Abstract
Combining genetic insights into the pathogenesis of Parkinson’s disease (PD) with findings from animal and cellular models of this disorder has advanced our understanding of the pathways that lead to the characteristic degeneration of dopaminergic neurons in the brain’s nigrostriatal pathway. This has fueled an increase in candidate compounds designed to modulate these pathways and to alter the processes underlying neuronal death in this disorder. Using mitochondrial quality control and the macroautophagy/lysosomal pathways as examples, we discuss the pipeline from a comprehensive genetic architecture for PD through to clinical trials for drugs targeting pathways linked to neurodegeneration in PD. We also identify opportunities and pitfalls on the road to a clinically effective disease-modifying treatment for this disease.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative movement disorder, primarily of old age, characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta of the brain and by the accumulation of intracellular proteinaceous inclusions called Lewy bodies (1). The loss of dopaminergic innervation results in a range of clinical features including tremor, bradykinesia and rigidity. The coming decades will see increasing numbers of people developing PD as the global population ages, representing a huge challenge for health care systems. At present, it is estimated that the cost of caring for patients with PD is in excess of $14 billion per year in the United States alone (2). Thus, there is an urgent need for therapeutic agents to combat this disease. Current drug strategies for PD focus on compensating for the lowered dopamine concentrations in the brain. This can be achieved by increasing the supply of dopamine precursors in the form of LDOPA, which potentiates dopamine signaling at synapses. Alternatively, degradation of dopamine or its reuptake can be blocked, or dopamine receptors can be directly activated with agonists (3). These approaches, while successful in dealing with some of the key symptoms of PD, do not alter the degenerative process underlying the disease and are associated with significant side effects, especially at higher doses. Crucially, strategies targeting the dopamine pathway have no impact on the duration of disease from onset until death.
To address the lack of disease-modifying therapies, the gap between understanding the neurochemical basis of the PD motor syndrome (a lack of dopamine) and understanding the pathological events resulting in this deficit needs to be bridged. By gaining insight into these pathological cascades, new interventions can be developed to compensate for, or even prevent, these events. Over the past thirty years, much progress has been made in this area of research, first through the discovery of environmental insults that directly lead to dopaminergic cell death, most notably the neurotoxin MPTP, the pesticide rotenone and the herbicide paraquat (4), and more recently through the identification of genetic variants that either cause PD or greatly enhance the risk of developing the disorder (5). In this Perspective, we explain how genetic discoveries in the field of PD have contributed to our understanding of the pathogenesis of this disorder and discuss the quest for new disease-modifying therapies.
THE GENETIC ARCHITECTURE OF PD
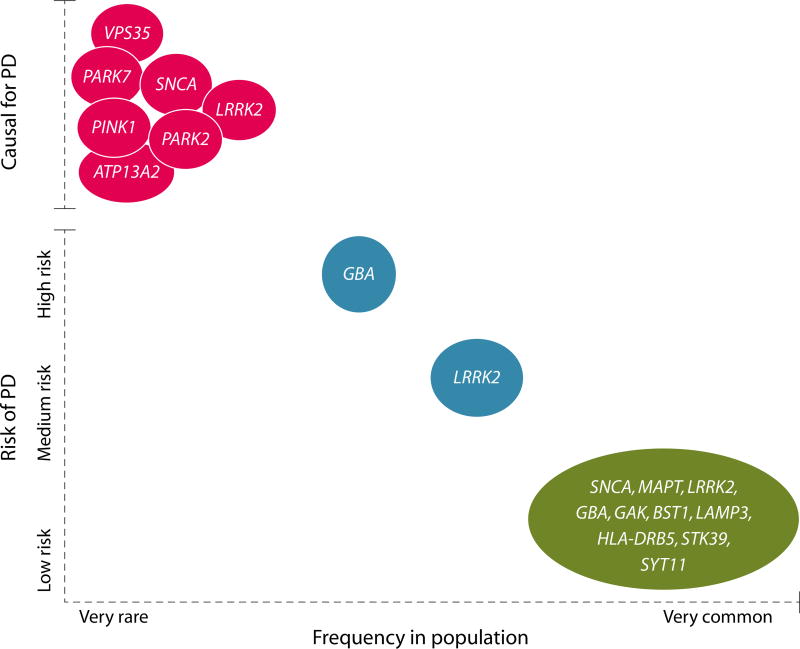
The first Mendelian gene for PD was identified in 1997, with the discovery of a point mutation in SNCA linked to an autosomal dominant form of the disease (6). Since then, over a dozen Mendelian loci have been implicated in familial PD, parkinsonism, and syndromes where parkinsonism is a prominent presenting symptom (Table 1) (5). More recently, advances in genomic technology have facilitated the identification of risk loci for PD through genomewide association studies (GWAS) (7). These studies have provided geneticists with an extensive list of loci that contribute to genetic risk for idiopathic (that is, non-Mendelian, genetically complex) PD. Whereas the majority of these loci are associated with very small increases in risk, the identification of genes within risk loci has the potential to uncover new druggable pathways that lead to the death of dopaminergic neurons in PD. By combining data from classical linkage studies, exome analysis and GWAS, it has now become possible to construct an overarching genetic architecture for PD. This architecture ranges from low-frequency high-risk variants in genes such as SNCA and LRRK2, through more frequent variants in genes such as GBA and LRRK2 that confer a greatly increased risk of disease but are not directly causative, to high-frequency low-risk variants in genes such as SNCA, MAPT, LRRK2 and at the HLA locus (Fig. 1). What is surprising about this architecture is that there are loci that span the spectrum of risk allele frequency and severity. For example, some point mutations in LRRK2 are causative for PD, whereas coding polymorphisms in the gene are strong risk factors and additional higher frequency variants at the LRRK2 locus contribute to a small increase in risk of developing PD (8). These data support a model in which there is a continuum in risk at certain loci, and importantly suggests that understanding the pathogenesis of at least some of the major Mendelian loci for PD has implications for the more common, idiopathic forms of the disease. Notably, emerging evidence suggests that LRRK2 interacts with the protein products of at least two GWAS hits, RAB7L1 and GAK (9), again linking PD-related genes and both monogenic and complex forms of PD into a cohesive network. The key challenge for the PD research community is to find the still missing links in these functional networks and to understand exactly how these genetic variants result in the preferential degeneration of dopaminergic neurons in the substantia nigra pars compacta. To achieve this, the cellular impact of these variants needs to be clarified.
