Summary
The Impaired Response Inhibition and Salience Attribution (iRISA) model proposes that impaired response inhibition and salience attribution underlie drug seeking and taking. To update this model, we systematically reviewed 105 task-related neuroimaging studies (N>15/group) published since 2010. Results demonstrate specific impairments within six large-scale brain networks (reward, habit, salience, executive, memory and self-directed network) during drug cue exposure, decision making, inhibitory control and social-emotional processing. Addicted individuals demonstrated increased recruitment of these networks during drug-related, but a blunted response during non drug-related processing, with the same networks also being implicated during resting-state. Associations with real-life drug use, relapse and therapeutic interventions and the relevance to initiation of drug use during adolescence support the clinical relevance of the results. While the salience and executive network showed impairments throughout the addiction cycle, the reward network was dysregulated at later stages of abuse. Effects were similar in alcohol, cannabis and stimulant addiction.
Keywords: fMRI, craving, marijuana, nicotine, smoking, cocaine, heroin, opioid, dependence, youth
In Brief
Zilverstand et al. find that behaviors in drug addiction are closely tied to brain impairments underlying drug cue reactivity, decision making, inhibitory control and social-emotional processing. Neuroimaging biomarkers can also be used to predict initiation and progression of drug addiction.
Introduction
Drug addiction is a disorder that encompasses excessive drug seeking and taking, as well as fundamental changes in cognition and emotional processing. Its core clinical symptoms and behavioral manifestations comprise of a chronically relapsing cycle of intoxication, bingeing, withdrawal and craving that propels uncontrollable drug use despite adverse consequences and a reduction in the pleasure derived from the drug. While much of the early research on drug addiction focused on understanding the rewarding properties of drugs, more recent research has made it increasingly clear that subtle cognitive and emotional impairments support the initiation, escalation and maintenance of this cycle. One model that describes the contribution of emotional and cognitive processes to drug addiction is the Impaired Response Inhibition and Salience Attribution (iRISA) model (see Goldstein and Volkow, 2002 with an update in 2011). This model proposed that impairments of two broad neuropsychological functions – response inhibition and salience attribution – and their underlying neural substrates contribute to craving, intoxication, bingeing and withdrawal across a broad range of substance addictions, including nicotine, alcohol and illicit drug addictions (Goldstein and Volkow, 2002, 2011). Its main hypotheses were based both on early neuroimaging work and seminal findings in animals suggesting that both the loss of inhibitory control and enhanced incentive salience of the drug propel drug seeking and taking (Jentsch and Taylor, 1999; Robinson and Berridge, 1993). The current review was conducted to update the iRISA model with the most recent evidence from the neuroimaging literature to characterize impairments of inhibition and salience processing across different drug addictions. It includes all neuroimaging research that used any task paradigm to investigate an addicted population since the year 2010. The included task paradigms ranged from reinforcer/reward related processing (e.g., drug cue exposure and monetary rewards), to decision making (e.g., choice and gambling paradigms), to inhibitory control (e.g., Stop Signal Task, Go/NoGo, Stroop tasks and cognitive reappraisal), to social-emotional tasks (both passive viewing and active), sensory processing, attention, working memory and learning tasks. To summarize this literature, we assessed brain function in drug addiction across a number of large-scale networks (introduced below), including findings from whole-brain analyses of significant group differences or group by task interaction effects. For a comprehensive review of the literature on animal studies, which is beyond the scope of this review, we refer the reader to three recent review papers on drug addiction endophenotypes (including cognitive/behavioral impairments) that are based on rodent and non-human primate models (Belin et al., 2016; Dalley et al., 2011; Everitt and Robbins, 2016).
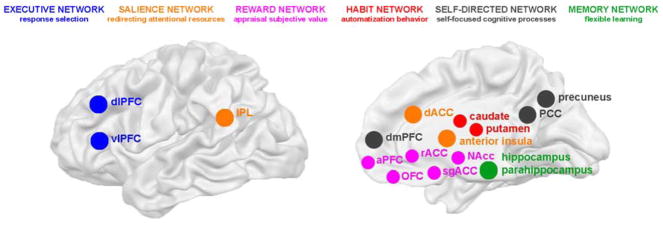
We organized the results across six large-scale brain networks that showed impairment of brain function in addiction in the studies summarized in this review: a) the “reward network”, which includes subcortical and cortical brain regions activated during the appraisal of subjective value [Nucleus Accumbens (NAcc)/ventral striatum, subgenual/rostral anterior cingulate (sgACC/rACC), anterior prefrontal cortex (aPFC) and orbitofrontal cortex (OFC)]; b) the striatal “habit network”, which underlies learning of automatized behavior (dorsal caudate and putamen); c) the “salience network”, regions involved in (re)directing attentional resources toward salient stimuli [anterior insula, dorsal anterior cingulate (dACC) and inferior parietal lobule (IPL); often also named the ventral frontoparietal attention network]; and d) the “executive network”, which supports the selection of possible behavioral responses [ventrolateral prefrontal cortex (vlPFC) and dorsolateral prefrontal cortex (dlPFC), often also named the inhibitory control network]. Two additional networks, which were found to be relevant to brain function in drug addiction, but were not discussed in our prior reviews of the iRISA model are e) the “self-directed network”, which is activated during self-directed/referential cognitive processes [dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC) and precuneus, often also named the Default Mode Network]; and f) the “memory network” involved in flexible, multi-cue learning and memory (hippocampus, parahippocampus, rhinal and retrosplenial cortex). As these brain networks encompass impaired brain function in addiction on a whole-brain level, this review is the first systematic attempt to integrate what we know about the function of each of these networks into a comprehensive model underlying drug addiction symptomatology. All studied brain networks are summarized in Figure 1.
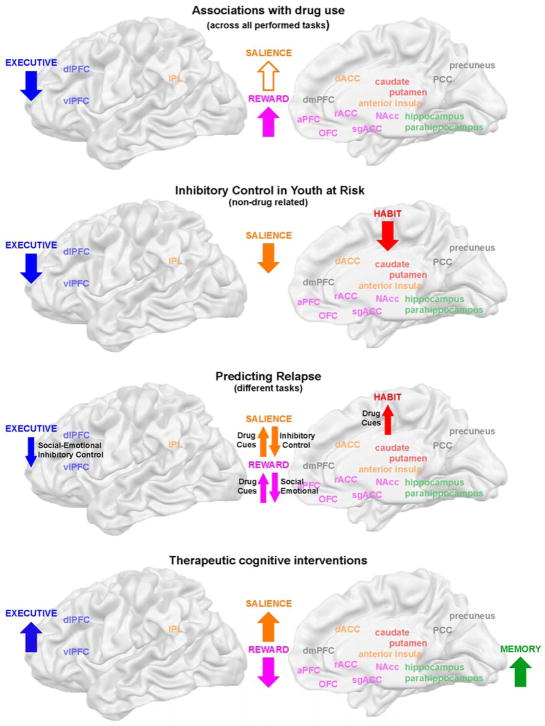
Figure 1. Aberrant brain networks in addiction.
Results were integrated for six large-scale brain networks that showed aberrant activation levels in individuals with addiction (color coding is the same for all figures and tables). Here, we also indicate which task aspect each brain network supports based on our current understanding of the basic neuroscience literature on animals and healthy humans (as described in the introduction).
The delineation of these six brain networks was in part driven by the patterns of co-activation observed in our review (generally the regions within the defined networks were found to be consistently co-activated, showing either hypo- or hyperactivation depending on the task context), but was foremost informed by our current understanding of the underlying function of each of these networks according to the basic neuroscience literature on brain function in animal models and healthy humans. We postulate that each brain network supports a certain task-related dimension, independent of the specific task performed (e.g., the visual system always supports visual scene analysis, whereas the reward network supports the appraisal of subjective value, independent of the task performed). As the reviewed tasks were highly complex, hence involving many brain networks, we interpret aberrant activation in a specific brain network as an impairment of that specific task aspect (e.g., aberrant activation of the reward network was interpreted to suggest impaired subjective value appraisal, independent of the actual task performed, such as for example a drug cue exposure or a monetary reward gambling task). Understanding the core function of each brain network was thus crucial to our understanding of the impairments observed in drug addiction, and is hence described here in some detail.
The neuroscience literature suggests that the reward network (NAcc/sgACC/rACC/OFC/aPFC) supports the appraisal of subjective value by integrating incentive motivational value in the NAcc (Milton and Everitt, 2012), computing subjective value and reward expectations or, more broadly, estimating expected outcomes in the rostral cingulate (sgACC/rACC) and OFC (Chase et al., 2015; Koechlin and Hyafil, 2007; Schoenbaum et al., 2016; Sescousse et al., 2013) and representing abstract subjective value (e.g., secondary rewards) in the aPFC (Badre, 2008; Koechlin and Hyafil, 2007; Sescousse et al., 2013). In human neuroimaging studies, the reward network shows a stronger response to salient positive as compared to negative events, in contrast to the salience network (insula/dACC), which reacts strongly to any salient stimulus, independent of valence (Bartra et al., 2013). In drug addiction, the reward network has been implicated in reinforcement learning (NAcc; Milton and Everitt, 2012), delay discounting (NAcc/OFC/sgACC; Robbins et al., 2012) and drug cue exposure, with its activation levels correlating with self-reported craving and urge to use (NAcc/rACC; Kühn and Gallinat, 2011; Wilson and Sayette, 2014), in line with a primary function of this network in integrating and representing subjective value.
The habit network (dorsal caudate/putamen) has been shown to underlie stimulus-response learning (Milton and Everitt, 2012), and as such drives the automatization of behavior (Everitt and Robbins, 2005), with the caudate being implicated in the initiation of behavior and the putamen specifically supporting habit learning (Grahn et al., 2008). In addicted individuals, an increased involvement of the habit network seems to underlie the transition from voluntary goal-directed behavior to compulsive drug-seeking and taking (Everitt and Robbins, 2005, 2016), consistent with a primary role of this network in strengthening non-voluntary, automatic behavior.
The salience network (insula/dACC/IPL) encompasses brain regions that react to highly relevant stimuli (Corbetta et al., 2008), independent of their valence (Bartra et al., 2013), with the insula most likely integrating interoceptive information (Naqvi and Bechara, 2009; Sutherland et al., 2012) and the dACC and IPL having a core function in the allocation of attentional control (Corbetta et al., 2008; Shenhav et al., 2013). In drug addiction, activation levels in this network correlate with self-reported craving (insula/dACC/IPL; Kühn and Gallinat, 2011) as well as the urge for drug seeking (Naqvi and Bechara, 2009), supporting an involvement of this network in redirecting attentional resources.
