Abstract
Background
Post-mastectomy reconstruction is a critical component of high-quality breast cancer care. Prior studies demonstrate socioeconomic disparity in receipt of reconstruction. Our objective was to evaluate trends in receipt of immediate reconstruction and examine socio-economic factors associated with reconstruction in a contemporary cohort.
Methods
Using the National Cancer Database, we identified women < 75 years of age with stage 0-1 breast cancer treated with mastectomy (n=297,121). Trends in immediate reconstruction rates (2004-2013) for the overall cohort and stratified by socioeconomic factors were examined using Join-point regression analysis. Annual percent change (APC) was calculated. We then restricted our sample to a contemporary cohort (2010-2013, n=145,577). Multivariable logistic regression identified socioeconomic factors associated with immediate reconstruction. Average adjusted predicted probabilities of receiving reconstruction were calculated.
Results
Immediate reconstruction rates increased from 27% to 48%. Although absolute rates of reconstruction for each stratification group increased, similar APC’s across strata led to persistent gaps in receipt of reconstruction. On multivariable logistic regression using our contemporary cohort, race, income, education, and insurance type were all strongly associated with immediate reconstruction. Patients with the lowest predicted probability of receiving reconstruction were patients with Medicaid who live in areas with the lowest rates of high school graduation (black 42.4 (40.5-44.3)%, white 45.7 (43.9-47.4)%).
Conclusions
Although reconstruction rates have increased dramatically over the past decade, lower rates persist for disadvantaged patients. Understanding how socioeconomic factors influence receipt of reconstruction and identifying modifiable factors are critical next steps towards identifying interventions to reduce disparities in breast cancer surgical care.
INTRODUCTION
Post-mastectomy reconstruction is a critical component of comprehensive, high-quality breast cancer care and has been associated with improved quality of life.1–4 This includes not only improved satisfaction with the appearance of the breast,3,5 but also better sexual, psychosocial and physical functioning.3,4 Post-mastectomy reconstruction is recognized as a breast cancer care quality measure. The National Accreditation Program of Breast Centers endorses a standard that all patients considering mastectomy be offered a consultation with a plastic surgeon.6 The importance of reconstruction is also reflected in health policy. For example, the 1998 Women’s Health and Cancer Rights Act mandated that insurance companies cover reconstructive procedures after mastectomy7,8 and New York Bill S6993A requires that surgeons discuss the option of reconstruction with all patients.9
There has been a steady increase in the rate of post-mastectomy reconstruction over the past decade. Drivers of this trend are multifactorial; in addition to the Women’s Health and Cancer Rights Act, patients benefit from advances in reconstructive techniques, expanded availability of plastic surgeons, and growing comfort of breast surgeons in pursuing immediate reconstruction at the time of the initial cancer surgery.10–14 However, although overall rates of reconstruction have increased, substantial variation exists regarding who undergoes reconstruction.10–24 Breast reconstruction following mastectomy is an elective component of breast cancer treatment and reconstruction is not the right choice for everyone. Patient preference or clinical factors rendering the patient a poor candidate for the procedure (i.e. smoking, obesity, recommended post-mastectomy radiation) can drive the decision to forego immediate and/or ever reconstruction. Variation driven by patient preference or clinical factors is expected and reasonable. However, prior studies evaluating patients diagnosed with breast cancer in the early 2000’s have suggested disparities in the receipt of immediate reconstruction by socioeconomic factors such as race, income, education-level and insurance.10–24 The objective of this study was to evaluate national rates of immediate post-mastectomy reconstruction and examine socioeconomic factors associated with receipt of reconstruction in a contemporary cohort.
