Abstract
Study Objectives
To quantify the association between insomnia or poor sleep with objective short sleep duration and incident cardiovascular disease (CVD) and mortality in the general population.
Methods
We conducted a time-to-event analysis of Sleep Heart Health Study data. Questionnaires and at-home polysomnography (PSG) were performed between 1994 and 1998. Participants were followed for a median of 11.4 years (Q1-Q3, 8.8–12.4 years) until death or last contact. The primary exposure was insomnia or poor sleep with short sleep defined as follows: difficulty falling asleep, difficulty returning to sleep, early morning awakenings, or sleeping pill use, 16–30 nights per month; and total sleep of <6 hr on PSG. We used proportional hazard models to estimate the association between insomnia or poor sleep with short sleep and CVD, as well as all-cause mortality.
Results
Among 4994 participants (mean age: 64.0 ± 11.1 years), 14.1 per cent reported insomnia or poor sleep, of which 50.3 per cent slept <6 hr. Among 4437 CVD-free participants at baseline, we observed 818 incident CVD events. After propensity adjustment, there was a 29 per cent higher risk of incident CVD in the insomnia or poor sleep with short sleep group compared with the reference group (HR: 1.29, 95% CI: 1.00, 1.66), but neither the insomnia or poor sleep only nor short sleep only groups were associated with higher incident CVD. Insomnia or poor sleep with objective short sleep was not associated with all-cause mortality (HR: 1.07, 95% CI: 0.86, 1.33).
Conclusions
Insomnia or poor sleep with PSG-short sleep was associated with higher risk of incident CVD. Future studies should evaluate the impact of interventions to improve insomnia with PSG-short sleep on CVD.
Keywords: insomnia, short sleep duration, cardiovascular disease, mortality, epidemiology
Statement of Significance.
Cardiovascular disease (CVD) remains the leading cause of death in the world, despite efforts to control known risk factors. Identifying novel risk factors may further improve CVD outcomes. Previous work suggests that insomnia with objective short sleep represents a more severe phenotype than short sleep occurring without insomnia symptoms, or insomnia symptoms occurring without short sleep. However, whether insomnia with objective short sleep confers a higher risk of major cardiovascular events, such as heart attack and stroke, is unknown. The present study is the first to quantify the association between insomnia or poor sleep with polysomnographically measured short sleep duration and incident CVD. As such, it seeks to determine whether the concurrent presence of these two sleep traits affects CVD event rates.
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality for both men and women worldwide. In the United States, CVD accounted for 28 per cent of all deaths in 2014 [1], despite public health initiatives addressing established risk factors. As such, there has been a call to rebalance efforts to mitigate CVD risk factors as well as to redirect attention towards examining novel lifestyle factors, such as sleep disturbance [2].
Insomnia and short sleep duration are associated with pathophysiological mechanisms underlying CVD [3–5], and each has been associated with prevalent and incident CVD [3, 6–8]. Insomnia symptoms, evaluated independent of sleep duration, also have been associated with hypertension, diabetes, and increased risk for coronary artery disease and congestive heart failure [3, 9, 10]. Although most epidemiological studies have evaluated each exposure (insomnia; short sleep) without consideration of the other, seminal work by Vgontzas, Fernandez-Mendoza, et al. suggests that the co-occurrence of insomnia with objective short sleep duration represents the most biologically severe phenotype of insomnia disorder [11, 12]. Of particular relevance to CVD, data derived from several experimental and community-based studies have indicated that insomnia with objective short sleep duration is associated with activation of the hypothalamic–pituitary–adrenal axis [11, 13–15] and increased neurocognitive-physiological arousal [13, 16–18]. The clinical importance of these findings is reflected in epidemiological data, mainly from a single cohort, which suggest that combining polysomnography (PSG) data on sleep duration with information on insomnia can identify individuals at higher risk for diabetes [19] and hypertension [20, 21], and men at increased risk of all-cause mortality [22].
Given the literature indicating that insomnia plus PSG-measured short sleep duration is associated with an increased burden of CVD risk factors, we hypothesized that this phenotype would also confer higher risk of incident CVD. Therefore, in this study, we quantified the association between self-reported insomnia symptoms or poor sleep with objective short sleep duration and incident CVD in a sample of 4994 men and women, participating in the Sleep Heart Health Study (SHHS), representing the largest, prospective cohort with information on insomnia and PSG-determined sleep duration to date.
Given that only one previous study examined the association between insomnia with PSG-short sleep and all-cause mortality, we also evaluated the association of this phenotype with all-cause mortality. Due to the greater availability of self-reported sleep duration compared with PSG sleep duration, we also explored whether self-reported short sleep and self-reported insomnia symptoms or poor sleep were associated with incident CVD. Finally, we explored whether any associations differed by sex.