The executive network (vlPFC, dlPFC) has been suggested to play a primary role in the representation and maintenance of goals during motivated behavior, hence supporting the selection of a behavioral response (Badre, 2008; Koechlin and Hyafil, 2007; Miller and Cohen, 2001). In animals, as well as humans, this network supports the inhibition of motor and cognitive responses and as such is also involved in tasks requiring cognitive flexibility (Aron et al., 2014; Jentsch and Taylor, 1999; Robbins et al., 2012). Both the dlPFC and vlPFC have been implicated in impaired inhibitory control in drug addiction, showing hypo-activation during motor response inhibition tasks (Luijten et al., 2014) and cognitive self-regulation (Zilverstand et al., 2017), which is in line with a crucial role of this network in response selection.
The self-directed network (dmPFC/PCC/precuneus) has been linked to self-focused processing, particularly to supporting higher cognitive regulatory functions such as self-awareness and self-reflection (Murray et al., 2012; Northoff et al., 2006). Evidence implicates the dmPFC in the representation of self-relevant stimuli (Abraham, 2013; Murray et al., 2012), whereas PCC and precuneus may have a more general function in evaluating familiarity and personal relevance (Qin and Northoff, 2011). In addicted individuals, activation levels in this network have been shown to correlate with self-reported craving and the urge to use (Kühn and Gallinat, 2011; Wilson and Sayette, 2014), aberrant activity in this network has been linked to a decreased ability to efficiently engage in non-self-directed cognitively demanding tasks (Sutherland et al., 2012) and to deficient tagging of self-relevance and impaired self-awareness (Lucantonio et al., 2014; Moeller and Goldstein, 2014), stressing the role of this network in self-regulatory/referential processes.
The memory network (hippocampus, parahippocampus, rhinal and retrosplenial cortex) represents flexible, relational memory (Doll et al., 2015; Ritchey et al., 2015), by supporting associative learning of multiple, rather than single cues (Eichenbaum et al., 1990). This network is involved in the flexible use of learned information as compared to repetitive behaviors (Eichenbaum et al., 1990; Packard, 2009; Schwabe, 2013), supporting adaptive higher cognitive functions (Schwabe, 2013) and playing an important role in voluntary, goal-directed behavior (Doll et al., 2015). While the involvement of this network in drug addiction has been studied to a lesser extent than the other described brain networks, there is evidence suggesting that stress impacts the relative use of this system, such that it shifts learning and memory away from these more flexible, “cognitive” systems towards the more reflexive, “habitual” processes supported by the habit network (Schwabe, 2013, 2017).
The current review summarizes the findings across these six brain networks and integrates the recent literature into the iRISA model. First, the iRISA model predicts that individuals with addiction demonstrate increased recruitment of relevant brain networks during drug-related processing, while showing decreased engagement of relevant brain networks when drug-related stimuli are absent (Goldstein and Volkow, 2011). Second, the model proposes that impaired salience attribution (drug > all other non-drug reinforcers) would be associated with an abnormal engagement of the reward, salience and habit networks concomitantly associated with impairments in response inhibition, highlighting the importance of the executive network in drug addiction (Goldstein and Volkow, 2011).
Results
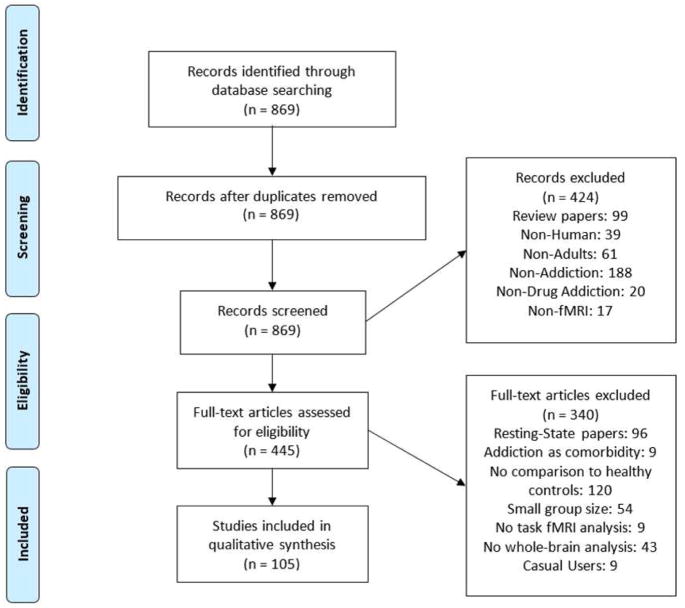
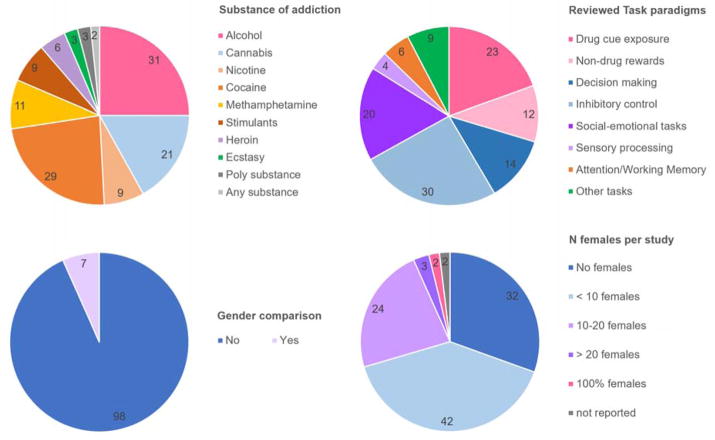
After an extensive literature search (869 studies were identified through Pubmed/Medline), we identified 105 large studies (n>15 per group) using a task paradigm, which included a comparison with a control group and performed corrected whole-brain analyses (see Methods and PRISMA Flow Diagram, Figure 2). The search was limited to recently published articles (2010 – 2017). We reviewed a total of 118 between-group analyses, as 13 studies used multiple task paradigms (Suppl. Table 1 + 2, for a complete reference list see Suppl. Material 3). About half (47%) of the studies investigated stimulant addiction (substances: 23% cocaine, 9% methamphetamine, 7% any stimulant use, 7% nicotine) with the other half being divided to alcohol (25%), cannabis (17%), heroin (5%), ecstasy (2%) or other substance addictions (4%) (Figure 3). Considering the prevalence of use and harm inflicted at both the individual and societal levels, it is clear that nicotine and opiate addiction are disproportionally underrepresented in the recent literature [prevalence current use nicotine: 23.5% of US population, NSDUH 2016: https://www.samhsa.gov; >50% of overdose deaths involve opioids (Rudd et al., 2016)]. Remarkably, while 51 studies on nicotine addiction were identified in the search, the majority (59%) lacked a comparison to healthy controls and another 22% had a small sample size or did not perform a whole-brain analysis. Further, smokers were generally included based on cigarette count per day (>12 cigarettes), rather than diagnostic interviews. Similarly, research into cannabis addiction used less stringent inclusion criteria; only 10% of the studies included cannabis users diagnosed with “dependence” using standard clinical interviews. Across all addicted populations women were grossly undersampled, with – on average - females constituting only 25% of the included participants. Overall, 30% of studies did not include any females, 40% included one to nine females, so in total 70% of studies included less than ten females in their study design (Figure 3). Only 7% of the reviewed studies conducted gender comparisons, and only three of the reviewed studies were well powered for conducting gender comparisons (N>20 females). Further, there were strong geographic biases in the studied populations (Suppl. Table 2). The large majority of studies on illicit stimulant use (78% of studies on cocaine, methamphetamine or any stimulant use), but none of the recent research on heroin addiction and only a minority of studies on alcohol (32%) and cannabis (38%) abuse, were performed in the United States (other countries with a large number of studies included China, Korea, the United Kingdom, the Netherlands, Germany and Spain). These geographic biases are likely linked to differences in socio-cultural-economic factors (e.g., health care), genetic predispositions (e.g., alcohol flush response in Asians) and recruitment strategies per country (e.g., biases in sampled socioeconomic status). Moreover, geographic biases were often much stronger when studies were split according to the task paradigms (e.g., all alcohol and cannabis studies on attention and working memory were performed in Germany/the Netherlands, whereas US research studied attention in smokers only; in contrast, all studies on sensory processing were performed in individuals with illicit stimulant addiction in the US). Finally, drug use status on the day of scanning differed systematically depending on the primary drug of choice. Alcohol users were generally scanned abstinent, whereas smokers were often imaged immediately after smoking. Individuals with heroin addiction usually participated in methadone maintenance programs, whereas cocaine users, similarly to the alcohol users, were imaged abstinent. Studies on cannabis and stimulants other than cocaine were distributed evenly between abstinent and non-abstinent individuals.
Figure 2. PRISMA Flow Diagram.
The search in Pubmed/Medline identified 869 studies. We included 105 large studies (n>15 per group) that used a task paradigm comparing an addicted population to a control group through corrected whole-brain analyses.
Figure 3. Investigated Populations and Task Paradigms.
The majority of studies investigated stimulant addiction and the most frequently used task paradigm was inhibitory control. Only 7% of the reviewed studies conducted gender comparisons, with only three studies well powered for conducting such comparisons (N> 20 females).
The main focus of the current review was thus to integrate the findings per task paradigm and across addicted populations, focusing on converging effects between different addicted populations rather than on comparisons between them. Overall, the selected studies used a wide array of tasks, most often investigating inhibitory control (26%), then drug cue exposure (19%), social-emotional task paradigms (16%), decision making (12%) and non-drug reward processing (10%), while fewer studies looked into attention or working memory (5%), sensory processing (3%) and other (often learning) tasks (9%) (Figure 3). With the exception of studies investigating “other tasks”, the results are summarized below (see Suppl. Table 1 for the results on the network level and Suppl. Table 2 for the results per brain region).
