardiovascular risk factor control interventions. While it is difficult to calculate specific attributable risk estimates, the hypertension control efforts initiated in the 1970s appears to have had the most substantial influence on the accelerated stroke mortality decline. Although implemented later in the time period, diabetes and dyslipidemia control and smoking cessation programs, particularly in combination with hypertension treatment, also appear to have contributed to the stroke mortality decline. Telemedicine and stroke systems of care, while showing strong potential effects, have not been in place long enough to show their influence on the decline. Other factors had probable effects, but additional studies are needed to determine their contributions.
Conclusion
The decline in stroke mortality is real and represents a major public health and clinical medicine success story. The repositioning of stroke from 3rd to 4th leading cause of death is the result of true mortality decline and not an increase of chronic lung disease mortality, which is now the 3rd leading cause of death in the United States. There is strong evidence the decline can be attributed to a combination of interventions and programs based on scientific findings and implemented with the purpose to reduce stroke risks, the most likely being improved hypertension control. Thus, research studies and the application of their findings to develop intervention programs have improved the health of the population. The continued application of aggressive evidence-based public health programs and clinical interventions are expected to result in further declines in stroke mortality.
Keywords: stroke risks, risk factors, hypertension, diabetes, hyperlipidemia
Introduction
The remarkable decline in stroke mortality was acknowledged as one of the ten great public health achievements for the United States (US) in the 20th century. Along with the associated decline in ischemic heart disease mortality, stroke was one of the few diseases explicitly identified.1 This decline has continued over the past decade, and dropping stroke mortality was again identified as one of the ten great public health achievements for the decade bridging 2001 to 2010.2 Stroke has now fallen from the third to fourth leading cause of death in the US.3–6
While both stroke and ischemic heart disease mortality have declined substantially, the patterns of their decline stand in stark contrast. (Figure 1) In 1900, the number of deaths from stroke and diseases of the heart were approximately equal.7 Between that time and 1968, deaths from stroke have shown a steady and (nearly) monotonic decrease, falling from over 150 per 100,000 to approximately 50 per 100,000. Stroke mortality has been declining slowly throughout most of the 20th century, approximately 1/2 % per year. Then, in the 1970s, the rate of decline accelerated to approximately 5 % per year. This is in contrast to deaths from diseases of the heart, when between 1900 and approximately 1968, there was a steady increase, with the striking decline only since that time. Improvements in the ICD-coding system allowed the identification and reporting of deaths from coronary heart disease starting in the mid 1950s. The differences in these patterns suggest that either shifts in the underlying risk factors with a differential impact on heart disease and stroke (for example, blood pressure (or atrial fibrillation) with a larger and more immediate impact on stroke than heart disease, to lipids with a larger impact on heart disease than stroke), or that coding of deaths from these diseases were changing over time.
Figure 1. Age-adjusted death rates for cerebrovascular disease and chronic lower respiratory disease, by year—United States, 1979–2010.
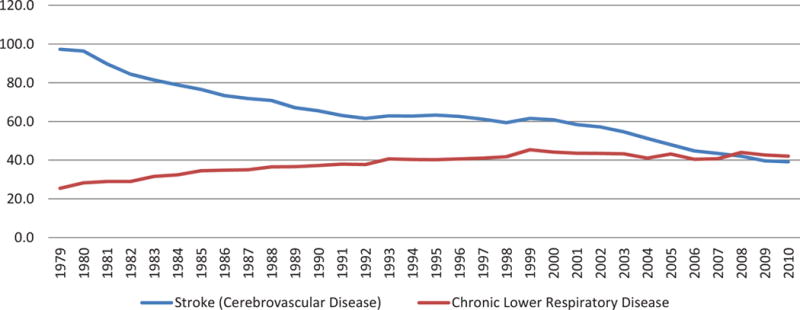
Rates per 100,000 population, standardized to the U.S. 2000 standard population Diseases were classisfied to the International Classification of Disease codes in use at the time the deaths were reported.
Studies have suggested differential rates of decline in stroke mortality by race and gender. A study of race-specific trends in organ and disease-specific mortality rates in the US from 1996 to 2005 revealed that despite a 23% decline in age-adjusted stroke death rates, stroke remained the second leading cause of death in blacks.8 Among whites, on the other hand, the 26% decline in stroke age-adjusted death rates resulted in stroke moving from the second to the fourth leading cause of death after ischemic heart disease, lung cancer, and chronic lower respiratory disease. Sex differences were also noted in that study. In men, stroke age-adjusted death rates fell by 28%, and stroke dropped from being the third to the fifth leading cause of death after ischemic heart disease, lung cancer, accidents, and chronic lower respiratory diseases. Among women, although the stroke age-adjusted death rates declined by 24%, stroke remained the second leading cause of death. In addition to disparities in rates of decline, the differences in rankings by gender and race were also due to differences in starting points; that is, the absolute rates, as well as competing causes of mortality. Gillum et al noted geographic differences in race-specific stroke mortality rates from 1999 to 2007, although overall rates declined in both African American and non-Hispanic whites.9
The Centers for Disease Control and Prevention (CDC) WONDER (Wide-ranging Online Data for Epidemiologic Research) system4 and historical reports from the National Vital Statistics System (NVSS)3 can be used to describe these patterns of change in death rates from stroke. Figure 2 illustrates cerebrovascular mortality by mutually-exclusive race-ethnic groups between 1999 and 2008, showing that this decline in mortality continues to be shared by all in the US (although potentially to different extents), as noted by Gillum.9 Where data are available on temporal patterns in incidence10, 11 and hospitalization rates,12 the data seem to reflect that these mortality declines are at least in part associated with declining incidence of stroke.13 Thus the trends in stroke mortality are influenced by the lower stroke incidence and improved case fatality rates.
Figure 2. Age-adjusted death rates for cerebrovascular disease by race, by year—United States, 1999–2010.
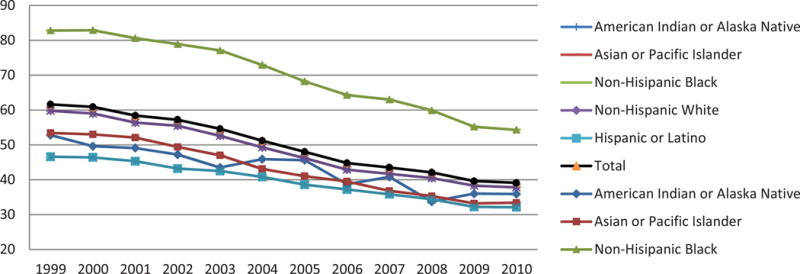
Rates per 100,000 population, standardized to the U.S. 2000 standard population
These remarkable declines in the US must also be interpreted in the context of an associated worldwide decline in stroke documented primarily in Western countries.14–26 This paper assesses factors and interventions that have been proposed to affect stroke mortality. The influence attributed to the different factors is described, and the potential contributions are quantified when possible.
Use and Limitations of Stroke Mortality and Ranking as an Indicator of Population Health and Risk
Mortality statistics commonly rely on sources such as the Compressed Mortality File (CMF) compiled by the National Center for Health Statistics (NCHS)27 and from death certificate information on the underlying or single condition that is the most relevant cause of death. In assessing stroke mortality over time, it is important to note changes in the definition of stroke that might affect classification, advances in technology (such as the advent of imaging) that may affect diagnosis, revisions to the International Classification of Diseases (ICD), modification of the coding instructions within the ICD system, recognition of other competing causes of death and changes in instructions on vital statistics coding from death certificates.28
The National Vital Statistics System (NVSS) is the most commonly used source for geographic and demographic mortality data in the United States. The classification and coding of cause of death listed on death certificates, including selection of the underlying cause of death is based on ICD.29 New versions of the ICD have been implemented nearly every decade since 1900 as medical knowledge has increased.28 Reclassification allows refinements to the coding system that account for advances in medical science and discovery of new diseases. From 1968–1979, ICDA-8 was used; from 1979–1998, ICD-9 was used; and since 1999, ICD-10 has been used. Changes in versions of the ICD can affect the interpretation of mortality trends over time.
The NCHS has produced reports documenting the comparability of different versions of the ICD for major disease categories. Comparability ratios can be applied to assess trends in mortality for a disease. This provides a more accurate assessment of the actual trend over time and corrects for ICD version changes. A comparability ratio of 1 for ICD-9 to ICD-10 for a disease would reflect that the change resulted in no increase or decrease of cases in the definition for that disease. A comparability ratio of >1 would imply that a coding change from ICD-9 to ICD-10 resulted in more cases of a particular disease attributable to the coding change alone in ICD-10 compared to ICD-9. The comparability ratio from ICD-8 to 9 was: 1.004930 for cerebrovascular disease (ICD-8 and ICD-9 codes 430–438); the ratio from ICD-9 to ICD-10 was 1.0588 (ICD-9 codes 430–438 and ICD-10 codes I60–I69).31 According to the NCHS, this nearly 6% increase for cerebrovascular disease was primarily due to a coding rule change that moved many cases that would have been classified pneumonia as the underlying cause of death in ICD-9 to cerebrovascular disease as the underlying cause of death in ICD-10.31
A limitation of use of the NVSS data is the lack of detailed information in recording cause of death. For example, of the 131,079 deaths in 2008 from cerebrovascular disease (I60–I69), 70,114 (53%) were coded as “Stroke, not specified as infarction or hemorrhage” (I64) while only 6,440 (5%) were identified as “Cerebral infarction” (I63).4 Even among the 6,440 coded as Cerebral infarction (I63), 3,526 (55%) were coded as “Cerebral infarction, unspecified” (I63.9), while only 912 (14%) were coded to be “Cerebral infarction due thrombosis of cerebral arteries” (I63.3) and 895 (14%) were coded to be “Cerebral infarction due to embolism of cerebral arteries” (I63.4).4 This substantial lack of specificity implies that responsible reporting of stroke mortality statistics based on NVSS data must be limited largely to “Cerebrovascular disease” (I60–I69), which not only will include deaths from secondary causes associated with stroke but also limits the ability to assess if changes in mortality are equally affecting deaths from infarction versus hemorrhagic stroke. Similarly, changes in diagnostic technology and neuroimaging can affect stroke diagnosis. For example, the number of TIAs which would be classified as minor strokes if all individuals with a TIA had neuroimaging as part of their work up.32
In addition, a high error rate in the certification of cause of death on death certificates is well known. In the REasons for Geographic and Racial Differences in Stroke Study (REGARDS), a comparison of cause of death in a cohort aged 45 and over at baseline showed that stroke death based on death certificate and compared to physician adjudication had a sensitivity of 52% and a specificity of 99%.33 Similar work in the Cardiovascular Health Study (CHS) performed in an older cohort (all age 65+ at baseline) showed the sensitivity of nosologist-coded stroke with physician adjudication was 68%, while the specificity was 95%.34 With such a high specificity and lower sensitivity, it is possible that the number of deaths from stroke may be systematically underreported. The reliability of vital statistics data depends on the accuracy of the death certificate, and inaccuracies are more likely to result from insufficient knowledge of the person’s medical history rather than from problems with the Vital Statistics coding system.35 However, key to the interpretation of secular trends is whether there have been temporal changes in the coding of stroke. To our knowledge, the possibility of such a temporal change in the coding of deaths from stroke has not been investigated except in a more recent time period; however, it has been suggested that such changes are not present for coronary diseases.36, 37 Others, however, have raised a concern that such a temporal change in cause-of-death and hospital discharge coding may weaken efforts to accurately assess secular changes in causes of death.38
Mortality rates are typically reported as age-adjusted death rates. The standard population for age-adjustment from 1940–1999 was the 1940 standard population. Beginning in 1999, the standard population for age-adjustment was the 2000 standard population. The population shift between 1940 and 2000 to a distribution with a greater proportion of elderly individuals in the population can produce very different results for mortality rates for cerebrovascular disease for identical years. In particular, the age-adjusted death rate for diseases associated with more deaths at advanced ages (such as ischemic stroke) will tend to be substantially higher when standardized to the 2000 population standard, which is used in this report. For example, the age-adjusted stroke death rate is 26.7 deaths per 100,000 standard population using the 1940 standard but is 63.9 using the year 2000 standard, which corresponds to a 2.4-fold difference.39
Figure 3 includes similar stroke mortality trends with a different scale in order to clearly present the changes between years and time periods. Assessing the trend in stroke mortality, there has been an overall decline in stroke mortality from 1968 through 2010. The slight increase from 1998 to 1999 is reflective of the change from ICD-9 to ICD-10, but there has been a continued downward trend from 1999 through 2009.40 The changes from ICD-9 to ICD-10 have been modest in comparison to the overall 50-year trend. In summary, a change in the ranking of a specific cause of death, such as cerebrovascular disease over time, is influenced by the comparability ratios for other diseases as well as the comparability ratio for cerebrovascular disease and changes in classification and coding of other diseases over time. In addition, it is not clear to what extent increases in stroke mortality reflects a poor quality of care or an increased appreciation for the role of patient preference in end-of-life decision making. Thus, it is possible that a steeper than appreciated decline in mortality is actually attenuated by an increasing trend toward palliative care in patients with severely disabling strokes.
Figure 3. Age-adjusted death rates for diseases of the heart, cerebrovascular disease, and chronic lower respiratory disease, by year—United States, 1900–2010.
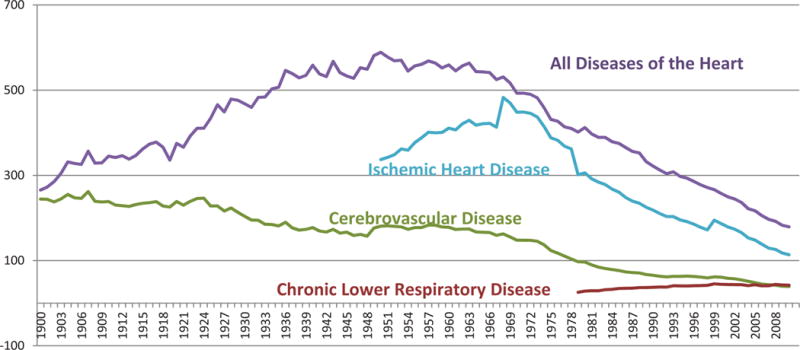
Rates per 100,000 population, standardized to the U.S. 2000 standard population
Diseases were classisfied to the International Classification of Disease codes in use at the time the deaths were reported.
| ICD 1 | 1900–1909 |
| ICD 2 | 1910–1920 |
| ICD 3 | 1921–1929 |
| ICD 4 | 1930–1938 |
| ICD 5 | 1939–1948 |
| ICD 6 | 1949–1959 |
| ICD 7 | 1960–1967 |
| ICD 8 | 1968–1978 |
| ICD 9 | 1979–1998 |
| ICD 10 | 1999–2009 |
Recurrent and Incident Strokes as a Factor in the Decline of Stroke Mortality
Overview of recurrence rates
Recurrent strokes represent 23% of the 800,000 strokes that occur each year in the United States5 and are associated with higher mortality rates, greater levels of disability, and increased costs as compared with first stroke events.41 Recurrent stroke rates within the first year have been shown to range from 5% to 15%.42–47 The 30-day case-fatality rate is almost double for a recurrent stroke as compared with the index stroke.48 Population-based epidemiologic studies found that early mortality is more commonly related to the index or recurrent stroke, whereas later mortality is generally related to cardiovascular causes.49–56 Age appears to play a significant role in the cause of death after a recurrent stroke with a greater proportionate mortality due to recurrent stroke rather than cardiac causes of death. This may be enhanced due to the greater mean age; that is, the older are more likely to die than younger people from the event. The impact of index stroke on future events has led to the recommendation that ischemic stroke be included in cardiac risk assessment models and instruments, because stroke survivors are at increased risk and more likely to die from a cardiovascular event.43, 53, 56, 57 It is important to note that the assessment of stroke mortality includes a mix of case fatality, in-hospital mortality, 30-day mortality, 1-year morality and other categories with the definitions of mortality often varying across studies. Likewise, it should be noted the indicator of stroke occurrence as incidence, recurrence and prevalence when assessing disease rates.
Trends in recurrence and incidence rates
Recurrent stroke rates have been decreasing over time. Hong et al,58 using a novel approach to identify trends in recurrent stroke in the US, found that recurrent stroke has declined substantially over the last 5 decades. Looking at the control arms of randomized, controlled trials of secondary stroke prevention interventions, they found that event rates for recurrent stroke and fatal stroke declined each decade from 1960 to 2009, with almost a 50% reduction in recurrent stroke rates in the 1990s and 2000s as compared with the 1960s. A systematic review of 13 studies from hospital-based or community based stroke registries found a temporal reduction in 5-year risk of stroke recurrence from 32% to 16.2%, but reported substantial differences across studies in terms of case mix and definition of stroke recurrence.59 Studies examining temporal trends in recurrent events are limited, but also report decreases in recurrent events over time.60–62 Data from the initial cohort from the Framingham Heart Study (FHS) beginning in 1949 and followed for 26 years found a recurrent stroke rate of 28% among survivors, including 2nd and 3rd strokes. The five-year cumulative recurrence rate was 42% for men and 24% for women.63 Results from epidemiological studies have shown a decline in first-ever stroke rates from 20% to 40% attributed to the improvement of risk factor control.58 Similar and even greater reductions are associated with recurrent stroke rates. Similar to first strokes, the risk of recurrent stroke are affected by differences in geography, race, socio-economic status, and type of care.47, 64–68
Clinical trials completed over the past 5 decades have demonstrated the benefit of secondary stroke prevention therapies. Evidence from these trials has demonstrated secondary stroke prevention benefits from vascular prevention therapies including antihypertensive therapy,69, 70 statin,71 and aspirin.72, 73 That secondary prevention has decreased recurrent stroke through improved blood pressure control, increased use of antiplatelet and anticoagulant medications, statins, and decreased smoking rates has been found by others to be associated with a decrease in coronary heart disease during 1980–2000.74 Because individuals participating in clinical trials may not be representative of the general population, some caution should be urged in the generalization of these findings to the more broad population. Further improvements in secondary prevention could reduce recurrent vascular events in stroke patients by as much as 80%.75 These findings suggest that a significant proportion of recurrent strokes can be prevented.76 It is important to recognize stroke in secondary prevention as a manifestation of multiple heterogeneous disorders including cardioembolism, small vessel disease, and large artery atherosclerosis. Clinical trial evidence from Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMPRIS)77 as compared to Warfarin-Aspirin Symptomatic Intracranial Disease Study (WASID)78, 79 indicated a reduction of secondary stroke risk with aggressive medical management in patients with intracranial atherosclerotic stenosis Results from the Secondary Prevention of Small Subcortical Strokes (SPS3) identified a declining risk of recurrence of small vessel disease compared to expected rates estimated from natural history studies,80 and findings from the prospective Oxford Vascular Study demonstrated declining risk of stroke due to carotid stenosis.81 These decreases in secondary stroke would likely contribute to the decline in stroke mortality.
A systematic review of worldwide stroke incidence studies from the 1970s through 2008 found that the age-adjusted stroke incidence rates in high-income countries declined 42% overall with declines in each subsequent decade of the study. These trends were found across age groups, with a greater decline in those aged 75 years and older. They also noted the early (up to 1 month) case fatality rate declined in high-income countries, but incidence and case fatality increased in low to middle income countries.82 Data from the FHS found significant declines in stroke incidence in both men and women when comparing 3 time periods (1950–1977, 1978–1989, and 1990–2004). It was also noted that the 30-day case fatality declined significantly in men but not women.83 The Greater Cincinnati Northern Kentucky Stroke Study (GCNKSS) reported that the annual incidence of strokes declined in 2005 compared to 1993–1994 and 1999 among whites but not blacks. The GCNKSS also found no change in the incidence of intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) or in case fatality rates across the study periods.13
Stroke incidence rates over time are subject to changes in the clinical definition of stroke and influenced by changes in technology that refine the diagnosis of stroke. Leary and Saver estimate that in 1998, approximately 770,000 persons experienced a symptomatic stroke and 11 million experienced an asymptomatic stroke.84 These changes would also affect rates of recurrent stroke over time, but the effect of that bias is unclear. Likewise it is important to recognize the impact of silent stroke which is estimated between 5% and 28% based on MRI scans.85, 86 The rates of silent stroke vary by hypertension and smoking status with highest rates in the older population.87 Similarly diffuse white matter disease affects a high proportion of the elderly with a mix of vascular and Alzheimer’s pathology.88 It is thus important to recognize that silent stroke and diffuse white matter disease are aspects of cerebrovascular disease with major effects on risk of cognitive impairment and dementia contributing to mortality in the aging population. Stroke severity is an important influence of stroke mortality, and with occurrence, affects overall rates. The different risk factors associated with stroke mortality, reduce stroke occurrence separately from those factors that influence mortality once a stroke has occurred.89 Thus, factors can be categorized in reducing stroke occurrence and/or stroke severity with different effects on mortality. For example, antiplatelet therapy and anti-hypertension therapy have different impact on reducing stroke occurrence and stroke severity.90
Increased application of advanced neuroimaging such as MRI might be improving the diagnosis of milder less fatal strokes over time. This would result in an apparent decline in the stroke case fatality rate, due solely to improved detection. However, this should not result in a change in stroke mortality over time unless technological advances improved the diagnosis of more severe, fatal strokes too, which seems unlikely. Likewise stroke subtype is a major consideration. For example, the incidence of intracerebral hemorrhage increases with age and has not decreased between 1980 and 2006.91, 92 Thus, future studies should include and address the different subtypes of stroke and corresponding stroke severity. This scientific evidence is essential to carefully and quickly identify the most effective and appropriate treatments for stroke patients.
In summary, the evidence suggests that there has been a decline in recurrent stroke and a possible decline in stroke incidence. This may be more pronounced by gender and in certain racial/ethnic groups. The trends in the declining rate of recurrent stroke and of stroke mortality seem to follow similar timelines, suggesting that secondary stroke prevention strategies may have impacted overall stroke mortality rates for both outcomes. The exact proportion of the stroke mortality decline that can be attributed to recurrent stroke is unclear and requires additional studies and trials specific to recurrent stroke. While stroke systems of care have improved the initiation of medications for secondary stroke prevention during the hospitalization, there are currently no nationwide systematic efforts aimed at ensuring control of risk factors after stroke.
Changes in Pulmonary and Lung Disease on the Assessment of Stroke Mortality Trends and Ranking
From 1979–1999, the mortality for chronic lower respiratory disease (CLRD) increased at a slow but steady rate and then showed a minimal decline from 2000–2008.93, 94 Mortality rates for CLRD have again declined in 2009 and in the preliminary mortality data for 2010.94, 95 In contrast, stroke mortality rates have declined steadily over the past 100 years, particularly in the last 50 years and at a much faster rate than for CLRD mortality. (Figure 4) These mortality trends for CLRD and stroke resulted in a change in the ranking of causes of death. Stroke which had been the 3rd ranking cause of death fell to the 4th ranking cause of death while CLRD rose from the 4th to the 3rd ranking cause of death. It should be noted that coding changes in both stroke and CLRD played a small role in the shift in causes of death. In 2008, there was a coding change that moved many cases not previously classified as CLRD into the CLRD classification. Chronic lower respiratory disease includes ICD-10 codes J40–J47. The following conditions were recoded to J44.0 (chronic obstructive pulmonary disease (COPD) with acute lower respiratory infection) in 2008: pneumonia (J12–J16, J18), other acute lower respiratory infections (J20–J22), and unspecified COPD (J44.9). Therefore, the mortality rates for CLRD through 2007 may not be comparable with rates from 2008 and beyond. According to the NVSS, stroke mortality rates declined 3.6% from 2007 to 2008, while mortality from CLRD increased 7.8%. The mortality rates for both stroke and CLRD have declined from 2008 to 2009 and from 2009 to 2010 (preliminary data) with slightly greater declines in stroke than in CLRD. Also in 2008, a coding change resulted in some deaths that would have previously been coded as subarachnoid hemorrhage (SAH) (ICD-10 I60) reassigned to vascular dementia (ICD-10 F01). The age-adjusted mortality rate for SAH declined from 1.81 in 2007 to 1.73 in 2008 and 1.68 in 2009.96 The mortality changes due to ICD-10 coding changes in 2008 were followed by similar declines in both CLRD and stroke mortality rates based on mortality data for 2009.95
Figure 4. Age-adjusted death rates for cerebrovascular disease and chronic lower respiratory disease, by year—United States, 1979–2010*.
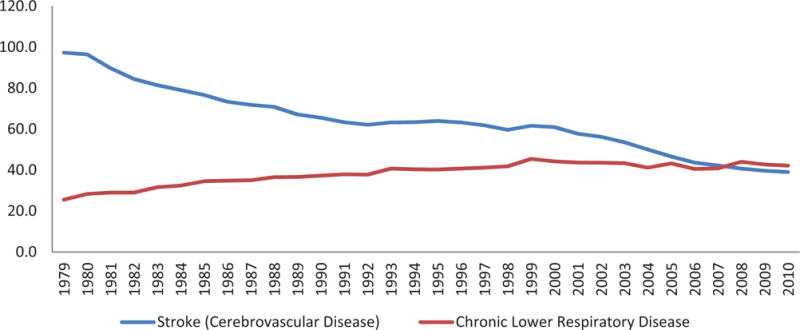
Per 100,000 population, standardized to the U.S. 2000 standard population Diseases were classified to the International Classification of Disease codes in use at the time the deaths were reported.