METHODS
The National Cancer Data Base (NCDB), a joint program of the American College of Surgeons Commission on Cancer and the American Cancer Society, is a large national cancer registry database that captures approximately 70% of all newly diagnosed cancers in the United States.25 We used the NCDB to identify women with newly diagnosed stage 0 or stage 1 breast cancer in 2004-2013 and treated with mastectomy. We excluded patients with stage 2 or 3 breast cancer, as these patients may be more likely to have a clinical contraindication for reconstruction, either due to tumor (i.e. inflammatory cancer) or treatment-related (i.e. need for post-mastectomy radiation) factors. We also excluded women over the age of 75 years because of the very low rate of reconstruction observed in this cohort (3.5%). The final sample size was 297,121.
The outcome variable of interest was the receipt of immediate breast construction (yes/no). The NCDB captures immediate breast reconstruction after mastectomy, i.e. reconstruction planned as part of the initial course of treatment. Covariates included sociodemographic (age, urban/rural residence, race, insurance, zip code level median household income and education), Charlson Comorbidity Index, diagnosis (stage), treatment (bilateral mastectomy, and whether patients received post-mastectomy radiation and/or systemic therapy), and reporting facility type, all ascertained at the time of diagnosis and first course treatment.
Descriptive statistics for all sociodemographic, diagnosis/treatment, and facility factors were generated. Unadjusted temporal trends in annual reconstruction rates were assessed using Join-point regression software (National Cancer Institute, Bethesda, MD); overall trends were assessed as well as trends stratified by socioeconomic factors (race, income, insurance, and education).26–29 Join-point regression models allowed for an assessment of the observed unadjusted changes in reconstruction that occurred each year during the study time period (i.e. the annual percent change [APC]). 95% confidence intervals were also estimated.
Because of the anticipated strong time trends for rates of immediate reconstruction,10–14 we restricted our assessment of the factors associated with reconstruction to a contemporary cohort of women diagnosed between 2010 and 2013 (sample size of 145,577). Multivariable logistic regression was then used to assess whether socioeconomic factors were associated with immediate reconstruction, controlling for patient age, comorbidities, tumor and treatment factors. Average adjusted predicted probabilities of receiving immediate reconstruction based on these models were then estimated, assuming a patient was <55 years of age, had a Charlson Comorbidity Index of 0, did not receive radiation or systemic therapy, and underwent a unilateral mastectomy at a metropolitan academic center in 2013. These values were intentionally selected because they are associated with higher rates of reconstruction, thereby facilitating comparison of the impact of socioeconomic factors on receipt of reconstruction.
This project is considered to be exempt by the University of Wisconsin Institutional Review Board.
RESULTS
Time Trends in Rates of Immediate Reconstruction between 2004-2013
We identified 297,121 women with stage 0 or 1 breast cancer who underwent mastectomy between 2004 and 2013. Patient demographics for the overall cohort are presented in Table 1. For this cohort, the rate of reconstruction across the entire study period was 40%. The rate of reconstruction increased steadily from 27% in 2004 to 48% in 2013. This corresponds to an APC of 2.3% (95% confidence interval 2.0-2.6%).
Table 1.