Drug cue exposure
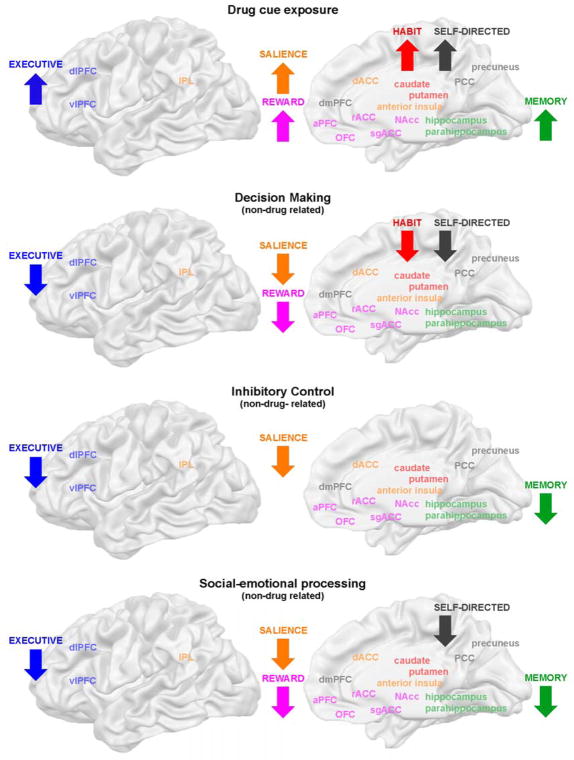
In total, 23 studies investigated the effect of presenting drug-related cues as compared to neutral stimuli (generally visually presented) to individuals with alcohol, cannabis, cocaine, ecstasy, heroin, methamphetamine or nicotine addiction. While most studies employed passive viewing paradigms, some integrated drug cue exposure with a decision making or inhibitory control task. The large majority of studies reported significant behavioral effects, such as increased subjective craving, increased endorsement of the drug or increased approach behavior during drug cue presentation in individuals with addiction (Suppl. Table 1). Further, 70% of studies reported significant group differences in brain function at a whole-brain level, most consistently reporting an upregulation of the reward network (ventral striatum, rACC/sgACC, dmPFC, OFC and aPFC; 10 out of 16 studies with any whole-brain effects) during drug cue exposure (Suppl. Table 1). This upregulation of the reward network was often accompanied by a significant increase of activation levels in the salience, habit, executive, self-directed and memory networks, with the effects in the salience network being second most consistent (anterior insula, dACC and IPL; reported in 50% of studies with significant results at the whole-brain level; see Figure 4a for a visual summary of the effects). This pattern of upregulation of multiple brain networks during drug cue exposure was found in cannabis, cocaine and heroin users, with effects being most evident in heroin users, independent of drug use status (3 out of 4 studies; Li et al., 2012, 2015; Tabatabaei-Jafari et al., 2014; Wang et al., 2014). In alcohol and nicotine addiction effects seemed to be somewhat weaker, as many studies did not report any significant findings or only reported upregulation of the reward network (Suppl. Table 1).
Figure 4. Aberrant brain activation patterns during task performance.
We found consistent impairments in brain function in six large-scale brain networks during four different tasks, suggesting broad multi-faceted impairments of multiple domains across tasks. While the involvement of specific brain networks was task-specific, we generally observed increased engagement of relevant brain networks during exposure to drug cues, but a blunted brain response during non-drug related tasks, as predicted by the iRISA model. These aberrant brain activation patterns were observed across addictions, independent of use status.
In summary, individuals with drug addiction demonstrated hyperactivation of the reward, salience, habit, self-directed, memory and executive networks during drug cue exposure (Figure 4a), networks which are involved in the appraisal of the subjective value of the drug cue encountered, automatization of the reaction towards this cue, directing attentional resources towards processing a cue, selecting or inhibiting a behavioral response, activating self-focused cognitive processes, as well as flexible learning and memory. We propose that these networks engage each other when an addicted individual encounters a drug-related cue, in accordance with the main hypothesis of the iRISA model, which states that drug-related processing is associated with an increased engagement of relevant brain networks. Interestingly, the large number of networks involved demonstrates the complexity of the individual’s reaction during passive viewing. Specifically, our results indicate that even a “simple” craving provocation task involves brain systems beyond the networks mostly studied in addiction research [e.g., brain regions involved in value appraisal, habit learning and response inhibition (Belin et al., 2016; Everitt and Robbins, 2016)] to encompass brain systems supporting higher cognitive processes (Goldstein and Volkow, 2011). Results were in accordance with previous neuroimaging meta-analyses, which have reported that the cue reactivity effect is anchored in the ventral striatum, but extends to multiple large-scale brain networks (Chase et al., 2011; Kühn and Gallinat, 2011). Given the involvement of multiple “higher cognitive” brain systems, this result underscores the need for more research into the functional role of networks such as the self-directed and memory network in addiction.
Non-drug rewards
The response to non-drug rewards (usually monetary rewards) was investigated by 12 studies conducted in individuals with alcohol, cannabis or cocaine addiction. As recent research demonstrates a possible impairment of decision making processes in addiction (Verdejo-Garcia et al., 2017), we separately reviewed non-drug related reward processing without (reviewed here) or with a decision making framework (reviewed below). The ”non-decision making” reward tasks reviewed here generally consisted of an anticipation phase, during which win or loss expectancies were created, and an outcome phase, during which win or loss outcomes were realized. The most commonly used task was the Monetary Incentive Delay (MID) task, which signals to participants which phase they are in (win or loss) and determines the outcome of each trial depending on how quickly participants respond to a short target with variable onset (Knutson et al., 2000). The majority of the reviewed studies (9 out of 12) did not report significant differences in behavioral performance between addicted individuals and healthy controls (Suppl. Table 1). Moreover, only 50% of the reviewed studies reported any significant effects on brain function at the whole-brain level, with mostly contradictory results (Suppl. Table 1). None of the significant brain effects reported during either task phases (anticipation vs. outcome) or reward condition (win vs. loss) were reliably (without contradiction) replicated across two studies.
Decision making
Non-drug reward processing (monetary rewards) within a decision making framework was investigated by another 14 studies, conducted in alcohol, cannabis, cocaine, heroin, methamphetamine or stimulant addiction. All tasks again included an anticipation and outcome phase, as well as win and loss conditions, but now also incorporated a choice component that allowed for assessing gambling behavior. The most commonly used task paradigm was the Iowa Gambling Task (IGT), which required participants to choose between smaller immediate monetary rewards with occasional small losses that yield a net gain and larger immediate rewards with occasional large losses that yield a net loss (Bechara et al., 1994). Overall, the quality of the reviewed studies was high, with the large majority of studies using well matched (narrow) control conditions (e.g., contrasting low with high reward, or low with high risk conditions). In comparison to monetary reward tasks without a choice component, the majority of the studies reviewed here (10 out of 14) reported significant behavioral effects (Suppl. Table 1). Addicted individuals reacted more slowly, were slower to learn reward contingencies, incurred significantly greater losses, showed less adaptation of their behavior after losses, selected more risky choices after losses and selected more risky choices overall. The most robust findings were greater overall losses and a tendency to choose more risky options (Arcurio et al., 2015; Beylergil et al., 2017; Gowin et al., 2017; Koester et al., 2013; Kohno et al., 2014; Tanabe et al., 2013; Wesley et al., 2011; Yamamoto et al., 2014). Further, 100% of the reviewed studies reported significant brain effects at a whole-brain level. While only three studies reported (inconsistent) effects related to win anticipation or when realizing win outcomes, observed brain patterns when anticipating or realizing negative outcomes were very consistent. Specifically, during loss anticipation, when realizing losses or tracking prediction errors, individuals with addiction demonstrated a disengagement of the salience (anterior insula, dACC and IPL), reward (ventral striatum, rACC/sgACC, dmPFC, OFC and aPFC) and executive networks (vlPFC/dlPFC), with some studies additionally finding reduced recruitment of the habit (dorsal striatum) and self-directed (PCC/precuneus) networks (Suppl. Table 1, Figure 4b). Such a pattern of disengagement was consistently observed across all studies that reported any significant brain effects for these phases (Beylergil et al., 2017; Gowin et al., 2017; Gradin et al., 2014; Harle et al., 2014; Stewart et al., 2014; Tanabe et al., 2013; Wesley et al., 2011; Worhunsky et al., 2017). Findings converged between “traditional” contrast analyses and more sophisticated computational approaches that investigated brain regions involved in tracking prediction errors by Bayesian modelling (Beylergil et al., 2017; Tanabe et al., 2013). Results were further consistent across the range of addictions studied, with no evidence for an influence of drug use status (Suppl. Table 1). Finally, while there were few effects during the decision making phase, two studies reported disengagement of the executive network (vlPFC/dlPFC) during high risk or difficult decisions (Kohno et al., 2014; Meade et al., 2017).
In summary, we found that gambling with monetary rewards led to a disengagement of brain networks particularly during the anticipation and realization of monetary loss. The current review thus points to an intriguing specificity of (strong) group differences for risky or difficult decisions and negative outcomes. The observed pattern of disengagement reported involved the reward, salience and executive networks - suggesting an impairment of value appraisal, salience attribution and response inhibition - but extended to the habit and self-directed networks (Figure 4b), thus also involving brain systems that are crucial in evaluating behavioral outcomes, learning and self-regulation. While we did not observe consistent brain effects during win anticipation or positive outcomes, a recent, more focused meta-analysis that specifically looked at low-risk trials with positive outcomes in monetary gambling tasks, demonstrated a similarly blunted brain response during anticipation of positive outcomes in drug addicted individuals (Luijten et al., 2017). Taken together, results suggest that a blunted brain response in situations of uncertainty – when making decisions - and after realized negative outcomes may potentially underlie persistent drug use despite catastrophic consequences in addiction. Overall, the results also confirm the predictions made by the iRISA model, with the reduced engagement of crucial brain networks when addicted individuals were gambling for monetary rewards (non-drug related processing) standing in stark contrast to the hyper-engagement of the same brain networks during drug-related processing. Our results support a multi-faceted account of the functional impairment in drug addiction, involving multiple brain systems beyond the ones studied most intensively to date. As such, the results presented here converge with accounts that conceptualize decision making as a multistage process that entails the subjective assessment of value and self-relevance, the modelling of a possible outcome space, the selection and inhibition of potential responses and instrumental as well as flexible learning processes (Doll et al., 2015; Lucantonio et al., 2012; Schultz, 2000; Shohamy and Daw, 2015). More refined computational modelling approaches (which may require larger sample sizes than in the reviewed studies) may help to disentangle the specific processes involved in the future.
