Abstract
Study Objectives
To evaluate the utility of multimodal low-cost approaches including actigraphy, a wrist-worn device monitoring rest/activity cycles, in identifying patients with idiopathic REM sleep behavior disorder (iRBD).
Methods
Seventy patients diagnosed with sleep disorders causing different motor manifestations during sleep (iRBD, sleep apnea, restless legs syndrome) and 20 subjects without any relevant motor manifestation during sleep, underwent video-polysomnography (vPSG) and 2 week actigraphy, completed six validated RBD screening questionnaires, and sleep apps use was assessed. Actigraphy was analyzed automatically, and visually by seven blinded sleep medicine experts who rated as “no,” “possible,” and “probable” RBD.
Results
Quantitative actigraphy analysis distinguished patients from controls, but not between patients with different types of motor activity during sleep. Visual actigraphy rating by blinded experts in sleep medicine using pattern recognition identified vPSG confirmed iRBD with 85%–95% sensitivity, 79%–91% specificity, 81%–91% accuracy, 57.7% ± 11.3% positive predictive value, 95.1% ± 3.3% negative predictive value, 6.8 ± 2.2 positive likelihood ratio, 0.14 ± 0.05 negative likelihood ratio and 0.874–0.933 area under the ROC curve (AUC). AUC of the best performing questionnaire was 0.868. Few patients used sleep apps; therefore, their potential utility in the evaluated patients’ groups is limited.
Conclusions
Visual analysis of actigraphy using pattern recognition can identify subjects with iRBD, and is able to distinguish iRBD from other motor activities during sleep, even when patients are not aware of the disease in contrast to questionnaires. Therefore, actigraphy can be a reliable screening instrument for RBD potentially useful in the general population.
Keywords: RBD, screening method, sleep, alpha-synuclein, parkinsonism
Statement of Significance.
This study evaluated the utility of actigraphy as widely available low-cost method to screen for idiopathic REM sleep behavior disorder (iRBD). Video-polysomnography is the gold standard and is required for a definite diagnosis of iRBD; however, it is expensive and not always available. Currently used screening questionnaires present many limitations when used outside the context of validation studies, so that alternatives for iRBD screening are needed. We showed that visual analysis of actigraphy using pattern recognition can distinguish not only between iRBD and controls, but also between iRBD and patients with other motor manifestations during sleep. Our results suggest that visual analysis of actigraphy may potentially be useful as screening method in the general population.
Introduction
REM sleep behavior disorder (RBD) is characterized by abnormal behaviors during REM sleep [1]. More than 80 per cent of patients with idiopathic RBD (iRBD) develop later on an overt α-synucleinopathy [2, 3]. Moreover, a recent study reported the presence of α-synucleinopathy biomarkers in patients with longstanding iRBD [4] challenging the concept of “idiopathic” RBD. Therefore, the alternative term “isolated RBD” has been proposed [5]. As iRBD represents in most cases an early stage α-synucleinopathy, early recognition is of outmost importance, particularly if disease modifying therapies will be available.
For a definite diagnosis of RBD, video polysomnography (vPSG) is required [1] since clinical history alone can be misleading due to several confounding entities, for example sleep apnea (SA) related movements [6], periodic leg movements (PLM) [7], other parasomnias [8], or nocturnal frontal lobe epilepsy [9]. Another challenge in diagnosing iRBD by history is the lack of self-awareness in many patients [10]. However, as vPSG is costly and not widely available, population-based studies of iRBD with vPSG confirmation are scarce [11–15]. Questionnaires are the most used screening method, but they showed low diagnostic value outside the context of validation studies, in which participants were already aware of investigated symptoms [16–18].
Actigraphy is similar to a wrist-worn watch and monitors rest/activity cycles; it is simple, low-cost, and widely available. Few studies in Parkinson disease (PD) showed possible usefulness of actigraphy in screening for RBD [19, 20]. Another easy tool, already widely used, is apps for the evaluation of sleep. We aimed to evaluate the usefulness of a multimodal low-cost approach as screening tool for RBD potentially useful in the general population.
Methods
Participants
Participants were recruited among patients undergoing vPSG at the Sleep Laboratory, Department of Neurology, Medical University of Innsbruck. A total of 90 participants were recruited: 20 patients with iRBD; 20 patients with restless legs syndrome (RLS); 20 patients with untreated SA with an apnea/hypopnea index (AHI) >15/h; 10 patients with RLS and treated SA (AHI < 15/h); and 20 controls, recruited among patients referring to our sleep laboratory for suspected sleep disorder (n = 11 suspected sleep-related breathing disorder, n = 6 excessive daytime sleepiness, n = 3 insomnia), which was not confirmed. Diagnoses of sleep disorders were made according to standard criteria [1, 21]. Any ongoing pharmacological treatment for RBD or RLS was continued during the study.
All participants were ≥18 years. Participants with shift work during the evaluation period were excluded from the study. Patients with RBD and concomitant SA were included only if the AHI was <15/h. As periodic leg movements during sleep (PLMS) are common in patients with RBD [22], their presence did not represent an exclusion criterion.
The aim of this study was to evaluate actigraphy as a screening method for iRBD potentially useful in the general population, so we sought to evaluate it in real-life conditions. Therefore, intake of ongoing pharmacological treatment before sleep was allowed.
The study was approved by the ethics committee of the Medical University of Innsbruck. All participants signed the informed consent before inclusion in the study.
Clinical and demographic data
Sleep history and demographic data were collected during an interview with a trained sleep medicine expert. Clinical data included the presence of a bed partner as well as questions addressing the presence of the most common sleep complaints (Table S1).
Video-polysomnography
Each participant underwent at least one night of 8 hour vPSG at the sleep laboratory, Department of Neurology, of the Medical University of Innsbruck. VPSG was performed according to international standards [23] using the SINBAR electromyography (EMG) montage with both flexor digitorum superficialis muscles [22], and was recorded on a OSG BrainRT PSG device (OSG Belgium; http://www.osg.be).