Table 1.
Genetic loci implicated in Mendelian PD
| Locus | Chromosome | Gene | Inheritance |
|---|---|---|---|
| PARK1 | 4q | SNCA (point mutation) | AD |
| PARK2 | 6q | PARK2 | AR |
| PARK3* | 2p | ? | AD |
| PARK4 | 4q | SNCA (multiplication) | AD |
| PARK5* | 4p | UCHL1 | ? |
| PARK6 | 1p | PINK1 | AR |
| PARK7 | 1p | DJ-1 | AR |
| PARK8 | 12p-q | LRRK2 | AD |
| PARK9 | 1p | ATP13A2 | AR |
| PARK10* | 1p | ? | ? |
| PARK11* | 2q | GIGYF2 | ? |
| PARK12* | Xq | ? | X-linked |
| PARK13* | 2p | HTRA2 | ? |
| PARK14 | 22q | PLA2G6 | ? |
| PARK15 | 22q | FBXO7 | AR |
| PARK16 | 1q | ? | ? |
Loci indicated with an asterisk require validation.
AD, autosomal dominant; AR, autosomal recessive.
Fig. 1. The genetic architecture of PD.
Mendelian genes are shown in red, strong risk factors are shown in blue, and weak risk factors identified by GWAS are shown in green. Examples of Mendelian genes include SNCA, PARK2, and PINK1, with GBA variants being a standout example of a strong risk factor. GAK and HLA-DRB5 are examples of low-risk, high-frequency gene variants recently identified by GWAS. Some genes, such as LRRK2, are found in several or all of the categories.
CELLULAR PATHWAYS AND PROTEIN DYSFUNCTION IN PD
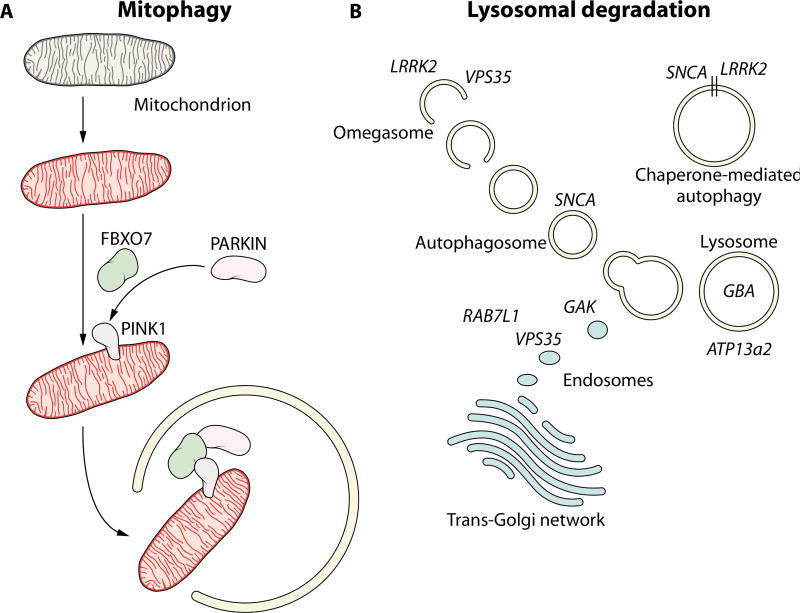
Discovery of the A53T mutation in the SNCA gene seventeen years ago led to the realization that aggregates of the protein α-synuclein (encoded by SNCA) are a key component of Lewy bodies, the pathological hallmark of PD. Since then, a huge research effort has been directed toward unpicking the mechanisms that connect this mutation, and subsequent mutations discovered in SNCA and other genes, to the death of dopaminergic neurons (10). These efforts have taken advantage of cell and animal models, ranging from simple organisms such as yeast through to rodent models and human neurons derived from induced pluripotent stem cells or embryonic stem cells (11). There are a number of recurring themes that have been identified across these models and with the various loci linked to PD (12). Two examples of such themes—mitochondrial quality control and the macroautophagy/lysosomal pathway—will be examined in detail as case studies of a molecular genetics approach to developing new PD drugs (Fig. 2).