Methods
Study sample
Data were derived from the SHHS, a multicenter, community-based prospective cohort study designed to evaluate associations between sleep-disordered breathing and CVD. Between 1995 and 1998, 6441 participants were enrolled from existing community-based cohorts, as described previously [23]. Eligible participants were at least 40 years old and not receiving active treatment for obstructive sleep apnea (e.g. continuous positive airway pressure, oral appliance, and oxygen therapy) [24]. Habitual snorers of less than 65 years old and persons of ethnic minorities were oversampled. The samples used in this analysis consist data from four “parent” cohorts (Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Framingham Heart Study, and Tucson Epidemiologic Study of Airway Obstructive Disease and the Health and Environment Study), and are publicly available (National Sleep Research Resource; sleepdata.org).
Our analytical sample included all SHHS participants who successfully completed baseline PSG. Of these 5061 participants, 67 were excluded for missing covariate data, leaving 4994 participants for analyses. For the incident CVD analyses, we excluded an additional 557 participants with prevalent CVD at baseline.
Protocol
At baseline, participants completed a Sleep Habits Questionnaire that elicited standardized information on sleep symptoms and patterns. Trained research technicians conducted interviews querying about cardiovascular health, health habits, and medication use; performed anthropometry and blood pressure measurements; and obtained at-home single-night unattended PSG [25]. PSG consisted of a portable monitor (Compumedics P-series, Abbotsford, Victoria, Australia) recording of electroencephalogram, electrooculogram, electrocardiogram, chin electromyogram, pulse oximetry, chest and abdominal excursion by inductance plethysmography, airflow by thermal sensor, and body position channels. Scoring was performed at central reading center [26, 27]. Additional covariate data were provided by the parent cohorts and included cardiovascular event and mortality surveillance through May 2011. Our analyses were considered exempt by our Institutional Review Board based on 45CFR46.101(b)(4).
Incident cardiovascular disease and mortality outcomes
Incident CVD was defined as the date of first event of nonfatal or fatal myocardial infarction, angina pectoris, revascularization procedure (i.e. percutaneous transcutaneous angioplasty, coronary stent placement, or coronary artery bypass grafting), or stroke between date of PSG and end of follow-up. CVD surveillance was performed by the parent cohorts according to cohort-specific protocols that have been previously described [28]. The cohorts conducted annual questionnaires or telephone follow-up and periodic research examinations [29]. When a participant was not able to be reached, all known contacts were called to determine the participant’s vital status. All potential events, including deaths, were investigated through review of medical records. Information from hospital records included electrocardiograms, stress tests, heart catheterizations, cardiac surgery, angioplasty, echocardiography, nuclear medicine scans, radiographs, computed tomography and magnetic resonance imaging, cerebral angiograms, lumbar punctures, pathology reports, death certificates, autopsy reports, and laboratory tests. Searches of community obituaries and local death registries linked to the Social Security Administration Death Master File [29] were also performed. Event classifications were reviewed by standing committees and adjudicated according to predefined protocols [30–32].
Assessment of the primary exposure: insomnia or poor sleep with short sleep duration
We classified participants as having “insomnia or poor sleep” based upon self-report of at least one of the following four questionnaire items, with affirmative responses occurring 16–30 nights per month: “Have trouble falling asleep,” “Wake up during the night and have difficulty getting back to sleep,” “Wake up too early in the morning and be unable to get back to sleep,” or “Take sleeping pills or other medication to help you sleep.” A self-report of diagnosed insomnia was not included in this definition. PSG-measured sleep duration was derived from the overnight sleep study, scored using Rechtschaffen and Kales criteria at a centralized reading center by trained research technicians blinded to all clinical data [25]. The primary definition of short sleep duration was defined as total sleep time of less than 6 hr on PSG, a threshold commonly used to define short sleep in adults in the epidemiological literature, including in the Penn State cohort [6, 33]. In secondary analyses, self-reported average sleep duration was based on responses to the questions: “How many hours of sleep do you usually get at night (or your main sleep period) on weekdays or workdays?” and “How many hours of sleep do you usually get at night (or your main sleep period) on weekends or your non-workdays?” Weekday hours were weighted in a 5:2 ratio with weekend hours. Given our interest in evaluating risk associations with phenotypes described by both the presence and absence of short sleep and the presence and absence of insomnia or poor sleep, we classified each person according to the four categories: (1) neither insomnia or poor sleep nor short sleep duration (reference group); (2) insomnia or poor sleep only; (3) short sleep duration only; and (4) insomnia or poor sleep with short sleep duration.