Sleep and PLMS were scored according to standard criteria [23]. Automatic scoring of PLMS was performed using a validated software integrated in the PSG system [24].
RBD questionnaires
All participants completed the following validated RBD questionnaires in this sequence: the RBD screening questionnaire (RBDSQ) [25], the Innsbruck RBD inventory (RBD-I) [26], the Hong-Kong RBD questionnaire (RBDQ-HK) [27], the Mayo sleep questionnaire [28], the Innsbruck RBD summary question [26], and the RBD single-question screen (RBD1Q) [29]. Patients filled in the questionnaires and single questions alone, in the presence of a sleep medicine expert who answered eventual questions.
Actigraphy
All participants were asked to undergo 2 weeks of actigraphy (a minimum of 7 days was required), worn on the dominant hand. The MicroMini-Motionlogger (Ambulatory Monitoring, NY) was used for this study, with set epoch length of 30 seconds, and recorded: (1) activity, through a piezoelectric tri-axial accelerometer; (2) distal temperature; (3) environmental light; and (4) participant-set lights off and lights on events, representing begin and end of sleep time. Actigraphy data were imported using the Motionlogger WatchWare software version 1.94.2.0, analyzed and edited using the Action-W Version 2.7.1 software.
Manual analysis was performed to determine the nocturnal rest period (NRP) based on motor activity, environmental light, and distal temperature. The onset of the NRP was defined as the first epoch with lights off and a sudden decrease in wrist activity, with less than 100 zero crossing counts per 30 seconds. The NRP ended with the first epoch of sustained motor activity above 100 zero crossing counts per 30 seconds, occurring before or coinciding with environmental light exposure. Epochs with sudden light exposures and motor activity above 100 zero crossing counts per 30 seconds during the NRP were defined as probable wakefulness episodes and excluded from analysis, until light was switched off and the first epoch of motor activity below 100 zero crossing counts per 30 seconds appeared.
Quantitative analysis of the NRPs was performed, comprising: (1) duration of the NRP; (2) mean motor activity score (the mean movement counts/30 seconds); (3) activity index, indicating the percentage of 30 seconds epochs with an activity score above 0; (4) the acceleration index, measuring changes in activity speed during the NRP; (5) the brief wake ratio, defined as the number of awakenings (estimated using the Cole–Kripke algorithm [30]) of ≤1 minute divided by the number of awakenings of any length; (6) the short burst inactivity index, calculated as the number of episodes of zero recorded activity lasting exactly 1 minute divided by the number of episodes of zero activity lasting any amount of time, expressed as percentage, which is an indirect measure of inactivity fragmentation; and (7) the number of epochs characterized as probable wakefulness episodes during the NRP.
In addition, 2 week actigraphy readout on a single page (Figure S1) was visually classified by seven sleep medicine experts (EB, AH, AI, JS, FSD, CT, AV) blinded to sleep diagnosis, as “no RBD”, “possible RBD”, “probable RBD”. After a short introduction about the graphic representation of the visual variables of the MicroMini-Motionlogger (activity, light, temperature, probable wakefulness episodes) in order to allow a correct interpretation of the data, actigraphy data were shown in a randomized order to each rater separately. For each participant, visual readout of actigraphy was first shown without any additional information and subsequently once more with additional information including demographic data (age, gender, body mass index), information from the clinical history (presence of snoring or breathing pauses by history, presence of RLS), and information from scales and questionnaires (Epworth Sleepiness Scale, Innsbruck RBD-I, Innsbruck RBD summary question). For visual rating, the Innsbruck RBD-I and the Innsbruck RBD summary question were selected post hoc as these showed the highest sensitivity and specificity (see results) for iRBD in the current cohort. The raters classified each actigraphy pattern first without any clinical information, then with clinical and questionnaires information, as indicated above.
Apps use
As part of the study, each participant was asked to use a sleep app of his or her choice for 2 weeks, to assess if apps could be a potential screening tool in these patients’ groups. Teaching of app download and use was provided if required. Data about sleep apps use were collected.
If participants were unwilling to use apps, information about the reason was collected with a standardized interview.
Statistics
Statistical analysis was performed using SPSS 24 (SPSS, Inc., Chicago, IL). Data were tested for normal distribution using the Kolmogorov–Smirnov test. Descriptive statistics are given as numbers (percentages) as well as medians (range) and interquartile range (IQR), as data were not normally distributed. Nonparametric statistics were applied. For group comparison, one way ANOVA and Tukey post hoc test have been used for scalar variables, Pearson X2, Cramer’s V, and Fischer’s exact test (the latter for comparison between two groups) for nominal variables. For the questionnaires and single questions, as well as for the visual actigraphy analysis, sensitivity and specificity have been calculated. In addition, for the visual actigraphy analysis false-positive rate, false-negative rate, positive and negative predictive value, accuracy, likelihood ratio positive and negative have been calculated and receiver operating characteristic (ROC) analysis have been performed to calculate the area under the ROC curve (AUC). Comparison of AUCs was performed using MedCalc version 17.9.7. Fleiss’ kappa and intraclass correlation coefficients (ICCs) have been calculated to evaluate inter-rater reliability and agreement between raters, respectively. Cronbach’s alpha was calculated to evaluate internal consistency. P-values <0.05 were considered significant. In case of multiple comparisons, correction for Bonferroni was performed, and p-values were set accordingly.