*Data for 2010 is preliminary
Despite an aging population, the actual numbers of stroke deaths have declined each year since 2000, while the numbers of actual deaths from CLRD fluctuated during this time. Mortality data showed a similar decline in total deaths from stroke and CLRD from 2008 to 2009.95 These more recent changes in the ranking causes of death for stroke and CLRD may be subject to further changes as the current rate of decline in stroke mortality has slowed over the previous five years and the mortality rate from CLRD has shown little decline since 2000. Both conditions are sensitive to tobacco use, which will be discussed in a later section.
A recent study assessed whether there had been changes in mortality attribution methods over time that might explain the recent change in ranking of causes of death for stroke and CLRD using data from the NVSS. Determinations of disease-specific mortality rely on a complex and annually reevaluated algorithm to select the “underlying cause of death” from the up to 20 causes listed on a death certificate. Therefore, systematic changes in the classification of stroke as the underlying cause of death could occur through changes in the underlying algorithms and/or changes in death certificate completion patterns. In an analysis by Burke et al,36 mortality data from 2000–2008 was used to compare changes in reporting of stroke as underlying cause of death with changes in death certificates reporting any mention of stroke. Similar comparisons were also made for the six leading organ and disease-specific causes of death including CRLD. If stroke mortality was underestimated by the system of mortality attribution, a greater decline in stroke as an underlying cause of death relative to any mention of stroke on the death certificate would have occurred. The authors found that age-adjusted death rates for stroke as an underlying cause of death and for stroke mentioned anywhere on the death certificate both declined by 33% from 2000 to 2008 and that the ratio of these death rates for stroke did not change over time (0.595 in 2000 vs. 0.598 in 2008). Changes in the same ratio for CRLD were too small (from 0.49 to 0.52) to explain stroke’s decline as a leading cause of death. The authors concluded that based on the data changes in mortality attribution methodology are not likely responsible for stroke’s decline as a leading cause of death.
In summary, there have been significant changes in COPD risks during the study period. However, these changes in lung disease do not offset or diminish the decline in stroke mortality. It will remain important to consider the epidemiology of COPD in future research and surveillance studies.
Hypertension as a Factor in the Decline in Stroke Mortality
The association of blood pressure (BP) levels and the risk of stroke were first recognized by the Society of Actuaries in the 1920s.97 In the 1960’s, early clinical studies identified clear benefits of lowering blood pressure on reducing stroke deaths.98 In the VA clinical trials for those with severe hypertension (115–129 mm Hg DBP) the effect was dramatic. After just 18 months those receiving placebo were having strokes at such an increased rate the trial was stopped and all participants were given antihypertensive drugs.99, 100 Other blood pressure lowering clinical trials were published showing a consistent pattern of benefit.101, 102 (see section on clinical trials below) The evidence for the benefits of lower blood pressures and reduced stroke risks is strong, continuous, graded, consistent, independent, predictive, and etiologically significant for those with and without coronary heart disease.103, 104 This information was used to launch and then implement on a long term basis the National High Blood Pressure Education (NHBPEP) program regarding the benefits of treating hypertension among the public, patients and physicians. The messages were heard; hypertension screenings increased and physicians began treating patients. Hypertension has become the most common primary diagnosis in America and antihypertensive medications are among the most commonly prescribed.105 Thus, lowering high blood pressure is proposed as a major factor for the reduction in stroke death rates during the last half of the 20th century and early 21st century.106 Specifically, the US age-adjusted stroke mortality rate reduction from 88 in 1950 to 23/100,000 in 2010, with consistent reductions in mortality for all age, race, and sex groups in the US,98 as well as other countries is consistent with high blood pressure recognition and reduction campaigns initiated during the same period.107 These BP reduction strategies included clinical interventions for hypertension and public health efforts focused on lifestyle for the shifting of blood pressure distributions. Although the decline in stroke mortality in the US began at the beginning of the 20th century, decades before hypertension treatment,108 the slope of the decline in mortality significantly accelerated after the introduction of tolerable antihypertensive drug therapy in the 1960s.109 It has been suggested the slight decline in stroke mortality in the first half of the 20th century is a statistical aberration perhaps from classification and attribution methodology.
Epidemiological studies have shown elevated blood pressure is the most important determinant of the risk of stroke. The risk is almost linear beginning at relatively low levels of systolic and diastolic blood pressures.110 Risk factors for high blood pressure, such as obesity, increased waist circumference, higher alcohol intake, and greater sodium intake are also associated with increased risks for stroke.111 It is estimated that the overwhelming majority of strokes each year could be prevented through awareness and optimal management of hypertension, and through lifestyle changes to healthier diets, greater physical activity, and smoking cessation. These four factors plus waist-to-hip ratio account for 82% and 90% of the population-attributable risk for ischemic stroke and for hemorrhagic stroke, respectively.112
Prevalence of high blood pressure and blood pressure distribution
Most recent estimates from the National Health and Nutrition Examination Survey (NHANES) identify 68 million or more Americans with high blood pressure warranting some form of monitoring or treatment.113–115 Global hypertension prevalence estimates of 1 billion individuals, with an estimated 7.1 million deaths per year may be attributable to hypertension.116 As the population ages, the number of individuals with elevated blood pressure increases.113, 117, 118 The substantial and increasing prevalence of elevated blood pressure combined with the evidence-based benefit of hypertension treatment have lead to the prioritization of prevention and control programs among federal, professional and voluntary agencies. Considerable success has been achieved in the past in meeting the goals of these programs. The percentage of patients with hypertension receiving treatment has increased to where more than 90 % of the population knows the relationship between high blood pressure (HBP) and stroke, nearly 70 percent of the adult hypertensive population are treated, and 46 percent of those treated for HBP are controlled to below 140/90 mmHg.115, 119 The mean systolic BP (SBP) for the US adult population declined from 131 mm Hg in 1960 to 122 mm in 2008.113, 120, 121 (Table 1) Figure 5 presents the smooth weighted frequency distributions of systolic BP from national population based surveys including National Health Interview Surveys and NHANES I, II, III, and 1999–2010. Between 1959 and 2010, median and 90th percentile systolic BP declined by approximately 16 mmHg. This declining shift in BP distributions was consistent for different age groups, including 18–29 years, 18–39 years, 30–59 years and 60–74 years. (Figure 5) These population wide changes in reduced blood pressures seen within the last five decades have been associated with the large accelerated reductions in stroke mortality. The shift in mean arterial blood pressure is more pronounced in older Americans who have a greater prevalence, who are more likely to visit physicians and who are on blood pressure treatment, than in younger people, even though they may be less likely to achieve goal blood pressure. This suggests there is an opportunity to reduce stroke rates even further. Goff et al described a gradual downward shift of the entire distribution of BP levels in the general population going back to the early 1900’s, suggesting one of the few risk factors where documentation of such a long-term change could contribute to the beginning of the decline in stroke mortality over the same century.122 The identification and recognition of elevated BP as a risk factor appears to have affected blood pressure levels and subsequent stroke mortality risks. While the decline in stroke mortality and lowering BP may have appeared to be evident before this recognition and treatment of hypertension, the effects of lowered blood pressures is most evident after the population-based campaigns.118 Hypertension treatment and control rates have consistently increased since the early 1970s. While there are age, race and gender disparities, this improvement is seen in all subsets of the population. Further demonstrating the impact of treatment, systolic blood pressures are lower for treated hypertensives than untreated for all groups. All populations have shown significant improvements during the time period. Likewise a reduction in mean SBP has been observed for all age, race and gender groups. The 90th percentile SBP levels have been lowered over the past decades suggesting significant impact of hypertension treatment and control. Similarly the 10th percentiles have also been lower through the past years. (Figure 5) The reduction in these lower BP levels is most likely the result of lifestyle and nonpharmacologic interventions and public health activities.
Table 1.
Mean Systolic Blood Pressure by Time Period NHANES I–IV
| 1960–62 | 131 mm hg |
|---|---|
| 1971–74 | 129 mm hg |
| 1976–81 | 126 mm hg |
| 1988–91 | 119 mm hg |
| 1988–94 | 121 mm hg |
| 1999–04 | 123 mm hg |
| 2001–08 | 122 mm hg |
Figure 5.
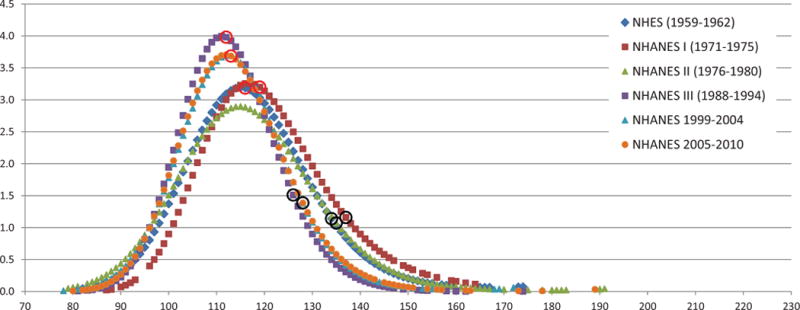
Smoothed weighted frequency distribution, median, and 90th percentile of SBP: US 1959–2010, age 18–29
Although pharmacological treatment of BP focuses on individuals with hypertension, currently defined as BP greater than 140/90,110, 123 epidemiological data demonstrate that the risk of stroke begins at BP below 140/90 mmHg levels. In a meta-analysis of 61 prospective, observational studies conducted by Lewington et al involving one million adults with no previous vascular disease at baseline, the researchers found that between the ages of 40–69 years, beginning with SBP of 115mm Hg and DBP of 75 mmHg, each incremental rise of 20 mmHg SBP and 10 mmHg DBP was associated with a two-fold increase in death rates from stroke.110 This effect is seen in all decades of life.
In addition, age- related rise in SBP is primarily responsible for an increase in both incidence and prevalence of hypertension.124 Further, Framingham Heart Study investigators reported the lifetime risk of hypertension to be approximately 90 percent for men and women who were nonhypertensive at 55 or 65 years and survived to age 80–85.125 Thus, if people live long enough virtually all will become hypertensive. Even after adjusting for competing mortality, that is death from other causes which would preclude a death from hypertension, the remaining lifetime risks of hypertension were 86–90% in women and 81–83% in men.125 Such lifetime risk estimates can be used in calculating the impact of BP reduction for stroke mortality declines.126 The increase of BP to hypertensive levels with age is evident by patterns and trends indicating that the 4-year rates of progression to hypertension are 50 percent for those 65 years and older with BP in the 130–139/85–89 mmHg range and 26 percent for those with BP between 120–129/80–84 mmHg range.127
As indicated, the reduced stroke mortality rates are evident in all categories of hypertension and BP levels. Great benefits of BP reduction are evident in the malignant or severe category of elevated BP levels.128, 129 These extreme BP levels are more prevalent among the high stroke risk populations, especially African Americans, but the values have been reduced with treatment with corresponding risk reduction.130, 131 However, hypertension emergencies, crises and malignant hypertension represent a small percent of the population with HBP. Up to 2% of patients with hypertension develop a hypertensive crisis at some point in their lifetime.132, 133 Thus, the lowering of these extreme high blood pressure levels have impact on the decline of stroke mortality but should be considered less of a contributor to overall stroke mortality decline because there are relatively fewer patients with this condition.
Observational studies
Cohort studies have demonstrated increased attributable risks associated with elevated BP levels.110, 134, 135 HBP was identified as responsible for the largest number of cardiovascular and stroke deaths in the US.136 The INTERSTROKE study concluded the contribution of various risk factors to the burden of stroke worldwide to be 34.6% for hypertension (CI 30.4–39.1).112 In addition, it was estimated that among treated hypertensives, approximately 45% of all strokes might be attributed to uncontrolled BP.137 Such risk estimates are consistent for all components of the population with significant population-attributable risk for elevated BP and stroke mortality.112, 137 The relationship between BP and risk of CVD events is demonstrated over time, continuous, consistent, and independent of other risk factors. The linear relationship holds true for all demographics indicating the higher the BP the greater the risk of stroke mortality.
Clinical trials
The benefit of hypertension treatment to reduce stroke risks is evident with the effective number-needed-to treat (NNT) estimates. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) states, “In clinical trials, antihypertensive therapy has been associated with reductions in stroke incidence averaging 35–40 percent; myocardial infarction, 20–25 percent; and heart failure, more than 50 percent.138 It is estimated that among patients with stage 1 hypertension (SBP 140–159 mmHg and/or DBP 90–99 mmHg) and additional cardiovascular risk factors, achieving a sustained 12 mmHg reduction in SBP over 10 years will prevent 1 cardiovascular event for every 11 patients treated. In the presence of CVD or target organ damage, only 9 patients would require such BP reductions to prevent a death”.139 Clinical trials have demonstrated that control of isolated systolic hypertension reduces total and stroke mortality.140–142 Reducing SBP even if BP control levels are not achieved improves risk and outcomes. In the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) and the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) Trial, DBP control rates exceeded 90%, but SBP control rates were considerably less (60–70%).143, 144
Data from the Hypertension Detection and Follow-up Program (HDFP) showed that reductions of 4.7 mm Hg reduced stroke mortality by 17.6%.145 Numerous other trials have provided evidence of hypertension treatment with blood pressure reduction and subsequent reduced stroke risks.146–160 The trials include placebo, comparison and efficacy designs with similar results indicating a benefit of blood pressure reduction and stroke risks. The studies also included different ages, races and both genders as well as different time-periods with consistent findings of stroke risk reduction with hypertension treatment. Further a recent meta-analysis of 32 randomized trials confirmed hypertension treatment in reducing stroke risks.161 Another meta-analysis reported substantial stroke risk reduction with tight BP control and lowered BP levels.162 Likewise a meta-analysis of 147 trials determined a 41% reduction in stroke risks with systolic BP reductions of 10 mm Hg.163 Another overview of evidence from observational epidemiologic studies and randomized controlled trials determined an average reduction of 12 to 13 mm Hg in systolic blood pressure over 4 years of follow-up was associated with a 37% reduction in stroke mortality.164 While there remains some questions about the specific BP treatment levels for stroke reduction due to trial design and study sample size,165 the clinical trial results are clear with regards to the benefit of BP reduction and stroke risks.166 With no exception, every large-scale well conducted clinical trial of BP lowering has shown the clear benefits of this maneuver. The decrease in blood pressure with drug therapy as assessed in clinical trials appears to be the major determinant for reduction in the risk of stroke and stroke deaths.167 Nonetheless, specific blood pressure reduction target goals below 140/90 mm Hg remain somewhat unclear. Further studies are required to determine the optimal BP goal and timing of achieving this goal after a stroke.
Several studies focused on secondary prevention, including an early study of US veterans.168 The Dutch TIA Trial Study169 and other major trials have shown significant lower rates of recurrent stroke with lower blood pressures. Most recently, the blood pressure reduction component of the Secondary Prevention of Small Subcortical Strokes (SPS3) Trial showed targeting a systolic blood pressure < 130 mm Hg is likely to reduce recurrent stroke by about 20% (p=0.08) and significantly reduced intracerebral hemorrhage by two thirds.170 The ongoing Systolic Blood Pressure Intervention Trial (SPRINT) is a 2-arm, multicenter, randomized clinical trial designed to test whether a treatment program aimed at reducing SBP to a lower goal than currently recommended will reduce cardiovascular disease and stroke risk as well as cognitive function.171
Hypertension treatment guidelines
Since 1977, NHBPEP’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) has recognized the high impact of elevated blood pressures and published guidelines on the diagnosis, prevention and management of hypertension.117, 123, 172–176 The treatment guidelines have included recommendations focused on the reduction of hypertension-related conditions including stroke. The guidelines have evolved as evidence about the benefits of treating to lower BP levels becomes available as well as study results which differentiate the effectiveness of the different classes of treatment. A major contribution of the JNC guidelines remains the definition of hypertension and BP treatment goals. With each set of JNC guidelines, the BP level for treatment and goals have typically been lowered. Specifically, the JNC 7 report has recognized then emphasized the need to treat systolic blood pressure, especially in older people. These recommendations may have impact population BP levels as systolic blood pressures have been lower with the evolving guidelines. (Table 1) These guidelines recommendations for clinical management are also used for public health hypertension control efforts. The implementation of the guidelines to address the populations at risks is designed to impact the disease risk including stroke mortality.177 A recent paper identified potential high impact of hypertension guidelines on the high risk population with high blood pressure.178
Structured programs
The impact of elevated blood pressure on the population has led to the establishment of prevention and management strategies for hypertension as major public health objectives.179–181 The premise is that if the elevation of blood pressure with age can be prevented or reduced, stroke and stroke mortality will be affected. This concept has led to the implementation of public health strategies and programs to reduce blood pressure in the population as an effort to lower stroke risks. Risk factors of interest include excess body weight; excess dietary sodium intake; suboptimal physical activity; inadequate intake of fruits, vegetables, and potassium; and excess alcohol intake.182, 183 These programs are aimed at working with manufacturers and restaurants as well as food procurement policies to reduce salt in the prepared and processed food, encouraging the consumption of more fresh fruits and vegetables, increasing community participation in physical activity, detecting and tracking high blood pressure at churches, worksites and community events and public education campaigns.184–187
This population-based approach employs a public health strategy which complements the clinical hypertension treatment and management. Primary prevention strategies are implemented to reduce the BP levels in the population, particularly in individuals with the pre-hypertension category (<140/90 mm Hg). This approach serves to decrease the blood pressure levels in the general population by relative modest amounts but in large populations has the potential to substantially reduce stroke morbidity and mortality, and to delay the onset of hypertension.188 Stamler and colleagues estimated two decades ago that a 5 mmHg reduction of SBP in the adult population would result in a 14 percent overall reduction in mortality due to stroke.189 As presented in Figures 1 and 2 and Table 1, the reduction in systolic blood pressure is consistent with the decline in stroke mortality, and corresponds to the predicted lower stroke mortality rates.
In the 1970s, as a strategy to increase public knowledge and screening for high blood pressure, the National Heart, Lung and Blood Institute (NHLBI) provided funding and technical assistance to develop state hypertension education and control programs. States and territories organized hypertension coalitions comprised of voluntary agencies such as the American Heart Association, the American Red Cross, local medical and nursing societies, and representatives from nearby hospitals. More than 2000 community groups and coalitions were developed and began hypertension screening and education programs. Programs developed patient tracking systems to determine what became of those who were screened. These efforts demonstrated a sharp increase in hypertension control rates and a marked decline in stroke mortality.190–194 This was later corroborated by data from the Department of Veterans Administration.195
As state health department epidemiologists began assembling hypertension prevalence rates from data collected during the screenings, it became apparent some states, particularly those in the Southeast experienced greater hypertension prevalence and more severe hypertension than others. These data prompted the examination of the NHANES blood pressure regional data and stroke mortality and subsequently two landmark studies were published, identifying 11 contiguous states in the southeast that had higher stroke mortality than the rest of the nation. These states were identified as the “Stroke Belt”.196, 197
Subsequently NHLBI and partners developed structured education efforts in the Southeast. Contracts were issued to the “Stroke Belt” state health departments to increase the intensity of education activities.198 Blood pressure screening programs were conducted using models from activities in barbershops. Mass media campaigns increased encouraging people to know their numbers, visit their doctor, reduce salt consumption, and increase physical activity. Two professional and advocacy societies were established, The Consortium for Southeast Hypertension Control (COSHEC) and the International Society for Hypertension in Blacks (ISHIB). Both focused their efforts on continuing medical education and community outreach. In addition, the Southern Medical Society increased their continuing medical education programs to focus on hypertension. Likewise, the American Society of Hypertension organized regional chapters including the Carolinas-Georgia-Florida Chapter to address specific regional risks.199–201 The pharmaceutical industry assisted by reprinting program materials or providing unrestricted education grants for regional continuing education conferences. Workshops were conducted to determine why the SE United States had higher stroke mortality than the rest of the nation.202 This compendium of structured community and professional activities was associated with a reduction in stroke mortality in the Southeast.
Other structured programs such as those at worksites,203, 204 and subsequently the Health and Human Services Million Hearts™ initiative and the American Heart Association’s Get with the Guidelines Programs, the Citizens for the Treatment of High Blood Pressure were developed and maintained under the premise of high blood pressure prevention, treatment and control as a means to reduce stroke mortality risk.205 These programs addressed the clinical and public health efforts and demonstrated an essential partnership to reduce the population burden from stroke.206
Hypertension research gaps and considerations
While the evidence for the hypertension management and stroke risks is strong, several research gaps should be addressed in order for the development of the most effective primary, secondary and tertiary prevention interventions. For example, a substantial fall in hypertension-associated ICH over the past 25 years has been well documented, but not in the overall number of cases of ICH in older age-groups due in part due to an increase in antithrombotic use.207 With an expected increase in prevalence of amyloid angiopathy among the ageing population, an increase in the number of cases of ICH might be projected. Likewise study of cerebral microbleeds (CVB) and hypertension with increased stroke risks has potential high impact as an important emerging imaging biomarker with the potential to provide insights into ICH pathophysiology, prognosis, and disease progression, as well as therapeutic strategies.208 Such studies also facilitate disparities as significant racial differences in CVB prevalence in ICH.209 While the benefit of BP reduction is well documented, the management of hypertension is complicated with several uncertain clinical questions.210 BP management in acute ischemic stroke remains problematic such as when to initiate antihypertensives and the level of BP reduction as well as the class of agents to be used.
In summary, multiple evidence sources identify the impact of BP reduction on stroke mortality decline. Epidemiological and observational studies over the past 5 decades consistently identify a significant association of BP level and stroke mortality for all genders, races and cultures, as well as all age groups. Higher BP equals greater risk for stroke. Clinical trials have confirmed the consistent findings of reduced BP and lower stroke mortality rates. The trends in stroke risks with BP level identified from the observational epidemiologic studies are consistent with the evidence for the levels of BP reduction from clinical trials. The evidence is strong such that clinical guidelines and intervention programs focus on BP management and lower BP levels for primary and secondary stroke prevention. These comprehensive components of population risk reduction are ideal models for the clinical medicine and population health partnership. The accelerated decline in stroke mortality beginning in the 1970s is consistent with the aggressive hypertension treatment and control strategies implemented in that time period. In addition, with an aging and heavier population, the pool of at-risk individuals has increased substantially during this time period. Yet, the stroke mortality rates continued to decline, which is consistent with the improved hypertension prevention and control rates, and declines in mean arterial BP rates in populations. The decrease in blood pressure with drug therapy as assessed in clinical settings and widespread public health interventions in the general population appears to be the major determinant for reduction in the risk of stroke and stroke deaths.167
Contribution of Diabetes Treatment and Control on Decline in Stroke
Diabetes mellitus is a risk factor for stroke and stroke mortality.211, 212 The prevalence of diabetes has been steadily increasing in the US and throughout the world.213, 214 Sparse data are available regarding trends in population prevalence of diabetes treatment or treatment intensity. As such, the temporal effect of changes in diabetes treatment on risk of stroke death cannot be determined.
Although the incidence of ischemic stroke has been declining in the US in recent years, the proportion of individuals with ischemic stroke with comorbid diabetes has increased. A recent analysis of nationwide trends in acute ischemic stroke (AIS) hospitalizations in the US from 1997 to 2006 revealed that the absolute number of AIS hospitalizations declined by 17% (from 489,766 in 1997 to 408,378 in 2006); however, the absolute number of AIS hospitalizations with comorbid type 2 diabetes rose by 27% [from 97,577 (20%) in 1997 to 124,244 (30%) in 2006, p <0.001]. The rise in comorbid diabetes over time was more pronounced in patients who were relatively younger, Black or ‘other’ race, on Medicaid, or admitted to hospitals located in the South. Factors independently associated with higher odds of diabetes in AIS patients were Black or ‘other’ versus White race, congestive heart failure, peripheral vascular disease, history of myocardial infarction, renal disease and hypertension.215
During the last two decades, the main thrust of diabetes treatment research has been to investigate whether tight glucose control would improve long-term outcomes, especially related to the development of both microvascular and macrovascular complications. Microvascular complications include retinopathy, nephropathy, and neuropathy (peripheral and autonomic), while macrovascular outcomes include cardiovascular events and cerebrovascular events. Regarding tight glucose control in the outpatient setting, observational studies have shown a positive correlation between measures of glycemic control and reduced rates of developing micro- and macro-vascular outcomes.216–218 The current guidelines for the management of diabetes emphasize patient-tailored goals for diabetic patients and related co-morbid conditions.219
For patients with Type 1 diabetes, the Diabetes Control and Complications Trial (DCCT) tested intensive glucose control vs. standard care among typically young patients with Type 1 diabetes (mean age was 27 years at trial enrollment). Subjects were treated for a mean of 6.5 years between 1983 and 1993 and a substantial benefit was seen in reducing microvascular complications. The number of macrovascular events was very small, as might be expected for typical, young Type 1 patients. A follow-up study tracked patients to 11 years after enrollment and found a significant reduction in macrovascular events for subjects in the intensive treatment arm.220
A recent meta-analysis of five interventional trials in both type 1 or type 2 diabetes examined the effect of tight glucose control on reducing macrovascular events and all-cause mortality221 and found that tight glucose control does provide benefit for reducing myocardial infarction and coronary heart disease events. No consistent effect was found for stroke; tight glucose control was neither beneficial nor harmful. There was a suggestion that mortality may be increased with tight glucose control, driven primarily by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial results.222
With any strategy of glucose lowering (acutely/intensively vs. chronically) there is a risk of symptomatic hypoglycemia and other side effects, and thus it is imperative that a benefit for such strategies be robust. The ACCORD study is worth discussing as it is one of the most recent glucose management trials in the outpatient setting and also one of the largest.