Additional covariates
Sociodemographic characteristics and health habits included baseline age, sex, race/ethnicity (non-Hispanic white, other), and smoking status (current, former, never). Apnea–hypopnea index (AHI) was derived from the PSG [25]. AHI was defined as the average number of obstructive apneas plus hypopneas associated with ≥3 per cent oxygen desaturation per hour of sleep. Antidepressants use within 2 weeks of the exam was identified by standardized recording of medication usage. Cardiometabolic factors included body mass index (BMI) determined by measured weight and height, hypertension, and total cholesterol and high-density lipoprotein (HDL). Blood pressure was measured via a standardized protocol. Hypertension was defined as an average systolic blood pressure (SBP) of >140 mm Hg or average diastolic blood pressure (DBP) of >90 mm Hg, or use of antihypertensive medications. Diabetes status was determined by self-report or use of insulin or hypoglycemic medications. Total cholesterol and HDL cholesterol values were measured by the parent cohorts (n = 4568).
Statistical analysis
Analyses were performed using SAS (v. 9.3, Cary, NC). For each analysis, survival time was defined from date of baseline PSG until date of first event or date of censor. Censoring time was the time of last known event-free status for those without incident events. Baseline characteristics are reported for each of the four insomnia or poor sleep and/or short sleep duration combinations. Differences in baseline characteristics across groups were tested using Pearson’s chi-squared tests for categorical variables and ANOVA for continuous variables. Unadjusted time-to-mortality (or CVD) event analysis was conducted by plotting the Kaplan–Meier cumulative incidence curves for each group and computing the log-rank statistic. To adjust for confounding in the time-to-event analysis and to improve model efficiency, a multiple propensity approach was used [34]. Briefly, multiple propensity scores were created in a multinomial logistic regression of age, sex, race, smoking status, AHI, BMI, antidepressant use, and history of CVD (for mortality analyses only) on the four groups (dependent variable). After obtaining the propensity scores from this model, a propensity-adjusted Cox Proportional Hazards model was formed using our exposure of interest (four groups), along with the multiple propensity scores, which were fitted with restricted cubic spline functions. Box-and-whisker plots of the distributions of the multiple propensity scores were examined to ensure adequate overlap between scores. Propensity model balance was confirmed with all model covariates having p > .05 when re-running the propensity model with the addition of the multiple propensity scores. The proportional hazards assumption was tested using a time-dependent interaction term between the four exposure combinations and follow-up time; we found no evidence that the relationship between our exposures and either outcome varied over time during the follow-up period (CVD, p = .61; all-cause mortality, p = .82). Additive interaction was assessed using the relative excess risk due to interaction (RERI) [35]. Wald chi-squared p-values for the multiplicative and additive interaction terms, as well as for the three exposure groups versus the reference group, were used to determine significance at the p < .05 level, and hazard ratios and corresponding 95% confidence intervals were determined.
In prespecified secondary analyses, we examined the relationship between insomnia or poor sleep with self-reported (rather than objective PSG) sleep duration and incident CVD and mortality. We also examined models that included hypertension, diabetes, total cholesterol, and HDL (potential mediators). We repeated these procedures in sensitivity analyses for the outcome, all-cause mortality. We performed tests of interaction to examine potential sex differences. Using similar procedures, we conducted sensitivity analyses examining the relationships between insomnia or poor sleep with objective short sleep duration and “hard” CVD outcomes (i.e., myocardial infarction and CVD death only) as well as coronary heart disease (CHD: myocardial infarction, revascularization, and CHD death only). We performed additional sensitivity analyses using less than 5 hr as our cutoff for short sleep duration and ran additional models excluding participants reporting antidepressant use and hypnotic use for both outcomes of interest, separately. Lastly, we excluded participants from studies where battery limitations resulted in termination of the PSG recording before final awakening (n = 1713).
Results
Baseline characteristics of the participants by self-reported insomnia or poor sleep and objective short sleep duration category are displayed in Table 1. Participants with insomnia or poor sleep were of similar age to participants without insomnia or poor sleep within strata of sleep duration. As expected, participants with insomnia or poor sleep tended to be female and, within strata of sleep duration, reported higher rates of smoking and antidepressant use, compared with participants without insomnia or poor sleep. Hypertension was also more prevalent among participants with insomnia or poor sleep within sleep duration strata as well as among short sleepers with and without insomnia or poor sleep.
Table 1.