Results
Clinical and demographic data
Ninety patients (74.4 per cent male, median age 54 years) were included in the study. Sex distribution was different between groups, but the sex distribution in the RBD group did not differ from that of all other groups. Age was different between groups, with RBD patients being older than patients with RLS, SA, and controls (Table S1). The ESS did not differ between groups (F = 0.625, p = 0.646) neither between iRBD and any other group (all p-values >0.9). Four patients with iRBD had an ongoing pharmacological treatment for RBD with Clonazepam (two patients were taking clonazepam 0.25 mg and two 0.5 mg). None of the iRBD patients included in this study were treated with melatonin. Ongoing RLS treatment was also continued during the study. In the iRBD groups, one patient was under therapy with α-2-δ-ligands, one patient with dopaminergic drugs. In the RLS group, two patients were taking α-2-δ-ligands and four patients dopaminergic drugs. Among patients with RLS and treated SA, one was under treatment with α-2-δ-ligands, three with dopaminergic drugs, and one with opioids. Other ongoing therapies with influence on sleep are listed in Table S2.
RBD screening questionnaires
In this study, sensitivity, specificity, and positive predictive values **for iRBD were: 80, 70, and 43 per cent for the RBDSQ; 85, 84, and 61 per cent for the Innsbruck RBD-I; 60, 86, and 55 per cent for the HK-RBD; 65, 97, and 87 per cent for the Innsbruck RBD summary question; and 60, 91, and 67 per cent for the RBD1Q (Table 1). Among the six questionnaires used in this study, the Innsbruck RBD-I demonstrated the best performance as the full questionnaire, the Innsbruck RBD summary question as the single question.
Table 1.
REM Sleep Behavior Disorder Questionnaires
| Total (n = 90) |
A. iRBD (n = 20) |
B. RLS (n = 20) |
C. RLS+ SA (n = 10) |
D. SA (n = 20) |
E. Controls (n = 20) |
X
2 / F (Cramér’s V) |
p | |
|---|---|---|---|---|---|---|---|---|
| RBDSQ, score | 4 (0–11) IQR 2–6 |
6 (3–11) IQR 5–9 |
5.5 (1–11) IQR 4–7.8 |
3 (0–11) IQR 2–4.5 |
2 (1–4) IQR 1–3 |
2.5 (0–7) IQR 0–4.8 |
13.493 | <0.001* A vs B 0.704 A vs C 0.017 A vs D <0.001* A vs E <0.001* |
| RBDSQ, pos N (%) | 37 (41.1) | 16 (80) | 14 (70) | 2 (20) | 0 (0) | 5 (25) | 37.336 (0.644) |
<0.001* A vs B 0.716 A vs C 0.004* A vs D <0.001* A vs E 0.001* |
| Innsbruck RBD-I, score | 0 (0–1) IQR 0–0.4 |
0.7 (0–1) IQR 0.4–0.8 |
0.2 (0–1) IQR 0–0.24 |
0 (0–1) IQR 0–0.31 |
0 (0–0.25) IQR 0–0.15 |
0 (0–0.6) IQR 0–0 |
15.435 | <0.001* A vs B <0.001* A vs C <0.001* A vs D <0.001* A vs E <0.001* |
| Innsbruck RBD-I, pos N (%) |
28 (31.1) | 17 (85) | 5 (25) | 3 (30) | 1 (5) | 2 (10) | 37.975 (0.650) |
<0.001* A vs B < 0.001* A vs C 0.005* A vs D <0.001* A vs E <0.001* |
| RBDQ-HK | 10 (0–66) IQR 1.5–18.5 |
24 (0–61) IQR 11–41 |
11.5 (0–66) IQR 4–18.8 |
0.5 (0–29) IQR 0–11.5 |
4 (0–17) IQR 0–8.8 |
10 (0–30) IQR 2–15.8 |
8.982 | <0.001* A vs B 0.017 A vs C <0.001* A vs D <0.001* A vs E 0.001* |
| RBDQ-HK, pos N (%) | 22 (24.4) | 12 (60) | 5 (25) | 1 (10) | 0 (0) | 4 (20) |
X
2 23.058 (0.509) |
<0.001* A vs B 0.025 A vs C 0.008* A vs D <0.001* A vs E 0.010 |
| Innsbruck RBD summary question, pos N (%) | 15 (16.7) | 13 (65) | 2 (10) | 0 (0) | 0 (0) | 0 (0) |
X
2 44.280 (0.701) |
<0.001* A vs B 0.001* A vs C 0.001* A vs D <0.001* A vs E <0.001* |
| RBD1Q, pos N (%) | 18 (20) | 12 (60) | 3 (15) | 1 (10) | 0 (0) | 2 (10) |
X
2 27.188 (0.550) |
<0.001* A vs B 0.008* A vs C 0.017 A vs D <0.001* A vs E 0.002* |
The Mayo Sleep Questionnaire is not shown as data are available only for patients with a bed partner.
Results of the RBD questionnaires. IQR, interquartile range; iRBD, idiopathic REM sleep behavior disorder; RBD1Q, REM sleep behavior disorder single-question screen; RBDQ-HK, Hong-Kong REM sleep behavior disorder questionnaire; RBD-I, REM sleep behavior disorder inventory; RBDSQ, REM sleep behavior disorder screening questionnaire; RLS, restless legs syndrome; SA, sleep apnea.
*Statistically significant after correction for multiple comparisons.
Quantitative actigraphy analysis
NRP, acceleration index, and probable wakefulness epochs did not differ between the groups, whereas activity score, activity index, and short burst inactivity index differed between groups. All these three parameters were higher in iRBD patients compared with controls; the activity index was significantly higher in iRBD also compared with SA patients (Table 2). Probable wakefulness epochs accounted for far below 10 per cent of the NRP.
Table 2.