Blood pressure reduction and modification of other risk factors has been shown to be beneficial for stroke reduction in patients with type 2 diabetes. In a pre-planned sub-study of ACCORD, patients with type 2 diabetes were found to have reduced risk for stroke if blood pressure is tightly controlled;223 this same effect was also seen in the UKPDS study.224 Patients with type 2 diabetes and chronic kidney disease are among those with the greatest risk for stroke. In the STENO-2 study, these patients had reduced all-cause mortality and stroke incidence in both the short and long-term when randomized to a comprehensive interventional health program compared to usual care. This program involved a regimented plan for managing diabetes as well as high blood pressure and high cholesterol, and also included wellness programs such as an exercise program, smoking cessation, and dietary intervention.225, 226 Collectively, these studies suggest that multi-factorial risk factor intervention, and especially tight blood pressure control, lowers risk for macrovascular events including stroke in patients with diabetes. An increasing obese population will present with increasing diabetes, further driving up BP prevalence and increasing stroke mortality. Preventing and controlling hypertension and diabetes particularly in this population mitigates stroke risk.
An additional line of research is whether intensive glucose control (typically defined as a glucose goal ≤ 150 mg/dL obtained using an insulin infusion during part or all of the ICU stay) during the acute stroke hospitalization improves outcomes and mortality. For this question, research is as yet inconclusive and ongoing in a large Phase III clinical trial (Stroke Hyperglycemia Insulin Network Effort; SHINE)227 in relation to stroke mortality, with a glucose goal of 80–130 mg/dL in the intensive arm. Prior studies did not show a benefit on post-stroke mortality,228–230 but were either pilot/safety studies or were underpowered due to difficulties with recruitment. This line of research is supported by trials of acute intensive glucose management that showed a reduced ICU mortality and length of stay,231 although a meta-analysis of all ICU based glucose management trials did NOT find a similar benefit.232 The Insulin Resistance Intervention after Stroke (IRIS) trial is a randomized, double-blind, placebo-controlled study that is seeking to test the effectiveness of pioglitazone for lowering the risk for stroke after ischemic stroke or TIA. As insulin resistance is estimated to affect 50% of stroke patients, these results may have impact for secondary stroke prevention.233
In summary, research continues to study the benefit of intensive glucose lowering in the setting of acute stroke hospitalization. At this time, there is insufficient evidence to know if this treatment is beneficial for reducing mortality or improving outcome and more data are needed. Tight glucose control did reduce stroke incidence for type 1 diabetic patients in one RCT; however the impact on overall stroke mortality in the population would be small given that type 1 diabetes is much less prevalent than type 2 diabetes and thus may have a relatively small impact on stroke mortality. Tight glucose control for type 2 diabetic patients has not been shown to reduce mortality from stroke (based on a meta-analysis) and in fact led to higher mortality in one large RCT. Multi-factorial risk factor intervention in diabetic patients, especially blood pressure control, has been shown to reduce mortality and macrovascular events including stroke in multiple RCTs. Data on the prevalence of diabetes over the last century are sparse.234
Contribution of Atrial Fibrillation (AF) Treatment and Control on Decline in Stroke Mortality
AF is a significant factor for stroke with an attributable risk of 1.5% for persons age 50–59 rising to 23.5% for 80–89 year olds.235 Data on secular trends in age-adjusted prevalence of AF are limited. Trends based on hospital discharge data are limited by ascertainment bias because telemetry and serial EKGs have become more common in hospitals over time. Results from a community-based study in Rochester, Minnesota showed a significant secular trend of increased atrial fibrillation prevalence from 1960 to 1989 among both stroke cases and controls of both genders, but it was not possible to quantify the contribution of ascertainment bias to the observed trends.236
In the Framingham Study, it was possible to identify secular trends based on biennial clinic examinations alone and based on all sources, including biennial examinations, private physician records and interim hospitalizations.237 Among men aged 65–84 years, the age-adjusted prevalence of atrial fibrillation at the biennial examination showed a suggestion of an increase from 2% in 1968–1970 to 5.3% in 1987–1989 (p=.08). No secular trend for an increased prevalence of AF was identified among women. Results based on AF detected from all sources showed an increase among men from 3.2% in 1968–1970 to 9.1% in 1987–1989 (p=.0002), but, again, no trend among women. Thus, limited available evidence suggests that age-adjusted atrial fibrillation rates are not decreasing over time, and may be increasing among men. In part, this may be attributable to better survival of cardiac conditions, including myocardial infarction.236, 237
Randomized clinical controlled trials comparing warfarin to aspirin in nonvalvular atrial fibrillation were not powered to detect differences in stroke mortality. Even a meta-analysis of available data published before 1999 was underpowered for this endpoint; the pooled estimate of the effect of warfarin on stroke mortality for six trials was 0.74 (95% confidence interval 0.39–1.40).238 A subsequent trial conducted among persons with atrial fibrillation, ages 75 and older,239 also showed a trend towards decreased stroke mortality for warfarin treated patients (relative risk 0.59, 95% confidence interval 0.27–1.24).
Data from a large administrative dataset240 shows a significant reduction in the composite end point of stroke or mortality for patients on warfarin with a CHADS2 greater or equal to 1. The clinical prediction rule for estimating the risk of stroke in patients with non-rheumatic atrial fibrillation includes: C (Congestive heart failure=1); H (Hypertension: blood pressure consistently above 140/90 mmHg/or treated hypertension on medication=1); A (Age ≥75 years=1); D (Diabetes mellitus=1); S2 (Prior Stroke or TIA or Thromboembolism=2). However, it should be noted that observational data of this type is subject to the potential bias that warfarin might be prescribed to healthier patients and aspirin to more frail patients.
The evidence is very strong that anticoagulation with warfarin for atrial fibrillation patients reduces fatal and non-fatal stroke by approximately 50%.241 There is a reduction in case fatality rates in patients taking warfarin compared to those not taking the medication.242
Since 1989, numerous trials have shown a benefit of warfarin treatment over antiplatelet therapy among patients with atrial fibrillation.241 Available evidence supports an increase in use of anticoagulation therapy for treatment of atrial fibrillation since the publication of these trials. A study of Medicare patients with atrial fibrillation from1992–2002243 showed that warfarin use increased significantly for each year examined, from 24.5% to 56.3%. A comparison of treatment trends among 569,883 ischemic stroke admissions to Get with the Guidelines-Stroke hospitals between 2003 and 2009 showed that the percent of stroke patients with atrial fibrillation treated with anticoagulation increased from 28% to 69% and that the percent of stroke patients with a contraindication to anticoagulation declined from 58% to 27%.244
In summary, there is evidence from observational studies that treatment of atrial fibrillation with warfarin has increased during the past 40 years, especially within the past two decades. Although data from randomized clinical trials show a 26% – 41% reduction of stroke mortality with warfarin, the estimated effect is not statistically significant due to relatively small numbers of fatal stroke events. However, there is strong data indicating that incident stroke, which does impact stroke mortality rates in the population, has been reduced. More importantly, there is evidence from a single observational study suggesting that the age-adjusted prevalence of atrial fibrillation may be increasing.
There is a need for further data on temporal trends in age-adjusted atrial fibrillation prevalence. In addition, it will be important for post-marketing surveillance to monitor the prevalence of use and clinical outcomes of the newer oral anticoagulants245 since available data suggests that they will have an even stronger effect than warfarin on reducing stroke mortality in atrial fibrillation. Thus, it is unclear whether changes in atrial fibrillation prevalence or management have contributed to changes in stroke mortality due to competing effects of increasing prevalence of atrial fibrillation, improved treatment of atrial fibrillation and that warfarin as a routine treatment of atrial fibrillation was not actively used until the last decade.
Contribution of Hyperlipidemia Treatment and Control on Decline in Stroke Mortality
Recent trends in dyslipidemia prevalence in the United States
Over the past 30 years, there have been improvements in awareness, treatment and control of dyslipidemia;246–249 however, dyslipidemia remains highly prevalent in the United States. Data from the National Health and Nutrition Examination Surveys (NHANES), cross-sectional samples of the US population, showed a decrease in the proportion of individuals with total cholesterol ≥240 mg/dL from 20.5% in 1988–1994 to 14.2% in 2005–2010.248 Age-adjusted mean total cholesterol levels decreased from 210 mg/dL in 1976–1980 to 200 mg/dL in 1999–2006 and mean low density lipoprotein cholesterol (LDL-C) levels declined from 134 to 119 mg/dL during the same time frame.249 On the other hand, the prevalence of adults with untreated desirable total cholesterol levels (<200 mg/dL) remained unchanged from 1988 to 2010 (46.8% in 1988–1994 to 46.0% in 2005–2010)248 and an estimated 33.5 million US adults >20 years currently have total serum cholesterol levels ≥240 mg/dL.5
The mild improvements in total cholesterol and LDL-C levels have likely been due to population-wide behavioral and environmental factors in addition to an increase in dyslipidemia awareness and medication use. An analysis of the distribution of total cholesterol levels across US birth cohorts from 1959 to 1994 revealed that the entire distribution of total cholesterol concentrations shifted to lower levels in the United States.250 The shift was more pronounced in the upper range of the distribution, likely reflecting changes in treatment and control of hypercholesterolemia. The decrease in the lower end of the distribution was likely due to population influences such as reduced consumption of dietary saturated fatty acid and cholesterol intake.250 Studies have shown improvements in awareness and control of hypercholesterolemia in the United States. The self-reported history of “high cholesterol” increased from 17% in 1988–1994 to 27% in 1999–2006, self-reported lipid medication use by those with high cholesterol increased from 16% to 38%,249 and LDL-C control increased from 4.0% in 1988–1994 to 25.1% in 1999–2004 among those with high LDL-C.247 More recent data shows that treatment of high LDL-C increased to 48% and control of LDL-C among those with high LDL-C was 33.2% in 2005–2008.251
Improvements in dyslipidemia control have not been uniform across demographic strata. Rates of LDL-C control were lower among adults aged 20–49 years compared with those ≥65 years (13.9% vs. 30.3%), non-Hispanic blacks and Mexican-Americans compared with non-Hispanic whites (17.2% and 16.5% vs. 26.9%), and men compared with women (22.6% vs. 28.0%).247 In addition, in the Framingham Heart Study, the proportion of those treated for high LDL-C was 41% in insured men but only 7% in uninsured men (OR of treatment 0.12; p<0.001). Control of LDL-C was achieved in only 7% of uninsured men with elevated LDL-C versus 31% in insured men (OR of control 0.17; p=0.004).252
High density lipoprotein cholesterol (HDL-C) and triglyceride levels - components of the metabolic syndrome - have not shown clear improvements over the past 30 years. Mean HDL-C increased from 50 mg/dL in 1976–1980 to 53 mg/dL in 1999–2006, but this change was likely due to alterations in measurement method. Mean triglyceride levels worsened from 130 to 146 mg/dL over the same time frame, coincident with an increase in mean BMI from 26 to 29 kg/m2.249 Obese individuals were nearly four times as likely (OR 3.7, 95% CI 3.4–4) and overweight individuals were over two times as likely (OR 2.4, 95% CI 2.2–2.6) to have elevated triglycerides as those with BMI <25 kg/m2, after adjusting for numerous variables.
Association between dyslipidemia and stroke incidence
Although high LDL-C and low HDL-C levels are clearly established risk factors for coronary artery disease, there is a less consistent association between dyslipidemia and stroke risk.253–255 The lack of an overall association likely conceals a positive association with ischemic stroke and negative association with hemorrhagic stroke.253 In addition, there is an unclear association between dyslipidemia and ischemic stroke risk, likely due to the heterogeneity of ischemic stroke mechanisms.
Some cohort and case-control studies have found an association between high total cholesterol, high LDL-C levels, high triglyceride levels, and low HDL-C levels and ischemic stroke,256–261 while others have shown weak or inconsistent associations.254, 255, 262, 263 Grouping all ischemic strokes together may conceal valuable information regarding the association between dyslipidemia and stroke subtypes: dyslipidemia is a risk factor for large vessel intra- and extracranial atherosclerosis258, 263–266 and lacunar stroke,258 but is not an established risk factor for cardioembolic stroke.
Case-control and cohort studies have shown that serum total cholesterol levels are inversely related with intracerebral hemorrhage (ICH),258, 267 however, one study did not show an independent association.268 The lipid fraction(s) responsible for the association with ICH risk are unclear,255, 260, 262, 269 but low LDL-C270 and triglyceride levels260, 270, 271 may drive the increased risk of ICH.
Analyses of the association between lipid levels and stroke risk should take into account the use of cholesterol-lowering medications, changes in lipid levels, and time from laboratory testing until event. An analysis of 2,940 individuals from the population-based prospective cohort study, Northern Manhattan Study (NOMAS), revealed that high LDL-C and non–HDL cholesterol levels were paradoxically associated with lower stroke risk.272 This paradoxical effect was likely due to the fact that treatment with cholesterol-lowering medications modified the effect of elevated LDL-C levels on stroke. After excluding individuals taking cholesterol-lowering medications, the paradoxical effect disappeared and there was a trend toward an increased risk of ischemic stroke with an LDL-C level >130 mg/dL.272
Association between dyslipidemia and stroke severity and mortality
Dyslipidemia has been associated with lower mortality after ischemic stroke.273, 274 In a recent study of 274,988 ischemic stroke patients admitted to 1036 hospitals participating in Get with the Guidelines-Stroke Program, history of dyslipidemia was associated with lower risk of in-hospital mortality (OR 0.68, 95% CI 0.64–0.71).273 This inverse association with stroke mortality could be due to the fact that dyslipidemia is associated with non-cardioembolic strokes, which are less severe and have better prognoses than cardioembolic strokes.273–275 Supporting this theory, the Copenhagen Stroke Study revealed an inverse and almost linear independent association between concentrations of total serum cholesterol and stroke severity. Smaller infarcts were associated with lower stroke severity. An increase of 1 mmol/L in cholesterol resulted in lower mortality (HR 0.89, 95% CI, 0.82 to 0.97).274 An alternative explanation is that a history of dyslipidemia is associated with use of medications such as statins, which may impact the severity or the prognosis of the stroke.273
Low total cholesterol, triglyceride, and LDL-C levels are also associated with a higher risk of death after ICH.276–278 This may be due to cholesterol’s protective effect against hematoma growth,279 or its importance for maintaining vessel integrity and resistance to rupture.280
On the other hand, one study revealed higher stroke mortality rates among those with dyslipidemia. Among individuals with diabetes who participated in NHANES, those with higher serum levels of non-HDL-C (composite marker of several atherogenic lipoproteins, including LDL, very-low-density lipoprotein, intermediate-density lipoprotein, and lipoprotein a) had a higher risk of death from stroke (RR 3.37 95% CI 0.95–11.90 and RR 5.81, 95% CI 1.96–17.25) for non-HDL-C concentrations of 130–189 mg/dL and 190–403 mg/dL, respectively (p=0.001 for linear trend) compared to participants with serum non-HDL-C concentrations of 35 to 129 mg/dL, after adjustment for demographic characteristics and selected risk factors.281
Effects of lipid-lowering therapy on stroke incidence and mortality
An estimated 24 million adults were taking statins in 2003–4 representing a steady increase in use from previous decade.282 Lipid-lowering therapy with statins reduces ischemic stroke incidence; however, the benefit is less robust among individuals with established cerebrovascular disease and may be counterbalanced by higher rates of ICH in statin users. A 2008 systematic review of studies investigating the effect of statins in 8,832 patients with a history of cerebrovascular disease showed a pooled relative risk of ischemic stroke of 0.80 (95% CI 0.70 to 0.92) and of hemorrhagic stroke of 1.73 (95% CI 1.19–2.50), suggesting that the beneficial impact of statins on ischemic stroke might be partially offset by the increased risk of hemorrhagic stroke.283 In addition, a 2009 Cochrane review reported a marginal benefit in reducing subsequent cerebrovascular events in those with a previous history of stroke or transient ischemic attack (OR 0.88, 95% CI 0.77–1.00) and a lack of association with all-cause mortality or sudden death (OR 1.00, 95% CI 0.83–1.20).284 There was an increase in the odds of hemorrhagic stroke with statin therapy (OR, 1.72; 95% CI 1.20–2.46); however, only two trials in the review specifically assessed the risk of hemorrhagic stroke.284 One of these trials, SPARCL, randomized 4,731 individuals with recent stroke or transient ischemic attack to high dose atorvastatin vs. placebo and showed a 5-year absolute reduction in risk of stroke of 2.2% (adjusted HR 0.84, 95% CI 0.7–0.99;P = 0.03; unadjusted p= 0.05). This result included a reduction in ischemic stroke, but an excess of hemorrhagic stroke. The overall mortality rate was similar.285 A post-hoc analysis of the trial found that treatment with atorvastatin was independently associated with an increased risk of hemorrhagic stroke (HR 1.68; 95% CI 1.09 – 2.59). Subjects enrolled with an index hemorrhagic stroke had a >5-fold increase in risk of recurrent hemorrhage.286
The association between statin therapy and ICH, however, is not consistent. A 2012 meta-analysis of 31 randomized controlled trials of statin therapy (91,588 subjects included in the active group and 91,215 in the control group) revealed no significant difference in incidence of ICH observed in the active treatment group versus control (OR, 1.08; 95% CI 0.88–1.32); however the meta-analysis only included two secondary stroke prevention trials.287 Total stroke (OR 0.84; 95% CI 0.78–0.91) and all-cause mortality (OR 0.92; CI 0.87– 0.96) were reduced in the active therapy group.287 In addition, a 2011 systematic review and meta-analysis of 23 randomized trials and 19 observational studies (248,391 patients) revealed that statins were not associated with an increased risk of ICH in randomized trials (RR 1.10; 95% CI 0.86–1.41), cohort studies (RR 0.94; 95% CI 0.81–1.10), or case-control studies (RR 0.60; 95% CI 0.41–0.88).288 A subsequent retrospective cohort study found no association between statin use and subsequent ICH among individuals with previous ischemic stroke.289
In a meta-analysis of 5 trials of more versus less intense therapy with statins (39,612 individuals; median follow-up 5.1 years) and 21 trials of statin versus control (129,526 individuals; median follow-up 4.8 years), all-cause mortality was reduced by 10% per 1.0 mmol/L LDL reduction (RR 0.90, 95% CI 0.87–0.93), largely reflecting significant reductions in deaths due to coronary heart disease and other cardiac causes, with no significant effect on deaths due to stroke or other vascular causes.290 Specifically, there were no significant effects on mortality from first stroke, mortality from first ischemic or first hemorrhagic stroke, or incidence of first non-fatal hemorrhagic stroke. There was, however, a significant reduction in first nonfatal ischemic stroke, corresponding to a 23% (99% CI 15–30) reduction per 1.0 mmol/L reduction in LDL cholesterol. Compared to the less intensive regimens, more intensive regimens produced a 16% further reduction in ischemic stroke (RR 0.84, 99% CI 0.71–0.99) and non-significant increase in risk of hemorrhagic stroke (RR 1.21, 99% CI 0.76–1.91).290
Statin therapy in the acute setting after stroke may have a beneficial impact on survival. A population-based prospective cohort study found that new post-stroke statin therapy was associated with both early and late survival, compared with statin untreated patients (OR for death 0.12, CI 0.03–0.54 at 7 days; OR 0.19, CI 0.07–0.48 at 90 days; OR 0.26, CI 0.12–0.55 at 1 year), after adjusting for age, prestroke disability, NIHSS score, hypertension, and aspirin. Similar findings were seen for statin therapy before stroke onset (adjusted OR for death compared with statin-untreated-patients, 0.04; 95% CI 0.00–0.33 at 7 days; OR 0.23; 95% CI 0.09–0.58 at 90 days; OR 0.48; 95% CI 0.23–1.01 at 1 year).291 A meta-analysis of 15 randomized placebo-controlled trials using various statins assessed the risk of strokes for patients with a history of coronary disease identifying significantly reduced recurrent ischemic stroke risk (RR=0.74; 95% CI, 0.64–0.86) with one recurrence of ischemic stroke prevented for every 110 coronary disease patients treated with a statin.292
The effect of fibrate therapy on stroke incidence and mortality is unclear, but a recent meta-analysis revealed a reduction in risk of vascular events among individuals with high triglycerides and/or low HLD-C treated with fibrates. Compared to placebo, fibrate therapy reduced the risk of vascular events in 7389 subjects with high triglycerides, (RR 0.75, 95% CI 0.65 to 0.86); in 5068 subjects with both high triglycerides and low HDL-C (RR 0.71, 95% CI 0.62 to 0.82), and in 15,303 subjects with low HDL-C (RR 0.84, 95% CI 0.77 to 0.91).293
In summary, over the past thirty years, there have been improvements in awareness, treatment and control of dyslipidemia; however, dyslipidemia remains highly prevalent and levels of control remain suboptimal. Declines in stroke were underway for more than a half century prior to the documentation of the potent role of blood lipids on heart disease (and potentially stroke) risk. The relationship between dyslipidemia and stroke risk and mortality remain equivocal, likely due to the differential impact of various lipid fractions on ischemic and hemorrhagic stroke risk. In addition, the heterogeneity of ischemic stroke mechanisms further complicates the picture. Currently, there is unclear evidence that dyslipidemia treatment and control have contributed to the decline in stroke mortality. Further randomized controlled trials are needed to assess the impact of treatment for dyslipidemia on stroke incidence, outcomes, and mortality, stratified by stroke subtype.
Contribution of Aspirin and Other Antiplatelet Drugs to Decline in Stroke Mortality
Aspirin and other antithrombotics reduce stroke mortality by preventing thrombotic ischemic strokes and by reducing the case fatality rate when started after acute ischemic stroke.
A systematic review and meta-analysis of randomized controlled trials showed that aspirin reduced the risk of incident stroke when used for secondary prevention after previous ischemic stroke (OR 0.77, 95% CI 0.69–0.85)294 or for secondary prevention after other major vascular events (ORs 0.62 to 0.73, all p<0.05).294 By contrast, when used in men and women for primary prevention aspirin did not reduce the overall stroke incidence (OR 0.95, 95% CI 0.85–1.06).295 This was because a significant reduction in incident ischemic stroke (OR 0.86, 95% CI 0.74–1.00) was offset by an increased incidence of hemorrhagic stroke (OR 1.32, 95% CI 1.00–1.75), and with no difference in the risk of stroke of unknown type which accounted for approximately one third of stroke events in the randomized trials.295 The risk of fatal stroke was non-significantly increased (OR 1.21, 95% CI 0.84–1.74).295 There is evidence that these effects differ by sex, however, with women experiencing more benefit than men. In a systematic review and meta-analysis of clinical trials of aspirin for primary stroke prevention in women, aspirin for primary prevention reduced the overall incidence of stroke (OR 0.83 95% CI 0.70–0.97), driven by a reduction in ischemic stroke (OR 0.76, 95% CI 0.63–0.93) that outweighed a non-significant increase in hemorrhagic stroke (OR 1.07, 95% CI 0.42–2.69) in the study sample.296 However, the largest randomized controlled trial in women found a non-significant increase in fatal stroke (OR 1.04, 95% CI 0.58 to 1.86) despite a reduction in overall (fatal and non-fatal) stroke incidence (OR 0.83, 95% CI 0.69–0.99), probably because hemorrhagic stroke, which was more frequent in aspirin users compared to placebo, had a higher case fatality rate than ischemic stroke, which was less frequent in aspirin users compared to placebo.297 Therefore aspirin does not appear to reduce fatal stroke in either men or women when used for primary prevention.