Inhibitory control
The most commonly used task paradigm in the reviewed literature was response inhibition. A total of 30 studies investigated inhibitory control in individuals with alcohol, cannabis, cocaine, ecstasy, methamphetamine, nicotine or stimulant addiction. The large majority of these studies measured brain function during inhibition of a motor response, using the Stroop, Flanker, Go/NoGo or Stop Signal Task. These button press tasks generally create a conflict condition, in which withholding a motor response is difficult and hence requires inhibitory control. In some studies, task difficulty was increased by using drug-related stimuli during the conflict condition. Further, two studies investigated cognitive inhibitory control with an emotion reappraisal paradigm (Albein-Urios et al., 2012; Zimmermann et al., 2017). Out of the 30 reviewed studies, only 13 studies reported a significantly impaired performance in individuals with addiction, measured either by slower responses and lower accuracy in button-press tasks or by self-report during cognitive reappraisal tasks (Suppl. Table 1). Nevertheless, 77% of the studies reported significant brain effects at the whole-brain level. During the conflict/reappraisal condition, individuals with addiction consistently showed significantly decreased recruitment of the salience network (anterior insula, dACC, IPL; Albein-Urios et al., 2012; Czapla et al., 2017; Fryer et al., 2013; Harle et al., 2014; Jan et al., 2014; Kober et al., 2014; Luijten et al., 2013; Moeller et al., 2014a, 2014b), often accompanied by significantly decreased engagement of the executive (vlPFC/dlPFC) and memory (hippocampus/parahippocampus) networks (Suppl. Table 1, Figure 4c). Importantly, studies that did not contrast the conflict condition with a neutral control condition (when looking at group differences) generally reported negative findings or findings that did not converge with other studies (Hester et al., 2013; Moeller et al., 2012; Zimmermann et al., 2017), whereas well controlled studies or studies using computational modelling approaches reported more extensive effects implicating the salience, executive and memory networks during conflict/conflict anticipation (Harle et al., 2014; Hu et al., 2015; Kober et al., 2014), and the executive and memory network during successful stops (Harle et al., 2014). This overall pattern was similar across different types of drug addiction, with the exception of one study conducted in young, highly-educated ecstasy users, who did not demonstrate a behavioral impairment of inhibitory control and showed an increased engagement of the executive and salience networks, potentially indicating a preserved compensatory response (Roberts and Garavan, 2010). Similar effects were reported independent of drug use status.
The hypoactivation of relevant brain networks during inhibitory control provides yet another example of a blunted brain response during non-drug related processing (when participants are not engaged by drug cues), as predicted by the iRISA model. The results suggest impairments in re-directing attentional resources, response selection (inhibition) and flexible learning during response inhibition. Hence, the empirical evidence again supports multifaceted impairments in addiction, even for relatively simple tasks. In contrast to the previous tasks, the reward network was not implicated during inhibitory control, possibly because most studies did not employ drug stimuli or other reinforcers and thus did not entail an appraisal aspect. Studies in which drug stimuli were integrated did show aberrant involvement of the reward network (Czapla et al., 2017; Tomasi et al., 2010). Overall, results were consistent with previous neuroimaging reviews and meta-analyses that have implicated either the executive network (Zilverstand et al., 2017), or both the salience and the executive networks as the anchors of an impaired inhibitory control effect in drug addiction (Goldstein and Volkow, 2011; Luijten et al., 2014). They also corroborate reviews of the animal literature, which have suggested that both the salience and executive network support motor response inhibition (Dalley et al., 2011; Ersche et al., 2012a). However, in contrast to previous reviews and meta-analyses, the results presented here additionally suggest an involvement of the memory network in impaired inhibitory control, potentially underlying impaired learning during task performance.
Social-emotional processing
Twenty studies explored brain function by employing social-emotional stimuli (faces or interactive scenes) to provoke an emotional reaction in individuals with alcohol, cannabis, cocaine, heroin, methamphetamine and nicotine addiction. The large majority of studies used passive (picture viewing) or relatively easy tasks (e.g., evaluating if a picture was taken indoors). While all included studies investigated reactivity to aversive stimuli, eight research groups additionally included stimuli with positive valence or drug stimuli (reviewed above). As many studies did not ask participants to rate the emotional valence of the presented stimuli, only four studies reported significant group differences in perceived emotional intensity, with addicted individuals showing higher distress, state anxiety and lower positive emotion (Daughters et al., 2017; Lee et al., 2013; Schmidt et al., 2014; Seo et al., 2013; Suppl. Table 1). Significant brain effects at the whole-brain level were found in 95% of studies. Independent of the task (passive viewing, non-emotional or emotional) and valence of the stimuli (pleasant or unpleasant), studies consistently reported a downregulation of the reward (ventral striatum, rACC/sgACC, dmPFC, OFC and aPFC), salience (anterior insula, dACC, IPL) and executive (vlPFC/dlPFC) networks during social-emotional processing, often accompanied by reduced recruitment of the memory (hippocampus/parahippocampus) and self-directed (PCC/precuneus) networks (Asensio et al., 2010; Caldwell et al., 2015; Canterberry et al., 2016; Costumero et al., 2017; Gilman et al., 2010; Hong et al., 2017; Kim et al., 2014; Landi et al., 2011; Maurage et al., 2012; Payer et al., 2012, 2011; Roberts and Garavan, 2013; Seo et al., 2013; Wesley et al., 2016; Suppl. Table 1, Figure 4d). Well-controlled studies using narrow contrasts and active task paradigms (Caldwell et al., 2015; Maurage et al., 2012) or highly salient stimuli (e.g., infant cries or erotic stimuli: Costumero et al., 2017; Landi et al., 2011) generally showed the most extensive effects implicating several brain networks. Compared to nicotine/cocaine addiction, brain effects in alcohol/cannabis addiction were somewhat less extensive, with specifically the reward network being less consistently implicated (Suppl. Table 1). No effects were reported in the single study investigating heroin addiction (Suppl. Table 1). As with other tasks, effects did not seem to be modulated by drug use status.
The observed disengagement of multiple brain networks during social-emotional processing presents a third example of a blunted response during non-drug related processing, as suggested by the iRISA model. Again, individuals with addiction demonstrated reduced engagement of multiple brain circuits involved in the appraisal of subjective valence, assessment of self-relevance, redirection of attention, selection (inhibition) of an appropriate response and flexible learning. Slight differences between different addicted populations (which need to be evaluated in direct comparisons in the future) suggest that differences in the preferred primary drug of choice (e.g., stimulants vs. other drugs) may particularly affect social-emotional processing, as previously proposed in a review of the animal literature, which concluded that opioids may have a less interruptive effect on social interaction as compared to other drugs (Blanco-Gandía et al., 2015). Overall, the results corroborate the role of affective dysregulation in the maintenance of drug addiction (Cheetham et al., 2010; Mantsch et al., 2016; Sinha, 2008; Zilverstand et al., 2017) and suggest that the impairment in brain function in drug addicted individuals during social—emotional processing is characterized by a shift away from non-drug related rewards (such as social rewards) to drug-related rewards (Goldstein and Volkow, 2011; Heilig et al., 2016).
Sensory processing
Four studies investigated sensory processing in methamphetamine and stimulant addiction. Two studies employed a breathing paradigm, which creates aversive sensations, while the other two studies used a soft touch paradigm, inducing pleasant sensations. Brain function during the sensory stimulation phase was compared with a resting phase. In all four studies, individuals with addiction did not report differences in the subjective sensory experience (Suppl. Table 1). However, there were significant brain effects, indicating decreased engagement of the sensory cortices, the salience (anterior insula, dACC, IPL) and executive (vlPFC/dlPFC) networks (May et al., 2013; Stewart et al., 2014, 2015) in addicted individuals. During aversive sensory processing, reduced activation levels in the reward network (ventral striatum, dmPFC, aPFC) were also observed (Stewart et al., 2014, 2015; Suppl. Table 1). In summary, observed impairments were again characterized by a disengagement of brain networks during non-drug related processing, specifically in regions supporting valence appraisal, attentional processes and response selection.
Attention and Working Memory
We identified six studies investigating attention or working memory in individuals with alcohol, cannabis or nicotine addiction. The low number of studies was surprising, particularly as no recent studies were performed in illicit drug users (beyond cannabis). From the six reviewed studies, only one reported an effect of chronic drug use on behavioral performance (Suppl. Table 1), with smokers showing improved accuracy during an attention task compared to non-smoking controls (Rose et al., 2010). Further, none of the studies reported alterations in brain function that were specific to a task condition which required increased attention or working memory load (Suppl. Table 1).
Associations with drug use
Across all different task paradigms reviewed, activation levels in the reward network (ventral striatum, rACC/sgACC, dmPFC, OFC and aPFC) showed significant associations with drug use in 24% of the studies with significant whole-brain effects, even when results from region-of-interest analyses were excluded (only regions from significant whole-brain analyses were included in Suppl. Table 1). Engagement of the reward network was consistently positively correlated with craving, addiction severity, use duration, frequency of use and relapse in alcohol, cannabis, cocaine, heroin and nicotine addiction, independent of the employed task paradigm (Costumero et al., 2017; Cousijn et al., 2013a, 2013b, 2014; Filbey et al., 2016; Fryer et al., 2013; Gowin et al., 2017; Hong et al., 2017; Kim et al., 2014; Kober et al., 2014; Li et al., 2015; Seo et al., 2013; Worhunsky and Stevens, 2013; Suppl. Table 1, see Figure 5a for a visual summary). Further, participation in cognitive-behavioral therapy reduced activation levels in the reward network (Worhunsky et al., 2013; Suppl. Table 1). These effects were consistently reported across all different addictions with the exception of methamphetamine dependence (studies in this population did not report investigating brain-drug use correlations). While correlations between reward network activations and drug use were found during cue exposure, decision making, inhibitory control, attention, working memory and social-emotional tasks, the cue exposure task seemed to be the best suited to probe associations based on the number of studies reporting these correlations in whole-brain analyses. Beyond the reward network, the second most consistent link between drug use and brain function was found for the executive network (vlPFC/dlPFC). Severity of addiction was linked to a disengagement of this network (Cousijn et al., 2012; Harle et al., 2014; Vollstädt-Klein et al., 2011), while abstinence and treatment upregulated it (Costumero et al., 2017; Gradin et al., 2014; Kober et al., 2014; Tabatabaei-Jafari et al., 2014; Worhunsky and Stevens, 2013; Suppl. Table 1, Figure 5a). This effect again spanned addictions, being reported in cannabis, cocaine, heroin, nicotine and stimulant users. Beyond the reward and executive networks, activation levels in the salience network (anterior insula, dACC, IPL) were consistently increased with more frequent use in cannabis addiction (Cousijn et al., 2013b, 2014; Ford et al., 2014; Suppl. Table 1, Figure 5a). Reports on the effect of craving, withdrawal, lifetime use and addiction severity on the engagement of the salience network were inconsistent, however, strikingly only observed in cannabis, nicotine and stimulant users, indicating that there may be a drug-specific effect (Suppl. Table 1). This effect may be non-linear; while recent cocaine and cannabis abstainers (< 6 months) showed increased engagement of the salience network (Costumero et al., 2017; Gowin et al., 2017; Kober et al., 2014), a longitudinal study demonstrated that this effect reversed over time, showing decreased engagement with longer abstinence at 1-year follow up (Kober et al., 2014). The remaining three brain networks were less consistently linked to real-life drug use. The self-directed network (PCC/precuneus) was upregulated during craving (Filbey et al., 2016; Fryer et al., 2013; Seo et al., 2013), but links to addiction severity, lifetime use, abstinence and treatment were not consistently reported (Suppl. Table 1, Figure 5a). Surprisingly, the habit network (dorsal striatum) was not consistently implicated in brain-drug use correlations. While correlations with craving, frequency of use, addiction severity and abstinence, and a modulation by participation in cognitive behavioral therapy were found, these effects generally did not replicate across studies (Suppl. Table 1). Similarly, associations between engagement of the memory network (hippocampus/parahippocampus) and craving, lifetime use, addiction severity, treatment and abstinence were not replicated across studies (Suppl. Table 1), which may in part be due to underreporting (lack of analyses) for brain regions less often implicated in the addiction literature.