Quantitative Actigraphy Analysis
| Total (n = 90) | A. iRBD (n = 20) |
B. RLS (n = 20) |
C. RLS+ SA (n = 10) |
D. SA (n = 20) |
E. Controls (n = 20) |
F, p | |
|---|---|---|---|---|---|---|---|
| NRP, min | 472 (333–641) IQR 436–502 |
486 (420–641) IQR 452–517 |
467 (333–554) IQR 430–497 |
482 (404–600) IQR 439–510 |
460 (358–579) IQR 429–483 |
467 (373–616) IQR 436–511 |
F 1.501, p = 0.209 A vs B 0.357 A vs C 0.986 A vs D 0.192 A vs E 0.759 |
| Activity score | 7.3 (2.7–43.4) IQR 5.6–9.9 |
10.3 (4.8–32.8) IQR 7.4–15.6 |
7.2 (3.9–43.4) IQR 5.4–9.6 |
7.7 (3.3–12.8) IQR 6.4–9.5 |
6.4 (3.5–28.4) IQR 5–11.1 |
6.7 (2.7–8.8) IQR 5.4–7.3 |
F 3.601, p = 0.009* A vs B 0.154 A vs C 0.250 A vs D 0.193 A vs E 0.003* |
| Activity index % | 22.4 (8.4–66.6) IQR 17.1–31.7 |
33.5 (15.7–62.8) IQR 28.6–49.1 |
22.7 (11–66.6) IQR 15.7–31.7 |
24.3 (10.1–32.9) IQR 13.4–31.4 |
19.3 (11.9–63.1) IQR 16.2–26.5 |
17.8 (8.4–33.9) IQR 16.4–22.2 |
F 6.783, p < 0.001* A vs B 0.011 A vs C 0.019 A vs D 0.005* A vs E <0.001* |
| Acceleration index | 0.04 (−0.5 to 0.4) IQR −0.1 to 0.1 |
0.07 (−0.4 to 0.3) IQR 0–0.1 |
0 (−0.5 to 0.3) IQR −0.15 to 0.07 |
0.2 (−0.2 to 0.4) IQR −0.1 to 0.3 |
0.04 (−0.2 to 0.3) IQR −0.04 to 0.2 |
0 (−0.2 to 0.3) IQR −0.07 to 0.13 |
F 1.759, p = 0.153 A vs B 0.933 A vs C 0.254 A vs D 1.000 A vs E 0.994 |
| Brief wake ratio | 0.4 (0.2–0.7) IQR 0.4–0.5 |
0.4 (0.3–0.5) IQR 0.3–0.5 |
0.5 (0.2–0.7) IQR 0.4–0.5 |
0.4 (0.3–0.5) IQR 0.3–0.5 |
0.4 (0.2–0.7) IQR 0.4–0.5 |
0.5 (0.2–0.7) IQR 0.4–0.6 |
F 2.579, p = 0.043 A vs B 0.478 A vs C 0.949 A vs D 0.886 A vs E 0.023 |
| Short burst inactivity index | 43.4 (19.4–78.9) IQR 35–54.6 |
56.9 (33.9–74.6) IQR 50.3–63 |
43.4 (25.7–67.3) IQR 34.7–51.3 |
49.8 (19.4–78.9) IQR 29.4–58 |
42 (25.7–78.3) IQR 36–47.7 |
36.7 (19.7–58) IQR 31.3–40.9 |
F 6.526, p = <0.001* A vs B 0.015 A vs C 0.118 A vs D 0.029 A vs E <0.001* |
Results of the quantitative analysis of actigraphy variables. IQR, interquartile range; iRBD, idiopathic REM sleep behavior disorder; NRP, nocturnal rest period; RLS, restless legs syndrome; SA, sleep apnea.
*Statistically significant after correction for multiple comparisons.
Visual actigraphy analysis
For all statistical analysis, we considered both “possible” and “probable” RBD as expert-ratings positive. Without clinical information, sensitivity to identify iRBD ranged between rates from 65 to 90 per cent, specificity from 64 to 86 per cent, and accuracy from 67 to 84 per cent.
In conjunction with provided clinical information, mean sensitivity was 87.9 ± 3.9 per cent, specificity 85.9 ± 4.8 per cent, positive predictive value 57.7 ± 11.3 per cent, negative predictive value 95.1 ± 3.3 per cent, accuracy 86.4 ± 3.8 per cent, positive likelihood ratio 6.8 ± 2.2 and negative likelihood ratio 0.14 ± 0.05 (Table 3).
Table 3.
Performance of the Blinded Raters’ Visual Actigraphy Analysis with Clinical Information
| SN % | SP % | FP rate | FN rate | PPV | NPV | Accuracy % | LR + | LR − | |
|---|---|---|---|---|---|---|---|---|---|
| Rater 1 | 85 | 80 | 20 | 15 | 55 | 88 | 81 | 4.25* | 0.19** |
| Rater 2 | 85 | 86 | 14 | 15 | 63 | 95 | 86 | 6.07** | 0.17** |
| Rater 3 | 90 | 91 | 9 | 10 | 75 | 97 | 91 | 10*** | 0.11** |
| Rater 4 | 95 | 79 | 21 | 5 | 56 | 98 | 82 | 4.52* | 0.06*** |
| Rater 5 | 90 | 87 | 13 | 10 | 50 | 97 | 88 | 6.92** | 0.11** |
| Rater 6 | 85 | 91 | 9 | 15 | 40 | 96 | 90 | 9.44** | 0.16** |
| Rater 7 | 85 | 87 | 13 | 15 | 65 | 95 | 87 | 6.54** | 0.17** |
FN, false negative; FP, false positive; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; SN, sensitivity; SP, specificity.
*Fair likelihood ratio.