Fig. 2. Examples of aberrant cellular pathways in PD.
(A) Shown is the involvement of Parkin, Pink1, and FBXO7 in the regulation of mitophagy. Damaged mitochondria (red) are identified and tagged for degradation through the process of mitophagy, which is disrupted in individuals carrying mutations in these proteins. In the absence of functional mitophagy, cellular damage and eventual cell death results from the accumulation of dysfunctional mitochondria. (B) Potential involvement of Mendelian loci and genetic risk factors for PD in the autophagy/lysosomal pathway. Mutations and genetic risk variants in genes such as those encoding LRRK2, VPS35, GBA, RAB7L1, and GAK may result in disruption of protein degradation pathways and accumulation of misfolded or aggregated proteins, leading to cytotoxicity and neuronal cell death.
Mitochondrial quality control, and in particular the cellular process of mitophagy (the selective targeting and recycling of damaged mitochondria) has been strongly linked to a number of recessive juvenile onset forms of parkinsonism (Fig. 2A). PARK2, PINK1, and FBXO7 (recessive loci with loss of function causing young onset parkinsonism) clearly cooperate in the regulation of mitophagy, with mutations in these genes acting to disrupt this process. A number of notable advances have been made in this area over the past year with the uncovering of ubiquitin phosphorylation as a key step in the pathway linking Parkin and Pink1 (13–16), as well as the identification of several genes that provide opposing or compensatory actions to Parkin (17, 18). How this process and these proteins relate to the idiopathic form of PD is unclear. Notably, the neuropathological data that does exist is equivocal as to whether mutations in Parkin, Pink1, and FBXO7 are associated with α-synuclein pathology, with some cases reported to have Lewy bodies and some not (19). Although interpreting the pathological events resulting from mutation of these genes is difficult, these findings demonstrate that detectable α-synuclein aggregates are not a sine qua non for selective dopaminergic cell death and parkinsonism. It is also notable that the loci containing these genes have not been found in GWAS for sporadic PD, contrary to some of the genes linked to dominant mutations.
In parallel to the identification of recessive mutations in genes implicated in mitophagy, a number of autosomal dominant mutations have been identified in genes that are causative or strongly linked to PD, where the majority of cases have evidence of Lewy body pathology. These genes have been implicated in vesicle trafficking pathways that terminate in lysosomal degradation (Fig. 2B) (20). α-Synuclein itself (both wildtype and mutant forms) has been linked to inhibition of macroautophagy and chaperone-mediated autophagy, two pathways that culminate in lysosomal degradation of proteins and organelles (21, 22), although in both cases the precise mechanism of the interaction is unclear. More recently, LRRK2 has been implicated in the initiation of macroautophagy and has been reported to be degraded by chaperone-mediated autophagy (23–25). Intriguingly, VPS35 and GBA also appear to fit into this schema (Figs. 1, 2B). VPS35 is involved in endosomal trafficking, and has recently been linked to alterations in the trans-Golgi network that may have consequences for lysosomal biology (26). The D620N mutation in VPS35 has also been reported to have a negative impact on the initiation of autophagy (27). GBA is a lysosomal hydrolase, homozygous mutations in which cause the lysosomal storage disorder Gaucher’s disease. The same mutations in the heterozygous state increase the risk of developing PD by about fivefold. (28). A number of studies using a variety of pathological samples and model systems suggest that GBA and α-synuclein form a bidirectional loop, with dysfunction of either of these proteins impacting the other and initiating a vicious circle resulting in decreased lysosomal function and increased α-synuclein aggregation (29–32). ATP13a2, recessive mutations in which cause a juvenile onset parkinsonian syndrome, is a lysosomal ATPase that has been suggested to have a close pathological relationship with α-synuclein (33–35). Intriguingly, two of the genes identified in GWAS for PD, GAK and RAB7L1, also appear to be linked to endosomal sorting and could conceivably fit into a pathway containing LRRK2 and VPS35, although the details of this pathway are only just starting to be elucidated (26, 36).
All of the genes discussed above in the context of mitophagy and the macroautophagy/lysosomal pathway have also been linked to a large number of other cellular pathways and molecular events. Parkin, for example, has been linked to a number of important roles in the cytosol (37, 38) including alternative pathways that could be targeted in a disease setting. LRRK2 has been implicated in a wide range of cellular pathways [see (8) for a more detailed review, and (42) for LRRK2’s potential role in inflammation]. There is an extensive literature on the biochemical properties of α-synuclein and how these properties are impacted by mutations and, most intriguingly, how this protein can spread in a prionlike manner from cell-to-cell (8, 11, 40). Each of these pathways/phenomena represent further case studies emphasizing the value of taking a genetics-based pathways approach to PD.