Figure 5. Clinical relevance of impaired brain function.
The reviewed results suggest a crucial role of the habit network in phases of marked behavioral change, such as during the initiation of drug use and relapse. In contrast, impairments in the salience and executive networks were not only linked to the prediction of early abuse in adolescence and relapse; they also correlated with current drug use and were modulated by therapeutic interventions. The association between salience network activation and drug use was only observed in individuals with cannabis and stimulant addiction (non-filled arrow). The reward network showed a strong upregulation with frequent and long term drug use, which was also predictive of relapse and reversed during therapeutic interventions. The memory network was specifically involved during therapeutic cognitive interventions.
In summary, we found consistent associations between real-life drug use and activation levels of the reward and executive networks, independent of the primary drug of choice, with reward network activation being positively correlated with almost any drug use variables investigated, and reduced recruitment of the executive network being linked to increased addiction severity and inability to remain abstinent. This consistent link with drug use duration and frequency may represent an important biomarker of drug use (potentially reflecting direct effects of a drug, and amenable for targeting for intervention purposes). In particular, the quantitative relation between drug consumption and upregulation of the reward network may constitute an important biomarker to predict escalation of drug use. For the salience network, we found a modulation by use frequency, which interestingly seemed restricted to cannabis and stimulant users. The observed effects in the reviewed studies support a potential ‘incubation of craving’ effect in this network, mirroring results from earlier preclinical work (Pickens et al., 2011) and a recent event-related potential study in cocaine addicted humans (Parvaz et al., 2016). In this study, we reported that an objective marker of motivated attention towards drug cues increased over time (at one and six months of abstinence), before decreasing again (at one year of abstinence) (Parvaz et al., 2016). Finally, we found that activation levels in the self-directed network were correlated with subjective craving and that there was no consistent link between drug use and the engagement of the habit and memory networks, suggesting that the impairments in these networks may not be a direct consequence of drug use. We hence speculate that, as the habit and memory networks both subserve learning, their engagement may be better indicative of a behavioral change (e.g., initiation of drug use or therapeutic treatment). Importantly, however, the strong link between engagement of the reward, salience and executive networks and daily drug use supports the second hypothesis of the iRISA model, whereby impairments in value assessment, redirection of attentional control and response inhibition are the core mechanisms involved in the maintenance of drug addiction.
Adolescents at risk for drug use
Another route to investigating the behavioral relevance of impairments in brain function is by understanding which impairments may reflect a risk factor to the initiation of drug use. Here, we highlight three larger studies that employed neuroimaging in adolescents to investigate brain function before the onset of substance abuse (selection criteria: n>15, corrected whole-brain analysis, drug-naïve adolescents). All three studies used an inhibitory control task. Youth with a family history of alcoholism demonstrated reduced engagement of the habit (dorsal striatum), salience (dACC) and executive (vlPFC) networks at 7 – 12 years of age in the conflict condition (Hardee et al., 2014). In substance-naïve youth, decreased recruitment of the habit (dorsal striatum) and executive (vlPFC/dlPFC) networks during inhibitory control predicted heavy alcohol drinking four to five years later (Norman et al., 2011; Wetherill et al., 2013; see Figure 5b). Together, these three studies suggest that a decreased recruitment of the habit, salience and executive networks during inhibitory control, and hence impairments in instrumental learning, attentional control and response selection (inhibition), may be a precursor and risk factor for drug use. Interestingly, this points to a specific role of impaired instrumental learning in early onset drug use, as the habit network did not show aberrant function in adults who performed inhibitory control tasks. Impairments in brain systems supporting attentional control and response inhibition, in contrast, were found both in at risk youth and adults, modulated by drug use frequency in the latter, indicating that these impairments may be both a precursor and a consequence of drug use. These neuroimaging studies hence converge with earlier preclinical findings and research on familial risk suggesting that impaired inhibitory control may be a neurocognitive endophenotype for addiction, contributing to the vulnerability to become addicted (Belin et al., 2008; Ersche et al., 2012b; Robbins et al., 2012) and further showing exacerbation with actual drug use (Jentsch and Pennington, 2014; Jentsch et al., 2014).
Predicting relapse
Equally important, longitudinal neuroimaging designs may be used to investigate whether brain function predicts success in maintaining abstinence in addicted adults. Here, we summarize results from five studies employing the reviewed tasks (n>15, corrected whole-brain analysis, longitudinal with or without a control group). Increased activation levels in the reward network (ventral striatum) when watching drug cues predicted relapse in heroin users up to 90 days later (Li et al., 2015). In cocaine users, increased engagement of the salience (anterior insula) and habit (dorsal putamen) networks during drug cue exposure was predictive of having a positive urine screen a week later (Prisciandaro et al., 2013). Together these two studies suggest that increased involvement of these brain networks during drug-related processing is predictive of relapse (Figure 5c), again pointing to a possible role for instrumental learning (engagement of the habit network) in periods of behavioral change. In contrast to these findings for approach behavior, a disengagement of the reward (rACC) and executive (vlPFC) networks during social-emotional processing (stressful cues) indicated the likelihood of relapse up to 1 year later in individuals with alcohol addiction, with the brain being a better predictor than behavioral measures of craving and use history (Seo et al., 2013). Similarly, decreased engagement of the executive network (vlPFC) and salience (dACC) networks during inhibitory control was linked to failure to remain abstinent for up to eight weeks (Worhunsky et al., 2013) or three months (Luo et al., 2013) in cocaine users, and relapse at one year follow-up in cannabis users (Kober et al., 2014; Figure 5c). These results converge with recent reviews of neuroimaging studies in humans (which also included region of interest studies), implicating the reward (ventral striatum), habit (dorsal striatum), salience (dACC) and executive (vlPFC) networks in relapse prediction (Courtney et al., 2016; Moeller et al., 2016), as well as with seminal work on reinstatement of drug seeking in animals, which has implicated the reward network (Mantsch et al., 2016; Pickens et al., 2011). Importantly, as the same brain networks (except for the reward network) were also found to underlie vulnerability in youth at risk, brain function in the habit, salience and executive networks may predict crucial transitions throughout the drug addiction cycle (onset of substance abuse, transition to addiction, chronic relapse). These results again support the second hypothesis of iRISA on the crucial role of impaired response inhibition and salience attribution in underlying the chronically relapsing nature of the cycle of addiction.
Change through treatment
Yet another avenue to study brain function in drug addiction is to follow the potential recovery of the brain once an individual undergoes treatment. Several comprehensive reviews on the neural correlates of therapeutic change have recently been published. We previously conducted a meta-analysis on the effects of pharmacological (e.g., agonists: nicotine patch or methadone; cognitive enhancers: modafinil or methylphenidate) and cognitive therapeutic interventions (e.g., cognitive-behavioral therapy, self-regulation techniques, motivational interventions) in alcohol, nicotine, opioid, and stimulant addiction (Konova et al., 2013). We concluded that the common neural targets of established and efficacious pharmacological and cognitive interventions are the reward (ventral striatum, OFC) and executive (vlPFC) networks, with cognitive interventions showing more extensive engagement of the executive network (vlPFC, dlPFC) and additional involvement of the salience (dACC) and self-directed (precuneus) networks. While we could not investigate directionality of the effects using this method, the reward and executive networks showed a dose-response effect and were implicated independently of the primary drug of use (Konova et al., 2013). In a second more recent review we investigated the directionality of effects induced by cognitive interventions (Zilverstand et al., 2016). We found that the cognitive regulation of craving consistently increased activation levels in the executive (vlPFC) and salience (dACC) networks, while it reduced the engagement of the reward network (ventral striatum, OFC). Second, we found that motivational interventions upregulated the executive (vlPFC) and memory (parahippocampus) networks. Third, mindfulness training reduced activation levels in the reward network (sgACC) and fourth, neurofeedback training aimed at downregulating activation levels of the reward network (sgACC) reduced craving (see Figure 5d for summary) (Zilverstand et al., 2016). Finally, in a third review on treatment mechanisms we focused on cognitive inhibitory control (across psychiatric disorders, including drug addiction), concluding that reduced engagement of the executive network (vlPFC/dlPFC) is the main impairment during cognitive reappraisal in individuals with impaired mental health (Zilverstand et al., 2017). Taken together these reviews suggest the importance of the reward, salience and executive networks in therapeutic change in general, further highlighting that other networks such as the memory network may be targeted only by specific interventions (e.g., motivational interventions) (Zilverstand et al., 2016). Importantly, all three reviews suggest that therapeutic interventions can normalize (to a certain extent) the impairments of brain function in drug addicted individuals.