**Very good likelihood ratio.
***Excellent likelihood ratio.
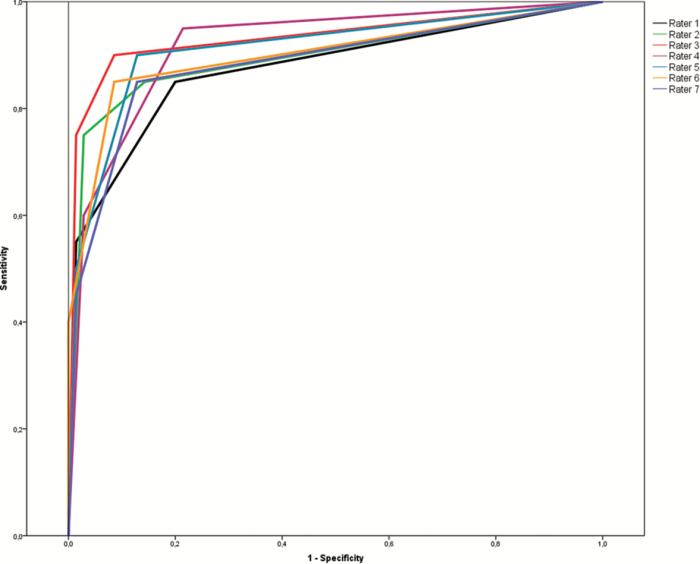
Area under the ROC curve (AUC) for each rater, with and without clinical information, for the whole sample and excluding the control group, is shown in Table 4 and Figure 1. Raters performed better than questionnaires in identifying iRBD. Comparison of the AUC between raters and questionnaires is shown in Table 5.
Table 4.
Area Under the ROC Curve Visual Actigraphy Analysis
| AUC wo information [CI] | AUC w information [CI] |
AUC wo information wo controls [CI] |
AUC w information wo controls [CI] |
|
|---|---|---|---|---|
| Rater 1 | 0.807 [0.681–0.933] | 0.874 [0.771–0.977] | 0.805 [0.677–0.933] | 0.867 [0.760–0.974] |
| Rater 2 | 0.720 [0.585-.0855] | 0.895 [0.795–0.995] | 0.712 [0.572–0.852] | 0.886 [0.781–0.990] |
| Rater 3 | 0.832 [0.711–0.954] | 0.933 [0.851–1.000] | 0.827 [0.703–0.951] | 0.926 [0.841–1.000] |
| Rater 4 | 0.821 [0.708–0.935] | 0.919 [0.847–0.991] | 0.790 [0.665–0.914] | 0.896 [0.813–0.979] |
| Rater 5 | 0.848 [0.736–0.960] | 0.911 [0.826–0.996] | 0.834 [0.717–0.950] | 0.901 [0.811–0.991] |
| Rater 6 | 0.792 [0.666–0.918] | 0.899 [0.802–0.996] | 0.776 [0.644–0.907] | 0.895 [0.796–0.994] |
| Rater 7 | 0.736 [0.604–0.868] | 0.884 [0.784–0.983] | 0.703 [0.561–0.844] | 0.867 [0.762–0.972] |
AUC, area under the ROC curve; CI, confidence interval; ROC, receiver operating characteristics; w, with; wo, without.
Figure 1.
ROC curve of the visual actigraphy analysis for each blinded rater. Each colored line represents one rater.
Table 5.
Comparison of the AUCs of Raters and Questionnaires
| RBDSQ | RBD-I | RBDQ-HK | Innsbruck summary question | RBD1Q | |
|---|---|---|---|---|---|
| Rater 1 | |||||
| p | 0.0035 | 0.1220 | 0.0046 | 0.0822 | 0.0101 |
| Δ areas | 0.154 | 0.0564 | 0.155 | 0.0974 | 0.152 |
| SE | 0.0528 | 0.0365 | 0.0546 | 0.0560 | 0.0592 |
| 95% CI | 0.0507–0.258 | −0.0151 to 0.128 | 0.0476–0.261 | −0.0124 to 0.207 | 0.0363–0.268 |
| Z | 2.921 | 1.546 | 2.831 | 1.738 | 2.574 |
| Rater 2 | |||||
| p | 0.0102 | 0.2812 | 0.0053 | 0.0642 | 0.0084 |
| Δ areas | 0.149 | 0.0515 | 0.150 | 0.0925 | 0.147 |
| SE | 0.0581 | 0.0478 | 0.0537 | 0.0500 | 0.0559 |
| 95% CI | 0.0354–0.263 | −0.0422 to 0.145 | 0.0444–0.255 | −0.00544 to 0.190 | 0.0378–0.257 |
| Z | 2.570 | 1.078 | 2.786 | 1.851 | 2.637 |
| Rater 3 | |||||
| p | 0.0001 | 0.0086 | 0.0008 | 0.0126 | 0.0012 |
| Δ areas | 0.187 | 0.0895 | 0.188 | 0.130 | 0.185 |
| SE | 0.0462 | 0.0340 | 0.0557 | 0.0523 | 0.0571 |
| 95% CI | 0.0967–0.278 | 0.0227–0.156 | 0.0784–0.297 | 0.0280–0.233 | 0.0734–0.297 |
| Z | 4.053 | 2.628 | 3.368 | 2.495 | 3.245 |
| Rater 4 | |||||
| p | 0.0005 | 0.0704 | 0.0013 | 0.0230 | 0.0015 |
| Δ areas | 0.176 | 0.0782 | 0.176 | 0.119 | 0.174 |
| SE | 0.0504 | 0.0432 | 0.0548 | 0.0524 | 0.0548 |
| 95% CI | 0.0772–0.275 | −0.00652 to 0.163 | 0.0689–0.284 | 0.0164–0.222 | 0.0666–0.282 |
| Z | 3.493 | 1.809 | 3.216 | 2.273 | 3.174 |
| Rater 5 | |||||
| p | 0.0005 | 0.0526 | 0.0027 | 0.0396 | 0.0036 |
| Δ areas | 0.166 | 0.0680 | 0.166 | 0.109 | 0.164 |
| SE | 0.0479 | 0.0351 | 0.0554 | 0.0530 | 0.0564 |
| 95% CI | 0.0719–0.260 | −0.000776 to 0.137 | 0.0575–0.275 | 0.00520–0.213 | 0.0534–0.274 |
| Z | 3.463 | 1.938 | 2.998 | 2.058 | 2.908 |
| Rater 6 | |||||
| p | 0.0065 | 0.2292 | 0.0029 | 0.0528 | 0.0047 |
| Δ areas | 0.152 | 0.0538 | 0.152 | 0.0947 | 0.150 |
| SE | 0.0556 | 0.0447 | 0.0509 | 0.0489 | 0.0530 |
| 95% CI | 0.0424–0.261 | 0.0339–0.141 | 0.0521–0.252 | −0.00116 to 0.191 | 0.0458–0.253 |
| Z | 2.723 | 1.203 | 2.983 | 1.936 | 2.826 |
| Rater 7 | |||||
| p | 0.0192 | 0.3934 | 0.0325 | 0.2141 | 0.0510 |
| Δ areas | 0.136 | 0.0387 | 0.137 | 0.0797 | 0.135 |
| SE | 0.0583 | 0.0454 | 0.0640 | 0.0642 | 0.0690 |
| 95% CI | 0.0222–0.251 | −0.0502 to 0.128 | 0.0114–0.262 | −0.0460 to 0.205 | −0.000609 to 0.270 |
| Z | 2.341 | 0.854 | 2.139 | 1.242 | 1.951 |
Statistically significant values are indicated in bold. CI, confidence interval; RBD, REM sleep behavior disorder; RBDSQ, REM sleep behavior disorder screening questionnaire; RBD-I, Innsbruck RBD inventory; RBDQ-HK, Hong-Kong RBD questionnaire; RBD1Q, RBD single-question screen; SE, standard error.