Finally, there are some genes implicated in PD where the link to pathogenesis is less clear, many of which have been identified by GWAS. Standout examples include MAPT, which encodes the microtubule-associated protein tau, and HLA-DRB5, a major histocompatibility locus (7). Tau, of course, has been strongly associated with neurodegeneration as the major constituent of neurofibrillary tangles in Alzheimer’s disease and a subset of the frontotemporal dementias (41). Tangle pathology, with a few rare exceptions, is not pathognomonic for PD and so how genetic variation at the MAPT locus is associated with risk for PD remains a mystery (39). The association of the HLA locus, however, contributes to a growing body of evidence supporting a role for the immune system in the etiology of PD. This has led to an increasing volume of research aimed at defining precisely how immune function may contribute to PD (42).
The strength of adopting a pathways approach is that it has the potential to allow identified genetic risk loci to become more than the sum of their individual values as drug targets. This can occur through the identification of key steps in these pathways that are not necessarily mutated in familial forms of a disease. An excellent example of how this can work in practice is provided by the amyloid precursor protein (APP) processing pathway in Alzheimer’s disease. By identifying the proteolytic production of β-amyloid peptide from APP as the key event in Alzheimer’s disease, researchers were able to identify proteins critical to this process beyond those directly linked to disease by mutations (APP, PSEN1, and PSEN2). In this way, β-secretase (BACE) and α-secretase (ADAM-10) were revealed as potential therapeutic targets (43–45). The example of the APP pathway, however, also acts as a cautionary tale, highlighting the major challenges presented by the task of developing drugs for treating neurodegenerative diseases. Compounds targeting γ-secretase (in which presenilin 1 and 2 form key components) have been taken to phase III clinical trials with disappointing results (46).
HURDLES IN PD DRUG DEVELOPMENT
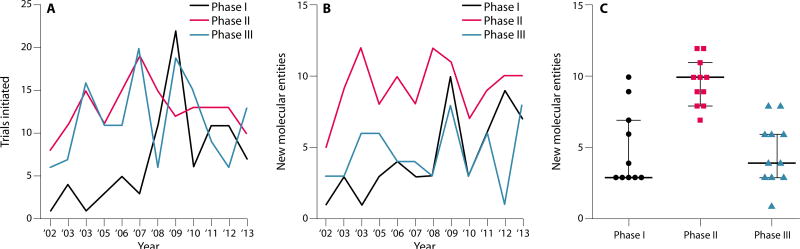
The drug development pipeline for PD has been, and remains, largely focused on symptomatic treatment. Progress in this area has been steady over the past 10 years (47, 48). The number of phase I interventional trials—defined for the purposes of this Perspective as drug, biologic, or genetic therapy aimed at modifying symptoms or disease course—shows a trend toward increasing year over year (Fig. 3A). The number of phase II and phase III clinical studies has fluctuated from year-to-year but remains essentially stable (Fig. 3A). A better gauge of the health of the PD drug pipeline, however, comes from examining the number of new molecular entities entering the pipeline annually (Fig. 3B). Over the last decade, there has been an average of 4.7 new molecular entities entering phase I and phase III clinical trials each year, and 9.6 new molecular entities entering phase II clinical trials annually (Fig. 3C). However, it should be noted that these data do not discriminate between drugs designed to modify symptoms and those that aim to modify disease trajectory.
Fig. 3. The drug pipeline in PD.
(A) The number of interventional phase I trials (black) for new molecular entities that are disease-modifying or that alleviate symptoms shows a trend upwards over the last 10 years. The number of phase II (red, mean = 12.9 SD = 2.8) and phase III (blue, mean = 11.58 SD = 5.0) trials has remained stable. (B) The number of new molecular entities entering interventional PD trials each year. Phase I (mean = 4.7 SD = 2.8), phase II (mean = 9.6 SD = 1.6), phase III (mean = 4.7 SD = 2.2). (C) Ten-year average for new molecular entities in phase I, phase II and phase III trials. Data are from www.clinicaltrials.gov. Interventional (symptom or disease-modifying) PD clinical trials using a drug, biologic, or gene therapy were selected; PET molecular imaging studies were excluded.
Feeding into the drug development pipeline, genetic advances have already helped to identify new candidate drug targets and pathways, although these have yet to be successfully translated into disease-modifying therapies. Several of the genetic loci directly implicated in PD are currently the subject of drug development efforts. For example, inhibitors have been developed that target LRRK2 kinase activity, antibodies have been raised against α-synuclein with the goal of using passive immunotherapy as a treatment, and compounds have been developed that modulate GBA activity (49–51). The majority of these therapies are in preclinical development. Notable exceptions include an antibody-based drug developed by AFFiRiS targeting α-synuclein that has undergone a phase I trial (52), a similar antibody-based approach targeting α-synuclein (designated BIIB037) by Biogen, currently undergoing a phase I trial, and an α-synuclein aggregation inhibitor (NPT200-11) under development by Neuropore and UCB pharmaceuticals. Importantly, the rationale underpinning these approaches is a modification of disease course.