When started within 48 hours of acute ischemic stroke, aspirin may reduce the risk of all-cause death at 1–3 months (OR 0.93, 95% CI 0.86 to 1.01) compared to placebo, according to a Cochrane systematic review and meta-analysis.298
The use of regular daily aspirin has increased from the 1970s to 2003. In 1980, following seminal randomized controlled trials in the 1970s299, 300 aspirin was approved by the FDA for secondary prevention of stroke post TIA. It was recommended by professional guidelines for prevention of stroke in 1994,301 and recommended for primary prevention in high-risk women and men by the U.S. Preventive Services Task Force in 2002 and 2009.302 Longitudinal nationally representative data on changing aspirin use over time are available, but are limited by differences in sampling and in ascertainment of aspirin use. Survey data from the National Health and Nutrition Examination Survey (NHANES) I indicate that aspirin use for analgesic purposes was common and increased from 59% in 1971–1975 to 78% in 1976–1980; however the majority of use was intermittent and the frequency of daily use was not determined.303 In 1998–1999, aspirin was used frequently, either daily or within with last week, by 18–30% of the U.S. population.304, 305 The growth of daily or every other day aspirin use in adults with stroke or cardiovascular disease (CVD) has been even higher. According to data from the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in outpatients with cardiovascular disease, initiation or continuation of aspirin was recommended in 5% of outpatient visits in 1980, 26% of outpatient visits in 1996, 33% of outpatient visits in 2003 and 47% of outpatient visits in 2007–2008.306–308 However, the true prevalence of aspirin use in patients might be underestimated from these physician surveys because patients may be taking aspirin without the recommendation or knowledge of the physician. Indeed, data from the Behavioral Risk Factor Surveillance System (BRFSS) surveys show that the prevalence of frequent aspirin use among persons with known stroke or cardiovascular disease is much higher, 61% in 1999 and 69% in 2003.304
There are a growing number of antithrombotic alternatives to aspirin. Ticlopidine reduced the risk of major vascular events compared to aspirin294 but was never widely used because of safety concerns. Clopidogrel reduced the risk of incident stroke in patients with cardiovascular disease or stroke compared to aspirin (RR 0.93, 95% CI 0.81–1.06) in one study sample; this reduction was similar to that observed in the trial for all major vascular events (RR 0.91) but was not statistically significant because of the small number of incident strokes.309 Likewise, the reduction in vascular death was similar (RR 0.92, 95% CI 0.80–1.07) but also not significant due to few deaths.309 One randomized controlled trial suggested the combination of aspirin and sustained-release dipyridamole reduced the risk of recurrent ischemic stroke compared to aspirin alone when used for secondary prevention of stroke (RR 0.77 95% CI 0.64–0.91),310 but another similar sized randomized controlled trial found a non-significant reduction in risk (HR 0.84, 95% CI 0.64–1.10),311 with neither trial finding that aspirin and dipyridamole reduced the risk of vascular or all-cause death compared to aspirin.310, 311 In a subsequent randomized controlled trial, aspirin and sustained-release dipyridamole was not superior to clopidogrel for secondary prevention of recurrent ischemic stroke, with higher risk of intracranial hemorrhage.312 A meta-analysis showed that the combination of aspirin and clopidogrel reduced the risk of stroke compared to aspirin alone in patients with cardiovascular diseases (RR 0.84, 95% CI 0.72–0.96).313 However, the combination of aspirin and clopidogrel increased the risk of major bleeding and did not reduce all-cause mortality; fatal strokes were not reported separately.313 A randomized controlled trial of aspirin and clopidogrel to prevent recurrent stroke in ischemic stroke patients showed no benefit compared to clopidogrel alone.314 Integrating all the evidence, current professional guidelines from the American Stroke Association suggest that either aspirin, clopidogrel or the combination of aspirin and sustained-release dipyridamole are reasonable alternatives for secondary prevention of ischemic stroke, although aspirin has the highest rated supporting evidence, and recommended against the use of the combination of aspirin and clopidogrel.76 Nationally representative data on the choice of aspirin, clopidogrel, or the combination of aspirin and clopidogrel for secondary prevention of stroke are not available, nor are data on longitudinal changes over time. Data from a large hospital registry from 2003 to 2008 showed that aspirin monotherapy remains the most common choice for patients discharged after admission for ischemic stroke, possibly based on its good side-effect profile and low cost.315
Results from the Secondary Prevention of Small Subcortical Strokes Trial (SPS3) indicated a combination of clopidogrel plus aspirin in patients with small vessel stroke led to reduction in stroke, but was not significantly better than aspirin alone and reported an increase in hemorrhagic complications.80 These findings were consistent for the risk of recurrent stroke; dual antiplatelet therapy (2.5% per year) vs. aspirin alone (2.7% per year) (hazard ratio, 0.92; 95% confidence interval [CI], 0.72 to 1.16), as well as the risk of recurrent ischemic stroke (hazard ratio, 0.82; 95% CI, 0.63 to 1.09) and disabling/or fatal stroke (hazard ratio, 1.06; 95% CI, 0.69 to 1.64). In addition, two ongoing trials may provide additional evidence regarding the benefit of aspirin and antithrombotic therapy. The Clopidogrel in High-risk Patients With Acute Non-disabling Cerebrovascular Events (CHANCE) trial was designed to assess the effects of a 3-month regimen of clopidogrel versus a 3-month regimen of aspirin alone on reducing the 3-month risk of any stroke in high-risk patients with TIA or minor stroke in China.316 Also, the Platelet-Oriented Inhibition in New TIA and minor ischemic stroke (POINT) Trial is a randomized, double-blind trial to determine whether clopidogrel is effective in improving survival free from major ischemic vascular events.317
The use of aspirin has a substantial global impact as well with different particular high risk in developing countries.82 In additional to risk levels stroke type varies as well as the prevalence of co-morbid conditions representing a significant future study need.
In summary, increased use of aspirin for secondary prevention of ischemic stroke in persons with history of ischemic stroke or ischemic heart disease has probably had a moderate impact on reducing stroke mortality, mediated by a reduction in the incidence of both fatal and non-fatal recurrent strokes. However, increased use of aspirin in persons without known cardiovascular disease has probably had no effect on stroke mortality, because reductions in first-ever fatal ischemic stroke are balanced by increases in fatal hemorrhagic stroke, with overall no net reduction in fatal stroke. Increased use of aspirin for acute ischemic stroke is expected to have had a mild impact on stroke mortality by reducing the stroke case fatality rate. The increased use of alternatives to aspirin, such as clopidogrel or the combination of aspirin and sustained-release dipyridamole has probably had no impact on reducing stroke mortality because their effects on fatal stroke are so similar to aspirin. In contemporary practice, the use of aspirin in acutely hospitalized ischemic stroke patients is widespread without much opportunity for further improvement; however, in the outpatient setting, a substantial portion of patients with known cardiovascular disease are not taking aspirin, even though they probably should be.
Contribution of Neurological and Technical Advances in Stroke Treatment on Decline in Stroke Mortality
Advances in the technological and medical treatment of stroke, as well as the systems for delivering care, have affected stroke mortality rates. While the advances are addressed in the individual sections of this report, the history and timing of the specific developments are important when considering the potential impact on the decline in stroke mortality.
One of the first major advances in stroke diagnosis occurred during the 1920s and 1930s involving the development of contrast angiography. The technique provided contrasted x-ray cerebral angiography for the diagnosis of stroke and distinction from other neurological conditions. Cerebral angiography was enhanced with the Seldinger technique which provides a safer procedure for access with less potential damage.318 Since its development, angiography technology has become a critical component in the diagnosis and management of stroke.
The measurement and assessment of cerebral flow metabolism (CBF) developed in the 1940s and 1950s represents a significant advancement in the treatment of stroke. This assessment represents a minimal risk and cost-effective resource easily used in the clinical setting. The first well-recognized studies performed by Kety and Schmidt in the 1940s used nitrous oxide gas resulting in the development of rate equations for blood flow, and the elucidation of the physiologic relationships between cerebral blood flow, blood pressure and blood volume.319 Several recently completed trials in acute stroke have used the principles of mismatch between blood flow and tissue injury (diffusion-perfusion mismatch) to identify patients with territory at risk who might benefit from reperfusion therapies.
In the 1950s, carotid endarterectomy was developed and introduced as a procedure that reduced the incidence of stroke from carotid stenosis. It was proven beneficial in randomized clinical trials among patients with symptomatic and asymptomatic carotid artery stenosis, leading to inclusion of the procedure in randomized clinical trials.320 These trial results have lead to the development of protocols for selection of the procedure for maximum benefits. Also in the 1950s, prosthetic heart valves were implanted in patients with rheumatic heart disease to reduce the risk for embolic stroke.321
Doppler ultrasonography was introduced in the 1960s.322 This technology has been substantially enhanced during the 1980s to effectively assess blood flow velocity and routinely screen for and follow carotid artery stenosis. Further enhancements that permitted the use of transcranial sonography permit insonation of intracranial vessels, and have proven useful in stroke prevention among patients with sickle cell disease.323 The procedures have been developed into practice protocols in an effort to prevent stroke risks.324 During the 1970s, computed tomography (CT) was first introduced, and has become the main diagnostic tool available for the evaluation of brain injury, and the identification of acute ischemic stroke used in the assessment of stroke and stroke risks.325 With the approval of intravenous thrombolysis and catheter-based embolectomy devices as critical stroke therapies, CT has become an essential component of all acute treatment protocols.326 While MRI imaging has improved the accuracy of stroke diagnosis, there is insufficient evidence as to whether or not it has had any relationship to stroke mortality.
During the 1990s, the FDA approved the use of tissue plasminogen activator (tPA) to treat stroke in the first three hours. As discussed in the dedicated section (Section XI) in this paper, while IV tPA has demonstrated time-dependent improvements in functional independence at 3 months and 1 year post stroke, it has not yet convincingly shown a reduction in stroke mortality. Likewise, three trials determined that endovascular therapy has not been found to reduce mortality from stroke.327–329
In summary, the numerous advancements in the diagnosis, management and prevention strategies have the potential for significant impacts on stroke mortality. Based on the timeframe for implementation into practice, it seems reasonable that in the most recent decades, changes to the organization of stroke care delivery may have the greatest impact on the decline on stroke mortality.
Contribution of Tissue Plasminogen Activator (tPA) Use on Decline in Stroke Mortality
The increasing use of intravenous tPA for acute ischemic stroke since 1996 is unlikely to have contributed to the overall decline in stroke mortality. Randomized controlled trials failed to show that intravenous tPA treatment prevents post-stroke death, although it does prevent post-stroke disability.330, 331 In a pooled analysis of patients treated within 4.5 hours, there was a slight non-significant excess of deaths at 90 days in the tPA-treated group (170/1273, 13.3%) compared to placebo (162/1277, 12.7%, p=0.68).332 Additionally, only a small proportion of ischemic stroke patients are treated with tPA. Administrative data suggests that treatment rates are low but have increased modestly, from 1.1% in 2004–2005 to 3.4% in 2009.333, 334 Endovascular therapies are used in less than 1% of acute ischemic stroke patients335 and are also unlikely to have reduced ischemic stroke mortality.
In summary, evidence from randomized controlled trials suggests that increasing tPA use cannot account for reductions in ischemic stroke mortality.
Contribution of Stroke Systems of Care (Telemedicine, Stroke Units/Teams, Primary and Secondary Stroke Centers) on Decline in Stroke Mortality
A Cochrane systematic review of 26 trials showed that compared with alternative services, stroke unit care reduced the odds of death recorded at final (median one year) follow-up (OR 0.86; 95% CI 0.76 to 0.98; P=0.02), the odds of death or institutionalized care (0.82; 0.73 to 0.92; P=0.0006) and death or dependency (0.82; 0.73 to 0.82; P=0.001).336 Outcomes were independent of patient age, sex and stroke severity. A nationwide population-based cohort study found no association between the number of stroke patients treated and mortality, despite a higher quality of early stroke care and fewer days in the hospital compared with patients in low-volume units.337 The results from data analyses indicate a positive impact of a policy of stroke unit care on case fatality rates.338 The positive stroke outcomes are part of the interactions of stroke systems of care, stroke units, and telemedicine.
Primary or Comprehensive Stroke Centers and Stroke Systems of Care
More recent US data suggests that Primary Stroke Centers (PSC) hospitals may have lower rates of mortality at discharge or beyond compared to non-stroke centers. A study of Medicare beneficiaries discharged with a primary diagnosis of ischemic stroke in 2006 evaluated risk standardized mortality rates (RSMR) in stroke discharges from 315 JC-certified PSC and 4,231 non-certified hospitals. Mean overall 30-day RSMR was 10.9% ± 1.7%. The RSMRs of JC-certified PSCs were lower than in non-certified hospitals (10.7% ± 1.7% vs 11.0% ± 1.7%), although the differences were small. Almost half of JC-certified PSC hospitals had RSMRs lower than the national average compared with 19% of non-certified hospitals.339 The same analysis technique applied to hemorrhagic strokes in 2006 revealed that unadjusted in-hospital mortality (SAH: 27.5% versus 33.2%, P<0.0001; ICH: 27.9% versus 29.6%, P=0.003) and 30-day mortality (SAH: 35.1% versus 44.0%, P<0.0001; ICH: 39.8% versus 42.4%, P<0.0001) were lower in JC-PSC hospitals. Risk-adjusted 30-day mortality was 34% lower (OR, 0.66; 95% CI, 0.58–0.76) after SAH and 14% lower (OR, 0.86; 95% CI, 0.80–0.92) after ICH for patients discharged from JC-PSC-certified hospitals.340 However, a retrospective analysis at 2002 data showed that JC PSC-certified hospitals had better outcomes than non-certified hospitals even before the program began.341 In summary, the evidence that certification itself produced these mortality reductions remains inconclusive.
Using data from 34 academic medical in the US, Douglas et al342 examined the 11 major criteria for establishing the PSC and found 4 of the criteria (written care protocols, integrated emergency medical services, organized emergency departments, and continuing medical/public education in stroke) were associated with increased use of tPA and that hospitals with additional criteria (acute stroke team, a stroke unit, or rapid neuroimaging) tended to have higher tPA use. However, none of the 11 criteria were associated with changes in stroke mortality.
Stroke Units/Stroke Teams
In a systematic review examining the impact of acute stroke units, 13 out of 14 studies found the percent of patients with stroke who died was lower when patients were treated in stroke units and the effect was primarily during the first 1 to 4 weeks after the index stroke.343 More recently, Langhorne et al.338 used a national comprehensive dataset from Scotland, to examine the 6-month mortality for patients treated in organized inpatient stroke units versus those not treated in an organized stroke unit, and found the impact of the stroke unit was absolute risk reduction in case fatality of 3%. It is important to note that the organized stroke unit is a heterogeneous “intervention” and that many of these studies are based in Europe and Canada, where the health systems are organized differently than in the US. In the US, one academic medical center implemented a multidisciplinary stroke team care consultation service. Following the implementation of the stroke team involvement, median length of stay decreased and there were fewer urinary tract infections. However, there was no change in the rate of aspiration pneumonia and mortality did not change344 Several hospitals described the implementation of acute stroke teams, but provided few comparisons of patient outcomes before and after the implementation.345, 346 In Australia, Hamidon347 reported that implementation of an acute stroke team led to reduced time from door to CT scan and reduced LOS, but no significant change in mortality. Xian used data from the New York Statewide Planning and Research Cooperative System and found that among 30,947 patients with acute ischemic stroke, 15,297 (49.4%) were admitted to designated stroke centers. Treatment in a designated stroke center, using the instrumental variable analysis, was associated with lower 30-day all-cause mortality (10.1% vs 12.5%). Differences in mortality also were observed at 1-day, 7-day, and 1-year follow-up.348
While the above description of future systems of care is promising, there are few papers and empirical data examining the impact of stroke systems of care.339–341, 348 Experts strongly believe that an organized system for stroke care is necessary to deliver high-quality stroke care.349–351 Several teams352 have described their experiences implementing stroke centers and systems, two reported increased use of tissue plasminogen activator (tPA) for thrombolysis;353, 354 only one study from Europe reported patient outcomes.354
Telemedicine
A barrier to effective stroke care has been the limited availability of stroke specialists on a 24/7 basis in all geographic regions. Telemedicine enables the assessment of individuals presenting with acute stroke by a stroke specialist who are located remotely. Use of telemedicine during the hyperacute/emergency, acute care and sub-acute phases of care is a strategy that could increase the number of patients who may be assessed and treated by a stroke specialists.355 In 2009, Schwamm et al356 reviewed the evidence for use of telemedicine for stroke care, including whether telemedicine was effective and a feasible alternative to bedside care when stroke specialist are not available. It was concluded that 1) high-quality video teleconferencing is reasonable for performing a general neurological exam and a non-acute NIHSS assessment, 2) FDA-approved teleradiology systems are recommended for timely review of CT scans, and 3) if a stroke specialist is not immediately available, high-quality video teleconferencing can facilitate appropriate use of intravenous tPA and in the acute setting, high-quality video teleconferencing is recommended if a stroke specialist is not immediately available. There is a need for future research focused on telemedicine and primary prevention of stroke.
In summary, given that the stroke systems of care have only recently been developed, the impact of these systems on reductions of stroke mortality prior to 2010 is unknown but likely small.
Contribution of Smoking and Other Respiratory Conditions on Decline in Stroke Mortality
The effect of cigarette smoking on stroke risk and mortality is well known and has been previously summarized.357, 358 Cigarette smoking is an established independent risk factor for stroke.358, 359 The estimated relative risk of stroke associated with current cigarette smoking (versus non-smokers) varies across epidemiologic studies and also by stroke type (ischemic, intracerebral hemorrhage, subarachnoid hemorrhage) but has been estimated to be 1.5 for total stroke in meta-analyses pooling results across studies.358 Among current smokers, there is a dose response relationship between the number of cigarettes smoked and stroke risk.358 Smoking cessation results in a reduction in stroke risk.359–361 Data from the Framingham Heart Study demonstrated that among former smokers stroke risk decreased by two years after smoking cessation and was at the level of non-smokers by five years after cessation.359 Similar to studies of incidence, studies focused on stroke mortality have demonstrated associations with smoking.362–364 In the Multiple Risk Factor Intervention Trial (MRFIT), after 10 years of follow-up, the relative risk of stroke mortality associated with current smoking was 2.5.364 In the Cancer Prevention Study II (CPS-II), risk of stroke mortality for current versus never smokers was 1.7 in men and 2.2 in women after accounting for demographics and additional risk factors in a multivariable model.362 In addition, abstention from smoking after stroke is associated with better outcomes. A retrospective analysis of individuals who participated in NHANES from 1988–1994 with mortality assessment through 2000 revealed that among individuals with history of stroke, abstaining from smoking was associated with lower all-cause mortality, after adjusting for demographic factors, medical comorbidities and lifestyle habits (HR 0.57, CI 0.34 to 0.98).365
Although studies directly assessing the role that cigarette smoking has played in declining stroke mortality rates are lacking, assuming that the stroke mortality risk associated with cigarette smoking has remained constant over time, any decline in the prevalence of smoking would contribute to the decline in stroke mortality. The prevalence of cigarette smoking in United States adults has declined substantially over time decreasing from 42% in 1965 to 19% in 2010. More recently, declines in the prevalence of smoking have slowed with a 24% relative change in current cigarette smoking for the time period 1995 through 2010 and an 8% relative change for the time period 2005 to 2010.366 In addition, smoking intensity or the number of cigarettes smoked per day among smokers has decreased over time.367
Using estimates of prevalence of current smoking over time, the confounder adjusted hazard ratios for stroke mortality comparing current versus never smokers from the CPS-II, and the number of stroke deaths from the National Center for Health Statistics,362 the stroke deaths attributable to smoking for successive years can be determined. These data show that from 2000 to 2009 the number of stroke deaths attributed to smoking has clearly declined, with notable differences across age and sex subgroups. The numbers of stoke deaths attributable to smoking would have been considerably greater for the time period from 1965 through the late eighties when smoking prevalence ranged from 30% to 40%.
It seems logical to assume the decline in smoking from approximately 4,500 cigarettes per capita in 1965 to approximate 2,000 cigarettes per capita in 2000 is a substantial contributor to declining stroke mortality rates for this time period.367 However, stroke mortality was also steadily declining between 1900 and 1965 (Figures 1 and 2), the same period that per capita consumption of cigarettes increased from nearly non-existent to the 4,500 level noted above. In addition, smoking prevalence has also been relatively constant since 2000, a period where stroke mortality has continued to decline. As such, the longer-term patterns in tobacco use do not fully correlate with the pattern of declining stroke mortality in the first half of the twentieth century. This discordance is in contrast to the patterns for heart disease which seem to better align with the trends in tobacco use perhaps due to the differing ages of onset for these two diseases.
Aside from active cigarette smoking, recent decades have seen an increase in research investigating the association of exposure to secondhand smoke (also termed passive smoking and environmental tobacco smoke) and stroke risk. Two meta-analyses on this topic have been published.368, 369 In 2006, Lee et al reported a pooled relative risk of 1.25 (95% CI: 1.16–1.36) for exposure to spousal smoking (or nearest equivalent) and risk of stroke.368 In a subsequent meta-analysis published in 2011, which included several new studies, the pooled relative risk was the same.369 Both reports also supported a dose response relationship between exposure to secondhand smoke and stroke risk. These results suggest that exposure to secondhand smoke may be a risk factor for stroke and therefore for stroke mortality, but as most studies relied on self-reported exposure recall bias is a concern as is publication bias. Of note, prospective studies using cotinine assessed exposure to secondhand smoke have not reported positive associations with stroke risk.370, 371
Similar to the prevalence of active smoking, exposure to secondhand smoke has declined substantially overtime suggesting a possible role in the decline in stroke mortality. Data from NHANES suggests that the proportion of non-smokers exposed to secondhand smoke decreased from 88% in 1988–1991 to 43% in 2001–2002.372, 373 This decline in exposure to secondhand smoke is likely a combination of the decline in active smoking among the population described earlier as well as an increase in the enactment of smoke-free policies in work and public settings. Some ecologic work has been done to investigate the impact of smoke-free policies on stroke.374–376 Some374, 376 but not all375 of these studies have suggested an association between implementation of smoking bans and stroke hospital admissions, but more definitive studies which account for individual-level confounding factors and assess incident stroke or stroke mortality as the endpoint are lacking.
Aside from smoking, other respiratory-related conditions may have contributed to declining stroke mortality over time, although there is more limited data to inform these hypotheses. Pneumonia is common after stroke. A recent meta-analysis of 87 studies estimated the rate of pneumonia in stroke patients to be 10%.377 In studies conducted in the United States, pneumonia is associated with increased stroke case fatality with estimates on the order of two to threefold increased risk.378, 379 Thus, any reduction in the occurrence of post-stroke pneumonia or in its impact on case fatality over time would translate into improvements in stroke mortality. The incidence of post-stroke pneumonia and/or its impact on case-fatality have likely improved over time due to earlier detection and treatment among stroke patients as well as the availability and increased uptake of pneumococcal vaccines particularly among the elderly.380 A recent analysis of stroke admissions identified from the Nationwide Inpatient Sample suggested no change in the percent of in-hospital cases of pneumonia for the relative short time period 1997–1998 to 2005–2006;381 however, longer term temporal trend data are not available. Similarly, data on changes in the impact of pneumonia on stroke case fatality over time are not available.
Sleep disordered breathing is common in the United States both in the general population and in stroke patients. Obstructive sleep apnea (OSA), one form of sleep disordered breathing, is increasingly prevalent in the US and has been identified as an independent risk factor for ischemic stroke and for the combined endpoint of stroke and death in prospective studies.382, 383 In the Sleep Heart Health Study, OSA measured by the apnea-hypopnea index (AHI) was associated with risk of incident ischemic stroke in men after multivariable adjustment for confounders (p=0.016 for linear trend associated with quartiles of AHI). Compared with men in the lowest quartile of AHI, men in the highest quartile had an adjusted hazard ratio of 2.86 (95% CI: 1.1–7.4). A similar dose-response pattern was not observed in women.382 OSA is also associated with post-stroke mortality.384–386 The prevalence of OSA among middle-aged adults has been estimated to be 4% for men and 2% for women (measured as an AHI>5 and sleepiness) or 24% in men and 9% in women based on AHI alone.387 The prevalence of OSA has likely increased over time due to the increasing prevalence of obesity;388 however, long-term trend data is not available. An analysis from the National Ambulatory Medical Care Survey reported a 12-fold increase in the diagnosis of sleep apnea in outpatients between 1990 and 1998.389 Countering the increase in prevalence in 1981, treatment for OSA with continuous positive air pressure (CPAP) became available.390, 391 Treatment with CPAP reduces cardiovascular events and thus could impact stroke risk.392 However, OSA is underdiagnosed393 and compliance with CPAP treatment, particularly among stroke patients, is suboptimal394, 395 making it difficult to assess the potential impact of OSA and its treatment on stroke mortality trends.
In summary, given the known link between cigarette smoking and stroke mortality from prospective cohort studies, the large decrease in the prevalence of active smoking in the US in the second half of the twentieth century likely contributed to the decline in stroke mortality during this time period although this hypothesis has not been formally tested. The data presented show that smoking related stroke deaths decreased from 2000 to 2009 due to the declining prevalence of smoking for this time period, with declines undoubtedly larger in previous decades when an even greater falloff in smoking prevalence was achieved suggesting a moderate impact of smoking on the overall decline in stroke mortality. However, a paradox in the relationship of smoking with the decline in stroke mortality is the period up to the mid-1960s during which smoking rates were dramatically increasing while stroke mortality was rapidly declining. The role of secondhand smoke in declining stroke mortality remains unclear due to the inconclusive evidence for a causal effect of secondhand smoke on stroke mortality. Other respiratory related conditions including pneumonia and obstructive sleep apnea may have impacted the decline in stroke mortality given their associations with stroke risk and/or post-stroke mortality but epidemiologic data is not sufficient to determine their impact.
Air Pollution and Environmental Factors on Decline in Stroke Mortality
As a result of the Clean Air Act and the implementation and periodic revision of clean air standards, air pollution levels in the United States have decreased considerably over time.396 Declines have been substantial for particulate matter (PM), a pollutant with known links to cardiovascular disease (see below); thus, this section will focus on the impact of PM on declining stroke mortality.
PM is a “complex mixture of extremely small particles and liquid droplets.”397 Particles are measured by their size in microns, and this size is related to their potential to cause health problems.397 Both PM10 (particles 10 microns in diameter) and PM2.5 (smaller particles 2.5 microns in diameter) are criteria pollutants meaning they are regulated by the Environmental Protection Agency (EPA) given their potential to cause harm to humans.398 There have been several revisions to the standards for PM in the last forty years based on mounting epidemiologic evidence linking exposure to PM to morbidity and mortality.396 The first standards for this pollutant were based on total suspended particles with a revision in 1987 to focus the standards on PM10. Standards for PM2.5 were added in 1997 with an update to reflect stricter allowable levels for this pollutant (for 24-hour period) in 2006. A national monitoring network for PM2.5 began in 1999 and was fully implemented in 2000.