Characteristics of Patient Cohort
| % (N=297,121) | |
|---|---|
|
| |
| Age | |
| <=45 | 19% (56,018) |
| >45 and <=55 | 30% (86,649) |
| >55 and <=65 | 30% (86,457) |
| >65 and <=75 | 21% (62,489) |
|
| |
| Stage | |
| 0 | 33% (97,225) |
| 1 | 67% (194,388) |
|
| |
| Charlson Comorbidity Index | |
| 0 | 86% (249,995) |
| 1 | 12% (34,819) |
| 2 | 2% (6,799) |
|
| |
| Post-mastectomy radiation | |
| No | 94% (274,590) |
| Yes | 6% (17,023) |
|
| |
| Chemotherapy | |
| No | 76% (220,491) |
| Yes | 24% (71,122) |
|
| |
| Race | |
| White | 84% (245,200) |
| Black | 10% (28,985) |
| Other | 5% (14,454) |
|
| |
| Bilateral mastectomy | |
| No | 70% (202,964) |
| Yes | 30% (88,649) |
|
| |
| Facility type | |
| Academic | 32% (87,959) |
| Comprehensive community | 50% (136,829) |
| Community | 10% (25,739) |
| Integrated | 8% (21,649) |
|
| |
| Insurance | |
| Private | 66% (191,998) |
| Medicare | 25% (72,899) |
| Medicaid | 5% (15,374) |
| Uninsured | 2% (5,232) |
| Unknown | 2% (6,110) |
|
| |
| Geographic Region | |
| New England | 19% (53,104) |
| South Atlantic | 23% (61,480) |
| Mid-west | 25% (67,201) |
| South | 16% (44,010) |
| West | 17% (46,688) |
|
| |
| Rural-Urban | |
| Metropolitan | 86% (244,239) |
| Urban | 12% (34,077) |
| Rural | 2% (4,520) |
|
| |
| Income* | |
| $63,000+ | 40% (114,927) |
| $48,000-62,999 | 26% (74,794) |
| $38,000-$47,999 | 20% (58,564) |
| <$38,000 | 14% (40,355) |
|
| |
| % with high school education* | |
| <7% | 31% (89,895) |
| 7-13% | 32% (93,413) |
| 13-20% | 23% (65,846) |
| >=21% | 14% (39,585) |
Median household income and percentage of patients with high school education in zipcode area
We then generated unadjusted Join-point lines to represent the rates of immediate reconstruction associated with specified socioeconomic factors over time (Figure 1). The absolute rate of immediate reconstruction has increased during the study period for all patient groups. Further, the APC for each stratification group has remained relatively similar between 2004 and 2013, as demonstrated by the overlapping 95% confidence intervals. This, in conjunction with the pre-existing absolute differences across strata, has resulted in a persistent gap in receipt of reconstruction across strata.
Figure 1.
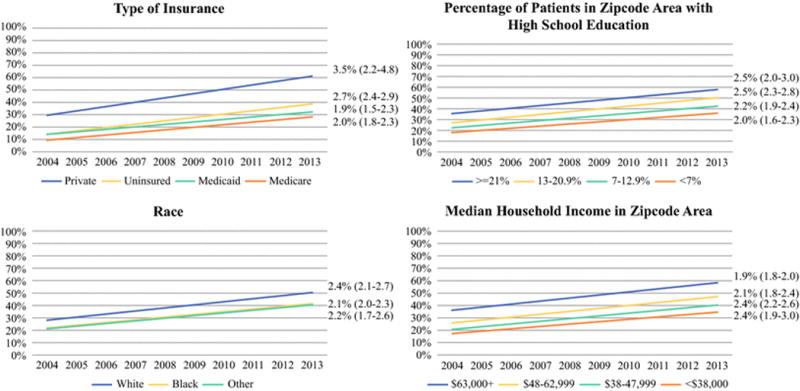
Trends in Rates of Immediate Reconstruction for Socioeconomic Factors
Factors Associated with Receipt of Immediate Reconstruction in a Contemporary Cohort
Our contemporary cohort of women diagnosed between 2010 and 2013 consisted of 145,577 patients. After controlling for age and clinical factors (comorbidities, tumor and treatment), the socioeconomic factors of race, type of insurance, income, and level of education were all strongly associated with receipt of reconstruction (Table 2).
Table 2.