Baseline characteristics of participants from the Sleep Heart Health Study, 1995–1998 (n = 4994)
| Characteristic | No insomnia/poor sleep | Insomnia or poor sleep | Overall n = 4994 |
P* | ||
|---|---|---|---|---|---|---|
| Normal sleep duration (n=2,489, 49.8%) | Short sleep duration (n=1,801, 36.1%) | Normal sleep duration (n=350, 7.0%) |
Short sleep duration (n=354, 7.1%) |
|||
| Age (years), mean ± SD | 62.1 ± 11.1 | 66.3 ± 10.7 | 63.2 ± 11.0 | 66.1 ± 11.2 | 64.0 ± 11.1 | <.0001 |
| Race, n (%) | .83 | |||||
| White |
2179 (87.5%) | 1566 (87.9%) | 301 (86.0%) | 307 (86.7%) | 4353 (87.2%) | |
| Black/other | 310 (12.5%) | 235 (13.1%) | 49 (14.0%) | 47 (13.3%) | 641 (12.8%) | |
| Female, n (%) | 1,412 (56.7%) | 789 (43.8%) | 268 (76.6%) | 205 (57.9%) | 2,674 (53.5%) | <.0001 |
| Smoking status, n (%) | <.001 | |||||
| Current | 217 (8.7%) | 177 (9.8%) | 38 (10.9%) | 48 (13.6%) | 480 (9.6%) | |
| Former | 1,054 (42.4%) | 851 (41.3%) | 148 (42.3%) | 153 (43.2%) | 2206 (44.2%) | |
| Never | 1218 (48.9%) | 773 (42.9%) | 164 (46.8%) | 153 (43.2%) | 2308 (46.2%) | |
| Antidepressant usage, n (%) | 140 (5.6%) | 96 (5.3%) | 82 (23.4%) | 55 (15.5%) | 373 (7.5%) | <.0001 |
| Weekday,self-reported sleep duration (hr/night), mean ± SD | 7.3 ± 1.0 | 7.0 ± 1.2 | 6.4 ± 1.4 | 5.9 ± 1.5 | 7.1 ± 1.2 | <.0001 |
| Weekend, self-reported sleep duration (hr/night), mean ± SD | 7.7 ± 1.1 | 7.4 ± 1.2 | 6.7 ± 1.6 | 6.2 ± 1.6 | 7.4 ± 1.3 | <.0001 |
| AHI (events/hr), median (IQR) | 8.5 (3.5,17.2) | 11.0 (5.2, 22.0) | 7.6 (3.3,16.0) | 9.8 (4.2,19.0) | 9.4 (4.0,19.1) | <.0001 |
| BMI (kg/m2), median (IQR) | 27.4 (24.7,30.6) | 28.0 (24.9,31.2) | 27.6 (24.9,30.9) | 28.0 (24.8,31.4) | 27.6 (24.8,30.9) | <.01 |
| Hypertension, n (%) | 860 (34.6%) | 833 (46.3%) | 137 (39.1%) | 186 (52.5%) | 2,016 (40.4%) | <.0001 |
| Baseline cardiovascular disease, n (%) | 319 (12.8%) | 313 (17.4%) | 35 (10.0%) | 69 (19.5%) | 736 (14.7%) | <.0001 |
| Total cholesterol (mg/dL), mean ± SD | 205 ± 37 | 205 ± 38 | 213 ± 38 | 212 ± 40 | 207 ± 38 | .0001 |
| HDL-C (mg/dL), mean ± SD | 51.0 ± 15.3 | 49.5 ± 15.8 | 54.0 ± 17.1 | 50.3 ± 15.9 | 50.6 ± 15.7 | <.0001 |
| Diabetes, n (%) | 158 (6.3%) | 168 (9.3%) | 18 (5.1%) | 27 (7.6%) | 371 (7.4%) | <.01 |
AHI = apnea–hypopnea index; BMI = body mass index; HDL-C = high-density lipoprotein-cholesterol.
*Pearson’s chi-squared test for categorical characteristics; ANOVA for continuous characteristics.
Incident CVD
Among 4437 CVD-free participants at baseline, we observed 818 incident CVD events, including 99 participants with angina pectoris, 222 myocardial infarctions, 250 coronary revascularization procedures, 159 strokes, and 88 CVD deaths during a median follow-up period of 11.4 years (IQR, 8.8–12.4 years). The unadjusted rate of incident CVD was 15.5 events per 1000 person-years of follow-up in the reference group (no insomnia or poor sleep, sleep duration >6 hr), 14.4 events per 1000 person-years in the insomnia or poor sleep only group, 21.8 events per 1000 person-years in the short sleep duration only group, and 25.8 events per 1000 person-years in the insomnia or poor sleep with short sleep duration group.