In terms of temporal trends, in the United States PM10 emissions levels (amount of pollutant released into atmosphere) peaked in 1950, with a sharp decline beginning in 1970, when the EPA was established and clean air standards were initiated,399 and a subsequent leveling off of levels in the nineties.400 Concentrations of PM in the air are impacted by emission levels as well as weather; thus, the declining emissions have translated into declining PM concentrations. From 1990 through 2010, there was a 38% decrease in the national average of PM10 concentrations (based on annual 2nd maximum 24-hour average), and from 2000 through 2010 there was a 27% decrease in the national average of PM2.5 concentrations (based on seasonally weighted annual averages).401 Although monitoring data is not available, declines in PM concentrations were undoubtedly larger prior to 1990 given the large declines in emission levels.
The influence of air pollution on cardiovascular disease was summarized in a 2004 scientific statement published by the American Heart Association402 with an updated statement focused specifically on PM published in 2010 given the growing body of literature for this pollutant published after the 2004 report.403 As summarized in the 2010 report, there is evidence from epidemiologic studies of both acute effects and long-term effects of exposure to PM on stroke risk and mortality. Briefly, time-series studies conducted in the United States, where levels are lower than those seen in many other parts of the world, have demonstrated significant associations between acute exposure to PM10 and stroke hospital admissions.404–406 Similarly, results from a time-series study and a case-crossover study in the United States have demonstrated significant associations between acute exposure to PM2.5 and stroke risk407, 408 and stroke mortality.409 Importantly, the case-crossover approach controls for potential confounders of the PM-stroke association, which is a limitation of most time series studies; however, this case-crossover study was limited to one geographic area.408 As described in the AHA report, there is also supporting evidence of the PM-stroke association from outside the United States.403
With regard to long-term effects, there is less published information although this area of research is growing. Investigators from the Women’s Health Initiative (WHI) found an increased risk of both fatal (HR=1.83, 95% CI: 1.11–3.00) cerebrovascular disease and non-fatal (HR=1.28, 95% CI: 1.02–1.61) stroke with increasing exposure to PM2.5 after accounting for confounding factors410 but similar results were not found in the Cancer Prevention Study II (CPS II) or in the Health Professionals Follow-up Study.411, 412 In the later, associations were also not found between PM10 and stroke mortality.411 In the California Teacher’s Study, a large prospective study of women, PM10 (HR=1.06, 95% CI: 1.00–1.13) was associated with incident stroke. A borderline association of PM2.5 (HR=1.14; 95% CI: 0.99–1.32) with incident stroke was identified in the entire cohort; with a significant association noted among the subset of postmenopausal women.413 Associations with stroke mortality, however, were not found. Note these studies varied in their exposure ascertainment, study populations, and adjustment for confounding factors making comparisons across the studies challenging.
Although not specific to stroke mortality, Laden et al considered whether declines in PM over time have contributed to declines in cardiovascular mortality.414 In this follow-up study of the Harvard Six Cities Study, PM2.5 concentrations and cardiovascular mortality were ascertained for two time periods, 1974–1989 (period 1) and 1990–1998 (period 2). The study found that PM2.5 exposure was associated with cardiovascular mortality to a similar extent for the two time periods and that improved cardiovascular mortality over time was associated with decreased mean concentrations of PM2.5 between the two time periods after accounting for confounding factors. Specifically, controlling for PM2.5 in period 1, each 10 reduction in mean PM2.5 concentrations in period 2 was associated with a reduction in cardiovascular mortality risk (RR=0.73, 95% CI: 0.57–0.95). If PM is linked to stroke mortality, we can conjecture that decreasing concentrations levels have also contributed to declining stroke mortality rates overtime although this has not been formally evaluated.
In summary, although large decreases in PM concentrations in the United States have occurred in recent years, the role of PM air pollution in declining stroke mortality remains unclear due to inconclusive epidemiologic evidence of a causal effect of PM on stroke mortality. This association is made more complex by the period from the beginning of the 1900s to the 1970s, during which pollution levels were increasing while stroke mortality was declining. While studies conducted in the United States have demonstrated associations between short-term PM exposures and stroke risk and mortality, almost all have been ecologic in nature leaving open the potential for confounding by individual-level factors. Studies of long-term PM exposures conducted in the United States have not been consistent with respect to an association with stroke risk and/or mortality.
Contribution of Exercise on Decline in Stroke Mortality
Associations between higher levels of physical activity and reduced all-cause mortality415, 416 and cardiovascular disease mortality417–419 have been found in large prospective cohort studies and reported in meta-analyses. Fewer studies have examined the role of physical activity and stroke mortality. This section reviews the literature focused on the potential impact of physical activity and cardiorespiratory fitness on stroke incidence and mortality.
Hu et al420 analyzed data from the Nurses’ Health Study, which followed 72,488 female nurses for 8 years and collected physical activity levels 3 times during the study period. Physical activity, including moderate-intensity exercise (i.e., walking), was associated with a substantial reduction in the risk of incident total stroke and ischemic stroke in a dose-response relationship. Relative risks for experiencing a stroke in the lowest to highest metabolic equivalent tasks quintiles were 1. 00, 0.98, 0.82, 0.74, and 0.66 (p for trend = 0.005). As part of the Women’s Health Study, a cohort of 37,636 women was followed for 10 years,421 and healthy lifestyle self-reported data (abstinence from smoking, low body mass index, moderate alcohol consumption, regular exercise, and healthy diet) were collected. The overall healthy lifestyle index, derived from the self-reported data, was associated with a significantly reduced risk of incident total and ischemic stroke, but not hemorrhagic stroke. In the National Runners’ Health Study, Williams422 reported that the risk for having a stroke was substantially reduced for men and women who reported vigorous physical activity. Men and women who ran ≥ 2 km/d (exceeding the guideline physical activity level) had significantly lower risk than those who ran less (P=0.05), and those who ran ≥ 4 km/d had significantly lower risk than those who ran 2 to 3.9 km/d (P=0.02). Men and women who ran ≥ 8 km/d were at 60% lower risk than those who ran < 2 km/d (P < 0.01).
Several studies examined physical activity or fitness and stroke mortality. In the Nord-Trondelag Health Survey in Norway, Ellekjaer423 it was reported that physical activity was associated with reduced risk of death from stroke among women aged 50 and older (n=14,101) who were followed for 10 years. For all women, the adjusted relative risk of risk of stroke death was 0.77 (95% CI, 0.61–0.98) for medium physical activity and 0.52 (95% CI, 0.38–0.72) for high levels of physical activity. When data were stratified by age group, women 50 to 69 years had reduced risk for medium and high physical activity and women 70 to 79 years had a reduced risk of stroke death for high physical activity. Another cohort study followed 31,023 men and 42,242 women in Japan, aged 40 to 79 years, for 10 years after a physical activity survey was completed. The multivariate-adjusted hazard ratios for the highest versus the second lowest categories of walking or sports participation was 0.71 (95% CI, 0.54–0.94) for ischemic stroke death. When data were stratified by sex, the inverse relationship remained, but the results were not statistically significant.424
Lee and Blair368 used data from the Aerobics Center Longitudinal Study, a US-based prospective cohort study of 16,878 men who were followed an average of 10 years. Moderate and high levels of cardiorespiratory fitness were associated with lower risk of stroke mortality in this sample. High-fit men had a 68% (95% CI: 0.12, 0.82) lower risk of stroke mortality and moderate-fit men had 63% (95% CI: 0.17, 0.83) lower risk of stroke mortality when compared with low-fit men. Hooker’s425 cohort study included 46,405 men and 15,282 women who completed a maximal treadmill exercise test between 1970 and 2001. Cardiorespiratory fitness was grouped as quartiles of the sex-specific distribution of maximal metabolic equivalents achieved. Mortality follow-up based on the National Death Index was conducted through December 31, 2003, and nonfatal stroke, defined as physician-diagnosed stroke, was obtained from surveys through 2004. Significant inverse associations between cardiorespiratory fitness and age-adjusted fatal, nonfatal, and total stroke rates were found for women and men.
For stroke survivors who have functional limitations, reduced physical activity post stroke may lead to an increased mortality risk. For example, among a nationally representative sample of the US population with previous stroke who were followed for 6 to 12 years, exercising regularly was associated with reduced all-cause mortality (hazard ratio = 0.66, 95% CI, 0.44 to 0.99).365
The prospective cohort studies described above suggest that higher levels of physical activity could reduce stroke mortality. The question about whether physical activity is a factor that contributed to the observed decline in stroke mortality between 1970 and 2010 requires data showing physical activity among the US population increased during that time. According to the Centers for Disease Control and Prevention, the proportion of the US population that reported no leisure-time physical activity decreased from approximately 31% in 1988 to 28% in 1998 and 25% in 2008.74 More detailed data from the Behavioral Risk Factor Surveillance System surveys found the percent of individuals who engaged in recommended levels of activity increased slightly from 24% in 1990 to 25% in 1998. The percent of those reporting insufficient activity increased from 45% in 1990 to 46% in 1998, and those reporting no physical activity decreased from 31% in 1990 to 29% in 1998.426 The reduction in physical activity during the last 50 years has been tied to workplace technological changes leading to a decline of physically active occupations, changes in the home with the increased availability and use of labor-saving devices, and changes in transportation systems with widespread use automobiles.427
In summary, the available data suggest small increases in the amount of physical activity in the last 20 years, and thus the effect of physical activity on the decline in stroke mortality may be minimal.
Effect of Obesity and Body Mass Patterns on Decline in Stroke Mortality
Obesity prevalence rates are rising in the US. It is estimated that 36% of US adults are obese and 33% are overweight.428 Although increased body weight is an established risk factor for primary stroke,429–431 the relationship of body size and obesity, defined as a body mass index (BMI) >30 kg/m2, with stroke mortality is more complex. A large prospective study of 18,403 middle-aged London-based male government employees reported that, compared to normal weight subjects, overweight or obese men who were free of coronary heart disease (CHD) at study’s initiation had an increased risk of mortality, including stroke mortality (OR=1.64; 95% CI=1.17 to 2.28).432 The association between increased weight and mortality was largely mediated through risk factors, such as blood pressure and plasma levels of cholesterol and glucose.432 Several large studies in the general US population have consistently identified a link between obesity and increased cardiovascular disease (CVD) mortality,248, 433, 434 but few have investigated stroke mortality specifically. In a collaborative meta-analysis of 57 prospective studies with 894,576 participants, mostly in western Europe and North America (61% male, mean recruitment age 46 years), there was no evidence of an association between BMI and stroke mortality in the lower BMI range (15–25 kg/m2). However, in the upper BMI range (25–50 kg/m2), each 5 kg/m2 higher BMI was associated with about 40% higher stroke mortality, irrespective of stroke subtype. This association was mostly accounted for by the effects of BMI on blood pressure and was much stronger in middle than in old age.435 Similarly, risk is increased with the association of body mass and increased metabolic risk factors indicating the benefit of lifestyle modification.136
Most studies examining the association of BMI with stroke mortality have been conducted in Asian populations, where BMI tends to be lower and stroke incidence higher than in the US. In a prospective study of 212,000 Chinese men, aged 40 to 79 years, with no history of CVD, there was a significant excess risk of stroke deaths among individuals with BMI>25 kg/m2. This association was largely accounted for by a higher blood pressure in these subjects and did not differ by major stroke subtypes.436 BMI was linearly related to increased stroke mortality in a prospective study of 154,736 Chinese men and women, aged 40 years and older. In this study, the relationship between BMI and stroke mortality was stronger among participants aged ≤60 years.437 In a prospective cohort of 3321 Korean post-menopausal women, obesity was associated with an increased risk of total stroke mortality and hemorrhagic stroke mortality, and with an increased risk of ischemic stroke mortality among ever-smokers but not never-smokers.438 In a prospective cohort of 43,913 Japanese adults, aged 40 to 79 years with no history of cancer, stroke and ischemic heart disease, both obesity, defined as self-reported BMI>27.5 kg/m2, and underweight, defined as self-reported BMI<18.5 kg/m2, were associated with all stroke mortality. A trend for a U-shaped association was observed for both hemorrhagic and ischemic stroke mortality, but only associations between increased hemorrhagic mortality and lower BMI and between increased ischemic stroke mortality and higher BMI were statistically significant.439 An association of total and hemorrhagic stroke mortality with underweight has also been reported in a study of 104,928 Japanese adults, aged 40 to 79, free of stroke, CHD, and cancer at enrollment.440 Fatal hemorrhagic stroke was also more frequent in lean men than in overweight and obese men in the Physician’s Health Study of 21,414 US male physicians.429
The association of body fat distribution with stroke mortality has not been widely addressed. In a study of middle-aged male civil servants free of CVD in Israel, subscapular skinfold thickness, a measure of subcutaneous trunk fat, and subscapular to triceps skinfold thickness, a measure of trunk versus peripheral body fat distribution, were associated with increased long-term stroke mortality. The association between central adiposity and stroke mortality was independent of BMI but was, at least partially, mediated by blood pressure.441
The complex relationship between body weight and stroke mortality is also manifest in patients with established stroke. Mounting evidence suggests that overweight and obese patients with established CVD have a more favorable prognosis than leaner patients.442 In a Danish stroke registry of 21,884 hospitalized stroke patients with BMI data (mean age 72.3 years), total post-stroke mortality was inversely related to BMI even after accounting for CVD risk factors.443 Overweight and obese patients had a 27% and 16% lower mortality rate, respectively, than normal-weight patients, while underweight patients had the highest mortality rate. This association was independent of age and smoking status and was similar for hemorrhagic and ischemic stroke patients. A study of the association between BMI category and all-cause mortality in 644 stroke survivors from the Third National Health and Nutrition Examination Surveys (NHANES III), a nationally representative survey of non-institutionalized civilian US population aged 25 or older, showed that after multivariable analysis, overall risk for all-cause mortality increased per kg/m2 of higher BMI (P=0.030), but an interaction between age and BMI (P=0.009) revealed that the association of higher BMI with mortality risk was strongest in younger individuals and declined linearly with increasing age, such that in the elderly, overweightness and obesity had a protective effect. The results were similar for the cardiovascular mortality outcome. Obese/overweight individuals under age 70 were more likely to die from cardiovascular or all causes than their normal weight counterparts. However, elderly stroke patients who were overweight or obese had a decreased rate of all-cause and cardiovascular mortality compared to normal-weight individuals of the same age.444 Similar findings of a protective effect of overweight/obesity on all-cause mortality after stroke have been reported in 2785 first-ever acute stroke patients from Greece.445 The risk of 10-year total mortality was lower by 18% and 29% in overweight and obese patients, respectively, compared to those with normal BMI. Overweight and obese patients also had better early (1 week) survival rates.
The effect of weight change on cardiovascular mortality in obese/overweight individuals with cardiovascular disease and/or diabetes mellitus has been recently reported in the Sibutramine Cardiovascular OUTcomes (SCOUT) trial, which assessed the effects of weight loss by lifestyle intervention and pharmacotherapy on cardiovascular morbidity and mortality, including stroke.446 Modest weight loss over short-term (6 weeks) and longer-term (up to 12 months) periods was associated with a lower cardiovascular mortality. In those with severe CVD, there was a U-shaped association between cardiovascular mortality and weight change.
There are data to suggest that adhering to a combination of healthy lifestyle practices in consideration of body size can lower both stroke incidence421, 447 and mortality after stroke.365 In a nationally representative sample of the US population (n=15 299) with previous stroke (n=649) followed from survey participation (1988–1994) through to mortality assessment (2000), the relationship between five factors (eating ≥5 servings of fruits/vegetables per day, exercising >12 times/month, having a body mass index of 18.5–29.9 mg/kg2, moderate alcohol use [1 drink/day for women and 2 drinks/day for men] and not smoking) and all-cause and cardiovascular mortality was assessed.365 Combinations of healthy lifestyle factors were associated with lower all-cause and cardiovascular mortality in a dose dependent fashion. All-cause mortality decreased with higher numbers of healthy behaviors (1–3 factors vs none: HR 0.12, CI 0.03 to 0.47; 4–5 factors vs none: HR 0.04, CI 0.01 to 0.20; 4–5 factors vs 1–3 factors: HR 0.38, CI 0.22 to 0.66; trend p=0.04). Similar effects were observed for cardiovascular mortality (4–5 factors vs none: HR 0.08, CI 0.01 to 0.66; 1–3 factors vs none: HR 0.15, CI 0.02 to 1.15; 4–5 factors vs 1–3 factors: HR 0.53, CI 0.28 to 0.98; trend p=0.18).
In summary, the impact of obesity and body mass patterns on stroke mortality likely differ in the contexts of health or pre-existing stroke or CVD, stroke subtypes, age, and other risk factors. The pathophysiological mechanisms underlying these complex relationships are incompletely understood and highlight the need for well-designed prospective and interventional studies to clarify the role of body size and composition, nutritional status, and their change overtime on stroke mortality and outcome. While obesity is associated with increasing prevalence of hypertension and diabetes, the increase in prevalence of obesity does not appear to influence the decline in stroke mortality rates. This most likely is due to treatment effects of hypertension and diabetes.
Impact of Research and Program Funding on Decline in Stroke Mortality: A Case Study
The 1965 Report of the Commission on Heart Disease, Cancer, and Stroke calls for a nationwide increase in screening and treatment of hypertension. Though actuarial studies showed a clear relationship between rising blood pressure and increase risk of death, and epidemiology studies showed a high prevalence of hypertension, no public health initiative was created. At that time there was no clear evidence of the benefit of lowering blood pressure. The evidence became available with the publication of the 1967 and 1969 Veterans Administration Cooperative Study on the Treatment of Hypertension.99, 100, 448 Armed with this information, Secretary of then the Department of Health, Education and Welfare, Elliot Richardson, created the National High Blood Education Program (NHBPEP).119 The NHBPEP was designed to raise public awareness and stimulate screening and treatment throughout the nation. The NHBPEP included the 50 state health departments, 2000 community groups, seven federal agencies, and a Coordinating Committee comprised of 45 national voluntary health organizations and professional societies.119 In addition, throughout the 1970’s, 1980’s, and beyond, several national profession associations such as American Society of Hypertension, the International Society of Hypertension in Blacks, the American Heart Association Council of High Blood Pressure Research, American Stroke Association, Consortium of Southeast High Blood Pressure Control, National Hypertension Association, the National Stroke Association, the American Heart Association with its many programs such as Get with the Guidelines, Million Hearts™, Power to End Stroke, assorted stroke prevention guidelines, as well as other professional medical and nursing societies began to increase their activities on hypertension research service and education. These NHBPEP partners used mass media campaigns, patient education programs, developed clinical guidelines, produced national and regional conferences on the detection and management of hypertension, and stimulated the development of hypertension detection and control programs at the local level. Hypertension quickly became fashionable and in the public eye. Within one decade, the percent of people aware of their hypertension has substantially increased, treatment rates doubled and control rates increased to over half within the last four decades.119–121 Concomitantly, mean arterial blood pressures have fallen precipitously. (Figure 5) In addition to increasing the public’s awareness about hypertension, the NHBPEP also appears to have interested scientists and clinical investigators to seek more information about the condition. The number of citations from the National Library of Medicine’s Pub Med data base accessed by using the search terms hypertension and clinical trials has increased from less than 50 in 1972, to 1200 per year in 2002.119
The decline of stroke mortality represents a success indicator and metric for programs and strategies specifically designed and implemented to reduce risks. Stroke mortality has been recognized for decades as an outcome with significant racial disparities with particular high risk among African Americans. The decline in stroke mortality for all race groups has reduced the magnitude of the race gap in stroke mortality risks.449 Likewise the variation in stroke mortality by geographic area with particular emphasis on the southeastern region of the US termed the “Stroke Belt”.202, 450–452 While the racial and geographic stroke risk disparities are evident, the factors associated with the patterns appear to originate in early life.453–455 These disparities and excess disease risks have been the stimulus for research and intervention programs focused on the identification of factors associated with these disparities. Included in these decades of funded efforts is the REGARDS study focused on the identification of factors associated with racial and geographic differences in stroke risks.456–458 This large cohort has confirmed many of the parameters for the differences in stroke risks including elevated blood pressure and diabetes, as well as identifying additional factors. These epidemiological studies have contributed to the effective intervention programs for reducing the disparity gaps in stroke risks.459–461
In summary, driven by solid research findings on stroke risk and prevention, funding of studies and intervention program has made a significant contribution to the reduction in stroke mortality as well as the narrowing of the racial and geographic disparities in risks. For the nation as a whole, control rates for hypertension have improved six fold during the last four decades driven by an increase in public awareness and treatment. The campaign has stimulated scientists to identify more refined contributing factors of stroke as well as high impact intervention programs.
Other Factors
The factors and parameters associated with the decline in stroke mortality previously described represent the traditional influences with the highest evidence and studies. There are other conditions that could also contribute to the decline in stroke and serve as mediating factors, which reduce stroke risk factors.
Sickle cell disease has long been recognized with increased risks of stroke among young African Americans.462, 463 Treatment guidelines and protocols have been implemented over the past 2 decades including use of transcranial Doppler and transfusion therapy have significantly reduced the stroke risks for this population.324 Nonetheless, these interventions among sickle cell patients affect a relatively low number of persons and probably have minimal impact on the overall historical and large stroke declines.
Salt intake is a cardiovascular risk factor with reduced sodium intake associated with reduced stroke rates,464–466 which is independent of other risk factors such as hypertension. The potential impact of sodium reduction is reported on the individual and population levels.467–471 Good measures of population wide consumption of sodium over long time periods are needed to determine its contribution to long term stroke mortality trend data. Reducing sodium consumption, however, can reduce BP levels and is adjunctive therapy for most and definitive therapy for some hypertensive patients.123
Adherence to medical regimens has served as a mediating factor in the decline in stroke mortality rates. The impact is seen through the reduction in stroke risk factors. Within the last few decades hypertension control rates109, 119, 123, 472 and smoking cessation rates7, 367 have substantially improved suggesting that patients are adhering more to their medical regimens.
Diet is also a mediating factor in the decline of stroke mortality. The Dietary Approaches to Stop Hypertension eating plan, which uses a diet that is low in sodium and is rich in potassium and calcium, has been shown to reduce blood pressure levels.473, 474 Higher dietary potassium and magnesium intake are associated with lower rates of stroke particularly among hypertensive women.475 The impact on steady long-term stroke mortality decline requires more data.
In summary, these parameters are reasonable considerations as factors influencing the stroke mortality decline and represent very important components of stroke prevention. The factors are strongly associated with other risk variables for stroke. More research is needed to quantify their direct effect and influence on stroke mortality.
Conclusion and Discussion
Stroke has now moved from the 3rd to the 4th leading cause of death in the United States. Within the last 5 decades, the decline in stroke mortality represents a major success for public health and clinical medicine. The decline is seen among both genders, all races and age groups. In addition to reduced overall risks, the reduced mortality for the under-65 year olds contributes significantly to the improved reduced years of potential life lost. The decline is considered valid and real, and not an artifact of competing conditions as cause of death or recurrent stroke rates or a marked increase in death rates from respiratory disease. While the precise attribution of specific factors is not possible, the Panel was able to assess multiple factors and interventions associated with the decline. Most likely, the combination of the different parameters and programs contribute to the significant decline. However, the available evidence indicates some factors have a greater impact. Clinical trial evidence demonstrates that lowering BP reduces strokes and stroke deaths. Observational and epidemiology studies demonstrate levels of BP are associated with stroke mortality risk, i.e. .the higher the BP the greater the risk for stroke. National probability survey data has shown a significant improvement in BP control and reduction in population systolic pressures. These factors are associated with a very significant and accelerated decline in stroke deaths. Treatment and control of diabetes and hyperlipidemia, have contributed to the stroke mortality declines, however the onset of these interventions is more recent and thus their impact is less clear. Systems of care, tPA use, smoking cessation, air pollution, exercise, atrial fibrillation and other factors may play a role, but additional studies are needed to determine their impact on population stroke deaths.
The decline in stroke mortality is one of the major public health successes of the past 50 years. With the implementation of evidence-based primary, secondary and tertiary stroke prevention strategies, these trends should continue.