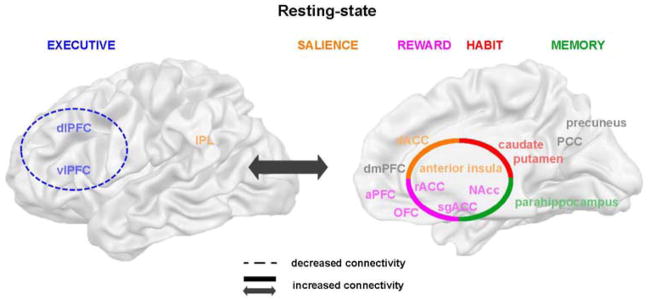
Resting-state as a biomarker
While task-based neuroimaging paradigms may be well suited to understanding the behavioral, emotional, and cognitive mechanisms underlying drug addiction, neuroimaging brain function at rest may be better suited to assess longitudinal change, as the “resting-state” paradigm allows the measure of brain function unbiased by (changing) task performance. Hence, we evaluated here if brain function during rest may reflect similar impairments as seen during task performance. The earliest systematic review of resting-state brain patterns in drug addiction was done by Sutherland and colleagues (2012) who proposed, based on the literature on nicotine addiction, that the salience (anterior insula, dACC) and executive (dlPFC) networks are tightly coupled during smoking, while they “uncouple” during abstinence (Sutherland et al., 2012). They further suggested that this effect is observed because the salience network “engages” the executive network during acute nicotine administration, orienting the individual’s attention towards drug use, while the disengagement of these two networks during abstinence impairs non-drug related processing (Sutherland et al., 2012). We reached a very similar conclusion in another comprehensive and recent review, encompassing 23 studies on chronic stimulant use (Zilverstand et al., 2018). We found tightened coupling between the reward (ventral striatum, rACC/sgACC, OFC), salience (anterior insula, dACC), habit (dorsal striatum) and memory (hippocampus) networks in chronic stimulant users, as well as enhanced coupling of all of these networks with the extended executive network (vlPFC, dlPFC, premotor, supplementary motor area and superior parietal lobe) (Figure 6). We concluded that the observed hyper-engagement of the executive network by the salience network during drug (nicotine) administration may solidify into a chronic state during long-term stimulant use, potentially underlying the impairments in brain function during task performance reviewed above. Importantly, five of the six brain networks implicated during task performance (all but the self-directed network) also showed abnormal coupling during resting-state (Zilverstand et al., 2018), suggesting that the resting-state paradigm (that can be administered to subjects who cannot perform selected tasks) may indeed be optimal for tracking brain function in these networks along the different phases (inclusive of intoxication and withdrawal) of the addiction cycle.
Figure 6. Aberrant functional connectivity during resting-state.
In chronic stimulant users, the reward, salience, habit and memory networks demonstrated enhanced coupling with each other, as well as with the executive network, while a decreased coupling was observed within the executive control network. Five of the six networks implicated in aberrant task performance (excluding the self-directed network) showed altered resting-state connectivity.
Conclusion
The current review summarizes the neuroimaging literature on cognitive and emotional impairments in individuals with drug addictions. We found consistent impairments in brain function in six large-scale brain networks during four different tasks, suggesting broad multi-faceted impairments of multiple dimensions across tasks - consistent with reports in the literature on widespread impairment of cognitive and emotional function in drug addiction (Spronk et al., 2013) and widespread changes in brain function in controlled longitudinal animal studies investigating chronic drug use (Beveridge et al., 2008). While the involvement of specific brain networks was task-specific, we generally observed increased engagement of relevant brain networks during exposure to drug cues, but a blunted brain response during non-drug related tasks, as predicted by the iRISA model (Goldstein and Volkow, 2011). However, we also found an intriguingly systematic pattern – across tasks – in the way these changes related to daily drug use. The reviewed results suggest a crucial role of the habit network in phases of marked behavioral change, such as during the initiation of drug use and relapse, consistent with theories that implicate this network in the “transition from voluntary, recreational drug use to compulsive drug-seeking habits” (Everitt and Robbins, 2016). In contrast, impairments in the salience and executive networks were not only linked to the prediction of early abuse in adolescence and relapse; they also correlated with current drug use and were modulated by therapeutic interventions, consistent with a central role of impaired response inhibition and salience attribution throughout the addiction cycle as proposed by the iRISA model (Goldstein and Volkow, 2011). The reward network showed a strong upregulation with frequent and long term drug use, demonstrating a dose-response relationship with excessive drug use, which was also predictive of relapse and reversed during therapeutic interventions. This upregulation of the reward network is consistent with the notion of dysregulated appraisal of the subjective value of the addictive substance in later stages of abuse as a direct effect of drug use, as suggested by all major theories on addiction (e.g., Everitt and Robbins, 2016; Goldstein and Volkow, 2011; Koob and Volkow, 2016), but particularly within decision making frameworks on addition (Schoenbaum et al., 2016). Somewhat less expected, the memory network was specifically involved during cognitive therapeutic interventions, which may be related to the need to reverse habitual responding by engaging the memory network to support flexible learning. The basic neuroscience literature supports this, as it suggests that the brain systems for habit learning and flexible learning compete with each other (White et al., 2013). Hence, our findings on this network may be seen as an extension of existing theories on the role of habitual responding in addiction, and may be related to recent research suggesting that reduced hippocampal engagement may constitute a risk factor for addiction (Everitt and Robbins, 2016). Importantly, our previous reviews on the mechanisms of therapeutic interventions suggest that it is possible, to a certain extent, to normalize brain function of the impaired networks, such as the memory network (Konova et al., 2013; Zilverstand et al., 2016, 2017). Finally, the self-directed network was implicated in subjective craving, consistent with a role in higher cognitive self-regulatory processes (Moeller and Goldstein, 2014). Overall, we conclude that the recent findings of the neuroimaging literature support the hypotheses of the iRISA model and other leading theories on addiction, and demonstrate the usefulness of a combined empirical-theoretical approach in understanding this complex disorder.
Strengths, limitations and recommendations
A possible strength of the current review encompasses the stringent selection criteria that we used to filter the results; only results from corrected whole-brain analyses were included due to concerns about false positives in the neuroimaging literature (Eklund et al., 2015). This was possible as the statistical rigor of the neuroimaging studies performed since our last review in 2011 (Goldstein and Volkow) has increased considerably, which contributed substantially to the observed convergence of effects across high quality studies. Second, we did not perform a meta-analysis, as this allowed us to aggregate the data on the level of brain networks. Considering the diverse literature, this approach allowed the meaningful integration of results across heterogeneous populations and study designs (and note concordance with previous meta-analyses on specific subtopics). For delineation of the discussed brain networks we used well-cited basic neuroscience literature, ensuring that the inclusion or exclusion of regions in networks (e.g., the premotor cortex and supplementary motor area may be considered to be part of the executive network) did not affect any of the general conclusions. Our systematic review was based on the consistency with which certain brain networks were implicated in group comparisons, discussing all brain networks of which any region was named in more than 30% of the studies (with the exception of the visual network, which was named in 40% of studies, but is exceptionally large). One brain region that was surprisingly not implicated by this approach was the amygdala (also not in social-emotional tasks, see Suppl. Table 2). This negative finding may be due in part to our systematic review approach, which biases results against brain regions with small effects sizes such as the amygdala (due to its small size and susceptibility artifacts in neuroimaging). However, of note is that using very similar methods in a previous review, we did find consistent effects for amygdala function in individuals with depression (Zilverstand et al., 2017).
Using this systematic review approach, six brain networks were most often implicated across tasks. While the involvement of the reward (ventral striatum, rACC/sgACC, dmPFC, OFC and aPFC), habit (dorsal striatum), salience (anterior insula, dACC, IPL), and executive (vlPFC, dlPFC) networks followed expectations (considering the overwhelming focus of the neuroimaging addiction literature on these four networks), the strong implication of the self-directed (PCC/precuneus) and memory (hippocampus, parahippocampus) networks deserves special mention. We previously emphasized the importance of deficits in self-directed processing and self-awareness in understanding decision making and choice in drug addiction [see Goldstein and colleagues (2009), Moeller and Goldstein (2014) for reviews]; nevertheless, this topic is still novel in addiction research. We and others also previously discussed the importance of learning and memory processes to drug addiction (Everitt and Robbins, 2016; Goldstein and Volkow, 2002); and although the involvement of the hippocampus/parahippocampus system in extinction of drug-related conditioning (Konova et al., 2017) and recovery from addiction (Lee et al., 2017) has been recently recognized, there is little research in drug addiction with a focus on flexible learning as supported by this brain network.
Some of the negative findings in this review, such as the lack of effects in monetary reward processing outside of a decision making context, or the lack of working memory effects in the studies employing a classic “N-back task” paradigm, suggest that novel task paradigms integrating these neuropsychological functions into a relevant behavioral context may be advantageous. Further, given that the behavioral literature on drug addiction clearly demonstrates replicable attention and working memory deficits in alcohol and cannabis users (Fernández-Serrano et al., 2011), as well as stimulant and opioid users (Baldacchino et al., 2012; Woicik et al., 2009), the paucity of neuroimaging studies (during the reviewed period) on working memory, attentional processing and learning was surprising and calls for more research on these topics. Also, while the reviewed studies on sensory processing provided very promising results, the number of studies on this topic precluded us from drawing any conclusions.
Another research gap revealed by this review was the scarcity of group-controlled whole-brain studies in nicotine addiction, as well as the overall low number of studies in opioid addiction. These gaps in research were somewhat offset, however, by the convergence of the general results across different substance addictions, or at least across the three major addictions reviewed: alcohol, cannabis and illicit stimulant addiction. However, because our methods were biased towards finding converging effects, a direct, and more valid, comparison between different addicted populations would comprise an exciting future research, particularly as the literature suggests that there may be differences in strength of impairments of certain task dimensions between opioid and stimulant addiction (Badiani et al., 2011). Similarly, neuroimaging studies allowing well-powered gender comparisons are very much needed. Also, the interesting but limited incursion into therapeutic interventions in addiction clearly merits more investigation (e.g., of dose effects including training duration and generalizability, individual differences) for the purpose of precision medicine in addiction. Further, while there is a substantial number of studies that aims at predicting clinical outcomes based on brain activation, many studies do not use methods beyond correlational approaches and hence cannot evaluate the unique contribution of select predictors. Last, the literature reviewed here was heavily biased towards cross-sectional research, calling for more within study designs to investigate interesting phenomena such as incubation of craving, and longitudinal studies on larger time scales, which could help differentiate the precursors from consequences of substance addiction and interrogate theoretical models of addiction that describe how recreational drug use may turn into addictive behavior (Everitt and Robbins, 2016).