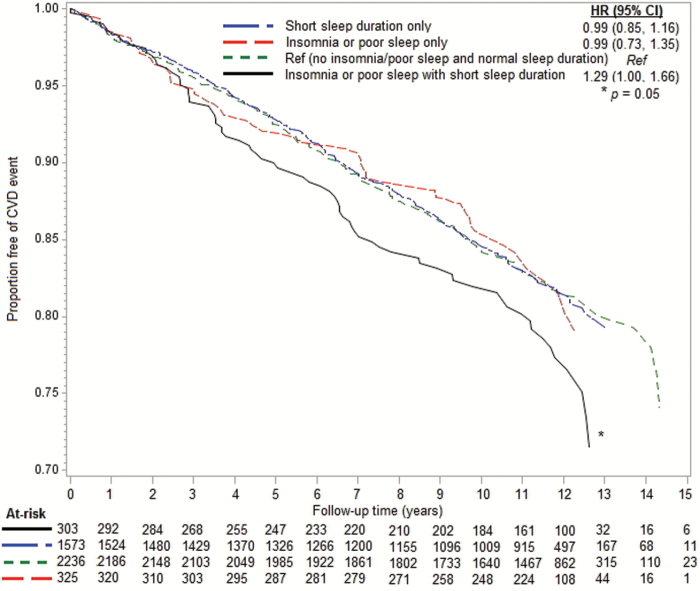
Figures 1 and 2 display the adjusted CVD risk associated with insomnia or poor sleep and sleep duration status. In propensity score adjusted models, there was a 29 per cent higher rate of incident CVD in the insomnia or poor sleep with short sleep duration group compared with the reference group (HR: 1.29, 95% CI: 1.00, 1.66). Neither the insomnia or poor sleep only group (HR: 0.99, 95% CI: 0.73, 1.35) nor the short sleep duration only group (HR: 0.99, 95% CI: 0.85, 1.16) differed from the reference group. Among participants with short sleep duration, insomnia or poor sleep conferred a 30 per cent higher risk of CVD (HR: 1.30, 95% CI: 1.01, 1.67). However, neither the test of multiplicative interaction (p = .18) nor a measure of additive interaction (RERI: 0.30, 95% CI: −0.52, 1.12; p = .48) was statistically significant. Test for interaction by sex was nonsignificant (p = .24).
Figure 1.
Propensity-adjusted cardiovascular disease event-free survival by insomnia or poor sleep and short sleep duration status.
Figure 2.
Propensity-adjusted hazard ratios for incident CVD by insomnia or poor sleep and sleep duration status.
Prespecified secondary analyses suggested similar although less precise estimates for the association between insomnia or poor sleep with self-reported short sleep duration and CVD (insomnia or poor sleep with self-reported short sleep: adjusted HR, 1.28; 95% CI, 0.94, 1.80). We found no evidence that hypertension mediated the association between insomnia or poor sleep with objective short sleep duration and CVD. Results did not change with adjustment for additional CVD risk factors (i.e. diabetes, total cholesterol, and HDL) or including both hypertension and diabetes in the model (Table 2). Results were similar in our sensitivity analyses when we defined our outcomes as CHD only or “myocardial infarction and CVD death only”, excluded participants with antidepressant use, excluded participants with hypnotic use, and excluded participants with truncated PSG recordings related to battery life. When we redefined our exposure as insomnia or poor sleep with PSG-short sleep duration (<5 hr), the hazard ratio increased, but this estimate was less precise (HR: 1.52, 95% CI: 1.08, 2.14).
Table 2.
Hazards ratios (95% CI) for incident CVD analyses
| Model | No insomnia/poor sleep | Insomnia or poor sleep | ||
|---|---|---|---|---|
| Normal sleep duration (n = 2236, 50.4%) | Short sleep duration (n = 1573, 35.5%) | Normal sleep duration (n = 325, 7.3%) | Short sleep duration (n = 303, 6.8%) | |
| Model 1: Age-sex adjusted | 1.0 (ref) | 1.00 (0.87, 1.17) | 1.02 (0.76, 1.38) | 1.31 (1.02, 1.68) |
| Model 2: Propensity score adjusted | 1.0 (ref) | 0.99 (0.85, 1.16) | 0.99 (0.73, 1.35) | 1.29 (1.00, 1.66) |
| Model 2 + Hypertension | 1.0 (ref) | 0.97 (0.83, 1.13) | 0.97 (0.71, 1.31) | 1.22 (0.96, 1.58) |
| Model 2 + Hypertension + diabetes | 1.0 (ref) | 0.97 (0.83, 1.13) | 1.01 (0.74, 1.37) | 1.19 (0.91, 1.54) |
| Model 2 + Hypertension + diabetes + lipids | 1.0 (ref) | 0.96 (0.81, 1.14) | 1.03 (0.72, 1.43) | 1.29 (0.97, 1.71) |
Mortality
Among 4994 participants at baseline, we observed 1163 deaths, including 355 CVD deaths, during a median follow-up period of 11.6 years (IQR: 10.2–12.4 years). The rate of death (per 1000 person-year) was 17.3 in the reference group (no insomnia or poor sleep, sleep duration >6 hours), 19.1 in the insomnia or poor sleep only group, 27.8 in the short sleep duration only group, and 26.6 in the insomnia or poor sleep with short sleep duration group.