Factors Associated with Receipt of Post-Mastectomy Reconstruction between 2010-2013
| Proportion with Reconstruction (N=145, 577) | Odds Ratio+ (95% Confidence Interval) | |
|---|---|---|
|
| ||
| Patient clinical and treatment factors | ||
|
| ||
| Age | ||
| <=45 | 63% | Ref |
| >45 and <=55 | 57% | 0.81 (0.78-0.85) |
| >55 and <=65 | 41% | 0.48 (0.46-0.50) |
| >65 and <=75 | 22% | 0.26 (0.25-0.27) |
|
| ||
| Charlson Comorbidity Index | ||
| 0 | 47% | Ref |
| 1 | 37% | 0.86 (0.83-0.89) |
| 2 | 24% | 0.58 (0.53-0.63) |
|
| ||
| Stage | ||
| 0 | 49% | Ref |
| 1 | 44% | 0.82 (0.78-0.87) |
|
| ||
| Bilateral mastectomy | ||
| No | 36% | Ref |
| Yes | 63% | 2.3 (2.3-2.4) |
|
| ||
| Post-mastectomy radiation | ||
| No | 46% | Ref |
| Yes | 35% | 0.54 (0.51-0.57) |
|
| ||
| Chemotherapy | ||
| No | 45% | Ref |
| Yes | 45% | 0.83 (0.81-0.86) |
|
| ||
| Patient Non-Clinical Factors | ||
|
| ||
| Race | ||
| White | 47% | Ref |
| Black | 38% | 0.88 (0.84-0.91) |
| Other | 38% | 0.60 (0.57-0.63) |
|
| ||
| Insurance | ||
| Private | 56% | Ref |
| Medicare | 25% | 0.65 (0.63-0.68) |
| Medicaid | 35% | 0.54 (0.51-0.57) |
| Uninsured | 30% | 0.43 (0.40-0.48) |
|
| ||
| Income* | ||
| $63,000+ | 55% | Ref |
| $48,000-62,999 | 44% | 0.75 (0.72-0.77) |
| $38,000-$47,999 | 37% | 0.64 (0.61-0.67) |
| <$38,000 | 32% | 0.58 (0.55-0.62) |
|
| ||
| % with high school education* | ||
| <7% | 55% | Ref |
| 7-13% | 47% | 0.94 (0.91-0.97) |
| 13-20% | 39% | 0.91 (0.87-0.95) |
| >=21% | 33% | 0.87 (0.83-0.92) |
|
| ||
| Rural-Urban | ||
| Metropolitan | 47% | Ref |
| Urban | 34% | 0.73 (0.70-0.76) |
| Rural | 30% | 0.65 (0.58-0.72) |
|
| ||
| Other | ||
|
| ||
| Facility type | ||
| Academic | 49% | Ref |
| Comp community | 43% | 0.88 (0.85-0.90) |
| Community | 28% | 0.53 (0.50-0.55) |
| Integrated | 53% | 1.3 (1.2-1.3) |
|
| ||
| Year of Diagnosis | ||
| 2010 | 43% | Ref |
| 2011 | 45% | 1.1 (1.0-1.1) |
| 2012 | 46% | 1.1 (1.1-1.18) |
| 2013 | 48% | 1.2 (1.16-1.24) |
Controlled for geographic region
All variables strongly associated with receipt of reconstruction at a p<0.0001
Median household Income and percentage of patients with high school education in zipcode area
Adjusted average predicted probabilities based on the multivariable logistic regression model facilitated interpretation of model results (Table 3). Patients who were white, had private insurance, and who lived in an area with the highest rates of high school education had the highest average predicted probability of reconstruction at 64.2% (95% confidence interval 63.2-65.1%). The lowest predicted probability of reconstruction was for patients with Medicaid insurance who lived in areas with the lowest rates of high school graduation; for these patients, predicted probability was 45.7% (43.9-47.4%) if white and 42.4% (40.5-44.3%) if black.
Table 3.
Predicted Probabilities of Receiving Immediate Reconstruction Based on Non-Clinical Factors
| Predicted Probability of Reconstruction % (95% Confidence Interval) | |||
|---|---|---|---|
| Highest Category of Education* | Lowest Category of Education* | ||
| Type of Insurance and Race | Private insurance, white |
64.2% (63.2-65.1) |
61.0% (59.8-62.3) |
| Private insurance, black |
61.1% (59.7-62.4) |
57.8% (56.3-59.4) |
|
| Medicaid insurance, white |
49.0% (47.4-50.7) |
45.7% (43.9-47.4) |
|
| Medicaid insurance, black |
45.7% (43.9-47.6) |
42.4% (40.5-44.3) |
|
Predicted probability of undergoing reconstruction, assuming a patient was <55 years of age, had a Charlson comorbidity score of 0, did not receive radiation or chemotherapy, and underwent a unilateral mastectomy at a metropolitan academic center in 2013.