Part of the challenge in developing a disease-modifying therapeutic lies in our incomplete understanding of the relationship of genetic targets, like LRRK2 and α-synuclein, to the pathways that drive neuronal death. This incomplete understanding of the mechanism of action means that we have yet to develop cellular or animal models that appropriately mimic the human disease process. Although it is not plausible to completely derisk any therapeutic candidate early in the drug development process, the current models have poor predictive power to determine if a drug candidate is likely to modify PD progression. This gap in understanding shift s considerable risk from the earlier (cheaper, faster) stages of drug development to later stage phase II and III clinical trials (expensive, slow), where evidence of a disease-modifying effect would be observed. The shift of the majority of risk to the most expensive and time-consuming part of drug development is unfortunately a common barrier to drug development for neurodegenerative diseases in general, reflecting the current, incomplete state of our understanding of the mechanisms leading to neuronal death and the dearth of physiologically relevant animal models.
CLINICAL TRIALS IN PD
It is, therefore, crucial that PD clinical trial design be as efficient and robust as possible to ensure the effective identification of any disease-modifying compound. Designing a PD clinical trial for a disease-modifying therapeutic agent depends on the clinical end point being targeted and ensuring that it is also acceptable to regulatory bodies. In 2007, the European Medicines Agency (EMA) issued draft guidance for PD trials seeking a disease-modifying claim (EMA/CHMP/330418/2012 rev. 2; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129601.pdf). While recognizing that no universal study design could be recommended, the EMA proposed that clinical trials should be randomized, placebo-controlled, and long-term. Clearly, in established PD cases, the primary goal is to slow further decline in motor function and progression of disability, and to prevent motor and nonmotor complications. Below we consider two possible trial designs to determine a disease-modifying effect.
Change in rate of functional decline according to the UPDRS
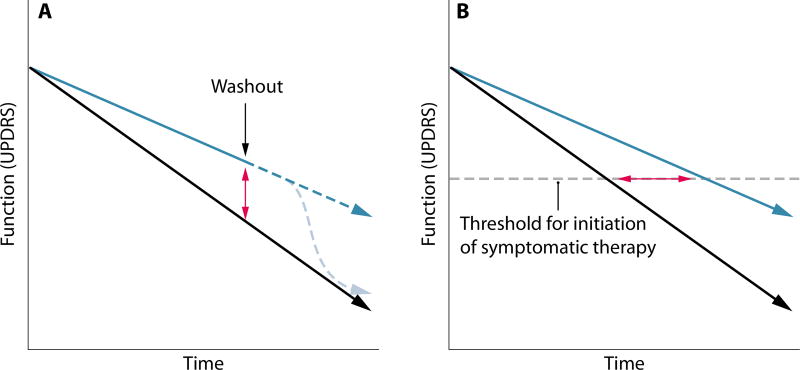
The Unified Parkinson’s Disease Rating Scale (UPDRS) is the “gold standard” among clinical end points, with broad recognition and acceptance by US and European regulatory agencies as a registration end point. Successful symptomatic therapeutic interventions that modulate the dopamine pathway have had a profound and rapid effect on this metric leading to smaller clinical trials of shorter duration (53). However, due to the slow rate of PD progression, observing a disease-modifying change in the rate of decline in the UPDRS requires longer duration trials of 2 to 5 years and a consensus regarding what is meant by a clinically meaningful change in the UPDRS (54). Another drawback of the UPDRS is that, by its very nature, it is a discrete rather than a linear measure of decline. This reduces its utility in the context of trying to uncover a disease-modifying impact during a clinical trial. It would also be advantageous for disease-modifying trials to rule out any purely symptomatic benefit by observing the trajectory of disease after washout of the drug (Fig. 4A). Controlling for population heterogeneity during the enrollment and duration of such trials is critical, given the variable rates of decline, disease stages, and the likelihood that some or all of the subjects will receive symptomatic treatment. The potential benefits of using carefully designed functional decline as an outcome measure are illustrated by the recent Eli Lilly trial of solanezumab in Alzheimer’s disease, which, despite the modest impact of the drug treatment, provides a proof-of-principle for trials of this type (55).
Fig. 4. Measuring efficacy of disease-modifying strategies in PD.
(A) Assessing disease modification versus symptomatic benefit using compound washout. Black line represents placebo, blue line represents a disease-modifying therapeutic, dashed light blue line represents a symptomatic therapeutic such as L-DOPA, red arrow represents a change in UPDRS. (B) Using time to initiation of symptomatic treatment as an end point in PD clinical trials. Black line represents placebo, blue line represents a disease-modifying therapeutic, red arrow represents the change in time required for the initiation of symptomatic treatment.