Figure S3 (supplementary materials) displays the adjusted mortality risk associated with insomnia or poor sleep and sleep duration status. In propensity score–adjusted models, there was a 14 per cent higher rate of death in the short sleep duration group compared with reference group (HR: 1.14, 95% CI: 1.01, 1.30). Neither the insomnia or poor sleep only group (HR: 0.99, 95% CI: 0.77, 1.26) nor the insomnia or poor sleep with short sleep duration group (HR: 1.07, 95% CI: 0.86, 1.33) differed from the reference group. Neither the test of multiplicative interaction (p = .75) nor the measure of additive interaction (RERI: −0.18, 95% CI: −0.60, 0.24; p = .48) was statistically significant. Test for interaction by sex was nonsignificant (p = .62).
In prespecified secondary analyses, results did not differ substantively when we explored the association between insomnia or poor sleep with self-reported short sleep duration and mortality (HR: 1.03, 95% CI: 0.80, 1.33). Furthermore, we found no evidence that hypertension or diabetes mediated the association between insomnia or poor sleep with objective short sleep duration and mortality. Results were similar when we excluded participants with hypnotic use or truncated PSG recordings. When we redefined our exposure using insomnia or poor sleep with PSG-short sleep duration (<5 hr), the hazard ratio increased, but this estimate was less precise (HR: 1.25, 95% CI: 0.94, 1.65).
Discussion
In the largest prospective cohort study of insomnia or poor sleep with objective short sleep duration to date, we found that participants with insomnia or poor sleep characterized by symptoms occurring 16–30 times per month, and a PSG-defined sleep duration of less than 6 hr had a 29% higher risk of incident CVD compared with participants without insomnia or poor sleep and an objective sleep duration of at least 6 hr. Presence of insomnia or poor sleep only (with at least 6 hr of PSG sleep duration) or short sleep only (without insomnia or poor sleep) was not associated with incident CVD. Results from secondary analyses suggest that insomnia or poor sleep with self-reported short sleep duration is similarly associated with increased CVD incidence, though estimates were less precise and not statistically significant. Lastly, the associations between insomnia or poor sleep with objective short sleep duration and CVD were not attenuated after adjusting for hypertension or diabetes.
Our findings are consistent with prior studies that showed higher risk of CVD risk factors in association with insomnia with PSG-determined short sleep, but not insomnia with normal objective sleep duration [19–21, 36]. The only other prospective cohort study examining insomnia with objective short sleep duration and CVD risk to date (the Penn State community–based cohort study) observed a higher incidence of hypertension with insomnia and PSG short sleep compared with the reference group (no insomnia; sleep duration >6 hr) [19]. We extend these findings by showing that this phenotype is also associated with a higher risk of incident, major cardiovascular events, further supporting the clinical relevance of this phenotype. Thus, our findings support the hypothesis, suggested by Vgontzas and colleagues, that insomnia with objective short sleep duration is a unique phenotype that is associated with higher CVD risk. Previous clinical and experimental studies investigating potential mechanisms underlying this association have suggested that patients with insomnia and objective short sleep duration display dysregulation of the hypothalamic–pituitary–adrenal axis and glucose metabolism [11, 14, 37] and alterations in cardiovascular autonomic control [16, 17], as well as abnormal brain metabolism [17]. Furthermore, our findings suggest that future investigations of sleep-related CVD risk should consider insomnia symptoms and sleep duration concurrently, as considering only one feature may provide an incomplete characterization of clinically relevant sleep phenotypes and their impact on health outcomes.
In contrast to our findings regarding incident fatal or nonfatal CVD, we found that short sleep duration only, but not insomnia or poor sleep with PSG-defined short sleep, was significantly associated with all-cause mortality. In contrast, the Penn State Cohort previously reported that insomnia with objective short sleep was associated with increased mortality in men [22]. These discrepant findings may be due to cohort differences (Penn State vs SHHS) in the following: (1) variation in the definition of insomnia, with Penn State defining insomnia as a self-reported complaint of insomnia with a duration of at least 1 year; (2) differences in PSG (in-lab for Penn State and in-home for SHHS); (3) analytic approaches (logistic regression vs survival analysis); (4) prevalence of diabetes (21.1% in men vs 7.4% overall), which may mediate or modify the effect of insomnia (or poor sleep) with short sleep duration on mortality; (5) number of deaths observed (Penn State 145 men/103 women vs SHHS 619 men/544 women); and (6) mean age of each cohort when first studied (42 years vs 64 years for the Penn State vs SHHS, respectively). In regard to this difference, the observed effects of insomnia or sleep disturbance on mortality may vary by age of the cohort, and this may be attributable to variation in insomnia or sleep disturbance by age, competing risks, or differential baseline mortality rates. The health impact of insomnia also may be more salient when chronicity is accounted for, as was done in the Penn State cohort, or characterized by daytime impairment. Although our definition of insomnia or poor sleep required a high symptom frequency (16–30 days), our definition did not include criteria related to daytime impairment, chronicity, or formal diagnostic assessment, and therefore, our classification of insomnia or poor sleep may represent a less severe phenotype.