Acknowledgments
We appreciate the contribution of Figure 5 provided by Cathleen Gillespie, MS, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Connie Land and Anne Leonard, American Heart Association, and several thoughtful anonymous reviewers.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Ten great public health achievements–United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48:241–243. [PubMed] [Google Scholar]
- 2.Ten great public health achievements–United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Health, United States, 2010: With special features on death and dying. Hyattsville, MD: 2011. Table 31. Available at http://www.cdc.gov/nchs/hus.htm. Accessed June 19, 2012. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC WONDER Online Database, compiled from Compressed Mortality File 1999–2009. 2012. Compressed Mortality File 1999–2009. Series 20 No. 2O. [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2012 update: a report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 7.Decline in deaths from heart disease and stroke–United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48:649–656. [PubMed] [Google Scholar]
- 8.Towfighi A, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke. 2010;41:499–503. doi: 10.1161/STROKEAHA.109.571828. [DOI] [PubMed] [Google Scholar]
- 9.Gillum RF, Kwagyan J, Obisesan TO. Ethnic and geographic variation in stroke mortality trends. Stroke. 2011;42:3294–3296. doi: 10.1161/STROKEAHA.111.625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ergin A, Muntner P, Sherwin R, He J. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117:219–227. doi: 10.1016/j.amjmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Wolf PA, D’Agostino RB, O’Neal MA, Sytkowski P, Kase CS, Belanger AJ, Kannel WB. Secular trends in stroke incidence and mortality. The Framingham Study. Stroke. 1992;23:1551–1555. doi: 10.1161/01.str.23.11.1551. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Alderman MH, Keenan NL, Croft JB. Declining US stroke hospitalization since 1997: National Hospital Discharge Survey, 1988–2004. Neuroepidemiology. 2007;29:243–249. doi: 10.1159/000112857. [DOI] [PubMed] [Google Scholar]
- 13.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre C, Curioni CC, Braga da Cunha C, Veras R. Progressive decline in stroke mortality in Brazil from 1980 to 1982, 1990 to 1992, and 2000 to 2002. Stroke. 2006;37:2784–2789. doi: 10.1161/01.STR.0000244768.46566.73. [DOI] [PubMed] [Google Scholar]
- 15.Ashton C, Bajekal M, Raine R. Quantifying the contribution of leading causes of death to mortality decline among older people in England, 1991–2005. Health Stat Q. 2010:100–127. doi: 10.1057/hsq.2010.6. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj A, Schernhammer ES, Haidinger G, Waldhor T. Trends in mortality from stroke in Austria, 1980–2008. Wien Klin Wochenschr. 2010;122:346–353. doi: 10.1007/s00508-010-1394-1. [DOI] [PubMed] [Google Scholar]
- 17.Bejot Y, Aouba A, de Peretti C, Grimaud O, Aboa-Eboule C, Chin F, Woimant F, Jougla E, Giroud M. Time trends in hospital-referred stroke and transient ischemic attack: results of a 7-year nationwide survey in France. Cerebrovasc Dis. 2010;30:346–354. doi: 10.1159/000319569. [DOI] [PubMed] [Google Scholar]
- 18.Cifkova R, Skodova Z, Bruthans J, Holub J, Adamkova V, Jozifova M, Galovcova M, Wohlfahrt P, Krajcoviechova A, Petrzilkova Z, Lanska V. Longitudinal trends in cardiovascular mortality and blood pressure levels, prevalence, awareness, treatment, and control of hypertension in the Czech population from 1985 to 2007/2008. J Hypertens. 2010;28:2196–2203. doi: 10.1097/HJH.0b013e32833d4451. [DOI] [PubMed] [Google Scholar]
- 19.Curioni C, Cunha CB, Veras RP, Andre C. The decline in mortality from circulatory diseases in Brazil. Rev Panam Salud Publica. 2009;25:9–15. doi: 10.1590/s1020-49892009000100002. [DOI] [PubMed] [Google Scholar]
- 20.Islam MS, Anderson CS, Hankey GJ, Hardie K, Carter K, Broadhurst R, Jamrozik K. Trends in incidence and outcome of stroke in Perth, Western Australia during 1989 to 2001: the Perth Community Stroke Study. Stroke. 2008;39:776–782. doi: 10.1161/STROKEAHA.107.493643. [DOI] [PubMed] [Google Scholar]
- 21.Kazlauskas H, Raskauskiene N, Radziuviene R, Janusonis V. Stroke mortality trends in the population of Klaipeda from 1994 to 2008. Medicina (Kaunas) 2011;47:512–519. [PubMed] [Google Scholar]
- 22.Kita Y, Turin TC, Ichikawa M, Sugihara H, Morita Y, Tomioka N, Rumana N, Okayama A, Nakamura Y, Abbott RD, Ueshima H. Trend of stroke incidence in a Japanese population: Takashima stroke registry, 1990–2001. Int J Stroke. 2009;4:241–249. doi: 10.1111/j.1747-4949.2009.00293.x. [DOI] [PubMed] [Google Scholar]
- 23.Koton S, Tanne D, Bornstein NM. Burden of stroke in Israel. Int J Stroke. 2008;3:207–209. doi: 10.1111/j.1747-4949.2008.00203.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunst AE, Amiri M, Janssen F. The decline in stroke mortality: exploration of future trends in 7 Western European countries. Stroke. 2011;42:2126–2130. doi: 10.1161/STROKEAHA.110.599712. [DOI] [PubMed] [Google Scholar]
- 25.Lewsey JD, Jhund PS, Gillies M, Chalmers JW, Redpath A, Kelso L, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, MacIntyre K. Age- and sex-specific trends in fatal incidence and hospitalized incidence of stroke in Scotland, 1986 to 2005. Circ Cardiovasc Qual Outcomes. 2009;2:475–483. doi: 10.1161/CIRCOUTCOMES.108.825968. [DOI] [PubMed] [Google Scholar]
- 26.Tu JV, Nardi L, Fang J, Liu J, Khalid L, Johansen H. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180:E118–125. doi: 10.1503/cmaj.081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Compressed Mortality File (CMF) on CDC WONDER Online Database. Available at http://wonder.cdc.gov/mortSQL.html. Accessed June 7, 2012.
- 28.Moriyama IM, Loy RM, Robb-Smith AHT. History of the statistical classification of diseases and causes of death. In: Rosenberg HM, Hoyert DL, editors. Hyattsville, MD: National Center for Health Statistics; 2011. Available at: http://www.cdc.gov/nchs/data/misc/classification_diseases2011.pdf. Accessed June 6, 2012. [Google Scholar]
- 29.Hetzel AM. History and organization of the vital statistics system. National Center for Health Statistics; 1977. Available at: http://www.cdc.gov/nchs/data/misc/usvss.pdf. Accessed June 6, 2012. [Google Scholar]
- 30.Klebba AJ, Scott JH. Monthly Vital Statistics Report. 11 Supplement. Vol. 28. Hyattsville, Maryland: National Center for Health Statistics; Feb 29, 1980. Estimates of Selected Comparability Ratios Based on Dual Coding of 1976 Death Certificates by the Eighth and Ninth Revisions of the International Classification of Diseases. 1980. Available at: http://www.cdc.gov/nchs/data/mvsr/supp/mv28_11s.pdf. Accessed June 6, 2012. [Google Scholar]
- 31.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. National vital statistics reports. 2. Vol. 49. Hyattsville, Maryland: National Center for Health Statistics; 2001. Comparability of cause of death between ICD–9 and ICD–10: Preliminary estimates. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_02.pdf. Accessed June 6, 2012. [PubMed] [Google Scholar]
- 32.Saver JL, Kidwell C. Neuroimaging in TIAs. Neurology. 2004;62:S22–25. doi: 10.1212/wnl.62.8_suppl_6.s22. [DOI] [PubMed] [Google Scholar]
- 33.Halanych JH, Shuaib F, Parmar G, Tanikella R, Howard VJ, Roth DL, Prineas RJ, Safford MM. Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol. 2011;173:1319–1326. doi: 10.1093/aje/kwr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J Am Geriatr Soc. 2009;57:133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DL, Al-Senani F, Lisabeth LD, Farnie MA, Colletti LA, Langa KM, Fendrick AM, Garcia NM, Smith MA, Morgenstern LB. Defining cause of death in stroke patients: The Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2007;165:591–596. doi: 10.1093/aje/kwk042. [DOI] [PubMed] [Google Scholar]
- 36.Burke JF, Lisabeth LD, Brown DL, Reeves MJ, Morgenstern LB. Determining stroke’s rank as a cause of death using multicause mortality data. Stroke. 2012;43:2207–2211. doi: 10.1161/STROKEAHA.112.656967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenfant C, Friedman L, Thom T. Fifty years of death certificates: the Framingham Heart Study. Ann Intern Med. 1998;129:1066–1067. doi: 10.7326/0003-4819-129-12-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 38.Mahonen M, Salomaa V, Keskimaki I, Moltchanov V, Torppa J, Molarius A, Tuomilehto J, Sarti C. The feasibility of combining data from routine Hospital Discharge and Causes-of-Death Registers for epidemiological studies on stroke. Eur J Epidemiol. 2000;16:815–817. doi: 10.1023/a:1007697720131. [DOI] [PubMed] [Google Scholar]
- 39.Anderson RN, Rosenberg HM. National Vital Statistics Reports. 3. Vol. 47. Hyattsville, Maryland: National Center for Health Statistics; 1998. Age Standardization of Death Rates: Implementation of the Year 2000 Standard. [PubMed] [Google Scholar]
- 40.Kochanek KD, Xu JQ, Murphy SL, et al. National vital statistics reports. 4. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: Preliminary data for 2009. [PubMed] [Google Scholar]
- 41.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–349. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 42.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 44.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44:626–634. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 45.El-Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K, Cushman M, Kronmal R. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke. 2006;37:1975–1979. doi: 10.1161/01.STR.0000231453.98473.67. [DOI] [PubMed] [Google Scholar]
- 46.Lisabeth LD, Smith MA, Brown DL, Moye LA, Risser JM, Morgenstern LB. Ethnic differences in stroke recurrence. Ann Neurol. 2006;60:469–475. doi: 10.1002/ana.20943. [DOI] [PubMed] [Google Scholar]
- 47.Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, Lichtman JH. Geographic variation in one-year recurrent ischemic stroke rates for elderly medicare beneficiaries in the USA. Neuroepidemiology. 2010;34:123–129. doi: 10.1159/000274804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16(Suppl 1):14–19. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 49.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 50.Peltonen M, Stegmayr B, Asplund K. Time trends in long-term survival after stroke: the Northern Sweden Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) study, 1985–1994. Stroke. 1998;29:1358–1365. doi: 10.1161/01.str.29.7.1358. [DOI] [PubMed] [Google Scholar]
- 51.Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry. 1990;53:824–829. doi: 10.1136/jnnp.53.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, Stewart-Wynne EG. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. 2000;31:2080–2086. doi: 10.1161/01.str.31.9.2080. [DOI] [PubMed] [Google Scholar]
- 53.Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 54.Prencipe M, Culasso F, Rasura M, Anzini A, Beccia M, Cao M, Giubilei F, Fieschi C. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke. 1998;29:126–132. doi: 10.1161/01.str.29.1.126. [DOI] [PubMed] [Google Scholar]
- 55.Vernino S, Brown RD, Jr, Sejvar JJ, Sicks JD, Petty GW, O’Fallon WM. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. 2003;34:1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
- 56.Brown DL, Lisabeth LD, Roychoudhury C, Ye Y, Morgenstern LB. Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack. Stroke. 2005;36:1285–1287. doi: 10.1161/01.STR.0000165926.74213.e3. [DOI] [PubMed] [Google Scholar]
- 57.Lackland DT, Elkind MS, D’Agostino R, Sr, Dhamoon MS, Goff DC, Jr, Higashida RT, McClure LA, Mitchell PH, Sacco RL, Sila CA, Smith SC, Jr, Tanne D, Tirschwell DL, Touze E, Wechsler LR. Inclusion of Stroke in Cardiovascular Risk Prediction Instruments: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2012;43:1998–2027. doi: 10.1161/STR.0b013e31825bcdac. [DOI] [PubMed] [Google Scholar]
- 58.Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation. 2011;123:2111–2119. doi: 10.1161/CIRCULATIONAHA.109.934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42:1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 60.Lewsey J, Jhund PS, Gillies M, Chalmers JW, Redpath A, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, MacIntyre K. Temporal trends in hospitalisation for stroke recurrence following incident hospitalisation for stroke in Scotland. BMC Med. 2010;8:23. doi: 10.1186/1741-7015-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardie K, Jamrozik K, Hankey GJ, Broadhurst RJ, Anderson C. Trends in five-year survival and risk of recurrent stroke after first-ever stroke in the Perth Community Stroke Study. Cerebrovasc Dis. 2005;19:179–185. doi: 10.1159/000083253. [DOI] [PubMed] [Google Scholar]
- 62.Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, Lichtman JH. Trends in one-year recurrent ischemic stroke among the elderly in the USA: 1994–2002. Cerebrovasc Dis. 2010;30:525–532. doi: 10.1159/000319028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13:290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 64.Feng W, Hendry RM, Adams RJ. Risk of recurrent stroke, myocardial infarction, or death in hospitalized stroke patients. Neurology. 2010;74:588–593. doi: 10.1212/WNL.0b013e3181cff776. [DOI] [PubMed] [Google Scholar]
- 65.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year survival after first-ever stroke in the perth community stroke study. Stroke. 2003;34:1842–1846. doi: 10.1161/01.STR.0000082382.42061.EE. [DOI] [PubMed] [Google Scholar]
- 66.Hier DB, Foulkes MA, Swiontoniowski M, Sacco RL, Gorelick PB, Mohr JP, Price TR, Wolf PA. Stroke recurrence within 2 years after ischemic infarction. Stroke. 1991;22:155–161. doi: 10.1161/01.str.22.2.155. [DOI] [PubMed] [Google Scholar]
- 67.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015–2020. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- 68.Sheinart KF, Tuhrim S, Horowitz DR, Weinberger J, Goldman M, Godbold JH. Stroke recurrence is more frequent in Blacks and Hispanics. Neuroepidemiology. 1998;17:188–198. doi: 10.1159/000026172. [DOI] [PubMed] [Google Scholar]
- 69.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 70.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 71.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 73.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 74.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 75.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881–1885. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 76.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 77.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL, Jr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benesch CG, Chimowitz MI. Best treatment for intracranial arterial stenosis? 50 years of uncertainty. The WASID Investigators. Neurology. 2000;55:465–466. doi: 10.1212/wnl.55.4.465. [DOI] [PubMed] [Google Scholar]
- 79.Howlett-Smith HA, Cruz-Flores S, Belden JR, Sauerbeck LR, Levine RL, Stern BJ, Chimowitz MI, the WASID Study Group Management of intracranial arterial stenosis in a heterogeneous population. Neurology. 1998;50:A295–A296. [Google Scholar]
- 80.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke. 2010;41:e11–17. doi: 10.1161/STROKEAHA.109.561837. [DOI] [PubMed] [Google Scholar]
- 82.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 83.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 84.Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- 85.Lee SC, Park SJ, Ki HK, Gwon HC, Chung CS, Byun HS, Shin KJ, Shin MH, Lee WR. Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension. 2000;36:73–77. doi: 10.1161/01.hyp.36.1.73-a. [DOI] [PubMed] [Google Scholar]
- 86.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, Anton-Culver H, O’Leary DH. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 87.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, Yoshita M, D’Agostino RB, Sr, DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 89.Appelros P, Nydevik I, Seiger A, Terent A. Predictors of severe stroke: influence of preexisting dementia and cardiac disorders. Stroke. 2002;33:2357–2362. doi: 10.1161/01.str.0000030318.99727.fa. [DOI] [PubMed] [Google Scholar]
- 90.Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34:122–126. doi: 10.1161/01.str.0000047852.05842.3c. [DOI] [PubMed] [Google Scholar]
- 91.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 92.Kleindorfer D. The bad news: stroke incidence is stable. Lancet Neurol. 2007;6:470–471. doi: 10.1016/S1474-4422(07)70111-4. [DOI] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed Mortality File 1979–1998. CDC WONDER On-line Database, compiled from Compressed Mortality File CMF 1968–1988, Series 20, No. 2A, 2000 and CMF 1989–1998, Series 20, No. 2E, 2003. Available at http://wonder.cdc.gov/cmf-icd9.html. Accessed on May 16, 2012.
- 94.Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999–2009 on CDC WONDER Online Database, released 2012. Data for year 2009 are compiled from the Multiple Cause of Death File 2009, Series 20 No. 2O, 2012, Data for year 2008 are compiled from the Multiple Cause of Death File 2008, Series 20 No. 2N, 2011, data for year 2007 are compiled from Multiple Cause of Death File 2007, Series 20 No. 2M, 2010, data for years 2005–2006, data are compiled from Multiple Cause of Death File 2005–2006, Series 20, No. 2L, 2009, and data for years 1999–2004, are compiled from the Multiple Cause of Death File 1999–2004, Series 20, No. 2J, 2007. Available at http://wonder.cdc.gov/ucd-icd10.html. Accessed on May 16, 2012.
- 95.Kochanek KD, Xu JQ, Murphy SL, et al. National vital statistics reports. 4. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: Preliminary data for 2009. [PubMed] [Google Scholar]
- 96.Miniño AM, Murphy SL, Xu JQ, Kochanek KD. National vital statistics reports. 10. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2011. Deaths: Final data for 2008. [PubMed] [Google Scholar]
- 97.Society of Actuaries: Build and Blood Pressure Study. Vol. 1. Chicago, IL: Society of Actuaries; 1959. [Google Scholar]
- 98.Freis ED. The role of hypertension. American Journal of Hypertension. 1960;50:11–13. doi: 10.2105/ajph.50.3_pt_2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 100.Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 101.Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 102.Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1982;247:633–638. [PubMed] [Google Scholar]
- 103.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 104.Flack JM, Neaton J, Grimm R, Jr, Shih J, Cutler J, Ensrud K, MacMahon S. Blood pressure and mortality among men with prior myocardial infarction. Multiple Risk Factor Intervention Trial Research Group. Circulation. 1995;92:2437–2445. doi: 10.1161/01.cir.92.9.2437. [DOI] [PubMed] [Google Scholar]
- 105.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002:1–32. [PubMed] [Google Scholar]
- 106.National Heart, Lung, and Blood Institute. Morbidity & mortality: 2012 chartbook on cardiovascular, lung and blood diseases. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 2012. [Google Scholar]
- 107.Bonita R, Stewart A, Beaglehole R. International trends in stroke mortality: 1970–1985. Stroke. 1990;21:989–992. doi: 10.1161/01.str.21.7.989. [DOI] [PubMed] [Google Scholar]
- 108.Kuller L, Seltser R, Paffenbarger RS, Jr, Krueger DE. Trends in cerebrovascular disease mortality based on multiple cause tabulation of death certificates 1930–1960. A comparison of trends in Memphis and Baltimore. Am J Epidemiol. 1968;88:307–317. doi: 10.1093/oxfordjournals.aje.a120890. [DOI] [PubMed] [Google Scholar]
- 109.Moser M. A decade of progress in the management of hypertension. Hypertension. 1983;5:808–813. doi: 10.1161/01.hyp.5.6.808. [DOI] [PubMed] [Google Scholar]
- 110.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 111.Kuller LH. Epidemiology and prevention of stroke, now and in the future. Epidemiol Rev. 2000;22:14–17. doi: 10.1093/oxfordjournals.epirev.a018011. [DOI] [PubMed] [Google Scholar]
- 112.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 113.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 114.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 115.Vital signs: prevalence, treatment, and control of hypertension–United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–108. [PubMed] [Google Scholar]
- 116.World Health Organization. The World Health Report 2002: Reducing risks, promoting healthy life. Geneva, Switzerland: Available at: http://www.who.int/whr/2002/. Accessed July 23, 2012. [Google Scholar]
- 117.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 118.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 119.Roccella EJ. The National High Blood Pressure Education Program. In: Oparil Susan, Weber Michael., editors. Hypertension: A Companion to Brenner and Rector’s The Kidney. 2nd. Elsevier Saunders; Philadelphia, Pa: [Google Scholar]
- 120.Wright JD, Hughes JP, Ostchega Y. National health statistics reports. 35. Hyattsville, MD: National Center for Health Statistics; 2011. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. [PubMed] [Google Scholar]
- 121.Yoon SS, Ostchega Y, Louis T. NCHS Data Brief. 48. Hyattsville, MD: National Center for Health Statistics; 2010. Recent trends in the prevalence of high blood pressure and its treatment and control, 1999–2008. [PubMed] [Google Scholar]
- 122.Goff DC, Howard G, Russell GB, Labarthe DR. Birth cohort evidence of population influences on blood pressure in the United States, 1887–1994. Ann Epidemiol. 2001;11:271–279. doi: 10.1016/s1047-2797(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 123.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 124.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 125.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 126.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 127.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 128.Lip GY, Beevers M, Beevers DG. Complications and survival of 315 patients with malignant-phase hypertension. J Hypertens. 1995;13:915–924. doi: 10.1097/00004872-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 129.Phillips SJ, Whisnant JP. Hypertension and the brain. The National High Blood Pressure Education Program. Arch Intern Med. 1992;152:938–945. [PubMed] [Google Scholar]
- 130.Saunders E. Hypertension in African-Americans. Circulation. 1991;83:1465–1467. doi: 10.1161/01.cir.83.4.1465. [DOI] [PubMed] [Google Scholar]
- 131.Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N Engl J Med. 1992;327:776–781. doi: 10.1056/NEJM199209103271107. [DOI] [PubMed] [Google Scholar]
- 132.Zampaglione B, Pascale C, Marchisio M, Cavallo-Perin P. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144–147. doi: 10.1161/01.hyp.27.1.144. [DOI] [PubMed] [Google Scholar]
- 133.Shayne PH, Pitts SR. Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41:513–529. doi: 10.1067/mem.2003.114. [DOI] [PubMed] [Google Scholar]
- 134.Lackland DT, Keil JE, Gazes PC, Hames CG, Tyroler HA. Outcomes of black and white hypertensive individuals after 30 years of follow-up. Clin Exp Hypertens. 1995;17:1091–1105. doi: 10.3109/10641969509033654. [DOI] [PubMed] [Google Scholar]
- 135.Gazes PC, Lackland DT, Mountford WK, Gilbert GE, Harley RA. Comparison of cardiovascular risk factors for high brachial pulse pressure in blacks versus whites (Charleston Heart Study, Evans County Study, NHANES I and II Studies) Am J Cardiol. 2008;102:1514–1517. doi: 10.1016/j.amjcard.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li C, Engstrom G, Hedblad B, Berglund G, Janzon L. Blood pressure control and risk of stroke: a population-based prospective cohort study. Stroke. 2005;36:725–730. doi: 10.1161/01.STR.0000158925.12740.87. [DOI] [PubMed] [Google Scholar]
- 138.Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 139.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–543. doi: 10.1161/01.hyp.35.2.539. [DOI] [PubMed] [Google Scholar]
- 140.Kostis JB, Davis BR, Cutler J, Grimm RH, Jr, Berge KG, Cohen JD, Lacy CR, Perry HM, Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 141.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 142.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 143.Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH, Jr, Hansson L, Lacouciere Y, Muller J, Sleight P, Weber MA, White WB, Williams G, Wittes J, Zanchetti A, Fakouhi TD, Anders RJ. Baseline Characteristics and Early Blood Pressure Control in the CONVINCE Trial. Hypertension. 2001;37:12–18. doi: 10.1161/01.hyp.37.1.12. [DOI] [PubMed] [Google Scholar]
- 144.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 145.Lackland DT, Egan BM, Mountford WK, Boan AD, Evans DA, Gilbert G, McGee DL. Thirty-year Survival for Black and White Hypertensive Individuals in the Evans County Heart Study and the Hypertension Detection and Follow-up Program. J Am Soc Hypertens. 2008;2:448–454. doi: 10.1016/j.jash.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-Terris M, Carr AA, Kappagoda T, Rocco MV, Schnaper HW, Sowers JR, Bond MG. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA. 1996;276:785–791. [PubMed] [Google Scholar]
- 147.Devereux RB, Dahlof B, Kjeldsen SE, Julius S, Aurup P, Beevers G, Edelman JM, de Faire U, Fyhrquist F, Helle Berg S, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H. Effects of losartan or atenolol in hypertensive patients without clinically evident vascular disease: a substudy of the LIFE randomized trial. Ann Intern Med. 2003;139:169–177. doi: 10.7326/0003-4819-139-3-200308050-00006. [DOI] [PubMed] [Google Scholar]
- 148.Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, Lanke J, de Faire U, Dahlof B, Karlberg BE. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- 149.Julius S, Alderman MH, Beevers G, Dahlof B, Devereux RB, Douglas JG, Edelman JM, Harris KE, Kjeldsen SE, Nesbitt S, Randall OS, Wright JT., Jr Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy: the LIFE study. J Am Coll Cardiol. 2004;43:1047–1055. doi: 10.1016/j.jacc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 150.Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn SM, Harris KE, Wedel H. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J Clin Hypertens (Greenwich) 2005;7:152–158. doi: 10.1111/j.1524-6175.2005.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 152.Mancia G, Brown M, Castaigne A, de Leeuw P, Palmer CR, Rosenthal T, Wagener G, Ruilope LM. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT) Hypertension. 2003;41:431–436. doi: 10.1161/01.HYP.0000057420.27692.AD. [DOI] [PubMed] [Google Scholar]
- 153.Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, Ohta M, Yamada T, Ogawa K, Kanae K, Kawai M, Seki S, Okazaki F, Taniguchi M, Yoshida S, Tajima N. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- 154.Reims HM, Oparil S, Kjeldsen SE, Devereux RB, Julius S, Brady WE, Fyhrquist F, Ibsen H, Lindholm LH, Omvik P, Wedel H, Beevers G, de Faire U, Kristianson K, Lederballe-Pedersen O, Nieminen MS, Dahlof B. Losartan benefits over atenolol in non-smoking hypertensive patients with left ventricular hypertrophy: the LIFE study. Blood Press. 2004;13:376–384. doi: 10.1080/08037050410016483. [DOI] [PubMed] [Google Scholar]
- 155.Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 156.Trenkwalder P, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Papademetriou V, Skoog I, Zanchetti A. The Study on COgnition and Prognosis in the Elderly (SCOPE) - major CV events and stroke in subgroups of patients. Blood Press. 2005;14:31–37. doi: 10.1080/08037050510008823. [DOI] [PubMed] [Google Scholar]
- 157.Weber MA, Bakris GL, Jamerson K, Weir M, Kjeldsen SE, Devereux RB, Velazquez EJ, Dahlof B, Kelly RY, Hua TA, Hester A, Pitt B. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010;56:77–85. doi: 10.1016/j.jacc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 158.Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper-DeHoff R, Pepine CJ. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension. 2004;44:637–642. doi: 10.1161/01.HYP.0000143851.23721.26. [DOI] [PubMed] [Google Scholar]
- 159.Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–1498. doi: 10.1001/jama.288.12.1491. [DOI] [PubMed] [Google Scholar]
- 160.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 161.Czernichow S, Zanchetti A, Turnbull F, Barzi F, Ninomiya T, Kengne AP, Lambers Heerspink HJ, Perkovic V, Huxley R, Arima H, Patel A, Chalmers J, Woodward M, MacMahon S, Neal B. The effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trials. J Hypertens. 2011;29:4–16. doi: 10.1097/HJH.0b013e32834000be. [DOI] [PubMed] [Google Scholar]
- 162.Lee M, Saver JL, Hong KS, Hao Q, Ovbiagele B. Does achieving an intensive versus usual blood pressure level prevent stroke? Ann Neurol. 2012;71:133–140. doi: 10.1002/ana.22496. [DOI] [PubMed] [Google Scholar]
- 163.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 165.Zanchetti A, Mancia G, Black HR, Oparil S, Waeber B, Schmieder RE, Bakris GL, Messerli FH, Kjeldsen SE, Ruilope LM. Facts and fallacies of blood pressure control in recent trials: implications in the management of patients with hypertension. J Hypertens. 2009;27:673–679. doi: 10.1097/HJH.0b013e3283298ea2. [DOI] [PubMed] [Google Scholar]
- 166.Grassi G, Arenare F, Trevano FQ, Dell’Oro R, Mancia AG. Primary and secondary prevention of stroke by antihypertensive treatment in clinical trials. Curr Hypertens Rep. 2007;9:299–304. doi: 10.1007/s11906-007-0055-x. [DOI] [PubMed] [Google Scholar]
- 167.Hansson L. Future goals for the treatment of hypertension in the elderly with reference to STOP-Hypertension, SHEP, and the MRC trial in older adults. Am J Hypertens. 1993;6:40S–43S. doi: 10.1093/ajh/6.3.40s. [DOI] [PubMed] [Google Scholar]
- 168.Freis ED. The Veterans Administration cooperative study on antihypertensive agents. Implications for stroke prevention. Stroke. 1974;5:76–77. doi: 10.1161/01.str.5.1.76. [DOI] [PubMed] [Google Scholar]
- 169.A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 170.Benavente OR, McClure LA, Coffey CS, Conwit R, Pergola PE, Hart RG. The Secondary Prevention of Small Subcortical Strokes (SPS3) Trial: results of the blood pressure intervention. International Stroke Conference. 2012 [Google Scholar]
- 171.ClinicalTrials.gov. Systolic Blood Pressure Intervention Trial (SPRINT) Available at: http://www.clinicaltrials.gov/ct2/show/NCT01206062?term=SPRINT&rank=3. Accessed April 8, 2013.