When clinical information was given, absolute agreement was good (intraclass correlation coefficient 0.754, Cronbach’s alpha 0.956). The inter-rater reliability showed substantial agreement for “no” and “probable” RBD (Fleiss’ kappa 0.650 and 0.670, respectively), and fair agreement for “possible” RBD (Fleiss’ kappa 0.297). With clinical information (including questionnaires information), the AUC of visual actigraphy analysis of each rater was better than the AUC of the best questionnaire (RBD-I, AUC 0.868), also when repeating analysis without controls (RBD-I, AUC 0.856).
Eight out of 20 patients with RBD were negative for at least one of the two questionnaires that were presented as clinical information. Raters recognized five to seven of them as positive. Eleven out of 70 patients without RBD resulted positive for at least one of presented questionnaire. Of them, 10–11 patients were scored as “no/possible” RBD, 5–9 patients as “no” RBD.
Apps
Sixty-three out of 90 participants (70 per cent) indicated to own a smartphone. There was a difference between groups (p < 0.001). Among participants having a smartphone, only 11 (17.5 per cent) were aware of apps for sleep analysis and 1 (1.6 per cent was currently using a sleep app. Forty-six patients with smartphone (73 per cent) agreed to use sleep apps for this study at the time of inclusion, whereas only 25 (39.7 per cent) de facto did so during the study. Of them, eight (32 per cent) needed help for finding and downloading the app. Median age of the 25 patients who used a sleep app was 53 (24–69) years. Apps used included “sleep better,” “sleep as android,” “SleepBot,” “sleep cycle,” “sleep sound,” “sleep time,” and “UP by Jawbone.” Among patients with smartphone not willing to use sleep apps for this study (n = 17), the most frequent reason was that they did not wish to use sleep apps (n = 13, 76.5 per cent). Other reasons were: too complicated (n = 2, 11.8 per cent), no internet connection (n = 1, 5.9 per cent) or not willing to have the smartphone close to the body during sleep (n = 1, 5.9 per cent).
Discussion
IRBD represents an early-stage α-synucleinopathy [5, 31–33]. Current screening methods for RBD are questionnaire-based; however, several recent studies showed that questionnaires are not reliable screening instruments for iRBD, as they showed low diagnostic value outside the context of validation studies, in which participants were already aware of investigated symptoms [13, 16–18]. We aimed to evaluate widely available, simple, and low-cost instruments, including actigraphy and smartphone sleep apps for identifying PSG-confirmed RBD. Visual actigraphy rating performed by experts in sleep medicine revealed high sensitivity, specificity, accuracy, and AUC in identifying iRBD, in particular if additional limited clinical information was available. This study shows that visual analysis of actigraphy could be a good screening instrument for iRBD in the general population.
As the most challenging differential diagnosis of iRBD based on evaluation of motor activity during sleep is movement related to SA and PLM, we designed this study to include these patients’ groups. Moreover, PLMS are common in RBD [22] and both SAS and PLMS are common in the elderly population [1, 33]. Therefore, these disorders can represent a major confounder when analyzing motor activity during sleep. As a suitable screening instrument would need to distinguish between these sleep disorders and RBD, we evaluated visual analysis of actigraphy excluding the healthy controls. This sub-analysis confirmed high performance of expert visual analysis of actigraphy.
According to our results, selected quantitative actigraphy parameters (activity score, activity index, short burst inactivity index) can be useful to distinguish RBD patients from controls but not from patients with other sleep-related motor activities, so that quantitative actigraphy analysis is according to our data not useful as screening method for RBD in the general population. Future actigraphy-based algorithm specifically developed for RBD, based on pattern recognition, would probably show a better performance. Quantitative actigraphy parameters for diagnosis of iRBD have been evaluated in two studies in PD patients [21, 34]. They reported high number of periods scored as wakefulness during the night in PD patients with RBD compared with PD patients without RBD. However, this “wake” time could represent not only RBD behaviors but also awakenings or other sleep-related motor activities. To reduce a possible bias, we excluded from analysis episodes manually scored as wakefulness based on light and motor activity.