We also explored whether insomnia or poor sleep with self-reported short sleep yielded similar associations with CVD compared with short sleep defined by single-night PSG. Although there is only weak correlation (r = 0.16) between self-reported and PSG-defined sleep duration in this cohort, insomnia or poor sleep with self-reported short sleep suggested comparable, although less precise, estimates of CVD risk compared with insomnia or poor sleep and PSG-defined short sleep. Insomnia or poor sleep with short self-reported sleep duration has previously been shown in several [38–41], but not all, prospective studies to predict CVD and mortality [42, 43]. Additionally, one cross-sectional study that used diagnostic criteria for insomnia disorder observed higher risk of stroke and myocardial infarction in participants with insomnia with self-reported short sleep [44]. The possibly stronger and unique information provided by PSG-measured sleep duration is consistent with recent findings by Bathgate et al., who showed that hypertension was associated with insomnia with PSG-short sleep, but not with insomnia with diary-reported short sleep [20].
It should also be noted that each measurement approach has different strengths and weaknesses and may identify somewhat different subgroups. Although PSG is the gold-standard for assessing sleep, its costs and burden preclude its widespread use in epidemiological and clinical studies. Data from a single night study may also underestimate sleep duration, a phenomenon referred to as a “first night effect.” Although commonly considered a source of measurement error, it is possible that individuals with a greater “first night effect” (or shorter sleep duration) comprise a group with heightened arousal responses that place them at increased risk for adverse health outcomes [45]. Supporting this notion, studies that have >1 night of in-laboratory PSG [46, 47] and at-home actigraphy [13, 16] have observed physiological hyperarousal in insomniacs with short sleep duration. Alternatively, self-reported sleep, which is relatively easy to assess and is widely used in the epidemiological literature, only modestly correlates with prospective sleep diaries and correlates weakly with actigraphy-derived sleep duration [41–44]. Nonetheless, self-reported information describes patient-centered concerns, which increasingly are recognized as relevant for clinical care and for directing therapeutic intervention, and may provide a more representative measure of chronic sleep patterns. Although further elucidation of possible differences in the mechanisms that relate alternative sleep phenotypes to chronic health conditions is needed, our results suggest that identifying short sleep by either self-report or PSG may improve characterization of a subgroup with insomnia or poor sleep at increased CVD risk.
We analyzed our exposures as both unique phenotypes as well as tested for statistical interactions between each “exposure.” We did not demonstrate significant statistical interactions between these variables, possibly due to limited power. In contrast, we showed distinct associations for categorical definitions of insomnia or poor sleep with/without short sleep, consistent with the effect of a unique “short sleep/insomnia” phenotype.
Our study has several strengths, including the prospective design, relatively large sample of men and women, ability to adjust for sleep-disordered breathing, and rigorous adjudication of CVD events. Nevertheless, there are also several limitations that merit consideration. Objective sleep duration was determined by a single-night PSG and may not represent habitual sleep, but rather may identify individuals who have high levels of stress reactivity in response to PSG. Our PSG data were derived from at-home recordings, and though patients may be more comfortable in their own homes than in-laboratory, patients with insomnia may experience improved sleep in the laboratory setting (i.e. reverse night effects) [48] and are expected to display more variability in sleep in the home setting [49]. It should also be noted that in our study, insomnia or poor sleep was defined using self-reported questions ascertaining information on symptoms and sleep medication use, and not on clinical assessment or diagnostic criteria, and therefore, our exposed group did not uniformly represent those with insomnia disorder. The requirement for daytime impairment or use of clinical diagnostic assessment may identify a more severe group than we characterized. Nonetheless, the questionnaires were administered using standardized procedures and our definition of insomnia included specific questions on symptoms that comprise much of the current diagnostic criteria. We did not have sufficient information to classify the chronicity or severity of insomnia or poor sleep, which may have further improved characterization. Although we adjusted for a number of key potential confounders, we did not have consistent data on alcohol use and only limited data were available to assess depression or other mood disorders. Data were not consistently available on medication use on the night of the PSG, precluding an assessment of acute medication effects on PSG sleep parameters.