Highest and lowest quartiles of percentage of patients in zipcode area with high school education
DISCUSSION
In this study of a national cancer registry, we demonstrated a continued increase in the rate of immediate reconstruction between 2004 and 2013. However, disparities in receipt of reconstruction for subgroups of patients persisted across the study period without evidence of improvement. In our adjusted model, being non-white, not having private insurance, and living in an area with lower median income and with lower rates of high school graduation were all strongly associated with lower likelihood of receiving immediate reconstruction. Importantly, we also determined that race and education were less significant contributors to receipt of reconstruction than other socioeconomic factors such as income and type of insurance.
Although the increases in the rate of immediate breast reconstruction observed in our study are promising, the ongoing disparities in the receipt of reconstruction for socioeconomically disadvantaged patients is significant. Our findings extend other studies that have identified disparities in receipt of breast reconstruction10–24 by demonstrating not only that the gap in receipt of reconstruction is evident in a contemporary patient cohort but also that the disparity has not narrowed over time. Given the known benefits associated with receipt of immediate breast reconstruction,1–4 it is critical that we as a surgical community move beyond simply describing that sociodemographic factors are associated with disparate care to understanding how these factors lead to the gaps in care in order to improve the quality of breast cancer care for disadvantaged patient populations.30
To provide a framework for understanding disparities in receipt of reconstruction, we developed a conceptual model based on the findings of this study and the literature to date.10–24 Our model, adapted from the Behavioral Change Wheel,31 posits that whether or not a patient undergoes reconstruction is determined by her Capability, Motivation, and Opportunity (Figure 2). Capability includes clinical factors that determine whether a patient is a good candidate for reconstruction, such as recommendation for post-mastectomy radiation or active smoking. However, this domain also includes education, as patients must have the knowledge that post-mastectomy reconstruction is a choice available to them, and empowerment, as patients must have the confidence to interact with their health care team to influence decision-making for reconstruction. Motivation reflects individual preferences for reconstruction based on factors such as body image but also more conscious decision-making around the feasibility for reconstruction based on competing priorities in a patient’s life. Finally, Opportunity reflects the context within which reconstruction is being considered, and includes the social and economic resources available to a patient (i.e. ability to leave work for frequent visits, finances for travel expenses, available child care) and access to specialty care (i.e. receiving a referral to see a plastic surgeon, having a plastic surgeon that accepts varied insurance types). Combined, these factors determine whether a patient undergoes breast reconstruction.
Figure 2.
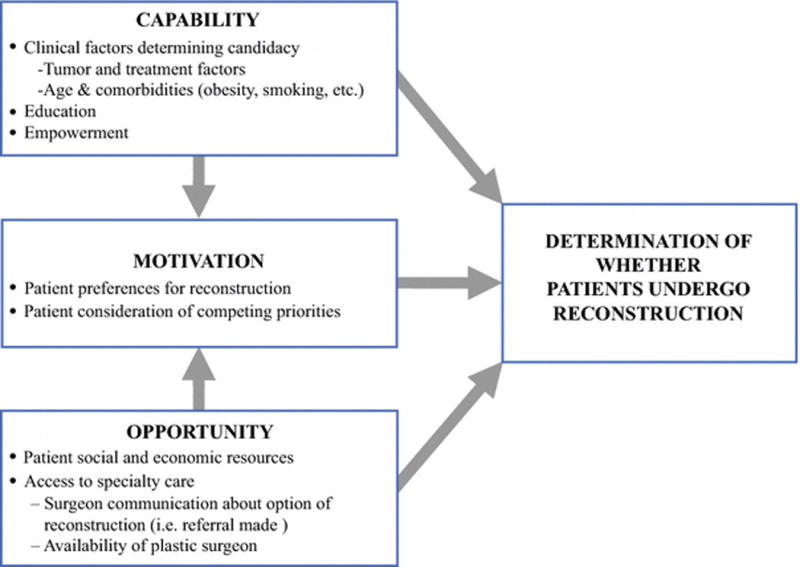
Proposed Framework for Understanding Disparities in Immediate Breast Reconstruction
This conceptual model provides a framework for the comprehensive evaluation of how socioeconomic factors influence receipt of immediate reconstruction and lead to disparities in care. Importantly, not all of these factors will be modifiable through interventions directed at the patient, provider or health care system. Understanding how these socioeconomic factors influence the receipt of breast reconstruction and determining which are modifiable through multi-level interventions are essential, albeit complex, steps towards improving the quality of care and reducing disparities in care for disadvantaged populations.