Time to initiation of symptomatic therapy
Because of drug complications, symptomatic treatment usually in the form of L-DOPA is often delayed until the severity of motor symptoms results in functional impairment. Unlike the ambiguity in what magnitude change in UPDRS is clinically meaningful, the initiation of symptomatic treatment is considered an early indicator of disease progression and a “real-life” end point (Fig. 4B). The time between baseline assessment and the end point (need for dopamine replacement therapy) can also be conceptualized as “survival” and is amenable to Kaplan-Meier statistical analysis (56). A confound of this end point is that when symptomatic therapy should be initiated is largely based on a physician’s subjective assessment of the patient’s status, and may be subject to considerable variation between clinicians.
Patient stratification and clinical trials
A critical challenge for the successful appraisal and validation of potential therapeutic compounds for PD is the assessment of such compounds in an appropriate clinical population. The eventual goal of any drug development program directed at PD is the isolation of a compound or compounds that are efficacious for the majority of people with the disease. The pipeline from the identification of putative drug targets using human genetics, however, presents us with an intermediate step. A compound developed to target a specific protein that is genetically associated with PD could be tested in human populations at increased risk of PD who possess variants in this target gene. This is exemplified by recent developments in the Alzheimer’s disease field where major efforts are afoot to test drugs targeting the product of aberrant processing of APP in patient cohorts carrying mutations in PSEN1, the gene encoding presenilin, which is a key component of the proteolytic complex responsible for APP processing (57). This stratified approach provides the greatest chance of success for a candidate compound prior to the more challenging terrain of a full phase III clinical trial, while also presenting opportunities for longitudinal assessment of disease progression and preclinical states (58).
As our understanding of the genetics of PD has progressed, several such patient populations have emerged. Most notable among these are carriers of mutations in LRRK2 and in GBA. The relatively high frequency of these mutations in human populations means that there are large numbers of individuals either currently with PD or at a greatly increased risk of developing the disorder. For drugs specifically designed to target either LRRK2 or GBA, these individuals are an ideal test of whether these compounds elicit a clinically meaningful change to disease progression. Although these compounds are not yet in clinical trials, efforts are already under way to assemble well-characterized cohorts of individuals carrying mutations in these genes. Examples include a cohort of North Africans carrying the G2019S mutation in LRRK2 (PD patients from the Barbary coast—Morocco, Tunisia, and Algeria—have an almost 40% chance of carrying this mutation) and Ashkenazi Jewish cohorts with either the G2019S mutation or risk variants at the GBA locus.
A confounding factor for patient cohorts with mutations or risk variants at LRRK2 or GBA loci is that these are not directly deterministic for PD. In the case of LRRK2 G2019S mutation carriers, penetrance is estimated to be ~80% at age 80 in some populations (59), with an odds ratios of up to 48.6 (in North African populations, 95% CI, 11.2–211.0) (60). For GBA mutations, the odds ratio has been estimated to be 5.43 (95% CI, 3.89 to 7.57) in a multicenter study (61). A recent Kaplan-Meier analysis estimated the penetrance for GBA heterozygous cases in a series from New York to be ~10% at age 85 (62). In this context, there would be value in developing a global cohort of individuals carrying mutations in SNCA. Although challenging due to the rarity of these mutations, the high penetrance and direct link between α-synuclein dysfunction and disease in these cases would provide a powerful means for testing therapies explicitly targeting α-synuclein. It should be emphasized, however, that GBA and LRRK2 mutation carriers, by virtue of their frequency in human populations worldwide, provide the most effective cohort for testing early interventions in PD. While progress is being made in early diagnosis of idiopathic PD (63), currently there are significant challenges in identifying patients ahead of motor symptom onset. Progress in this arena awaits advances in biomarker identification, which is a big focus of PD research worldwide (64). An excellent example of this is the Parkinson’s Progression Marker Initiative (PPMI)—a consortium of academic groups, PD charities, government bodies and pharmaceutical firms—which is following a cohort of PD patients longitudinally and recording changes in key molecules such as α-synuclein that are putative biomarkers (65). The power of this approach, especially when combined with careful genetic characterization, is demonstrated by a recent study combining biomarker and genetic information to model PD diagnosis (66).