Due to the characteristic temporal patterns distinctive of REM sleep during the night, we hypothesized that sleep experts could recognize RBD according to patterns of motor activity during sleep, for example based on (1) the presence of pseudo-periodic clusters of motor activity during sleep, in line with expected temporal patterns of REM sleep occurrence, and (2) the absence of motor activity during the first hour of nocturnal rest time. Such patterns of motor activity in the arm are expected to be different from those of SA and RLS. The usefulness of evaluating arm movements using EMG to achieve a correct diagnosis of RBD has been extensively demonstrated, as it improves the detection of RBD [22, 35–37]. Moreover, 65 per cent of REM-sleep-related behavioral events would be missed without recording of the upper-extremity EMG [35, 37]. Our hypothesis that wrist actigraphy can be useful in identifying motor patterns typical of RBD was confirmed as raters were able to recognize such patterns visually scoring actigraphy with high sensitivity and specificity. This study demonstrates that visual analysis of actigraphy indeed represents an easy, low-cost and useful screening instrument for RBD that can be applied to general population studies. Moreover, as opposed to questionnaires, actigraphy allows identification of RBD even in patients who are not aware of the abnormal behaviors. Visual recognition of activity patterns related to RBD allowed differentiation not only between RBD and controls, but also between RBD and other types of motor activities during sleep (e.g. PLM or movements related to SA), as shown by statistical analysis performed excluding the control group. Moreover, raters performed better than questionnaires in recognizing iRBD (Table 5), and could identify some iRBD patients that scored falsely negative with questionnaires. Therefore, visual analysis of actigraphy is able to recognize even patients with subclinical iRBD, not aware of the symptoms.
Other easy available and low-cost instruments, sleep apps, were used only by few participants. The majority of the participants in this study would not be willing to use apps as a screening tool. Moreover, as sleep assessment is based on the absence of motor activity, apps cannot currently be considered suitable in diagnosis of motor disorders of sleep and probably need further technical improvement before being studied as a screening method for a disease with such complex and protean motor manifestations.
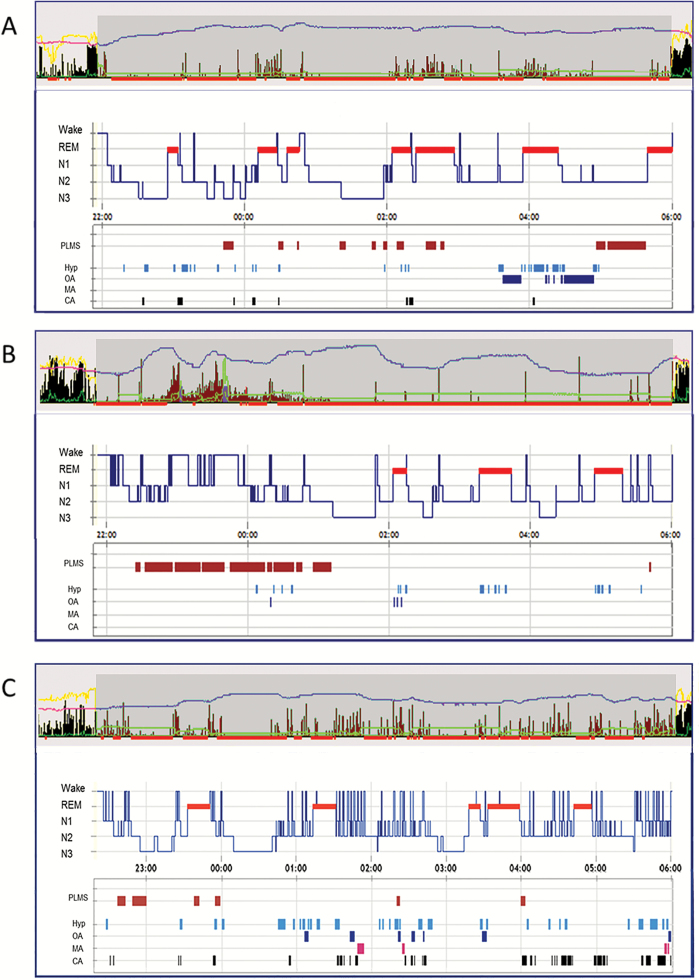
One potential limitation of this study is the age difference between groups, as patients’ groups were not matched. However, we think this is not a drawback as we aimed to compare different motor activities during sleep, which have per se different age prevalence. While we showed that providing simple clinical information is useful, we did not evaluate the impact of each single clinical variable. Actigraphy was not systematically performed during vPSG, so that registered activity could not be systematically compared with electromyographic activity during vPSG. This was done only in some patients, and examples are shown in Figure 2. Visual analysis performed by raters who are not experts in sleep medicine would probably not be as accurate, and it may require a specific training. Strengths of the study are the inclusion of patients with different types of sleep-related motor activities as well as the availability of vPSG data for all participants, allowing to confirm or rule out RBD.
Figure 2.
Examples of actigraphy patterns in patients with different type of motor activity during sleep. (A) Patient with REM sleep behavior disorder. The appearance of wrist activity measured by actigraphy (upper panel) is associated with episodes of REM sleep (PSG, lower panel). Activity during non-REM sleep is very low, even in the presence of PLM during sleep (PLMS) or respiratory events. (B) Patient with RLS. Wrist activity measured by actigraphy is associated with PLM during wakefulness (PLMW) and PLMS, occurring during the first half of the night. Of note, activity associated with PLMW has higher amplitude than activity associated with PLMS. During the few respiratory events as well as during REM sleep, no wrist activity has been registered. (C) Patient with SA. Wrist activity measured by actigraphy is associated with respiratory events, in REM and non-REM sleep. High-amplitude activity appears to be associated with arousals. In each part of the figure, the upper panel shows the wrist activity measured by actigraphy. Activity measured with zero crossing mode is represented by a black/brown histogram. Activity measured with proportional integral mode is represented by a green line. The yellow line represents the measured environmental light; the pink/violet line represents the measured distal body temperature. Red bars represent periods automatically marked as probable sleep time. The manually determined nocturnal rest period is represented by the darker grey box. The lower panel represents PSG data, including hypnogram (representing the sleep stages during the night), PLMS, and respiratory parameters. CA, central apneas; Hyp, hypopneas; MA, mixed apneas; OA, obstructive apneas; PLM, periodic leg movements; PLMS, periodic leg movements during sleep; PLMW, periodic leg movements during wakefulness; PSG, polysomnography; RBD, REM sleep behavior disorder; RLS, restless legs syndrome; SA, sleep apnea.