LIMITATIONS
Our study is limited by the specificity of the patient-level sociodemographic variables included in the NCDB. The NCDB reports education and income at the zipcode area level. Although this provides a general view of area-level socioeconomic factors, it may not accurately reflect socioeconomic conditions for an individual patient. We also were not able to assess how distance to reconstructive care may influence receipt of reconstruction. Finally, we cannot determine from the NCDB whether a referral to see a plastic surgeon was offered, whether a patient was offered reconstruction but declined, or whether delayed reconstruction was received.
CONCLUSIONS
Although rates of immediate reconstruction have increased dramatically over the past decade, lower rates persist for subgroups of disadvantaged patients. This gap in care quality is likely mediated by a mix of fixed and modifiable factors that may operate at the patient, provider and system levels. Understanding how socioeconomic factors influence receipt of immediate reconstruction and determining which are modifiable critical next steps towards identifying interventions that will improve the quality of breast cancer surgical care for disadvantaged populations.
SYNOPSIS.
Although immediate reconstruction rates have increased, lower rates persist for disadvantaged patients. Understanding how socioeconomic factors influence receipt of reconstruction and determining which are modifiable are critical steps towards identifying interventions to reduce disparities in breast cancer surgical care.
Acknowledgments
Financial Support: Research reported in this manuscript was funded through the Building Interdisciplinary Research Careers in Women’s Health Scholar Program (NIH K12 HD055894) and MT-DIRC Fellowship (R25CA171994). Additional funding came from NIH surgical oncology training award (T32CA090217).
References
- 1.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plastic and reconstructive surgery. 2000 Oct;106(5):1014–1025. doi: 10.1097/00006534-200010000-00010. discussion 1026-1017. [DOI] [PubMed] [Google Scholar]
- 2.Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Annals of surgery. 2008 Jun;247(6):1019–1028. doi: 10.1097/SLA.0b013e3181728a5c. [DOI] [PubMed] [Google Scholar]
- 3.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery. 2013 Aug;132(2):201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 4.Elder EE, Brandberg Y, Bjorklund T, et al. Quality of life and patient satisfaction in breast cancer patients after immediate breast reconstruction: a prospective study. Breast. 2005 Jun;14(3):201–208. doi: 10.1016/j.breast.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Nano MT, Gill PG, Kollias J, Bochner MA, Malycha P, Winefield HR. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ journal of surgery. 2005 Nov;75(11):940–947. doi: 10.1111/j.1445-2197.2005.03517.x. [DOI] [PubMed] [Google Scholar]
- 6.American College of Surgeons. National Accreditation Program for Breast Centers Standards Manual. Chicago, IL: American College of Surgeons; 2014. [Google Scholar]
- 7.Women’s Health and Cancer Rights Act (WHCRA) HR 4328. 1998 [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. Women’s Health and Cancer Rights Act (WHCRA) https://www.cms.gov/CCIIO/Programs-and-Initiatives/Other-Insurance-Protections/whcra_factsheet.html. Accessed April 7, 2017.