CONCLUSIONS AND FUTURE PERSPECTIVES
These are exciting times for researchers working to develop disease-modifying therapies for PD. Genetic advances and cellular pathway discoveries are beginning to provide a list of putative drug targets. In addition to the pathways described above, there is increasing insight into cell-to-cell transmission of protein aggregates as a mechanism enabling pathology to spread through the central nervous system. There is also a growing awareness of immune function as a central player in the etiology of PD and of a potential role for transcriptional/translational control in initiating disease (67). As our understanding of the biology of PD advances, the ability to rationally target the root causes of the changes in brain function that result in this disease will improve. It is important, however, not to underestimate the challenges that we face in developing drugs for PD. A major, and long appreciated, hurdle is that for drugs to specifically engage targets within the brain, they first must cross the blood-brain barrier. It is also clear from drug development efforts targeting other chronic neurodegenerative disorders that early intervention will be crucial for success. Thus, improving early diagnosis—and indeed moving to a diagnosis prior to the emergence of movement impairment—will be key to developing effective new treatments for PD. A further challenge, and one highlighted by a recent study examining LRRK2 inhibitors in nonhuman primates (68), is that there may be serious biological consequences to long-term inhibition or manipulation of genetically-derived PD drug targets. This may emerge as a major obstacle given the likelihood that any disease-modifying drug for PD will need to be taken on a continual, life-long basis. Given these challenges, it is critical that the barriers that may exist between the different stages of drug development and between academia and industry are eliminated—through joint funding programs, new consortia (perhaps using the template of the PPMI) and increased exchange of investigators and knowledge between basic research, clinical medicine and translational research/industry. This much is owed to the patients living with PD, and to the many others destined to develop the disease.
Acknowledgments
Funding: P.A.L. is a Parkinson’s UK research fellow (grant F1002). This work was supported in part by the Wellcome Trust/Medical Research Council (MRC) Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson’s Disease Consortium (UKPDC) whose members include University College, London Institute of Neurology, the University of Sheffield and the MRC Protein Phosphorylation Unit at the University of Dundee, and MRC grant MR/L010933/1.
Footnotes
Competing interests: T.G. is listed on patent no. EP1802749 (A2) “KASPP (LRRK2) gene, its production and use for the detection and treatment of neurodegenerative disorders.”
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disorders. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J, Poewe W. Therapies in Parkinson’s disease. Curr. Opin. Neurol. 2012;25:433–447. doi: 10.1097/WCO.0b013e3283542fc2. [DOI] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C. The genetics of Parkinson’s syndromes: A critical review. Curr. Opin. Genet. Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 7.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simón-Sánchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, Stefánsson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB N. W. Wood-International Parkinson Disease Genomics Consortium. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paisan-Ruiz C, Lewis P, Singleton A. LRRK2: Cause, risk, and mechanism. J. Parkinson’s Dis. 2013;3:85–103. doi: 10.3233/JPD-130192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manzoni C, Denny P, Lovering RC, Lewis PA. Computational analysis of the LRRK2 interactome. Peer J. 2015;3:e778. doi: 10.7717/peerj.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara E, Lewis PA, Ling H, Proukakis C, Houlden H, Hardy J. α-Synuclein mutations cluster around a putative protein loop. Neurosci. Lett. 2013;546:67–70. doi: 10.1016/j.neulet.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy J. Genetic analysis of pathways to Parkinson disease. Neuron. 2010;68:201–206. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 2014;460:127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 16.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510:370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 18.Yun J, Puri R, Yang H, Lizzio MA, Wu C, Sheng ZH, Guo M. MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. eLife. 2014;3:e01958. doi: 10.7554/eLife.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124:325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzoni C, Lewis PA. Dysfunction of the autophagy/lysosomal degradation pathway is a shared feature of the genetic synucleinopathies. FASEB J. 2013;27:3424–3429. doi: 10.1096/fj.12-223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC. α-Synuclein impairs macroautophagy: Implications for Parkinson’s disease. J. Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 23.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM. Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzoni C, Mamais A, Dihanich S, Abeti R, Soutar MP, Plun-Favreau H, Giunti P, Tooze SA, Bandopadhyay R, Lewis PA. Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim. Biophys. Acta. 2013;1833:2900–2910. doi: 10.1016/j.bbamcr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, Ding J, Nalls MA, International Parkinson’s Disease Genomics Consortium; North American Brain Expression Consortium. Olszewski M, Hauser DN, Kumaran R, Lozano AM, Baekelandt V, Greene LE, Taymans JM, Greggio E, Cookson MR. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2626–2631. [Google Scholar]