- 172.Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. A cooperative study. JAMA. 1977;237:255–261. [PubMed] [Google Scholar]
- 173.The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140:1280–1285. [PubMed] [Google Scholar]
- 174.The 1988 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1988;148:1023–1038. [PubMed] [Google Scholar]
- 175.The 1984 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1984;144:1045–1057. [PubMed] [Google Scholar]
- 176.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 177.Handler J, Lackland DT. Translation of hypertension treatment guidelines into practice: a review of implementation. J Am Soc Hypertens. 2011;5:197–207. doi: 10.1016/j.jash.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 178.Bertoia ML, Waring ME, Gupta PS, Roberts MB, Eaton CB. Implications of new hypertension guidelines in the United States. Hypertension. 2012;60:639–644. doi: 10.1161/HYPERTENSIONAHA.112.193714. [DOI] [PubMed] [Google Scholar]
- 179.National Center for Health Statistics. Healthy People 2000 Final Review. Hyattsville, Maryland: Public Health Service; 2001. Available at: http://www.cdc.gov/nchs/data/hp2000/hp2k01.pdf. Accessed August 2, 2012. [Google Scholar]
- 180.Centers for Disease Control and Prevention. High Blood Pressure. Available at: http://www.cdc.gov/bloodpressure/. Accessed August 1, 2012.
- 181.National Heart, Lung and Blood Institute. What is High Blood Pressure? Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hbp/. Accessed August 1, 2012.
- 182.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 183.He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension. 2003;42:1093–1099. doi: 10.1161/01.HYP.0000102864.05174.E8. [DOI] [PubMed] [Google Scholar]
- 184.Centers for Disease Control and Prevention. Division for Heart Disease and Stroke Prevention. Available at: http://www.cdc.gov/dhdsp/. Accessed October 25, 2012.
- 185.American Heart Association. Lifestyle Changes. Available at: http://www.heart.org/HEARTORG/Conditions/HeartAttack/PreventionTreatmentofHeartAttack/Lifestyle-Changes_UCM_303934_Article.jsp. Accessed October 25, 2012.
- 186.National Heart Blood and Lung Institute. National High Blood Pressure Education Program. Available at: http://www.nhlbi.nih.gov/about/nhbpep/index.htm. Accessed October 25, 2012.
- 187.Sister to Sister. The Women’s Heart Health Foundation. Available at: http://www.sistertosister.org/. Accessed October 25, 2012.
- 188.Dannenberg AL, Drizd T, Horan MJ, Haynes SG, Leaverton PE. Progress in the battle against hypertension. Changes in blood pressure levels in the United States from 1960 to 1980. Hypertension. 1987;10:226–233. doi: 10.1161/01.hyp.10.2.226. [DOI] [PubMed] [Google Scholar]
- 189.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 190.Itskovitz HD, Kochar MS, Anderson AJ, Rimm AA. Patterns of blood pressure in Milwaukee. JAMA. 1977;238:864–868. [PubMed] [Google Scholar]
- 191.Wheeler FC, Lackland DT, Mace ML, Reddick A, Hogelin G, Remington PL. Evaluating South Carolina’s community cardiovascular disease prevention project. Public Health Rep. 1991;106:536–543. [PMC free article] [PubMed] [Google Scholar]
- 192.Lackland DT, Weinrich MC, Wheeler FC, Shepard DM. Sodium in drinking water in South Carolina. Am J Public Health. 1985;75:772–774. doi: 10.2105/ajph.75.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Freeman DH, Jr, Ostfeld AM, Hellenbrand K, Richards VA, Tracy R. Changes in the prevalence distribution of hypertension: Connecticut adults 1978–79 to 1982. J Chronic Dis. 1985;38:157–164. doi: 10.1016/0021-9681(85)90088-8. [DOI] [PubMed] [Google Scholar]
- 194.D’Atri DA, Fitzgerald EF, Freeman DH, Jr, Vitale JN, Ostfeld AM. The Connecticut high blood pressure program: a program of public education and high blood pressure screening. Prev Med. 1980;9:91–107. doi: 10.1016/0091-7435(80)90061-4. [DOI] [PubMed] [Google Scholar]
- 195.Perry HM, Freis ED, Frohlich ED. Department of Veterans Affairs hypertension meeting: a proposal for improved care. Hypertension. 2000;35:853–857. doi: 10.1161/01.hyp.35.4.853. [DOI] [PubMed] [Google Scholar]
- 196.Roccella EJ, Lenfant C. Differences Between Blacks and Whites in High Blood Pressure Control, the United States Experience. Tropical Cardiology, Dakar, Senegal, West Africa. 1986 Nov;XIII:33–44. [Google Scholar]
- 197.Roccella EJ, Lenfant C. Regional and racial differences among stroke victims in the United States. Clin Cardiol. 1989;12:IV18–22. doi: 10.1002/clc.4960121306. [DOI] [PubMed] [Google Scholar]
- 198.National Heart, Lung and Blood Institute. Stroke Belt Initiative. Available at: http://www.nhlbi.nih.gov/health/prof/heart/other/sb_spec.pdf. Accessed April 8, 2013.
- 199.Egan BM, Lackland DT, Basile JN. American Society of Hypertension regional chapters: leveraging the impact of the clinical hypertension specialist in the local community. Am J Hypertens. 2002;15:372–379. doi: 10.1016/s0895-7061(01)02323-8. [DOI] [PubMed] [Google Scholar]
- 200.Egan BM, Lackland DT, Igho-Pemu P, Hendrix KH, Basile J, Rehman SU, Okonofua EC, Quarshie A, Oduwole A, Onwuanyi A, Reed J, Obialo C, Ofili EO. Cardiovascular risk factor control in communities–update from the ASH Carolinas-Georgia Chapter, the Hypertension Initiative, and the Community Physicians’ Network. J Clin Hypertens (Greenwich) 2006;8:879–886. doi: 10.1111/j.1524-6175.2006.05677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lackland DT, Egan BM. Community-Based Management Programs: A model for improving cardiovascular health. In: Izzo SL, Black HR, editors. Hypertension Primer. American Heart Association; 2008. [Google Scholar]
- 202.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 203.Alderman MH, Davis TK. Blood pressure control programs on and off the worksite. J Occup Med. 1980;22:167–170. [PubMed] [Google Scholar]
- 204.Foote A, Erfurt JC. Hypertension control at the work site. Comparison of screening and referral alone, referral and follow-up, and on-site treatment. N Engl J Med. 1983;308:809–813. doi: 10.1056/NEJM198304073081404. [DOI] [PubMed] [Google Scholar]
- 205.National High Blood Pressure Education Program. Summary Report: The National High Blood Pressure Education Program Coordinating Committee Meeting; December 17–18, 2002; National Press Club and Grand Hyatt Hotel, Washington, DC. Bethesda, MD: National High Blood Pressure Education Program, National Heart, Lung, and Blood Institute; 2002. pp. 1–37. [Google Scholar]
- 206.McGinnis JM. Can public health and medicine partner in the public interest? Health Aff (Millwood) 2006;25:1044–1052. doi: 10.1377/hlthaff.25.4.1044. [DOI] [PubMed] [Google Scholar]
- 207.Lovelock CE, Molyneux AJ, Rothwell PM. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6:487–493. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 208.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 209.Copenhaver BR, Hsia AW, Merino JG, Burgess RE, Fifi JT, Davis L, Warach S, Kidwell CS. Racial differences in microbleed prevalence in primary intracerebral hemorrhage. Neurology. 2008;71:1176–1182. doi: 10.1212/01.wnl.0000327524.16575.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aiyagari V, Gorelick PB. Management of blood pressure for acute and recurrent stroke. Stroke. 2009;40:2251–2256. doi: 10.1161/STROKEAHA.108.531574. [DOI] [PubMed] [Google Scholar]
- 211.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 212.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 213.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 214.Resnick HE, Shorr RI, Kuller L, Franse L, Harris TB. Prevalence and clinical implications of American Diabetes Association-defined diabetes and other categories of glucose dysregulation in older adults: the health, aging and body composition study. J Clin Epidemiol. 2001;54:869–876. doi: 10.1016/s0895-4356(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 215.Towfighi A, Markovic D, Ovbiagele B. Current national patterns of comorbid diabetes among acute ischemic stroke patients. Cerebrovasc Dis. 2012;33:411–418. doi: 10.1159/000334192. [DOI] [PubMed] [Google Scholar]
- 216.Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001;322:15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Standl E, Balletshofer B, Dahl B, Weichenhain B, Stiegler H, Hormann A, Holle R. Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich General Practitioner Project. Diabetologia. 1996;39:1540–1545. doi: 10.1007/s001250050612. [DOI] [PubMed] [Google Scholar]
- 218.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Diabetes care. Introduction. Diabetes Care. 2013;36(Suppl 1):S1–2. doi: 10.2337/dc13-S001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 222.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 225.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 226.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 227.Bruno A, Durkalski VL, Hall CE, Juneja R, Barsan WG, Janis S, Meurer WJ, Fansler A, Johnston KC. The Stroke Hyperglycemia Insulin Network Effort (SHINE) trial protocol: a randomized, blinded, efficacy trial of standard vs. intensive hyperglycemia management in acute stroke. Int J Stroke. 2013 doi: 10.1111/ijs.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 229.Gray CS, Hildreth AJ, Sandercock PA, O’Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 230.Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke. 2009;40:3804–3809. doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 233.ClinicalTrials.gov. Insulin Resistance Intervention After Stroke Trial (IRIS) Available at: http://clinicaltrials.gov/ct2/show/NCT00091949. Accessed April 9, 2013.
- 234.Centers for Disease Control and Prevention. Diabetes Data and Trends. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/figage.htm. Accessed November 20, 2012.
- 235.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 236.Tsang TS, Petty GW, Barnes ME, O’Fallon WM, Bailey KR, Wiebers DO, Sicks JD, Christianson TJ, Seward JB, Gersh BJ. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 237.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 238.Taylor FC, Cohen H, Ebrahim S. Systematic review of long term anticoagulation or antiplatelet treatment in patients with non-rheumatic atrial fibrillation. BMJ. 2001;322:321–326. doi: 10.1136/bmj.322.7282.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 240.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk–treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97:2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 241.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 242.O’Donnell M, Oczkowski W, Fang J, Kearon C, Silva J, Bradley C, Guyatt G, Gould L, D’Uva C, Kapral M, Silver F. Preadmission antithrombotic treatment and stroke severity in patients with atrial fibrillation and acute ischaemic stroke: an observational study. Lancet Neurol. 2006;5:749–754. doi: 10.1016/S1474-4422(06)70536-1. [DOI] [PubMed] [Google Scholar]
- 243.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10-year perspective (1992 to 2002) Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 244.Reeves MJ, Grau-Sepulveda MV, Fonarow GC, Olson DM, Smith EE, Schwamm LH. Are quality improvements in the Get with The Guidelines-Stroke Program related to better care or better data documentation? Circ Cardiovasc Qual Outcomes. 2011;4:503–511. doi: 10.1161/CIRCOUTCOMES.111.961755. [DOI] [PubMed] [Google Scholar]
- 245.Granger CB, Armaganijan LV. Newer oral anticoagulants should be used as first-line agents to prevent thromboembolism in patients with atrial fibrillation and risk factors for stroke or thromboembolism. Circulation. 2012;125:159–164. doi: 10.1161/CIRCULATIONAHA.111.031146. discussion 164. [DOI] [PubMed] [Google Scholar]
- 246.Arnett DK, Jacobs DR, Jr, Luepker RV, Blackburn H, Armstrong C, Claas SA. Twenty-year trends in serum cholesterol, hypercholesterolemia, and cholesterol medication use: the Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation. 2005;112:3884–3891. doi: 10.1161/CIRCULATIONAHA.105.549857. [DOI] [PubMed] [Google Scholar]
- 247.Hyre AD, Muntner P, Menke A, Raggi P, He J. Trends in ATP-III-defined high blood cholesterol prevalence, awareness, treatment and control among U.S. adults. Ann Epidemiol. 2007;17:548–555. doi: 10.1016/j.annepidem.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 248.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. Jama. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010;106:969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 250.Goff DC, Jr, Labarthe DR, Howard G, Russell GB. Primary prevention of high blood cholesterol concentrations in the United States. Arch Intern Med. 2002;162:913–919. doi: 10.1001/archinte.162.8.913. [DOI] [PubMed] [Google Scholar]
- 251.Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol–United States, 1999–2002 and 2005–200. MMWR Morb Mortal Wkly Rep. 2011;60:109–114. [PubMed] [Google Scholar]
- 252.Brooks EL, Preis SR, Hwang SJ, Murabito JM, Benjamin EJ, Kelly-Hayes M, Sorlie P, Levy D. Health insurance and cardiovascular disease risk factors. Am J Med. 2010;123:741–747. doi: 10.1016/j.amjmed.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet. 1995;346:1647–1653. [PubMed] [Google Scholar]
- 254.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 255.Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002;56(Suppl 1):i19–24. doi: 10.1136/jech.56.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, Cheng JF, Paik MC, Shea S, Berglund L. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. Jama. 2001;285:2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 258.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 259.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. Jama. 2008;300:2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 260.Bonaventure A, Kurth T, Pico F, Barberger-Gateau P, Ritchie K, Stapf C, Tzourio C. Triglycerides and risk of hemorrhagic stroke vs. ischemic vascular events: The Three-City Study. Atherosclerosis. 2010;210:243–248. doi: 10.1016/j.atherosclerosis.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 261.Curb JD, Abbott RD, Rodriguez BL, Masaki KH, Chen R, Popper JS, Petrovitch H, Ross GW, Schatz IJ, Belleau GC, Yano K. High density lipoprotein cholesterol and the risk of stroke in elderly men: the Honolulu heart program. Am J Epidemiol. 2004;160:150–157. doi: 10.1093/aje/kwh177. [DOI] [PubMed] [Google Scholar]
- 262.Psaty BM, Anderson M, Kronmal RA, Tracy RP, Orchard T, Fried LP, Lumley T, Robbins J, Burke G, Newman AB, Furberg CD. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: The Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:1639–1647. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 263.Bang OY, Saver JL, Liebeskind DS, Pineda S, Ovbiagele B. Association of serum lipid indices with large artery atherosclerotic stroke. Neurology. 2008;70:841–847. doi: 10.1212/01.wnl.0000294323.48661.a9. [DOI] [PubMed] [Google Scholar]
- 264.Fine-Edelstein JS, Wolf PA, O’Leary DH, Poehlman H, Belanger AJ, Kase CS, D’Agostino RB. Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology. 1994;44:1046–1050. doi: 10.1212/wnl.44.6.1046. [DOI] [PubMed] [Google Scholar]
- 265.Salonen R, Seppanen K, Rauramaa R, Salonen JT. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arteriosclerosis. 1988;8:788–792. doi: 10.1161/01.atv.8.6.788. [DOI] [PubMed] [Google Scholar]
- 266.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 267.Suzuki K, Izumi M, Sakamoto T, Hayashi M. Blood pressure and total cholesterol level are critical risks especially for hemorrhagic stroke in Akita, Japan. Cerebrovasc Dis. 2011;31:100–106. doi: 10.1159/000321506. [DOI] [PubMed] [Google Scholar]
- 268.Suh I, Jee SH, Kim HC, Nam CM, Kim IS, Appel LJ. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet. 2001;357:922–925. doi: 10.1016/S0140-6736(00)04213-6. [DOI] [PubMed] [Google Scholar]
- 269.Nakaya N, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H. Large-scale cohort study on the relationship between serum lipid concentrations and risk of cerebrovascular disease under low-dose simvastatin in Japanese patients with hypercholesterolemia: sub-analysis of the Japan Lipid Intervention Trial (J-LIT) Circ J. 2005;69:1016–1021. doi: 10.1253/circj.69.1016. [DOI] [PubMed] [Google Scholar]
- 270.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–2725. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 271.Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2011;31:2982–2989. doi: 10.1161/ATVBAHA.111.234948. [DOI] [PubMed] [Google Scholar]
- 272.Willey JZ, Xu Q, Boden-Albala B, Paik MC, Moon YP, Sacco RL, Elkind MS. Lipid profile components and risk of ischemic stroke: the Northern Manhattan Study (NOMAS) Arch Neurol. 2009;66:1400–1406. doi: 10.1001/archneurol.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, Hernandez AF, Peterson ED, Fonarow GC, Schwamm LH. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines-Stroke Program. Circulation. 2010;122:1496–1504. doi: 10.1161/CIRCULATIONAHA.109.932822. [DOI] [PubMed] [Google Scholar]
- 274.Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all-cause mortality: ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38:2646–2651. doi: 10.1161/STROKEAHA.107.490292. [DOI] [PubMed] [Google Scholar]
- 275.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 276.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Munteis E, Bohm P. Serum lipid levels and in-hospital mortality in patients with intracerebral hemorrhage. Neurology. 2005;65:1198–1202. doi: 10.1212/01.wnl.0000180968.26242.4a. [DOI] [PubMed] [Google Scholar]
- 277.Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Doi M, Izumi Y, Ohta H. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation. 2009;119:2136–2145. doi: 10.1161/CIRCULATIONAHA.108.795666. [DOI] [PubMed] [Google Scholar]
- 278.Ramirez-Moreno JM, Casado-Naranjo I, Portilla JC, Calle ML, Tena D, Falcon A, Serrano A. Serum cholesterol LDL and 90-day mortality in patients with intracerebral hemorrhage. Stroke. 2009;40:1917–1920. doi: 10.1161/STROKEAHA.108.536698. [DOI] [PubMed] [Google Scholar]
- 279.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, Skolnick BE, Davis SM. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 280.Bang OY, Saver JL, Liebeskind DS, Starkman S, Villablanca P, Salamon N, Buck B, Ali L, Restrepo L, Vinuela F, Duckwiler G, Jahan R, Razinia T, Ovbiagele B. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology. 2007;68:737–742. doi: 10.1212/01.wnl.0000252799.64165.d5. [DOI] [PubMed] [Google Scholar]
- 281.Li C, Ford ES, Tsai J, Zhao G, Balluz LS, Gidding SS. Serum non-high-density lipoprotein cholesterol concentration and risk of death from cardiovascular diseases among U.S. adults with diagnosed diabetes: the Third National Health and Nutrition Examination Survey linked mortality study. Cardiovasc Diabetol. 2011;10:46. doi: 10.1186/1475-2840-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 283.Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke. 2008;39:497–502. doi: 10.1161/STROKEAHA.107.488791. [DOI] [PubMed] [Google Scholar]
- 284.Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev. 2009:CD002091. doi: 10.1002/14651858.CD002091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 286.Goldstein MR, Mascitelli L, Pezzetta F. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2009;72:1448. doi: 10.1212/01.wnl.0000346751.39886.01. author reply 1448–1449. [DOI] [PubMed] [Google Scholar]
- 287.McKinney JS, Kostis WJ. Statin Therapy and the Risk of Intracerebral Hemorrhage: A Meta-Analysis of 31 Randomized Controlled Trials. Stroke. 2012 doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 288.Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, Donner A, Mamdani M, Douketis JD, Arima H, Chalmers J, MacMahon S, Tirschwell DL, Psaty BM, Bushnell CD, Aguilar MI, Capampangan DJ, Werring DJ, De Rango P, Viswanathan A, Danchin N, Cheng CL, Yang YH, Verdel BM, Lai MS, Kennedy J, Uchiyama S, Yamaguchi T, Ikeda Y, Mrkobrada M. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124:2233–2242. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- 289.Hackam DG, Austin PC, Huang A, Juurlink DN, Mamdani MM, Paterson JM, Hachinski V, Li P, Kapral MK. Statins and intracerebral hemorrhage: a retrospective cohort study. Arch Neurol. 2012;69:39–45. doi: 10.1001/archneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- 290.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ni Chroinin D, Callaly EL, Duggan J, Merwick A, Hannon N, Sheehan O, Marnane M, Horgan G, Williams EB, Harris D, Kyne L, McCormack PM, Moroney J, Grant T, Williams D, Daly L, Kelly PJ. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: the North Dublin Population Stroke Study. Stroke. 2011;42:1021–1029. doi: 10.1161/STROKEAHA.110.596734. [DOI] [PubMed] [Google Scholar]
- 292.Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med. 2003;163:669–676. doi: 10.1001/archinte.163.6.669. [DOI] [PubMed] [Google Scholar]
- 293.Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis. 2011;217:492–498. doi: 10.1016/j.atherosclerosis.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 294.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. Jama. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 297.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 298.Sandercock PA, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2008:CD000029. doi: 10.1002/14651858.CD000029.pub2. [DOI] [PubMed] [Google Scholar]
- 299.A randomized trial of aspirin and sulfinpyrazone in threatened stroke. The Canadian Cooperative Study Group. N Engl J Med. 1978;299:53–59. doi: 10.1056/NEJM197807132990201. [DOI] [PubMed] [Google Scholar]
- 300.Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Stroke. 1977;8:301–314. doi: 10.1161/01.str.8.3.301. [DOI] [PubMed] [Google Scholar]
- 301.Feinberg WM, Albers GW, Barnett HJ, Biller J, Caplan LR, Carter LP, Hart RG, Hobson RW, 2nd, Kronmal RA, Moore WS, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation. 1994;89:2950–2965. doi: 10.1161/01.cir.89.6.2950. [DOI] [PubMed] [Google Scholar]
- 302.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 303.Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24:3177–3184. [PubMed] [Google Scholar]
- 304.Ajani UA, Ford ES, Greenland KJ, Giles WH, Mokdad AH. Aspirin use among U.S. adults: Behavioral Risk Factor Surveillance System. Am J Prev Med. 2006;30:74–77. doi: 10.1016/j.amepre.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 305.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 306.George MG, Tong X, Sonnenfeld N, Hong Y. Recommended use of aspirin and other antiplatelet medications among adults–National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey, United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):11–18. [PubMed] [Google Scholar]
- 307.Stafford RS. Aspirin use is low among United States outpatients with coronary artery disease. Circulation. 2000;101:1097–1101. doi: 10.1161/01.cir.101.10.1097. [DOI] [PubMed] [Google Scholar]
- 308.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353. doi: 10.1371/journal.pmed.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 310.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 311.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 312.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhou YH, Wei X, Lu J, Ye XF, Wu MJ, Xu JF, Qin YY, He J. Effects of combined aspirin and clopidogrel therapy on cardiovascular outcomes: a systematic review and meta-analysis. PLoS One. 2012;7:e31642. doi: 10.1371/journal.pone.0031642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ, investigators M. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 315.Menon BK, Frankel MR, Liang L, LaBresh KA, Ellrodt G, Hernandez AF, Fonarow GC, Schwamm LH, Smith EE. Rapid change in prescribing behavior in hospitals participating in Get With The Guidelines-Stroke after release of the MATCH clinical trial results. Stroke. 2010;41:2094–2097. doi: 10.1161/STROKEAHA.110.584151. [DOI] [PubMed] [Google Scholar]
- 316.ClinicalTrials.gov. Clopidogrel in High-risk Patients With Acute Non-disabling Cerebrovascular Events (CHANCE) Available at: http://clinicaltrials.gov/show/NCT00979589. Accessed April 9, 2013.