- 9.Assembly NYS. Assembly Bill A1009fB. 2010 https://www.nysenate.gov/legislation/bills/2009/A10094/amendment/B. Accessed April 7, 2017.
- 10.Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast cancer research and treatment. 2012 Nov;136(2):535–545. doi: 10.1007/s10549-012-2273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Mar 20;32(9):919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruper L, Holt A, Xu XX, et al. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Annals of surgical oncology. 2011 Aug;18(8):2158–2165. doi: 10.1245/s10434-011-1580-z. [DOI] [PubMed] [Google Scholar]
- 13.Sisco M, Du H, Warner JP, Howard MA, Winchester DP, Yao K. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. Journal of the American College of Surgeons. 2012 Nov;215(5):658–666. doi: 10.1016/j.jamcollsurg.2012.07.008. discussion 666. [DOI] [PubMed] [Google Scholar]
- 14.Yang RL, Newman AS, Lin IC, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013 Jul 1;119(13):2462–2468. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 15.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Annals of surgery. 2006 Feb;243(2):241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Nov 10;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2014 May-Jun;24(3):e261–269. doi: 10.1016/j.whi.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roughton MC, DiEgidio P, Zhou L, Stitzenberg K, Meyer AM. Distance to a Plastic Surgeon and Type of Insurance Plan Are Independently Predictive of Postmastectomy Breast Reconstruction. Plastic and reconstructive surgery. 2016 Aug;138(2):203e–211e. doi: 10.1097/PRS.0000000000002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng JF, Kronowitz SJ, Sun CC, et al. The effect of ethnicity on immediate reconstruction rates after mastectomy for breast cancer. Cancer. 2004 Oct 01;101(7):1514–1523. doi: 10.1002/cncr.20529. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Pappas L, Neumayer L, Agarwal J. An analysis of immediate postmastectomy breast reconstruction frequency using the surveillance, epidemiology, and end results database. The breast journal. 2011 Jul-Aug;17(4):352–358. doi: 10.1111/j.1524-4741.2011.01105.x. [DOI] [PubMed] [Google Scholar]
- 21.Offodile AC, 2nd, Tsai TC, Wenger JB, Guo L. Racial disparities in the type of postmastectomy reconstruction chosen. The Journal of surgical research. 2015 May 01;195(1):368–376. doi: 10.1016/j.jss.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. American journal of surgery. 2009 Aug;198(2):237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Wexelman B, Schwartz JA, Lee D, Estabrook A, Ma AM. Socioeconomic and geographic differences in immediate reconstruction after mastectomy in the United States. The breast journal. 2014 Jul-Aug;20(4):339–346. doi: 10.1111/tbj.12274. [DOI] [PubMed] [Google Scholar]
- 24.Yang RL, Newman AS, Reinke CE, et al. Racial disparities in immediate breast reconstruction after mastectomy: impact of state and federal health policy changes. Annals of surgical oncology. 2013 Feb;20(2):399–406. doi: 10.1245/s10434-012-2607-9. [DOI] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Annals of surgical oncology. 2008 Mar;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Statistics in medicine. 2009 Dec 20;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frasier LL, Holden S, Holden T, et al. Temporal Trends in Postmastectomy Radiation Therapy and Breast Reconstruction Associated With Changes in National Comprehensive Cancer Network Guidelines. JAMA oncology. 2016 Jan;2(1):95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer. 2015 Mar 01;121(5):747–757. doi: 10.1002/cncr.29134. [DOI] [PubMed] [Google Scholar]
- 29.Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958-2004) International journal of cancer. 2009 Jan 15;124(2):443–448. doi: 10.1002/ijc.23911. [DOI] [PubMed] [Google Scholar]
- 30.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. American journal of public health. 2006 Dec;96(12):2113–2121. doi: 10.2105/AJPH.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation science : IS. 2011 Apr 23;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]