In summary, our data indicate that experts in sleep medicine are able to recognize the activity patterns of iRBD with simple visual analysis of actigraphy. Actigraphy is a promising screening method, which outperforms questionnaires alone according to our data. Actigraphy in conjunction with little clinical information might prove useful as a first step to identify iRBD in the general population, to select patients who will undergo vPSG for confirming diagnosis of early-stage α-synucleinopathy.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
The authors thank Heinz Hackner for his excellent realization of video-polysomnographies.
Funding
This study was supported by a grant from the Austrian Science Fund (FWF) to Birgit Högl, I 2120-B27. Ambra Stefani and Marc Guaita report support from the Austrian Science Fund, project number I 2120-B27.
Notes
Conflict of interest statement. None declared.
References
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Schenck CH, et al. Delayed emergence of a Parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):744–748. [DOI] [PubMed] [Google Scholar]
- 3. Iranzo A, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–453. [DOI] [PubMed] [Google Scholar]
- 4. Iranzo A, et al. ; SINBAR (Sleep Innsbruck Barcelona) group Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology. 2017;89(3):242–248. [DOI] [PubMed] [Google Scholar]
- 5. Högl B, et al. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat Rev Neurol. 2018;14(1):40–55. [DOI] [PubMed] [Google Scholar]
- 6. Iranzo A, et al. Severe obstructive sleep apnea/hypopnea syndrome mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. [DOI] [PubMed] [Google Scholar]
- 7. Gaig C, et al. Periodic limb movements during sleep mimicking REM sleep behavior disorder: a new form of periodic limb movement disorder. Sleep. 2017;40(3). [DOI] [PubMed] [Google Scholar]
- 8. Oudiette D, et al. Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep. 2009;32(12):1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derry CP. The sleep manifestations of frontal lobe epilepsy. Curr Neurol Neurosci Rep. 2011;11(2):218–226. [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Arcos A, et al. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep. 2016;39(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu HF, et al. Sleep-related injury in the elderly–an epidemiological study in Hong Kong. Sleep. 2000;23(4):513–517. [PubMed] [Google Scholar]
- 12. Kang SH, et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pujol M, et al. Idiopathic REM sleep behavior disorder in the elderly Spanish community: a primary care center study with a two-stage design using video-polysomnography. Sleep Med. 2017;40:116–121. [DOI] [PubMed] [Google Scholar]
- 14. Postuma RB, et al. Screening for prodromal Parkinson’s disease in the general community: a sleep-based approach. Sleep Med. 2016;21:101–105. [DOI] [PubMed] [Google Scholar]
- 15. Bušková J, et al. Screening for REM sleep behavior disorder in the general population. Sleep Med. 2016;24:147. [DOI] [PubMed] [Google Scholar]
- 16. Frauscher B, et al. A prospective questionnaire study in 100 healthy sleepers: non-bothersome forms of recognizable sleep disorders are still present. J Clin Sleep Med. 2014;10(6):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stiasny-Kolster K, et al. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson’s disease. Sleep Med. 2015;16(1):186–189. [DOI] [PubMed] [Google Scholar]
- 18. Stefani A, et al. Accordance of the REM-sleep behaviour disorder (RBD) screening questionnaire (RBDSQ): a population-based 2-year follow-up study. Mov Disord Clin Pract. 2017;4:403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louter M, et al. Actigraphy as a diagnostic aid for REM sleep behavior disorder in Parkinson’s disease. BMC Neurol. 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naismith SL, et al. The relationship between actigraphically defined sleep disturbance and REM sleep behaviour disorder in Parkinson’s disease. Clin Neurol Neurosurg. 2010;112(5):420–423. [DOI] [PubMed] [Google Scholar]
- 21. Allen RP, et al. ; International Restless Legs Syndrome Study Group Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. [DOI] [PubMed] [Google Scholar]
- 22. Frauscher B, et al. ; SINBAR (Sleep Innsbruck Barcelona group) Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31(5):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL and Vaughn BVfor the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasmnet.org. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 24. Stefani A, et al. Validation of a leg movements count and periodic leg movements analysis in a custom polysomnography system. BMC Neurol. 2017;17(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stiasny-Kolster K, et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord. 2007;22(16):2386–2393. [DOI] [PubMed] [Google Scholar]
- 26. Frauscher B, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27(13):1673–1678. [DOI] [PubMed] [Google Scholar]
- 27. Li SX, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med. 2010;11(1):43–48. [DOI] [PubMed] [Google Scholar]
- 28. Boeve BF, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12(5):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Postuma RB, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cole RJ, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 31. Högl B, et al. Rapid eye movement sleep behavior disorder and other rapid eye movement sleep parasomnias. Continuum (Minneap Minn). 2017;23(4, Sleep Neurology):1017–1034. [DOI] [PubMed] [Google Scholar]
- 32. Iranzo A, et al. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016;15(4):405–419. [DOI] [PubMed] [Google Scholar]
- 33. Sixel-Döring F, et al. The evolution of REM sleep behavior disorder in early Parkinson disease. Sleep. 2016;39(9):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fantini ML, et al. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59(12):1889–1894. [DOI] [PubMed] [Google Scholar]
- 35. Iranzo A, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12(3):284–288. [DOI] [PubMed] [Google Scholar]
- 36. Frauscher B, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernández-Arcos A, et al. Diagnostic value of isolated mentalis versus mentalis plus upper limb electromyography in idiopathic REM sleep behavior disorder patients eventually developing a neurodegenerative syndrome. Sleep. 2017;40(4). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.