Method details
Study selection
We performed a systematic literature search to identify fMRI studies investigating task performance in drug addiction. The Medline/Pubmed search term was comprised of the language (“English”), a term for selecting neuroimaging papers (“fMRI” OR “functional magnetic resonance imaging” OR “functional imaging”), a term defining the experimental paradigm (reward OR “cue reactivity” OR “cue exposure” OR “drug cue” OR craving OR cognitive OR executive OR inhibition OR regulation OR reappraisal OR attention OR learning OR extinction OR memory OR insight OR meta-cognition OR decision OR choice OR judgment OR risk OR gambling) and a term defining the population (marijuana OR cannabis OR “THC” OR cocaine OR methamphetamine OR amphetamine OR stimulant OR ecstasy OR “MDMA” OR heroin OR opiate OR “opioid addiction” OR polysubstance OR “drug abuse” OR “drug addiction” OR “drug dependence” OR “substance abuse” OR “substance addiction” OR “substance dependence” OR “nicotine dependence” OR “nicotine addiction” OR “chronic smokers” OR “lifetime smokers” OR “alcohol abuse” OR “alcohol dependence” OR “alcohol addiction”). Only studies adhering to the following criteria were included in the systematic review (the brain function during resting-state and in youth at risk were evaluated based on our previous reviews on these topics):
Studies published in English, in a peer-reviewed journal, since 2010 until 2017
Task-related original research studies performed in adult human subjects that compared individuals with substance dependence to healthy subjects
Studies with a minimal group size of N=15 after exclusion that computed whole-brain contrasts using adequate thresholding procedures (e.g., cluster thresholding, FDR/FWE correction) (Eklund et al., 2015)
See Figure 2 for the PRISMA Flow Diagram on the number of found and included studies.
Data extraction
We summarized the methods used in the reviewed studies in the supplementary tables, including a) the studied population b) drug use status, c) the sample size, d) employed task paradigm, e) important interventions, f) behavioral effects, g) brain effects (per network in Suppl. Table 1 and per region in Suppl. Table 2), h) associations with drug use, treatment, or relapse, g) study site, h) percentage of women included and i) performed gender comparisons. We reported a) group differences in activation level independent of the task condition (e.g., marked as “A>HC” or “HC>A”), b) group differences in activation level for a specific task condition [e.g., marked as “A>HC (reward)” or “HC>A (reward)”] and c) group differences in activation level for a specific task condition as contrasted against a control condition [e.g., marked as “G*T(↑A)(punishment)”, see table legends] (Suppl. Tables 1+2). As findings may not necessarily be comparable across different studies given the use of different labelling systems, we relabeled all originally reported results using a single labeling system based on the MRIcron Brodmann template (see Suppl. Material 4 for the complete list of labels). When required, we transformed the originally reported peak coordinates into MNI space using Brett’s Talairach to MNI algorithm (http://www.sdmproject.com/utilities/). Similarly, we used consistent labels for the different conditions across different tasks (e.g., the “incongruent” condition of the Flanker task and the “NoGo” condition of the Go/NoGo task were both relabeled as “conflict” condition, see Suppl. Tables 1+2).
Supplementary Material
Results listed by brain networks
Results listed by single brain regions
Reference list of all included studies
Labelling of brain regions
Highlights.
Systematic review of 105 task neuroimaging studies on addiction since 2010
Impairments of the salience and executive networks throughout the addiction cycle
Dysregulated reward network with dose-response effects at later stages of abuse
Habit network predicts initiation of use, memory network is linked to recovery
Acknowledgments
This work was supported by a fellowship from the Netherlands Organisation for Scientific Research (Rubicon 446-14-015 to A.Z.) and by grants from the National Institute on Drug Abuse (U01DA041174; and R01DA041528 to R.Z.G; and R01MH090134 to NAK).
Footnotes
Author Contributions
Conceptualization, A.Z. and R.Z.G.; Formal Analysis, A.Z. and A.S.H; Writing – Original Draft, A.Z.; Writing – Review and Editing, A.S.H., N.A.-K. and R.Z.G; Funding Acquisition, A.Z., N.A.-K. and R.Z.G.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A. The World According to Me: Personal Relevance and the Medial Prefrontal Cortex. Front Hum Neurosci. 2013;7:1–4. doi: 10.3389/fnhum.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albein-Urios N, Verdejo-Román J, Asensio S, Soriano-Mas C, Martínez-González JM, Verdejo-García A. Re-appraisal of negative emotions in cocaine dependence: Dysfunctional corticolimbic activation and connectivity. Addict Biol. 2012;19:415–426. doi: 10.1111/j.1369-1600.2012.00497.x. [DOI] [PubMed] [Google Scholar]
- Arcurio LR, Finn PR, James TW. Neural mechanisms of high-risk decisions-to-drink in alcohol-dependent women. Addict Biol. 2015;20:390–406. doi: 10.1111/adb.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack Ra. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ. Altered neural response of the appetitive emotional system in cocaine addiction: An fMRI Study. Addict Biol. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Baldacchino a, Balfour DJK, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012;36:2056–2068. doi: 10.1016/j.neubiorev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damageto human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High Impulsivity Predicts the Switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: Insights from preclinical research. Genes, Brain Behav. 2016;15:74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Beveridge TJR, Gill KE, Hanlon CA, Porrino LJ. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylergil SB, Beck A, Deserno L, Lorenz RC, Rapp MA, Schlagenhauf F, Heinz A, Obermayer K. Dorsolateral prefrontal cortex contributes to the impaired behavioral adaptation in alcohol dependence. NeuroImage Clin. 2017;15:80–94. doi: 10.1016/j.nicl.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Gandía MC, Mateos-García A, García-Pardo MP, Montagud-Romero S, Rodríguez-Arias M, Miñarro J, Aguilar MA. Effect of drugs of abuse on social behaviour: a review of animal models. Behav Pharmacol. 2015;26:541–570. doi: 10.1097/FBP.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Harenski CL, Harenski KA, Fede SJ, Steele VR, Koenigs MR, Kiehl KA. Abnormal frontostriatal activity in recently abstinent cocaine users during implicit moral processing. Front Hum Neurosci. 2015;9:565. doi: 10.3389/fnhum.2015.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canterberry M, Peltier MR, Brady KT, Hanlon CA. Attenuated neural response to emotional cues in cocaine-dependence: a preliminary analysis of gender differences. Am J Drug Alcohol Abuse. 2016;42:577–586. doi: 10.1080/00952990.2016.1192183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Kumar P, Eickhoff SB, Dombrovski AY. Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci. 2015;15:435–459. doi: 10.3758/s13415-015-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Yücel M, Lubman DI. The role of affective dysregulation in drug addiction. Clin Psychol Rev. 2010;30:621–634. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costumero V, Bustamante JC, Rosell-Negre P, Fuentes P, Llopis JJ, Ávila C, Barrós-Loscertales A. Reduced activity in functional networks during reward processing is modulated by abstinence in cocaine addicts. Addict Biol. 2017;22:479–489. doi: 10.1111/adb.12329. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-Bias Predicts Development of Cannabis Problem Severity in Heavy Cannabis Users: Results from a Prospective FMRI Study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, Van Den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol. 2013a;18:570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, Van Den Brink W, Veltman DJ, Porrino LJ, Goudriaan AE. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict Biol. 2013b;18:1013–1023. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: A prospective fMRI study. Hum Brain Mapp. 2014;35:2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla M, Baeuchl C, Simon JJ, Richter B, Kluge M, Friederich HC, Mann K, Herpertz SC, Loeber S. Do alcohol-dependent patients show different neural activation during response inhibition than healthy controls in an alcohol-related fMRI go/no-go-task? Psychopharmacology (Berl) 2017;234:1001–1015. doi: 10.1007/s00213-017-4541-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Ross TJ, Bell RP, Yi JY, Ryan J, Stein EA. Distress tolerance among substance users is associated with functional connectivity between prefrontal regions during a distress tolerance task. Addict Biol. 2017;22:1378–1390. doi: 10.1111/adb.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Shohamy D, Daw ND. Multiple memory systems as substrates for multiple decision systems. Neurobiol Learn Mem. 2015;117:4–13. doi: 10.1016/j.nlm.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RGM. Hippocampal Representation in Place Learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. Can parametric statistical methods be trusted for fMRI based group studies? 2015 arXiv:1511.01863. [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012a;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal Brain Structure Implicated in Stimulant Drug Addiction. Science (80- ) 2012b;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T. fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Hum Brain Mapp. 2016;37:3431–3443. doi: 10.1002/hbm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Wammes M, Neufeld R, Mitchell D, Theberge J, Williamson P, Osuch E. Unique functional abnormalities in youth with combined marijuana use and depression: An fMRI study. Front Psychiatry. 2014;5:1–11. doi: 10.3389/fpsyt.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, DWT, Shanbhag H, Krystale JH, Mathalon DH. Differential Brain Response to Alcohol Cue Distractors across Stages of Alcohol Dependence. Biol Psychol. 2013;92:282–291. doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Davis MB, Hommer DW. Greater activation in left hemisphere language-related regions during simple judgment tasks among substance-dependent patients in treatment for alcoholism. Alcohol Clin Exp Res. 2010;34:331–341. doi: 10.1111/j.1530-0277.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig aDB, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, May AC, Wittmann M, Tapert SF, Paulus MP. Doubling Down: Increased Risk-Taking Behavior Following a Loss by Individuals With Cocaine Use Disorder Is Associated With Striatal and Anterior Cingulate Dysfunction. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:94–103. doi: 10.1016/j.bpsc.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Baldacchino A, Balfour D, Matthews K, Steele JD. Abnormal Brain Activity During a Reward and Loss Task in Opiate Dependent Patients Receiving Methadone Maintenance Therapy. Neuropsychopharmacology. 2014;39:1–35. doi: 10.1038/npp.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM. Development of impulse control circuitry in children of alcoholics. Biol Psychiatry. 2014;76:708–716. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle KM, Shenoy P, Stewart JL, Tapert SF, Yu AJ, Paulus MP. Altered Neural Processing of the Need to Stop in Young Adults at Risk for Stimulant Dependence. J Neurosci. 2014;34:4567–4580. doi: 10.1523/JNEUROSCI.2297-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016;17:592–599. doi: 10.1038/nrn.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Bell RP, Foxe JJ, Garavan H. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend. 2013;133:86–93. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, Kim SM, Jung HY, Kang KD, Min KJ, Han DH. Cognitive avoidance and aversive cues related to tobacco in male smokers. Addict Behav. 2017;73:158–164. doi: 10.1016/j.addbeh.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Sinha R, Li CSR. Conflict anticipation in alcohol dependence - A model-based fMRI study of stop signal task. NeuroImage Clin. 2015;8:39–50. doi: 10.1016/j.nicl.