- 27.Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 2014;5:3828. doi: 10.1038/ncomms4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi JH, Stubblefield B, Cookson MR, Goldin E, Velayati A, Tayebi N, Sidransky E. Aggregation of α-synuclein in brain samples from subjects with glucocerebrosidase mutations. Mol. Genet. Metab. 2011;104:185–188. doi: 10.1016/j.ymgme.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J. Biol. Chem. 2011;286:28080–28088. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, Dickson DW, Mazzulli JR, Bardgett ME, Haik KL, Ekhator O, Chava AK, Howard J, Gannon M, Hof man E, Chen Y, Prasad V, Linn SC, Tamargo RJ, Westbroek W, Sidransky E, Krainc D, Shull GE. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum. Mol. Genet. 2013;22:2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J. Neuroscience. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC, Kim D, Tessarollo L, Dawson VL, Dawson TM. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat. Neurosci. 2013;16:1392–1400. doi: 10.1038/nn.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray S, Lewis PA. A tangled web—Tau and sporadic Parkinson’s disease. Front. Psychiat. 2010;1:150. doi: 10.3389/fpsyt.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- 41.Ittner LM, Götz J. Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 42.Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, Saad M, Bras JM, Bettella F, Nicolaou N, Simón-Sánchez J, Mittag F, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefánsson H, Majamaa K, Gasser T, Heutink P, Wood NW, Martinez M, Singleton AB, Nalls MA, Hardy J, Morris HR, Williams NM International Parkinson’s Disease Genomics Consortium. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum. Mol. Genet. 2013;22:1039–1049. doi: 10.1093/hmg/dds492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahrenholz F, Postina R. Alpha-secretase activation—an approach to Alzheimer’s disease therapy. Neurodegener. Dis. 2006;3:255–261. doi: 10.1159/000095264. [DOI] [PubMed] [Google Scholar]
- 44.Kandalepas PC, Vassar R. Identification and biology of β-secretase. J. Neurochem. 2012;120(suppl. 1):55–61. doi: 10.1111/j.1471-4159.2011.07512.x. [DOI] [PubMed] [Google Scholar]
- 45.De Strooper B, Vassar R, Golde T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Neurology. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS, Siemers E, Sethuraman G, Mohs R Alzheimer’s Disease Cooperative Study Steering Committee, Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 47.Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Movement Disorders. 2015;30:114–120. doi: 10.1002/mds.26078. [DOI] [PubMed] [Google Scholar]
- 48.Fox SH, et al. The Movement Disorder Society evidence-based medicine review update: Treatments for the motor symptoms of Parkinson’s disease. Movement Disorders. 2011;26(suppl. 3):S2–S41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 49.Taymans JM. Can the increasing number of newly developed leucine-rich repeat kinase 2 inhibitors validate or invalidate a potential disease-modifying therapeutic approach for Parkinson’s disease? Expert Opin. Therap. Patents. 2014;24:727–730. doi: 10.1517/13543776.2014.915945. [DOI] [PubMed] [Google Scholar]
- 50.Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, Lee VM. Α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Reports. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, Santic R, Meindl S, Vigl B, Smrzka O, Schneeberger A, Mattner F, Masliah E. Next-generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127:861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pahwa R, Lyons KE, Hauser RA, Fahn S, Jankovic J, Pourcher E, Hsu A, O’Connell M, Kell S S. GuptaAPEX-PD Investigators. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:142–148. doi: 10.1016/j.parkreldis.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br. J. Clin. Pharmacol. 2012;74:267–283. doi: 10.1111/j.1365-2125.2012.04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA, Demattos R. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 56.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: Good practice and pitfalls. Lancet. 2002;359:1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- 57.Mullard A. Sting of Alzheimer’s failures of set by upcoming prevention trials. Nat. Rev. Drug Discov. 2012;11:657–660. doi: 10.1038/nrd3842. [DOI] [PubMed] [Google Scholar]
- 58.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S J. C. MorrisDominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW International LRRK2 Consortium. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lesage S, Dürr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, BriceFrench A Parkinson’s Disease Genetics Study Group. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 61.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: Information for genetic counseling. Genetics Med. 2013;15:146–149. doi: 10.1038/gim.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T. Identifying prodromal Parkinson’s disease: Pre-motor disorders in Parkinson’s disease. Movement Disorders. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 64.Schapira AH. Recent developments in biomarkers in Parkinson disease. Curr. Opin. Neurol. 2013;26:395–400. doi: 10.1097/WCO.0b013e3283633741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parkinson I. The Parkinson Progression Marker Initiative (PPMI) Prog. Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nalls MA, McLean CY, Rick J, Eberly S, Hutten SJ, Gwinn K, Sutherland M, Martinez M, Heutink P, Williams NM, Hardy J, Gasser T, Brice A, Price TR, Nicolas A, Keller MF, Molony C, Gibbs JR, Chen-Plotkin A, Suh E, Letson C, Fiandaca MS, Mapstone M, Federoff HJ, Noyce AJ, Morris H, Van Deerlin VM, Weintraub D, Zabetian C, Hernandez DG, Lesage S, Mullins M, Conley ED, Northover CA, Frasier M, Marek K, Day-Williams AG, Stone DJ, Ioannidis JP, Singleton AB Parkinson’s Disease Biomarkers Program and Parkinson’s Progression Marker Initiative investigators. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: A population-based modelling study. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taymans JM, Nkiliza A, Chartier-Harlin MC. Deregulation of protein translation control, a potential game-changing hypothesis for Parkinson’s disease pathogenesis. Trends Mol. Med. 2015;21:466–472. doi: 10.1016/j.molmed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, Lewin-Koh S-C, Lin T, Liu X, Liu S, Lyssikatos JP, O’Mahony J, Reichelt M, Roose-Girma M, Sheng Z, Sherer T, Smith A, Solon M, Sweeney ZK, Tarrant J, Urkowitz A, Warming S, Yaylaoglu M, Zhang S, Zhu H, Estrada AA, Watts RJ. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 2015;7:273ra15. doi: 10.1126/scitranslmed.aaa3634. [DOI] [PubMed] [Google Scholar]