Conclusions
Our study indicates that insomnia or poor sleep with objective short sleep duration reflects a vulnerable phenotype associated with a higher risk of CVD. Our findings support the need to characterize both objective sleep duration and insomnia symptoms in risk assessments. Further research aimed at elucidating the metabolic and vascular correlates of insomnia or poor sleep and short sleep will improve our understanding of the CVD risk associated with these findings. The study supports studies of interventions targeting both sleep problems as levers for minimizing CVD.
Supplementary Material
Supplementary material is available at SLEEP online.
Funding
This work was supported by grants U01HL53916, U01HL53931, U01HL53934, U01HL53937, U01HL53938, U01HL53940, U01HL53941, and U01HL64360 from the National Institutes of Health (NIH) and supported in part by NIH R35 HL135818 and R24HL114473.
Supplementary Material
Work Performed: Beth Israel Deaconess Medical Center and Tulane University School of Public Health and Tropical Medicine
References
- 1. Kochanek KD MS, et al. Deaths: Final data for 2014.National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 2. Mozaffarian D, et al. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 2008;117(23):3031–3038. [DOI] [PubMed] [Google Scholar]
- 3. Javaheri S, et al. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levenson JC, et al. The pathophysiology of insomnia. Chest. 2015;147(4):1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. St-Onge MP, et al. ; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cappuccio FP, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, et al. Sleep duration and chronic diseases among U.S. adults age 45 years and older: evidence from the 2010 behavioral risk factor surveillance system. Sleep. 2013;36(10):1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Consensus Conference P, et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11:931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laugsand LE, et al. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–1393. [DOI] [PubMed] [Google Scholar]
- 10. Laugsand LE, et al. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. [DOI] [PubMed] [Google Scholar]
- 11. Vgontzas AN, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63. [DOI] [PubMed] [Google Scholar]
- 13. Floam S, et al. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. [DOI] [PubMed] [Google Scholar]
- 14. D’Aurea C, et al. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 2015;73(6): 516–519. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, et al. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34(2):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castro-Diehl C, et al. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39(11):1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller CB, et al. An objective short sleep insomnia disorder subtype is associated with reduced brain metabolite concentrations in vivo: a preliminary magnetic resonance spectroscopy assessment. Sleep. 2017. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015. Mar;65(3): 644–650. [DOI] [PubMed] [Google Scholar]
- 19. Vgontzas AN, et al. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bathgate CJ, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez-Mendoza J, et al. Insomnia with objective short sleep duration and incident hypertension: the penn state cohort. Hypertension. 2012;60(4):929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vgontzas AN, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nieto EJ, et al. The sleep heart health study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 24. Group SHHSR. Sleep Heart Health Study Manual of Operation. Seattle, WA: SHHS Coordinating Center; 1996. [Google Scholar]
- 25. Redline S, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 26. Redline S, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 27. Whitney CW, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21(7):749–757. [DOI] [PubMed] [Google Scholar]
- 28. Gottlieb DJ, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Punjabi NM, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Investigators A. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 31. Cupples LA. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, eds. The Framingham Heart Study. DARAEIoCDW, D.C: Government Printing Office; 1987: 9–13. [Google Scholar]
- 32. Ives DG, et al. Surveillance and ascertainment of cardiovascular events. The cardiovascular health study. Ann Epidemiol. 1995;5(4):278–285. [DOI] [PubMed] [Google Scholar]
- 33. Cappuccio FP, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spreeuwenberg MD, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care. 2010;48(2):166–174. [DOI] [PubMed] [Google Scholar]
- 35. Rothman K. GSMEneP. PA: Lippincott Williams & Wilkins; 1998. 340–342. [Google Scholar]
- 36. Johann AF, et al. Insomnia with objective short sleep duration is associated with longer duration of insomnia in the Freiburg Insomnia Cohort compared to insomnia with normal sleep duration, but not with hypertension. PLoS One. 2017;12(7):e0180339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vasisht KP, et al. Differences in insulin secretion and sensitivity in short-sleep insomnia. Sleep. 2013;36(6):955–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chandola T, et al. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33(6):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rod NH, et al. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4):e91965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sands-Lincoln M, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22(6):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sivertsen B, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chien KL, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Helbig AK, et al. Symptoms of insomnia and sleep duration and their association with incident strokes: findings from the population-based MONICA/KORA Augsburg Cohort Study. PLoS One. 2015;10(7):e0134480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalmbach DA, et al. DSM-5 insomnia and short sleep: comorbidity landscape and racial disparities. Sleep. 2016;39(12):2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandez-Mendoza J, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vgontzas AN, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. [DOI] [PubMed] [Google Scholar]
- 47. Vgontzas AN, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45(1):21–31. [DOI] [PubMed] [Google Scholar]
- 48. Hauri PJ, et al. Reverse first night effect in insomnia. Sleep. 1989;12(2):97–105. [DOI] [PubMed] [Google Scholar]
- 49. Edinger JD, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.