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan RK, Lin JC, McLaren DG, Kirk IJ, Kydd RR, Russell BR. The effects of methylphenidate on cognitive control in active methamphetamine dependence using functional magnetic resonance imaging. Front Psychiatry. 2014;5:1–18. doi: 10.3389/fpsyt.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Pennington ZT. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76:479–486. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Han DH, Min KJ, Kim BN, Cheong JH. Brain activation in response to cravingand aversion-inducing cues related to alcohol in patients with alcohol dependence. Drug Alcohol Depend. 2014;141:124–131. doi: 10.1016/j.drugalcdep.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kober H, DeVito EE, Deleone CM, Carroll KM, Potenza MN. Cannabis Abstinence During Treatment and One-Year Follow-Up: Relationship to Neural Activity in Men. Neuropsychopharmacology. 2014;39:1–11. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior Prefrontal Function and the Limits of Human Decision-Making. Science (80- ) 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koester P, Volz KG, Tittgemeyer M, Wagner D, Becker B, Gouzoulis-Mayfrank E, Daumann J. Decision-making in Polydrug Amphetamine-type Stimulant Users: an fMRI Study. Neuropsychopharmacology. 2013;38:1377–1386. doi: 10.1038/npp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky Decision Making, Prefrontal Cortex, and Mesocorticolimbic Functional Connectivity in Methamphetamine Dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Goldstein RZ. Common and distinct neural targets of treatment: Changing brain function in substance addiction. Neurosci Biobehav Rev. 2013;37:2806–2817. doi: 10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova ABAB, Parvaz MAMA, Bernstein V, Zilverstand A, Moeller SJSJ, Delgado MRMR, Alia-Klein N, Goldstein RZRZ. Neural mechanisms of extinguishing drug and pleasant cue associations in human addiction: role of the VMPFC. Addict Biol online. 2017 Sep 5; doi: 10.1111/adb.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJV, Mencl WE, Worhunsky PD, Potenza MN, Mayes LC. Maternal neural responses to infant cries and faces: Relationships with substance use. Front Psychiatry. 2011;2:1–13. doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Ku J, Jung YC, Lee H, An SK, Kim KR, Yoon KJ, Namkoong K. Neural evidence for emotional involvement in pathological alcohol craving. Alcohol Alcohol. 2013;48:288–294. doi: 10.1093/alcalc/ags130. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D. An Update on Memory Reconsolidation Updating. Trends Cogn Sci. 2017;21:531–545. doi: 10.1016/j.tics.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, Wu N, Chang H, Zheng Y, Qin W, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: An event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, Zheng Y, Zhang D, Wang L, Li Y, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: An event-related functional magnetic resonance imaging study. Addict Biol. 2015;20:968–978. doi: 10.1111/adb.12182. [DOI] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-chaudhary S, Shaham Y, Lupica CR, Schoenbaum G. Orbitofrontal activation restores insight lost after cocaine use. Nat Neurosci. 2014;17:1092–1099. doi: 10.1038/nn.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, Smits M, Nijs IMT, Pepplinkhuizen L, Franken IHA. The role of dopamine in inhibitory control in smokers and non-smokers: A pharmacological fMRI study. Eur Neuropsychopharmacol. 2013;23:1247–1256. doi: 10.1016/j.euroneuro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Luijten M, Machielsen MWJ, Veltman DJ, Hester R, de Haan L, Franken IHA. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 2014;39:149–169. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G. Disruption of Reward Processing in Addiction. JAMA Psychiatry. 2017;74:387. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Hong K, Sinha R, Mazure CM, Li CR. Error processing and gender-shared and -specifi neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P. Disrupted Regulation of Social Exclusion in Alcohol-Dependence: An fMRI Study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine Dependent Individuals Show Attenuated Brain Response to Pleasant Interoceptive Stimuli. Drug Alcohol Depend. 2013;131:238–246. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Hobkirk AL, Towe SL, Chen N, Bell RP, Huettel SA. Cocaine dependence modulates the effect of HIV infection on brain activation during intertemporal decision making. Drug Alcohol Depend. 2017;178:443–451. doi: 10.1016/j.drugalcdep.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Froboese MI, Konova AB, Misyrlis M, Parvaz MA, Goldstein RZ, Alia-Klein N. Common and distinct neural correlates of inhibitory dysregulation: Stroop fMRI study of cocaine addiction and intermittent explosive disorder. J Psychiatr Res. 2014a;58:55–62. doi: 10.1016/j.jpsychires.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz Ma, Woicik Pa, Volkow ND, Goldstein RZ. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. 2014b;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Bederson L, Alia-Klein N, Goldstein RZ. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Elsevier B.V; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Selfreferential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Packard MG. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Res. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry. 2016;73:1127. doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural Correlates of Affect Processing and Aggression in Methamphetamine Dependence. Arch Gen Psychiatry. 2011;68:271. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Nurmi EL, Wilson SA, McCracken JT, London ED. Effects of methamphetamine abuse and serotonin transporter gene variants on aggression and emotion-processing neurocircuitry. Transl Psychiatry. 2012;2:e80. doi: 10.1038/tp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, Mcrae-clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network ? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: The PMAT framework. Elsevier B.V; 2015. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Roberts GMP, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage. 2010;52:429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Roberts GMP, Garavan H. Neural mechanisms underlying ecstasy-related attentional bias. Psychiatry Res - Neuroimaging. 2013;213:122–132. doi: 10.1016/j.pscychresns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Kurup PK, Stein EA. Nicotine modulation of information processing is not limited to input (attention) but extends to output (intention) Psychopharmacology (Berl) 2010;209:291–302. doi: 10.1007/s00213-010-1788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden MR. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. Morb Mortal Wkly Rep [MMWR] 2016;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Schmidt AA, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Roessler A, Smieskova R, Lang UE, Walter M. Acute effects of heroin on negative emotional processing: Relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chang CY, Lucantonio F, Takahashi YK. Thinking Outside the Box: Orbitofrontal Cortex, Imagination, and How We Can Treat Addiction. Neuropsychopharmacology. 2016;41:2966–2976. doi: 10.1038/npp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple Reward Signals in the Brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schwabe L. Stress and the Engagement of Multiple Memory Systems: Integration of Animal and Human Studies. Hippocampus. 2013;23:1035–1043. doi: 10.1002/hipo.22175. [DOI] [PubMed] [Google Scholar]
- Schwabe L. Memory under stress: from single systems to network changes. Eur J Neurosci. 2017;45:478–489. doi: 10.1111/ejn.13478. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted Ventromedial Prefrontal Function, Alcohol Craving, and Subsequent Relapse Risk. JAMA Psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldu X, Segura B, Dreher JC. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Daw ND. Integrating memories to guide decisions. Curr Opin Behav Sci. 2015;5:85–90. [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 2014;142:110–119. doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Tapert SF, Paulus MP. Hyperactivation to pleasant interoceptive stimuli characterizes the transition to stimulant addiction. Drug Alcohol Depend. 2015;154:264–270. doi: 10.1016/j.drugalcdep.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein Ea. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei-Jafari H, Ekhtiari H, Ganjgahi H, Hassani-Abharian P, Oghabian MA, Moradi A, Sadighi N, Zarei M. Patterns of Brain Activation During Craving in Heroin Dependents Successfully Treated by Methadone Maintenance and Abstinence-Based Treatments. J Addict Med. 2014;8:123–129. doi: 10.1097/ADM.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, Banich MT. Reduced neural tracking of prediction error in Substance-dependent individuals. Am J Psychiatry. 2013;170:1356–1363. doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik Pa, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:1–10. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Chong TTJ, Stout JC, Yücel M, London ED. Stages of dysfunctional decision-making in addiction. Pharmacol Biochem Behav. 2017;46:142–153. doi: 10.1016/j.pbb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Kobiella A, Bühler M, Graf C, Fehr C, Mann K, Smolka MN. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol. 2011;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang H, Li W, Zhu J, Gold MS, Zhang D, Wang L, Li Y, Yan X, Cheng J, et al. Reduced responses to heroin-cue-induced craving in the dorsal striatum: Effects of long-term methadone maintenance treatment. Neurosci Lett. 2014;581:120–124. doi: 10.1016/j.neulet.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res - Neuroimaging. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lile JA, Hanlon CA, Porrino LJ. Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl) 2016;233:1035–1044. doi: 10.1007/s00213-015-4180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF. Atypical Neural Activity during Inhibitory Processing in Substance-Naïve Youth Who Later Experience Alcohol-Induced Blackouts. Drug Alcohol Depend. 2013;128:243–249. doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Packard MG, Mcdonald RJ. Dissociation of Memory Systems: The Story Unfolds. Behav Neurosci. 2013;127:813–834. doi: 10.1037/a0034859. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette Ma. Neuroimaging craving: Urge intensity matters. Addiction. 2014:195–203. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang G, Volkow ND, Goldstein RZ. The Neuropsychology of Cocaine Addiction: Recent Cocaine Use Masks Impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional Brain Networks Associated with Cognitive Control, Cocaine Dependence and Treatment Outcome. Psychol …. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Potenza MN, Rogers RD. Alterations in functional brain networks associated with loss-chasing in gambling disorder and cocaine-use disorder. Drug Alcohol Depend. 2017;178:363–371. doi: 10.1016/j.drugalcdep.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Reynolds J, Krmpotich T, Banich MT, Thompson L, Tanabe J. Temporal profile of fronto-striatal-limbic activity during implicit decisions in drug dependence. Drug Alcohol Depend. 2014;136:108–114. doi: 10.1016/j.drugalcdep.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MAMA, Moeller SJSJ, Goldstein RZRZ. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. Prog Brain Res. 2016;224:285–304. doi: 10.1016/bs.pbr.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MAMA, Goldstein RZRZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review Neuroimage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, O’Halloran R, Goldstein RZ. Resting-state and structural brain connectivity in individuals with stimulant addiction. A systematic review. In: Pickard H, Ahmed S, editors. The Routledge Handbook of the Philosophy and Science of Addiction. Routledge; 2018. in press. [Google Scholar]
- Zimmermann K, Walz C, Derckx RT, Kendrick KM, Weber B, Dore B, Ochsner KN, Hurlemann R, Becker B. Emotion regulation deficits in regular marijuana users. Hum Brain Mapp. 2017;38:4270–4279. doi: 10.1002/hbm.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results listed by brain networks
Results listed by single brain regions
Reference list of all included studies
Labelling of brain regions