- 317.ClinicalTrials.gov. Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial. doi: 10.1111/ijs.12129. Available at: http://clinicaltrials.gov/ct2/show/NCT00991029. Accessed April 9, 2013. [DOI] [PMC free article] [PubMed]
- 318.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 319.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man; theory, procedure and normal values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, Caplan LR, Day A, Goldstone J, Hobson RW, 2nd, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc Committee, American Heart Association. Stroke. 1995;26:188–201. doi: 10.1161/01.str.26.1.188. [DOI] [PubMed] [Google Scholar]
- 321.Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:407–416. doi: 10.1056/NEJM199608083350607. [DOI] [PubMed] [Google Scholar]
- 322.Satomura S, Kaneko Z. Ultrasonic blood rheograph. Proceedings of the 3rd International Conference on Medical Electronics. 1960:254–8. [Google Scholar]
- 323.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 324.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, Bonds DR, Brambilla D. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 325.Axel L. Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology. 1980;137:679–686. doi: 10.1148/radiology.137.3.7003648. [DOI] [PubMed] [Google Scholar]
- 326.Kaste M. Reborn workhorse, CT, pulls the wagon toward thrombolysis beyond 3 hours. Stroke. 2004;35:357–359. doi: 10.1161/01.STR.0000115165.43847.ED. [DOI] [PubMed] [Google Scholar]
- 327.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer R, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TA. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JL. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 331.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 332.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 333.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schumacher HC, Bateman BT, Boden-Albala B, Berman MF, Mohr JP, Sacco RL, Pile-Spellman J. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. 2007;50:99–107. doi: 10.1016/j.annemergmed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 335.Qureshi AI, Suri MF, Nasar A, He W, Kirmani JF, Divani AA, Prestigiacomo CJ, Low RB. Thrombolysis for ischemic stroke in the United States: data from National Hospital Discharge Survey 1999–2001. Neurosurgery. 2005;57:647–654. discussion 647–654. [PubMed] [Google Scholar]
- 336.Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD000197.pub2. Art. No.: CD000197. Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858CD000197.pub2/abstract. Accessed Nov 9, 2012. [DOI] [PubMed]
- 337.Svendsen ML, Ehlers LH, Ingeman A, Johnsen SP. Higher stroke unit volume associated with improved quality of early stroke care and reduced length of stay. Stroke. 2012;43:3041–3045. doi: 10.1161/STROKEAHA.111.645184. [DOI] [PubMed] [Google Scholar]
- 338.Langhorne P, Lewsey JD, Jhund PS, Gillies M, Chalmers JWT, Redpath A, Briggs A, Walters M, Capewell S, McMurray JJV, MacIntyre K. Estimating the impact of stroke unit care in a whole population: an epidemiological study using routine data. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81:1301–1305. doi: 10.1136/jnnp.2009.195131. [DOI] [PubMed] [Google Scholar]
- 339.Lichtman JH, Jones SB, Wang Y, Watanabe E, Leifheit-Limson E, Goldstein LB. Outcomes after ischemic stroke for hospitals with and without Joint Commission-certified primary stroke centers. Neurology. 2011;76:1976–1982. doi: 10.1212/WNL.0b013e31821e54f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lichtman JH, Jones SB, Leifheit-Limson EC, Wang Y, Goldstein LB. 30-day mortality and readmission after hemorrhagic stroke among Medicare beneficiaries in Joint Commission primary stroke center-certified and noncertified hospitals. Stroke. 2011;42:3387–3391. doi: 10.1161/STROKEAHA.111.622613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lichtman JH, Allen NB, Wang Y, Watanabe E, Jones SB, Goldstein LB. Stroke patient outcomes in US hospitals before the start of the Joint Commission Primary Stroke Center certification program. Stroke. 2009;40:3574–3579. doi: 10.1161/STROKEAHA.109.561472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Douglas VC, Tong DC, Gillum LA, Zhao S, Brass LM, Dostal J, Johnston SC. Do the Brain Attack Coalition’s criteria for stroke centers improve care for ischemic stroke? Neurology. 2005;64:422–427. doi: 10.1212/01.WNL.0000150903.38639.E1. [DOI] [PubMed] [Google Scholar]
- 343.Collaboration UT. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke Unit Trialists Collaboration. Stroke. 1997;28:2139–2144. doi: 10.1161/01.str.28.11.2139. [DOI] [PubMed] [Google Scholar]
- 344.Webb DJ, Fayad PB, Wilbur C, Thomas A, Brass LM. Effects of a specialized team on stroke care. The first two years of the Yale Stroke Program. Stroke. 1995;26:1353–1357. doi: 10.1161/01.str.26.8.1353. [DOI] [PubMed] [Google Scholar]
- 345.Nazir FS, Petre I, Dewey HM. Introduction of an acute stroke team: an effective approach to hasten assessment and management of stroke in the emergency department. Journal of Clinical Neuroscience. 2009;16:21–25. doi: 10.1016/j.jocn.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 346.Taylor R, Benton C, Buckner A, Jones R, Schneider A. Mission Hospital’s code stroke team: implications for an aging population. North Carolina Medical Journal. 2009;70:301–306. [PubMed] [Google Scholar]
- 347.Hamidon BB, Dewey HM. Impact of acute stroke team emergency calls on in-hospital delays in acute stroke care. Journal of Clinical Neuroscience. 2007;14:831–834. doi: 10.1016/j.jocn.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 348.Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, Chappel AR, Peterson ED, Friedman B. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP. Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34:2200–2207. doi: 10.1161/01.STR.0000086528.32334.06. [DOI] [PubMed] [Google Scholar]
- 350.Meyers PM, Schumacher HC, Connolly ES, Jr, Heyer EJ, Gray WA, Higashida RT. Current status of endovascular stroke treatment. Circulation. 2011;123:2591–2601. doi: 10.1161/CIRCULATIONAHA.110.971564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bardach NS, Zhao S, Gress DR, Lawton MT, Johnston SC. Association between subarachnoid hemorrhage outcomes and number of cases treated at California hospitals. Stroke. 2002;33:1851–1856. doi: 10.1161/01.str.0000019126.43079.7b. [DOI] [PubMed] [Google Scholar]
- 352.Cramer SC, Stradling D, Brown DM, Carrillo-Nunez IM, Ciabarra A, Cummings M, Dauben R, Lombardi DL, Patel N, Traynor EN, Waldman S, Miller K, Stratton SJ. Organization of a United States county system for comprehensive acute stroke care. Stroke. 2012;43:1089–1093. doi: 10.1161/STROKEAHA.111.635334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Demaerschalk BM, Bobrow BJ, Paulsen M. Development of a metropolitan matrix of primary stroke centers: the Phoenix experience. Stroke. 2008;39:1246–1253. doi: 10.1161/STROKEAHA.107.500678. [DOI] [PubMed] [Google Scholar]
- 354.Perez de la Ossa N, Millan M, Arenillas JF, Sanchez-Ojanguren J, Palomeras E, Dorado L, Guerrero C, Davalos A. Influence of direct admission to Comprehensive Stroke Centers on the outcome of acute stroke patients treated with intravenous thrombolysis. Journal of Neurology. 2009;256:1270–1276. doi: 10.1007/s00415-009-5113-7. [DOI] [PubMed] [Google Scholar]
- 355.Silva GS, Schwamm LH. Use of telemedicine and other strategies to increase the number of patients that may be treated with intravenous thrombolysis. Current Neurology & Neuroscience Reports. 2012;12:10–16. doi: 10.1007/s11910-011-0235-6. [DOI] [PubMed] [Google Scholar]
- 356.Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, Handschu R, Jauch EC, Knight WAt, Levine SR, Mayberg M, Meyer BC, Meyers PM, Skalabrin E, Wechsler LR, American Heart Association Stroke C, Interdisciplinary Council on Peripheral Vascular D. Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, Handschu R, Jauch EC, Knight WAt, Levine SR, Mayberg M, Meyer BC, Meyers PM, Skalabrin E, Wechsler LR. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40:2616–2634. doi: 10.1161/STROKEAHA.109.192360. [DOI] [PubMed] [Google Scholar]
- 357.Centers for Disease Control and Prevention. 2004 Surgeon General’s Report - The Health Consequences of Smoking. 2004. [PubMed] [Google Scholar]
- 358.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298:789–794. doi: 10.1136/bmj.298.6676.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wolf PA, D’Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA. 1988;259:1025–1029. [PubMed] [Google Scholar]
- 360.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Smoking cessation and the risk of stroke in middle-aged men. JAMA. 1995;274:155–160. [PubMed] [Google Scholar]
- 361.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269:232–236. [PubMed] [Google Scholar]
- 362.Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA. 2000;284:706–712. doi: 10.1001/jama.284.6.706. [DOI] [PubMed] [Google Scholar]
- 363.Hart CL, Hole DJ, Smith GD. Risk factors and 20-year stroke mortality in men and women in the Renfrew/Paisley study in Scotland. Stroke. 1999;30:1999–2007. doi: 10.1161/01.str.30.10.1999. [DOI] [PubMed] [Google Scholar]
- 364.Kuller LH, Ockene JK, Meilahn E, Wentworth DN, Svendsen KH, Neaton JD. Cigarette smoking and mortality. MRFIT Research Group. Prev Med. 1991;20:638–654. doi: 10.1016/0091-7435(91)90060-h. [DOI] [PubMed] [Google Scholar]
- 365.Towfighi A, Markovic D, Ovbiagele B. Impact of a healthy lifestyle on all-cause and cardiovascular mortality after stroke in the USA. J Neurol Neurosurg & Psychiatry. 2012;83:146–151. doi: 10.1136/jnnp-2011-300743. [DOI] [PubMed] [Google Scholar]
- 366.Centers for Disease Control and Prevention. Smoking and Tobacco Use. Available at: http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm. Accessed September 6, 2012.
- 367.American Lung Association. American Lung Association Research and Program Services Epidemiology and Statistics Unit (ALARaPSEaS Unit) Trends in tobacco use. 2011 Jul; Available at: http://www.lung.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf. Accessed June 26, 2012.
- 368.Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc. 2002;34:592–595. doi: 10.1097/00005768-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 369.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health. 2011;33:496–502. doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 370.Jefferis BJ, Lawlor DA, Ebrahim S, Wannamethee SG, Feyerabend C, Doig M, McMeekin L, Cook DG, Whincup PH. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart. 2010;96:854–859. doi: 10.1136/hrt.2009.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Whincup PH, Gilg JA, Emberson JR, Jarvis MJ, Feyerabend C, Bryant A, Walker M, Cook DG. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ. 2004;329:200–205. doi: 10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 374.Herman PM, Walsh ME. Hospital admissions for acute myocardial infarction, angina, stroke, and asthma after implementation of Arizona’s comprehensive statewide smoking ban. Am J Public Health. 2011;101:491–496. doi: 10.2105/AJPH.2009.179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Juster HR, Loomis BR, Hinman TM, Farrelly MC, Hyland A, Bauer UE, Birkhead GS. Declines in hospital admissions for acute myocardial infarction in New York state after implementation of a comprehensive smoking ban. Am J Public Health. 2007;97:2035–2039. doi: 10.2105/AJPH.2006.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Naiman A, Glazier RH, Moineddin R. Association of anti-smoking legislation with rates of hospital admission for cardiovascular and respiratory conditions. CMAJ. 2010;182:761–767. doi: 10.1503/cmaj.091130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 379.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 380.Influenza and pneumococcal vaccination coverage levels among persons aged > or = 65 years–United States, 1973–1993. MMWR. 1995;44:506–507. 513–505. [PubMed] [Google Scholar]
- 381.Towfighi A, Tai W, Markovic D, Ovbiagele B. Sex-specific temporal trends in in-hospital mortality after stroke among middle-age individuals in the United States. Stroke. 2011;42:2740–2745. doi: 10.1161/STROKEAHA.110.612648. [DOI] [PubMed] [Google Scholar]
- 382.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 384.Parra O, Arboix A, Montserrat JM, Quinto L, Bechich S, Garcia-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:267–272. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 385.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, Franklin KA. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 386.Turkington PM, Elliott MW. Sleep disordered breathing following stroke. Monaldi Arch Chest Dis. 2004;61:157–161. doi: 10.4081/monaldi.2004.695. [DOI] [PubMed] [Google Scholar]
- 387.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 388.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 389.Namen AM, Dunagan DP, Fleischer A, Tillett J, Barnett M, McCall WV, Haponik EF. Increased physician-reported sleep apnea: the National Ambulatory Medical Care Survey. Chest. 2002;121:1741–1747. doi: 10.1378/chest.121.6.1741. [DOI] [PubMed] [Google Scholar]
- 390.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 391.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 392.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 393.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 394.Brown DL, Chervin RD, Kalbfleisch JD, Zupancic MJ, Migda EM, Svatikova A, Concannon M, Martin C, Weatherwax KJ, Morgenstern LB. Sleep Apnea Treatment After Stroke (SATS) Trial: Is It Feasible? J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg & Psychiatry. 2006;77:1143–1149. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.United States Environmental Protection Agency. Technology Transfer Network. National Ambient Air Quality Standards (NAAQS) Available at: http://www.epa.gov/ttn/naaqs/standards/pm/s_pm_history.html. Accessed September 6, 2012.
- 397.United States Environmental Protection Agency. Particulate Matter. Available at: http://www.epa.gov/pm/. Accessed September 6, 2012.
- 398.United States Environmental Protection Agency. Six Common Air Pollutants. Available at: http://www.epa.gov/air/urbanair/. Accessed September 6, 2012.
- 399.United States Environmental Protection Agency. Clean Air Act. Available at: http://epa.gov/oar/caa/caa_history.html. Accessed September 6, 2012.
- 400.United States Environmental Protection Agency. National Air Pollution Emission Trends, 1900 – 1998. 2000 [Google Scholar]
- 401.United States Environmental Protection Agency. Particulate Matter. Available at: http://www.epa.gov/airtrends/pm.html#pmloc. Accessed September 6, 2012.
- 402.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 403.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 404.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 406.Low RB, Bielory L, Qureshi AI, Dunn V, Stuhlmiller DF, Dickey DA. The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, September 11, 2001, and day of the week. Stroke. 2006;37:951–957. doi: 10.1161/01.STR.0000214681.94680.66. [DOI] [PubMed] [Google Scholar]
- 407.Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, Smith MA, Morgenstern LB. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 410.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 411.Puett RC, Hart JE, Suh H, Mittleman M, Laden F. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect. 2011;119:1130–1135. doi: 10.1289/ehp.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 413.Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, Smith DF, Garcia C, Chang ET, Bernstein L. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 416.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- 417.Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, Willett WC, Manson JE. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 418.Wisloff U, Nilsen TIL, Droyvold WB, Morkved S, Slordahl SA, Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? ‘The HUNT study, Norway’. Eur J Cardiovasc Prev & Rehabil. 2006;13:798–804. doi: 10.1097/01.hjr.0000216548.84560.ac. [DOI] [PubMed] [Google Scholar]
- 419.Vatten LJ, Nilsen TIL, Holmen J. Combined effect of blood pressure and physical activity on cardiovascular mortality. J Hypertens. 2006;24:1939–1946. doi: 10.1097/01.hjh.0000244941.49793.f9. [DOI] [PubMed] [Google Scholar]
- 420.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 421.Kurth T, Moore SC, Gaziano JM, Kase CS, Stampfer MJ, Berger K, Buring JE. Healthy lifestyle and the risk of stroke in women. Arch Intern Med. 2006;166:1403–1409. doi: 10.1001/archinte.166.13.1403. [DOI] [PubMed] [Google Scholar]
- 422.Williams PT. Reduction in incident stroke risk with vigorous physical activity: evidence from 7.7-year follow-up of the national runners’ health study. Stroke. 2009;40:1921–1923. doi: 10.1161/STROKEAHA.108.535427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ellekjaer H, Holmen J, Ellekjaer E, Vatten L. Physical activity and stroke mortality in women. Ten-year follow-up of the Nord-Trondelag health survey, 1984–1986. Stroke. 2000;31:14–18. doi: 10.1161/01.str.31.1.14. [DOI] [PubMed] [Google Scholar]
- 424.Noda H, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A, Group JS Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol. 2005;46:1761–1767. doi: 10.1016/j.jacc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 425.Hooker SPP, Sui XMD, Colabianchi NP, Vena JP, Laditka JP, LaMonte MJP, Blair SNPED Cardiorespiratory Fitness as a Predictor of Fatal and Nonfatal Stroke in Asymptomatic Women and Men. Stroke. 2008;39:2950–2957. doi: 10.1161/STROKEAHA.107.495275. [DOI] [PubMed] [Google Scholar]
- 426.Centers for Disease Control and Prevention. Physical Activity Trends – United States, 1990–1998. MMWR. 2001;50:166–169. [Google Scholar]
- 427.Brownson RC, Boehmer TK. Patterns and Trends in Physical Activity, Occupation, Transportation, Land Use and Sedantary Behaviors. TRB Special Report 282: Does the Built Environment Influence Physical Activity? Examining the Evidence. Available at: http://onlinepubs.trb.org/onlinepubs/archive/downloads/sr282papers/sr282Brownson.pdf. Accessed Nov 8, 2012.
- 428.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 429.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162:2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 430.Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, Speizer FE, Manson JE. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 431.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 432.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Davey Smith G. Obesity and overweight in relation to disease-specific mortality in men with and without existing coronary heart disease in London: the original Whitehall study. Heart. 2006;92:886–892. doi: 10.1136/hrt.2005.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 434.Yan LL, Daviglus ML, Liu K, Stamler J, Wang R, Pirzada A, Garside DB, Dyer AR, Van Horn L, Liao Y, Fries JF, Greenland P. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- 435.Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Zhou M, Offer A, Yang G, Smith M, Hui G, Whitlock G, Collins R, Huang Z, Peto R, Chen Z. Body mass index, blood pressure, and mortality from stroke: a nationally representative prospective study of 212,000 Chinese men. Stroke. 2008;39:753–759. doi: 10.1161/STROKEAHA.107.495374. [DOI] [PubMed] [Google Scholar]
- 437.Bazzano LA, Gu D, Whelton MR, Wu X, Chen CS, Duan X, Chen J, Chen JC, He J. Body mass index and risk of stroke among Chinese men and women. Ann Neurol. 2010;67:11–20. doi: 10.1002/ana.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yi SW, Odongua N, Nam CM, Sull JW, Ohrr H. Body mass index and stroke mortality by smoking and age at menopause among Korean postmenopausal women. Stroke. 2009;40:3428–3435. doi: 10.1161/STROKEAHA.109.555144. [DOI] [PubMed] [Google Scholar]
- 439.Funada S, Shimazu T, Kakizaki M, Kuriyama S, Sato Y, Matsuda-Ohmori K, Nishino Y, Tsuji I. Body mass index and cardiovascular disease mortality in Japan: the Ohsaki Study. Prev Med. 2008;47:66–70. doi: 10.1016/j.ypmed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 440.Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Wada Y, Inaba Y, Tamakoshi A, Group JS Body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke. 2005;36:1377–1382. doi: 10.1161/01.STR.0000169925.57251.4e. [DOI] [PubMed] [Google Scholar]
- 441.Tanne D, Medalie JH, Goldbourt U. Body fat distribution and long-term risk of stroke mortality. Stroke. 2005;36:1021–1025. doi: 10.1161/01.STR.0000162584.39366.1c. [DOI] [PubMed] [Google Scholar]
- 442.Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42:3646–3650. doi: 10.1161/STROKEAHA.111.619163. [DOI] [PubMed] [Google Scholar]
- 443.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- 444.Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke. 2009;40:2704–2708. doi: 10.1161/STROKEAHA.109.550228. [DOI] [PubMed] [Google Scholar]
- 445.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 446.Caterson ID, Finer N, Coutinho W, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Legler UF, Shepherd GM, Rode RA, Perdok RJ, Renz CL, James WP, on behalf of the SI Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 447.Myint PK, Luben RN, Wareham NJ, Bingham SA, Khaw KT. Combined effect of health behaviours and risk of first ever stroke in 20,040 men and women over 11 years’ follow-up in Norfolk cohort of European Prospective Investigation of Cancer (EPIC Norfolk): prospective population study. BMJ. 2009;338:b349. doi: 10.1136/bmj.b349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Effects of treatment on morbidity in hypertension. 3. Influence of age, diastolic pressure, and prior cardiovascular disease; further analysis of side effects. Circulation. 1972;45:991–1004. doi: 10.1161/01.cir.45.5.991. [DOI] [PubMed] [Google Scholar]
- 449.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 450.Lanska DJ. Geographic distribution of stroke mortality in the United States: 1939–1941 to 1979–1981. Neurology. 1993;43:1839–1851. doi: 10.1212/wnl.43.9.1839. [DOI] [PubMed] [Google Scholar]
- 451.Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997;28:1639–1647. doi: 10.1161/01.str.28.8.1639. [DOI] [PubMed] [Google Scholar]
- 452.Lanska DJ, Kryscio R. Geographic distribution of hospitalization rates, case fatality, and mortality from stroke in the United States. Neurology. 1994;44:1541–1550. doi: 10.1212/wnl.44.8.1541. [DOI] [PubMed] [Google Scholar]
- 453.Lackland DT, Egan BM, Jones PJ. Impact of nativity and race on “Stroke Belt” mortality. Hypertension. 1999;34:57–62. doi: 10.1161/01.hyp.34.1.57. [DOI] [PubMed] [Google Scholar]
- 454.Fang J, Madhavan S, Alderman MH. The association between birthplace and mortality from cardiovascular causes among black and white residents of New York City. N Engl J Med. 1996;335:1545–1551. doi: 10.1056/NEJM199611213352101. [DOI] [PubMed] [Google Scholar]
- 455.Barker DJ, Lackland DT. Prenatal influences on stroke mortality in England and Wales. Stroke. 2003;34:1598–1602. doi: 10.1161/01.STR.0000077257.27430.7E. [DOI] [PubMed] [Google Scholar]
- 456.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 457.Yang D, Howard G, Coffey CS, Roseman J. The confounding of race and geography: how much of the excess stroke mortality among African Americans is explained by geography? Neuroepidemiology. 2004;23:118–122. doi: 10.1159/000075954. [DOI] [PubMed] [Google Scholar]
- 458.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Judd SE, McClure LA, Howard VJ, Lackland DT, Halanych JH, Kabagambe EK. Heavy drinking is associated with poor blood pressure control in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Int J Environ Res Public Health. 2011;8:1601–1612. doi: 10.3390/ijerph8051601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4:32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Cummings DM, Doherty L, Howard G, Howard VJ, Safford MM, Prince V, Kissela B, Lackland DT. Blood pressure control in diabetes: temporal progress yet persistent racial disparities: national results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2010;33:798–803. doi: 10.2337/dc09-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Adams RJ, McKie VC, Brambilla D, Carl E, Gallagher D, Nichols FT, Roach S, Abboud M, Berman B, Driscoll C, Files B, Hsu L, Hurlet A, Miller S, Olivieri N, Pegelow C, Scher C, Vichinsky E, Wang W, Woods G, Kutlar A, Wright E, Hagner S, Tighe F, Waclawiw MA, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110–129. doi: 10.1016/s0197-2456(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 463.Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, McKie K, Figueroa R, Litaker M, Weiner S, Brambilla D. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- 464.Appel LJ, Angell SY, Cobb LK, Limper HM, Nelson DE, Samet JM, Brownson RC. Population-wide sodium reduction: the bumpy road from evidence to policy. Ann Epidemiol. 2012;22:417–425. doi: 10.1016/j.annepidem.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 466.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 468.Sodium intake among adults - United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2010;59:746–749. [PubMed] [Google Scholar]
- 469.CDC grand rounds: dietary sodium reduction - time for choice. MMWR Morb Mortal Wkly Rep. 2012;61:89–91. [PubMed] [Google Scholar]
- 470.Perry IJ, Beevers DG. Salt intake and stroke: a possible direct effect. J Hum Hypertens. 1992;6:23–25. [PubMed] [Google Scholar]
- 471.Consensus Action on Salt and Health (CASH) doi: 10.7748/ns.29.40.30.s31. Available at: http://www.actiononsalt.org.uk/awareness/Salt%20and%20stroke%202012/salt%20and%20stroke:%20the%20facts/59737.html. Accessed October 23, 2012. [DOI] [PubMed]
- 472.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 473.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 474.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 475.Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174:35–43. doi: 10.1093/aje/kwr051. [DOI] [PubMed] [Google Scholar]


